Abstract
Introduction
Pain, comorbid fatigue and sleep disturbances are common and distressing symptoms for patients with advanced cancer, negatively impacting their quality of life. Clinical guidelines recommend non-pharmacological interventions, including acupuncture and massage, for pain management in adult patients with cancer in adjunct to conventional care. However, high-quality evidence about the comparative effectiveness and long-term durability of these therapies for symptom management is limited.
Methods and analysis
We describe the design of a two-arm, parallel group, multicentre randomised controlled trial that investigates the use of acupuncture versus massage for musculoskeletal pain among 300 patients with diverse types of advanced cancer. The primary aim is to evaluate the long-term effectiveness (26 weeks from randomisation) of acupuncture vs massage for pain (primary outcome) and comorbid symptoms (fatigue, sleep disturbance and quality of life). The secondary aim is to identify patient-level demographic characteristics (eg, sex, race, age), clinical factors (eg, insomnia, pain severity) and psychological attributes that are associated with a greater reduction in pain for either acupuncture or massage. Patients will receive weekly acupuncture or massage treatments for 10 weeks, followed by monthly booster sessions up to 26 weeks. The primary endpoint will be the change in worst pain intensity score from baseline to 26 weeks. We will collect validated patient-reported outcomes at multiple time points over 26 weeks.
Ethics and dissemination
The Institutional Review Board at Memorial Sloan Kettering Cancer Center in New York approved this protocol. Results will be disseminated via peer-reviewed scientific journals and conference presentations. Our findings will help patients and healthcare providers make informed decisions about incorporating non-pharmacological treatments to manage pain for patients with advanced cancer.
Trial registration number
Keywords: pain management, complementary medicine, cancer pain
Strengths and limitations of this study.
This study represents the largest randomised controlled trial to date comparing the effectiveness of acupuncture versus massage for pain management among patients with advanced cancer.
By recruiting a diverse population in terms of race/ethnicity and cancer types, this study will offer insight into the sociodemographic, clinical factors and physiological attributes that can inform and help predict factors to personalise treatment.
All participants will be followed up to 26 weeks.
The study design does not include a control group comparing the standard of care for pain management as prescribed by the clinical team.
The study design does not allow crossover between the acupuncture and massage groups.
Introduction
Cancer is a leading cause of morbidity and mortality, second only to heart disease.1 Because of recent innovations in cancer therapeutics, the definition for advanced cancer is challenging because some patients with metastatic cancer can now be ‘cured’ or at least enter long-term remission leaving them to often live with symptomatic sequelae. Compared with the general population, patients with advanced cancer are at a greater risk for chronic physical and psychological symptoms.2–5 Among patients with advanced cancer, symptoms of pain, fatigue and insomnia are the most commonly reported, often clustered together, and are generally not well managed.4–10 Previous studies have shown prevalence rates of pain as high as 66% among patients with advanced cancer.11 12
Historically, pain management in cancer has predominantly relied on drug therapies; however, increasing clinical evidence suggesting the potential harm over time of long-term opioid therapy for chronic cancer pain, not to mention the current opioid abuse epidemic sweeping the USA, underscore a need for additional treatments.13 14 As more individuals with advanced cancer live longer, patient-centred pain management integrating non-pharmacological interventions based on research evidence has strong potential to improve the quality of pain management for this population. Hence, clinical guidelines and leading medical organisations recommend non-pharmacological interventions, including acupuncture and massage, in conjunction with drug therapies for pain management.14–19
Acupuncture, a therapy of traditional Chinese medicine, involves penetrating the skin with thin, solid, metallic needles that are manipulated by hand or electrical stimulation.20 With respect to the efficacy of acupuncture for chronic pain in cancer populations, a systematic review and meta-analysis found that when acupuncture is incorporated into conventional cancer care, it is more effective than conventional drug management alone for cancer pain.21 A recent comparative effectiveness randomised controlled trial (RCT) found that electroacupuncture and auricular acupuncture were significantly more efficacious for pain reduction than usual care among diverse cancer survivors (N=360).22 Further, there is some evidence suggesting that acupuncture may improve sleep disturbances, fatigue and anxiety in patients with cancer experiencing pain.23 24
Massage, which involves the manual manipulation of muscles and other soft tissue areas of the body, is one of the earliest known forms of pain relief. Since massage therapy techniques promote joint flexibility, relieve muscular tension and improve range of motion, massage therapy has mechanistic plausibility for addressing musculoskeletal pain in patient populations.13 25 In a recent meta-analysis conducted by the Evidence for Massage Therapy Working Group, massage therapy was effective at treating pain compared with other controls (such as reading, usual care or active attention) in cancer populations.26 In addition to pain management, massage therapy may improve fatigue, sleep and anxiety in cancer populations.26–30
Despite acupuncture and massage therapy both being widely available and commonly used as non-pharmacological treatments for pain,13 31 there is currently a gap in the evidence regarding the comparative effectiveness of these options as well as the long-term durability of their treatment effects among patients living with advanced cancer. We planned an RCT to evaluate the long-term comparative effectiveness of acupuncture vs massage for pain in patients living with advanced cancer. Our primary aim is to compare the long-term effectiveness (26 weeks from randomisation) of acupuncture versus massage for pain (primary outcome) and comorbid symptoms (fatigue, sleep disturbance and quality of life (QoL)) in patients living with advanced cancer. Our secondary aim is to identify patient-level demographic characteristics (eg, sex, race, age), clinical factors (eg, insomnia, pain severity) and psychological attributes (ie, outcome expectation) that are associated with a greater reduction in pain for either acupuncture or massage.
Methods and analysis
Study design
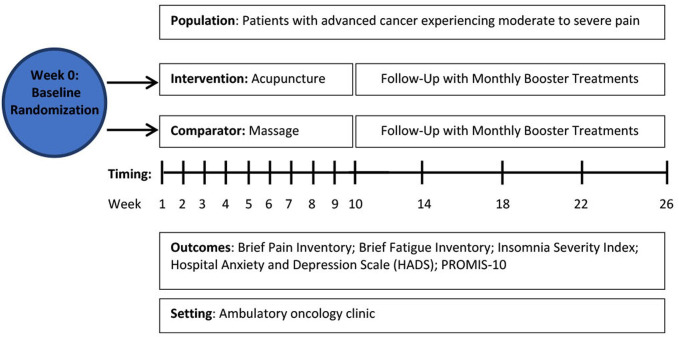
The Integrative Medicine for Pain in Patients with Advanced Cancer Trial (IMPACT) is a two-arm, parallel group RCT to compare the effectiveness of acupuncture and massage for pain and comorbid symptoms in a heterogeneous sample of 300 patients living with advanced cancer who have been experiencing moderate to severe pain (figure 1). Eligible patients will be randomly assigned in a 1:1 ratio to acupuncture or massage. Patients will receive weekly acupuncture or massage treatments for 10 weeks followed by monthly booster sessions up to week 26. All patients will continue to receive their standard medical care and pain management as prescribed by their physicians. The primary endpoint will be the change in worst pain intensity score (as assessed by the Brief Pain Inventory (BPI)) from baseline to 26 weeks. We will also collect validated patient-reported outcome measures of pain and comorbid symptoms at seven timepoints over 26 weeks (table 1).
Figure 1.
Study schema for the Integrative Medicine for Pain in Patients with Advanced Cancer Trial. PROMIS, Patient-Reported Outcomes Measurement Information System.
Table 1.
Schedule of data collection
| Outcome | Active intervention | Follow-up | |||||
| Week 0 | Week 4 | Week 10 | Week 14 | Week 18 | Week 22 | Week 26 | |
| Primary outcome—pain | |||||||
| Brief Pain Inventory | X | X | X | X | X | X | X |
| Secondary outcomes—fatigue, sleep, anxiety and quality of life | |||||||
| Brief Fatigue Inventory | X | X | X | X | |||
| Insomnia Severity Index | X | X | X | X | |||
| Hospital Anxiety and Depression Scale | X | X | X | X | |||
| PROMIS-10 Global Health | X | X | X | X | |||
| Patients’ Global Impression of Change | X | X | X | ||||
| Covariates | |||||||
| Demographics (eg, age, sex, race/ethnicity) | X | X | |||||
| Clinical characteristics (eg, tumour type, stage, cancer therapy) | X | X | |||||
| Pain medication diary | X | X | X | X | X | ||
| Predictive variables | |||||||
| Mao expectancy of treatment effects | X | X | |||||
PROMIS-10, Patient-Reported Outcomes Measurement Information System.
Participants
We will recruit study participants in the USA through Memorial Sloan Kettering Cancer Center (MSK), a National Cancer Institute-designated comprehensive cancer centre, with a main campus in Manhattan and numerous regional sites in New York (Westchester County and Long Island) and New Jersey (Bergen, Monmouth and Basking Ridge). We will also recruit patients from the Baptist Health Miami Cancer Institute (MCI), which is an affiliate of MSK’s strategic alliance. For MSK-affiliated patients, we will use a population-based method by mailing out letters to potentially eligible patients identified through a data query of MSK’s electronic health records. We will also use stakeholders and partnering clinicians to publicise the study and provide referrals. The target accrual goal is 300 participants. Enrolment began in October 2019 and study participant assessments are scheduled to be completed by July 2022.
Inclusion and exclusion criteria
Patients will be eligible for the study if they are English or Spanish-speaking, over 18 years old, and able to walk with only occasional assistance (Karnofsky functional score of ≥60). They must also have a diagnosis of the following: stage III or IV lung cancer; any stage pancreatic cancer; unresectable cholangiocarcinoma; unresectable liver cancer; unresectable ampullary or periampullary cancer or other stage IV gastrointestinal cancer; stage III or IV ovarian or fallopian tube cancers or other stage IV gynecologic cancer; stage IV breast cancer; stage IV genitourinary cancer; stage III or IV sarcoma; stage IV melanoma; stage III or IV head/neck cancer; stage IV endocrine cancer; or haematological malignancies (lymphoma, myeloma and leukaemia). Patients will need to have an expected prognosis of 6 months or greater from their treating physician or the study clinician.
To be eligible, patients must also report ongoing musculoskeletal pain, defined as regional (eg, joints, extremities, back, neck) or more generalised (ie, fibromyalgia) pain, as their primary source of pain. The pain must be present for at least 1 month and occur for at least 15 days of the preceding 30 days. In addition, patients must report that their pain is four or greater on a numerical rating scale of 0–10. Non-musculoskeletal pain syndromes (eg, headache, facial pain, chest pain or visceral abdominal pain) may be present if musculoskeletal pain is the primary source of pain. Patients will be excluded from the study if they have a blood platelet count of less than 15 000 platelets per microliter.
Procedure
All potential participants will undergo an initial screening with a research coordinator in person or over the telephone. At this initial contact, the research coordinator will explain the study goals and procedures and screen participants for eligibility. Next, a study healthcare provider will meet with screened and interested patients to confirm eligibility. Once deemed eligible, patients will complete the informed consent and undergo randomisation. Patients will complete assessments online using Research Electronic Data Capture (REDCap), a data management software system, at seven time points: weeks 0, 4, 10, 14, 18, 22 and 26. To encourage adherence to the study procedures, participants will receive reminders to complete study assessments. Additionally, all participants will be compensated with a US$40 gift card for completion of the week 10 visit and a US$60 gift card for the completion of the week 26 visit, for a total of US$100.
Randomisation
We will randomise 300 participants to acupuncture or massage using MSK’s Clinical Research Database (CRDB), a secure computer system that ensures full allocation concealment. Randomisation will be performed by the method of random permuted block stratified by any current opioid use (yes vs no) and by accrual site (MSK main campus, MSK regional sites vs MCI). Given the nature of the interventions, patients and providers will not be blinded to treatment assignments. The principal investigator (PI), study statisticians and outcome assessment clinical research coordinator will be blinded to treatment assignments.
Primary outcome
The short-form BPI is one of the most widely used instruments to measure pain and has been demonstrated to be a reliable, valid and responsive measure (Cronbach’s alpha 0.77–0.91).32 The BPI contains four pain severity items and seven pain interference items, all rated on a scale from 0 to 10 (higher ratings indicate worse pain intensity/interference). A pain interference subscale can be computed by taking the average rating of the seven pain interference items. A pain severity subscale score can similarly be computed for the four pain severity items; however, the Worst Pain severity item and the Average Pain severity item are often examined separately from the pain intensity subscale in clinical research because they tend to be more sensitive indicators of changes in patients’ perceived pain. The primary outcome of this study will be the patient’s rating of their Worst Pain in the past week with response choices of 0 ‘no pain’ to 10 ‘pain as bad as you can imagine.’ The Average Pain rating in the past week and the pain interference subscale will be used as secondary pain outcomes.
Secondary outcomes
The Patients’ Global Impression of Change (PGIC) is a one-item survey used to define a clinically important change in pain from the patient’s perspective.33 34 The PGIC can be used as an anchor to derive anchor-based minimally important differences for pain measures like the BPI. Participants will be asked ‘How would you describe your pain since the first clinical visit? I am: very much worse, much worse, a little worse, the same, a little improved, much improved, very much improved.’ Subjects reporting ‘much improved’ or ‘very much improved’ will be considered responders.
The Brief Fatigue Inventory is a nine-item instrument designed to assess fatigue severity and has been shown to be reliable and valid in multiple languages and diverse populations.35 36 Three items ask patients to rate the severity of their fatigue at its ‘worst,’ ‘usual,’ and ‘now’ during normal waking hours, with 0 being ‘no fatigue’ and 10 being ‘fatigue as bad as you can imagine.’ Six items ask patients to rate the amount that fatigue has interfered with different aspects of their life during the past 24 hours, with 0 being ‘does not interfere’ and 10 being ‘completely interferes.’35 A composite fatigue severity score can be found by averaging the nine-item scores.
The Insomnia Severity Index is a reliable and valid seven-item scale used to assess subjective insomnia severity.37 38 The items are scored on a five-point Likert response scale (eg, 0=no problem; 4=very severe problem), yielding a total score ranging from 0 to 28 with higher scores representing more severe insomnia symptoms. Established cutoffs are: <8, no clinically significant insomnia; 8–14, subthreshold insomnia; 15–21, clinical insomnia (moderate severity); >21, clinical insomnia (severe).37 A reduction of eight points is considered to be clinically meaningful improvement among those with insomnia.39
The Hospital Anxiety and Depression Scale is a 14-item scale with seven items measuring depression and seven items measuring anxiety that has been shown to be both reliable and valid.40 41 Each item is answered by the patient on a four-point (0–3) response category so possible scores range from 0 to 21 for anxiety and depression, with higher scores indicating higher symptomatology. Established cutoffs are: 0–7 not significant; 8–10 subclinical, and 11–21 clinically significant depression/anxiety.42
The Patient-Reported Outcomes Measurement Information System Scale V.1.2—Global Health is a brief instrument composed of 10 items that demonstrates adequate reliability and validity43 44 as a measure of health-related QOL in general and clinical populations.45 46 The measure yields two scores for physical health and mental health with higher scores indicative of better QOL.
Assessment of outcome expectancy as a predictive variable for treatment response
Outcome expectancy has long been considered an important predictor of treatment outcomes and has gained increasing recognition in massage and acupuncture clinical trials.47 48 The Mao Expectancy of Treatment Effects,49 originally developed as the Acupuncture Expectancy Scale,50 is a four-item instrument to measure outcome expectancy for various interventions (eg, acupuncture, herbs, cognitive behavioural therapies)49 51 and has demonstrated reliability and validity.50 The score ranges from 4 to 20, with a higher score indicating greater expectancy. We will use this measure to explore whether expectancy predicts treatment outcomes and may impact the observed differences between groups.
Covariates
We will collect specific demographic (eg, age, sex, race/ethnicity) and other relevant historical medical data (eg, cancer treatment). We will also track participants’ self-reported use of analgesic medications (eg, acetaminophen, non-steroidal anti-inflammatory drugs, opioids and adjuvants for neuropathic pain) by having participants complete weekly pain medication diaries to calculate weekly average analgesic medication usage throughout the study time period.52 As pain often results in increased healthcare utilisation, we will track emergency department visits and hospitalisations via the patient’s electronic health record. Additionally, we will collect participants’ reasons for either stopping treatment or dropping out of the clinical trial, such as treatment adverse events, disease complications or scheduling issues with work.
Interventions
Licensed and oncology-experienced acupuncturists and massage therapists will deliver all treatments. All acupuncturists and massage therapists will be given a manual with the specific treatment protocols for acupuncture and massage (see online supplemental appendices 1 and 2) and will be trained by the PI and/or lead acupuncturist and massage therapist. For quality assurance, the lead therapists will audit at least two charts for each therapist per week for adherence to treatment protocol and documentation standards, and all therapists will be recertified twice yearly. We have extensive experience in conducting integrative medicine symptom trials including ensuring the quality of interventions.22 23 29 53–55
bmjopen-2021-058281supp001.pdf (109.2KB, pdf)
bmjopen-2021-058281supp002.pdf (102.1KB, pdf)
For the acupuncture intervention, we will use a treatment protocol developed and tested by our group that has demonstrated improvements in pain, fatigue and sleep among patients with cancer.22 23 54–56 After sterilising the skin, the acupuncturist will place between 10 and 20 needles at a minimum of four local points around the body area with the most pain and at individual points depending on the participant’s comorbid symptoms. The acupuncture needles will be inserted to appropriate depths depending on the location on the body and body type of the participant.57 The acupuncturist will manipulate the needles to achieve the ‘De Qi’ sensation for the participants. ‘De Qi’ is a local sensation of soreness, numbness, or distension that accompanies the insertion and manipulation of needles during acupuncture.58 The needles at the four local points for pain will be electrically stimulated at 2 Hz by connecting to a TENS unit. The acupuncturist will leave the needles in place for 20 min with brief manipulation at the beginning and end of the treatment.
For the massage intervention, we will use a treatment protocol developed and tested by our group that has shown improvements in pain and fatigue among patients with cancer undergoing chemotherapy.29 Consistent with oncology massage practice, therapists will administer compressions with light to moderate pressure and will use any of the following oncology massage techniques: compression; muscle stripping; active/passive range of motion, post-isometric stretching; effleurage (gliding); myofascial release; positional release; and trigger/tender point release.59 60 Therapists will start with a 5 min protocol including guided diaphragmatic breathing exercise, rib mobilisations and occipital release to increase parasympathetic tone. Next, depending on the participant’s primary area of pain, the therapist will focus 20 min of massage on that specific body area followed by effleurage toward the heart. The massage therapist will focus on the following identified areas of pain: head/jaw; cervical spine; thoracic spine; shoulder; upper extremity; lumbar; sacral; pelvic; hip and lower extremity.
Before each massage or acupuncture treatment, the massage therapist or acupuncturist will review the participant’s current health status and modify his/her techniques if needed. In the case of acupuncture, shallow needling with minimal stimulation will be used, and needles will only be placed in the extremities. For participants with electronically charged medical devices, no stimulation will be used. In the case of massage, light touch will be used, and areas of bruising will be avoided. The massage therapist or acupuncturist will document any treatment modifications and the medical reason for the modification, which will allow us to systematically capture participants who received a modified treatment. Patients will be monitored for side effects at each visit. Adverse events related to the administration of either acupuncture or massage will be collected each week before and after each treatment by the acupuncturist/massage therapist or clinical research coordinator. The Common Terminology Criteria for Adverse Events V.5 will be used for toxicity evaluation.
Analytical approach
We will perform the analysis for each aim following the intention-to-treat (ITT) principle (ie, participants will be analysed according to the treatment group to which they will be randomly allocated regardless of drop-out or treatment adherence status). For all specific aims, our main analytic tool will be linear mixed-effects models (LMMs) because our primary outcome (worst pain severity) and secondary outcomes are repeated continuous outcomes over time.61 The general template of each LMM will model the outcome as a function of treatment arm and assessment time, controlling for the randomisation stratification variables (baseline opioid use and accrual site), and including a subject-specific random intercept and slope.
For aim 1, we will plot the outcome measure trajectories by randomisation arm over time and summarise each outcome measure at each assessment time by treatment arm using descriptive statistics. Tests of ITT differences between randomisation arms with respect to changes in outcomes will be based on coefficients from specific time-by-arm interactions added to the general LMM template described above. Our primary effectiveness comparison will focus on changes in BPI Worst Pain from baseline to 26 weeks between acupuncture versus massage. Aim 1 secondary outcomes (eg, fatigue, insomnia, QOL) will be analysed using the same methods described above. We will also perform responder analyses by considering those who experienced 30% or greater reduction in BPI Worst Pain at end of treatment (week 10) as responders.34 62 63 We will compare the proportion of responders in acupuncture and massage at the end of the intervention period using descriptive cross-tabulations and logistic regression adjusting for the randomisation strata.
For aim 2, we will conduct exploratory, hypothesis-generating heterogeneity of treatment effect (HTE) analyses to identify patient-level factors associated with treatment response to either acupuncture or massage by incorporating relevant variables (eg, sex, race/ethnicity, expectation, opioid use) and variable-by-intervention interaction terms in linear regression models predicting week 26 worst pain controlling for baseline worst pain and stratification factors. Each variable of interest will be assessed for HTE in a separate model. For these exploratory regression analyses, we will guard against inflated type I error due to multiple testing by adjusting the variable-by-intervention interaction p values for the false discovery rate.64 65 Our current focus on evaluating and reporting HTE will be based on the approach proposed by Kent et al.66 However, we will also apply promising emerging Bayesian67 68 and machine learning69 70 methods, which can identify HTE and subgroups based on multiple variables simultaneously and are potentially more powerful than traditional univariate methods. Since our inclusion criteria allows for patients with various cancer types, we will also perform exploratory subgroup analysis to see if there is any difference in treatment effect (both primary and secondary outcomes) among patients with solid tumour cancers vs blood cancers. Because our trial will enrol patients with advanced cancer, interventions may need to be modified for patient safety issues such as for those with low platelets or bruising in the area where there is pain. We will conduct exploratory analyses to examine if there are any differences in outcomes for those patients who received non-modified treatments versus those who had modified treatments. We will also conduct exploratory analyses to see whether individuals with low platelet counts experienced more adverse events compared with patients with normal platelet counts.
To address missing data, we will perform sensitivity analyses (eg, assess impact on results of adjusting for patient disease progression or death) and apply data analysis strategies that are as robust as possible to data losses. We will first explore whether missingness is associated with observed variables (eg, randomisation arm and the baseline outcome measures) by comparing participants with complete and incomplete data. Of note, the LMMs described above validly include participants with incomplete data under the missing at random assumption. However, our exploration of the data may deem the missing at random assumption to be inappropriate; hence, we will perform sensitivity analyses to evaluate the robustness of our LMM results by refitting the models after imputing the missing week 26 outcomes using multiple imputation.
Power analysis and sample size
For our sample size/power considerations, we calculated the smallest standardised effect size (ie, Cohen’s d) we will be able to detect with 80% power, given our sample size of 300 and other assumptions. Using the ‘power.mmrm’ function from the R package ‘longpower,’ we applied the formulas in Lu et al,71 to derive the smallest detectable effect size for the coefficient of the time-by-arm interaction term in our LMM, given our study design and assumptions, which we transformed to represent the standardised mean difference (ie, Cohen’s d) between the two arms at 26 weeks postrandomisation. Based on our prior experience72 73 and given that patients living with advanced cancer may have unanticipated health issues (eg, hospitalisations, death), we conservatively anticipate lost to follow-up to be 20% by 26 weeks. Assuming this 20% lost to follow-up, correlation between baseline and 26-week BPI Worst Pain of 0.5, and two-sided alpha of 0.05, and with 150 participants in each of the two active intervention arms, we will have 80% power to detect an effect size of 0.35 (standardised mean difference, Cohen’s d) at 26 weeks post-randomisation between acupuncture vs massage. Based on our own preliminary data in patients with stage IV cancer who experienced moderate to severe pain (N=284), the mean BPI Worst Pain score was 6.3 with SD of 1.7. A difference of 1 on the BPI Worst Pain score (considered a clinically meaningful difference in pain) based on SD of 1.7 equals an effect size (Cohen’s d) of 0.59. In this study, we have 99% power to detect this clinically meaningful mean difference of 1 point (Cohen’s d of 0.59) on the BPI Worst Pain score. Our trial is more than sufficiently powered to detect a clinically meaningful difference between acupuncture and massage at 26 weeks.
Patient and public involvement
Recognising the value of incorporating feedback from patients and their families, we organised a formal patient/stakeholder advisory board composed of 10 members (ie, patients, caregivers and stakeholders from advocacy and cancer organisations) to contribute to the study design, optimal delivery of interventions, recruitment and retention strategies, and implementation and dissemination efforts. By collaborating with patient/stakeholder partners, the patient perspective is included and helps to ensure that the research conducted is relevant and not unduly burdensome for patients. Our patient/stakeholder advisory board members helped generate the research questions, choose the comparison groups, develop patient-centred inclusion and exclusion criteria, determine the timing of the primary endpoint, refine the research protocol, choose the most appropriate outcomes, decide on specific measurement tools and create patient-friendly recruitment materials. Throughout the project, our patient/stakeholder partners will have specific roles in recruitment activities and will help to ensure that our trial is accessible to participants from diverse communities. Additionally, patient/stakeholder partners’ involvement will contribute to effectively translating and disseminating the study findings to patient, family, stakeholder and research audiences to effect real-world change.
Discussion
Pain and comorbid fatigue and sleep disturbance are among the most common and distressing symptoms for patients living with advanced cancer.4–9 These co-occurring symptoms also negatively impact patients’ QoL and functional performance.10 74 75 Unlike drug therapies that mostly focus on treating one symptom, acupuncture and massage can address multiple symptoms during treatment, which makes them potentially beneficial not only for pain but also for its related comorbid symptoms (eg, fatigue and sleep disturbance) among patients with advanced cancer. Acupuncture and massage are both widely available and commonly used nonpharmacological treatments for pain and other comorbid symptoms in cancer populations. Therefore, this RCT study will provide high quality evidence of the comparative effectiveness and durability of acupuncture versus massage that can be readily incorporated into clinical care to improve patient-centred decision making for pain management.
Ethics and dissemination
The institutional review board at MSK Cancer Center (MSK) approved the study protocol; most recent version of the protocol approved on 19 May 2021. For this trial, we will adhere to the guidelines from the Consolidated Standards of Reporting Trials76 and Standards for Reporting Interventions in Clinical Trials of Acupuncture.77 This trial is funded by the Patient-Centered Outcomes Research Institute (SMPAI-2018C2-12883).
The results of this study will be presented at national and international meetings, and a manuscript will be submitted for publication in a peer-reviewed journal. This research will inform which therapy (acupuncture or massage) is more effective for reducing pain and comorbid fatigue and insomnia in patients living with advanced cancer. Such information will lead to evidence-based and patient-centred decision making to incorporate these approaches for optimal pain management for the growing population of individuals living with advanced cancer. By collaborating with patient/stakeholder partners, patient/stakeholder partners help to interpret both expected and unexpected study findings in a way that is culturally sensitive and relevant to patients’ lived experiences. Patient/stakeholder partners’ active involvement will contribute to effectively translating and disseminating the study findings to patient, family, stakeholder and research audiences to effect real-world change by providing education and awareness of the benefits of integrative, non-pharmacological options for pain management in people with advanced cancer.
Supplementary Material
Acknowledgments
The authors would like to thank the patients, healthcare providers, clinical staff, research staff and patient/stakeholder partners at all study sites for their contributions to this study.
Footnotes
Contributors: SADR, REB, KP, JMac, DW, MB-B, JMao, AE: conceptualisation. REB, KP, JMao: data curation. REB, KP, JF: formal analysis. JMao: funding acquisition. SADR, NE, JMao: investigation. SADR, NE, JMao: project administration. JMao: resources. SADR, NE, KL, GD, HX, JMao: supervision. SADR, NE, JMao: writing-original draft. All authors: writing-review and editing.
Funding: Research reported in this article was funded through a Patient-Centered Outcomes Research Institute (PCORI) award (SMPAI-2018C2-12883). The statements presented in this work are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee. It was also supported in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30 CA008748) and the Translational and Integrative Medicine Research Fund (award/grant number not applicable) at Memorial Sloan Kettering Cancer Center.
Disclaimer: The funders do not have any role in study design; collection, management, analysis, and interpretation of data; writing of the report; and decision to submit for publication.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.American Cancer Society . Cancer facts and statistics. American Cancer Society, 2017. [Google Scholar]
- 2.Delgado-Guay MO, Ferrer J, Ochoa J, et al. Characteristics and outcomes of advanced cancer patients who received palliative care at a public hospital compared with those at a comprehensive cancer center. J Palliat Med 2018;21:678–85. 10.1089/jpm.2017.0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cleeland CS, Zhao F, Chang VT, et al. The symptom burden of cancer: evidence for a core set of cancer-related and treatment-related symptoms from the eastern cooperative Oncology Group symptom outcomes and practice patterns study. Cancer 2013;119:4333–40. 10.1002/cncr.28376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnsen AT, Petersen MA, Pedersen L, et al. Symptoms and problems in a nationally representative sample of advanced cancer patients. Palliat Med 2009;23:491–501. 10.1177/0269216309105400 [DOI] [PubMed] [Google Scholar]
- 5.Kirkova J, Rybicki L, Walsh D, et al. The relationship between symptom prevalence and severity and cancer primary site in 796 patients with advanced cancer. Am J Hosp Palliat Care 2011;28:350–5. 10.1177/1049909110391464 [DOI] [PubMed] [Google Scholar]
- 6.Dong ST, Butow PN, Costa DSJ, et al. Symptom clusters in patients with advanced cancer: a systematic review of observational studies. J Pain Symptom Manage 2014;48:411–50. 10.1016/j.jpainsymman.2013.10.027 [DOI] [PubMed] [Google Scholar]
- 7.Dong ST, Butow PN, Tong A, et al. Patients' experiences and perspectives of multiple concurrent symptoms in advanced cancer: a semi-structured interview study. Support Care Cancer 2016;24:1373–86. 10.1007/s00520-015-2913-4 [DOI] [PubMed] [Google Scholar]
- 8.Walsh D, Donnelly S, Rybicki L. The symptoms of advanced cancer: relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer 2000;8:175–9. 10.1007/s005200050281 [DOI] [PubMed] [Google Scholar]
- 9.Walsh D, Rybicki L. Symptom clustering in advanced cancer. Support Care Cancer 2006;14:831–6. 10.1007/s00520-005-0899-z [DOI] [PubMed] [Google Scholar]
- 10.Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum 2001;28:465–70. [PubMed] [Google Scholar]
- 11.van den Beuken-van Everdingen MHJ, Hochstenbach LMJ, Joosten EAJ, et al. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage 2016;51:e1079:1070–90. 10.1016/j.jpainsymman.2015.12.340 [DOI] [PubMed] [Google Scholar]
- 12.Porta-Sales J, Nabal-Vicuna M, Vallano A, et al. Have we improved pain control in cancer patients? A multicenter study of ambulatory and hospitalized cancer patients. J Palliat Med 2015;18:923–32. 10.1089/jpm.2015.29002.jps [DOI] [PubMed] [Google Scholar]
- 13.Tick H, Nielsen A, Pelletier KR, et al. Evidence-based nonpharmacologic strategies for comprehensive pain care: the Consortium pain Task force white paper. Explore 2018;14:177–211. 10.1016/j.explore.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 14.Paice JA, Portenoy R, Lacchetti C, et al. Management of chronic pain in survivors of adult cancers: American Society of clinical oncology clinical practice guideline. J Clin Oncol 2016;34:3325–45. 10.1200/JCO.2016.68.5206 [DOI] [PubMed] [Google Scholar]
- 15.Swarm RA, Abernethy AP, Anghelescu DL, et al. Adult cancer pain. J Natl Compr Canc Netw 2013;11:992–1022. 10.6004/jnccn.2013.0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Network NCC . NCCN clinical practice guidelines in oncology: adult cancer pain version I, 2018. Available: https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf [Accessed 22 Jun 2018]. [DOI] [PubMed]
- 17.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain-United States, 2016. JAMA 2016;315:1624–45. 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Commission TJ . R3 report issue 11: pain assessment and management standards for hospitals, 2018. Available: https://www.jointcommission.org/r3_issue_11/ [Accessed 25 Jun 2018].
- 19.Qaseem A, Wilt TJ, McLean RM. Clinical guidelines committee of the American College of P. noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2017;166:514–30. [DOI] [PubMed] [Google Scholar]
- 20.Mao JJ, Kapur R. Acupuncture in primary care. Prim Care 2010;37:105–17. 10.1016/j.pop.2009.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu HY, Hsieh YJ, Tsai PS. Systematic review and meta-analysis of acupuncture to reduce cancer-related pain. Eur J Cancer Care 2017;26:12457. 10.1111/ecc.12457 [DOI] [PubMed] [Google Scholar]
- 22.Mao JJ, Liou KT, Baser RE, et al. Effectiveness of electroacupuncture or auricular acupuncture vs usual care for chronic musculoskeletal pain among cancer survivors: the peace randomized clinical trial. JAMA Oncol 2021;7:720–7. 10.1001/jamaoncol.2021.0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao JJ, Farrar JT, Bruner D, et al. Electroacupuncture for fatigue, sleep, and psychological distress in breast cancer patients with aromatase inhibitor-related arthralgia: a randomized trial. Cancer 2014;120:3744–51. 10.1002/cncr.28917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hershman DL, Unger JM, Greenlee H, et al. Effect of acupuncture vs sham acupuncture or Waitlist control on joint pain related to aromatase inhibitors among women with early-stage breast cancer: a randomized clinical trial. JAMA 2018;320:167–76. 10.1001/jama.2018.8907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buckenmaier C, Cambron J, Werner R, et al. Massage therapy for pain-call to action. Pain Med 2016;17:1211–4. 10.1093/pm/pnw092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyd C, Crawford C, Paat CF, et al. The impact of massage therapy on function in pain Populations-a systematic review and meta-analysis of randomized controlled trials: part II, cancer pain populations. Pain Med 2016;17:1553–68. 10.1093/pm/pnw100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kutner JS, Smith MC, Corbin L, et al. Massage therapy versus simple touch to improve pain and mood in patients with advanced cancer: a randomized trial. Ann Intern Med 2008;149:369–79. 10.7326/0003-4819-149-6-200809160-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez G, Liu W, Milbury K, et al. The effects of oncology massage on symptom self-report for cancer patients and their caregivers. Support Care Cancer 2017;25:3645–50. 10.1007/s00520-017-3784-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao JJ, Wagner KE, Seluzicki CM, et al. Integrating oncology massage into chemoinfusion suites: a program evaluation. J Oncol Pract 2017;13:e207–16. 10.1200/JOP.2016.015081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cassileth BR, Vickers AJ. Massage therapy for symptom control: outcome study at a major cancer center. J Pain Symptom Manage 2004;28:244–9. 10.1016/j.jpainsymman.2003.12.016 [DOI] [PubMed] [Google Scholar]
- 31.Yun H, Sun L, Mao JJ. Growth of integrative medicine at leading cancer centers between 2009 and 2016: a systematic analysis of NCI-Designated comprehensive cancer center websites. J Natl Cancer Inst Monogr 2017;2017:4. 10.1093/jncimonographs/lgx004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap 1994;23:129–38. [PubMed] [Google Scholar]
- 33.Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther 2004;27:26–35. 10.1016/j.jmpt.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 34.Farrar JT, Young JP, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–58. 10.1016/S0304-3959(01)00349-9 [DOI] [PubMed] [Google Scholar]
- 35.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the brief fatigue inventory. Cancer 1999;85:1186–96. [DOI] [PubMed] [Google Scholar]
- 36.Lin C-C, Chang A-P, Chen M-L, et al. Validation of the Taiwanese version of the brief fatigue inventory. J Pain Symptom Manage 2006;32:52–9. 10.1016/j.jpainsymman.2005.12.019 [DOI] [PubMed] [Google Scholar]
- 37.Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med 2001;2:297–307. 10.1016/s1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 38.Savard M-H, Savard J, Simard S, et al. Empirical validation of the insomnia severity index in cancer patients. Psychooncology 2005;14:429–41. 10.1002/pon.860 [DOI] [PubMed] [Google Scholar]
- 39.Morin CM, Belleville G, Bélanger L, et al. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34:601–8. 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith AB, Selby PJ, Velikova G, et al. Factor analysis of the hospital anxiety and depression scale from a large cancer population. Psychol Psychother 2002;75:165–76. 10.1348/147608302169625 [DOI] [PubMed] [Google Scholar]
- 41.Moorey S, Greer S, Watson M, et al. The factor structure and factor stability of the hospital anxiety and depression scale in patients with cancer. Br J Psychiatry 1991;158:255–9. 10.1192/bjp.158.2.255 [DOI] [PubMed] [Google Scholar]
- 42.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 43.Hays RD, Bjorner JB, Revicki DA, et al. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res 2009;18:873–80. 10.1007/s11136-009-9496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Revicki DA, Kawata AK, Harnam N, et al. Predicting EuroQol (EQ-5D) scores from the patient-reported outcomes measurement information system (PROMIS) global items and domain item banks in a United States sample. Qual Life Res 2009;18:783–91. 10.1007/s11136-009-9489-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothrock NE, Hays RD, Spritzer K, et al. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the patient-reported outcomes measurement information system (PROMIS). J Clin Epidemiol 2010;63:1195–204. 10.1016/j.jclinepi.2010.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barile JP, Reeve BB, Smith AW, et al. Monitoring population health for healthy people 2020: evaluation of the NIH PROMIS(R) global health, CDC healthy days, and satisfaction with life instruments. Qual Life Res 2013;22:1201–11. 10.1007/s11136-012-0246-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colagiuri B, Smith CA. A systematic review of the effect of expectancy on treatment responses to acupuncture. Evid Based Complement Alternat Med 2012;2012:857804. 10.1155/2012/857804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalauokalani D, Cherkin DC, Sherman KJ, et al. Lessons from a trial of acupuncture and massage for low back pain: patient expectations and treatment effects. Spine 2001;26:1418–24. 10.1097/00007632-200107010-00005 [DOI] [PubMed] [Google Scholar]
- 49.Keefe JR, Amsterdam J, Li QS, et al. Specific expectancies are associated with symptomatic outcomes and side effect burden in a trial of chamomile extract for generalized anxiety disorder. J Psychiatr Res 2017;84:90–7. 10.1016/j.jpsychires.2016.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mao JJ, Armstrong K, Farrar JT, et al. Acupuncture expectancy scale: development and preliminary validation in China. Explore 2007;3:372–7. 10.1016/j.explore.2006.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garland SN, Gehrman P, Barg FK, et al. Choosing options for insomnia in cancer effectively (CHOICE): design of a patient centered comparative effectiveness trial of acupuncture and cognitive behavior therapy for insomnia. Contemp Clin Trials 2016;47:349–55. 10.1016/j.cct.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 52.Robinson-Papp J, George MC, Wongmek A, et al. The quantitative analgesic questionnaire: a tool to capture patient-reported chronic pain medication use. J Pain Symptom Manage 2015;50:381–6. 10.1016/j.jpainsymman.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen LX, Mao JJ, Fernandes S, et al. Integrating acupuncture with exercise-based physical therapy for knee osteoarthritis: a randomized controlled trial. J Clin Rheumatol 2013;19:308–16. 10.1097/RHU.0b013e3182a21848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mao JJ, Xie SX, Farrar JT, et al. A randomised trial of electro-acupuncture for arthralgia related to aromatase inhibitor use. Eur J Cancer 2014;50:267–76. 10.1016/j.ejca.2013.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liou KT, Baser R, Romero SAD, et al. Personalized electro-acupuncture versus auricular-acupuncture comparative effectiveness (PEACE): a protocol of a randomized controlled trial for chronic musculoskeletal pain in cancer survivors. Medicine 2020;99:e20085. 10.1097/MD.0000000000020085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mao JJ, Bruner DW, Stricker C, et al. Feasibility trial of electroacupuncture for aromatase inhibitor--related arthralgia in breast cancer survivors. Integr Cancer Ther 2009;8:123–9. 10.1177/1534735409332903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng X. Chinese acupuncture and moxibustion. Beijing, China: Foreign Languages Press, 1987. [Google Scholar]
- 58.Mao JJ, Farrar JT, Armstrong K, et al. De qi: Chinese acupuncture patients' experiences and beliefs regarding acupuncture needling sensation-an exploratory survey. Acupunct Med 2007;25:158–65. 10.1136/aim.25.4.158 [DOI] [PubMed] [Google Scholar]
- 59.MacDonald G. Medicine hands: massage therapy for people with cancer. Simon and Schuster, 2014. [Google Scholar]
- 60.Walton T. Medical conditions and massage therapy: a decision tree approach. Lippincott Williams & Wilkins, 2010. [Google Scholar]
- 61.Tango T. Power and sample size for the S:T repeated measures design combined with a linear mixed-effects model allowing for missing data. J Biopharm Stat 2017;27:963–74. 10.1080/10543406.2017.1293083 [DOI] [PubMed] [Google Scholar]
- 62.Farrar JT, Portenoy RK, Berlin JA, et al. Defining the clinically important difference in pain outcome measures. Pain 2000;88:287–94. 10.1016/S0304-3959(00)00339-0 [DOI] [PubMed] [Google Scholar]
- 63.Farrar JT, Pritchett YL, Robinson M, et al. The clinical importance of changes in the 0 to 10 numeric rating scale for worst, least, and average pain intensity: analyses of data from clinical trials of duloxetine in pain disorders. J Pain 2010;11:109–18. 10.1016/j.jpain.2009.06.007 [DOI] [PubMed] [Google Scholar]
- 64.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B Methodol 1995;57:289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 65.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann. Statist. 2001;29:1165–88. 10.1214/aos/1013699998 [DOI] [Google Scholar]
- 66.Kent DM, Rothwell PM, Ioannidis JPA, et al. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials 2010;11:85. 10.1186/1745-6215-11-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Henderson NC, Louis TA, Wang C, et al. Bayesian analysis of heterogeneous treatment effects for patient-centered outcomes research. Health Serv Outcomes Res Methodol 2016;16:213–33. 10.1007/s10742-016-0159-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang C, Louis TA, Henderson NC, et al. beanz: an R package for Bayesian analysis of heterogeneous treatment effects with a graphical user interface. J Stat Softw 2018;85:31. 10.18637/jss.v085.i07 [DOI] [Google Scholar]
- 69.Imai K, Ratkovic M. Estimating treatment effect heterogeneity in randomized program evaluation. Ann Appl Stat 2013;7:443–70. 10.1214/12-AOAS593 [DOI] [Google Scholar]
- 70.Wager S, Athey S. Estimation and inference of heterogeneous treatment effects using random forests. J Am Stat Assoc 2017:1–15. [Google Scholar]
- 71.Lu K, Luo X, Chen P-Y. Sample size estimation for repeated measures analysis in randomized clinical trials with missing data. Int J Biostat 2008;4:Article 9. 10.2202/1557-4679.1098 [DOI] [PubMed] [Google Scholar]
- 72.Mao JJ, Bowman MA, Xie SX, et al. Electroacupuncture versus gabapentin for hot flashes among breast cancer survivors: a randomized placebo-controlled trial. J Clin Oncol 2015;33:3615–20. 10.1200/JCO.2015.60.9412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mao JJ, Liou KT, Baser RE, et al. Effectiveness of electroacupuncture or auricular acupuncture vs usual care for chronic musculoskeletal pain among cancer survivors. JAMA Oncol 2021;7:720. 10.1001/jamaoncol.2021.0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dodd MJ, Cho MH, Cooper BA, et al. The effect of symptom clusters on functional status and quality of life in women with breast cancer. Eur J Oncol Nurs 2010;14:101–10. 10.1016/j.ejon.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miaskowski C, Cooper BA, Paul SM, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum 2006;33:E79–89. 10.1188/06.ONF.E79-E89 [DOI] [PubMed] [Google Scholar]
- 76.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg 2012;10:28–55. 10.1016/j.ijsu.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 77.MacPherson H, Altman DG, Hammerschlag R, et al. Revised standards for reporting interventions in clinical trials of acupuncture (stricta): extending the CONSORT statement. PLoS Med 2010;7:e1000261. 10.1371/journal.pmed.1000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-058281supp001.pdf (109.2KB, pdf)
bmjopen-2021-058281supp002.pdf (102.1KB, pdf)