Abstract
Background
Gabapentin and pregabalin are commonly prescribed medications to treat pain in patients with diabetic neuropathy. Gabapentin and pregabalin can cause fluid retention, which is hypothesized to be associated with cardiovascular diseases. However, whether long-term use of gabapentin and pregabalin is associated with adverse cardiovascular diseases remains unknown. This study aims to examine the association between gabapentin use, pregabalin use and several adverse cardiovascular events.
Methods
This retrospective cohort study used propensity score matching within patient electronic health records (EHRs) from a multicenter database with 106 million patients from 69 health care organizations in the US. The study population comprised 210,064 patients who had a diagnosis of diabetic neuropathy and were prescribed diabetic neuropathy medications in their EHRs. The exposure cohort comprised patients who were prescribed gabapentin or pregabalin to treat diabetic neuropathy. The comparison cohort comprised patients who were not prescribed either gabapentin or pregabalin but were prescribed other drugs to treat diabetic neuropathy. The outcomes of interest were myocardial infarcts, strokes, heart failure, peripheral vascular disease, and venous thromboembolic events. We calculated hazard ratios (HRs) and 95% confidence intervals (CIs) for 3-month and 5-year risk for adverse cardiovascular events between the propensity score-matched cohorts.
Results
Both gabapentin and pregabalin were associated with increased risk of 5-year adverse cardiovascular events compared with the comparison group. In patients prescribed gabapentin, the highest risk was observed for deep venous thrombosis (HR: 1.58, 95% CI 1.37–1.82), followed by pulmonary embolism (HR: 1.5, 95% CI 1.27–1.76), peripheral vascular disease (HR: 1.37, 95% CI 1.27–1.47), stroke (HR: 1.31, 95% CI 1.2–1.43), myocardial infarction (HR: 1.25, 95% CI 1.14–1.38) and heart failure (HR: 1.14, 95% CI 1.07–1.21). In patients prescribed pregabalin, the highest risk was observed for deep venous thrombosis (HR: 1.57, 95% CI 1.31–1.88), followed by peripheral vascular disease (HR: 1.35, 95% CI 1.22–1.49), myocardial infarction (HR: 1.29, 95% CI 1.13–1.47), pulmonary embolism (HR: 1.28, 95% CI 1.04–1.59), stroke (HR: 1.26, 95% CI 1.12–1.42), and heart failure (HR: 1.2, 95% CI 1.11–1.3). There were significant associations between short-term (3 month) gabapentin use and heart failure, myocardial infarction, peripheral vascular disease, deep venous thrombosis, and pulmonary embolism. Short-term (3 month) pregabalin use was associated with deep venous thrombosis, peripheral vascular disease.
Conclusion
In patients with diabetic neuropathy who were prescribed gabapentin and pregabalin, there is an increased risk for heart failure, myocardial infarction, peripheral vascular disease, stroke, deep venous thrombosis, and pulmonary embolism with long-term use. Our findings suggest that increased risk for adverse cardiovascular events, along with other side effects, the efficacy of pain control and the degree of tolerance of the patient, should be considered when prescribing gabapentin and pregabalin long-term in patients with diabetic neuropathy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-022-01610-9.
Keywords: Adverse cardiovascular events, Gabapentin, Pregabalin, Diabetic neuropathy, Cohort study
Introduction
The American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) provide annual guidance for clinicians. The standard of medical care in 2022 highlights cardiovascular disease management in patients with diabetes [1], as well as management of complications of diabetes, including diabetic neuropathy [2]. Neuropathic pain in diabetes can be severe and can impact quality of life [2]. Pregabalin and gabapentin are recommended as pharmacologic treatments for diabetic neuropathy [2]. While both medications were initially developed as anticonvulsants, pregabalin was approved to treat diabetic neuropathy in 2014 and gabapentin is often prescribed “off label” to treat diabetic neuropathy [3].
Both gabapentin and pregabalin can cause fluid retention by altering arterial myogenic tone [4]. Although fluid retention is not a recognized risk factor for cardiovascular disease, every medical condition that causes fluid retention—heart failure, nephrotic syndrome, cirrhosis, hypothyroidism, obstructive sleep apnea and Cushing’s syndrome—is associated with an increased risk of myocardial infarcts, strokes, and venous thromboembolic disease [5–19]. Moreover, previous studies have demonstrated that a number of medications that cause fluid retention are associated with an increased risk of adverse cardiovascular events. The list includes cyclo-oxygenase-2 inhibitors (COX-2 inhibitors) [20–23], nonselective non-steroidal anti-inflammatory drugs (NSAIDs) [22, 24, 25], hormone replacement therapy with estrogens and progestins [26, 27], oral contraceptives[28], insulins[29] and sulfonylureas [30, 31].
The consensus from multiple guidelines and systematic reviews is that gabapentinoids, serotonin norepinephrine reuptake inhibitors and tricyclic antidepressants have the best evidence to support their use in the treatment of diabetic neuropathic pain but comparative effectiveness studies to inform the best choice are lacking [32]. Opioid analgesics should be avoided due to their serious adverse effects and potential for addiction [3]. Long-term use of gabapentin and pregabalin is generally well tolerated and provides sustained efficacy [33, 34]. However, currently it remains unknown whether long-term use of gabapentin and pregabalin in patients with diabetic neuropathy is associated with increased risk for adverse cardiovascular events, including myocardial infarcts, strokes, heart failure, peripheral vascular disease, and venous thromboembolic events.
Methods
Database description
TriNetX research network is a de-identified population-level database with 106 million patients from 69 health care organizations, mostly large academic medical institutions with both inpatient and outpatient facilities at multiple locations across 50 states in the US, covering diverse geographic locations, age groups, racial and ethnic groups, income levels and insurance types [35]. Built-in statistical functions within TriNetX Analytics Platform perform statistical analyses on patient-level data and reports population level data and results without including protected health information (PHI) identifiers. MetroHealth System, Cleveland OH, Institutional Review Board (IRB) has determined that any research using TriNetX is not Human Subject Research and therefore exempt from IRB review.
Study population
The study population comprised of 210,064 patients who had a diagnosis of diabetic neuropathy and were prescribed with diabetic neuropathy medications in their EHRs. The study population was divided into two cohorts: (1) an Exposure cohort consisting of patients who were prescribed gabapentin or pregabalin to treat diabetic neuropathy (International Classification of Diseases (ICD-10) diagnosis code: E11.40 Type 2 diabetes mellitus with diabetic neuropathy, unspecified) and (2) a Comparison cohort consisting of patients who were not prescribed either gabapentin or pregabalin but were prescribed other commonly-prescribed drugs (topiramate, duloxetine, tapentadol, capsaicin, nortriptyline, carbamazepine, venlafaxine, amitriptyline, and mexiletine) to control diabetic neuropathy [36, 37]. Patients who took no prescription medication for diabetic neuropathy were excluded since they were likely to have relatively mild diabetic neuropathy. We controlled for severity of diabetic neuropathy by including only patients in both the exposure cohort and the comparison cohort who were taking at least one prescription medication for diabetic neuropathy. To investigate the long-term effect, the long-term users were defined with the additional requirement that they had another drug prescription recorded more than 3 years after the first prescription.
Statistical analysis
Outcome measures
The outcomes of interest were diagnoses of cardiovascular diseases including heart failure (ICD-10: I50 Heart failure), myocardial infarction (ICD-10: I21 Acute myocardial infarction), peripheral vascular disease (ICD-10: I73.9 Peripheral vascular diseases, unspecified), stroke (ICD-10: I63 Cerebral infarction), deep venous thrombosis (ICD-10: I82.40 Acute embolism and thrombosis of unspecified deep veins of lower extremity), and pulmonary embolism (ICD-10: I26 Pulmonary embolism).
Covariates
Covariates included demographics (age, gender, race, and ethnicity), risk factors of cardiovascular disease, initial drug indications including approved, and off-label uses, related medications, adverse socioeconomic circumstances, and specific risk factors for each disease.
The risk factors of cardiovascular diseases were hypertension (ICD-10: I10-I16 Hypertensive diseases), high cholesterol (ICD-10: E78.0 Pure hypercholesterolemia), obesity (ICD-10: E66 Overweight and obesity), diabetes (ICD-10: E08-E13 Diabetes mellitus), alcohol abuse (ICD-10: F10.1 Alcohol abuse), tobacco use (ICD-10: Z72.0 Tobacco use), use of NSAID (ICD-10: Z79.1 long-term (current) use of non-steroidal anti-inflammatories), obstructive sleep apnea [ICD-10: G47.33 Obstructive sleep apnea (adult) (pediatric)], end stage renal disease (ICD-10: N18.6 End stage renal disease), pre-existing heart failure (ICD-10: I50 Heart failure) and pre-existing peripheral vascular disease (ICD-10: I73.9 Peripheral vascular diseases, unspecified). Adverse socioeconomic circumstances were determined by incorporating ICD-10: Z55-Z65 Persons with potential health hazards related to socioeconomic and psychosocial circumstances. The medication list, presented in Additional file 1: Table S2, includes the medications for diabetic neuropathy, medications that can cause fluid retention and medications that can raise the blood pressure.
The initial indications were diabetic neuropathy (ICD-10: E11.40 Type 2 diabetes mellitus with diabetic neuropathy, unspecified), seizure (ICD-10: G40 Epilepsy and recurrent seizures), postherpetic neuralgia (ICD-10: B02.29 Other postherpetic nervous system involvement), fibromyalgia (ICD-10: M79.7 Fibromyalgia), pain (ICD-10: R52 Pain, unspecified), neuropathic pain (ICD-10: M79.2 Neuralgia and neuritis, unspecified), restless leg syndrome (ICD-10: G25.81 Restless leg syndrome).
The specific risk factors and complications for heart failure (ICD-10: I50 Heart failure) were myocardial infarction (ICD-10: I21 Acute myocardial infarction), myocarditis (ICD-10: I51.4 Myocarditis, unspecified), and arrhythmias (ICD-10: I49 Other cardiac arrhythmias); for myocardial Infarction (ICD-10: I21 Acute myocardial infarction) were sudden cardiac arrest (ICD-10: Z86.74 Personal history of sudden cardiac arrest), and metabolic syndrome (ICD-10: E88.81 Metabolic syndrome); for stroke (ICD-10: I63 Cerebral infarction) were atrial fibrillation (ICD-10: I48 Atrial fibrillation and flutter), arrhythmias (ICD-10: I49 Other cardiac arrhythmias), and depression (ICD-10: F32 Depressive episode), for peripheral vascular disease (ICD-10: I73.9 Peripheral vascular diseases, unspecified) were stroke (ICD-10: I63 Cerebral infarction), myocardial infarction (ICD-10: I21 Acute myocardial infarction), and atherosclerosis (ICD-10: I70 Atherosclerosis); for deep venous thrombosis (ICD-10: I82.40 Acute embolism and thrombosis of unspecified deep veins of lower extremity) were inflammatory bowel disease (ICD-10: K51.9 Ulcerative colitis, unspecified), and cancer (ICD-10: C80.1 Malignant (primary) neoplasm, unspecified); for pulmonary embolism (ICD-10: I26 Pulmonary embolism) was cancer (ICD-10: C80.1 Malignant (primary) neoplasm, unspecified). The full list of covariates, their standardized names, codes, and data types in TriNetX are described in Additional file 1: Table S2.
The Exposure cohort and Comparison cohort were propensity-score matched (1:1 matching using the nearest neighbor greedy matching algorithm) for covariates described above. Kaplan–Meier analysis was performed to estimate the risk of each cardiovascular adverse event in the Exposure and Comparison cohorts. The time index for the exposure and the comparison is the drug first prescription date. 3-month outcomes and 5-year outcomes are determined by following patients with studied outcomes within 3-month and 5-year time frame since the drug prescription. Hazard ratios, 95% confidence interval and p-value were calculated at significance set at p-value < 0.05 (two-sided).
Results
Patient characteristics
Table 1 and Additional file 1: Table S3 presents characteristics of the gabapentin and comparison groups for long-term use before and after propensity score matching for covariates of deep venous thrombosis. Data for pregabalin and patient characteristics for short-term drug use are in the Supplement (Additional file 1: Table S4–S6). Patients with diabetic neuropathy who were prescribed gabapentin were younger than those prescribed comparison drugs (60.5 versus 62.8), comprised fewer women, more African Americans, Hispanics and had fewer comorbidities. After propensity-score matching, the two cohorts were balanced.
Table 1.
The characteristics of the patients who were prescribed gabapentin and comparison drugs for long term before and after propensity-score matching for covariates of deep venous thrombosis
| Characteristics | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Cohort, No. (%) | Cohort, No. (%) | |||||
| Gabapentin | Comparison | SMD | Gabapentin | Comparison | SMD | |
| Cohort size | 38,603 | 8359 | 7050 | 7050 | ||
| Age at Index | 60.5 ± 12.3 | 62.8 ± 12.5 | 0.19* | 61.5 ± 11.7 | 62.2 ± 12.5 | 0.06 |
| Female | 54.0 | 61.7 | 0.16* | 63.5 | 61.6 | 0.04 |
| Race and ethnicity | ||||||
| White | 64.7 | 72.7 | 0.17* | 70.9 | 71.7 | 0.02 |
| African American | 26.5 | 16.4 | 0.25* | 19.4 | 17.6 | 0.05 |
| Unknown Race | 7.0 | 9.5 | 0.09 | 8.5 | 9.3 | 0.03 |
| Hispanic or Latino | 9.5 | 5.9 | 0.13* | 6.4 | 6.5 | 0.01 |
| Asian | 1.2 | 0.8 | 0.04 | 0.7 | 0.8 | 0.01 |
| Cardiovascular risk factors | ||||||
| Hypertensive diseases | 85.0 | 87.4 | 0.07 | 86.9 | 87.1 | 0.01 |
| Obesity | 40.1 | 47.2 | 0.14* | 47.3 | 47.1 | 0.00 |
| High cholesterol | 23.1 | 28.6 | 0.13* | 28.0 | 28.1 | 0.00 |
| Obstructive sleep apnea | 18.0 | 24.5 | 0.16* | 24.4 | 23.9 | 0.01 |
| Tobacco use | 4.1 | 6.7 | 0.11* | 5.9 | 6.5 | 0.03 |
| Pre-existing heart failure | 17.8 | 20.0 | 0.06 | 19.1 | 20.1 | 0.02 |
| Pre-existing peripheral vascular disease | 14.4 | 14.3 | 0.00 | 14.3 | 14.1 | 0.01 |
| End stage renal disease | 4.5 | 4.3 | 0.01 | 3.8 | 4.2 | 0.02 |
| Alcohol abuse | 4.0 | 3.7 | 0.01 | 4.1 | 3.8 | 0.01 |
| Long term use of NSAID | 1.4 | 2.6 | 0.09 | 2.0 | 2.3 | 0.02 |
| Adverse socioeconomic circumstances | 4.6 | 7.6 | 0.12* | 6.7 | 7.5 | 0.03 |
| Initial use | ||||||
| Postherpetic neuralgia | 0.6 | 0.4 | 0.03 | 0.5 | 0.5 | 0.01 |
| Fibromyalgia | 11.7 | 14.0 | 0.07 | 15.7 | 14.6 | 0.03 |
| Pain | 9.5 | 11.7 | 0.07 | 11.2 | 11.8 | 0.02 |
| Restless legs syndrome | 4.4 | 4.9 | 0.03 | 5.5 | 5.0 | 0.02 |
| Epilepsy and recurrent seizures | 2.7 | 6.3 | 0.17* | 5.3 | 5.7 | 0.02 |
| Neuralgia and neuritis, unspecified | 4.7 | 4.1 | 0.03 | 4.5 | 4.3 | 0.01 |
| Specific risk factor of deep venous thrombosis | ||||||
| Pre-existing deep venous thrombosis | 2.9 | 3.6 | 0.04 | 3.4 | 3.5 | 0.01 |
| Cancer | 1.2 | 2.0 | 0.07 | 1.8 | 2.0 | 0.01 |
| Inflammatory bowel disease | 0.4 | 0.6 | 0.03 | 0.5 | 0.6 | 0.02 |
SMD standardized mean differences
*SMD greater than 0.1, a threshold being recommended for declaring imbalance
Risk of 5-year adverse cardiovascular events associated with gabapentin and pregabalin in patients with diabetic neuropathy
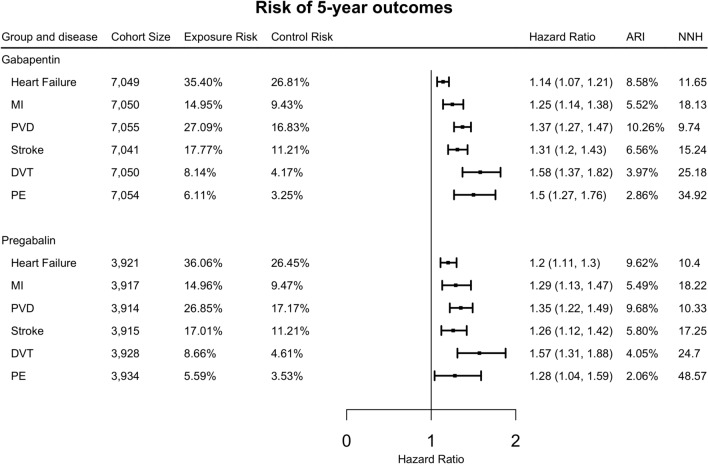
As shown in Fig. 1, both gabapentin and pregabalin were associated with increased risk of 5-year adverse cardiovascular events compared with the comparison group. In patients prescribed gabapentin, the highest risk was observed for deep venous thrombosis (HR: 1.58, 95% CI 1.37–1.82), followed by pulmonary embolism (HR: 1.5, 95% CI 1.27–1.76), peripheral vascular disease (HR: 1.37, 95% CI 1.27–1.47), stroke (HR: 1.31, 95% CI 1.2–1.43), myocardial infarction (HR: 1.25, 95% CI 1.14–1.38) and heart failure (HR: 1.14, 95% CI 1.07–1.21). In patients prescribed pregabalin, the highest risk was observed for deep venous thrombosis (HR: 1.57, 95% CI 1.31–1.88), followed by peripheral vascular disease (HR: 1.35, 95% CI 1.22–1.49), myocardial infarction (HR: 1.29, 95% CI 1.13–1.47), pulmonary embolism (HR: 1.28, 95% CI 1.04–1.59), stroke (HR: 1.26, 95% CI 1.12–1.42), and heart failure (HR: 1.2, 95% CI 1.11–1.3).
Fig. 1.
Risk of 5-year outcomes in propensity-score matched exposure groups and comparison groups in the patients who had taken the drugs long term. (ARI: absolute risk increase, NNH: number need to harm, MI: myocardial infarction, PVD: peripheral vascular disease, DVT: deep venous thrombosis, PE: pulmonary embolism)
Risk of 3-month adverse cardiovascular events associated with gabapentin and pregabalin in patients with diabetic neuropathy
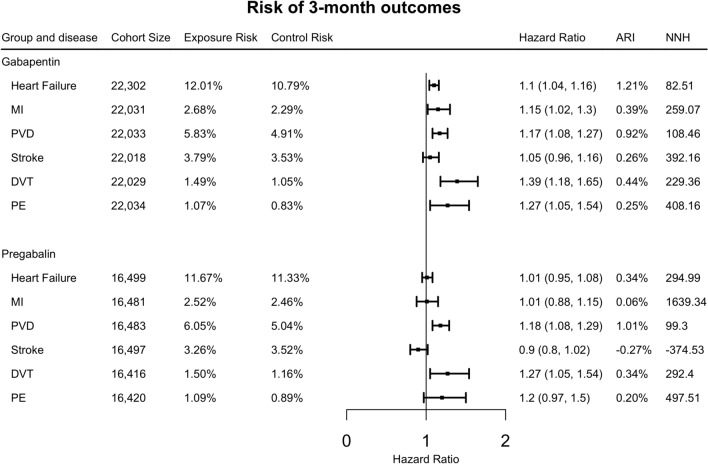
As shown in Fig. 2, gabapentin was associated with an increased risk of deep venous thrombosis (HR: 1.39, 95% CI 1.18–1.65), pulmonary embolism (HR: 1.27, 95% CI 1.05–1.54), peripheral vascular disease (HR: 1.17, 95% CI 1.08–1.27), myocardial infarction (HR: 1.15, 95% CI 1.02–1.3), and heart failure (HR: 1.1, 95% CI 1.04–1.16) compared with the comparison group within the 3-month time frame following the drug treatment. There was no significant association between gabapentin and risk of stroke (HR: 1.05, 95% CI 0.96–1.16). Pregabalin was associated with an increased risk of 3-month adverse cardiovascular events for deep venous thrombosis (HR: 1.27, 95% CI 1.05–1.54), peripheral vascular disease (HR: 1.18, 95% CI 1.08–1.29). There was no association between pregabalin and risk of 3-month adverse cardiovascular events for pulmonary embolism (HR: 1.2, 95% CI 0.97–1.5), myocardial infarction (HR: 1.01, 95% CI 0.88–1.15), stroke (HR: 0.9, 95% CI 0.8–1.02), and heart failure (HR: 1.01, 95% CI 0.95–1.08).
Fig. 2.
Risk of 3-month outcomes in propensity-score matched exposure groups and comparison groups in the patients who had ever taken the drugs. (ARI: absolute risk increase, NNH: number need to harm, MI: myocardial infarction, PVD: peripheral vascular disease, DVT: deep venous thrombosis, PE: pulmonary embolism)
Discussion
Using the large-scale nation-wide database of patient de-identified TriNetX EHRs, our study found that there is an increased risk of adverse cardiovascular events, including heart failure, myocardial infarction, peripheral vascular disease, stroke, deep venous thrombosis, and pulmonary embolism in patients with diabetic neuropathy, following long-term use of gabapentin and pregabalin, with highest risk for deep venous thrombosis. There were significant associations between short-term (3 month) gabapentin use and heart failure, myocardial infarction, peripheral vascular disease, deep venous thrombosis, and pulmonary embolism. Short-term (3 month) pregabalin use was associated with deep venous thrombosis and peripheral vascular disease.
Associations between gabapentin, pregabalin and heart failure have been reported in case reports [38, 39], but not in observational studies [40, 41]. These case reports and observational studies are limited to short-term follow up [38–41]. To the best of our knowledge, this study is among the first to report that long-term use of gabapentin and pregabalin is associated with increased risk for adverse cardiovascular events in patients with diabetic neuropathy. While the cardiovascular effects of gabapentin and pregabalin need to be verified in other populations that use these drugs, our findings call for thoughtful consideration of risk and benefit when deciding to use gabapentin and pregabalin long-term in patients with diabetic neuropathy, and possibly in other populations as well.
One possible explanation for the findings is that gabapentin and pregabalin can alter arterial myogenic tone and cause fluid retention [4]. Fluid retention causes either an increase in cardiac output or an increase in blood pressure [42]. Velocity of blood flow increases either way, thereby increasing turbulence of blood flow [43]. Increased turbulence reduces shear stress upon arterial walls, thereby increasing endothelial dysfunction, which is a well-established cardiovascular risk factor [44]. However, our finding cannot rule out other potential mechanisms. Future research is necessary to further understand the underlying mechanism of the observed increased risk of CVDs among patients with diabetic neuropathy who were prescribed gabapentin and pregabalin. Additional work is needed to determine if other medications that cause fluid retention are also associated with increased adverse cardiovascular events. Anti-hypertensive medications that cause fluid retention, notably calcium channel blockers, alpha receptor antagonists and hydralazine, have favorable cardiovascular effects. Aromatase inhibitors and androgen deprivation therapy cause fluid retention but increase the longevity of cancer patients. For medications that do not improve longevity, do not lower blood pressure (BP) and that are used long-term, such as androgens, corticosteroids (when used to treat non-life-threatening conditions), mineralocorticoids, pramipexole and ropinirole, patients and clinicians need to know if there is an increased risk of cardiovascular disease. For androgens, the research literature is inconclusive concerning cardiovascular risk: some studies have documented an increased risk of adverse cardiovascular events while other studies have failed to do so [45, 46]. Cardiovascular safety data is needed for medications that cause fluid retention but do not increase longevity or lower BP.
If an increased risk of adverse cardiovascular events is also associated with other medications that cause fluid retention, then our study has implications for the pharmaceutical industry and for the entities responsible for regulating pharmaceutical products: the Food and Drug Administration in the United States, the European Medicines Agency in the European Union, the Medicines and Healthcare Products Regulatory Agency in Britain, and the Health Products and Food Branch of Health Canada. If fluid retention is a risk factor for cardiovascular disease, then data regarding the fluid retention properties of medications would be relevant to the drug approval process. In that circumstance, for medications that do not extend life, do not lower BP and are intended to be used long term, regulatory agencies should require new drug applications to provide fluid retention data. Cardiovascular safety data would be warranted for medications that cause fluid retention.
Many patients take medications that cause fluid retention. One implication of our study is that it may be possible to mitigate the cardiovascular consequences of medications that cause fluid retention, at least in part, by simultaneously prescribing a diuretic. This is a potential area for future research.
Numerous risk factors for cardiovascular diseases (CVDs) have been recognized, including age, gender, smoking status, hypertension, diabetes, hypercholesterolemia, physical inactivity, obstructive sleep apnea and overweight [16, 47]. Findings from this study suggest that long-term use of gabapentin/pregabalin is another risk factor for CVDs in patients with diabetic neuropathy. Future research is needed to understand whether these risk factors act synergistically or independently in increasing the risk for CVDs in specific patient populations.
Several limitations of this study should be noted. First, the patients in the TriNetX EHR database represent people who had medical encounters with the 69 contributing health care systems. The generalizability of the results needs to be validated in other national data resources. Second, there is no data linkage between the drug prescriptions and diagnosis codes. We assumed that if the diagnosis of diabetic neuropathy happened before the drug prescription, then the patients were taking the drug for treating diabetic neuropathy. Nonetheless, there might be other indications for prescribing these drugs. We addressed this problem by controlling the other initial uses in propensity score matching. Third, to control for severity of diabetic neuropathy, we excluded patients not taking medications for treatment of diabetic neuropathy since they might have relatively mild diabetic neuropathy. The control cohort included patients taking other medications to treat diabetic neuropathy. The exposure group could also take other medications to treat diabetic neuropathy, in addition to gabapentin and pregabalin (“polypharmacy”). For propensity-score matching, the exposure and control cohorts were matched for specific drugs that are commonly prescribed to treat diabetic neuropathy. Fourth, due to the nature of EHR data, TriNetX platform captures patient information that is entered during patients’ encounter with healthcare organization. Consequently, there is no guarantee of completeness for specific research purposes. For example, other events (e.g., diet, certain lifestyle factors, employment) that happened outside of patient medical encounters may not be captured in patient EHRs. However, because we compared exposure and control cohorts all from TriNetX database, selection bias should not substantially affect the relative risk analyses. Though the exposure and control cohorts were extensively propensity-score matched for demographics, original disease indications, risk factors, and other commonly prescribed medications, we acknowledge the limitation of unmeasured confounders that are not included in patient EHRs. Fifth, drug duration was an important covariate. We used a computerized database of medical information to define exposure to gabapentin and pregabalin. The database identifies only that the medications were prescribed, not that they were consumed, and electronic health records cannot provide information about how long patients actually take any given drug. Nonetheless, automated pharmacy records are generally reliable sources of information on drug use [48, 49]. We addressed this problem by defining long-term users with an additional query for the patients who have another drug prescription recorded more than 3 years after the first drug prescription. Sixth, we could not determine the doses of gabapentin or pregabalin that were prescribed. It is possible that the cardiovascular risk of these medications is dose dependent such that low doses may not be associated with an increased risk of adverse cardiovascular events.
Conclusion
We identified an increased risk of adverse cardiovascular events associated with use of gabapentin and pregabalin in patients with diabetic neuropathy. Our findings need to be verified in other populations of gabapentin and pregabalin users and with other medications that cause fluid retention. If our results are generalizable, increased risk of adverse cardiovascular outcomes, along with other side effects, the efficacy of pain control and the degree of tolerance of the patient, should inform the decision-making process when prescribing gabapentin, pregabalin, and perhaps other medications that cause fluid retention.
Supplementary Information
Additional file 1: Table S1. Outcomes and their standardized names, codes and data types that are used in the TriNetX database. Table S2. Covariates and their standardized names, codes and data types that are used in the TriNetX database. Table S3. The characteristics of the patients who were prescribed gabapentin and comparison drugs for long term before and after propensity-score matching for covariates of deep venous thrombosis. Table S4. The characteristics of the patients who were prescribed pregabalin and comparison drugs for long term before and after propensity-score matching for covariates of deep venous thrombosis. Table S5. The characteristics of the patients who were prescribed gabapentin and comparison drugs before and after propensity-score matching for covariates of deep venous thrombosis. Table S6. The characteristics of the patients who were prescribed pregabalin and comparison drugs before and after propensity-score matching for covariates of deep venous thrombosis.
Acknowledgements
Not applicable.
Abbreviations
- EHRs
Electronic health records
- CVDs
Cardiovascular diseases
- HRs
Hazard ratios
- CIs
Confidence intervals
- NSAIDs
Nonselective non-steroidal anti-inflammatory drugs
- BP
Blood pressure
- SMD
Standardized mean difference
Author contributions
YP, RPB, and RX conceived and designed this study. YP implemented the statistical analysis. DCK facilitated access to TriNetX. RX supervised the study and obtained the funding. PBD critically contributed to study design, results interpretation, and manuscript preparation. All the authors read and approved the manuscript.
Funding
We acknowledge fundings from National Institute on Aging (Grants nos. AG057557, AG061388, AG062272, AG076649), National Institute on Alcohol Abuse and Alcoholism (Grant no. AA029831), the Clinical and Translational Science Collaborative (CTSC) of Cleveland (Grant no. TR002548), National Cancer Institute Case Comprehensive Cancer Center (CA221718, CA043703, CA2332216). The funders have no roles in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Availability of data and materials
The data that support the findings of this study are available from TriNetX [35] (https://trinetx.com/).
Declarations
Ethics approval and consent to participate
The MetroHealth System, Cleveland, Ohio, institutional review board has determined that any research using TriNetX is not human participants research and therefore is exempt from institutional review board review.
Consent for publication
Not applicable.
Competing interests
RPB has a patent for the concept that one can use markers of fluid retention to gauge the cardiovascular risk of a drug. No other disclosures were reported.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Robert P. Blankfield, Email: blankfieldmd@gmail.com
Rong Xu, Email: rxx@case.edu.
References
- 1.American Diabetes Association Professional Practice Committee 10. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(Supplement_1):S144–S174. doi: 10.2337/dc22-S010. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Professional Practice Committee 12. Retinopathy, neuropathy, and foot care: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(Supplement_1):S185–S194. doi: 10.2337/dc22-S012. [DOI] [PubMed] [Google Scholar]
- 3.Goodman CW, Brett AS. A clinical overview of off-label use of gabapentinoid drugs. JAMA Intern Med. 2019;179(5):695. doi: 10.1001/jamainternmed.2019.0086. [DOI] [PubMed] [Google Scholar]
- 4.Largeau B, et al. Gabapentinoid-induced peripheral edema and acute heart failure: a translational study combining pharmacovigilance data and in vitro animal experiments. Biomed Pharmacother. 2022;149:112807. doi: 10.1016/j.biopha.2022.112807. [DOI] [PubMed] [Google Scholar]
- 5.Hjalmarsson C, et al. Risk of stroke in patients with heart failure and sinus rhythm: data from the Swedish Heart Failure Registry. ESC Heart Fail. 2021;8(1):85–94. doi: 10.1002/ehf2.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg B, et al. Ejection fraction, B-type natriuretic peptide and risk of stroke and acute myocardial infarction among patients with heart failure. Clin Cardiol. 2019;42(2):277–284. doi: 10.1002/clc.23140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger JS, et al. Risk of ischemic stroke in patients newly diagnosed with heart failure: focus on patients without atrial fibrillation. J Cardiac Fail. 2019;25(6):436–447. doi: 10.1016/j.cardfail.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Kang S-H, et al. Risk of stroke in congestive heart failure with and without atrial fibrillation. Int J Cardiol. 2017;248:182–187. doi: 10.1016/j.ijcard.2017.07.056. [DOI] [PubMed] [Google Scholar]
- 9.Fanola CL, et al. Incident heart failure and long-term risk for venous thromboembolism. J Am Coll Cardiol. 2020;75(2):148–158. doi: 10.1016/j.jacc.2019.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Go AS, et al. Primary nephrotic syndrome and risks of ESKD, cardiovascular events, and death: the Kaiser permanente nephrotic syndrome study. JASN. 2021;32(9):2303–2314. doi: 10.1681/ASN.2020111583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol. 2012;7(3):513–520. doi: 10.2215/CJN.10131011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambrosino P, et al. The risk of venous thromboembolism in patients with cirrhosis. Thromb Haemost. 2017;26(01):139–148. doi: 10.1160/TH16-06-0450. [DOI] [PubMed] [Google Scholar]
- 13.Xiong J, et al. Cirrhosis and risk of stroke: a systematic review and meta-analysis. Atherosclerosis. 2018;275:296–303. doi: 10.1016/j.atherosclerosis.2018.06.876. [DOI] [PubMed] [Google Scholar]
- 14.Ning Y, et al. What is the association of hypothyroidism with risks of cardiovascular events and mortality? A meta-analysis of 55 cohort studies involving 1,898,314 participants. BMC Med. 2017;15(1):1–15. doi: 10.1186/s12916-017-0777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danescu LG, et al. Venous thromboembolism in patients hospitalized with thyroid dysfunction. Clin Appl Thromb Hemost. 2009;15(6):676–680. doi: 10.1177/1076029609336856. [DOI] [PubMed] [Google Scholar]
- 16.Shah NA, Yaggi HK, Concato J, Mohsenin V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep and Breathing. 2010;14(2):131–136. doi: 10.1007/s11325-009-0298-7. [DOI] [PubMed] [Google Scholar]
- 17.Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc. 2004;79(8):1036–1046. doi: 10.4065/79.8.1036. [DOI] [PubMed] [Google Scholar]
- 18.Lippi G, Mattiuzzi C, Franchini M. Sleep apnea and venous thromboembolism. Thromb Haemost. 2015;114(11):958–963. doi: 10.1160/TH15-03-0188. [DOI] [PubMed] [Google Scholar]
- 19.van der Pas R, Leebeek F, Hofland L, De Herder W, Feelders R. Hypercoagulability in C ushing’s syndrome: prevalence, pathogenesis and treatment. Clin Endocrinol. 2013;78(4):481–488. doi: 10.1111/cen.12094. [DOI] [PubMed] [Google Scholar]
- 20.Bombardier C, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343(21):1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 21.Nussmeier NA, Langford RM, Boyce SW. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005 doi: 10.1056/NEJMoa050330. [DOI] [PubMed] [Google Scholar]
- 22.McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296(13):1633–1644. doi: 10.1001/jama.296.13.jrv60011. [DOI] [PubMed] [Google Scholar]
- 23.Jüni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet. 2004;364:2021. doi: 10.1016/S0140-6736(04)17514-4. [DOI] [PubMed] [Google Scholar]
- 24.Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the american heart association. Circulation. 2007;115(12):1634–1642. doi: 10.1161/CIRCULATIONAHA.106.181424. [DOI] [PubMed] [Google Scholar]
- 25.Cooper C, et al. Safety of oral non-selective non-steroidal anti-inflammatory drugs in osteoarthritis: what does the literature say? Drugs Aging. 2019;36(S1):15–24. doi: 10.1007/s40266-019-00660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossouw JE. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 27.Anderson GL, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1775. [DOI] [PubMed] [Google Scholar]
- 28.Roach REJ, Helmerhorst FM, Lijfering WM, Stijnen T, Algra A, Dekkers OM. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD011054.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roumie CL, et al. Association between intensification of metformin treatment with insulin vs sulfonylureas and cardiovascular events and all-cause mortality among patients with diabetes. JAMA. 2014;311(22):2288. doi: 10.1001/jama.2014.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pantalone KM, et al. The risk of developing coronary artery disease or congestive heart failure, and overall mortality, in type 2 diabetic patients receiving rosiglitazone, pioglitazone, metformin, or sulfonylureas: a retrospective analysis. Acta Diabetol. 2009;46(2):145–154. doi: 10.1007/s00592-008-0090-3. [DOI] [PubMed] [Google Scholar]
- 31.Schramm TK, et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr077. [DOI] [PubMed] [Google Scholar]
- 32.Feldman EL, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):42. doi: 10.1038/s41572-019-0097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Putzke JD, Richards JS, Kezar L, Hicken BL, Ness TJ. Long-term use of gabapentin for treatment of pain after traumatic spinal cord injury. Clin J Pain. 2002;18(2):116–121. doi: 10.1097/00002508-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Onouchi K, Koga H, Yokoyama K, Yoshiyama T. An open-label, long-term study examining the safety and tolerability of pregabalin in Japanese patients with central neuropathic pain. J Pain Res. 2014;7:439–447. doi: 10.2147/JPR.S63028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.“TriNetX,” TriNetX. https://trinetx.com/. Accessed 1 Jun 2022.
- 36.Azhary H, Farooq MU, Bhanushali M, Majid A, Kassab MY. Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81(7):6. [PubMed] [Google Scholar]
- 37.Drugs used to treat Diabetic Peripheral Neuropathy. Drug.com. https://www.drugs.com/condition/diabetic-neuropathy.html?submitted=true&category_id=&include_rx=true&include_otc=true&show_off_label=true&only_generics=true#sortby. Accessed 1 June 2022.
- 38.Erdoğan G, Ceyhan D, Güleç S. Possible heart failure associated with pregabalin use: case report. Agri. 2011;23:80. [PubMed] [Google Scholar]
- 39.Murphy N, Mockler M, Ryder M, Ledwidge M, McDonald K. Decompensation of chronic heart failure associated with pregabalin in patients with neuropathic pain. J Cardiac Fail. 2007;13(3):227–229. doi: 10.1016/j.cardfail.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Lund M, Poulsen G, Pasternak B, Worm Andersson N, Melbye M, Svanström H. Use of pregabalin and worsening heart failure: a nationwide cohort study. Drug Saf. 2020;43(10):1035–1044. doi: 10.1007/s40264-020-00969-6. [DOI] [PubMed] [Google Scholar]
- 41.Ho JM-W, et al. Pregabalin and heart failure: a population-based study. Pharmacoepidemiol Drug Saf. 2017;26(9):1087–1092. doi: 10.1002/pds.4239. [DOI] [PubMed] [Google Scholar]
- 42.Blankfield RP. Implications of calculated intravascular volume changes upon atherosclerotic cardiovascular disease. Clin Hemorheol Microcirc. 2008;38(2):75–81. [PubMed] [Google Scholar]
- 43.Blankfield RP. Calculated effect of fluid retention upon velocity of blood flow and turbulence: implications for atherosclerosis. Clin Hemorheol Microcirc. 2011;47(2):79–86. doi: 10.3233/CH-2010-1369. [DOI] [PubMed] [Google Scholar]
- 44.Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1(3):183–198. [PMC free article] [PubMed] [Google Scholar]
- 45.Basaria S, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kloner RA, Carson C, Dobs A, Kopecky S, Mohler ER. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67(5):545–557. doi: 10.1016/j.jacc.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Wood D. Established and emerging cardiovascular risk factors. Am Heart J. 2001;141(2):S49–S57. doi: 10.1067/mhj.2001.109951. [DOI] [PubMed] [Google Scholar]
- 48.West SL, Savitz DA, Koch G, Strom BL, Guess HA, Hartzema A. Recall accuracy for prescription medications: self-report compared with database information. Am J Epidemiol. 1995;142(10):1103–1112. doi: 10.1093/oxfordjournals.aje.a117563. [DOI] [PubMed] [Google Scholar]
- 49.Leister KA, Drew Edwards WA, Christensen DB, Clark H. A comparison of patient drug regimens as viewed by the physician, pharmacist and patient”. Med Care. 1981;19:658–664. doi: 10.1097/00005650-198106000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Outcomes and their standardized names, codes and data types that are used in the TriNetX database. Table S2. Covariates and their standardized names, codes and data types that are used in the TriNetX database. Table S3. The characteristics of the patients who were prescribed gabapentin and comparison drugs for long term before and after propensity-score matching for covariates of deep venous thrombosis. Table S4. The characteristics of the patients who were prescribed pregabalin and comparison drugs for long term before and after propensity-score matching for covariates of deep venous thrombosis. Table S5. The characteristics of the patients who were prescribed gabapentin and comparison drugs before and after propensity-score matching for covariates of deep venous thrombosis. Table S6. The characteristics of the patients who were prescribed pregabalin and comparison drugs before and after propensity-score matching for covariates of deep venous thrombosis.
Data Availability Statement
The data that support the findings of this study are available from TriNetX [35] (https://trinetx.com/).