Abstract
Introduction
Current health claims recognize the ability of oat ß-glucan to lower blood cholesterol; however, its ability to improve glycemic control is less certain. We undertook a systematic review and meta-analysis of randomized controlled trials (RCTs) to update the evidence on the effect of oats and oat ß-glucan on glycemic control in individuals with diabetes.
Research design and methods
MEDLINE, EMBASE and Cochrane were searched (June 2021) for RCTs of ≥2 weeks investigating the effect of oat ß-glucan on glycemic control in diabetes. The outcomes were hemoglobin A1c (HbA1c), fasting glucose, 2-hour postprandial glucose (2h-PG) from a 75 g oral glucose tolerance test, homeostatic model assessment of insulin resistance (HOMA-IR) and fasting insulin. Independent reviewers extracted the data and assessed the risk of bias. Data were pooled using the generic inverse variance method. Heterogeneity was assessed (Cochran Q) and quantified (I2). Pooled estimates were expressed as mean difference (MD) with 95% CI. The certainty of evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluations approach.
Results
Eight trial comparisons (n=407) met the eligibility criteria. All trials were in adults with type 2 diabetes who were predominantly middle-aged, overweight and treated by antihyperglycemic medications or insulin. A median dose of 3.25 g of oat ß-glucan for a median duration of 4.5 weeks improved HbA1c (MD, −0.47% (95% CI −0.80 to −0.13), pMD=0.006), fasting glucose (−0.75 mmol/L (−1.20 to –0.31), pMD<0.001), 2h-PG (−0.42 mmol/L (−0.70 to –0.14), pMD=0.003) and HOMA-IR (−0.88 (−1.55 to –0.20), pMD=0.011). There was a non-significant reduction in fasting insulin (−4.30 pmol/L (−11.96 to 3.35), pMD=0.271). The certainty of evidence was high for fasting glucose, moderate for HOMA-IR and fasting insulin (downgraded for imprecision), and low for HbA1c and 2h-PG (downgraded for imprecision and inconsistency).
Conclusions
Consumption of oats and oat ß-glucan results in generally small improvements in established markers of fasting and postprandial glycemic control beyond concurrent therapy in adults with type 2 diabetes. The current evidence provides a very good indication for reductions in fasting glucose and less of an indication for reductions in HbA1c, 2h-PG, fasting insulin and HOMA-IR in this population.
Trial registration number
Keywords: Diabetes Mellitus, Type 2; Nutritional Sciences
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Oat ß-glucan has been recognized for its ability to lower cholesterol and reduce postprandial glycemic response, with approved health claims in Canada, USA and/or Europe.
Systematic reviews and meta-analyses of randomized controlled trials have shown beneficial effects of oats and oat ß-glucan on glycemic control in diabetes, but are out of date, limited in their measures of glycemic control, and lack dose response analyses and assessments of certainty of evidence.
WHAT THIS STUDY ADDS
Our synthesis of eight randomized controlled trial comparisons in 407 adults with type 2 diabetes who were predominantly middle-aged, overweight or obese and with moderately controlled diabetes treated by antihyperglycemic medications or insulin showed that oats or oat ß-glucan at a median ß-glucan dose of 3.25 g over a median study duration of 4.5 weeks resulted in small important improvements in hemoglobin A1c (HbA1c) and fasting glucose and more trivial improvements in 2-hour postprandial glucose (2h-PG), fasting insulin and homeostatic model assessment of insulin resistance (HOMA-IR) beyond concurrent therapy.
The certainty of evidence was high for fasting glucose, moderate for HOMA-IR and fasting insulin, and low for HbA1c and 2h-PG, owing to downgrades for imprecision and/or inconsistency.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Current evidence suggests that oats and oat ß-glucan improve fasting and postprandial glycemic profiles beyond concurrent therapy, suggesting that oats and oat ß-glucan may be a useful add-on therapy for diabetes management, supporting current diabetes recommendations and providing a basis for the development of new health claims.
Introduction
Sustained glycemic control in order to reduce the risk of long-term complications remains a diabetes management challenge but can be supported by dietary therapies.1 2 Viscous dietary fibers have been recognized as an important component of lifestyle management strategies for diabetes owing to their health benefits in improving glycemic control and reducing cardiovascular disease risk.3 The viscosity of fiber is also an important factor contributing to reducing postprandial glycemia.4 ß-glucan found in oats is a viscous fiber that has been particularly recognized for its ability to lower low-density lipoprotein cholesterol, with approved health claims from Health Canada, the US Food and Drug Administration and the European Food Safety Authority (EFSA).5–7 Recently, EFSA has also recognized oat ß-glucan for its ability to reduce postprandial glycemic response.8 Whether these postprandial reductions are sustainable and translate into longer term improvements in glycemic control is unclear.
Previous systematic reviews and meta-analyses have shown that viscous fiber supplements that include oat ß-glucan can improve glycemic control outcomes in type 2 diabetes. These syntheses, however, are now out of date, with the most recent census over 3 years old9 and have either not isolated the effect of oat ß-glucan9 or have been limited in the measures used to assess glycemic control.10 11 Important dose response meta-regression analyses and assessment of the certainty of evidence have also been lacking.10 11
To address these gaps, our aim was to conduct a systematic review and meta-analysis to synthesize the currently available evidence from randomized controlled trials (RCTs) on the effects of oats and oat ß-glucan fiber on measures of glycemic control, hepatic insulin sensitivity, whole body insulin sensitivity and beta-cell function, and assess the certainty of evidence using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach.
Methods
We followed the Cochrane Handbook for Systematic Reviews of Interventions12 to conduct this systematic review and meta-analysis and reported our results in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines13 (online supplemental table 1). The protocol is registered at ClinicalTrials.gov (NCT04631913).
bmjdrc-2022-002784supp001.pdf (1.8MB, pdf)
Data sources and searches
We searched MEDLINE, EMBASE and Cochrane Central Register of Controlled Trials databases through June 6, 2021. Online supplemental table 2 outlines the detailed search strategy. Validated filters from the McMaster University Health Information Research Unit were applied to limit the database search to controlled studies only.14 Manual searches of the reference lists of included studies supplemented the systematic search.
Study selection
We included RCTs in human adults with diabetes, with a study duration of ≥2 weeks, that investigated the effects of oats or oat ß-glucan compared with a suitable non-oat control (ie, non-viscous fiber, placebo or standard background diet without added oats or oat ß-glucan) on markers of glycemic control, insulin sensitivity and beta-cell function. No restrictions were placed on language.
Data extraction and quality assessment
Two reviewers (VC and AA) independently extracted relevant data from eligible studies. Extracted data included study design, randomization, blinding, setting, funding sources, study duration, number of participants, participant characteristics (age, body mass index (BMI), sex and health status), oat ß-glucan dose, oat ß-glucan molecular weight, intervention form (whole grain oat or oat ß-glucan), intervention food matrix, intervention and comparator energy comparison (isocaloric, hypocaloric or hypercaloric), available carbohydrate, food form, macronutrient profile, and outcome data. In the absence of numerical data, we extracted values from figures using a PlotDigitizer tool.15 If multiple control arms were reported with negative (usual care or no treatment) and positive (guideline-based treatment) controls, we selected the negative control arm as the comparator. If outcome data in a single trial were reported for multiple intervention study durations, we extracted data from the study duration that contained data for the most outcomes of interest; if this was the same, we used the longer study duration. When the ß-glucan content was not provided, we estimated ß-glucan dose from oats and oat bran at 5.0% and 6.9%, respectively.16 17 We extracted mean differences (MDs) and standard errors (SEs) between the intervention and comparator arms from each applicable trial comparison. When these were not provided, they were calculated from the available data using published formulas.12 MDs for change from baseline were preferred over change in end values. Authors were contacted if relevant data were missing from publications.
The same two investigators (VC and AA) assessed all included studies for risk of bias using the Cochrane Risk of Bias V.2.0 tool.18 We assessed risk of bias from five domains, namely randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome and selection of the reported results. The tool provides a judgment of ‘low risk of bias’, ‘some concerns’ or ‘high risk of bias’ for each domain based on responses to signaling questions. An overall risk of bias was determined based on judgments from each domain. We resolved discrepancies in data extraction and risk of bias by consensus and review with a third investigator (JLS).
Outcomes
Outcomes included established markers of glycemic control (hemoglobin A1c (HbA1c), fasting glucose, fasting insulin, 2-hour postprandial glucose (2h-PG) from a 75 g oral glucose tolerance test (OGTT)), and measures of hepatic insulin sensitivity (homeostatic model assessment of insulin resistance (HOMA-IR), hyperinsulinemic-euglycemic clamp), whole body insulin sensitivity (Matsuda OGTT-Insulin Sensitivity Index, frequently sampled intravenous glucose tolerance test, hyperinsulinemic-euglycemic clamp) and beta-cell function (Insulin Secretion-Sensitivity Index 2).
Data synthesis and analysis
STATA 16.1 was used for all analyses. We expressed the pooled effect estimates for all outcomes as MD with 95% CI. Data were pooled using the generic inverse variance method with DerSimonian and Laird random effect models.19 Fixed effects were used when less than five trial comparisons were available for an outcome.20 A paired analysis was applied for crossover designs and for within-arm mean differences in parallel designs with an assumed correlation coefficient of 0.5.21 22 To mitigate a unit-of-analysis error, when arms of trials with multiple intervention or control arms were used more than once, the corresponding sample size was divided accordingly.12
Heterogeneity was assessed using the Cochran Q statistics and quantified using the I2 statistics, where I2 ≥50% and pQ<0.100 were used as evidence of significant substantial heterogeneity.12 Potential sources of heterogeneity were explored using sensitivity analysis. Sensitivity analyses were done via two methods. We conducted an influence analysis by systematically removing one trial comparison at a time and recalculating the overall effect estimate and heterogeneity. We conducted a second sensitivity analysis by changing the assumed correlation coefficients used for paired analysis from 0.5 to 0.25 and 0.75. Linear dose response analyses were assessed by random effects with restricted maximum likelihood methods. Non-linear dose response was modeled with restricted cubic spline with three knots at Harrell’s recommended percentiles.23
If ≥10 trial comparisons were available, subgroup analyses were conducted using meta-regression (significance at p<0.05). A priori subgroup analyses were conducted by dose, comparator, intervention form, study duration, baseline level, design, body weight change, saturated fat intake, carbohydrate intake, protein intake, intervention food matrix, oat ß-glucan molecular weight and risk of bias. If ≥10 studies were available, publication bias was assessed by inspection of contour-enhanced funnel plots and formal testing with Egger’s and Begg’s tests (significance at p<0.10).24–26 If evidence of publication bias was suspected, the Duval and Tweedie trim-and-fill method was performed to adjust for funnel plot asymmetry by imputing missing study data and assess for small-study effects.27
Grading of evidence
We employed the GRADE system to assess the certainty of evidence. The GRADE Handbook28 and GRADEpro V.3.2 software29 were used. The overall certainty in the evidence was graded as either high, moderate, low or very low. Two investigators (VC and AZ) independently performed GRADE assessments for each outcome. RCTs are initially graded as high. Reasons to downgrade include study limitations (risk of bias), inconsistency of results (substantial unexplained interstudy heterogeneity, I2 >50% and pQ<0.10), indirectness of evidence, imprecision and publication bias. The results were considered imprecise if the 95% CI overlapped with the minimally important difference (MID) for each outcome, which was 0.3% for HbA1c, 0.5 mmol/L for fasting glucose, 5 pmol/L for fasting insulin, 0.5 mmol/L for 2h-PG from a 75 g OGTT and 1 for HOMA-IR. The evidence may be upgraded if a significant dose response gradient is detected.
Results
Search results
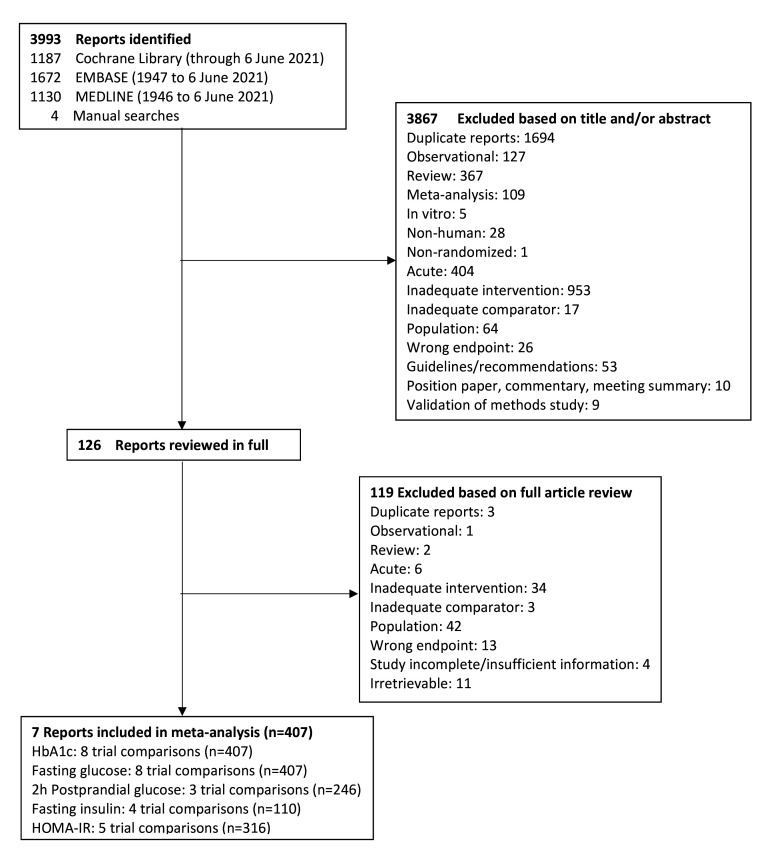
Figure 1 shows the flow of the literature. We identified 3993 reports through database and manual searches. A total of seven reports met the inclusion criteria and contained data for eight trial comparisons involving 407 participants. These included eight trial comparisons for HbA1c and fasting glucose, three for 2h-PG, four for fasting insulin and five for HOMA-IR. No trials were available for other measures of hepatic insulin sensitivity, whole body insulin sensitivity or beta-cell function. All trials included were in individuals with type 2 diabetes as no trials were identified in populations with type 1 diabetes.
Figure 1.
Flow of the literature on the effect of oats and oat ß-glucan on glycemic control and insulin sensitivity. HbA1c, hemoglobin A1c; HOMA-IR, homeostatic model assessment of insulin resistance.
Trial characteristics
Table 1 shows the characteristics of the included trials. The trials were conducted in a variety of locations, with most conducted in China and France (n=2 trial comparisons each), followed by Sweden, Greece, Scotland, Mexico and USA (n=1 each). One trial was conducted in both France and Sweden. The median study size was 35 participants (range 13–140). The median age of the participants was 59 years (range 53–67) and majority of the participants were overweight or obese, with a median BMI of 28.5 kg/m2 (range 25.2–31.5). Participants had a median baseline HbA1c of 7.4% (range 6.8–8.4), based on seven of eight trial comparisons that reported baseline HbA1c. Antihyperglycemic medications were reported in seven of eight trial comparisons, in which 88% of the participants were receiving pharmacotherapy with antihyperglycemic agents (59.9%), insulin (17.6%) or both (10.5%). The median duration of diabetes was 7.3 years (range 3.2–10.1), based on five of eight trial comparisons that reported diabetes duration. Five trial comparisons were parallel design and three were crossover design. Three studies were double-blinded, three were single-blinded and two were open-label. The median study duration was 4.5 weeks (range 3–8). The median oat ß-glucan dose was 3.25 g (range 2–5.5). The intervention was delivered as either whole oats (n=4), oat ß-glucan concentrate (n=3) or oat bran (n=1) and through various food matrices, most commonly porridge and cereal (n=3 each), followed by bread (n=2) and then soup, muffins, cereal bars and cake (n=1 each). The comparators included wheat cereal and/or bread (n=2), soup without added oat ß-glucan (n=1), egg (n=1), no dietary intervention (n=2) or standard dietary advice (n=1). Seven of the eight trials reported on total dietary fiber intake. Five trials were matched for total dietary fiber between the control and intervention groups. Li et al30 had a difference in fiber between the control and intervention groups attributed to the addition of the oat intervention (36.1±4.2 g in the intervention vs 22.1±4.0 g in the control and 39.0±4.8 g in the intervention vs 22.1±4.0 g in the control).
Table 1.
Trial characteristics
| Study, year | n | Design | Blinding | Study duration, weeks | Age, years | BMI, kg/m2 | Baseline HbA1c, % | Antihyperglycemic therapy (n participants) | Diabetes duration, years | Background diet |
Total fiber intake, g | Intervention dose, g | Description | Setting | Funding | Outcomes |
| Stevens et al, 198532 | 25 (M: 8, F: 17) |
P | OL | 6 | I: 53±10.8 |
NR | NR | None | I: 7.7±9.7 |
I: increased fiber diet | I: 11.0 | 3.5 | I: 50 g oat bran as hot cereal or in muffins | OP, USA | A | HbA1c, FG |
| C: 56±10.4 |
C: 3.2±2.8 |
C: usual | C: 11.0 | C: no dietary intervention | ||||||||||||
| Kabir et al, 200236 | 13 (M: 13, F: 0) |
CO | DB | 4 | 59±7.2 | 28±3.6 | I: 8.3±1.80 |
Medication* (12); diet (1) | NR | Usual | NR† | 3 | I: cereal with muesli containing 3 g oat ß-glucan | OP, France | A, IN | HbA1c, FG, FI |
| C: 8.1±1.08 |
NR† | C: wheat cereal and bread | ||||||||||||||
| Cugnet-Anceau et al, 201031 | 53 (M: 32, F: 21) |
P | DB | 8 | I: 62±9.1 |
I: 30.5±4.1 |
I: 7.3±0.92 |
Insulin or medication (9); diet and/or medication (20) | NR | Usual | I: 19.7±5.3 |
3.5 | I: oat ß-glucan-enriched soup | OP, Sweden and France | A | HbA1c, FG |
| C: 62±7.5 |
C: 29.0±4.1 |
C: 7.5±1.29 |
Insulin or medication (12); diet and/or medication (12) | C: 22.3±12 |
C: soup without oat ß-glucan | |||||||||||
| Liatis et al, 200934 | 41 (M: 23, F: 18) |
P | DB | 3 | I: 60±9.1 |
I: 29.6±4.8 |
I: 7.3±1.61 |
Medication* (19); diet (4) | I: 7.6±6 |
General dietary instruction | NR | 3 | I: oat ß-glucan-enriched bread | OP, Greece | NR | HbA1c, FG, FI, HOMA-IR |
| C: 67±8.9 |
C: 27.0±3.7 |
C: 6.9±1.50 |
Medication* (15); diet (3) | C: 10.1±8.2 |
NR | C: bread made with wheat flour | ||||||||||
| McGeoch et al, 201333 | 27 (M: 18, F: 9) |
CO | OL | 8 | 61±6.4 | 31.5±5.1 | 6.8±0.14 | NR | 3.1±0.7 | I: oat-enriched diet | I: 23.9 | 5.5 | I: carbohydrate content substituted for oat-based product | OP, Scotland | A | HbA1c, FG, FI, 2h-PG, HOMA-IR |
| C: healthy eating recommendations | C: 25.4 | C: standard dietary advice and avoid oats | ||||||||||||||
| Ballesteros et al, 201535 | 29 (M: 10, F: 19) |
CO | SB | 5 | 54±8.3 | I: 30.8±6.5 |
6.8±0.89 | Medication* (26); insulin (6) | NR | Usual | I: 27.5±9.2 |
2.0 | I: 40 g oatmeal | OP, Mexico | IN | HbA1c, FG, FI, HOMA-IR |
| C: 30.8±6.4 |
C: 26.0±10.1 |
C: 1 egg | ||||||||||||||
| Li et al, 201630 | 140 (M: 80, F: 60) |
P | SB | 4 | I: 59±6.1 |
I: 26.9±2.7 |
I: 8.4±1.44 |
Medication* (43); insulin (16); combined (15) | I: 8.3±6.3 |
I: low-fat and high-fiber | I: 36.1±4.2 |
2.65 | I: 50 g whole grain oats | IP, China | IN | HbA1c, FG, 2h-PG, HOMA-IR |
| C: 59±3.9 |
C: 25.2±0.9 |
C: 8.1±1.52 |
Medication* (32); insulin (12); combined (11) | C: 6.6±3.0 |
C: usual | C: 22.1±4 |
C: no dietary intervention | |||||||||
| 139 (M: 72, F: 67) |
P | SB | 4 | I: 59±6.8 |
I: 27.4±2.4 |
I: 8.3±1.35 |
Medication* (47); insulin (12); combined (14) | I: 7.9±6.4 |
I: low-fat and high-fiber | I: 39.0±4.8 |
5.30 | I: 100 g whole grain oats | IP, China | IN | HbA1c, FG, 2h-PG, HOMA-IR | |
| C: 59±3.9 |
C: 25.2±0.9 |
C: 8.1±1.52 |
Medication* (32); insulin (12); combined (11) | C: 6.6±3.0 |
C: usual | C: 22.1±4 |
C: no dietary intervention |
*Specified oral agent.
†Treatments matched for fiber.
A, agency; BMI, body mass index; C, control; CO, crossover; DB, double-blind; F, female; FG, fasting glucose; FI, fasting insulin; HbA1c, hemoglobin A1c; HOMA-IR, homeostatic model assessment of insulin resistance; 2h-PG, 2-hour postprandial glucose from a 75 g oral glucose tolerance test; I, intervention; IN, industry; IP, inpatient; M, male; NR, not reported; OL, open-label; OP, outpatient; P, parallel; SB, single-blind.
Risk of bias
Online supplemental tables 3–7 and online supplemental figures 1–5 show the risk of bias assessments of the individual trials by the Cochrane Risk of Bias V.2.0 tool. There were some concerns due to missing outcome data for one trial31 and randomization in another trial32 for HbA1c and fasting glucose. All other trials were determined to be low risk of bias across all domains.
Hemoglobin A1c
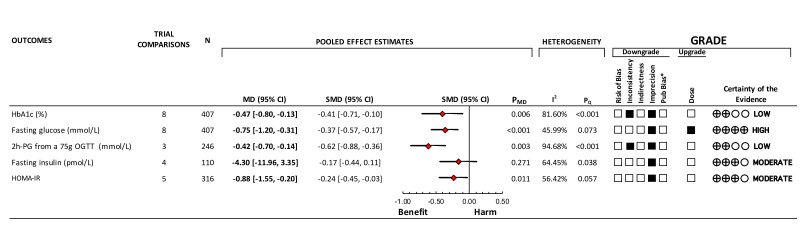
Figure 2 and online supplemental figure 6 show the effect of oats and oat ß-glucan on HbA1c. In eight trial comparisons involving 407 participants with a median study duration of 4.5 weeks, oats and oat ß-glucan reduced HbA1c (MD, −0.47% (95% CI −0.80 to −0.13), pMD=0.006), with significant substantial heterogeneity (I2=81.60%, pQ<0.001).
Figure 2.
Summary plot of the effect of oats and oat ß-glucan on glycemic control and insulin sensitivity. Data are expressed as weighted MD with 95% CI using the generic inverse variance method modeled by random effects (≥5 trials available) or fixed effects (<5 trials available). To allow for the pooled effect estimates for each outcome to be displayed on the same axis, MDs were transformed to SMDs. Pseudo-95% CIs for each transformed SMD were derived directly from the original MD and 95% CI. Between-study heterogeneity was assessed by the Cochran Q statistics, where p<0.100 is considered statistically significant, and quantified by the I2 statistics, where I2 ≥50% is considered evidence of substantial heterogeneity.60 The GRADE of randomized controlled trials is rated as ‘high’ certainty of evidence and can be downgraded by five domains and upgraded by one domain. The filled black squares indicate downgrade and/or upgrade for each outcome. *Unable to assess publication bias due to <10 studies per outcome. GRADE, Grading of Recommendations, Assessment, Development and Evaluations; HbA1c, hemoglobin A1c; HOMA-IR, homeostatic model assessment of insulin resistance; 2h-PG, 2-hour postprandial glucose; MD, mean difference; OGTT, oral glucose tolerance test; SMD, standardized mean difference.
Online supplemental figure 7 and online supplemental table 8 show the results of the sensitivity analysis for HbA1c. Removal of individual trials did not alter the magnitude, direction or significance of the effect estimate (range of MD, from −0.57% (95% CI −1.00 to −0.14) to −0.24% (95% CI −0.44 to −0.04)). Changing the correlation coefficient to 0.25 or 0.75 did not alter the magnitude, direction or significance of the effect estimate or the evidence for heterogeneity.
Online supplemental figure 8 shows the dose response analysis for HbA1c. No significant linear or non-linear dose response was observed (p≥0.05).
Fasting glucose
Figure 2 and online supplemental figure 9 show the effect of oats and oat ß-glucan on fasting glucose. In eight trial comparisons involving 407 participants with a median study duration of 4.5 weeks, oats and oat ß-glucan reduced fasting glucose (MD, −0.75 mmol/L (95% CI −1.20 to −0.31), pMD<0.001), with no evidence of substantial heterogeneity (I2=45.99%, pQ=0.073).
Online supplemental figure 10 and online supplemental table 8 show the results of the sensitivity analysis for fasting glucose. Removal of individual trials did not alter the magnitude, direction or significance of the effect estimate (range of MD, from −0.87 mmol/L (95% CI −1.37 to −0.37) to −0.61 mmol/L (95% CI −0.97 to −0.25)). Changing the correlation coefficient from 0.5 to 0.25 or 0.75 did not alter the magnitude, direction or significance of the effect estimate. Changing the correlation coefficient from 0.5 to 0.75 changed the evidence for heterogeneity from not substantial to substantial (I2=57.65%, pQ=0.021).
Online supplemental figure 11 shows the dose response analysis for fasting glucose. A significant linear dose response relationship was observed indicating a 0.39 mmol/L reduction in fasting glucose per 1 g oat ß-glucan (slope=−0.39 (95% CI −0.64 to –0.14), p<0.001). There was no evidence of a non-linear dose response (p=0.125).
2h-PG from a 75 g OGTT
Figure 2 and online supplemental figure 12 show the effect of oats and oat ß-glucan on 2h-PG. In three trial comparisons involving 246 participants with a median study duration of 4 weeks, oats and oat ß-glucan reduced 2h-PG (MD, −0.42 mmol/L (95% CI −0.70 to −0.14), pMD=0.003), with significant substantial heterogeneity (I2=94.68%, pQ<0.001).
Online supplemental figure 13 and online supplemental table 8 show the sensitivity analysis for 2h-PG. Removal of McGeoch et al33 did not alter the direction or significance of the effect, but did increase the magnitude (MD, −2.87 mmol/L (95% CI −3.70 to –2.04), p<0.001) and explained the heterogeneity (I2 <0.01%, pQ=0.605). Changing the correlation coefficient to 0.25 or 0.75 did not alter the magnitude, direction or significance of the effect estimate or the heterogeneity.
Online supplemental figure 14 shows the dose response analysis for 2h-PG. No significant linear dose response was observed. A non-linear dose response was not modeled due to an insufficient number of trial comparisons.
Fasting insulin
Figure 2 and online supplemental figure 15 show the effect of oats and oat ß-glucan on fasting insulin. In four trial comparisons involving 110 participants with a median study duration of 4.5 weeks, oats and oat ß-glucan reduced fasting insulin (MD, −4.30 pmol/L (95% CI −11.96 to 3.35), pMD=0.271), with significant substantial heterogeneity (I2=64.45%, pQ=0.038).
Online supplemental figure 16 and online supplemental table 8 show the results of the sensitivity analysis for fasting insulin. Removal of individual trials did not alter the magnitude, direction or significance of the effect estimate (range of MD, from −9.07 pmol/L (95% CI −23.39 to 5.26) to −2.23 pmol/L (95% CI −10.09 to 5.63)). Removal of Liatis et al34 explained the substantial heterogeneity (MD, −2.23 (95% CI −10.09 to 5.63), pMD=0.579, I2=39.42%, pQ=0.192). Changing the correlation coefficient from 0.5 to 0.25 or 0.75 did not alter the magnitude, direction or significance of the effect estimate or the evidence for heterogeneity.
Online supplemental figure 17 shows the dose response analysis for fasting insulin. No significant linear dose response was observed. A non-linear dose response was not modeled due to an insufficient number of trial comparisons.
Homeostatic model assessment of insulin resistance
Figure 2 and online supplemental figure 18 show the effect of oats and oat ß-glucan on HOMA-IR. In five trial comparisons involving 316 participants with a median study duration of 4 weeks, oats and oat ß-glucan reduced HOMA-IR (MD, −0.88 (95% CI −1.55 to −0.20), pMD=0.011), with significant substantial heterogeneity (I2=56.42%, pQ=0.057).
Online supplemental figure 19 and online supplemental table 8 show the results of the sensitivity analysis for HOMA-IR. Removal of individual trials did not alter the magnitude of the effect estimate or the direction or significance of the effect (range of MD, from −1.56 (95% CI −2.80 to −0.31) to −0.65 (95% CI −1.17 to −0.12)). Removal of Liatis et al34 explained the substantial heterogeneity (MD, −0.65 (95% CI −1.17 to −0.12), pMD=0.016, I2=41.82%, pQ=0.161). Changing the correlation coefficient to 0.25 or 0.75 did not alter the magnitude, direction or significance of the effect estimate or the heterogeneity.
Online supplemental figure 20 shows the dose response analysis for HOMA-IR. No significant linear dose response was observed. A non-linear dose response was not modeled due to an insufficient number of trial comparisons.
Subgroup analyses and publication bias
As <10 trial comparisons were available for each outcome, sources of heterogeneity were not explored in subgroup analyses and publication bias was not assessed.
GRADE assessment
Online supplemental table 9 shows the certainty of evidence for each outcome assessed by GRADE. The certainty of evidence was rated as low for HbA1c and 2h-PG due to inconsistency and imprecision of the pooled effect estimates, moderate for fasting insulin and HOMA-IR due to imprecision of the pooled effect estimates, and high for fasting glucose owing to a downgrade for imprecision of the pooled effect estimate and upgrade for a linear dose response gradient. We did not downgrade the evidence for serious inconsistency for either fasting insulin or HOMA-IR, as the evidence of substantial heterogeneity was explained through influence analysis with the removal of Liatis et al.34 Although we were able to explain the evidence of substantial heterogeneity by influence analysis for 2h-PG, there were insufficient trial comparisons to warrant not downgrading.
Discussion
We conducted a systematic review and meta-analysis of seven RCTs involving eight trial comparisons of the effect of oats and oat ß-glucan at a median oat ß-glucan dose of 3.25 g on markers of glycemic control and insulin sensitivity over a median study duration of 4.5 weeks in 407 adults with type 2 diabetes who were predominantly middle-aged, overweight or obese and with moderately controlled diabetes treated by antihyperglycemic medications or insulin. No data were available in type 1 diabetes. We showed that oat ß-glucan intake resulted in small important reductions in HbA1c and fasting glucose and more trivial reductions in 2h-PG, fasting insulin (not statistically significant) and HOMA-IR beyond concurrent therapy. There was a significant linear dose response gradient in fasting glucose indicating a 0.39 mmol/L reduction in fasting glucose per 1 g oat ß-glucan.
Results in the context of the literature
Three previous systematic reviews and meta-analyses assessed the role of viscous fiber from oats on glycemic markers in individuals with diabetes. The first two syntheses by Shen et al11 and Hou et al,10 which focused exclusively on oat ß-glucan, showed reductions in HbA1c and fasting glucose and non-significant reductions in fasting insulin and HOMA-IR. The magnitude of the reductions in HbA1c (MD, −0.42% and MD, −0.21% vs MD, −0.47%) and fasting glucose (MD, −0.39 mmol/L and MD, −0.52 mmol/L vs MD, −0.75 mmol/L) became greater and the reduction in HOMA-IR became significant in our updated synthesis which captured four more eligible trials30 32 33 35 than Shen et al11 and up to three more eligible trials30 32 33 35 in some glycemic control outcomes than Hou et al.10 The third synthesis by Jovanovski et al9 investigated the effects of total viscous fiber sources (ß-glucan from oats or barley, guar gum, konjac, psyllium, pectin, xanthan gum, locust bean gum, and alginate and agar) on glycemic control in individuals with diabetes. It showed no effect modification by viscous fiber type, suggesting a ‘class-effect’ such that the reductions seen in HbA1c, fasting glucose and HOMA-IR held across the different fiber types including ß-glucan.9 There was no effect of viscous fiber or effect modification by viscous fiber type on insulin. Three fewer trial comparisons in HbA1c and fasting glucose32 35 36 and two fewer trial comparisons in HOMA-IR30 35 were included in their pooled effect estimate as they explored only supplemental sources of ß-glucan.
Several other systematic reviews and meta-analyses have assessed the effects of oats and oat products on measurements of glycemic control in mixed populations with and without diabetes.37–39 These syntheses, some of which missed several eligible trials in individuals with diabetes,30–36 showed significant reductions in fasting insulin but failed to show consistent significant reductions in HbA1c, fasting glucose or HOMA-IR, suggesting the effects may be more evident in individuals with diabetes.
None of the previous systematic reviews and meta-analyses assessed the effect of oat ß-glucan on 2h-PG. As an important contributor to HbA1c and risk of diabetes complications,40 the reduction seen in 2h-PG in the present synthesis supports the reductions seen in other established markers of glycemic control in individuals with diabetes.
One of the eight included trials (Ballesteros et al35) used a comparator of eggs rather than a similar food without oat ß-glucan, no dietary intervention or standard dietary advice; sensitivity analyses demonstrated that the magnitude, direction and significance of the effect for all outcomes were not altered by the removal of Ballesteros et al (online supplemental figures 7, 10, 13, 19).
Total fiber interventions, including a combination of insoluble fiber and viscous soluble fiber, have been shown to improve markers of glycemic control.41 42 A systematic review and meta-analysis of RCTs demonstrated that increased dietary fiber significantly improved HbA1c by −0.66%, fasting glucose by −0.80 mmol/L, insulin by −11.67 pmol/L and HOMA-IR by −1.27 in individuals living with diabetes.42 Synthesis of RCTs has also shown that viscous soluble fiber supplementation significantly improved HbA1c by −0.58%, fasting glucose by −0.82 mmol/L and HOMA-IR by −1.89 in individuals living with diabetes.9 Our results for oat ß-glucan support the benefits provided by viscous soluble fibers.
The mechanism by which ß-glucan improves blood glucose control is thought to relate to its effect on postprandial absorption and metabolism of carbohydrates. Its ability to increase intestinal viscosity and consequently slow the rate of gastric emptying and the absorption of carbohydrate has the effect of decreasing the postprandial glycemic response to the carbohydrate contained in a meal.43 Systematic reviews and meta-analyses of acute RCTs have shown a strong linear relationship between reductions in postprandial glucose following a carbohydrate meal and an increase in the dose and molecular weight of ß-glucan.44 45 The alpha-glucosidase inhibitor acarbose provides important biological analogy as an oral prandial agent that effectively converts the diet to a low glycemic index (GI) diet by decreasing the absorption of the carbohydrate contained in a meal, thereby decreasing the acute postprandial glycemic response.46–49 Large RCTs and systematic reviews and meta-analyses of RCTs of acarbose have shown similar reductions in HbA1c and fasting glucose in individuals at risk of50 51 and with type 2 diabetes,52 53 which have translated into reductions in incident diabetes, hypertension, cardiovascular disease (CVD), myocardial infarction and stroke in individuals at risk of type 2 diabetes50 51 (although the data are only suggestive for cardiovascular benefit, as a recent trial failed to confirm the reduction in cardiovascular events with a lower dose of acarbose in Chinese adults who were at risk of diabetes and had pre-existing coronary disease54). Like low GI foods or acarbose, high ß-glucan foods which may also have lower GI can be expected to reduce diabetes incidence in individuals at risk of diabetes and improve management of HbA1c, and decrease CVD risk in individuals with established type 2 diabetes.
Strengths and limitations
Our systematic review and meta-analysis has several strengths. First, we completed a comprehensive systematic search of the available literature. Second, we included only RCTs, a study design which provides the greatest protection against systematic error. Third, there was a significant linear dose response gradient for fasting glucose, which increased our certainty of evidence for fasting glucose. Finally, we included a GRADE assessment to explore the certainty of available evidence.
Our analysis also revealed several limitations. There was evidence of inconsistency between the available trials for HbA1c and 2h-PG. We were not able to conduct subgroup analyses to further explore sources of inconsistency as <10 trial comparisons were available for each outcome. The evidence for HbA1c and 2h-PG was therefore downgraded for inconsistency. Another limitation was the serious imprecision in the pooled estimates across all outcomes with the 95% CIs overlapping the MID in each case, leading to further downgrades for imprecision. Finally, although publication bias was not suspected, we were unable to assess publication bias as <10 trial comparisons were available for each outcome.
Weighing these strengths and limitations, the certainty of evidence was assessed as high for fasting glucose due to a downgrade for imprecision and an upgrade for a significant dose relationship, moderate for fasting insulin and HOMA-IR due to a downgrade for imprecision, and low for HbA1c and 2h-PG due to downgrades for both inconsistency and imprecision.
Implications
Maintaining glycemic control is essential in the prevention of diabetes-related microvascular and, to a lesser extent, macrovascular complications. Nutrition therapy is the cornerstone of diabetes management which when optimized can have significant improvements in diabetes control.1 Dietary fibers, particularly viscous fibers, are highlighted as an important part of medical nutrition therapy.1 The average intake of dietary fiber, however, continues to fall more than 30% short of recommendations.55–57 The failure to achieve the recommended intake for health benefits extends to oats. Although oats have seen a 3.5-fold increase in production (as rolled oats) in the 9 years since the approval of the health claim for oats and cholesterol reduction,58 those who consume oatmeal on average consume one cup or 2 g of oat ß-glucan per day, which is well below both the dose required by the health claim (3 g) for cholesterol reduction and the median dose of intake identified in our synthesis (3.25 g) for an improvement in glycemic control.5–7 59 These low intakes suggest that most individuals have an important opportunity to realize the benefits of oats through an increase in intake of oats and oat ß-glucan. Our findings strengthen the indication for the use of whole oats and oat ß-glucan as add-on therapy in the management of people with type 2 diabetes, supporting current clinical practice guidelines1 2 and providing a basis for the future development of health claims for oat ß-glucan and glucose regulation.
Conclusion
Oats and oat ß-glucan consumption over the short-term to moderate-term results in improvements in established markers of fasting and postprandial glycemic control beyond concurrent therapy in adults with type 2 diabetes who were predominantly middle-aged, overweight or obese and with moderately controlled diabetes treated by antihyperglycemic medications or insulin. The available evidence provides a very good indication for small important reductions in fasting glucose and less of an indication for small important reductions in HbA1c and more trivial reductions in 2h-PG, fasting insulin and HOMA-IR in this population. The main sources of uncertainty in the evidence were imprecision and inconsistency. More large, high-quality RCTs are required to improve the precision of the pooled effect estimates and to allow for better exploration and understanding of the sources of inconsistency (heterogeneity) in the estimates between trials.
Footnotes
Contributors: VC acquired the data, performed the data analysis, interpreted the data and drafted the manuscript. AA acquired the data and assisted with data analysis and interpretation. TAK and FA-Y assisted with data analysis and interpretation. AZ, CWCK and JLS were responsible for the conception and design of the study, acquired funding for the study, and guided data analysis and interpretation. All authors contributed to the critical revision of the manuscript for important intellectual content. JLS was responsible for overall study supervision and is the study guarantor. All authors approved the final version of the manuscript. The corresponding author attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was supported by an unrestricted grant from the Quaker Oats Center of Excellence. VC was funded by a University of Toronto Undergraduate Summer Research Award and Toronto 3D Summer Student Award. AZ was funded by a Banting & Best Diabetes Centre Fellowship in Diabetes Care (funded by Eli Lilly). AA was funded by a Toronto 3D MSc Scholarship Award. TAK was funded by a Toronto 3D Postdoctoral Fellowship Award. LC was funded by a Mitacs Elevate Postdoctoral Fellowship Award. JLS was funded by a Diabetes Canada Clinician Scientist Award.
Competing interests: VC has received research support from the University of Toronto and Toronto 3D Knowledge Synthesis and Clinical Trials foundation. AZ is a part-time research associate at INQUIS Clinical Research, a contract research organization, and a consultant for Glycemic Index Foundation, a not-for-profit health promotion charity. She has received funding from the Banting & Best Diabetes Centre. AA has received funding from the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. TAK has received research support from the Canadian Institutes of Health Research (CIHR), the International Life Sciences Institute (ILSI) and the National Honey Board. He has been an invited speaker at the Calorie Control Council annual meeting for which he has received an honorarium. He was received funding from the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. FA-Y is a part-time research assistant at INQUIS Clinical Research, a contract research organization. LC was a Mitacs Elevate postdoctoral fellow jointly funded by the Government of Canada and the Canadian Sugar Institute. She was previously employed as a casual clinical coordinator at INQUIS Clinical Research (formerly Glycemic Index Laboratories), a contract research organization. DJAJ has received research grants from Saskatchewan Pulse Growers, the Agricultural Bioproducts Innovation Program through the Pulse Research Network, Advanced Foods and Materials Network, Loblaw Companies, Unilever, Barilla, Almond Board of California, Agriculture and Agri-Food Canada, Pulse Canada, Kellogg’s Company (Canada), Quaker Oats (Canada), Procter & Gamble Technical Centre, Bayer Consumer Care (Springfield, New Jersey, USA), Pepsi/Quaker, International Nut and Dried Fruit, Soyfoods Association of North America, Coca-Cola Company (investigator-initiated, unrestricted grant), Solae, Hain Celestial, Sanitarium Company, Orafti, International Tree Nut Council Nutrition Research and Education Foundation, Peanut Institute, Soy Nutrition Institute, Canola and Flax Councils of Canada, Calorie Control Council, Canadian Institutes of Health Research, Canada Foundation for Innovation, and Ontario Research Fund; has received in-kind supplies for trials as a research support from the Almond Board of California, Walnut Council of California, Peanut Institute, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (PepsiCo), Pristine Gourmet, Bunge, Kellogg Canada and WhiteWave Foods; has been on the speaker’s panel, served on the scientific advisory board or received travel support or honorariums from the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies, Griffin Hospital (for the development of the NuVal scoring system), Coca-Cola Company, EPICURE, Danone, Diet Quality Photo Navigation, Better Therapeutics (FareWell), Verywell, True Health Initiative, Institute of Food Technologists, Soy Nutrition Institute, Herbalife Nutrition Institute, Saskatchewan Pulse Growers, Sanitarium Company, Orafti, Almond Board of California, International Tree Nut Council Nutrition Research and Education Foundation, Peanut Institute, Herbalife International, Pacific Health Laboratories, Nutritional Fundamentals for Health, Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and the Netherlands, Solae, Kellogg, Quaker Oats, Procter & Gamble, Abbott Laboratories, Dean Foods, California Strawberry Commission, Hain Celestial, PepsiCo, Alpro Foundation, Pioneer Hi-Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, Advanced Foods and Materials Network, Canola and Flax Councils of Canada, Agriculture and Agri-Food Canada, Canadian Agri-Food Policy Institute, Pulse Canada, Saskatchewan Pulse Growers, Soyfoods Association of North America, Nutrition Foundation of Italy, Nutrasource Diagnostics, McDougall Program, Toronto Knowledge Translation Group (St Michael’s Hospital), Canadian College of Naturopathic Medicine, The Hospital for Sick Children, Canadian Nutrition Society, American Society for Nutrition, Arizona State University, Paolo Sorbini Foundation, and the Institute of Nutrition, Metabolism and Diabetes; has received an honorarium from the US Department of Agriculture to present the 2013 WO Atwater Memorial Lecture and the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council; has received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association; and is a member of the International Carbohydrate Quality Consortium. DJAJ’s wife, Alexandra L Jenkins, is a director and partner of Glycemic Index Laboratories, and his sister, Caroline Brydson, received funding through a grant from the St Michael’s Hospital Foundation to develop a cookbook for one of his studies. CWCK has received grants or research support from the Advanced Foods and Materials Network, Agriculture and Agri-Food Canada (AAFC), Almond Board of California, Barilla, Canadian Institutes of Health Research (CIHR), Canola Council of Canada, International Nut and Dried Fruit Council, International Tree Nut Council Research and Education Foundation, Loblaw Brands, Peanut Institute, Pulse Canada and Unilever. He has received in-kind research support from the Almond Board of California, Barilla, California Walnut Commission, Kellogg Canada, Loblaw Companies, Nutrartis, Quaker (PepsiCo), Peanut Institute, Primo, Unico, Unilever, and WhiteWave Foods/Danone. He has received travel support and/or honoraria from Barilla, California Walnut Commission, Canola Council of Canada, General Mills, International Nut and Dried Fruit Council, International Pasta Organisation, Lantmannen, Loblaw Brands, Nutrition Foundation of Italy, Oldways Preservation Trust, Paramount Farms, Peanut Institute, Pulse Canada, Sun-Maid, Tate & Lyle, Unilever and WhiteWave Foods/Danone. He has served on the scientific advisory board for the International Tree Nut Council, International Pasta Organisation, McCormick Science Institute and Oldways Preservation Trust. He is a founding member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD), is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the EASD and is a Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. JLS has received research support from the Canada Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research, Innovation and Science, Canadian Institutes of Health Research (CIHR), Diabetes Canada, PSI Foundation, Banting & Best Diabetes Centre (BBDC), American Society for Nutrition (ASN), INC International Nut and Dried Fruit Council Foundation, National Dried Fruit Trade Association, National Honey Board (the US Department of Agriculture (USDA) Honey ‘Checkoff’ Program), International Life Sciences Institute (ILSI), Pulse Canada, Quaker Oats Center of Excellence, The United Soybean Board (the USDA Soy ‘Checkoff’ Program), The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), and the Nutrition Trialists Fund at the University of Toronto (a fund established by an inaugural donation from the Calorie Control Council). He has received in-kind food donations to support a randomized controlled trial from the Almond Board of California, California Walnut Commission, Peanut Institute, Barilla, Unilever/Upfield, Unico/Primo, Loblaw Companies, Quaker, Kellogg Canada, WhiteWave Foods/Danone and Nutrartis. He has received travel support, speaker fees and/or honoraria from Diabetes Canada, Dairy Farmers of Canada, FoodMinds, International Sweeteners Association, Nestlé, Pulse Canada, Canadian Society of Endocrinology and Metabolism (CSEM), GI Foundation, Abbott, General Mills, Biofortis, ASN, Northern Ontario School of Medicine, INC Nutrition Research & Education Foundation, European Food Safety Authority (EFSA), Comité Européen des Fabricants de Sucre (CEFS), Nutrition Communications, International Food Information Council (IFIC), Calorie Control Council, and Physicians Committee for Responsible Medicine. He has or has had ad hoc consulting arrangements with Perkins Coie, Tate & Lyle, Wirtschaftliche Vereinigung Zucker eV, Danone and INQUIS Clinical Research. He is a member of the European Fruit Juice Association Scientific Expert Panel and former member of the Soy Nutrition Institute (SNI) Scientific Advisory Committee. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Obesity Canada/Canadian Association of Bariatric Physicians and Surgeons. He serves or has served as an unpaid scientific advisor for the Food, Nutrition, and Safety Program (FNSP) and the Technical Committee on Carbohydrates of ILSI North America. He is a member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His wife is an employee of AB InBev. SBM and LAL declare no competing interests.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Primary data files available upon request.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Sievenpiper JLC, C. B.; Dworatzek, P. D.; Freeze, et al. Clinical practice guidelines for the prevention and management of diabetes in Canada: nutrition therapy. Can J Diabetes 2018;2018:S64–79. [Google Scholar]
- 2.American Diabetes Association . 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019;42:S103–23. 10.2337/dc19-S010 [DOI] [PubMed] [Google Scholar]
- 3.Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med 2020;17:e1003053. 10.1371/journal.pmed.1003053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jenkins DJ, Wolever TM, Leeds AR, et al. Dietary fibres, fibre analogues, and glucose tolerance: importance of viscosity. Br Med J 1978;1:1392–4. 10.1136/bmj.1.6124.1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Food Directorate Health Product and Food Branch . Oat products and blood cholesterol lowering Ottawa: health Canada, 2010. Available: https://www.canada.ca/en/health-canada/services/food-nutrition/food-labelling/health-claims/assessments/products-blood-cholesterol-lowering-summary-assessment-health-claim-about-products-blood-cholesterol-lowering.html#shr-pg0
- 6.Food US, Administration D, Health, et al. Food labeling: health claims. Soluble Dietary Fiber From Certain Foods and Coronary Heart Disease [21 CFR Part 101 [Docket No. 01Q–0313]:[ 2002. https://www.govinfo.gov/content/pkg/FR-2002-10-02/pdf/02-25067.pdf [Google Scholar]
- 7.EFSA Panel on Dietetic Products, Nutrition and Allergies . Scientific opinion on the substantiation of a health claim related to oat beta-glucan and lowering blood cholesterol and reduced risk of (coronary) heart disease pursuant to article 14 of regulation (EC) NO 1924/2006. EFSA Journal 2010;8:1885. [Google Scholar]
- 8.EFSA Panel on Dietetic Products, Nutrition and Allergies . Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and “digestive function” (ID 850) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2011;9:2207. [Google Scholar]
- 9.Jovanovski E, Khayyat R, Zurbau A, et al. Should viscous fiber supplements be considered in diabetes control? results from a systematic review and meta-analysis of randomized controlled trials. Diabetes Care 2019;42:755–66. 10.2337/dc18-1126 [DOI] [PubMed] [Google Scholar]
- 10.Hou Q, Li Y, Li L, et al. The metabolic effects of oats intake in patients with type 2 diabetes: a systematic review and meta-analysis. Nutrients 2015;7:10369–87. 10.3390/nu7125536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen XL, Zhao T, Zhou Y, et al. Effect of oat β-glucan intake on glycaemic control and insulin sensitivity of diabetic patients: a meta-analysis of randomized controlled trials. Nutrients 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins J, Thomas J, Chandler J. Cochrane Handbook for systematic reviews of interventions version 6.2. Cochrane 2021. [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilczynski NL, Morgan D, Haynes RB, et al. An overview of the design and methods for retrieving high-quality studies for clinical care. BMC Med Inform Decis Mak 2005;5:20. 10.1186/1472-6947-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohatgi A. WebPlotDigitizer. 4.4 ed, 2020. [Google Scholar]
- 16.Chen WJ, Anderson JW. Soluble and insoluble plant fiber in selected cereals and vegetables. Am J Clin Nutr 1981;34:1077–82. 10.1093/ajcn/34.6.1077 [DOI] [PubMed] [Google Scholar]
- 17.Anderson JW, Bridges SR. Dietary fiber content of selected foods. Am J Clin Nutr 1988;47:440–7. 10.1093/ajcn/47.3.440 [DOI] [PubMed] [Google Scholar]
- 18.Sterne JAC, Savović J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-Analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 20.Tufanaru C, Munn Z, Stephenson M, Aromataris E, et al. Fixed or random effects meta-analysis? common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc 2015;13:196–207. 10.1097/XEB.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 21.Elbourne DR, Altman DG, Higgins JPT, et al. Meta-Analyses involving cross-over trials: methodological issues. Int J Epidemiol 2002;31:140–9. 10.1093/ije/31.1.140 [DOI] [PubMed] [Google Scholar]
- 22.Balk EM, Earley A, Patel K. AHRQ methods for effective health care. empirical assessment of Within-Arm correlation imputation in trials of continuous outcomes. Agency for Healthcare Research and Quality (US): Rockville (MD), 2012. [PubMed] [Google Scholar]
- 23.Harrell FEJ. Regressiong modelling strategies with applications to linear models, logistic regression, and survival analysis: Springer series in statistics. Springer 2001. [Google Scholar]
- 24.Peters JL, Sutton AJ, Jones DR, et al. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 2008;61:991–6. 10.1016/j.jclinepi.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begg CB, Mazumdar M. Operating characteristics of a RANK correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- 27.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. 10.1111/j.0006-341x.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 28.Schünemann HJ, Brożek J, Guyatt G. Grade Handbook for grading quality of evidence and strength of recommendations, 2013
- 29.GDT G. GRADEpro Guideline Development Tool [Software] McMaster University, 2020
- 30.Li X, Cai X, Ma X, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cugnet-Anceau C, Nazare J-A, Biorklund M, et al. A controlled study of consumption of beta-glucan-enriched soups for 2 months by type 2 diabetic free-living subjects. Br J Nutr 2010;103:422–8. 10.1017/S0007114509991875 [DOI] [PubMed] [Google Scholar]
- 32.Stevens J, Burgess MB, Kaiser DL, et al. Outpatient management of diabetes mellitus with patient education to increase dietary carbohydrate and fiber. Diabetes Care 1985;8:359–66. 10.2337/diacare.8.4.359 [DOI] [PubMed] [Google Scholar]
- 33.McGeoch SC, Johnstone AM, Lobley GE, et al. A randomized crossover study to assess the effect of an oat-rich diet on glycaemic control, plasma lipids and postprandial glycaemia, inflammation and oxidative stress in type 2 diabetes. Diabet Med 2013;30:1314–23. 10.1111/dme.12228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liatis S, Tsapogas P, Chala E, et al. The consumption of bread enriched with betaglucan reduces LDL-cholesterol and improves insulin resistance in patients with type 2 diabetes. Diabetes Metab 2009;35:115–20. 10.1016/j.diabet.2008.09.004 [DOI] [PubMed] [Google Scholar]
- 35.Ballesteros MN, Valenzuela F, Robles AE, et al. One egg per day improves inflammation when compared to an Oatmeal-Based breakfast without increasing other cardiometabolic risk factors in diabetic patients. Nutrients 2015;7:3449–63. 10.3390/nu7053449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kabir M, Oppert J-M, Vidal H, et al. Four-week low-glycemic index breakfast with a modest amount of soluble fibers in type 2 diabetic men. Metabolism 2002;51:819–26. 10.1053/meta.2002.33345 [DOI] [PubMed] [Google Scholar]
- 37.Bao L, Cai X, Xu M, et al. Effect of oat intake on glycaemic control and insulin sensitivity: a meta-analysis of randomised controlled trials. Br J Nutr 2014;112:457–66. 10.1017/S0007114514000889 [DOI] [PubMed] [Google Scholar]
- 38.He L-xia, Zhao J, Huang Y-sheng, LX H, Huang YS, et al. The difference between oats and beta-glucan extract intake in the management of HbA1c, fasting glucose and insulin sensitivity: a meta-analysis of randomized controlled trials. Food Funct 2016;7:1413–28. 10.1039/c5fo01364j [DOI] [PubMed] [Google Scholar]
- 39.Zou Y, Liao D, Huang H, et al. A systematic review and meta-analysis of beta-glucan consumption on glycemic control in hypercholesterolemic individuals. Int J Food Sci Nutr 2015;66:355–62. 10.3109/09637486.2015.1034250 [DOI] [PubMed] [Google Scholar]
- 40.Monnier L, Colette C. Postprandial and basal hyperglycaemia in type 2 diabetes: contributions to overall glucose exposure and diabetic complications. Diabetes Metab 2015;41:6s9–6s15. 10.1016/S1262-3636(16)30003-9 [DOI] [PubMed] [Google Scholar]
- 41.Anderson JW, Randles KM, Kendall CWC, et al. Carbohydrate and fiber recommendations for individuals with diabetes: a quantitative assessment and meta-analysis of the evidence. J Am Coll Nutr 2004;23:5–17. 10.1080/07315724.2004.10719338 [DOI] [PubMed] [Google Scholar]
- 42.Mao T, Huang F, Zhu X, et al. Effects of dietary fiber on glycemic control and insulin sensitivity in patients with type 2 diabetes: a systematic review and meta-analysis. Journal of Functional Foods 2021;82:104500. [Google Scholar]
- 43.Tosh SM, Bordenave N. Emerging science on benefits of whole grain oat and barley and their soluble dietary fibers for heart health, glycemic response, and gut microbiota. Nutr Rev 2020;78:13–20. 10.1093/nutrit/nuz085 [DOI] [PubMed] [Google Scholar]
- 44.Zurbau A, Noronha JC, Khan TA, et al. The effect of oat β-glucan on postprandial blood glucose and insulin responses: a systematic review and meta-analysis. Eur J Clin Nutr 2021;75:1540-1554. 10.1038/s41430-021-00875-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolever TMS, Mattila O, Rosa-Sibakov N, et al. Effect of varying molecular weight of oat β-glucan taken just before eating on postprandial glycemic response in healthy humans. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jenkins DJ, Taylor RH, Nineham R, et al. Manipulation of gut hormone response to food by soluble fiber and alpha-glucosidase inhibition. Am J Gastroenterol 1988;83:393–7. [PubMed] [Google Scholar]
- 47.Taylor RH, Jenkins DJ, Barker HM, et al. Effect of acarbose on the 24-hour blood glucose profile and pattern of carbohydrate absorption. Diabetes Care 1982;5:92–6. 10.2337/diacare.5.2.92 [DOI] [PubMed] [Google Scholar]
- 48.Jenkins DJ, Taylor RH, Goff DV, et al. Scope and specificity of acarbose in slowing carbohydrate absorption in man. Diabetes 1981;30:951–4. 10.2337/diab.30.11.951 [DOI] [PubMed] [Google Scholar]
- 49.Jenkins DJ, Taylor RH, Nineham R, et al. Combined use of guar and acarbose in reduction of postprandial glycaemia. Lancet 1979;2:924–7. 10.1016/s0140-6736(79)92622-9 [DOI] [PubMed] [Google Scholar]
- 50.Chiasson J-L, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359:2072–7. 10.1016/S0140-6736(02)08905-5 [DOI] [PubMed] [Google Scholar]
- 51.Chiasson J-L, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA 2003;290:486–94. 10.1001/jama.290.4.486 [DOI] [PubMed] [Google Scholar]
- 52.Hanefeld M, Cagatay M, Petrowitsch T, et al. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J 2004;25:10–16. 10.1016/s0195-668x(03)00468-8 [DOI] [PubMed] [Google Scholar]
- 53.van de Laar FA, Lucassen PL, Akkermans RP, et al. Alpha-Glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care 2005;28:154–63. 10.2337/diacare.28.1.154 [DOI] [PubMed] [Google Scholar]
- 54.Holman RR, Coleman RL, Chan JCN, et al. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2017;5:877–86. 10.1016/S2213-8587(17)30309-1 [DOI] [PubMed] [Google Scholar]
- 55.Casagrande SS, Cowie CC. Trends in dietary intake among adults with type 2 diabetes: NHANES 1988-2012. J Hum Nutr Diet 2017;30:479–89. 10.1111/jhn.12443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Canada H. Do Canadian adults meet their nutrient requirements through food intake alone, 2012. Available: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/fn-an/alt_formats/pdf/surveill/nutrition/commun/art-nutr-adult-eng.pdf [Accessed Available from].
- 57.U.S. Department of Health and Human Services and U.S. Department of Agriculture (2015) . 2015-2020 dietary guidelines for Americans, 2015. Available: https://health.gov/our-work/nutrition-physical-activity/dietary-guidelines/previous-dietary-guidelines/2015 [Accessed Dec 2015].
- 58.Canada S. Food availability, 2019, 2020. Available: https://www150.statcan.gc.ca/n1/daily-quotidien/200528/dq200528c-eng.htm
- 59.Musa-Veloso K, Fallah S, O'Shea M, et al. Assessment of intakes and patterns of cooked Oatmeal consumption in the U. S. Using Data from the National Health and Nutrition Examination Surveys. Nutrients 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guyatt G, Oxman AD, Akl EA, et al. Grade guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2022-002784supp001.pdf (1.8MB, pdf)
Data Availability Statement
Data are available upon reasonable request. Primary data files available upon request.