Abstract
Introduction
A new concept of ‘NeoRAS wild-type (WT)’, which means conversion of RAS status from RAS mutant to RAS WT after treatment, has been reported. Previous observational and proof-of-concept studies have demonstrated the efficacy of epidermal growth factor receptor inhibitors in patients with NeoRAS WT metastatic colorectal cancer (mCRC). Moreover, posthoc biomarker analyses of these studies have suggested that not only the RAS status in the circulating tumour DNA (ctDNA) but also other gene mutational status may be useful as biomarkers of epidermal growth factor receptor inhibitors for NeoRAS WT mCRC.
Methods and analysis
This trial is a multicentre, single-arm, phase II trial to assess the efficacy and safety of panitumumab plus irinotecan therapy for patients with NeoRAS mCRC. The key eligibility criteria include RAS mutant mCRC initially proven in tumour tissue refractory or intolerant to fluoropyrimidine, oxaliplatin and irinotecan; RAS WT in ctDNA (defined as plasma mutant allele frequencies of all RAS ≤0.1%) within 28 days before enrolment and Eastern Cooperative Oncology Group performance status ≤2. The primary endpoint is the response rate. The target sample size is 30 patients. Biomarker analyses are planned to be performed using next-generation sequencing-based ctDNA analysis.
Ethics and dissemination
This study was approved by the certified review board of National Cancer Center Hospital. The main results of the trial will be presented in international meetings and in medical journals.
Trial registration number
s031210565.
Keywords: chemotherapy, gastrointestinal tumours, gastrointestinal tumours
Strengths and limitations of this study.
The C-PROWESS trial is a prospective multicentre, single-arm, phase II trial.
The trial is designed to assess the efficacy and safety of panitumumab plus irinotecan therapy in patients with NeoRAS.
However, no comparator is a limitation.
Translational research of biomarkers using liquid biopsies at baseline and after discontinuation of the treatment is planned to be performed.
Introduction
RAS mutations (MTs) induce the activation of the protein kinase pathway and promote carcinogenesis and cancer growth.1 Patients with RAS MT metastatic colorectal cancer (mCRC) have a poorer prognosis than those with RAS wild-type (WT) mCRC.2 3 Epidermal growth factor receptor (EGFR) inhibitors (such as cetuximab and panitumumab), which are key drugs for mCRC, are ineffective for patients with RAS MT mCRC (KRAS/NRAS exons 2, 3 and 4).4–10
International guidelines recommend RAS genetic testing prior to the administration of an EGFR inhibitor in patients with mCRC.11–13 Repeat biopsies are not performed in routine clinical practice to monitor the RAS MT status;11–13 the consistency of the RAS MT status before and after chemotherapy remains unclear.
Recent advances in diagnostic technology for the detection of genetic MTs by liquid biopsy, especially circulating tumour DNA (ctDNA), have made minimally invasive, simple and repeatable testing possible.14–16 It is well known that RAS status can change before and after treatment. First reported was the identification of RAS MTs in ctDNA in EGFR inhibitor-resistant RAS WT mCRC patients.17 18 This involved acquired resistance to EGFR inhibitors, and several clinical trials have reported that remeasuring the RAS status before treatment is an important predictor of treatment efficacy when considering EGFR inhibitor rechallenge.19–21
On the other hand, there have been some reports that RAS MT observed at the initial diagnosis converted to RAS WT after treatment.22 These cases have been called ‘NeoRAS WT’ mCRC.22 The incidence of NeoRAS WT mCRC has been reported to range from 10.7% to 40% when assessed in tumour tissue samples,23 24 and from 18.8% to 83.3% in the ctDNA.25–31
There have been several reports of the use of EGFR inhibitors in patients with NeoRAS WT. Mohamed et al, in a proof-of-concept study of EGFR inhibitors in patients with NeoRAS WT,31 reported that the objective response rate (RR) was 55.6% and progression-free survival (PFS) was 9 months in patients with NeoRAS WT mCRC treated with fluorouracil, folinic acid, irinotecan and cetuximab.31 This result suggested that EGFR inhibitors may be effective in patients with NeoRAS WT mCRC.
Although retrospective analyses and proof-of-concept studies have indicated the potential efficacy of EGFR inhibitors in NeoRAS WT mCRC,22 31 the safety and efficacy have not been validated prospectively. Furthermore, the definition of NeoRAS WT mCRC has not been established.
Therefore, this trial will evaluate the efficacy of panitumumab and irinotecan in patients with NeoRAS WT mCRC confirmed in ctDNA after prior treatment.
Methods and analysis
Trial design
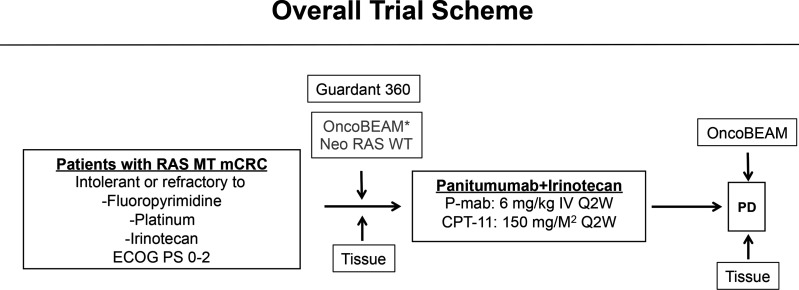
This trial is a multicentre, single-arm, phase II trial to investigate the safety and effiCacy of Panitumumab and iRinOtecan in NeoRAS Wild-type mEtaStatic colorectal cancer patientS (C-PROWESS trial). The overall trial scheme is illustrated in figure 1.
Figure 1.
Overall trial scheme. Liquid biopsies for the OncoBEAM RAS CRC kit and Guardant360 will be performed at baseline, and the OncoBEAM RAS CRC kit will be used after the discontinuation of the protocol treatment. OncoBEAM: OncoBEAM RAS CRC kit; *Substitution of results immediately before enrolment. CRC, colorectal cancer.
Patients
The key eligibility criteria are as follows: (1) RAS MT (KRAS/NRAS exons 2, 3 and 4) and BRAF V600E WT mCRC initially diagnosed in the tumour tissue; (2) refractory or intolerant to fluoropyrimidine, oxaliplatin and irinotecan; (3) RAS WT in ctDNA (mutant allele frequencies of all RAS ≤0.1%) within 28 days prior to enrolment; (4) an Eastern Cooperative Oncology Group performance status ≤2 and (5) preserved organ function. The details of the eligibility criteria are presented in box 1.
Box 1. Eligibility criteria for the C-PROWESS trial.
Inclusion criteria
Histologically proven diagnosis of colorectal adenocarcinoma.
Advanced or recurrent colorectal cancer (excluding appendix and anal canal cancer).
RAS mutation (MT) (KRAS/NRAS exons 2, 3 or 4 MT) confirmed by tumour histology prior to first-line chemotherapy.
Confirmation of refractoriness or intolerance to previous treatments with chemotherapy, including fluoropyrimidines, oxaliplatin or irinotecan (irinotecan is applied to refractory disease only), regardless of prior treatment with trifluridine tipiracil hydrochloride, regorafenib or angiogenesis inhibitors.
Confirmation of the RAS WT within 28 days from the test result date by ctDNA analysis using the OncoBEAM RAS CRC KIT.
At least one measurable lesion according to the RECIST V.1.1 criteria evaluated by CT or MRI within 28 days before registration.
Eastern Cooperative Oncology Group performance status ≤2.
Age ≥20 years.
-
Adequate organ function (bone marrow, liver and renal function) as defined by the following laboratory values obtained within 15 days prior to enrolment in the study:
Absolute neutrophil count ≥1500/mm3.
Platelets ≥75 000/mm3/mm3.
Serum total bilirubin ≤1.5 mg/dLd; serum AST (GOT) and ALT (GPT)≤100 U/L (except for patients with tumour involvement of the liver who must have AST and ALT ≤200 U/L).
A life expectancy of at least 60 days.
Written informed consent.
Exclusion criteria
Evidence of BRAF V600E MT by tumour histology.
Treated with blood transfusion, blood products or haematopoietic factor products such as Granulocyte Colony Stimulating Factor within 7 days prior to enrolment in this study.
A history of severe drug hypersensitivity or severe drug allergy.
Active infection (fever of 38°C or higher due to infection).
Ascites, pleural effusion or pericardial effusion requiring continuous drainage.
Uncontrolled diabetes mellitus.
Uncontrolled hypertension.
-
Patients who have been treated with any of the following treatments prior to starting the study drug:
Extensive surgery ≤4 weeks prior to starting the study drug (eg, surgical treatment with organ resection, excluding colostomy).
Proctocolectomy ≤2 weeks prior to starting the study drug.
Any chemotherapy ≤2 weeks prior to starting the study drug.
Radiotherapy ≤2 weeks prior to starting the study drug.
Clinically significant electrocardiographic abnormality or clinically significant cardiovascular accidents within 6 months prior to study enrolment, including myocardial infarction, severe unstable angina or New York Heart Association functional classification class III or IV congestive heart failure.
Patients with severe lung disease (interstitial pneumonia, pulmonary fibrosis or severe emphysema).
History of clinically significant mental disorder or central nervous system disorder.
Symptomatic brain metastasis or clinically suspected brain metastasis on symptoms.
Diarrhoea that interferes with daily life.
Intestinal paralysis, intestinal obstruction.
Coexisting active malignancies.
Pregnant or lactating women; women of childbearing potential or men with female partners of childbearing potential who are unwilling to use a highly effective method of contraception or avoid intercourse during and on completion of the study.
Patients who have been assessed by the site physician as inappropriate for this study.
-
Patients who have been treated with EGFR inhibitors prior to starting the study drug.
CRC, colorectal cancer; ctDNA, circulating tumour DNA; EGFR, epidermal growth factor receptor; WT, wild-type.
Treatment
Patients will receive panitumumab at 6 mg/kg and irinotecan at 150 mg/m2 biweekly until progressive disease, unacceptable toxicity, withdrawal of informed consent or death. The starting dose of irinotecan can be reduced to 120 mg/m2 according to UGT1A1 status (homozygosity/double heterozygosity).
Outcomes and statistical considerations
The primary endpoint of the C-PROWESS trial is RR, defined as the proportion of patients who achieve complete or partial response by the investigator’s assessment. The secondary endpoints include PFS, overall survival (OS), disease control rate, incidences of adverse events and the ratio of Neo RAS WT mCRC after failure of fluoropyrimidines, oxaliplatin and irinotecan. The response is evaluated according to the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1, using CT at 6 and 12 weeks after the start of the protocol treatment and repeated every 8 weeks thereafter. The RR threshold is set at 4%, according to the results of previous clinical trials with tipiracil/trifluridine (+bevacizumab)32–34 or regorafenib.35 The required sample size is 30, whereas an RR of 15% is deemed to be promising (one-sided α=0.10; β=0.2). The primary endpoint is planned to be analysed in all the patients who receive at least one dose of the protocol treatment and who satisfy all the inclusion and exclusion criteria. Participant enrolment started on 1 February 2022 and will end on 31 January 2023.
Biomarker analysis
Samples for liquid biopsies are planned to be collected at baseline and after discontinuation of the protocol treatment (figure 1). The ctDNA will be analysed using a highly sensitive digital PCR method, the OncoBEAM RAS CRC kit and targeted next-generation sequencing, Guardant360. The OncoBEAM RAS CRC kit, which has been approved in Japan to detect RAS MTs in the ctDNA derived from mCRC, detects 34 MTs in KRAS/NRAS codons 12, 13, 59, 61, 117 and 146 in plasma.36 Guardant360, a hybrid capture-based next-generation sequencing panel of the ctDNA developed by Guardant Health, detects other gene alterations.37 Exploratory analyses will be performed to identify the proportion of patients without RAS and other MTs related to resistance to anti-EGFR inhibitors (defined as ‘True NeoRAS WT’). The clinical outcomes (RR, PFS, OS and disease control rate) of patients with true NeoRAS WT receiving panitumumab and irinotecan combination therapy will be compared with those of patients without true NeoRAS WT and the overall study population. Moreover, the clinical outcomes will be compared according to the presence or absence of each genetic and epigenetic abnormality using exome sequencing and immunohistochemical staining in tissue samples and in ctDNA analysis before and after treatment with the protocol treatment using NGS, to explore the relationship with clinical outcomes and the mechanism of resistance.
Trial organisation
Eight core high-volume centres in Japan will participate in this trial.
Ethics and dissemination
This study was approved by the certified review board of the national cancer centre hospital (jRCT, s031210565; registration date, 20 January 2022.). The main results of the trial will be presented in international meetings and in medical journals.
Clinical questions
The clinical questions to be addressed in this study will be the definition of NeoRAS WT mCRC and the therapeutic effect of EGFR inhibitors on NeoRAS WT mCRC.
First, when ‘NeoRAS WT’ mCRC is defined by RAS MT status only, it is not possible to determine whether the RAS MT has completely disappeared or has not been measured due to a low volume of ctDNA. Therefore, we will perform NGS analysis using ctDNA before administering the protocol treatment to confirm the incidence of ‘True NeoRAS WT’ mCRC patients. ‘True NeoRAS WT’ mCRC is defined as the disappearance of RAS and the detection of other genetic MTs after treatment. If the proportion of ‘True NeoRAS WT’ mCRC patients is clarified, it may be even more useful in the enrichment of the population that will respond to treatment with EGFR inhibitors.
Second, our trial will evaluate the relationship between gene MTs other than RAS in ctDNA and the efficacy of EGFR inhibitors in NeoRAS WT mCRC. The ORR, PFS and OS may differ according to the presence or absence of some genetic abnormality that may lead to primary resistance to EGFR inhibitors, such as EGFR extracellular domain, Erb-B2 Receptor Tyrosine kinase 2, Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit alpha, among others, in the ctDNA (NGS) prior to protocol treatment.
In addition, we plan to measure the ctDNA RAS again after the administration of the protocol treatment. This will allow us to understand how frequently RAS MT reappears after treatment and whether the same RAS MT reappears or different variants of RAS MT newly appear. If the same RAS variant allele frequency increases after the administration of treatment, it may be due to tissue heterogeneity in which dominant clones with/out RAS mutation in tumours are changed by an EGFR inhibitor. Furthermore, the mechanism of resistance to EGFR inhibitors will be clarified.
Limitations
The limitations of this study are the small sample size and lack of a control arm.
Summary
Our trial will evaluate the efficacy of panitumumab and irinotecan in patients with NeoRAS WT mCRC to develop personalised therapeutic regimens based on the ctDNA results.
Supplementary Material
Acknowledgments
We thank the independent data monitoring committee (Atsushi Sato, Yasuo Hamamoto and Hidekazu Kuramochi) and the data managers (Yumiko Izoe, Tomoko Sanada, Hitomi Hannan and Yuki Horiike).
Footnotes
Contributors: HO, AT, KK, NB, KY and ES, as task managers, participated in the coordination of this trial, the design and the writing of the protocol, data collection, data analysis, data interpretation and writing of the manuscript. AT, YK, DN, MS, TD, KO, RS, KO and TW, as the protocol preparation committee, participated in this trial, including design and writing of the protocol, data collection, data analysis, data interpretation and preparation of the manuscript. NI, as the chief of statistical analysis, participated in the statistical setting of the trial, design and data analysis. All the authors have reviewed and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: KY has received honoraria from Daiichi-Sankyo.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. Ras oncogenes: weaving a tumorigenic web. Nat Rev Cancer 2011;11:761–74. 10.1038/nrc3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richman SD, Seymour MT, Chambers P, et al. Kras and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC focus trial. J Clin Oncol 2009;27:5931–7. 10.1200/JCO.2009.22.4295 [DOI] [PubMed] [Google Scholar]
- 3.Cremolini C, Loupakis F, Antoniotti C, et al. Folfoxiri plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 tribe study. Lancet Oncol 2015;16:1306–15. 10.1016/S1470-2045(15)00122-9 [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Köhne C-H, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408–17. 10.1056/NEJMoa0805019 [DOI] [PubMed] [Google Scholar]
- 5.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-Ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757–65. 10.1056/NEJMoa0804385 [DOI] [PubMed] [Google Scholar]
- 6.Douillard J-Y, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the prime study. J Clin Oncol 2010;28:4697–705. 10.1200/JCO.2009.27.4860 [DOI] [PubMed] [Google Scholar]
- 7.Loupakis F, Ruzzo A, Cremolini C, et al. Kras codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer 2009;101:715–21. 10.1038/sj.bjc.6605177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allegra CJ, Rumble RB, Hamilton SR, et al. Extended ras gene mutation testing in metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy: American Society of clinical oncology provisional clinical opinion update 2015. J Clin Oncol 2016;34:179–85. 10.1200/JCO.2015.63.9674 [DOI] [PubMed] [Google Scholar]
- 9.De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective Consortium analysis. Lancet Oncol 2010;11:753–62. 10.1016/S1470-2045(10)70130-3 [DOI] [PubMed] [Google Scholar]
- 10.Schirripa M, Cremolini C, Loupakis F, et al. Role of NRAS mutations as prognostic and predictive markers in metastatic colorectal cancer. Int J Cancer 2015;136:83–90. 10.1002/ijc.28955 [DOI] [PubMed] [Google Scholar]
- 11.Benson AB, Venook AP, Cederquist L, et al. Colon cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017;15:370–98. 10.6004/jnccn.2017.0036 [DOI] [PubMed] [Google Scholar]
- 12.Yoshino T, Arnold D, Taniguchi H, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, mos, Sso and TOS. Ann Oncol 2018;29:44–70. 10.1093/annonc/mdx738 [DOI] [PubMed] [Google Scholar]
- 13.Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for cancer of the colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 2020;25:1–42. 10.1007/s10147-019-01485-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 2017;14:531–48. 10.1038/nrclinonc.2017.14 [DOI] [PubMed] [Google Scholar]
- 15.Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 2017;17:223–38. 10.1038/nrc.2017.7 [DOI] [PubMed] [Google Scholar]
- 16.Osumi H, Shinozaki E, Yamaguchi K, et al. Clinical utility of circulating tumor DNA for colorectal cancer. Cancer Sci 2019;110:1148–55. 10.1111/cas.13972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Misale S, Yaeger R, Hobor S, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012;486:532–6. 10.1038/nature11156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siravegna G, Mussolin B, Buscarino M, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med 2015;21:795–801. 10.1038/nm.3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cremolini C, Rossini D, Dell’Aquila E, et al. Rechallenge for Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer With Acquired Resistance to First-line Cetuximab and Irinotecan. JAMA Oncol 2019;5:343–50. 10.1001/jamaoncol.2018.5080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sartore-Bianchi A, Pietrantonio F, Lonardi S, et al. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: the phase 2 CHRONOS trial. Nat Med 2022;28:1612–8. 10.1038/s41591-022-01886-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osawa H, Shinozaki E, Nakamura M, et al. Phase II study of cetuximab rechallenge in patients with Ras wild-type metastatic colorectal cancer: E-rechallenge trial. Annals of Oncology 2018;29:viii161. 10.1093/annonc/mdy281.029 [DOI] [Google Scholar]
- 22.Gazzaniga P, Raimondi C, Urbano F, et al. EGFR Inhibitor as Second-Line Therapy in a Patient With Mutant RAS Metastatic Colorectal Cancer: Circulating Tumor DNA to Personalize Treatment. JCO Precis Oncol 2018;2:1–6. 10.1200/PO.17.00277 [DOI] [PubMed] [Google Scholar]
- 23.Sugimachi K, Sakimura S, Kuramitsu S, et al. Serial mutational tracking in surgically resected locally advanced colorectal cancer with neoadjuvant chemotherapy. Br J Cancer 2018;119:419–23. 10.1038/s41416-018-0208-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li W, Qiu T, Guo L, et al. Major challenges related to tumor biological characteristics in accurate mutation detection of colorectal cancer by next-generation sequencing. Cancer Lett 2017;410:92–9. 10.1016/j.canlet.2017.09.014 [DOI] [PubMed] [Google Scholar]
- 25.Raimondi C, Nicolazzo C, Belardinilli F, et al. Transient disappearance of Ras mutant clones in plasma: a Counterintuitive clinical use of EGFR inhibitors in Ras mutant metastatic colorectal cancer. Cancers 2019;11:42. 10.3390/cancers11010042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moati E, Blons H, Taly V, et al. Plasma clearance of Ras mutation under therapeutic pressure is a rare event in metastatic colorectal cancer. Int J Cancer 2020;147:1185–9. 10.1002/ijc.32657 [DOI] [PubMed] [Google Scholar]
- 27.Henry J, Willis J, Parseghian CM, et al. NeoRAS: incidence of Ras reversion from Ras mutated to Ras wild type. J Clin Oncol 2020;38:180. 10.1200/JCO.2020.38.4_suppl.180 [DOI] [Google Scholar]
- 28.Fernández Montes A, Martinez Lago N, De la Cámara Gómez J, et al. Folfiri plus panitumumab as second-line treatment in mutated Ras metastatic colorectal cancer patients who converted to wild type Ras after receiving first-line FOLFOX/CAPOX plus bevacizumab-based treatment: phase II CONVERTIX trial. Ann Oncol 2019;30:iv23–4. 10.1093/annonc/mdz155.088 [DOI] [Google Scholar]
- 29.Sunakawa Y, Nakamura M, Ishizaki M, et al. RAS Mutations in Circulating Tumor DNA and Clinical Outcomes of Rechallenge Treatment With Anti-EGFR Antibodies in Patients With Metastatic Colorectal Cancer. JCO Precis Oncol 2020;4:898–911. 10.1200/PO.20.00109 [DOI] [PubMed] [Google Scholar]
- 30.Nicolazzo C, Barault L, Caponnetto S, et al. Circulating methylated DNA to monitor the dynamics of Ras mutation clearance in plasma from metastatic colorectal cancer patients. Cancers 2020;12:3633. 10.3390/cancers12123633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouchahda M, Saffroy R, Karaboué A, et al. Undetectable RAS-Mutant Clones in Plasma: Possible Implication for Anti-EGFR Therapy and Prognosis in Patients With RAS-Mutant Metastatic Colorectal Cancer. JCO Precis Oncol 2020;4:1070–9. 10.1200/PO.19.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–19. 10.1056/NEJMoa1414325 [DOI] [PubMed] [Google Scholar]
- 33.Kuboki Y, Nishina T, Shinozaki E, et al. Tas-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK force): an investigator-initiated, open-label, single-arm, multicentre, phase 1/2 study. Lancet Oncol 2017;18:1172–81. 10.1016/S1470-2045(17)30425-4 [DOI] [PubMed] [Google Scholar]
- 34.Pfeiffer P, Yilmaz M, Möller S, et al. Tas-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol 2020;21:412–20. 10.1016/S1470-2045(19)30827-7 [DOI] [PubMed] [Google Scholar]
- 35.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (correct): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12. 10.1016/S0140-6736(12)61900-X [DOI] [PubMed] [Google Scholar]
- 36.Diehl F, Schmidt K, Durkee KH, et al. Analysis of mutations in DNA isolated from plasma and stool of colorectal cancer patients. Gastroenterology 2008;135:489–98. 10.1053/j.gastro.2008.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odegaard JI, Vincent JJ, Mortimer S, et al. Validation of a plasma-based comprehensive cancer genotyping assay utilizing orthogonal tissue- and plasma-based methodologies. Clin Cancer Res 2018;24:3539–49. 10.1158/1078-0432.CCR-17-3831 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.