Abstract
Background
The triglyceride–glucose (TyG) index, which is a reliable surrogate marker of insulin resistance (IR), has been associated with cardiovascular diseases. However, evidence of the impact of the TyG index on the severity of coronary artery disease (CAD) is limited. This study investigated the relationship between the TyG index and CAD severity of individuals with different glucose metabolic statuses.
Methods
This study enrolled 2792 participants with CAD in China between January 1, 2018 and December 31, 2021. All participants were divided into groups according to the tertiles of the TyG index as follows: T1 group, TyG index < 6.87; T2 group, TyG index ≥ 6.87 to < 7.38; and T3 group, TyG index ≥ 7.38. The glucose metabolic status was classified as normal glucose regulation, pre-diabetes mellitus (pre-DM), and diabetes mellitus according to the standards of the American Diabetes Association. CAD severity was determined by the number of stenotic vessels (single-vessel CAD versus multi-vessel CAD).
Results
We observed a significant relationship between the TyG index and incidence of multi-vessel CAD. After adjusting for sex, age, body mass index, smoking habits, alcohol consumption, hypertension, estimated glomerular filtration rate, antiplatelet drug use, antilipidemic drug use, and antihypertensive drug use in the logistic regression model, the TyG index was still an independent risk factor for multi-vessel CAD. Additionally, the highest tertile of the TyG group (T3 group) was correlated with a 1.496-fold risk of multi-vessel CAD compared with the lowest tertile of the TyG group (T1 group) (odds ratio [OR], 1.496; 95% confidence interval [CI], 1.183–1.893; P < 0.001) in the multivariable logistic regression model. Furthermore, a dose–response relationship was observed between the TyG index and CAD severity (non-linear P = 0.314). In the subgroup analysis of different glucose metabolic statuses, the T3 group (OR, 1.541; 95% CI 1.013–2.344; P = 0.043) were associated with a significantly higher risk of multi-vessel CAD in individuals with pre-DM.
Conclusions
An increased TyG index was associated with a higher risk of multi-vessel CAD. Our study indicated that TyG as an estimation index for evaluating IR could be a valuable predictor of CAD severity, especially for individuals with pre-DM.
Keywords: Triglyceride–glucose index, Diabetes mellitus, Coronary artery disease
Background
Coronary artery disease (CAD), a cardiovascular disease (CVD) caused by coronary artery stenosis, is the main cause of death globally [1]. Because of aging and unhealthy lifestyle habits, morbidity and mortality caused by CAD are increasing; furthermore, CAD leads to a serious public health burden [2].
Recently, the incidences of type 2 diabetes mellitus (T2DM) and insulin resistance (IR) have increased remarkably with the improvement of living standards [3–5]. T2DM is a known risk factor that affects CAD progression and treatment strategies [6–8]. IR is a critical mechanism in the development of diabetes mellitus (DM) and has been broadly considered as a risk factor for atherosclerotic cardiovascular diseases [9–11]. The triglyceride–glucose (TyG) index, which is calculated as follows: Ln [fasting triglycerides (mg/dL) × fasting plasma glucose (mg/dL) / 2], is considered a dependable surrogate marker of IR [12, 13]. Previous studies have shown a significant relationship between the TyG index and incidence of CVDs, including coronary artery stenosis, coronary artery calcification, and carotid artery atherosclerosis, despite the presence of diabetes [14–17]. A recent large-scale study performed in Chinas suggested that an increased TyG index is independently correlated with a higher risk of myocardial infarction (MI) and emphasized the importance of monitoring the TyG index to distinguish individuals at high risk for MI [18].
Coronary angiography (CAG) as the gold standard for diagnosing CAD is an accurate and widely used imaging modality that aims to identify the number and degree of coronary artery stenosis. Participants with ≥ 50% lumen stenosis in at least one major coronary artery based on CAG findings were diagnosed with CAD [19]. Furthermore, the severity of CAD was based on the number of stenotic vessels and has a crucial role in the prognosis of CVD. Participants with multi-vessel CAD are at higher risk for CVD than those with single-vessel CAD, especially those with an abnormal glucose metabolic status [19, 20]. A recent study suggested that the TyG index is associated with the risk of multi-vessel CAD for the DM population, but not with that for individuals with pre-diabetes mellitus (pre-DM) or normoglycemia (NGR) [19]. However, the results might be attributable to a lack of power because of the small population enrolled in that study. Evidence of the effect of the TyG index on CAD severity is limited. Therefore, this study aimed to explore the relationship between the TyG index and CAD severity in a large cohort of participants with CAD and different glucose metabolic statuses.
Methods
Ethics statements
This retrospective observational cohort study conformed to the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Hospital. Written informed consents were obtained from all participants.
Study design and population
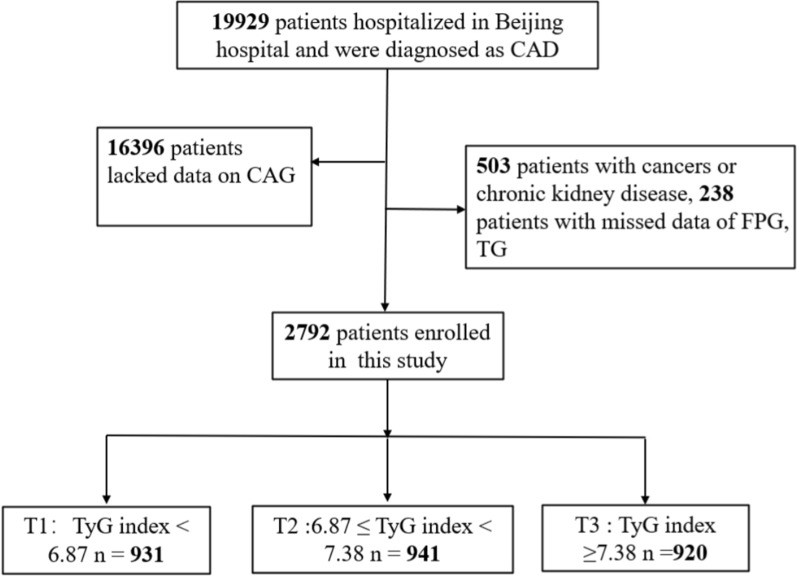
We enrolled 19,929 participants hospitalized at Beijing Hospital who were diagnosed with CAD from January 1, 2016 to December 30, 2021. We excluded 16,396 participants who lacked data regarding CAG, 503 participants with cancer or chronic kidney disease, and 238 participants with missing fasting plasma glucose (FPG) and triglyceride data (Fig. 1). Finally, 2792 participants were included in the final statistical analysis that investigated the relationship between the TyG index and CAD severity. We divided the enrolled participants into three groups according to the tertiles of the TyG index as follows: T1 group, TyG index < 6.87 (n = 931); T2 group, TyG index ≥ 6.87 to < 7.38 (n = 941); and T3 group, TyG index ≥ 7.38 (n = 920).
Fig. 1.
Flowchart of study participants. CAD, coronary artery disease; CAG, coronary angiography; FPG, fasting plasma glucose; TyG, triglyceride–glucose
Measurements and definitions
All sociodemographic characteristics, medical history, medical imaging data, and blood sample analysis results of the participants were collected from the medical records. Sociodemographic characteristics included age, sex, height, weight, smoking status, and drinking. The medical history included a history of chronic kidney disease, cancer, diabetes, and hypertension. Antihypertensive, antiplatelet, and antilipidemic medications were recorded. Blood samples from all participants were collected after at least 8 h of fasting. Laboratory parameters, including FPG, creatinine, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), were measured using the LABOSPECT 008 system (Hitachi, Tokyo, Japan), and the glycated hemoglobin A1c (HbA1c) level was determined using high-performance liquid chromatography (G8; TOSOH, Tokyo, Japan) in the laboratory of Beijing Hospital. The body mass index (BMI) was calculated as weight (kg) divided by the squared height (m2), and the estimated glomerular filtration rate (eGFR) was calculated according to the Chronic Kidney Disease Epidemiology Collaboration creatinine equation [21]. CAG was performed by experts who were blinded to the study protocol before an evaluation using percutaneous femoral arteriography.
The TyG index was calculated using the following equation: Ln [TG (mg/dL) × FPG (mg/dL) / 2].
CAD was referred to as at least one major coronary artery with ≥ 50% stenosis evaluated by CAG, including the left anterior descending, left circumflex, and right coronary arteries. The number of coronary arteries with ≥ 50% stenosis indicated the CAD severity [19]. Participants with one major coronary artery with ≥ 50% stenosis were defined as having single-vessel CAD, whereas multi-vessel CAD was considered when participants had more than two coronary arteries with ≥ 50% stenosis. According to the American Diabetes Association criteria, diabetes was diagnosed when participants had the following: an FPG level ≥ 7.0 mmol/L; 2-h plasma glucose level ≥ 11.1 mmol/L according to the oral glucose tolerance test; HbA1c ≥ 6.5%; or diabetes history. Pre-DM was diagnosed when participants without self-reported DM had an FPG level ranging from 5.6 to 6.9 mmol/L, 2-h plasma glucose level ranging from 7.8 to 11.0 mmol/L, or HbA1c level ranging from 5.7% to 6.4%. NGR was considered when participants did not have diabetes or pre-DM [22].
Statistical analysis
Continuous variables are described as the mean ± standard deviation or median and interquartile range (25%–75%). Categorical variables are described as the number or percentage. The one-way analysis of variance or Kruskal–Wallis test was used to compare the baseline variables of the TyG index tertiles when appropriate, and the chi-square test was performed to compare the categorical variables among groups.
To analyze the association between the TyG index and CAD severity (single-vessel CAD versus multi-vessel CAD), odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using a logistic regression analysis. Model 1 was unadjusted. Model 2 was adjusted for age and sex. Model 3 was adjusted for the variables in model 2 and further adjusted for antihypertensive medications, antidiabetic medications, FPG level, HDL-C level, LDL-level, triglyceride level, high-sensitivity C-reactive protein level, and eGFR. Moreover, restricted cubic splines were used to examine the shape of the associations between the baseline TyG index and CAD severity.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC) and R version 4.0.3 (R Foundation for Statistical Computing). Statistical significance was set at P < 0.05.
Results
Baseline characteristics
The average age of the 2792 participants with CAD was 66 ± 10 years, and 69.02% were men. Table 1 shows the baseline characteristics based on tertiles of the TyG index. Compared with the other two groups, the T3 group participants tended to be younger and male and had higher BMI, FPG, LDL-C, TC, and eGFR values (all P < 0.05). Moreover, participants in the T3 group tended to have a history of hypertension and smoking (both P < 0.05). The proportions of DM, use of antihypertensive drugs, and multi-vessel CAD were higher in the T3 group than in the other groups (all P < 0.05).
Table 1.
Baseline characteristics according to tertiles of the TyG index
| Total (n = 2792) | T1 (n = 931) | T2 (n = 941) | T3 (n = 920) | P-value | |
|---|---|---|---|---|---|
| Age (years) | 66 ± 10 | 68 ± 10 | 66 ± 10 | 63 ± 11 | < 0.01 |
| Male (n, %) | 1927 (69.02%) | 650 (69.82%) | 643 (68.33%) | 634 (68.91%) | < 0.01 |
| SBP (mmHg) | 136 ± 18 | 135 ± 18 | 136 ± 18 | 136 ± 19 | 0.11 |
| DBP (mmHg) | 77 ± 12 | 76 ± 12 | 77 ± 12 | 77 ± 12 | 0.03 |
| FPG (mmol/l) | 6.36 ± 2.22 | 5.29 ± 0.93 | 6.01 ± 1.40 | 7.81 ± 2.94 | < 0.01 |
| BMI (Kg/m2) | 25.71 ± 3.34 | 24.74 ± 3.23 | 25.94 ± 3.11 | 26.45 ± 3.43 | < 0.01 |
| HDL-C (mg/dL) | 1.04 ± 0.25 | 1.15 ± 0.27 | 1.03 ± 0.23 | 0.94 ± 0.21 | < 0.01 |
| LDL-C (mg/dL) | 2.12 ± 0.80 | 1.89 ± 0.71 | 2.12 ± 0.74 | 2.36 ± 0.88 | < 0.01 |
| TC (mg/dL) | 3.72 ± 0.93 | 3.45 ± 0.80 | 3.65 ± 0.84 | 4.07 ± 1.03 | < 0.01 |
| HbA1c (%) | 6.59 ± 1.23 | 6.17 ± 0.87 | 6.45 ± 1.06 | 7.16 ± 1.45 | < 0.01 |
| eGFR (ml/min) | 85.97 ± 17.08 | 86.24 ± 15.31 | 85.03 ± 16.33 | 86.65 ± 19.34 | 0.12 |
| Smoking (n, %) | 1299 (46.53%) | 406 (43.61%) | 432 (45.91%) | 461 (50.11%) | 0.02 |
| Drinking (n, %) | 1771 (63.43%) | 584 (62.73%) | 588 (62.49%) | 599 (65.11%) | 0.43 |
| Hypertension (n, %) | 1949 (69.81%) | 595 (63.91%) | 674 (71.63%) | 680 (73.91%) | < 0.01 |
| Glucose metabolism state | |||||
| NGR (n, %) | 530 (18.98%) | 275 (29.54%) | 175 (18.60%) | 80 (8.70%) | < 0.01 |
| Pre-DM (n, %) | 1038 (37.17%) | 394 (42.32%) | 399 (42.40%) | 245 (26.63%) | < 0.01 |
| DM (n, %) | 1224 (43.84%) | 262 (28.14%) | 367 (39.00%) | 595 (64.67%) | < 0.01 |
| Medications | |||||
| Antiplatelets (n, %) | 2643 (94.66%) | 883 (94.84%) | 883 (93.84%) | 877 (95.33%) | 0.34 |
| Antihypertensive drugs (n, %) | 2317 (82.99%) | 728 (78.20%) | 795 (84.48%) | 794 (86.30%) | < 0.01 |
| Antilipidemic drugs (n, %) | 2629 (94.16%) | 877 (94.20%) | 878 (93.30%) | 874 (95.0%) | 0.30 |
| Multi-vessel CAD (n, %) | 2159 (77.33%) | 688 (73.90%) | 736 (78.21%) | 735 (79.89%) | < 0.01 |
TyG triglyceride–glucose, SBP systolic blood pressure, DBP diastolic blood pressure, FPG fasting plasma glucose, BMI body mass index, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, TC total cholesterol, HbA1c glycated hemoglobin A1c, eGFR estimated glomerular filtration rate, NGR normoglycemia, Pre-DM pre-diabetes mellitus, DM diabetes mellitus, CAD coronary artery disease
Table 2 presents the baseline characteristics of those with single-vessel and those with multi-vessel CAD. A total of 2159 participants were diagnosed with multi-vessel CAD and evaluated using percutaneous CAG. Compared with participants with single-vessel CAD, those with multi-vessel CAD tended to be older and men. However, the eGFR and BMI values of participants with multi-vessel CAD were lower (both P < 0.05). Regarding the glucose metabolism statuses, the occurrence rate of multi-vessel CAD were 17.28%, 36.17%, and 46.55% for those with NGR, pre-DM, and DM, respectively. Moreover, the proportion of—antihypertensive drug use was higher for participants with multi-vessel CAD than for those with single-vessel CAD (both P < 0.05).
Table 2.
Baseline characteristics according to single-vessel or multi-vessel CAD
| Total (n = 2792) |
Single-vessel CAD (n = 633) |
Multi-vessel CAD (n = 2159) |
P-value | |
|---|---|---|---|---|
| Age (years) | 66 ± 10 | 65 ± 10 | 66 ± 10 | < 0.01 |
| Male (n,%) | 1927 (69.02%) | 359 (56.71%) | 1568 (72.62%) | < 0.01 |
| SBP (mmHg) | 136 ± 18 | 136 ± 18 | 135 ± 18 | 0.58 |
| DBP (mmHg) | 77 ± 12 | 77 ± 11 | 77 ± 12 | 0.56 |
| FPG (mmol/l) | 6.36 ± 2.22 | 5.92 ± 1.68 | 6.49 ± 2.34 | < 0.01 |
| BMI (Kg/m2) | 25.71 ± 3.34 | 25.79 ± 3.38 | 25.68 ± 3.32 | < 0.01 |
| HDL-C (mg/dL) | 1.04 ± 0.25 | 1.10 ± 0.27 | 1.03 ± 0.25 | 0.92 |
| LDL-C (mg/dL) | 2.12 ± 0.80 | 2.12 ± 0.78 | 2.12 ± 0.81 | 0.92 |
| TC (mg/dL) | 3.72 ± 0.93 | 3.77 ± 0.90 | 3.71 ± 0.94 | 0.17 |
| HbA1c (%) | 6.59 ± 1.23 | 6.33 ± 1.03 | 6.67 ± 1.27 | < 0.01 |
| eGFR | 85.97 ± 17.08 | 88.88 ± 14.02 | 85.11 ± 17.79 | < 0.01 |
| Smoking (n, %) | 1299 (46.53%) | 250 (39.49%) | 1049 (48.59%) | < 0.01 |
| Drinking (n, %) | 1771 (63.43%) | 394 (62.24%) | 1377 (63.78%) | 0.48 |
| Hypertension (n, %) | 1949 (69.81%) | 412 (65.09%) | 1537 (71.19%) | < 0.01 |
| Glucose metabolism state | ||||
| NGR (n, %) | 530 (18.98%) | 157 (24.80%) | 373 (17.28%) | < 0.01 |
| Pre-DM (n, %) | 1038 (37.8%) | 257 (40.60%) | 781 (36.17%) | < 0.01 |
| DM (n, %) | 1224 (43.84%) | 219 (34.60%) | 1005 (46.55%) | < 0.01 |
| Medications | ||||
| Antiplatelets (n, %) | 2643 (94.66%) | 595 (94.00%) | 2048 (94.86%) | 0.40 |
| Antihypertensive drugs (n, %) | 2317 (82.99%) | 491 (77.57%) | 1826 (84.58%) | < 0.01 |
| Antilipidemic drugs (n, %) | 2629 (94.16%) | 590 (93.21%) | 2039 (94.44%) | 0.25 |
SBP systolic blood pressure, DBP diastolic blood pressure, FPG fasting plasma glucose, BMI body mass index, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, TC total cholesterol, HbA1c glycated hemoglobin A1c, eGFR estimated glomerular filtration rate, NGR normoglycemia, Pre-DM pre-diabetes mellitus, DM diabetes mellitus, CAD coronary artery disease
Association between the TyG index and severity of CAD
Table 3 describes the results of the logistic regression analysis. The univariate logistic regression analysis indicated that the TyG index was not statistically correlated with multi-vessel CAD. However, the T2 group (OR, 1.268; 95% CI 1.025–1.569; P = 0.029) and T3 group (OR, 1.403; 95% CI 1.129–1.745; P = 0.002) were at higher risk for multi-vessel CAD. After adjusting for age and sex in model 2, the TyG index as a continuous variable was an independent predictor of multi-vessel CAD (OR, 1.398; 95% CI 1.197–1.633; P < 0.001). Using the T1 group as a reference, the multivariate logistic regression analysis indicated that the risk of multi-vessel CAD for the T2 and T3 groups was 1.330-fold higher (OR, 1.330; 95% CI 1.061–1.668; P = 0.013) and 1.578-fold higher (OR, 1.578; 95% CI 1.249–1.994; P < 0.001), respectively. After adjusting for sex, age, BMI, smoking, drinking, hypertension, eGFR, antiplatelet drug use, antihypertensive drug use, and antilipidemic drug use, we found that the TyG index as a continuous variable was still an independent hazard factor for multi-vessel CAD (OR, 1.355; 95% CI 1.154–1.591; P < 0.001). Compared with the T1 group, which was regarded as the reference, the T2 group had a 1.283-fold risk of multi-vessel CAD (OR, 1.283; 95% CI 1.024–1.607; P = 0.031 ) in the multivariate logistic regression model, whereas the T3 group had a 1.496-fold risk of multi-vessel CAD (OR, 1.496; 95% CI 1.183–1.893; P < 0.001).
Table 3.
Associations between the TyG index and severity of CAD
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| TyG index | 0.825 | 0.559–1.216 | 0.331 | 1.398 | 1.197–1.633 | < 0.001 | 1.355 | 1.154–1.591 | < 0.001 |
| T1 | Reference | Reference | Reference | ||||||
| T2 | 1.268 | 1.025–1.569 | 0.029 | 1.330 | 1.061–1.668 | 0.013 | 1.283 | 1.024–1.607 | 0.031 |
| T3 | 1.403 | 1.129–1.745 | 0.002 | 1.578 | 1.249–1.994 | < 0.001 | 1.496 | 1.183–1.893 | < 0.001 |
Model 1: unadjusted
Model 2: adjusted for age and sex
Model 3: adjusted for sex, age, BMI, smoking, drinking, hypertension, eGFR, antiplatelet drug use, antilipidemic drug use, and antihypertensive drug use
TyG triglyceride–glucose, CAD coronary artery disease, OR odds ratio, CI confidence interval, BMI body mass index, eGFR estimated glomerular filtration rate
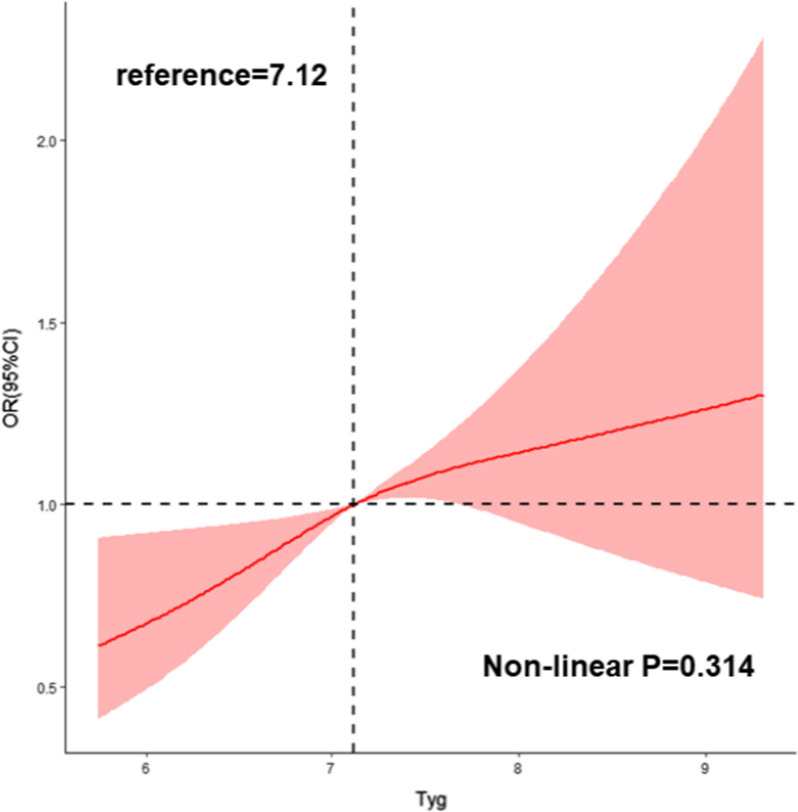
The results of the restricted cubic splines are presented in Fig. 2. We observed a dose–response relationship between the TyG index and risk of multi-vessel CAD (non-linear P = 0.314).
Fig. 2.
Restricted cubic splines for the odds ratio of multi-vessel CAD. CAD, coronary artery disease; OR, odds ratio; CI, confidence interval
Table 4 shows the relationship between the TyG index and CAD severity according to different diabetes statuses, including NGR, pre-DM, and DM. When adjusted for sex, age, BMI, smoking, drinking, hypertension, eGFR, antiplatelet drug use, antihypertensive drug use, and antilipidemic drug use in model 3, the TyG index as a continuous variable was an independent risk factor for multi-vessel CAD in subgroup of pre-DM (OR, 1.367; 95% CI 1.000–1.867; P = 0.049). In the pre-DM subgroup, the T3 group (OR, 1.541; 95% CI 1.013–2.344; P = 0.043) was associated with a significantly higher risk of multi-vessel CAD when the T1 group was used as the reference.
Table 4.
Associations between the TyG index and severity of CAD according to different diabetes statuses
| Glucose metabolism state | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| NGR | |||||||||
| TyG index | 0.825 | 0.559–1.216 | 0.331 | 0.929 | 0.617–1.398 | 0.723 | 0.846 | 0.551–1.299 | 0.444 |
| T1 | Reference | Reference | Reference | ||||||
| T2 | 1.206 | 0.787–1.848 | 0.389 | 1.246 | 0.759–2.045 | 0.385 | 1.167 | 0.710–1.916 | 0.543 |
| T3 | 0.660 | 0.393–1.109 | 0.117 | 0.739 | 0.400–1.366 | 0.335 | 0.684 | 0.371–1.262 | 0.225 |
| Pre-DM | |||||||||
| TyG index | 1.210 | 0.910–1.607 | 0.189 | 1.412 | 1.043–1.903 | 0.023 | 1.367 | 1.000–1.867 | 0.049 |
| T1 | Reference | Reference | Reference | ||||||
| T2 | 1.161 | 0.844–1.597 | 0.359 | 1.230 | 0.859–1.761 | 0.258 | 1.217 | 0.860–1.724 | 0.268 |
| T3 | 1.351 | 0.927–1.968 | 0.118 | 1.613 | 1.050–2.477 | 0.029 | 1.541 | 1.013–2.344 | 0.043 |
| DM | |||||||||
| TyG index | 1.162 | 0.931–1.449 | 0.185 | 1.230 | 0.982–1.542 | 0.072 | 1.214 | 0.963–1.530 | 0.100 |
| T1 | Reference | Reference | Reference | ||||||
| T2 | 1.282 | 0.857–1.918 | 0.226 | 1.341 | 0.871–2.064 | 0.182 | 1.265 | 0.849–1.884 | 0.249 |
| T3 | 1.300 | 0.901–1.875 | 0.161 | 1.390 | 0.934–2.069 | 0.104 | 1.321 | 0.921–1.914 | 0.141 |
Model 1: unadjusted
Model 2: adjusted for age and sex
Model 3: adjusted for sex, age, BMI, smoking, drinking, hypertension, eGFR, antiplatelet drug use, antilipidemic drug use, and antihypertensive drug use
TyG triglyceride–glucose, CAD coronary artery disease, OR odds ratio, CI confidence interval, BMI body mass index, eGFR estimated glomerular filtration rate, NGR normoglycemia, Pre-DM pre-diabetes mellitus, DM diabetes mellitus
Discussion
In our study, a significant relationship between the TyG index and the occurrence of multi-vessel CAD, which represents the CAD severity, was observed. After adjusting for potential risk factors, including sex, age, BMI, smoking, alcohol consumption, hypertension, eGFR, antiplatelet drug use, antihypertensive drug use, and antilipidemic drug use, the TyG index was still an independent risk factor for multi-vessel CAD. Additionally, the highest tertile of the TyG group (T3 group) was correlated with a 1.496-fold risk of multi-vessel CAD compared with the lowest tertile of the TyG group (T1 group). To our best knowledge, the present study is the first to observe a dose–response relationship between the TyG index and risk of multi-vessel CAD. Furthermore, an increased TyG index was correlated with a significantly higher risk of multi-vessel CAD, especially for individuals with pre-DM.
Compared with single-vessel CAD, multi-vessel CAD is correlated with a higher risk of a worse prognosis even after percutaneous coronary intervention therapy [23]. Multi-vessel CAD increases the difficulty of percutaneous coronary intervention, reflects the severity of CAD, and has received constant attention in clinical practice. In our study, participants with multi-vessel CAD tended to be male and older, and they were more likely to have DM, hypertension, and a history of smoking. A retrospective cohort study showed that T2DM was independently related to a higher risk of multi-vessel CAD and severe CAD [20]. As a critical mechanism of DM, IR has a strong relationship with the development and progression of atherosclerotic cardiovascular diseases, especially CAD [10, 25]. IR indicates that insulin cannot function properly in target tissues, including the skeletal muscle, adipose tissue, and hepatic tissue [24]. Simental-Mendía et al. first suggested that the TyG index could be a surrogate for the Homeostatic Model Assessment to evaluate IR (HOMA-IR) [12]. Recently, the TyG index has been verified as a simple and dependable estimate index for IR and is comparable to the euglycemic-hyperinsulinemic clamp method, which is considered the gold standard for evaluating IR [26].
A large-scale retrospective study performed in Korea indicated that the group with the highest TyG index was at higher risk for stroke and MI [27]. Previous studies have demonstrated that the TyG index could be a useful marker of arterial stiffness and atherosclerosis [28, 29]. Moreover, a cohort study observed that the TyG index was related to the stenosis severity and number of stenosed coronary arteries [30]. Consistent with these studies, our study suggested that a higher TyG index was notably correlated with CAD severity in a relatively large cohort of participants with CAD. This finding indicated that the TyG index is expected to be a useful predictor of CAD severity before CAG is performed in clinical practice.
The relationship between IR and the risk of cardiovascular diseases in the established DM population has been controversial [31, 32]. Recently, a retrospective study suggested that the TyG index was correlated with the risk of multi-vessel CAD in the DM subgroup [19]. In contrast, our study did not observe a strong association between the TyG index and multi-vessel CAD in the DM subgroup. However, the definition of DM in the aforementioned study [19] excluded the self-reported DM history, which was different from our study. The results might have been influenced by the definitions and limited to their small study population. Consistent with our study, a previous study indicated that the TyG index is a useful marker for identifying IR in those without diabetes [33]. Another study that enrolled 5764 participants reported that IR parameters including the TyG index and HOMA-IR were not related to the risk of obstructive CAD in the DM population [31]. It is well-known that DM is characterized by hyperglycemia and IR. It is speculated that the probable mechanism of severe CAD may be more related to the glycemic status than IR in established diabetes [31]. The accuracy of this hypothesis should be confirmed by future studies. Our results support that intensive glycemia control is still the important strategy for preventing CAD in established diabetes [34].
Associations between pre-DM and cardiovascular diseases may differ according to the pre-DM criteria and ethnic variations [35]. A meta-analysis of 53 prospective studies demonstrated that pre-DM was correlated with an increased risk of composite CVD, including CAD [36]. In our study, the subgroup analysis suggested that a higher TyG index was significantly associated with an increased risk of multi-vessel CAD in the pre-DM group. According to previous studies, pre-DM was closely related to diffuse coronary stenosis compared to DM and NGR, which increases the complexity of the percutaneous coronary intervention and leads to a worse prognosis [37, 38]. Although pre-DM can be regarded as a risk factor for future CVD, it should be mentioned that not every participant needs drug therapy [36]. Therefore, it is vital to identify the phenotypes that require pharmacological intervention in the pre-DM population. The American Diabetes Association suggests considering drug therapy for participants with one or more risk factors, including high triglyceride concentration, HbA1c level > 42 mmol/mol, reduced HDL-C concentration, and hypertension [39]. Our results support the suggestions of the American Diabetes Association and demonstrate that TyG as a combined index of triglycerides and FPG for evaluating IR could be a useful marker for identifying participants at risk for severe CAD with pre-DM. CAD severity gradually increased with NGR, pre-DM, and DM in the present study. Regarding the results of the NGR subgroup, the routine assessments of the FPG, HbA1c, and TyG index are considered equally important to the early detection of pre-DM and IR.
Strengths and limitations
This study included a relatively large cohort of participants with CAD. To the best of our knowledge, this is the first study to investigate the dose–response relationship of the TyG index and CAD severity of participants with CAD. However, this study also had several limitations. First, this study involved a single-center and only enrolled the Asian population; therefore, the results should be interpreted cautiously. Second, because of the inevitable inherent disadvantages of retrospective studies, we could not infer a causal relationship during this study; therefore, a prospective study is necessary to verify these findings in the future. Additionally, data regarding factors such as income, education, and the employment of participants were not collected during this study, which might have affected the results. Third, the medication durations and dosages of antiplatelet drugs, antilipidemic drugs, and antihypertensive drugs for this cohort were not collected, which might have resulted in bias for these factors in the logistic models.
Conclusions
Our results demonstrated that an increased TyG index was correlated with a higher risk of multi-vessel CAD. A dose–response relationship was observed between the TyG index and risk of multi-vessel CAD. Our study indicated that TyG as an estimation index for evaluating IR could be a useful predictor of CAD severity, especially in the pre-DM population.
Acknowledgements
The authors thank all the members of the Beijing hospital for their contribution.
Abbreviations
- CAD
Coronary artery disease
- CVD
Cardiovascular disease
- T2DM
Type 2 diabetes mellitus
- IR
Insulin resistance
- DM
Diabetes mellitus
- ASCVD
Atherosclerotic cardiovascular diseases
- TyG
Triglyceride–glucose
- TG
Triglyceride
- MI
Myocardial infarction
- CAG
Coronary angiography
- Pre-DM
Pre-diabetes mellitus
- NGR
Normoglycemia
- FPG
Fasting plasma glucose
- TC
Total cholesterol
- HDL-C
High-density lipoprotein cholesterol
- HOMA-IR
Homeostatic Model Assessment to evaluate IR
- LDL-C
Low-density lipoprotein cholesterol
- HbA1c
Glycated hemoglobin A1c
- BMI
Body mass index
- eGFR
Estimated glomerular filtration rate
- ADA
American Diabetes Association
- OR
Odds ratio
- CI
Confidence interval
- PCI
Percutaneous coronary intervention
- CHD
Coronary heart disease
Author contributions
XW, WX and QS designed the study, FW and PJ reviewed and revised the manuscript. QS coded and analyzed the data. WX wrote the manuscript. ZZ, XM, and YX collected the data. CX and CY interpreted the data. All authors read and approved the final manuscript.
Funding
This work was supported by National Key R&D Program of China (2020YFC2008100), National High Level Hospital Clinical Research Funding (No. BJ-2022–095) and National High Level Hospital Clinical Research Funding (No. BJ-2022–117).
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available due privacy and ethical restrictions but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This cohort study conformed to the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Hospital. Written informed consent was obtained from all the patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest regarding the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiang Wang, Wei Xu and Qirui Song contributed equally to this work
Contributor Information
Pengfei Jin, Email: j790101@163.com.
Fang Wang, Email: bjh_wangfang@163.com.
References
- 1.Sacco RL, Roth GA, Reddy KS, Arnett DK, Bonita R, Gaziano TA, Heidenreich PA, Huffman MD, Mayosi BM, Mendis S, et al. The heart of 25 by 25: achieving the goal of reducing global and regional premature deaths from cardiovascular diseases and stroke: a modeling study From the American heart association and world heart federation. Circulation. 2016;133(23):e674–690. doi: 10.1161/CIR.0000000000000395. [DOI] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation. 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 3.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Mi J, Shan XY, Wang QJ, Ge KY. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes (Lond) 2007;31(1):177–188. doi: 10.1038/sj.ijo.0803354. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z, Jin T, Weng J. A thorough analysis of diabetes research in China from 1995 to 2015: current scenario and future scope. Sci China Life Sci. 2019;62(1):46–62. doi: 10.1007/s11427-018-9377-y. [DOI] [PubMed] [Google Scholar]
- 6.Arnold SV, Bhatt DL, Barsness GW, Beatty AL, Deedwania PC, Inzucchi SE, Kosiborod M, Leiter LA, Lipska KJ, Newman JD, et al. Clinical management of stable coronary artery disease in patients with type 2 diabetes mellitus: a scientific statement from the American heart association. Circulation. 2020;141(19):e779–e806. doi: 10.1161/CIR.0000000000000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bragg F, Holmes MV, Iona A, Guo Y, Du H, Chen Y, Bian Z, Yang L, Herrington W, Bennett D, et al. Association between diabetes and cause-specific mortality in rural and Urban areas of China. JAMA. 2017;317(3):280–289. doi: 10.1001/jama.2016.19720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I et al: Diabetes mellitus, fasting glucose, and risk of cause-specific death. The New England journal of medicine 2011, 364(9):829–841. [DOI] [PMC free article] [PubMed]
- 9.Wu S, Liu W, Ma Q, Yu W, Guo Y, Zhao Y, Shi D, Liu Y, Zhou Z, Wang J, et al. Association between insulin resistance and coronary plaque vulnerability in patients with acute coronary syndromes: insights from optical coherence tomography. Angiology. 2019;70(6):539–546. doi: 10.1177/0003319718809931. [DOI] [PubMed] [Google Scholar]
- 10.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bersch-Ferreira AC, Sampaio GR, Gehringer MO, Torres E, Ross-Fernandes MB, da Silva JT, Torreglosa CR, Kovacs C, Alves R, Magnoni CD, et al. Association between plasma fatty acids and inflammatory markers in patients with and without insulin resistance and in secondary prevention of cardiovascular disease, a cross-sectional study. Nutr J. 2018;17(1):26. doi: 10.1186/s12937-018-0342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 13.Guerrero-Romero F, Villalobos-Molina R, Jimenez-Flores JR, Simental-Mendia LE, Mendez-Cruz R, Murguia-Romero M, Rodriguez-Moran M. Fasting triglycerides and glucose index as a diagnostic test for insulin resistance in young adults. Arch Med Res. 2016;47(5):382–387. doi: 10.1016/j.arcmed.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Irace C, Carallo C, Scavelli FB, De Franceschi MS, Esposito T, Tripolino C, Gnasso A. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract. 2013;67(7):665–672. doi: 10.1111/ijcp.12124. [DOI] [PubMed] [Google Scholar]
- 15.Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, Kim JH, Park JS. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42(8):1569–1573. doi: 10.2337/dc18-1920. [DOI] [PubMed] [Google Scholar]
- 16.Lee EY, Yang HK, Lee J, Kang B, Yang Y, Lee SH, Ko SH, Ahn YB, Cha BY, Yoon KH, et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. 2016;15(1):155. doi: 10.1186/s12944-016-0324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Silva A, Caldas APS, Hermsdorff HHM, Bersch-Ferreira AC, Torreglosa CR, Weber B, Bressan J. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol. 2019;18(1):89. doi: 10.1186/s12933-019-0893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian X, Zuo Y, Chen S, Liu Q, Tao B, Wu S, Wang A. Triglyceride-glucose index is associated with the risk of myocardial infarction: an 11-year prospective study in the Kailuan cohort. Cardiovasc Diabetol. 2021;20(1):19. doi: 10.1186/s12933-020-01210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su J, Li Z, Huang M, Wang Y, Yang T, Ma M, Ni T, Pan G, Lai Z, Li C, et al. Triglyceride glucose index for the detection of the severity of coronary artery disease in different glucose metabolic states in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol. 2022;21(1):96. doi: 10.1186/s12933-022-01523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu MM, Peng J, Guo YL, Wu NQ, Zhu CG, Gao Y, Dong Q, Li JJ. Impact of diabetes on coronary severity and cardiovascular outcomes in patients with heterozygous familial hypercholesterolaemia. Eur J Prev Cardiol. 2022;28(16):1807–1816. doi: 10.1093/eurjpc/zwab042. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(1):S67–74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorajja P, Gersh BJ, Cox DA, McLaughlin MG, Zimetbaum P, Costantini C, Stuckey T, Tcheng JE, Mehran R, Lansky AJ, et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J. 2007;28(14):1709–1716. doi: 10.1093/eurheartj/ehm184. [DOI] [PubMed] [Google Scholar]
- 24.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293–302. doi: 10.1038/nrendo.2014.29. [DOI] [PubMed] [Google Scholar]
- 25.Wassink AM, van der Graaf Y, Olijhoek JK, Visseren FL. Metabolic syndrome and the risk of new vascular events and all-cause mortality in patients with coronary artery disease, cerebrovascular disease, peripheral arterial disease or abdominal aortic aneurysm. Eur Heart J. 2008;29(2):213–223. doi: 10.1093/eurheartj/ehm582. [DOI] [PubMed] [Google Scholar]
- 26.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 27.Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18(1):361. doi: 10.1186/s12916-020-01824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambrinoudaki I, Kazani MV, Armeni E, Georgiopoulos G, Tampakis K, Rizos D, Augoulea A, Kaparos G, Alexandrou A, Stamatelopoulos K. The TyG index as a marker of subclinical atherosclerosis and arterial stiffness in lean and overweight postmenopausal women. Heart Lung Circ. 2018;27(6):716–724. doi: 10.1016/j.hlc.2017.05.142. [DOI] [PubMed] [Google Scholar]
- 29.Wu S, Xu L, Wu M, Chen S, Wang Y, Tian Y. Association between triglyceride-glucose index and risk of arterial stiffness: a cohort study. Cardiovasc Diabetol. 2021;20(1):146. doi: 10.1186/s12933-021-01342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thai PV, Tien HA, Van Minh H, Valensi P. Triglyceride glucose index for the detection of asymptomatic coronary artery stenosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19(1):137. doi: 10.1186/s12933-020-01108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho YR, Ann SH, Won KB, Park GM, Kim YG, Yang DH, Kang JW, Lim TH, Kim HK, Choe J, et al. Association between insulin resistance, hyperglycemia, and coronary artery disease according to the presence of diabetes. Sci Rep. 2019;9(1):6129. doi: 10.1038/s41598-019-42700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meigs JB, Larson MG, D'Agostino RB, Levy D, Clouse ME, Nathan DM, Wilson PW, O'Donnell CJ. Coronary artery calcification in type 2 diabetes and insulin resistance: the framingham offspring study. Diabetes Care. 2002;25(8):1313–1319. doi: 10.2337/diacare.25.8.1313. [DOI] [PubMed] [Google Scholar]
- 33.Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, Ko YL. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE. 2016;11(3):e0149731. doi: 10.1371/journal.pone.0149731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitazawa M, Fujihara K, Osawa T, Yamamoto M, Yamada MH, Kaneko M, Matsubayashi Y, Yamada T, Yamanaka N, Seida H, et al. Risk of coronary artery disease according to glucose abnormality status and prior coronary artery disease in Japanese men. Metabolism: Clin Exp. 2019;101:153991. doi: 10.1016/j.metabol.2019.153991. [DOI] [PubMed] [Google Scholar]
- 35.Eastwood SV, Tillin T, Sattar N, Forouhi NG, Hughes AD, Chaturvedi N. Associations between prediabetes, by three different diagnostic criteria, and incident CVD differ in South Asians and Europeans. Diabetes Care. 2015;38(12):2325–2332. doi: 10.2337/dc15-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ (Clin Res ed) 2016;355:i5953. doi: 10.1136/bmj.i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ertan C, Ozeke O, Gul M, Aras D, Topaloglu S, Kisacik HL, Demir AD, Aydogdu S, Ozin B. Association of prediabetes with diffuse coronary narrowing and small-vessel disease. J Cardiol. 2014;63(1):29–34. doi: 10.1016/j.jjcc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Zhou Y, Zhang T, Lin X, Ma X, Wang Z, Liu Y, Shi D, Zhou Z, Zhao Y. Fasting blood glucose and HbA(1c) correlate with severity of coronary artery disease in elective PCI patients with HbA(1c) 5.7% to 6.4. Angiology. 2020;71(2):167–174. doi: 10.1177/0003319719887655. [DOI] [PubMed] [Google Scholar]
- 39.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30(3):753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available due privacy and ethical restrictions but are available from the corresponding author on reasonable request.