Abstract
Background
Reproductive and sexual health (RSH) concerns are common and distressing for young adults diagnosed with breast and gynecologic cancer and their partners. This study evaluates the efficacy of a virtual couple-based intervention called Opening the Conversation (OC). The OC intervention is grounded in theory and evidence-based practice and was adapted to improve coping and communication specifically in relation to RSH concerns after cancer.
Methods
This Phase III trial is conducted in a fully remote setting and enrolls young adult couples (current age 18–44 years) with a history of breast or gynecologic cancer (stage 1–4, diagnosed under age 40) within the past 6 months to 5 years. Eligible dyads are recruited from across the USA. The target sample size is 100 couples. Dyads are randomly assigned to receive either the 5-session OC intervention or a 4-session active control intervention (Side by Side). The primary outcomes are change in reproductive distress and sexual distress. Secondary outcomes include communication about reproductive concerns, communication about sexual concerns, depressive symptoms, sexual function, relationship quality, relationship intimacy, sexual satisfaction, self-efficacy to communicate about sex and intimacy, and quality of life. An exploratory aim examines whether dyadic coping and communication quality mediate intervention effects on survivors’ and partners’ reproductive distress or sexual distress. Self-report outcome measures are assessed for both groups at baseline (T1), 2 weeks post-treatment (T2), and 3 months post-treatment (T3).
Discussion
Despite the importance of RSH for quality of life for young adult cancer survivors and their partners, evidence-based interventions that help couples navigate RSH concerns are lacking. This randomized controlled trial will determine the efficacy of a novel couple-based intervention to reduce distress related to RSH concerns for younger couples after breast or gynecologic cancer, in comparison to an active control intervention.
Trial registration
ClinicalTrials.gov NCT04806724. Registered on Mar 19, 2021.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-022-06665-3.
Keywords: Breast cancer, Gynecologic cancer, Young adult, Reproductive health, Sexual health, Randomized controlled trial
Administrative information
| Title | Opening the conversation: study protocol for a Phase III trial to evaluate a couple-based intervention to reduce reproductive and sexual distress among young adult breast and gynecologic cancer survivor couples |
| Trial registration | ClinicalTrials.gov, NCT04806724. Registered on Mar 19, 2021 |
| Protocol version | Version 1 dated 05/11/2021 |
| Funding | American Cancer Society RSG-19-123-01-CPPB |
| Author affiliations | Jessica R. Gorman, Oregon State University; Karen S. Lyons, Boston College; S. Marie Harvey, Oregon State University; Chiara Acquati, University of Houston; John M. Salsman, Wake Forest Baptist Comprehensive Cancer Center; Deborah A Kashy, Michigan State University; Julia H. Drizin, Oregon State University; Ellie Smith, Oregon State University; Lisa M. Flexner, Oregon State University-Cascades; Brandon Hayes-Lattin, OHSU Knight Cancer Institute; Jennifer B Reese, Fox Chase Cancer Center |
| Name and contact information for the trial sponsor |
American Cancer Society, Inc. Extramural Discovery Science Department Email: grants@cancer.org Phone: 404-329-7558 |
| Role of sponsor | N/A |
Background and rationale
Breast and gynecologic cancers (BGC) are among the most common cancers in young adults [1]. Survival rates in this population are high, as are the long-term burden of cancer and its treatment on physical and psychosocial outcomes [2–4]. Young BGC survivors are at particular risk for reproductive and sexual distress due to exposure to gonadotoxic treatment and/or treatment affecting pelvic nerves and organ structures as well as scarring, loss of breasts or feeling in the breast, and pain [5–7]. The reproductive and sexual health (RSH) consequences of cancer, including worries about infertility, body image, dating and intimate relationships, and sexual function [7–12], are among the most common and distressing aspects of survivorship for young BGC survivors and their partners [13–19]. Indeed, whereas sexual changes are common and distressing for patients of all ages, younger survivors and couples are at even greater risk of psychological distress due to such changes compared to their older counterparts [20–22]. In sum, RSH concerns can negatively affect intimate relationships, family building plans, and quality of life well after treatment ends [6, 12, 13].
Despite how important intimate relationships and reproductive health are in the quality of life for young adult survivors [23], there are no evidence-based interventions in place to help young BGC couples struggling with distress surrounding both reproductive and sexual health concerns. Additionally, most couple-based interventions were developed for heterosexual couples and have not focused on meeting the needs of LGBTQ+ survivors and their partners [24]. This is important because LGBTQ+ couples tend to be less satisfied with the care they receive, may not feel welcome in clinical and support group settings, and are at heightened risk for significant unmet survivorship care needs, particularly related to psychological and sexual health [25–28]. Available approaches also often neglect the needs of partners. Finally, supportive care to help couples with RSH concerns is not widely available, particularly in rural areas, and barriers accessing such care remain high [29, 30]. When available, services typically rely on in-person formats for delivery and involve therapists with specialized training, who are often constrained to serving clients in urban centers and large cancer centers.
Research demonstrates that psychosocial interventions targeted at couples can enhance dyadic coping (i.e., coping together as a team) and communication in the context of cancer [24, 31–33]. These skills are critical for managing distress and maintaining relationship health and quality of life [34–37]. Specifically, couple communication about reproductive and sexual health is increasingly recognized as an important predictor of relationship functioning [38–40]. Both partners can experience fear, uncertainty, and relationship difficulties due to fertility concerns [41, 42], suggesting that addressing such concerns for both partners would be important in an intervention. Regarding sexual concerns, the most effective approaches to addressing sexual health and reducing sexual distress after cancer actively engage partners [43–45]. Couple-focused interventions, therefore, represent a promising approach to managing RSH distress after cancer.
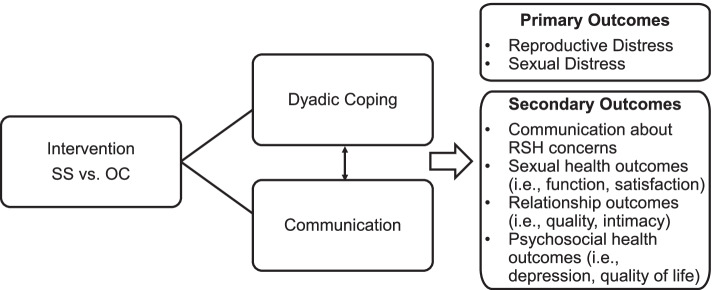
Increasingly, psychosocial interventions for cancer survivors have been taking on a virtual format delivered via videoconference. Because virtual delivery of psychosocial interventions can remove barriers to access among those in less well-resourced cancer care settings and rural areas [46, 47], this format offers a promising approach to fill a gap in supportive care for young couples from all geographic areas faced with navigating life after cancer [9, 14]. Prior research points to the feasibility of a virtual intervention format [47], particularly for recruiting more ethnically and geographically diverse patients as well as less healthy patient populations [48]. The primary objective of this Phase III trial is to evaluate the efficacy of a virtual psychosocial intervention, Opening the Conversation (OC), to reduce RSH distress among young BGC survivors and their partners. OC was developed through systematically adapting an intervention initially designed to help couples cope with and communicate about cancer distress in general, called Side by Side (SS). OC was designed to help young couples cope together and communicate effectively about RSH concerns specifically. SS and OC are grounded in methods of cognitive behavioral therapy [34] and Bodenmann’s Systemic Transactional Model of dyadic coping [36, 49–53]. SS was previously modified from CanCOPE [54] in a pilot trial [55], then adapted specifically for virtual delivery with young adult BGC survivor couples in preparation for the current study [56]. In a prior in-person randomized controlled trial of SS, conducted in Germany with 72 heterosexual couples (female survivors of breast or gynecologic cancer and their partners, median age 52 years), couples receiving the SS intervention reported less avoidance in dealing with cancer, more posttraumatic growth, better communication quality, and better dyadic coping than those in an attention control condition [49]. A virtual version of SS, which focuses on general cancer-related distress, serves as the comparison condition to the OC intervention. The study conceptual framework is shown in Fig. 1. This manuscript describes the study protocol for a Phase III randomized controlled trial comparing two parallel conditions, OC and SS.
Fig. 1.
Study conceptual framework
Objectives
The specific aims of this study are:
To evaluate whether the OC intervention leads to significantly greater improvement in reproductive distress and sexual distress reported by BGC survivors and their partners, as compared to the SS intervention (Aim 1a, primary outcomes)
To evaluate whether the OC intervention leads to significantly great improvement in cancer survivors’ and their partners’ relationship (relationship quality, relationship intimacy, sexual function, sexual satisfaction, self-efficacy to communicate about sex and intimacy) and psychosocial health (depressive symptoms, quality of life) outcomes, as compared to the SS intervention (Aim 1a, secondary outcomes)
In exploratory analyses, we will evaluate whether dyadic coping and communication quality mediate intervention effects on survivors’ and partners’ reproductive distress or sexual distress. (Aim 1b)
Methods
Trial design
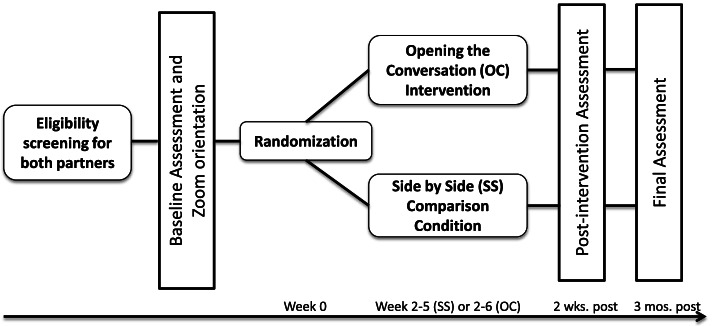
The study will enroll 100 dyads (couples), each comprised of a breast or gynecological cancer survivor (diagnosed between 18 and 39 years of age) and their identified intimate/romantic partner. This is a two-group randomized controlled trial design with one pre-test and two post-test quantitative assessments. Dyads will be randomized to either OC or SS, with equal allocation to each condition. OC includes 5 weekly sessions, 90 min each. SS includes 4 weekly sessions, 90 min each. Both OC and SS are delivered remotely by a trained interventionist to participants across the USA. Web-based assessments are collected at baseline (T1), 2 weeks post-intervention (T2), and 3-month follow-up (T3). This study protocol has followed the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement guidelines (See SPIRIT Checklist) [57]. The study will be conducted and reported according to the Consolidated Standards of Reporting Trials (CONSORT) criteria [58]. The study has been approved by the institutional review board (IRB) at Oregon State University (IRB 7621). The study flow is shown in Fig. 2.
Fig. 2.
Study flow diagram
Eligibility criteria
Cancer survivor participants are eligible if they meet the following criteria: (1) cancer diagnosis between the ages of 18 and 39, (2) current age 1844, (3) cancer diagnosis between 6 months and 5 years prior to enrollment date, (4) diagnosed with breast or gynecologic cancer, (5) self-reported cancer stages 1–4 at time of diagnosis, (6) self-reported ability to participate in a virtual intervention, (7) self-reported committed intimate partner who is willing to participate (any gender/sexual orientation), (8) English speaking, and (9) high-speed internet access via a smart phone, tablet, and/or computer.
Partners are eligible if they meet the following criteria: (1) age 18 or older, (2) English speaking, (3) self-reported ability to participate in a virtual intervention via videoconference, and (4) high-speed internet access via a smart phone, tablet, and/or computer.
Survivors and partners are excluded if either partner does not meet the eligibility criteria. Both partners must enroll in order to participate.
Procedures
Recruitment and informed consent
Study recruitment is multi-pronged in that it includes a range of sources of participant identification, including study advertisements online via social media platforms, outreach through community partners, and letters mailed to potentially eligible cancer survivors in the Oregon State Cancer Registry (OSCaR). This strategy was selected both to facilitate achieving recruitment targets and to reach a diverse sample of dyads nationwide in light of the differences in survivors found through these sources.
Consent is obtained electronically after both partners complete screening procedures and potential participants can discuss their questions with a member of the research staff. Once both members of the dyad have completed consent, the dyad is considered to be consented. Enrollment is determined at randomization, after both partners complete the baseline assessment.
Data collection
Quantitative data are collected using Research Electronic Data Capture (REDCap). Each member of the dyad will complete separate surveys via REDCap at T1, T2, and T3, which are expected to take approximately 30 min each. Individual participants can receive compensation for the study of up to $30 in gift cards, or $60 in gift cards per dyad for completing all assessments. Strategies to increase retention include web-based surveys, flexible scheduling of intervention sessions, and sending email reminders. If either member of the dyad is unable or chooses not to continue participating in the intervention, both may be asked if they would be willing to remain in the study for data collection only and we will attempt to collect data as though they had participated in the assigned intervention.
Study staff complete training in human subject protection, secure data management, and protocols and procedures. All assessments occur in REDCap, which could reduce error related to manual data entry. Routine data checks are in place to check for data quality. Data safety and monitoring will occur in monthly investigator meetings. The group will monitor recruitment, enrollment, data collection, procedures, and any adverse events or protocol deviations (tracked and reported as required by the IRB). Data security is maintained through password-protected files, restricted access to datasets, and de-identification of data when possible.
Randomization
The study uses 1:1 blocked randomization (groups of 4), stratified by cancer type (breast versus gynecologic) and sexual orientation reported by the cancer survivor (heterosexual versus other). The study statistician generates the randomization sequence, and assignment to condition occurs via REDCap after the dyad has completed both the baseline assessment and videoconference orientation call with study staff. Couples are notified of their intervention assignment after scheduling their first session with the study interventionist. At that time, they receive both a mailed version and link to an electronic version of the participant materials. As with most behavioral interventions, the study interventionist, intervention supervisor, and participants are not masked to condition. However, although participants know whether they are receiving OC or SS, they are not told which of these is the experimental condition. The condition is masked to the PI, biostatistician, and research assistants who will evaluate quantitative outcome data. Quantitative outcome data is also collected via REDCap to minimize contact with participants while collecting study data. Should a review of adverse events reveal significant between-group differences that could warrant unmasking of the study conditions by the study biostatistician.
Interventions
Intervention overview
Participants in both conditions receive a manualized virtual psychosocial intervention, either OC or SS. The OC intervention includes 5 weekly sessions, and the SS intervention (comparison) includes 4 weekly sessions. Both interventions are delivered by a trained interventionist following a standard intervention protocol and integrating participant handouts with educational material and skill-building exercises. Each session is approximately 90 min. All sessions are audio-recorded. There are no restrictions on receipt of concomitant care participants may receive while enrolled in the study.
Interventionist training and adherence
Interventionists have a master’s degree in a mental health field (e.g., social work, psychology) and experience working clinically with cancer survivors and their partners/family members. Training includes certification in delivery of both intervention conditions by two study investigators (JG, JR). Training is done remotely via a workshop for which trainees are asked to review materials ahead of time, including the study protocol (interventionist manuals, participant materials) and background readings on relevant topics such as young adult breast/gynecologic cancer, RSH after cancer, couple-based intervention, and theoretical foundations. The remote workshop includes a review of the protocols, key concepts from the required readings, specific skills, and discussions. This is followed by a mock session delivery with feedback. Interventionists complete a full test case with each condition and receive feedback from the supervisor (Co-I Reese, who is a licensed psychologist and has experience in delivering and supervising couples’ interventions in cancer). Training also emphasizes the importance of fidelity to the interventionist manuals, and strategies for developing and maintaining rapport and engagement via videoconference. Interventionists complete reports after each session to document adherence to the manual, to indicate whether there were any unplanned diversions, and to document any challenges faced. Supervision occurs throughout the study and includes review of sessions, audio recordings, and interventionist reports of adherence. Supervision also includes regular group meetings to discuss cases and problem solve. A trained independent reviewer not involved in intervention delivery will randomly select and review 15% of sessions to independently document adherence.
Study arms
Opening the Conversation: experimental intervention group
The intervention group, OC, receives 5 weekly sessions with a trained interventionist. Session content is outlined in Table 1. Couples learn coping and communication strategies that increase their support for one another, particularly related to reproductive and sexual distress. The comparison arm, SS, provided a foundation for the development of OC, but OC was adapted to specifically address coping and communication about RSH concerns after cancer. Modifications were based on an iterative process of interviews with couples and stakeholder feedback. Briefly, these included new educational material about RSH-related concerns after cancer for both partners, integration of opportunity to discuss RSH topics across sessions, and the addition of a fifth session to give couples the opportunity to use new skills learned to discuss an RSH topic of their choice. Details about the systematic adaptation process and resulting changes are available elsewhere [56].
Table 1.
Opening the Conversation (OC) and Side by Side (SS) session summaries
| Session | Opening the Conversation | Side by Side |
|---|---|---|
| 1 |
• Introduction and overview • Cancer’s impact on relationships and RSH • Introduce educational materials on RSH concerns after cancer • Introduce supportive communication, supportive behavior, mindfulness |
• Introduction and overview • Cancer’s impact on relationships • Introduce supportive communication, supportive behavior, mindfulness |
| 2 |
• Stressors and stress response, with focus on RSH • Coping strategies and self-talk, with examples related to RSH • Practice supportive communication, focusing on an RSH stressor |
• Stressors and stress response, with focus on cancer experience • Coping strategies and self-talk • Practice supportive communication, focusing on a cancer-related stressor |
| 3 |
• Practice supportive communication, focusing on an RSH stressor • Communication with family/friends, with examples related to RSH • Patient advocacy and healthcare provider communication about RSH concerns |
• Practice supportive communication • Communication with family/friends |
| 4 |
• Sexual health concerns, emotional and physical intimacy • Communication exercise to build shared understanding about intimacy • Identifying and scheduling activities to support intimacy • Setting goals to support intimacy |
• Emotional intimacy • Making time for activities to support relationship • Explore positive aspects of your cancer experience • Setting goals to support relationship |
| 5 |
• Reproductive health and family building concerns • Practice supportive communication with RSH topic • Skill review • Setting goals to support relationship |
None |
Side by Side: active comparison group
The comparison group, SS, receives 4 weekly sessions with a trained interventionist. Session content is outlined in Table 1. Couples learn coping and communication strategies that increase their support for one another as they navigate cancer-related stressors in general. SS includes a discussion and exercise focused on emotional, but not physical, aspects of intimacy. SS was selected as a comparator because of its grounding in theory and evidence-based practice along with its demonstrated effectiveness for improving relationship and psychosocial outcomes among BGC survivors [34, 36, 49]. Original content was updated for virtual delivery to our intended audience, including use of inclusive language for LGBTQ+ couples.
Measures
The outcome measures used are reliable and valid with cancer survivors. We selected short form versions, where available, to reduce participant burden. All outcome measures are assessed at 3 time points: baseline (immediately prior to intervention), 2 weeks after the last session, and 3 months after the last session. All outcome measures and assessment instruments are listed in Table 2.
Table 2.
Schedule of enrollment, interventions, and assessments
| Enrollment eligibility T0 |
Allocation baseline T1 |
Post-allocation | ||||
|---|---|---|---|---|---|---|
| Sessions 1–4 | Sessions 1–5 | T2 2 weeks | T3 3 months | |||
| ENROLLMENT | ||||||
| Eligibility screen | X | |||||
| Informed consent | X | |||||
| Allocation | X | |||||
| INTERVENTIONS | ||||||
| OC intervention | X | |||||
| SS intervention | X | |||||
| ASSESSMENTS | ||||||
| Primary outcomes | ||||||
|
Reproductive distress Fertility Problem Inventory (FPI)-Relationship Concern Domain |
X | X | X | |||
|
Sexual distress Sexual and Relationship Distress Scale (SaRDS) |
X | X | X | |||
| Secondary outcomes | ||||||
|
Relationship functioning Dyadic Adjustment Scale (DAS-7) |
X | X | X | |||
|
Relationship intimacy Miller Social Intimacy Scale (MSIS) |
X | X | X | |||
|
Communication about reproductive concerns Couples’ Illness Communication Scale (CSIS) |
X | X | X | |||
|
Communication about sexual concerns Couples’ Illness Communication Scale (CSIS) |
X | X | X | |||
|
Sexual function (female) Female Sexual Function Index (FSFI) |
X | X | X | |||
|
Sexual function (male) International Index of Erectile Function (IIEF) |
X | X | X | |||
|
Sexual satisfaction General Measure of Sexual Satisfaction (GMSEX) |
X | X | X | |||
|
Depressive symptoms Patient Health Questionnaire Depression Scale (PHQ-8) |
X | X | X | |||
|
Health-related quality of life PROMIS Global 10 v1.2 |
X | X | X | |||
|
Self-efficacya Self-Efficacy to Communicate about Sex and Intimacy (SECSI) |
X | X | X | |||
| Mediating variables | ||||||
| Dyadic coping | Dyadic Coping Inventory (DCI) | X | X | X | ||
| Communication quality | Communication Patterns Questionnaire- Short Form (CPQ-SF) | X | X | X | ||
aCancer survivor-only measure
Intervention acceptability and appropriateness and interventionist assessments are measured 2 weeks after the last session. Two process measures (perceived usefulness and session-specific evaluations) are measured during the intervention.
Primary outcome measures
Reproductive distress
Reproductive distress is measured using the Fertility Problem Inventory (FPI)-Relationship Concern Domain. This measure consists of 10 items assessing concerns about infertility’s impact on the relationship. Higher scores indicate a higher level of distress [59–61].
Sexual distress
Sexual and relationship distress is measured with the Sexual and Relationship Distress Scale (SaRDS), a 30-item, multidimensional scale that measures relationship distress within the context of sexual difficulties [62]. Item responses are summed to create a total summary score, with higher scores indicating more sexual distress.
Secondary outcome measures
Communication about reproductive concerns
Communication about reproductive concerns is assessed via the Couples’ Illness Communication Scale, a four-item scale adapted to measure couple communication about reproductive/fertility concerns [63]. Item responses are summed to create a total score, with higher scores indicating better reproductive-related couple communication.
Communication about sexual concerns
Communication about sexual concerns is also assessed via the Couples’ Illness Communication Scale [63], adapted to measure couple communication about sexual concerns. Higher scores indicate better sex-related couple communication.
Depressive symptoms
Depression is measured using the Patient Health Questionnaire Depression Scale (PHQ-8), an eight-item scale assessing depression severity [64, 65]. Higher scores reflect higher depressive symptoms.
Female sexual function
Female sexual function is measured via the Female Sexual Function Index (FSFI), a 19-item, multidimensional scale assessing the following domains of female sexual function including desire, arousal, lubrication, orgasm, pain, and satisfaction [66, 67]. Subscale scores are summed to create a total score, with higher scores indicating better functioning.
Male sexual function
Male sexual function is measured via the International Index of Erectile Function (IIEF), a 15-item, multidimensional scale assessing various domains of male sexual function [68, 69]. Item responses within each domain are summed to create subscale scores, and subscale scores are summed to create a total score, with higher scores indicating better functioning.
Relationship quality
Relationship functioning is measured with the Dyadic Adjustment Scale (DAS-7), a seven-item scale measuring relationship functioning [70]. Responses are summed to create a total score, with higher scores reflecting better relationship functioning [70–72].
Relationship intimacy
Intimacy is measured using the Miller Social Intimacy Scale, a 17-item scale that measures the level of intimacy currently experienced in an interpersonal relationship [73]. Responses are summed, with higher scores reflecting higher levels of intimacy.
Sexual satisfaction
Sexual satisfaction is measured with the General Measure of Sexual Satisfaction (GMSEX), a five-item scale assessing the sexual relationship, with higher scores indicating greater sexual satisfaction [74, 75].
Self-efficacy to communicate about sex and intimacy
Self-efficacy is measured for cancer survivors only using the Self-Efficacy to Communicate about Sex and Intimacy (SECSI) scale. This 10-item measure assesses cancer survivors’ self-efficacy for sexual health communication over the last month, with higher scores indicating higher self-efficacy [65].
Quality of life
Quality of life is assessed via the PROMIS (Patient-Reported Outcome Measurement Information Systems) Global 10-item scale, which measures aspects of physical and mental health. Scores are standardized to the general population [76, 77].
Intervention mediators
Dyadic coping
Dyadic coping is measured with the Dyadic Coping Inventory, a 37-item scale measuring perceived communication and dyadic coping among couples. This scale consists of several subscales measuring supportive, delegated, negative, and joint coping. Responses to each item are summed to create a total score, with higher scores indicating higher relationship quality [78, 79].
Communication quality
Communication quality is measured using the Communication Patterns Questionnaire-Short Form (CPQ-SF), an 11-item self-assessment measuring perceptions of relationship interactions. The CPQ-SF assesses different patterns of communication (non-constructive communication and constructive communication), with higher scores indicating more use of that communication pattern during conflict [80].
Demographics, cancer, and reproductive history
Socio-demographic characteristics including age at baseline, race, ethnicity, sexual orientation, gender identity, education level, number of children, and employment status are collected via self-report. Relationship, cancer, and reproductive characteristics are also assessed by self-report, including relationship status and duration, desire for children, cancer type, stage, and treatments, time since diagnosis, and reproductive history.
Acceptability, appropriateness, and feasibility
Intervention acceptability and appropriateness are assessed via AIM/FIM subscales [81] and a counselor assessment form. Perceived usefulness is assessed after the first session with items asking how logical the program seemed, how helpful they think the program will be, and how competent they believe the counselor is. Each week, participants are asked to assess the extent to which they interacted with and understood the program material.
Statistical plan
We will compare recruitment, retention, satisfaction, and completion of assessments for the intervention and comparison group. Our approach to recruitment and retention minimizes missing data by monitoring and actively following up with reminders for survey completion, and by incentivizing participation. Initial analyses will examine characteristics of non-completers. As recommended by Lang & Little [82], multiple imputation (using 50 imputed samples) will be used to impute missing values for analyses using multilevel modeling (MLM) and full information maximum likelihood (FIML) will be used for mediation analyses conducted within a structural equation modeling framework.
Basic descriptive data analyses will be conducted to examine means, standard deviations, and correlations among the study measures. Normality of outcomes will be assessed, and data transformations will be applied as needed.
Multilevel modeling (MLM; SPSS Version 27) with REML will be used to test Aim 1a for both the primary (reproductive and sexual distress) and secondary outcomes (relationship outcomes and psychosocial health outcomes). These models will examine mean differences in outcomes between OC and SS over time (baseline, 2 weeks, 3 months) and across role (i.e., survivor versus partner) for outcomes measured from both partners. In our primary analyses, time, treatment, and role will be treated as categorical, and models will include all main effects and interactions among these variables. Evidence of treatment efficacy will be assessed by the interaction between time and treatment as we expect no treatment differences at baseline, but we expect to see such differences after the intervention. These models will include random intercepts for survivors and partners, as well as the correlation between the intercepts (i.e., if a survivor is high in average distress across time, is the partner high as well?). They will also include a time-specific correlation between the partners’ residuals (i.e., if a survivor is especially distressed at a particular time point, is the partner especially distressed at that time as well?). In addition to being able to model the interdependence between survivors and partners, the MLM approach has the advantage that it does not delete participants with missing data at some time points and so this analysis utilizes all available data. Outcomes measured only for survivors or only for partners will also be analyzed using MLM, but in this case role will not be included as a predictor and the random effects will include only a random intercept and residual variance.
Covariates to be assessed include age, time since diagnosis, cancer stage, metastatic status, number of children, relationship duration, income, race, ethnicity, and sexual orientation. To determine which covariates should be included in the analyses, preliminary analyses will be conducted in which each outcome is predicted by the full set of qualifying covariates. Only those covariates that predict at least one outcome significantly will be included in the analyses. Once the set of covariates are determined in this way, the same set of covariates will be included in all analyses.
Exploratory Aim 1b posits a mediational model in which the effects of the intervention on both partners’ primary outcomes are mediated by both partners’ dyadic coping and communication. To examine mediation, the statistical package MPLUS will be used to estimate and test the indirect effects of the treatment on 3-month outcomes via the patient’s and partner’s dyadic coping and communication measured 2 weeks after the intervention. The same set of covariates used in tests of Aim 1a will be included.
Sample size estimate/ power calculations
A sensitivity power analysis was conducted using the program G*Power and anticipating an initial sample of 100 couples (N = 200 individuals), and an attrition rate of 20% at the final assessment. Given that the design has two levels of the treatment and three assessments, for outcomes that are assessed on only survivors (or only partners), the study has 90% power to detect small to moderate effects (i.e., d = .32) if there is no attrition. With the expected 20% attrition, the study has 80% power to detect effects greater than d = .36. Because both partners will complete most of the same outcomes, for these outcomes the effective sample size is larger than the number of dyads but smaller than the number of individuals. Specifically, assuming a cross-dyad correlation of r = .4 (e.g., the correlation between the two partners’ distress), the effective sample size to test the treatment by time interaction is 142 without attrition and 114 with attrition. Thus, for these outcomes, without attrition the study will have 90% power to detect effects of d > .27 and with attrition it will have 90% power to detect effects of d > .30.
Ethical aspects
This trial was approved by the Oregon State University Human Research Protections Program (Protocol 7621). All investigators and research staff have been trained in principles of ethical conduct of human subject research. Study participants complete informed consent procedures. Financial compensation ($10 per survey) is similar to that in other couple-based intervention studies and intended to express appreciation to participants for their time. As a minimal risk study, a Data and Safety Monitoring Board was not needed. Investigators meet monthly to monitor study progress and discuss data collection and study progress, including monitoring risks and benefits to participants. The study coordinator is responsible for tracking enrollment procedures and ensuring that data collection is complete. Adverse events are reported to the PI within 1 week and are summarized quarterly for the governing IRB. Protocol revisions will be approved by the governing IRB and reported to ClinicalTrials.gov (NCT04806724). Table 3 outlines trial registration data. In very rare cases where high distress is observed that is severe enough that continuing the study is judged to interfere with participants’ well-being, couples will be counseled to discontinue and provided resources and information encouraging follow-up care. As a minimal risk study, there are no provisions in place for ancillary, post-trial, or compensation for study-related harms.
Table 3.
Trial registration data
| Data category | Information |
|---|---|
| Primary registry and trial identifying number | ClinicalTrials.gov |
| Date of registration in primary registry | March 19, 2021 |
| Secondary identifying numbers | None |
| Source(s) of monetary or material support | American Cancer Society |
| Primary sponsor | American Cancer Society |
| Secondary sponsor(s) | None |
| Contact for public queries | JG, PhD, MPH [Jessica.Gorman@oregonstate.edu] |
| Contact for scientific queries | JG, PhD, MPH [Jessica.Gorman@oregonstate.edu] |
| Public title | Opening the Conversation |
| Scientific title | Opening the Conversation for Couples With Reproductive Health Concerns |
| Countries of recruitment | USA |
| Health condition(s) or problem(s) studied | Reproductive and sexual distress after cancer |
| Intervention(s) |
Experimental Intervention Group: Opening the Conversation intervention, 5 sessions Active Comparison Group: Side by Side intervention, 4 sessions |
| Key inclusion and exclusion criteria |
Ages eligible for study: ≥18 years to 44 years (cancer survivor, partner 1) and ≥18 years (partner 2) Sexes eligible for study: all Accepts healthy volunteers: no |
|
Inclusion criteria (partner 1): cancer diagnosis between the ages of 18 and 39; current age 18–44; cancer diagnosis between 6 months and 5 years prior to enrollment date; diagnosed with breast or gynecologic cancer; self-reported cancer stages 1–4 at time of diagnosis; committed partner who is willing to participate Inclusion criteria (partner 2): age 18 or older | |
| Exclusion criteria: survivors and partners are excluded if either partner does not meet the eligibility criteria; both partners must enroll in order to participate. | |
| Study type | Interventional |
| Allocation: randomized intervention. Parallel assignment masking: double blind (investigator, outcomes assessor) | |
| Primary purpose: supportive care | |
| Phase III | |
| Date of first enrolment | September 2021 |
| Target sample size | 100 dyads (200 individuals) |
| Recruitment status | Recruiting |
| Primary outcome(s) | Reproductive distress, Sexual distress (time frame: 3 months) |
| Key secondary outcomes | Communication about reproductive concerns, communication about sexual concerns, depressive symptoms, sexual function, relationship quality, relationship intimacy, sexual satisfaction, self-efficacy to communicate about sex and intimacy, and quality of life |
Discussion
Upon completion of this Phase III trial, we will have rigorously tested the efficacy of a novel theory-driven virtual intervention, called Opening the Conversation (OC), addressing a range of RSH concerns experienced by young BGC survivors and their partners, with attention to inclusivity for LGBTQ+ individuals. If efficacious, the OC intervention could help fill known gaps in care for young couples who are experiencing RSH-related distress [83–85]. We will also examine theoretically based mediators of OC intervention effects; these analyses are novel as there is little known about possible mechanisms underlying the effects of couple-based psychosocial interventions in cancer, and even less known about mechanisms of interventions focusing on RSH.
Novel aspects of OC include the focus on both reproductive and sexual concerns, which often co-occur for younger couples [85, 86], and inclusivity for LGBTQ+ couples. Furthermore, the intervention is grounded in proven cognitive behavioral therapy techniques [34], in valid theoretical models (i.e., Bodenmann’s Systematic Transactional Model of Dyadic Coping [51, 52]), and prior evidence-based interventions [50, 54, 87]. These aspects serve as important strengths of this intervention. Moreover, the OC intervention covers a broad range of RSH topics (i.e., contraception, fertility potential, pelvic health) and potential action steps (e.g., type of healthcare professional to talk to about the concern), making it well-suited to meeting the varied needs of BGC survivors and their partners. Finally, the intervention is designed for virtual delivery, which could increase reach and improve access for young couples [88, 94] and was preferred by couples in the formative research phase [35].
This study will be the first to evaluate the effect of a psychosocial intervention focused on reducing RSH distress in young cancer survivor couples. One strength of the study design is the use of an active comparison condition, SS, which has been previously tested and found to be effective in improving support and communication skills for couples experiencing cancer-related distress [49]. With the inclusion of this skills training content in the active control, we expect to see benefits for both OC and SS, but anticipate more significant reductions in RSH distress for those in the OC condition, where RSH-related issues are explicitly addressed. Furthermore, we designed the OC intervention with broader implementation and dissemination in mind, such as consideration of feasibility and practicality. We anticipate that planning an efficacy trial with later-stage implementation and dissemination in mind may help reduce barriers to future implementation in practice to facilitate adoption and use of the intervention in real-life practice [95, 96]. One key example of an effort taken to enhance real-world adoptability of OC is the use of master’s level interventionists, rather than PhD level interventionists, which aligns with the practice in most cancer centers. We also developed standardized training materials for study interventionists; if OC is found to be effective, these materials could be used to train other mental health professionals with a similar background as part of future dissemination efforts. Additionally, we include assessment of perceived feasibility and acceptability of the intervention, with the goal of identifying further modifications to facilitate its future implementation.
The current study design also has limitations. First, only individuals with a committed partner are able to participate, which necessarily excludes unpartnered individuals. Future studies should assess the needs of women with breast or gynecologic cancer with RSH concerns who are not partnered in order to design appropriate interventions for this population, as their needs may differ from those of partnered women. Second, because the intervention’s focus is on improvements at the individual and couple levels, it does not address other important social or structural determinants such as access to RSH care after cancer. Additional multilevel strategies may complement and reinforce couple-based approaches to improving equity in RSH care after cancer. Finally, although we purposefully engaged LGBTQ+ couples in the adaptation process for OC [56], based on prior research experience, and results of similar trials that do not limit enrollment by sexual orientation [97], it is likely that most participants will be heterosexual and cisgender. Although we would ideally be able to examine outcomes and potential differences based on sexual orientation and gender, we may have too small a sample size of LGBTQ+ couples to accomplish this. Thus, future studies should consider focusing efforts on these specific populations. Finally, the focus on BGC cancer couples specifically excludes young couples with other types of cancer who experience RSH concerns [5, 7, 98]. If OC proves effective, further adaptations could be made for other cancer populations. Despite these limitations, results of the study will contribute significantly to our understanding of the role of psychosocial interventions for reducing RSH distress after cancer.
A large number of young BGC couples experience RSH concerns after cancer, and these concerns often go unaddressed. Evidence-based interventions are needed to fill this gap in supportive care in survivorship. Results are expected to determine whether the OC intervention improves reproductive distress and sexual distress reported by BGC survivors and their partners, as compared to the SS intervention. It is also expected to yield important information about mechanisms of change underlying intervention-related improvement in outcomes and could document the needs of subgroups of survivors and partners that would help in further targeting the intervention. Upon completion of this trial, the study team will be prepared to consider next steps, which may include expanding the intervention to multiple sites to explore implementation, dissemination, and strategies for integration into comprehensive survivorship care and further intervention adaptation and evaluation with other cancer survivor populations.
Dissemination plans
The findings of this trial will be disseminated to researchers and the public through the study’s entry on ClinicalTrials.gov, through publication in peer-reviewed journals, and through presentation of the findings to the scientific community at scientific conferences. To reach a wider audience, summary results will also be shared widely with participants, advocacy groups, and community partners such as via social media platforms.
Trial status
This trial is actively recruiting participants. Recruitment began in September 2021 and is expected to continue through 2023. This manuscript describes the study protocol dated 05/11/2021.
Supplementary Information
Additional file 1. Model research consent form.
Acknowledgements
We thank the participants in our preliminary qualitative research and community advisors for their assistance with adapting the intervention materials in preparation for this trial. We are grateful to Tammy Winfield for her assistance with formatting/editing intervention materials. We also wish to thank Dr. Nina Heinrichs and Dr. Tanja Zimmermann for supporting our team’s adaptation of the Side by Side intervention.
Abbreviations
- BGC
Breast and gynecologic cancer
- CPQ-SF
Communication Patterns Questionnaire-Short Form
- CSIS
Couples’ Illness Communication Scale
- DAS-7
Dyadic Adjustment Scale-7 item
- DCI
Dyadic Coping Inventory
- FIML
Full information maximum likelihood
- FSFI
Female Sexual Function Index
- FPI
Fertility Problem Inventory
- GMSEX
General Measure of Sexual Satisfaction
- IIEF
International Index of Erectile Function
- MLM
Multilevel modeling
- MSIS
Miller Social Intimacy Scale
- OC
Opening the Conversation
- PHQ-8
Patient Health Questionnaire-8 item
- PROMIS
Patient-Reported Outcome Measurement Information System
- REML
Restricted maximum likelihood
- RSH
Reproductive and Sexual Health
- SaRDS
Sexual and Relationship Distress Scale
- SECSI
Self-Efficacy to Communicate about Sex and Intimacy
- SS
Side by Side
Authors’ contributions
Authorship was determined using the standards endorsed by the International Committee of Medical Journal Editors. There is no plan for the use of medical writers. JRG is the principal investigator of the study; she led the proposal and protocol development and wrote the first draft of this manuscript. KSL, SMH, and JBR are co-investigators and contributed to the drafting of the manuscript, study design, development of intervention materials, and selection of outcome measures. JBR also serves as the interventionist supervisor. Consultants JS and CA contributed to study design decisions, including selection of outcome measures, refining intervention protocols, and development of intervention materials. Statistician DAK contributed to study design and analytic planning. Graduate research assistants JHD and ES contributed to the design and implementation of procedures for study, data collection, randomization, recruitment, and consent, data monitoring, and modifications to the study protocol and procedures as needed. Consultant LMF played a key role in the development and refinement of the intervention materials. Consultant BHL contributed to medical aspects such as study eligibility criteria and review of intervention materials. All authors read and approved the final manuscript.
Funding
The study was funded by the American Cancer Society (PI: Gorman), RSG-19-123-01-CPPB.
Availability of data and materials
De-identified quantitative data will be stored electronically without any personal identifying information for open access. Data will be stripped of indirect and direct identifying information before sharing. All study data and relevant materials from the trial described in this manuscript will be retained and archived by the primary study site for a minimum of 3 years after study completion.
Declarations
Ethics approval and consent to participate
This study was approved by the Oregon State University Human Research Protection Program (Protocol 7621). Informed consent will be obtained by all study participants. Consent elements are standard for behavioral intervention trials, with this exception: sessions and interviews are audio recorded and the consent form stipulates this as necessary for participation. No biological specimens are collected as part of this protocol and as such no consent is necessary for the use of biological specimens. Consent is not obtained to use data in ancillary studies.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70(6):443–459. doi: 10.3322/caac.21637. [DOI] [PubMed] [Google Scholar]
- 2.Murphy BL, Day CN, Hoskin TL, Habermann EB, Boughey JC. Adolescents and young adults with breast cancer have more aggressive disease and treatment than patients in their forties. Ann Surg Oncol. 2019;26(12):3920–3930. doi: 10.1245/s10434-019-07653-9. [DOI] [PubMed] [Google Scholar]
- 3.Warner EL, Kent EE, Trevino KM, Parsons HM, Zebrack BJ, Kirchhoff AC. Social well-being among adolescents and young adults with cancer: a systematic review. Cancer. 2016;122(7):1029–1037. doi: 10.1002/cncr.29866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett M, McDonnell G, DeRosa A, Schuler T, Philip E, Peterson L, et al. Psychosocial outcomes and interventions among cancer survivors diagnosed during adolescence and young adulthood (AYA): a systematic review. J Cancer Surviv. 2016;10(5):814–831. doi: 10.1007/s11764-016-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schover LR, van der Kaaij M, van Dorst E, Creutzberg C, Huyghe E, Kiserud CE. Sexual dysfunction and infertility as late effects of cancer treatment. EJC Suppl. 2014;12(1):41–53. doi: 10.1016/j.ejcsup.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaz AF, Pinto-Neto AM, Conde DM, Costa-Paiva L, Morais SS, Pedro AO, et al. Quality of life and menopausal and sexual symptoms in gynecologic cancer survivors: a cohort study. Menopause. 2011;18(6):662–669. doi: 10.1097/gme.0b013e3181ffde7f. [DOI] [PubMed] [Google Scholar]
- 7.Karabulut N, Erci B. Sexual desire and satisfaction in sexual life affecting factors in breast cancer survivors after mastectomy. J Psychosoc Oncol. 2009;27(3):332–343. doi: 10.1080/07347330902979101. [DOI] [PubMed] [Google Scholar]
- 8.Fobair P, Hoppe RT, Bloom J, Cox R, Varghese A, Spiegel D. Psychosocial problems among survivors of Hodgkin’s disease. J Clin Oncol. 1986;4(5):805–814. doi: 10.1200/JCO.1986.4.5.805. [DOI] [PubMed] [Google Scholar]
- 9.Robinson L, Miedema B, Easley J. Young adult cancer survivors and the challenges of intimacy. J Psychosoc Oncol. 2014;32(4):447–462. doi: 10.1080/07347332.2014.917138. [DOI] [PubMed] [Google Scholar]
- 10.Bradford A, Fellman B, Urbauer D, Gallegos J, Meaders K, Tung C, et al. Assessment of sexual activity and dysfunction in medically underserved women with gynecologic cancers. Gynecol Oncol. 2015;139(1):134–140. doi: 10.1016/j.ygyno.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grover S, Hill-Kayser CE, Vachani C, Hampshire MK, DiLullo GA, Metz JM. Patient reported late effects of gynecological cancer treatment. Gynecol Oncol. 2012;124(3):399–403. doi: 10.1016/j.ygyno.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Carter J, Chi DS, Brown CL, Abu-Rustum NR, Sonoda Y, Aghajanian C, et al. Cancer-related infertility in survivorship. Int J Gynecol Cancer. 2010;20(1):2–8. doi: 10.1111/IGC.0b013e3181bf7d3f. [DOI] [PubMed] [Google Scholar]
- 13.Levin AO, Carpenter KM, Fowler JM, Brothers BM, Andersen BL, Maxwell GL. Sexual morbidity associated with poorer psychological adjustment among gynecological cancer survivors. Int J Gynecol Cancer. 2010;20(3):461–470. doi: 10.1111/IGC.0b013e3181d24ce0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson EG, Sansom-Daly UM, Wakefield CE, Ellis SJ, McGill BC, Doolan EL, et al. Sexual and romantic relationships: experiences of adolescent and young adult cancer survivors. J Adolesc Young Adult Oncol. 2016;5(3):286–291. doi: 10.1089/jayao.2015.0061. [DOI] [PubMed] [Google Scholar]
- 15.Ljungman L, Ahlgren J, Petersson LM, Flynn KE, Weinfurt K, Gorman JR, et al. Sexual dysfunction and reproductive concerns in young women with breast cancer: type, prevalence, and predictors of problems. Psychooncology. 2018;27(12):2770–2777. doi: 10.1002/pon.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wettergren L, Kent EE, Mitchell SA, Zebrack B, Lynch CF, Rubenstein MB, et al. Cancer negatively impacts on sexual function in adolescents and young adults: the AYA HOPE study. Psychooncology. 2017;26(10):1632–1639. doi: 10.1002/pon.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jing L, Zhang C, Li W, Jin F, Wang A. Incidence and severity of sexual dysfunction among women with breast cancer: a meta-analysis based on female sexual function index. Support Care Cancer. 2019;27(4):1171–1180. doi: 10.1007/s00520-019-04667-7. [DOI] [PubMed] [Google Scholar]
- 18.Olsson M, Steineck G, Enskär K, Wilderäng U, Jarfelt M. Sexual function in adolescent and young adult cancer survivors-a population-based study. J Cancer Surviv. 2018;12(4):450–459. doi: 10.1007/s11764-018-0684-x. [DOI] [PubMed] [Google Scholar]
- 19.Olsson M, Enskär K, Steineck G, Wilderäng U, Jarfelt M. Self-perceived physical attractiveness in relation to scars among adolescent and young adult cancer survivors: a population-based study. J Adolesc Young Adult Oncol. 2018;7(3):358–366. doi: 10.1089/jayao.2017.0089. [DOI] [PubMed] [Google Scholar]
- 20.Arndt V, Merx H, Sturmer T, Stegmaier C, Ziegler H, Brenner H. Age-specific detriments to quality of life among breast cancer patients one year after diagnosis. Eur J Cancer Care. 2004;40(5):673–680. doi: 10.1016/j.ejca.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Bidstrup PE, Christensen J, Mertz BG, Rottmann N, Dalton SO, Johansen C. Trajectories of distress, anxiety, and depression among women with breast cancer: Looking beyond the mean. Acta Oncol. 2015;54(5):789–796. doi: 10.3109/0284186X.2014.1002571. [DOI] [PubMed] [Google Scholar]
- 22.Acquati C, Kayser K. Dyadic coping across the lifespan: a comparison between younger and middle-aged couples with breast cancer. Front Psychol. 2019;10:404. doi: 10.3389/fpsyg.2019.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geue K, Schmidt R, Sender A, Sauter S, Friedrich M. Sexuality and romantic relationships in young adult cancer survivors: satisfaction and supportive care needs. Psychooncology. 2015;24(11):1368–1376. doi: 10.1002/pon.3805. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Chan CWH, Chow KM, Xiao J, Choi KC. A systematic review and meta-analysis of couple-based intervention on sexuality and the quality of life of cancer patients and their partners. Support Care Cancer. 2020;28(4):1607–1630. doi: 10.1007/s00520-019-05215-z. [DOI] [PubMed] [Google Scholar]
- 25.Seay J, Mitteldorf D, Yankie A, Pirl WF, Kobetz E, Schlumbrecht MP. Survivorship care needs among LGBT cancer survivors. J Psychosoc Oncol. 2018;36(4):393–405. doi: 10.1080/07347332.2018.1447528. [DOI] [PubMed] [Google Scholar]
- 26.Brown MT, McElroy JA. Unmet support needs of sexual and gender minority breast cancer survivors. Support Care Cancer. 2018;26(4):1189–1196. doi: 10.1007/s00520-017-3941-z. [DOI] [PubMed] [Google Scholar]
- 27.Hulbert-Williams NJ, Plumpton CO, Flowers P, et al. The cancer care experiences of gay, lesbian and bisexual patients: A secondary analysis of data from the UK Cancer Patient Experience Survey. Eur J Cancer Care. 2017;26(4):e12670. 10.1111/ecc.12670. [DOI] [PubMed]
- 28.Boehmer U, Glickman M, Winter M, Clark MA. Long-term breast cancer survivors’ symptoms and morbidity: differences by sexual orientation? J Cancer Surviv. 2013;7(2):203–210. doi: 10.1007/s11764-012-0260-8. [DOI] [PubMed] [Google Scholar]
- 29.Regan TW, Lambert SD, Girgis A, Kelly B, Kayser K, Turner J. Do couple-based interventions make a difference for couples affected by cancer? A systematic review. BMC Cancer. 2012;12:279. doi: 10.1186/1471-2407-12-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fredman SJ, Baucom DH, Gremore TM, Castellani AM, Kallman TA, Porter LS, et al. Quantifying the recruitment challenges with couple-based interventions for cancer: applications to early-stage breast cancer. Psychooncology. 2009;18(6):667–673. doi: 10.1002/pon.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traa MJ, De Vries J, Bodenmann G, Den Oudsten BL. Dyadic coping and relationship functioning in couples coping with cancer: a systematic review. Br J Health Psychol. 2015;20(1):85–114. doi: 10.1111/bjhp.12094. [DOI] [PubMed] [Google Scholar]
- 32.Badr H, Krebs P. A systematic review and meta-analysis of psychosocial interventions for couples coping with cancer. Psychooncology. 2013;22(8):1688–1704. doi: 10.1002/pon.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott JL, Kayser K. A review of couple-based interventions for enhancing women's sexual adjustment and body image after cancer. Cancer J. 2009;15(1):48–56. doi: 10.1097/PPO.0b013e31819585df. [DOI] [PubMed] [Google Scholar]
- 34.Epstein N, Baucom DH. Enhanced cognitive-behavioral therapy for couples: a contextual approach. Washington, DC: American Psychological Association; 2002. [Google Scholar]
- 35.Gorman JR, Smith E, Drizin JH, Lyons KS, Harvey SM. Navigating sexual health in cancer survivorship: a dyadic perspective. Support Care Cancer. 2020;28:5429–5439. doi: 10.1007/s00520-020-05396-y. [DOI] [PubMed] [Google Scholar]
- 36.Bodenmann G, Pihet S, Kayser K. The relationship between dyadic coping and marital quality: a 2-year longitudinal study. J Fam Psychol. 2006;20(3):485–493. doi: 10.1037/0893-3200.20.3.485. [DOI] [PubMed] [Google Scholar]
- 37.Bodenmann G. Dyadic coping and its significance for marital functioning. 2005. In: Ravenson TA, Kayser K, Bodnemann G, editors. Couples copign with stress: emerging persepctives on dyadic coping. Washington, DC: American Psychological Association; 2005. pp. 33–49. [Google Scholar]
- 38.Badr H. New frontiers in couple-based interventions in cancer care: refining the prescription for spousal communication. Acta Oncol. 2017;56(2):139–145. doi: 10.1080/0284186X.2016.1266079. [DOI] [PubMed] [Google Scholar]
- 39.Otto AK, Ketcher D, Heyman RE, Vadaparampil ST, Ellington L, Reblin M. Communication between advanced cancer patients and their family caregivers: relationship with caregiver burden and preparedness for caregiving. Health Commun. 2021;36(6):714–721. doi: 10.1080/10410236.2020.1712039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawkey A, Ussher JM, Perz J, Parton C. Talking but not always understanding: couple communication about infertility concerns after cancer. BMC Public Health. 2021;21(1):161. doi: 10.1186/s12889-021-10188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dryden A, Ussher JM, Perz J. Young women’s construction of their post-cancer fertility. Psychol Health. 2014;29(11):1341–1360. doi: 10.1080/08870446.2014.932790. [DOI] [PubMed] [Google Scholar]
- 42.Lehmann V, Nahata L, Ferrante AC, Hansen-Moore JA, Yeager ND, Klosky JL, et al. Fertility-related perceptions and impact on romantic relationships among adult survivors of childhood cancer. J Adolesc Young Adult Oncol. 2018;7(4):409–414. doi: 10.1089/jayao.2017.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hummel SB, van Lankveld JJDM, Oldenburg HSA, Hahn DEE, Kieffer JM, Gerritsma MA, et al. Efficacy of internet-based cognitive behavioral therapy in improving sexual functioning of breast cancer survivors: results of a randomized controlled trial. J Clin Oncol. 2017;35(12):1328–1340. doi: 10.1200/JCO.2016.69.6021. [DOI] [PubMed] [Google Scholar]
- 44.Schover LR, Yuan Y, Fellman BM, Odensky E, Lewis PE, Martinetti P. Efficacy trial of an internet-based intervention for cancer-related female sexual dysfunction. J Natl Compr Cancer Netw. 2013;11(11):1389–1397. doi: 10.6004/jnccn.2013.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carroll AJ, Baron SR, Carroll RA. Couple-based treatment for sexual problems following breast cancer: a review and synthesis of the literature. Support Care Cancer. 2016;24(8):3651–3659. doi: 10.1007/s00520-016-3218-y. [DOI] [PubMed] [Google Scholar]
- 46.Porter LS, Keefe FJ, Baucom DH, Olsen M, Zafar SY, Uronis H. A randomized pilot trial of a videoconference couples communication intervention for advanced GI cancer. Psychooncology. 2017;26(7):1027–1035. doi: 10.1002/pon.4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richardson LK, Frueh BC, Grubaugh AL, Egede L, Elhai JD. Current directions in videoconferencing tele-mental health research. Clin Psychol Sci Pr. 2009;16(3):323–338. doi: 10.1111/j.1468-2850.2009.01170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rupert DJ, Poehlman JA, Hayes JJ, Ray SE, Moultrie RR. Virtual versus in-person focus groups: comparison of costs, recruitment, and participant logistics. J Med Internet Res. 2017;19(3):e80. doi: 10.2196/jmir.6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heinrichs N, Zimmermann T, Huber B, Herschbach P, Russell DW, Baucom DH. Cancer distress reduction with a couple-based skills training: a randomized controlled trial. Ann Behav Med. 2012;43(2):239–252. doi: 10.1007/s12160-011-9314-9. [DOI] [PubMed] [Google Scholar]
- 50.Bodenmann G, Shantinath SD. The Couples Coping Enhancement Training (CCET): a new approach to prevention of marital distress based upon stress and coping. Fam Relat. 2004;53(5):477–484. doi: 10.1111/j.0197-6664.2004.00056.x. [DOI] [Google Scholar]
- 51.Bodenmann G, Randall AK, Falconier MK. Coping in couples: the Systemic Transactional Model (STM) In: Falconier MK, Randall AK, Bodenmann G, editors. Couples coping with stress: a cross-cultural perspective. New York: Routledge/Taylor & Francis Group; 2016. pp. 5–22. [Google Scholar]
- 52.Bodenmann G. Dyadic coping: a systemic-transactional view of stress and coping among couples: Theory and empirical findings. Eur Rev Appl Psychol. 1997;47(2):137–141. [Google Scholar]
- 53.Bodenmann G. A systemic-transactional conceptualization of stress and coping in couples. Swiss J Psychol. 1995;54(1):34–49. [Google Scholar]
- 54.Scott JL, Halford WK, Ward BG. United we stand? The effects of a couple-coping intervention on adjustment to early stage breast or gynecological cancer. J Consult Clin Psychol. 2004;72(6):1122–1135. doi: 10.1037/0022-006X.72.6.1122. [DOI] [PubMed] [Google Scholar]
- 55.Zimmermann T, Heinrichs N, Scott JL. CanCOPE “Schritt für Schritt”: Die Effektivität eines partnerschaftlichen Unterstützungsprogramms bei Frauen mit Brust- oder gynäkologischen Krebserkrankungen. [CanCOPE ‘Step by Step’: the effectiveness of a couple-based intervention program for women with breast or gynecological cancer] Verhaltenstherapie. 2006;16(4):247–255. doi: 10.1159/000096122. [DOI] [Google Scholar]
- 56.Gorman JR, Lyons KS, Reese JB, Acquati C, Smith E, Drizin JH, et al. Adapting a theory-informed intervention to help young adult couples cope with reproductive and sexual concerns after cancer. Front Psychol. 2022;13:813548. doi: 10.3389/fpsyg.2022.813548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulz KF, Altman DG, Moher D, Group C CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11:32. doi: 10.1186/1745-6215-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Armuand GM, Wettergren L, Rodriguez-Wallberg KA, Lampic C. Desire for children, difficulties achieving a pregnancy, and infertility distress 3 to 7 years after cancer diagnosis. Support Care Cancer. 2014;22(10):2805–2812. doi: 10.1007/s00520-014-2279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moura-Ramos M, Gameiro S, Canavarro MC, Soares I. Assessing infertility stress: re-examining the factor structure of the Fertility Problem Inventory. Hum Reprod. 2012;27(2):496–505. doi: 10.1093/humrep/der388. [DOI] [PubMed] [Google Scholar]
- 61.Newton CR, Sherrard W, Glavac I. The Fertility Problem Inventory: measuring perceived infertility-related stress. Fertil Steril. 1999;72(1):54–62. doi: 10.1016/S0015-0282(99)00164-8. [DOI] [PubMed] [Google Scholar]
- 62.Frost R, Donovan C. The development and validation of the Sexual and Relationship Distress Scale. J Sex Med. 2018;15(8):1167–1179. doi: 10.1016/j.jsxm.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 63.Arden-Close E, Moss-Morris R, Dennison L, Bayne L, Gidron Y. The Couples’ Illness Communication Scale (CICS): development and evaluation of a brief measure assessing illness-related couple communication. Br J Health Psychol. 2010;15(Pt 3):543–559. doi: 10.1348/135910709X476972. [DOI] [PubMed] [Google Scholar]
- 64.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1-3):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 65.Arthur EK, Wills CE, Browning K, Overcash J, Menon U. The Self-Efficacy to Communicate about Sex and Intimacy (SECSI) scale: psychometric assessment in women treated for cancer. Support Care Cancer. 2020;28(3):1449–1457. doi: 10.1007/s00520-019-04963-2. [DOI] [PubMed] [Google Scholar]
- 66.Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31(1):1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 67.Baser RE, Li Y, Carter J. Psychometric validation of the Female Sexual Function Index (FSFI) in cancer survivors. Cancer. 2012;118(18):4606–18. doi: 10.1002/cncr.26739. [DOI] [PubMed] [Google Scholar]
- 68.Rosen RC, Cappelleri JC, Gendrano N., 3rd The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res. 2002;14(4):226–244. doi: 10.1038/sj.ijir.3900857. [DOI] [PubMed] [Google Scholar]
- 69.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 70.Hunsley J, Best M, Lefebvre M, Vito D. The seven-item short form of the Dyadic Adjustment Scale: further evidence for construct validity. Am J Fam Ther. 2001;29(4):325–335. doi: 10.1080/01926180126501. [DOI] [Google Scholar]
- 71.Hendrick SS. A generic measure of relationship satisfaction. J Marriage Fam. 1988;50(1):93–98. doi: 10.2307/352430. [DOI] [Google Scholar]
- 72.Sharpley CF, Rogers HJ. Preliminary validation of the Abbreviated Spanier Dyadic Adjustment Scale - some psychometric data regarding a screening-test of marital adjustment. Educ Psychol Meas. 1984;44(4):1045–1050. doi: 10.1177/0013164484444029. [DOI] [Google Scholar]
- 73.Miller RS, Lefcourt HM. The assessment of social intimacy. J Pers Assess. 1982;46(5):514–518. doi: 10.1207/s15327752jpa4605_12. [DOI] [PubMed] [Google Scholar]
- 74.Mark KP, Herbenick D, Fortenberry JD, Sanders S, Reece M. A psychometric comparison of three scales and a single-item measure to assess sexual satisfaction. J Sex Res. 2014;51(2):159–169. doi: 10.1080/00224499.2013.816261. [DOI] [PubMed] [Google Scholar]
- 75.Lawrance K-aG, Byers ES. Development of the interpersonal exchange model of sexual satisfaction in long term relationships. Can J Hum Sex. 1992;1:123–128. [Google Scholar]
- 76.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18(7):873–880. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bodenmann G. Dyadic coping and the significance of this concept for prevention and therapy. Z Gesundheitspsychol. 2008;16(3):108–111. doi: 10.1026/0943-8149.16.3.108. [DOI] [Google Scholar]
- 79.Randall AK, Hilpert P, Jimenez-Arista LE, Walsh KJ, Bodenmann G. Dyadic coping in the US: psychometric properties and validity for use of the English Version of the Dyadic Coping Inventory. Curr Psychol. 2016;35(4):570–582. doi: 10.1007/s12144-015-9323-0. [DOI] [Google Scholar]
- 80.Futris TG, Campbell K, Nielsen RB, Burwell SR. The Communication Patterns Questionnaire-Short Form: a review and assessment. Fam J. 2010;18(3):275–287. doi: 10.1177/1066480710370758. [DOI] [Google Scholar]
- 81.Weiner BJ, Lewis CC, Stanick C, Powell BJ, Dorsey CN, Clary AS, et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci. 2017;12(1):108. doi: 10.1186/s13012-017-0635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lang KM, Little TD. Principled Missing Data Treatments. Prev Sci. 2018;19(3):284-94. 10.1007/s11121-016-0644-5. [DOI] [PubMed]
- 83.Gorman JR, Drizin JH, Smith E, Flores-Sanchez Y, Harvey SM. Patient-centered communication to address young adult breast cancer survivors’ reproductive and sexual health concerns. Health Commun. 2021;36(13):1743–1758. doi: 10.1080/10410236.2020.1794550. [DOI] [PubMed] [Google Scholar]
- 84.Keesing S, Rosenwax L, McNamara B. A dyadic approach to understanding the impact of breast cancer on relationships between partners during early survivorship. BMC Womens Health. 2016;16(1):57. doi: 10.1186/s12905-016-0337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hawkey AJ, Ussher JM, Perz J, Parton C, Patterson P, Bateson D, et al. The impact of cancer-related fertility concerns on current and future couple relationships: People with cancer and partner perspectives. Eur J Cancer Care. 2021;30(1):e13348. doi: 10.1111/ecc.13348. [DOI] [PubMed] [Google Scholar]
- 86.Luk BHK, Loke AY. Sexual satisfaction, intimacy and relationship of couples undergoing infertility treatment. J Reprod Infant Psychol. 2019;37(2):108–122. doi: 10.1080/02646838.2018.1529407. [DOI] [PubMed] [Google Scholar]
- 87.Hill EK, Sandbo S, Abramsohn E, Makelarski J, Wroblewski K, Wenrich ER, et al. Assessing gynecologic and breast cancer survivors’ sexual health care needs: Sexual Care Needs of Cancer Survivors. Cancer. 2011;117(12):2643–2651. doi: 10.1002/cncr.25832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kubo A, Altschuler A, Kurtovich E, Hendlish S, Laurent CA, Kolevska T, et al. A pilot mobile-based mindfulness intervention for cancer patients and their informal caregivers. Mindfulness. 2018;9(6):1885–1894. doi: 10.1007/s12671-018-0931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lengacher CA, Reich RR, Ramesar S, Alinat CB, Moscoso M, Cousin L, et al. Feasibility of the mobile mindfulness-based stress reduction for breast cancer (mMBSR(BC)) program for symptom improvement among breast cancer survivors. Psychooncology. 2018;27(2):524–531. doi: 10.1002/pon.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rosen KD, Paniagua SM, Kazanis W, Jones S, Potter JS. Quality of life among women diagnosed with breast cancer: a randomized waitlist controlled trial of commercially available mobile app-delivered mindfulness training. Psychooncology. 2018;27(8):2023–2030. doi: 10.1002/pon.4764. [DOI] [PubMed] [Google Scholar]
- 91.Russell L, Ugalde A, Milne D, Krishnasamy M, Seung Chul EO, Austin DW, et al. Feasibility of an online mindfulness-based program for patients with melanoma: study protocol for a randomised controlled trial. Trials. 2018;19:223. doi: 10.1186/s13063-018-2575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zernicke KA, Campbell TS, Speca M, McCabe-Ruff K, Flowers S, Carlson LE. A randomized wait-list controlled trial of feasibility and efficacy of an online mindfulness–based cancer recovery program: the etherapy for cancer applying mindfulness trial. Psychosom Med. 2014;76(4):257–267. doi: 10.1097/PSY.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 93.Trevino KM, Raghunathan N, Latte-Naor S, Polubriaginof FCG, Jensen C, Atkinson TM, et al. Rapid deployment of virtual mind-body interventions during the COVID-19 outbreak: feasibility, acceptability, and implications for future care. Support Care Cancer. 2021;29(2):543–546. doi: 10.1007/s00520-020-05740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baldwin-Medsker A, Holland J, Rodriguez ES. Access to Care: Using eHealth to limit location-based barriers for patients with cancer. Clin J Oncol Nurs. 2020;24:16–23. doi: 10.1188/20.CJON.S1.16-23. [DOI] [PubMed] [Google Scholar]
- 95.Klesges LM, Estabrooks PA, Dzewaltowski DA, Bull SS, Glasgow RE. Beginning with the application in mind: designing and planning health behavior change interventions to enhance dissemination. Ann Behav Med. 2005;29(Suppl):66–75. doi: 10.1207/s15324796abm2902s_10. [DOI] [PubMed] [Google Scholar]
- 96.Kerner J, Rimer B, Emmons K. Introduction to the special section on dissemination—dissemination research and research dissemination: how can we close the gap? Health Psychol. 2005;24(5):443–446. doi: 10.1037/0278-6133.24.5.443. [DOI] [PubMed] [Google Scholar]
- 97.Reese JB, Smith KC, Handorf E, Sorice K, Bober SL, Bantug ET, et al. A randomized pilot trial of a couple-based intervention addressing sexual concerns for breast cancer survivors. J Psychosoc Oncol. 2019;37(2):242–263. doi: 10.1080/07347332.2018.1510869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ljungman L, Eriksson LE, Flynn KE, Gorman JR, Stahl O, Weinfurt K, et al. Sexual dysfunction and reproductive concerns in young men diagnosed with testicular cancer: an observational study. J Sex Med. 2019;16(7):1049–1059. doi: 10.1016/j.jsxm.2019.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Model research consent form.
Data Availability Statement
De-identified quantitative data will be stored electronically without any personal identifying information for open access. Data will be stripped of indirect and direct identifying information before sharing. All study data and relevant materials from the trial described in this manuscript will be retained and archived by the primary study site for a minimum of 3 years after study completion.