Once considered mere “platelet dust,” extracellular vesicles (EVs) have become a substantial topic in physiology because of their role in disease (1). Most cell types can produce EVs and they are found in seemingly every major body fluid, including the blood and urine (2). EVs dissociate from their parent cells in three manners, which serve as their classifications. First, microparticles, microvesicles, and ectosomes form through the blebbing of the plasma membrane. Second, the fusion of multivesicular bodies to the plasma membrane release exosomes, and, third, apoptotic bodies form during apoptosis (2,3). Primarily composed of lipids and proteins, EVs can serve as packages of intracellular material, including proteins and RNAs (2). EVs potentially function to remove unwanted material, as some have suggested. However, others also suggest they have a much broader function and could play an essential role in transporting material from cell to cell and affect cell–cell signaling (2).
Outside of their potential role in cell communication, EVs may also have another critical use by acting as a marker for disease. With their prevalence in bodily fluids, EVs released from their parent cells could provide a noninvasive window into the conditions of their parent cells. Either looking at changes in content in urinary EVs (uEVs) or the number of uEVs could provide evidence of changes occurring, so this idea has led to the exploration of using uEVs as a diagnostic tool. For example, several studies have identified changes in uEVs in patients with primary aldosteronism (4–6). Changes such as these could help not only identify the various mechanisms for a disease, but also identify changes in normal physiology.
One challenge with using uEVs as a diagnostic marker for the composition of cells within the kidneys is the lack of clear characterization and the isolation method, with the ambiguity of size and phenotype for EVs in general (3). Additionally, sample handling has the potential for changing the characteristics (3). Besides the challenges of isolation and characterization, the question of how well the uEVs represent the environment left within the kidney remains. With the mechanism for secretion of proteins and RNAs in uEVs unknown, the cargo of uEVs may not accurately depict the cell membrane composition within the nephron.
Recently, however, other studies have used a large-scale proteomic assessment of uEVs to identify how they relate to kidney composition (7,8). Here, they identified protein abundances with uEVs, how these proteins and protein classes correlated to what was found in the kidneys, and if physiologic changes within the kidneys could be detected in various models (7). Within this study, several markers were identified, such as Alix, Tsg101, Cd63, and Cd81, as correlating well with levels within the kidneys, and they additionally identified protein transporters, especially those found on the apical side, to have the highest level of correlation (7). These transporters include Aqp1, Aqp2, sodium/phosphate cotransporter NaPi-2a, and cubilin (7). It ought to be noted that in many studies there remains high individual variability, suggesting differences in secretion and composition of uEVs (7)
As apical protein transporters may have the best ability to correlate to protein levels within the kidney, disease states that affect these protein levels have the potential to have their uEVs serve well as a diagnostic marker. Differences within the Renin-Angiotensin-Aldosterone System system and levels of various ions such as K+ or Na+ within the diet, with their effect on these channels, would serve well as potential diagnostic markers for changes occurring within the kidney (3,4,7–11). Accordingly, several studies have begun to look for these potential changes (4,7–11).
Some studies encountered difficulty identifying correlations between a high salt diet and sodium transporters, such as NKCC2 and NCC (9). One study found a correlation between excretion rates of these transporters and the levels within the kidneys in animal models, but had difficulty measuring the effect in human subjects because the levels of NKCC2 and NCC excreted did not correlate with urinary sodium reabsorption or blood pressure (9). This further identifies the challenge of using uEVs to characterize the phenotype within the kidney as levels of the transporters within the uEVs do not necessarily communicate their secretion rate or how many transporters are active within the membrane of the cells lining the nephron.
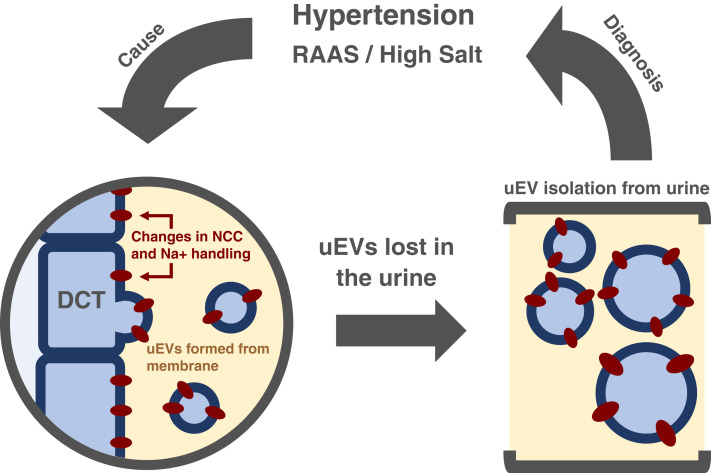
The study by Wu et al. (12), seeks to identify changes in NCC and phosphorylated NCC, the active form, within uEVs of patients with primary aldosteronism who received seated saline suppression testing, which involves an infusion of 0.9% saline. With seated saline suppression testing, those with primary aldosteronism do not see the typical suppression of renin and plasma aldosterone with the NaCl loading. NCC levels have been shown to be regulated by K+ levels and may play some role in hypertension. By investigating NCC and phosphorylated NCC levels within these parameters, the authors explore the potential to use uEVs as a method to take a snapshot of the renal epithelial cell function (Figure 1).
Figure 1.
Graphic abstract.
By using a noninvasive measurement of protein levels such as uEVs, there comes the potential for a greater understanding of the renal function within individuals. However, much remains unknown about uEVs. Questions remain about how secretion rates of uEVs are regulated and how these transporters sequester or do not sequester in the membranes of uEVs; if they are randomly incorporated or intentionally trafficked to the uEVs. If EVs could act in cell–cell signaling, do the uEVs have an outside purpose, such as increasing the number of transporters within other cells? Additionally, do uEVs experience changes in uptake into the cells around them? Overall, the type, sizes, and phenotypes of EVs shift with diseases as well. If these factors relate to EVs, they can potentially affect the composition and number of uEVs and shape how EVs will be used.
This study begins to answer these questions on the potential of using uEVs as a diagnostic tool. Future studies will undoubtedly elucidate the inner workings of uEVs and help find ways they can be used to identify diseases.
Disclosures
All authors have nothing to disclose.
Funding
None.
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the author(s).
Footnotes
See related article, “Acute intravenous NaCl and volume expansion reduces NCC abundance and phosphorylation in urinary extracellular vesicles,” on pages 910–921.
Author Contributions
K. Deck wrote the original draft; S. Mu was responsible for the funding acquisition, provided supervision, and reviewed and edited the manuscript.
References
- 1.Wolf P: The nature and significance of platelet products in human plasma. Br J Haematol 13: 269–288, 1967 [DOI] [PubMed] [Google Scholar]
- 2.Yáñez-Mó M, Siljander PRM, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O: Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4: 27066, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.La Salvia S, Gunasekaran PM, Byrd JB, Erdbrügger U: Extracellular vesicles in essential hypertension: Hidden messengers. Curr Hypertens Rep 22: 76, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Burrello J, Gai C, Tetti M, Lopatina T, Deregibus MC, Veglio F, Mulatero P, Camussi G, Monticone S: Characterization and gene expression analysis of serum-derived extracellular vesicles in primary aldosteronism. Hypertension 74: 359–367, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Barros ER, Rigalli JP, Tapia-Castillo A, Vecchiola A, Young MJ, Hoenderop JGJ, Bindels RJM, Fardella CE, Carvajal SA: Proteomic profile of urinary extracellular vesicles identifies AGP1 as a potential biomarker of primary aldosteronism. Endocrinol 162: bqab032, 2021 [DOI] [PubMed] [Google Scholar]
- 6.Luther M, Peng D, Vaidya A, McDonald H, Schey K, Adler GK. Abstract P237: Alterations in renal sodium handling proteins determined by urinary extracellular vesicle analysis during dietary sodium modulation and in primary aldosteronism. Hypertension 78: AP237–AP237, 2021 [Google Scholar]
- 7.Wu Q, Poulsen SB, Murali SK, Grimm PR, Su XT, Delpire E, Welling PA, Ellison DH, Fenton RA: Large-scale proteomic assessment of urinary extracellular vesicles highlights their reliability in reflecting protein changes in the kidney. J Am Soc Nephrol 32: 2195–2209, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabaratnam R, Geertsen L, Skjødt K, Højlund K, Dimke H, Lund L, Svenningsen P: In human nephrectomy specimens, the kidney level of tubular transport proteins does not correlate with their abundance in urinary extracellular vesicles. Am J Physiol Renal Physiol 317: F560–F571, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Esteva-Font C, Wang X, Ars E, Guillén-Gómez E, Sans L, González Saavedra I, Torres F, Torra R, Masilamani S, Ballarín JA, Fernández-Llama P: Are sodium transporters in urinary exosomes reliable markers of tubular sodium reabsorption in hypertensive patients? Nephron, Physiol 114: 25–34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi Y, Wang X, Rose KL, MacDonald WH, Zhang B, Schey KL, Luther JM: Activation of the endogenous renin-angiotensin-aldosterone system or aldosterone administration increases urinary exosomal sodium channel excretion. J Am Soc Nephrol 27: 646–656, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoorn EJ, Gritter M, Cuevas CA, Fenton RA: Regulation of the renal NaCl cotransporter and its role in potassium homeostasis. Physiol Rev 100: 321–356, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Wu A, Wolley MJ, Wu Q, Cowley D, Palmfeldt J, Welling PA, Fenton RA, Stowasser M: Acute intravenous nacl and vol expansion reduces NCC abundance and phosphorylation in urinary extracellular vesicles. Kidney360 3: 910–921, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]