Abstract
Purpose
Anorexia nervosa (AN) is the most frequent eating disorder (ED), whose cardiac complications may have life-threatening consequences for both the physical and psychological health of affected children. In this study, we reported and analysed the echocardiographic anomalies found in pediatric patients diagnosed with AN.
Methods
We reported the demographic and clinical characteristics of children aged 8 to 18 years, who were diagnosed with AN and underwent a complete cardiological evaluation at the Emergency Department of the Bambino Gesù Children's Hospital, IRCCS, Rome between the 1st January 2021 and the 30th June 2021. Furthermore, we compared the patients according to the presence of pericardial effusion and a BMI (body mass index) cut-off 14.5 kg/m2.
Results
Forty-nine patients were included in the study. The mean age was 15.1 years. Most patients were female (89.8%). The mean length of hospitalization was 18 days. The mean BMI at admission was 14.8 kg/m2, with a median weight loss of 9 kg in the last year. Eleven patients (22.4%) presented with cardiovascular signs or symptoms at admission. Most patients had pericardial effusion on heart ultrasound, with a mean thickness of 6 mm (SD ± 4). The LV (left ventricle) thickness over age was significantly higher in patients with pericardial effusion, with a Z score of −2.0 vs −1.4 (p = 0.014). The administration of psychiatric drugs was significantly more frequent in patients with a lower BMI (37.5% vs 12%, p = 0.038).
Conclusion
Our study suggests that a non-urgent baseline echocardiographic evaluation with focus on left-ventricular wall thickness and mass in children with anorexia nervosa is advisable.
Level III
Evidence obtained from cohort or case-control analytic studies.
Keywords: Anorexia nervosa, Feeding and eating disorders, Cardiovascular abnormalities, Child, Adolescent, Echocardiography
Introduction
Eating disorders (ED) are chronic illnesses with potentially life-threatening consequences for both physical and psychological health. Anorexia nervosa (AN) represents the most frequent eating disorder, with a lifetime prevalence of 0.1–3.6% [1] and a median age of onset of 18 years [2]. AN is characterized by body image distortion and incessant search for extreme thinness; this ideal condition could be achieved through a restriction of caloric intake/excessive exercise (restrictive subtype) or by means of periodic “binge eating” associated with emptying practices such as induction of vomiting and intake of diuretic or laxatives (bulimic/purgative subtype) [3, 4]. Among the psychiatric disorders encoded by the DSM-V classification, AN is the one characterized by the highest mortality rate (5.1 deaths/1000/year), with deaths related to both suicide and medical complications [5]. It is estimated that up to 5.9% of mortality in AN is caused by cardiovascular complications, mainly sudden cardiac death due to QTc prolongation leading to ventricular arrhythmias [6, 7]. This is remarkable, if one considers that 80% of anorexic patients develop cardiovascular abnormalities due to weight loss and malnutrition [8–12]. The specific physio-pathological mechanisms leading to the cardiac changes observed in AN are still not fully understood, as well as their correlation with BMI, amount of weight loss and laboratory markers (electrolytes, serum proteins, hormones like FT4 e IGF1) [13–16]. On the other hand, refeeding seems to lead to normalisation of such alterations in most affected people [17–21]. In addition, the increased stress caused by the COVID-19 pandemic and the collective restrictions imposed by the government in attempts to limit the spread of the disease has affected children’s mental health [22]. In this setting, the increased number of young people is requiring admission to health-care services, because of ED has been linked to the onset of a real “ED epidemic” within the COVID-19 pandemic [23, 24]. In particular, recent studies showed a more acute and severe ED onset during COVID-19 pandemic than in the recent past as well as an increased rate of psychiatric comorbidities and use of psychotropic drugs and a higher proportion of hospitalization [25, 26].
The purpose of the present study was to early assess ecocardiographic evaluation in patients diagnosed with AN admitted at ED during COVID-19 pandemic period and report the main echocardiographic anomalies correlating with the clinical and demographic characteristics of our sample.
Methods
We conducted a retrospective study on all pediatric patients with a diagnosis of AN (according to DSM-V criteria) who accessed the Emergency Department of the Bambino Gesù Children's Hospital, IRCCS, Rome between the 1st January 2021 and the 30th June 2021, during COVID-19 pandemic. Patient data were obtained by clinical chart review. We included patients aged 8–18 years with a diagnosis of restrictive or binge-eating/purging AN, who underwent a complete cardiological evaluation (electrocardiogram—ECG—and echocardiography with Doppler ultrasound) at admission to the Emergency Department of our Institution. All the patients included in the study performed an echocardiographic evaluation at admission in ED.
The exclusion criteria were: age < 8 years and > 18 years; diagnosis of AN without a complete cardiological evaluation at admission. For each patient, the following clinical data were reported: auxological parameters, including age, gender, length of hospitalization, BMI (both discrete value and relative percentile), reported weight loss before admission in the last year, reported duration of weight loss; use of psychiatric drugs; cardiovascular signs and symptoms, including syncope, thoracic pain, splitted II heart tone; vital signs, including heart rate, systolic (SAP) and diastolic (DAP) arterial pressure; QT interval corrected according to Bazett’s formula (QTc); heart ultrasound parameters, including presence and thickness of pericardial effusion, LV function expressed by shortening fraction (SF) related to age, right-ventricular (RV) function evaluated by tricuspid annular plane systolic excursion (TAPSE), LV thickness related to age, LV mass related to body surface area (BSA) according to Haycock, frequency of patients with a normal ventricular mass (VM), mitral valve prolapse (MVP), mitral valve arching, and mild mitral insufficiency; laboratory workup, with serum dosage of B-type natriuretic peptide (BNP), N-terminal pro-B-type natriuretic peptide (NT-pro BNP), high-sensitivity troponin (Hs-troponin), proteins, albumin, azotemia, creatinine, electrolytes, creatine phosphokinase (CPK), thyroid-stimulating hormone (TSH), and thyroxine (T4). All electrocardiographic and echocardiographic exams were performed and interpreted by the same cardiologist to reduce inter-operator variability. All data were gathered and stored in an electronic database.
We performed three different analyses on the gathered data. First, we conducted bivariate comparison between the heart ultrasound parameters reported and the remaining clinical and demographic variables of the patients. Second, we compared patients with and without pericardial effusion. Third, we conducted a comparison between patients with a BMI cut-off above and below 14.5 kg/m2. The software IBM SPSS version 23.0 was used for statistical analysis. Continuous normally distributed variables were expressed as means and standard deviations and analysed with the Student’s T test. Continuous non-normally distributed variables were expressed as medians and ranges and analysed with the Mann–Whitney U test. Categorical variables were expressed as proportions and percentages and analysed with the Chi-squared test or Fisher’s exact test (when appropriate). A p value less than 0.05 was considered statistically significant.
This study was approved by the Ethics Committee of our Children’s Hospital according to the Declaration of Helsinki (as revised in Seoul, Korea, October 2008).
Results
Forty-nine patients were included in the study. The demographic and clinical characteristics of the patients, as well as cardiovascular and laboratory parameters, are outlined in Table 1.
Table 1.
Clinical and demographic characteristics of study sample
| Total | 49 |
| Age—median (IQR) | 15.1 (3.0) |
| Females—no. (%) | 44 (89.8) |
| Male to female ratio | 1:8.8 |
| Length of hospitalization (days)—median (IQR) | 18 (19) |
| BMI (kg/m2)—mean (SD) | 14.8 (2.1) |
| BMI (percentile)—median (IQR) | 0.5 (5.5) |
| Weight loss (kg)—median (IQR) | 9.5 (8.8) |
| Weight loss (months)—median (IQR) | 3.0 (4.0) |
| Psychiatric drugs—no. (%) | 12 (24.5) |
| Aripiprazole | 9 (18.4) |
| Sertraline | 5 (10.2) |
| Benzodiazepines | 4 (8.2) |
| Olanzapine | 2 (4.1) |
| Mirtazapine | 1 (2.0) |
| Cardiovascular signs and symptoms—no. (%) | 11 (22.4) |
| Syncope—no. (%) | 8 (16.3) |
| Thoracic pain—no. (%) | 2 (4.1) |
| Splitted II heart tone—no. (%) | 1 (2.0) |
| Vital signs | |
| Heart rate—median (IQR) | 52 (12) |
| SAP (mmHg)—mean (SD) | 103 (9.5) |
| DAP (mmHg)—mean (SD) | 65 (7.6) |
| QTc—mean (SD) | 393 (27) |
| Heart ultrasound | |
| Pericardial effusion—no. (%) | 32 (65.3) |
| Pericardial effusion (mm)—mean (SD) | 6 (4) |
| LV function (SF, mm over age %)—mean (SD) | 37 (5) |
| RV function (TAPSE, mm)—mean (SD) | 21 (3) |
| LV thickness over age (Z score)—mean (SD) | −1.8 (0.8) |
| LV mass over BSA (Z score)—mean (SD) | −2.1 (0.9) |
| Normal VM—no. (%) | 22 (44.9) |
| PVM—no. (%) | 5 (10.2) |
| Arching—no. (%) | 10 (20.4) |
| Mild mitral insufficiency—no. (%) | 22 (44.9) |
| Laboratory workup | |
| BNP—mean (SD) | 89.9 (49.9) |
| NT-pro BNP—median (IQR) | 68.6 (169.1) |
| Hs-troponin—median (IQR) | 4.0 (3.2) |
| Proteins (g/dl)—mean (SD) | 7.0 (0.5) |
| Albumin (g/dl)—mean (SD) | 4.7 (0.4) |
| Azotemia (mg/dl)—mean (SD) | 13.6 (5.9) |
| Creatinine (mg/dl)—mean (SD) | 0.76 (0.18) |
| Sodium (mEq/l)—median (IQR) | 139 (2) |
| Potassium (mEq/l)—median (IQR) | 4.4 (0.5) |
| Calcium (mg/dl)—mean (SD) | 9.6 (0.4) |
| Magnesium (mg/dl)—median (IQR) | 2.2 (0.2) |
| CPK (U/l)—median (IQR) | 70 (41) |
| TSH (mcU/ml)—median (IQR) | 2.08 (1.97) |
| FT4 (ng/dl)—mean (SD) | 1.12 (0.24) |
The mean age was 15.1 years (IQR 3.0 years). Most patients were female (n = 44, 90%). The mean length of hospitalization was 18 days. The mean BMI at admission was 14.8 kg/m2, with a median weight loss of 9 kg in the last year. Twelve patients (25%) were taking psychiatric drugs at admission: the majority of them (n = 9, 18%) was taking aripiprazole. Eleven patients (22%) presented with cardiovascular signs or symptoms, mostly with syncope (n = 8, 16%). The median heart rate was 52 bpm (IQR 12). The values of the arterial blood pressure and QTc were within the range of normality. The majority of our population (n = 32, 65%) had pericardial effusion on heart ultrasound, with a mean thickness of 6 mm (SD ± 4). The mean LV function by SF was 37% (SD ± 5). The mean TAPSE was 21 mm (SD ± 3). The mean Z scores of the LV thickness over age and the LV mass over BSA was −1.8 (SD ± 0.8) and −2.1 (SD ± 0.9). The VM was normal in 22 patients (45%). MVP and mitral arching were recorded in 5 (10%) and 10 (20%) patients, respectively. A mild mitral insufficiency was found in 22 patients (45%). The evaluated laboratory tests were found normal in all cases.
The clinical and demographic characteristics of patients with and without pericardial effusion are summarised in Table 2.
Table 2.
Clinical and demographic characteristics of patients by pericardial effusion
| Pericardial effusion | Present | Absent | P value |
|---|---|---|---|
| Total | 32 | 17 | – |
| Age—median (IQR) | 15.0 (2.8) | 15.4 (3.4) | 0.737 |
| Females—no. (%) | 30 (93.8) | 14 (82.4) | 0.326 |
| Male to female ratio | 1:16 | 1:4.6 | – |
| Length of hospitalization (days)—median (IQR) | 18 (25) | 22 (18) | 0.338 |
| BMI (kg/m2)—mean (SD) | 14.9 (2.1) | 14.6 (2.0) | 0.639 |
| BMI (percentile)—median (IQR) | 0.8 (8.4) | 0.3 (2.7) | 0.573 |
| Weight loss (kg)—median (IQR) | 10 (9) | 9 (10) | 0.877 |
| Weight loss (months)—median (IQR) | 3 (5) | 2 (4) | 0.225 |
| Psychiatric drugs—no. (%) | 7 (21.9) | 5 (29.4) | 0.729 |
| Aripiprazole | 5 (15.6) | 4 (23.5) | 0.700 |
| Sertraline | 4 (12.5) | 1 (5.9) | 0.646 |
| Benzodiazepines | 1 (3.1) | 3 (17.6) | 0.114 |
| Olanzapine | 0 (0) | 2 (11.8) | 0.116 |
| Mirtazapine | 0 (0) | 1 (5.9) | 0,.347 |
| Cardiovascular signs and symptoms—no. (%) | 8 (25.0) | 3 (17.6) | 0.725 |
| Syncope—no. (%) | 6 (18.7) | 2 (11.8) | 0.696 |
| Thoracic pain—no. (%) | 2 (6.3) | 0 (0) | 0.537 |
| Splitted II heart tone—no. (%) | 0 (0) | 1 (5.9) | 0.347 |
| Vital signs | |||
| Heart rate—median (IQR) | 51 (11) | 54 (27) | 0.521 |
| PAS (mmHg)—mean (SD) | 104 (10) | 101 (9) | 0.212 |
| PAD (mmHg)—mean (SD) | 66 (7) | 63 (8) | 0.144 |
| QTc—mean (SD) | 399 (20) | 387 (36) | 0.247 |
| Heart ultrasound | |||
| LV function (mm over age %)—mean (SD) | 38 (4) | 36 (7) | 0.324 |
| RV function (TAPSE mm)—mean (SD) | 20 (3) | 21 (3) | 0.895 |
| LV thickness over age (Z score)—mean (SD) | −2.0 (0.8) | −1.4 (0.7) | 0.014 |
| LV mass over BSA (Z score)—mean (SD) | −2.1 (1.0) | −2.0 (0.8) | 0.721 |
| Normal VM—no. (%) | 15 (46.9) | 7 (41.2) | 0.702 |
| PVM—no. (%) | 4 (12.5) | 1 (5.9) | 0.646 |
| Arching—no. (%) | 8 (25.0) | 2 (11.8) | 0.459 |
| Mild mitral insufficiency—no. (%) | 15 (46.8) | 7 (41.2) | 0.702 |
| Laboratory workup | |||
| BNP—mean (SD)* | 95.5 (50.6) | 58.4 (38.2) | 0.245 |
| NT-pro BNP—median (IQR)* | 206.0 (183.5) | 35.7 (–) | 0.121 |
| Hs-troponin—median (IQR)* | 4.2 (3.9) | 3.9 (4.0) | 0.826 |
| Proteins (g/dl)—mean (SD) | 7.0 (0.5) | 7.0 (0.6) | 0.873 |
| Albumin (g/dl)—mean (SD) | 4.8 (0.4) | 4.7 (0.3) | 0.284 |
| Azotemia (mg/dl)—mean (SD) | 14.4 (6.2) | 12.0 (5.0) | 0.169 |
| Creatinine (mg/dl)—mean (SD) | 0.8 (0.2) | 0.7 (0.2) | 0.479 |
| Sodium (mEq/l)—median (IQR) | 140 (3) | 139 (2) | 0.905 |
| Potassium (mEq/l)—median (IQR) | 4.4 (0.6) | 4.4 (0.5) | 0.842 |
| Calcium (mg/dl)—mean (SD) | 9.6 (0.3) | 9.6 (0.6) | 0.902 |
| Magnesium (mg/dl)—median (IQR) | 2.2 (0.1) | 2.1 (0.2) | 0.052 |
| CPK (U/l)—median (IQR) | 74 (50) | 63 (48) | 0.136 |
| TSH (mcU/ml)—median (IQR) | 2.87 (2.19) | 1.60 (0.94) | 0.001 |
| FT4 (ng/dl)—mean (SD) | 1.09 (0.26) | 1.15 (0.21) | 0.532 |
Significant values are in bold
*Sparse data
A similar distribution of age was found in the two subgroups. Length of hospitalization, BMI, and weight loss were not significantly different between the two groups. We found no significant differences in the presence of cardiovascular signs and symptoms on admission between patients with and without pericardial effusion (25.0% vs 17.6%, respectively, p = 0.725). Heart rate, arterial blood pressures, and QTc did not differ significantly between the two groups. Among the heart ultrasound parameters analysed, only the LV thickness over age was significantly higher in patients with pericardial effusion, with a Z score of −2.0 vs −1.4 (p = 0.014). All the remaining variables were similar between the two groups, with the exception of the median TSH that was higher in patients with pericardial effusion (p = 0.001), although still within normal ranges.
The clinical and demographic characteristics of patients stratified according to a BMI cut-off of 14.5 kg/m2 are summarised in Table 3.
Table 3.
Clinical and demographic characteristics of patients by the BMI cut-off of 14.5
| BMI | < 14.5 | ≥ 14.5 | P value |
|---|---|---|---|
| Total | 24 | 25 | – |
| Age—median (IQR) | 14.5 (3.1) | 15.1 (3.3) | 0.363 |
| Females—no. (%) | 21 (87.5) | 23 (92.0) | 0.667 |
| Male to female ratio | 1:7 | 1:11.5 | |
| Length of hospitalization (days)—median (IQR) | 24 (21) | 18 (25) | 0.104 |
| Weight loss (kg)—median (IQR) | 9.5 (15) | 9.5 (7) | 0.758 |
| Weight loss (months)—median (IQR) | 3 (6) | 3 (5) | 0.802 |
| Psychiatric drugs—no. (%) | 9 (37.5) | 3 (12.0) | 0.038 |
| Aripiprazole | 6 (25.0) | 3 (12.0) | 0.289 |
| Sertraline | 3 (12.5) | 2 (8.0) | 0.667 |
| Benzodiazepines | 3 (12.5) | 1 (4.0) | 0.349 |
| Olanzapine | 2 (8.3) | 0 (0) | 0.235 |
| Mirtazapine | 1 (4.2) | 0 (0) | 0.490 |
| Cardiovascular signs and symptoms—no. (%) | 4 (16.7) | 7 (28.0) | 0.339 |
| Syncope—no. (%) | 4 (16.7) | 4 (16.0) | 1.000 |
| Thoracic pain—no. (%) | 0 (0) | 2 (8.0) | 0.490 |
| Splitted II heart tone—no. (%) | 0 (0) | 1 (4.0) | 1.000 |
| Vital signs | |||
| Heart rate—median (IQR) | 56 (18) | 50 (9) | 0.075 |
| PAS (mmHg)—mean (SD) | 101 (9) | 105 (9) | 0.126 |
| PAD (mmHg)—mean (SD) | 65 (7) | 65 (8) | 0.780 |
| QTc—mean (SD) | 396 (7) | 394 (24) | 0.862 |
| Heart ultrasound | |||
| Pericardial effusion—no. (%) | 13 (54.2) | 19 (76.0) | 0.140 |
| Pericardial effusion (mm)—mean (SD) | 5 (4) | 7 (3) | 0.221 |
| LV function (mm over age %)—mean (SD) | 38 (4) | 37 (7) | 0.702 |
| RV function (TAPSE mm)—mean (SD) | 20 (3) | 21 (3) | 0.187 |
| LV thickness over age (Z score)—mean (SD) | −1.8 (0.9) | −1.8 (0.7) | 0.967 |
| LV mass over BSA (Z score)—mean (SD) | −2.1 (0.8) | −2.0 (1.0) | 0.755 |
| Normal VM—no. (%) | 11 (45.8) | 11 (44.0) | 0.897 |
| PVM—no. (%) | 4 (16.7) | 1 (4.0) | 0.189 |
| Arching—no. (%) | 2 (8.3) | 8 (32.0) | 0.074 |
| Mild mitral insufficiency—no. (%) | 11 (45.8) | 11 (44.0) | 0.897 |
| Laboratory workup | |||
| BNP—mean (SD)* | 104 (48) | 81 (51) | 0.330 |
| NT-pro BNP—median (IQR)* | 206 (–) | 44 (172) | 0.289 |
| Hs-troponin—median (IQR)* | 4.1 (5.3) | 4.0 (2.8) | 0.510 |
| Proteins (g/dl)—mean (SD) | 6.8 (0.4) | 7.3 (0.5) | 0.001 |
| Albumin (g/dl)—mean (SD) | 4.7 (0.3) | 4.8 (0.4) | 0.337 |
| Azotemia (mg/dl)—mean (SD) | 13.8 (6.0) | 13.4 (5.9) | 0.781 |
| Creatinine (mg/dl)—mean (SD) | 0.7 (0.2) | 0.8 (0.2) | 0.043 |
| Sodium (mEq/l)—median (IQR) | 139 (1) | 140 (3) | 0.522 |
| Potassium (mEq/l)—median (IQR) | 4.4 (0.5) | 4.5 (0.5) | 0.294 |
| Calcium (mg/dl)—mean (SD) | 9.5 (0.4) | 9.8 (0.6) | 0.024 |
| Magnesium (mg/dl)—median (IQR) | 2.2 (0.1) | 2.2 (0.2) | 0.336 |
| CPK (U/l)—median (IQR) | 64 (37) | 88 (69) | 0.036 |
| TSH (mcU/ml)—median (IQR) | 2.04 (2.15) | 2.31 (2.37) | 0.291 |
| FT4 (ng/dl)—mean (SD) | 1.12 (0.20) | 1.11 (0.29) | 0.978 |
Significant values are in bold
*Sparse data
Demographic data, vital signs, cardiovascular signs and symptoms, and heart ultrasound parameters were not significantly different between the two groups. The administration of psychiatric drugs was significantly more frequent in patients with BMI below the cut-off (38% vs 12%, p = 0.038). We also found a significantly lower value of proteins, creatinine, calcium, and CPK in patients with a lower BMI, although still within normal ranges.
Discussion
Our results confirmed the strict connection between AN, weight loss, and the reduction of LV mass. In particular, we showed an LV wall thickness and LV mass decrease of −1.8 and −2.1 of Z score, respectively. These data were not significant when considering the differences between patients with a BMI below or above the chosen cut-off of 14.5.
This is in line with the literature, where a lower BMI reflects a lower LV mass index, as well as a smaller LV mass: in fact, a decrease in LV mass ranging from 30 to 50% has been described [27]. These data suggest that cardiac volume is initially reduced in severe AN, although this alteration is apparently reversible [28–30]
In addition, we found that the majority of patients with AN had pericardial effusion (65.3%), although this was not significantly associated with any of the reported variables, except for a reduced LV thickness over age (Z score −2.0 vs −1.4, p = 0.014). In the study by Docx et al. [26], 29 of the 128 (22.7%) adolescent girls with AN included had pericardial effusion, which was significantly associated with lower BMI and LV mass, contrary to our results. Similarly to our study, Kastner et al. [21] found a significant but little difference in the BMI of anorexic girls with or without pericardial effusion. Interestingly, the longitudinal evaluation of these patients proved that patients with pericardial effusion required longer hospitalization and had a remarkable, significant weight gain, compared to patients without pericardial effusion. This suggests a relationship of pericardial effusion with the difference between desired and actual BMI—rather than with the “point-of-care” BMI—that has not yet been proven. However, a proxy of this measure that we have included in our analysis, that is the severity and time of weight loss, also failed to correlate with pericardial effusion. A recent 20-year-long longitudinal study on 29 women diagnosed with AN during their adolescence did not report pericardial effusion among the echocardiographic variables (Fig. 1) [31].
Fig. 1.
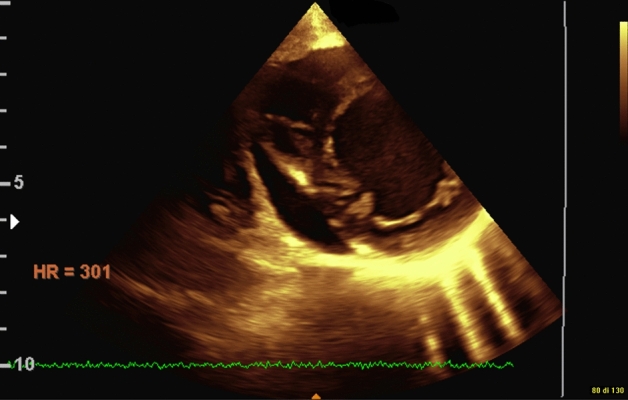
Pericardial effusion
The higher rate of pericardial involvement in our patients may be due to a more severe clinical status of these patients, owing to the delayed referral caused by the COVID-19 pandemic [25]. None of our patients reported cardiac tamponade, which is considered by the literature a very rare complication in this group of patients [32].
Moreover, a statistically significant difference was found between the presence of pericardial effusion in our AN population and increased TSH (p < 0.001), similar to previous research that reported this connection with T3 hormone levels [26]; however, we did not measure T3 hormone levels as this is increasingly converted to reverse T3 under semi-starvation [33].
Another cardiac complication documented in patients with AN has been mitral valve disease. Although an incidence of mitral valve prolapse in AN ranging from 33 to 60% [34] has been recorded in the literature, arching of the superior leaflet, which is the echocardiographic counterpart of angiographically defined prolapse [35], was reported in 20% of our sample. According to this definition, no mitral valve prolapse was detected by Borgia et al. in their sample of 38 children and adolescents with anorexia nervosa [36]. Nonetheless, a similar rate of mitral regurgitation (50%) was observed compared to our sample (45%) (Fig. 2).
Fig. 2.
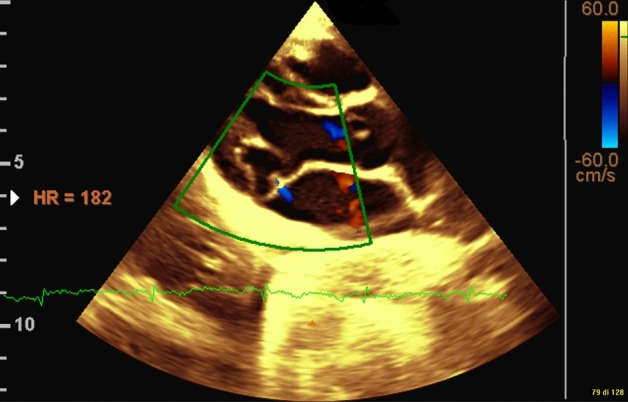
Mitral valve arching
AN and cardiac abnormalities have been linked to electrolyte alterations, such as hypophosphatemia, hypokalemia, and hypocalcemia. [35, 36]. Our results showed a reduction in average sodium, potassium, phosphate, and calcium values below or above the chosen BMI cut-off of 14.5, with no statistical differences. Early detections of these changes are fundamental to limit worsening of clinical status.
Although AN has been linked to cardiac complications and increased morbidity, these are reversible in most cases, with weight recovery [18]. Our study did not focus on the recovery aspects, and this may be called a limitation, yet our goal was to identify early cardiac involvement in AN to manage these patients early. Further long-term prospective studies on the effects of weight recovery in such patients should consider both echocardiographic and MRI parameters to investigate any long-term effect, such as myocardial fibrosis, as suggested in the literature [37]. Moreover, clinicians should be aware of refeeding syndrome and congestive heart failure, as described in previous findings [38].
Our study period goes from January 1, 2021 to June 30, 2021 and overlaps with the SARS-CoV-2 pandemic. Although recent reports have documented cardiac abnormalities during the pandemic, such as pericardial effusion and left-ventricular dysfunction [39], we could not confirmed these findings in our sample, as all of our patients were tested for SARS-CoV-2 and found negative at the time of admission.
Strengths and limitations
Our study described cardiologic abnormalities in a sample of pediatric patients with AN in an emergency setting, while most research has focused on adults and children, followed-up prospectively. We also limited inter-operator variability by recruiting a single pediatric cardiologist to perform all echocardiographic studies. Finally, patients included in our study were admitted to the emergency department during the COVID-19 pandemic, which may have contributed to the increased severity of presentation and the observed echocardiographic changes. However, all patients in our sample were screened for SARS-CoV-2 infection and proved negative, thus eliminating the bias of COVID-19 as a possible direct cause of the echocardiographic anomalies observed. However, our study is limited by its retrospective nature and the lack of sample size estimation, which may have underpowered our analysis.
Conclusion
Pediatric AN is known to be associated with cardiac complications that may require immediate, noninvasive evaluation upon admission to the emergency department and close monitoring after patient recovery and weight gain. Our study suggests that a non-urgent baseline echocardiographic evaluation with focus on left-ventricular wall thickness and mass in children with anorexia nervosa is advisable, as a putative association with cardiovascular complications and death may be proved in further prospective studies.
What is already known on this subject?
Cardiological complications of AN, evident electro- or echocardiographically, have been described in the literature as a consequence of both malnutrition and weight regain.
What does this study add?
This study emphasizes the importance of early assessment of cardiologic function in patients with AN as early as admission to the emergency department. It also describes cardiologic changes in a pediatric population with AN, adding data and information to the literature on this special group of patients.
We believe that our findings may also be of great use to the wide community of pediatricians and other specialists involved in this specific area of children's care.
Author contributions
All authors conceived the study concept. GS, AC, MR, MRM, UR, and AA designed the study, were major contributors in writing the manuscript, contributed to the interpretation of the data, reviewed and revised the manuscript; MR performed statistical analysis; PS, CM allowed for the acquisition of data, and reviewed and revised the manuscript; VZ, AD, AR, and AV revised the manuscript for important intellectual content, and reviewed and critically revised the manuscript for important intellectual content; AV coordinated the study; all authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Funding
None.
Availability of data and materials
The datasets are available from the corresponding author on reasonable request.
Code availability
The source codes are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Ethical approval was granted by the Research Ethics Board at the Bambino Gesù Children's Hospital, IRCCS, Rome, Italy.
Informed consent
Informed consent was not required, given the retrospective nature of the study and the completely anonymous treatment of the data.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Galmiche M, Déchelotte P, Lambert G, Tavolacci MP. Prevalence of eating disorders over the 2000–2018 period: a systematic literature review. Am J Clin Nutr. 2019;109:1402–1413. doi: 10.1093/ajcn/nqy342. [DOI] [PubMed] [Google Scholar]
- 2.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey replication. Biol Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5. Washington: American Psychiatric Association Publishing; 2013. [Google Scholar]
- 4.Hornberger LL, Lane MA, Adolescence CO. Identification and management of eating disorders in children and adolescents. Pediatrics. 2021;147:e2020040279. doi: 10.1542/peds.2020-040279. [DOI] [PubMed] [Google Scholar]
- 5.Arcelus JMA. Mortality rates in patients with anorexia nervosa and other eating disorders. A metanalysis of 36 studies. Arch Gen Psychiatry. 2011;68:724–731. doi: 10.1001/archgenpsychiatry.2011.74. [DOI] [PubMed] [Google Scholar]
- 6.Neumarker KJ (1997) Mortality and sudden death in anorexia nervosa. Int J Eat Disord 21:205–212. Doi: 10.1002/(sici)1098-108x(199704)21:3<205::aid-eat1>3.0.co;2-o [DOI] [PubMed]
- 7.Mehler PS, Watters A, Joiner T, Krantz MJ. What accounts for the high mortality of anorexia nervosa? Int J Eat Disord. 2022;55(5):633–636. doi: 10.1002/eat.23664. [DOI] [PubMed] [Google Scholar]
- 8.De Simeone G, Scalfi L, Galderisi M, Celentano A, Di Biase G, Tammaro P, Garofalo M, Mureddu GF, de Divitis O, Contaldo F. Cardiac abnormalities in young women with anorexia nervosa. Br Heart J. 1994;71:287–292. doi: 10.1136/hrt.71.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharp CW, Freeman CPL. The medical complication of anorexia nervosa. Br J Psychiatry. 1993;162:452–462. doi: 10.1192/bjp.162.4.452. [DOI] [PubMed] [Google Scholar]
- 10.Cooke RA, Chambers JB, Singh R, Todd GJ, Smeeton NC, Treasure J, Treasure T. QT interval in anorexia nervosa. Br Heart J. 1994;72:69–73. doi: 10.1136/hrt.72.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg SJ, Comerci GD, Feldman L. Cardiac output and regional myocardial contraction in anorexia nervosa. J Adolesc Health Care. 1988;9:15–21. doi: 10.1016/0197-0070(88)90013-7. [DOI] [PubMed] [Google Scholar]
- 12.Papadopoulos FC, Ekbom A, Brandt L, Ekselius L. Excess mortality, causes of death and prognostic factors in anorexia nervosa. Br J Psychiatry. 2009;194:10–17. doi: 10.1192/bjp.bp.108.054742. [DOI] [PubMed] [Google Scholar]
- 13.Sachs KV, Harnke B, Mehler PS, Krantz MJ. Cardiovascular complications of anorexia nervosa: a systematic review. Int J Eat Disord. 2016;49:238–248. doi: 10.1002/eat.22481. [DOI] [PubMed] [Google Scholar]
- 14.Spaulding-Barclay MA, Stern J, Mehler PS. Cardiac changes in anorexia nervosa. Cardiol Young. 2016;26:623–628. doi: 10.1017/S104795111500267X. [DOI] [PubMed] [Google Scholar]
- 15.Giovinazzo SSS. Anorexia nervosa and heart disease: a systematic review. Eat Weight Disord. 2018;24:199–207. doi: 10.1007/s40519-018-0567-1. [DOI] [PubMed] [Google Scholar]
- 16.Smythe JCC. Cardiac abnormalities identified with echocardiography in anorexia nervosa: systematic review and meta-analysis. Br J Psychiatry. 2020;219:477–486. doi: 10.1192/bjp.2020.1. [DOI] [PubMed] [Google Scholar]
- 17.Olivares JL, Vazquez M, Fleta J, Moreno LA, Pérez-Gonzàlez JM, Bueno M. Cardiac findings in adolescents with anorexia nervosa at diagnosis and after weight restoration. Eur J Pediatr. 2005;164:383–386. doi: 10.1007/s00431-005-1647-6. [DOI] [PubMed] [Google Scholar]
- 18.Mont L, Castro J, Herreros B, Paré C, Azqueta M, Magriña J, Puig J, Toro J, Brugada J. Reversibility of cardiac abnormalities in adolescents with anorexia nervosa after weight recovery. J Am Acad Child Adolesc Psychiatry. 2003;42:808–813. doi: 10.1097/01.CHI.0000046867.56865.EB. [DOI] [PubMed] [Google Scholar]
- 19.Roche F, Barthelemy JC, Mayaud N, Pichot V, Duverney D, Germain M, Lang F, Estour B. Refeeding normalizes the QT rate dependence of female anorexic patients. Am J Cardiol. 2005;95:277–280. doi: 10.1016/j.amjcard.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Ulger Z, Gurses D, Ozyurek AR, Arikan C, Levent E, Aydogdu S. Follow-up of cardiac abnormalities in female adolescents with anorexia nervosa after refeeding. Acta Cardiol. 2006;61:43–49. doi: 10.2143/AC.61.1.2005139. [DOI] [PubMed] [Google Scholar]
- 21.Kastner S, Salbach-Andrae H, Renneberg B, Pfeiffer E, Lehmkuhl U, Schmitz L. Echocardiographic findings in adolescents with anorexia nervosa at beginning of treatment and after weight recovery. Eur Child Adolesc Psychiatry. 2012;21:15–21. doi: 10.1007/s00787-011-0227-8. [DOI] [PubMed] [Google Scholar]
- 22.Walsh O, McNicholas F. Assessment and management of anorexia nervosa during COVID-19. Ir J Psychol Med. 2020;37:187–191. doi: 10.1017/ipm.2020.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solmi F, Downs JL, Nicholls DE. COVID-19 and eating disorders in young people. Lancet Child Adolesc Health. 2021;5:316–318. doi: 10.1016/S2352-4642(21)00094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otto AK, Jary JM, Sturza J, Miller CA, Prohaska N, Bravender T, Van Huysse J. Medical admissions among adolescents with eating disorders during the COVID-19 pandemic. Pediatrics. 2021;148:e2021052201. doi: 10.1542/peds.2021-052201. [DOI] [PubMed] [Google Scholar]
- 25.Spina G, Roversi M, Marchili MR, Raucci U, Fini F, Mirra G, Testa G, Guarnieri B, Clemente A, Diamanti A, Zanna V, Castiglioni MC, Vicari S, Reale A, Villani A. Psychiatric comorbidities and dehydration are more common in children admitted to the emergency department for eating disorders in the COVID-19 era. Eat Weight Disord. 2022;16:1–8. doi: 10.1007/s40519-022-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Docx MK, Gewillig M, Simons A, Vandenberghe P, Weyler J, Ramet J, Mertens L. Pericardial effusions in adolescent girls with anorexia nervosa: clinical course and risk factors. Eat Disord. 2010;18:218–225. doi: 10.1080/10640261003719484. [DOI] [PubMed] [Google Scholar]
- 27.St John Sutton MG, Plappert T, Crosby L, Douglas P, Mullen J, Reichek N. Effects of reduced left ventricular mass on chamber architecture, load, and function: a study of anorexia nervosa. Circulation. 1985;72:991–1000. doi: 10.1161/01.cir.72.5.991. [DOI] [PubMed] [Google Scholar]
- 28.Scheggi V, Castellini G, Vanni F, Menale S, Filardo C, Gironi V, Rinaldi A, Zoppetti N, Alterini B, Ricca V, Marchionni N. Echocardiographic abnormalities in adults with anorexia nervosa. Am J Cardiol. 2022;175:152–157. doi: 10.1016/j.amjcard.2022.03.061. [DOI] [PubMed] [Google Scholar]
- 29.Kuwabara M, Niwa K, Yamada U, Ohta D. Low body mass index correlates with low left ventricular mass index in patients with severe anorexia nervosa. Heart Vessels. 2018;33:89–93. doi: 10.1007/s00380-017-1051-y. [DOI] [PubMed] [Google Scholar]
- 30.Escudero CA, Potts JE, Lam PY, De Souza AM, Mugford GJ, Sandor GG. An echocardiographic study of left ventricular size and cardiac function in adolescent females with anorexia nervosa. Eur Eat Disord Rev. 2016;24:26–33. doi: 10.1002/erv.2409. [DOI] [PubMed] [Google Scholar]
- 31.Flamarique I, Vidal B, Plana MT, Andrés-Perpiñá S, Gárriz M, Sánchez P, Pajuelo C, Mont L, Castro-Fornieles J. Long-term cardiac assessment in a sample of adolescent-onset anorexia nervosa. J Eat Disord. 2022;10(1):12. doi: 10.1186/s40337-022-00533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kircher JN, Park MH, Cheezum MK, Hulten EA, Kunz JS, Haigney M, Atwood JE. Cardiac tamponade in association with anorexia nervosa: a case report and review of the literature. Cardiol J. 2012;19:635–638. doi: 10.5603/cj.2012.0117. [DOI] [PubMed] [Google Scholar]
- 33.Tamai H, Mori K, Maisubayashi S, Kiyohara K, Nakagawa T, Okimura MC, Walter RM, Kumagai LF, Nagataki S. Hypothalamic-pituitary-thyroidal dysfunctions in anorexia nervosa. Psychother Psychosom. 1986;46:127–131. doi: 10.1159/000287973. [DOI] [PubMed] [Google Scholar]
- 34.Johnson GL, Humphries LL, Shirley PB, Mazzoleni A, Noonan JA. Mitral valve prolapse in patients with anorexia nervosa and bulimia. Arch Intern Med. 1986;146:1525–1529. doi: 10.1001/archinte.1986.00360200083014. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert BW, Schatz RA, VonRamm OT, Behar VS, Kisslo JA. Mitral valve prolapse. Two-dimensional echocardiographic and angiographic correlation. Circulation. 1976;54:716–723. doi: 10.1161/01.cir.54.5.716. [DOI] [PubMed] [Google Scholar]
- 36.Borgia F, Cirillo P, Riccio MP, Raimondi F, Franco D, Scippa L, Franzese A, Esposito G, De Luca N, Bravaccio C. Anorexia nervosa-related cardiopathy in children with physical instability: prevalence, echocardiographic characteristics and reversibility at mid-term follow-up. Eur J Pediatr. 2021;180:3379–3389. doi: 10.1007/s00431-021-04130-y. [DOI] [PubMed] [Google Scholar]
- 37.Cariem AK, Lemmer ER, Adams MG, Winter TA, O'Keefe SJ. Severe hypophosphataemia in anorexia nervosa. Postgrad Med J. 1994;70:825–827. doi: 10.1136/pgmj.70.829.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davidson A, Anisman PC, Eshaghpour E. Heart failure secondary to hypomagnesemia in anorexia nervosa. Pediatr Cardiol. 1992;13:241–242. doi: 10.1007/BF00838786. [DOI] [PubMed] [Google Scholar]
- 39.Oflaz S, Yucel B, Oz F, Sahin D, Ozturk N, Yaci O, Polat N, Gurdal A, Cizgici AY, Dursun M, Oflaz H. Assessment of myocardial damage by cardiac MRI in patients with anorexia nervosa. Int J Eat Disord. 2013;46:862–866. doi: 10.1002/eat.22170. [DOI] [PubMed] [Google Scholar]
- 40.Schocken DD, Holloway JD, Powers PS. Weight loss and the heart. Effects of anorexia nervosa and starvation. Arch Intern Med. 1989;149:877–881. doi: 10.1001/archinte.1989.00390040085017. [DOI] [PubMed] [Google Scholar]
- 41.Cantarutti N, Battista V, Adorisio R, Cicenia M, Campanello C, Listo E, Campana A, Trocchio G, Drago F. Cardiac manifestations in children with SARS-COV-2 infection: 1-year pediatric multicenter experience. Children (Basel) 2021;8:717. doi: 10.3390/children8080717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets are available from the corresponding author on reasonable request.
The source codes are available from the corresponding author on reasonable request.


