Key Points
There are important national and center differences in the prescription of icodextrin, with the United States a clear outlier; across all countries, icodextrin was more likely to be used if membrane function tests indicated reduced ultrafiltration capacity to glucose.
This large, international observational study was unable to show patient or hemodialysis transfer advantages to icodextrin use.
Where use of icodextrin was low, this was compensated for by much greater use of high glucose and overall higher ultrafiltration volumes at each level of urine volume; this practice may confound associations between icodextrin and survival outcomes.
Keywords: diabetes and the kidney, dialysis modality transfer, icodextrin, patient survival, peritoneal dialysis, peritoneal membrane function
Visual Abstract
Abstract
Background
Icodextrin has been shown in randomized controlled trials to benefit fluid management in peritoneal dialysis (PD). We describe international icodextrin prescription practices and their relationship to clinical outcomes.
Methods
We analyzed data from the prospective, international PDOPPS, from Australia/New Zealand, Canada, Japan, the United Kingdom, and the United States. Membrane function and 24-hour ultrafiltration according to icodextrin and glucose prescription was determined at baseline. Using an instrumental variable approach, Cox regression, stratified by country, was used to determine any association of icodextrin use to death and permanent transfer to hemodialysis (HDT), adjusted for demographics, comorbidities, serum albumin, urine volume, transplant waitlist status, PD modality, center size, and study phase.
Results
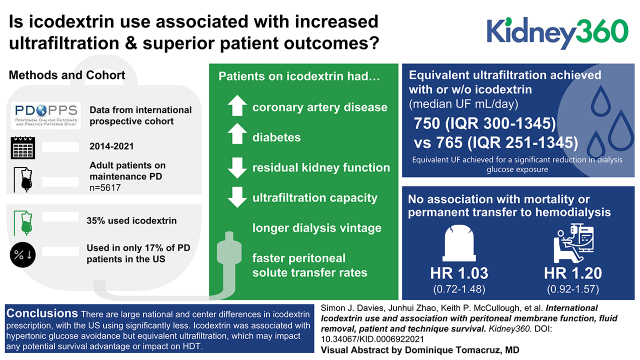
Icodextrin was prescribed in 1986 (35%) of 5617 patients, >43% of patients in all countries, except in the United States, where it was only used in 17% and associated with a far greater use of hypertonic glucose. Patients on icodextrin had more coronary artery disease and diabetes, longer dialysis vintage, lower residual kidney function, faster peritoneal solute transfer rates, and lower ultrafiltration capacity. Prescriptions with or without icodextrin achieved equivalent ultrafiltration (median 750 ml/d [interquartile range 300–1345 ml/d] versus 765 ml/d [251–1345 ml/d]). Icodextrin use was not associated with mortality (HR=1.03; 95% CI, 0.72 to 1.48) or HDT (HR 1.2; 95% CI, 0.92 to 1.57).
Conclusions
There are large national and center differences in icodextrin prescription, with the United States using significantly less. Icodextrin was associated with hypertonic glucose avoidance but equivalent ultrafiltration, which may affect any potential survival advantage or HDT.
Introduction
Volume overload is common in patients on peritoneal dialysis (PD) (1) and is linked to cardiovascular disease, the leading cause of death in kidney failure (2). Poor peritoneal ultrafiltration contributes to volume overload and is an important reason for hemodialysis (HD) transfer (HDT) in patients on PD (3,4). Icodextrin, a dialysis solution containing macromolecules, is able to maintain ultrafiltration for the long dwell (8–16 hours) and has been shown in clinical trials to improve volume status (5,6), increase ultrafiltration compared with glucose, and reduce episodes of overhydration in both continuous ambulatory peritoneal dialysis and automated peritoneal dialysis (APD) (7,8). A recent enriched meta-analysis (8) supports the theoretical prediction that icodextrin will have its greatest effect when the peritoneal solute transfer rate (PSTR) is above average, which is important, given the consistent finding that this aspect of membrane function has been associated with increased mortality on PD (9,10). Reduced net ultrafiltration and excess fluid reabsorption in the long exchange is one of the putative mechanisms whereby a faster PSTR leads to worse survival, and guidelines recommend icodextrin use under these circumstances (10).
Randomized controlled trials of icodextrin use have not shown a benefit in HDT and only a suggestion of a survival benefit (7,8). This may reflect a lack of power in these trials, which were never designed to investigate these outcomes, even when brought together in meta-analyses (7,8). As a result, reliance on robust observational data is necessary. The Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS), one of the largest international PD cohort studies to date, is a unique opportunity to understand how icodextrin prescription varies by country and by patient and center characteristics, and whether its use translates into clinical benefits, including enhanced ultrafiltration and superior patient outcomes. We sought to test the hypothesis that icodextrin use is associated with increased 24-hour peritoneal ultrafiltration and improvements in patient survival and HDT.
Materials and Methods
Study Population
PDOPPS is an international prospective cohort study in collaboration with the International Society for Peritoneal Dialysis (11). Patients ≥18 years of age receiving maintenance PD (excluding combination HD/PD hybrid therapy) were enrolled randomly from national samples of randomly selected PD facilities treating a minimum of 20 PD patients. Study approval was obtained by a central national or institutional review board. Additional study approval and patient consent were obtained as required by national and local ethics committee regulations. Further study details are provided at https://www.dopps.org/OurStudies/PeritonealDialysisPDOPPS.aspx.
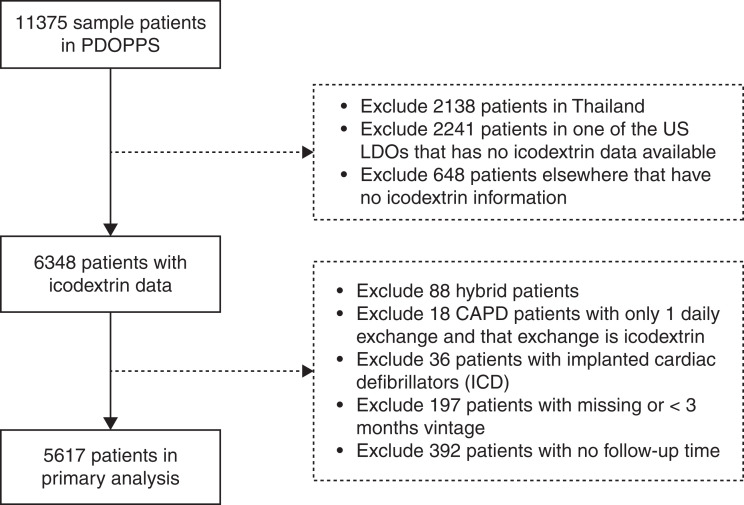
The current analysis was restricted to Australia/New Zealand, Canada, Japan, the United Kingdom, and the United States in PDOPPS phases 1–2 (phase 1: 2014–2018; phase 2: 2018–2021) because icodextrin was not available in Thailand. To avoid inclusion of patients with cardiorenal syndrome using icodextrin as an adjunct volume removal strategy, patients with a single icodextrin exchange only per day, or those with implanted cardiac defibrillators were excluded. Incident patients with <3 months on PD were excluded.
Study baseline was defined as the initial 4-month interval for each patient where information on icodextrin use or not was captured. Of those on icodextrin at baseline, 88% remained on it throughout the study, whereas 84% of those not on icodextrin at baseline continued to remain off icodextrin throughout the study.
Patient demographics and comorbidities were captured for each patient. Laboratory measurements, blood pressure, membrane function as determined from a peritoneal equilibration test (PET), including PSTR (dialysate/plasma creatinine ratio at 4 hours) and the ultrafiltration capacity (net ultrafiltration using glucose 2.5%, which was the concentration used in 94% of tests across all countries), dialysis prescription details, 24-hour urine, and ultrafiltration volumes were captured at baseline. Center-level practices of the frequency of undertaking PETs were obtained from a questionnaire administered to the nurse study coordinator at participating facilities. Data were obtained from manual medical chart extraction and entered into a web-based data collection tool, with the exception of US patients receiving care at large dialysis organization sites where data were imported from electronic health records. High glucose prescription was defined as any use of 2.27% or 3.86% solutions.
Statistical Analyses
Icodextrin use at baseline was the exposure of interest. Outcomes of interest included 24-hour ultrafiltration at baseline, permanent HDT, and all-cause mortality. Temporary HDT/hybrid where the patient did not return to PD within 12 weeks was also defined as HDT. Dying within 7 days of permanent or temporary HDT or hybrid therapy was considered a death (not HDT) outcome. To establish prescription practices, patient-level use of icodextrin was analyzed according to known clinical indications, including comorbidities, level of residual kidney function, dialysis glucose prescription, and, where available, peritoneal membrane function (PSTR and ultrafiltration capacity). The influence of center-level practices for the determination of membrane function on icodextrin use was also investigated.
Cox regression was used to analyze the association of icodextrin with death, HDT, and combined outcome of either death or HDT. Follow-up started at study baseline and ended at whichever came first: death, 7 days after modality switch, loss to follow-up, transplantation, or study end.
To reduce confounding by indication, all models were adjusted for patient-level confounders, including PDOPPS phase, patient age, sex, time on PD (PD vintage), comorbidities, urine volume, albumin, and transplant waiting list status. To adjust for possible center-level confounding, we also adjusted for center size and percentage APD use.
Instrumental Variable Analyses
In order to account partially for patient-level unmeasured confounders (e.g., markers of metabolic and/or volume management challenges) that may affect the relationship between icodextrin use and outcomes, we conducted analyses applying an instrumental variable approach that used the dialysis center as the instrument (12–15). The first stage used a linear model on dialysis center and patient factors listed above to predict icodextrin use. The second stage was a Cox model for survival outcomes such as mortality or HDT (16). The first-stage F statistic, used to reject the null hypothesis of weak instruments, was 10, with the interpretation that the instrumental variable estimates are less biased than standard regression (13,17,18).
Subgroup Analysis and Sensitivity Analysis
To explore if associations between icodextrin use and outcomes differed by patient/treatment characteristics, models were repeated by specified subgroups (see Supplementary Material): age, body mass index, PSTR, urine volume, diabetic status, PD modality, region, dialysis assistance, and use of biocompatible dialysis fluid. We calculated a P value for the interaction between icodextrin use and the subgroup variable (e.g., diabetes yes/no) using a likelihood ratio test, comparing the model with all main-effect adjustments and icodextrin use to the model with the indicated interaction with icodextrin use. Because the alternative to an icodextrin day dwell would be a glucose day dwell, a sensitivity analysis excluding dry-day patients on APD was also performed.
Treatment of Missing Data
Missing data were multiply imputed using the sequential regression multiple imputation method by IVEware. Results from 20 such imputed datasets were combined for the final analysis using Rubin’s formula. The proportion of missing data was <10% for all imputed covariates, with the exception of urine volume (33%) and transplant waiting list status (26%). Membrane function (80%) and 24-hour ultrafiltration (60%) were not imputed or used in models due to the amount of missing data.
Results
Icodextrin Practice Patterns
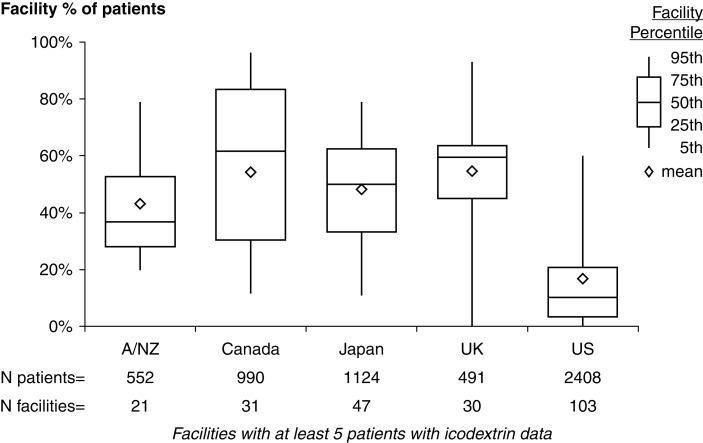
Icodextrin was used in 1986 of the 5617 (35%) patients included in the analysis (Figure 1), although this differed substantially by country: 43%–56% of patients in Australia/New Zealand, Canada, Japan, and the United Kingdom compared with 17% of patients in the United States. Across all countries, use of icodextrin was lower among patients on PD for <1 year (Supplemental Figure 1). Marked within-country center variation was observed within all PDOPPS regions (Figure 2). For example, the bottom 25th percentile of facilities in Canada used icodextrin for ≤30% of their patients compared with ≥83% in the upper 75th percentile. This center variation in practice was still apparent when the 1328 APD dry-day patients not eligible for icodextrin were excluded (Supplemental Figure 2).
Figure 1.
Patient inclusion and exclusion criteria.
Figure 2.
Distribution of the center-level proportion of patients using icodextrin by country, including all patients. A/NZ, Australia/New Zealand; UK, United Kingdom; US, United States. Note: icodextrin data are not available in one US large dialysis organization.
Icodextrin use according to patient and treatment characteristics is presented in Table 1. To clarify the country-level differences further, distributions of all covariates by country and icodextrin status are also presented in Table 2 and Supplemental Table 1. Patients prescribed icodextrin were more likely to have coronary artery disease and diabetes, have been on PD treatment longer, have lower residual 24-hour urine volume and kidney function, use more hypertonic glucose, and have a faster PSTR. These prescription practices were similarly observed across all countries regardless of the overall icodextrin use, with the exception that in the United States, there was no significant increase in icodextrin use with time on treatment (see Supplemental Figure 1), and its use was not associated with a reduction in the use of hypertonic (2.27% or 3.86%) glucose, which overall was higher than in other countries (Table 2). At a center level, there was considerable variation in the reported approach to routine membrane function testing. In the United States, 13% of centers reported a policy of routine membrane function testing in prevalent patients, whereas this proportion was 39%, 27%, 73%, and 68% for Australia/New Zealand, Canada, Japan, and the United Kingdom, respectively (Supplemental Figure 3). Overall, icodextrin was prescribed in 47% of patients treated in centers that do routine PETs compared with 37% that do not.
Table 1.
Patient characteristics by icodextrin use
| Characteristics | No Icodextrin | Icodextrin |
|---|---|---|
| Patient/treatment characteristics | N=3631 patients | N=1986 patients |
| Time on PD, yr | 1.1 (0.5–2.6) | 1.2 (0.5–2.9) |
| <1 | 48% | 46% |
| 1–1.9 | 20% | 18% |
| ≥2 | 33% | 37% |
| Age, yr | 60 (15) | 62 (15) |
| Men, % | 58% | 66% |
| US Black, % | 30% | 25% |
| Time with ESRD, yr | 1.5 (0.6–3.3) | 1.6 (0.6–3.5) |
| Body mass index, kg/m2 | 27 (6) | 28 (6) |
| PD modality | ||
| CAPD | 24% | 36% |
| APD | 76% | 64% |
| APD with wet day, % | 47% | 99% |
| APD with dry day, % | 53% | 1% |
| Caregiver(s) involved in PD exchanges, % | 15% | 19% |
| Systolic blood pressure, mm Hg | 138 (23) | 139 (23) |
| <110 | 9% | 9% |
| 110–149 | 61% | 60% |
| ≥150 | 30% | 32% |
| Comorbidities | ||
| Coronary artery disease | 19% | 25% |
| Cerebrovascular disease | 7% | 11% |
| Congestive heart failure | 10% | 13% |
| Peripheral vascular disease | 9% | 14% |
| Other cardiovascular disease | 12% | 15% |
| Hypertension | 82% | 90% |
| Diabetes | 46% | 51% |
| Gastrointestinal bleeding | 2% | 3% |
| Lung disease | 4% | 6% |
| Neurologic disease | 3% | 5% |
| Psychiatric disorder | 16% | 12% |
| Cancer (nonskin) | 9% | 12% |
| Recurrent cellulitis/gangrene | 1% | 2% |
| Transplant waitlisted, % | 42% | 37% |
| Laboratory values | ||
| Phosphorus, mg/dl | 5.3 (1.5) | 5.1 (1.3) |
| Hemoglobin, g/L | 11 (2) | 11 (2) |
| Albumin, g/dL | 3.5 (0.5) | 3.3 (0.5) |
| Sodium, mEq/L | 139 (4) | 136 (4) |
| Serum creatinine, mg/dl | 8.8 (3.8) | 8.8 (3.5) |
| 24-h urine volume, L | 0.9 (0.8) | 0.8 (0.7) |
| <0.5 | 34% | 41% |
| 0.5–0.9 | 24% | 26% |
| 1.0–1.4 | 22% | 18% |
| ≥1.5 | 21% | 16% |
| Peritoneal solute transfer ratea | 0.66 (0.13) | 0.74 (0.12) |
| Potassium, mEq/L | 4.3 (0.6) | 4.2 (0.7) |
| HbA1c in diabetic patients, % | 7 (1.5) | 7 (1.6) |
| Cared for at center that employs routine PET, % | 40% | 51% |
Results shown as prevalence, mean (standard deviation), or median (interquartile range). CAPD, continuous ambulatory peritoneal dialysis; APD, automated peritoneal dialysis; PD, peritoneal dialysis; PET: peritoneal equilibration test.
Expressed as 4-hour dialysate/plasma creatinine ratio.
Table 2.
Therapy characteristics by country and by icodextrin use
| Australia/New Zealand | Canada | Japan | United Kingdom | United States | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Non Icodextrin | Icodextrin | No Icodextrin | Icodextrin | No Icodextrin | Icodextrin | No Icodextrin | Icodextrin | No Icodextrin | Icodextrin |
| Number of patients | 317 | 238 | 439 | 551 | 622 | 521 | 240 | 267 | 2013 | 409 |
| Prescribed therapy volume, L | 9.5 (3.4) | 11 (4) | 9.2 (3.7) | 11 (4) | 6 (2.4) | 6.7 (2.7) | 8.9 (3.6) | 9.8 (4.2) | 11 (4) | 12 (4) |
| Nutrineal, % | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 5 | 0 | 0 |
| Neutral pH low GDP, % | 34 | 32 | 9 | 6 | 100 | 99 | 23 | 30 | a | a |
| PD solution type | ||||||||||
| Use of 2.27% but not 3.86% | 73% | 80% | 53% | 64% | 27% | 44% | 44% | 46% | 53% | 54% |
| Use of any 3.86% | 10% | 9% | 13% | 15% | 0% | 0% | 1% | 3% | 42% | 43% |
| Without any 2.27% or 3.86% use | 17% | 12% | 34% | 21% | 73% | 56% | 55% | 52% | 6% | 4% |
| Residual Kt/V urea | 1 (0.9) | 0.7 (0.7) | 0.9 (0.8) | 0.7 (0.6) | 0.9 (0.7) | 0.6 (0.5) | 1.2 (0.7) | 0.9 (0.6) | 0.9 (0.8) | 0.4 (0.5) |
| Peritoneal Kt/V urea | 1.3 (0.6) | 1.3 (0.5) | 1.2 (0.6) | 1.4 (0.5) | 1 (0.4) | 1.1 (0.4) | 1.1 (0.5) | 1.2 (0.5) | 1.5 (0.5) | 1.6 (0.5) |
| Total Kt/V urea | 2.3 (0.8) | 2.1 (0.8) | 2.1 (0.9) | 2.1 (0.7) | 1.9 (0.6) | 1.7 (0.4) | 2.4 (0.7) | 2.1 (0.6) | 2.3 (0.7) | 2.1 (0.5) |
Results shown as prevalence or mean (standard deviation). GDP, glucose degradation product; PD, peritoneal dialysis.
Low GDP, neutral-pH solutions are not commercially available in the United States.
Outcome: Peritoneal Ultrafiltration
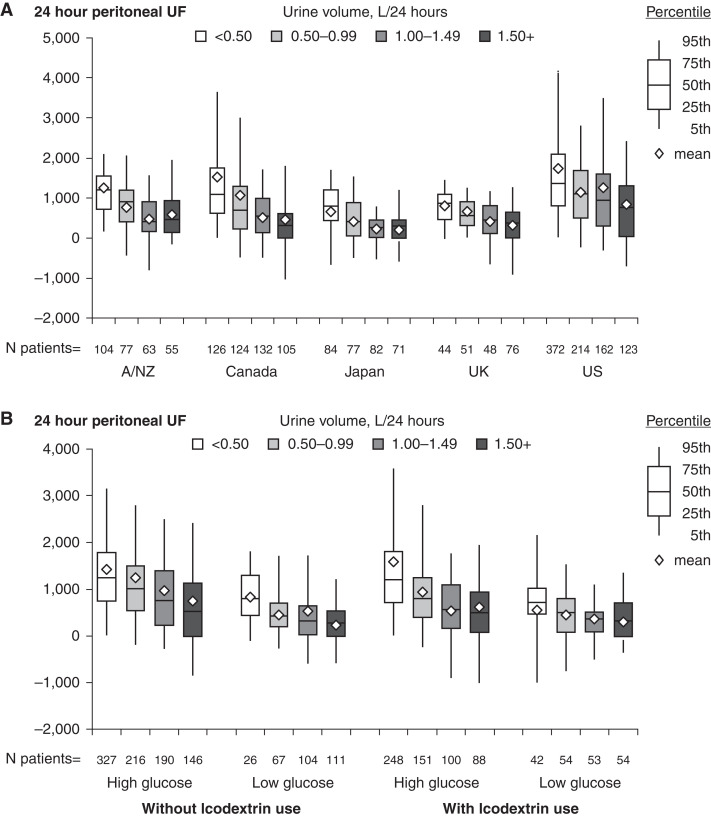
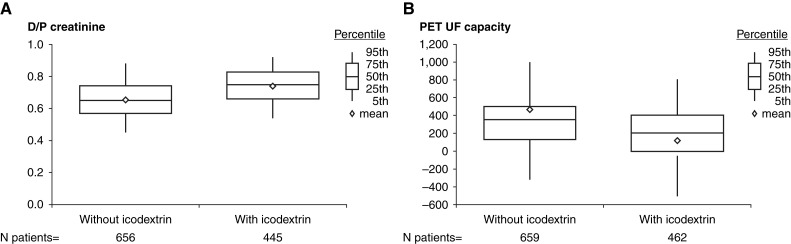
The median 24-hour peritoneal ultrafiltration was 765 ml/d (interquartile range [IQR] 251–1345) and 750 (IQR 300–1345) for users and nonusers of icodextrin, respectively. As would be expected, the achieved 24-hour peritoneal ultrafiltration was strongly affected by the residual 24-hour urine volume (Figure 3). Icodextrin use was not associated with increased ultrafiltration regardless of whether the concomitant use of high (any glucose concentration >1.36%) or low PD glucose prescriptions were used. However, where data were available, this demonstrated preferential use of icodextrin in patients with faster PSTR and substantially reduced ultrafiltration capacity. This was seen in all countries and translated into an equivalent amount of net 24-hour ultrafiltration stratified for urine volume when compared with patients not treated with icodextrin (Figures 3 and 4 and Supplemental Figure 4). Icodextrin use was also associated with an equivalent daily ultrafiltration volume, despite these patients using substantially less hypertonic glucose overall, especially in those with low urine volume. The patients for whom this additional information was available did not differ from the whole group in terms of demographics, although the data were relatively more complete for Japan and less complete for the United States.
Figures 3.
Twenty-four-hour peritoneal UF stratified by urine volume and country (upper panel), and by icodextrin and glucose prescriptions. High-glucose prescription was defined as any 2.27% or 3.86% solution use. Low-glucose prescription was defined as no 2.27% or 3.86% solution use. As urine volume decreases UF increases, more markedly in the US. Ultrafiltration according to icodextrin use is not different at equivalent categories of urine volume and glucose prescription. UF, ultrafiltration.
Figure 4.
Peritoneal solute transfer characteristics and UF capacity at study baseline by icodextrin use. (A) D/P creatinine. (B) PET UF capacity. D/P, dialysate to plasma creatinine ratio at 4 hours on the PET. PET, peritoneal equilibration test.
Outcomes: Patient Survival and HDT
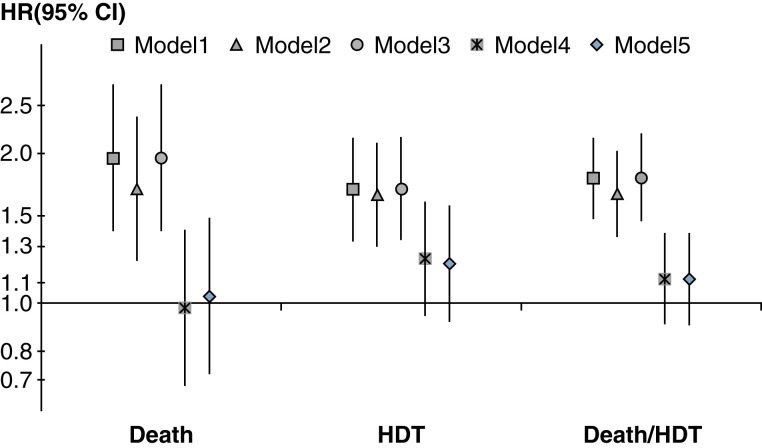
During a median follow-up of 1.14 years (IQR 0.62–1.86), 712 patients died, and 1167 patients had HDT events (the 11 patients who died within 7 days of HDT were counted as death). When using an instrumental variable analysis, icodextrin use was not significantly associated with the risk of death (hazard ratio [HR]=1.03; 95% confidence interval [95% CI], 0.72 to 1.48), HDT (HR=1.2; 95% CI, 0.92 to 1.57), or death/HDT (HR=1.12; 95% CI, 0.9 to 1.38; Figure 5, model 5). Omitting the dry-day patients on APD did not have substantial effects on the outcomes from the instrumental variable analyses (Supplemental Table 2). Infection was the most common cause of HDT, followed by either inadequate solute clearance or ultrafiltration and psychosocial/medical problems, among patients with or without icodextrin use (Supplemental Figure 5).
Figure 5.
Association between icodextrin and clinical outcomes by sequential adjustment. Instrumental variable models stratified by country and US large dialysis organization, accounting for center clustering. Model 1, adjusted for DOPPS phase only. Model 2, model 1+age, men, vintage. Model 3, model 2+comorbidities, transplant waiting list. Model 4, model 3+urine volume, albumin. Model 5, model 4+center size, center % APD. DOPPS, Dialysis Outcomes and Practice Patterns Study; APD, automated peritoneal dialysis.
Subgroup Analyses
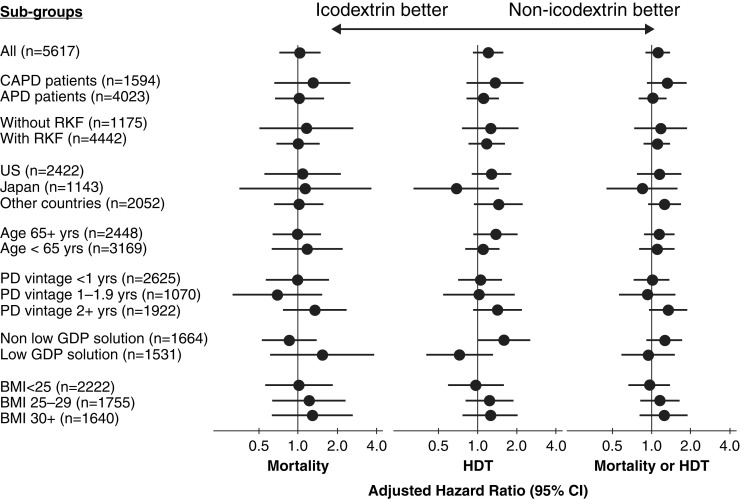
We analyzed the association between icodextrin use and outcomes among a number of subgroups of interest. None of the interactions investigated had a P value <0.05 after accounting for multiple comparisons by using the Benjamini–Hochberg false discovery rate correction, whether dry-day patients on APD were included or excluded (Figure 6 Supplemental Tables 3 and 4).
Figure 6.
Association between icodextrin and clinical outcomes. All models stratified by country, and adjusted for PDOPPS phase, age, sex, vintage, 13 comorbidities, transplant waiting list, center % APD, center size, albumin, urine volume, and accounting for center clustering. Number of events: mortality 712, HDT 1167, mortality/HDT 1879. Patients from Japan and the United States were excluded from the low GDP solution subgroup analysis because low-GDP solutions were not available in the US and were used exclusively in Japan. RKF was defined as 24-hour urine volume ≥0.2 L. Other countries included Australia/New Zealand, Canada, and the United Kingdom. CAPD, continuous ambulatory peritoneal dialysis; PD, peritoneal dialysis; GDP, glucose degradation product; BMI, body mass index. All P values for interactions are >0.05.
Discussion
In the largest global survey of icodextrin prescription practices to date, we found substantial country- and center-level variation in its use. In particular, practices in the United States were different from other countries in terms of both a lower frequency of icodextrin use and the lack of an increase in its prescription with time on treatment (Supplemental Table 5). The explanation of this marked difference is likely to be multifactorial, but some postulations can be made. The proportion of centers undertaking routine membrane function testing in prevalent patients was the lowest for the United States (Supplemental Figure 3), which may translate into a lower likelihood of identifying patients who might benefit from icodextrin, or conversely less membrane testing and icodextrin use, so as to keep down dialysis costs, reflecting the US reimbursement model. The ANZDATA registry recently reported that the main center-level factor associated with icodextrin use was the greater use of membrane function testing (19). Another likely relevant factor is the consistent finding that the United States appears to be an outlier in the amount of ultrafiltration and the use of hypertonic glucose. PDOPPS previously reported that the use of both 2.27%/2.5% and 3.86%/4.5% glucose/dextrose is substantially higher in the United States (20); this is confirmed in the present analysis in which 45% of patients used the 3.86%/4.5% concentration in at least one exchange, very few (4%–6%) having prescriptions without use of either 2.27% or 3.86%, and this is regardless of whether icodextrin is also being used. These differences in US practices translate into higher overall 24-hour ultrafiltration volumes compared with other countries, which when combined with urine volume result in equivalent total fluid removal (see Supplemental Table 1). The reasons for these country-level differences cannot be determined from this study but may include dietary and cultural factors, reflected here by the highest body mass index being observed in the US patients. Interestingly, US patients on HD tend to have higher interdialytic weight gains, pointing to higher fluid intake, which may be reflecting a common underlying cause, such as salt intake (21,22).
The variability in within-country facility-level prescription of icodextrin cannot be fully explained by this analysis, although again frequency of routine membrane function testing had some effect. By contrast, consistent patterns in patient-level prescription could be seen. Generally, it was more frequently prescribed when cardiovascular or diabetic comorbidity was present, when residual kidney function was lower, and when time on therapy was longer, suggesting a reactive rather than proactive approach to fluid management. Where membrane function was measured, patients on icodextrin had on average a faster PSTR in all countries (Table 2). This faster PSTR translated into an average reduction of 200-ml ultrafiltration capacity (using 2.27% glucose)—enough to be clinically significant and to necessitate the use of more hypertonic glucose if fluid reabsorption is to be avoided. Given that membrane function was only measured in a small subset of patients, it cannot be assumed that this targeting of icodextrin to patients with less efficient membranes applied to all patients, but this subgroup was representative of the study sample based on other similar characteristics. However, it is likely to contribute to the explanation of why patients on icodextrin did not achieve overall higher levels of ultrafiltration, when stratified for residual urine volume, than those treated with glucose alone. Randomized trials demonstrate that ultrafiltration with icodextrin is superior to glucose ≤2.27% in the long exchange (7,8), but this benefit would be offset by the use of higher concentrations of glucose across remaining exchanges as appears to be the case in this analysis. The most reasonable conclusion to draw from these data is that icodextrin was being used selectively to maintain sufficient ultrafiltration in those with less efficient membranes and more generally to avoid excessive use of hypertonic glucose. The exception to this was prescription practice in the United States.
This analysis did not find a beneficial association between icodextrin use and patient survival or HDT. Indeed, icodextrin use was associated with worse survival unless this was adjusted for urine volume and plasma albumin (Figure 5). This likely reflects its preferential use in patients with risk factors for worse volume status. Its failure to improve survival may well reflect our finding that icodextrin was not associated with more overall ultrafiltration compared with its nonuse in contrast to randomized trials in which it is one of the proposed mechanisms by which it confers clinical benefit (7,8). Given the known association between faster than average PSTR and worse survival, it might be postulated that the use of icodextrin may mitigate this survival disadvantage, especially as there is evidence that it was used preferentially in this half of the population. Unfortunately, there were insufficient measures of membrane function for this hypothesis to be tested here, despite the size of PDOPPS.
In an attempt to account for treatment by indication bias, we undertook an instrumental variable analysis. This technique depends on the assumption that the likelihood of a patient being prescribed icodextrin would in part be determined by the overall propensity of their PD center to prescribe icodextrin. In particular, this would take advantage of the marked differences, especially by country, but also by facilities within countries in the proportion of patients receiving icodextrin. Potentially this approach would have most benefited from the discrepancy in icodextrin prescription observed between the United States and other countries. Another confounding factor in this analytic approach is the other potential indications for prescribing icodextrin such as improved glycemic control in diabetics (23,24), reduced insulin resistance (25), and avoidance of other concerns that relate to glucose exposure such as weight gain and dialysate-associated membrane injury (26). These might explain the large country and center differences in prescribing that have not been examined here.
The lack of effect on overall risk of HDT was not unexpected. In this regard, the PDOPPS cohort is in keeping with previous randomized controlled trials (7,8). It might be anticipated that icodextrin use would have a positive effect on transfers to HD due to suboptimal salt and water removal, but this occurred in only 27% of overall HDT events, ranging from 15% in the United States to 41% in Japan. In another PDOPPS analysis, we recently found that greater center use of icodextrin was associated with an increased likelihood of cure after a peritonitis episode (27). This may relate to confounding based on icodextrin use and differences in peritonitis treatment practices across facilities. Previous studies have shown that icodextrin reduced biofilm biomass on PD catheters, probably due to the lack of glucose as carbon source compared with bicarbonate/lactate buffered glucose-based solution (28,29). However, a beneficial effect of icodextrin on HDT was not observed in the present study.
This study has limitations. As for all observational studies, especially when the proportion of prevalent subjects is high, the inferences with respect to cause and effect require testing in interventional studies, and this is still true, despite our use of statistical adjustment and instrumental variables approach to reduce bias. This is especially true if there are unmeasured clinical practices affecting clinical outcomes that also increase the likelihood of icodextrin use. As already indicated, a significant limitation is the missingness of data relating to membrane function, the achieved 24-hour ultrafiltration, and residual urine volume. The reason for this is that we have only included data for measurement of these parameters at 4 months of the study baseline at which the icodextrin prescription was documented. It is also clear that many facilities do not measure these variables routinely. Given that PDOPPS is a prevalent study, this component of the dataset was inevitably incomplete. We compared the characteristics of patients (see Table 2) who had these measures with those who did not, and we found that there were no differences in the patient populations, suggesting that the data missingness reflected local measurement practice rather than a selected patient effect. This did affect our ability to construct a survival model that included membrane function, known to be strongly associated with survival on peritoneal dialysis. Overall, data missingness was affected by country, being least in Japan and greatest in the United States. Furthermore, data were not available on icodextrin prescription from a large dialysis organization in the United States, which was excluded from this analysis. If anything, this may have increased the observed differences between the United States and other countries. The lack of an objective measurement of volume status (e.g., bioimpedance) or assessment of dietary salt and fluid intake is a further limitation.
In summary, there is variation in icodextrin use in the PDOPPS countries, with notably low use in the United States where there is much greater dependency on high glucose concentration solutions, resulting in overall greater net ultrafiltration. There is also considerable variability in icodextrin use between facilities in every country. Across all countries, icodextrin use is more common among patients who have less residual kidney function and membranes that are less efficient at removing fluid with glucose, and possibly because of this, the achieved daily ultrafiltration is not different between those using versus not. There is, however, considerable variability in routine membrane evaluation, which may be limiting the use of icodextrin. Icodextrin also tends to be used in those who have been on dialysis for longer and have more comorbidities, suggesting that further evaluation of a proactive approach to its use may be necessary earlier in the course of PD therapy. Use of icodextrin therefore appears to be targeted toward a group expected to have worse survival chances, and this cannot be completely accounted for by survival modeling. Its use can, however, avoid the excessive use of hypertonic glucose without detriment.
Disclosures
S.J. Davies received consulting fees from Baxter Healthcare and Ellen Medical, and honoraria from Fresenius Medical Care. T. Kanjanabuch received consultancy fees from VISTERA as a country investigator and current recipient of the National Research Council of Thailand and Innovation Fund Chulalongkorn University. received speaking honoraria from AstraZeneca and Baxter Healthcare. K.P. McCullough, R.L. Pisoni, B.M. Robinson, and J. Zhao are employees of Arbor Research Collaborative for Health, which funds the DOPPS. R. Mehrotra received consulting fee from Baxter Healthcare. J. Perl received grants from AHRQ; consulting fee from Baxter Healthcare, Davita, Fresenius Medical Care, and Otsuka; honoraria from Baxter Healthcare, Davita Healthcare Partners, DCI, Fresenius Medical Care, and US Renal Care; support for attending meetings and/or travel from Liberdi Dialysis; and stock or stock options from Liberdi dialysis. B.M. Robinson received consultancy fees or travel reimbursement since 2018 from AstraZeneca, GlaxoSmithKline, and Kyowa Kirin Co., all paid directly to his institution of employment. A.Y.-M. Wang participated on a Data Safety Monitoring Board or Advisory Board (DSMB ASPIRED TRIAL) and is International Society of Nephrology (ISN) Councilor and North and East Asia Regional Board Deputy Chair, ISN Education working group Deputy Chair Council Member of International Society of Peritoneal Dialysis, and President of International Society of Renal Nutrition and Metabolism. All remaining authors have nothing to disclose.
Funding
This manuscript was directly supported by Baxter Healthcare. The Dialysis Outcomes and Practice Patterns Study (DOPPS) Program is funded by a consortium of private industry, public funders, and professional societies. More information on DOPPS funding can be found here: https://www.dopps.org/AboutUs/Support.aspx. Specific grant support for UK patient involvement is from Kidney Research UK and the National Institutes of Health Research (all payments to the employing institution, Keele University). Funding for PDOPPS was provided by: the National Health and Medical Research Council (Australia); the National Institute for Health Research (United Kingdom); the National Institute of Diabetes and Digestive and Kidney Diseases (United States); the Patient-Centered Outcomes Research Institute (United States); the Japanese Society of Peritoneal Dialysis; the Canadian Institute for Health Research; Baxter International, Inc. (United States); the National Research Council of Thailand (2558-113); Rachadaphiseksompot Endorcement Fund (GCURS_59_12_30_03), Chulalongkorn University, Thailand; and the National Science and Technology Development Agency (NSTDA), Thailand.
Acknowledgments
We acknowledge and thank the following individuals for their contributions: PDOPPS Dialysis Prescription and Fluid Management Work Group Members: Angela Wang (Hong Kong), John Burkart (United States), Gillian Brunier (Canada), Hideki Kawanishi (Japan), Sunil Badve (Australia), Suchai Sritippayawan (Thailand), Sarinya Boongird (Thailand), Andreas Vychytil (Austria), Wim Van Biesen (Belgium), and Thyago de Moraes (Brazil). We also thank Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, who provided editorial assistance on this paper.
Footnotes
See related editorial, “Icodextrin in Peritoneal Dialysis: Implications on Clinical Practice and Survival Outcome,” on pages 793–795.
Author Contributions
S.V. Badve, S.J. Davies, T. Kanjanabuch, H. Kawanishi, K.P. McCullough, R. Mehrotra, J. Perl, R. Pisoni, B.M. Robinson, A.Y.-M. Wang and J. Zhao were responsible for the final approval of the manuscript to be published; S.J. Davies, T. Kanjanabuch, H. Kawanishi, Y.-L. Kim, K.P. McCullough, J. Perl, R. Pisoni, B.M. Robinson, A.Y.-M. Wang, and J. Zhao provided intellectual content of critical importance to the work described; S.J. Davies, Y.-L. Kim, K.P. McCullough, R. Mehrotra, J. Perl, R. Pisoni, B.M. Robinson, A.Y.-M. Wang, and J. Zhao were responsible for the conception or design, or analysis and interpretation of data, or both; and S.J. Davies, Y.-L. Kim, K.P. McCullough, J. Perl, R. Pisoni, B.M. Robinson, and J. Zhao were responsible for drafting the article or revising it.
Data Sharing Statement
The data that support the findings of this study are available from Arbor Research Collaborative for Health, but restrictions apply to the availability of these data which were used for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of Arbor Research Collaborative for Health.
Supplemental Material
This article contains supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0006922021/-/DCSupplemental.
Icodextrin use by peritoneal dialysis vintage and by country. Download Supplemental Figure 1, DOCX file, 256 KB (256KB, docx)
Distribution of the center-level proportion of patients using icodextrin by country, excluding automated peritoneal dialysis dry-day patients. Download Supplemental Figure 2, DOCX file, 256 KB (256KB, docx)
Percent of facilities routinely performing peritoneal equilibration test (PET) by country. Download Supplemental Figure 3, DOCX file, 256 KB (256KB, docx)
Twenty-four-hour peritoneal ultrafiltration by urine volume, icodextrin, and glucose, excluding dry-day patients. Download Supplemental Figure 4, DOCX file, 256 KB (256KB, docx)
Reasons for permanent switch to hemodialysis/hybrid by icodextrin use at baseline. Download Supplemental Figure 5, DOCX file, 256 KB (256KB, docx)
Patient characteristics by country and by icodextrin use. Download Supplemental Table 1, DOCX file, 256 KB (256KB, docx)
Proportion of patients started icodextrin by country and by months since start of peritoneal dialysis. Download Supplemental Table 2, DOCX file, 256 KB (256KB, docx)
Icodextrin use and clinical outcomes, excluding automated peritoneal dialysis dry-day patients. Download Supplemental Table 3, DOCX file, 256 KB (256KB, docx)
P values for interactions by model type and outcome, including all patients. Download Supplemental Table 4, DOCX file, 256 KB (256KB, docx)
P values for interactions by model type and outcomes, excluding automated peritoneal dialysis dry-day patients. Download Supplemental Table 5, DOCX file, 256 KB (256KB, docx)
References
- 1.Wang AY, Dong J, Xu X, Davies S: Volume management as a key dimension of a high-quality PD prescription. Perit Dial Int 40: 282–292, 2020. 10.1177/0896860819895365 [DOI] [PubMed] [Google Scholar]
- 2.Kim YL, Biesen WV: Fluid overload in peritoneal dialysis patients. Semin Nephrol 37: 43–53, 2017. 10.1016/j.semnephrol.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 3.Mujais S, Story K: Peritoneal dialysis in the US: Evaluation of outcomes in contemporary cohorts. Kidney Int Suppl 70: S21–S26, 2006. 10.1038/sj.ki.5001912 [DOI] [PubMed] [Google Scholar]
- 4.Jaar BG, Plantinga LC, Crews DC, Fink NE, Hebah N, Coresh J, Kliger AS, Powe NR: Timing, causes, predictors and prognosis of switching from peritoneal dialysis to hemodialysis: A prospective study. BMC Nephrol 10: 3, 2009. 10.1186/1471-2369-10-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, Bosselmann HP, Heimbürger O, Simonsen O, Davenport A, Tranaeus A, Divino Filho JC: Icodextrin improves the fluid status of peritoneal dialysis patients: Results of a double-blind randomized controlled trial. J Am Soc Nephrol 14: 2338–2344, 2003. 10.1097/01.ASN.0000083904.12234.27 [DOI] [PubMed] [Google Scholar]
- 6.Konings CJ, Kooman JP, Schonck M, Gladziwa U, Wirtz J, van den Wall Bake AW, Gerlag PG, Hoorntje SJ, Wolters J, van der Sande FM, Leunissen KM: Effect of icodextrin on volume status, blood pressure and echocardiographic parameters: A randomized study. Kidney Int 63: 1556–1563, 2003. 10.1046/j.1523-1755.2003.00887.x [DOI] [PubMed] [Google Scholar]
- 7.Htay H, Johnson DW, Wiggins KJ, Badve SV, Craig JC, Strippoli GF, Cho Y: Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Syst Rev 10: CD007554, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goossen K, Becker M, Marshall MR, Bühn S, Breuing J, Firanek CA, Hess S, Nariai H, Sloand JA, Yao Q, Chang TI, Chen J, Paniagua R, Takatori Y, Wada J, Pieper D: Icodextrin versus glucose solutions for the once-daily long dwell in peritoneal dialysis: An enriched systematic review and meta-analysis of randomized controlled trials. Am J Kidney Dis 75: 830–846, 2020. 10.1053/j.ajkd.2019.10.004 [DOI] [PubMed] [Google Scholar]
- 9.Mehrotra R, Ravel V, Streja E, Kuttykrishnan S, Adams SV, Katz R, Molnar MZ, Kalantar-Zadeh K: Peritoneal equilibration test and patient outcomes. Clin J Am Soc Nephrol 10: 1990–2001, 2015. 10.2215/CJN.03470315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morelle J, Stachowska-Pietka J, Öberg C, Gadola L, La Milia V, Yu Z, Lambie M, Mehrotra R, de Arteaga J, Davies S: ISPD recommendations for the evaluation of peritoneal membrane dysfunction in adults: Classification, measurement, interpretation and rationale for intervention. Perit Dial Int 41: 352–372, 2021. 10.1177/0896860820982218 [DOI] [PubMed] [Google Scholar]
- 11.Perl J, Davies SJ, Lambie M, Pisoni RL, McCullough K, Johnson DW, Sloand JA, Prichard S, Kawanishi H, Tentori F, Robinson BM: The Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS): Unifying efforts to inform practice and improve global outcomes in peritoneal dialysis. Perit Dial Int 36: 297–307, 2016. 10.3747/pdi.2014.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angrist JD, Imbens GW, Rubin DB: Identification of causal effects using instrumental variables. J Am Stat Assoc 91: 444–455, 1996. 10.1080/01621459.1996.10476902 [DOI] [Google Scholar]
- 13.Wooldridge J: Introductory Econometrics: A Modern Approach, 4th Ed., Nashville, NT, South-Western College Publishing, 2002, pp 506–545 [Google Scholar]
- 14.Newhouse JP, McClellan M: Econometrics in outcomes research: The use of instrumental variables. Annu Rev Public Health 19: 17–34, 1998. 10.1146/annurev.publhealth.19.1.17 [DOI] [PubMed] [Google Scholar]
- 15.Greenland S: An introduction to instrumental variables for epidemiologists. Int J Epidemiol 29: 722–729, 2000. 10.1093/ije/29.4.722 [DOI] [PubMed] [Google Scholar]
- 16.Pisoni RL, Arrington CJ, Albert JM, Ethier J, Kimata N, Krishnan M, Rayner HC, Saito A, Sands JJ, Saran R, Gillespie B, Wolfe RA, Port FK: Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: An instrumental variable analysis. Am J Kidney Dis 53: 475–491, 2009. 10.1053/j.ajkd.2008.10.043 [DOI] [PubMed] [Google Scholar]
- 17.Stock JH, Wright JH, Yogo M: A survey of weak instruments and weak identification in generalized method of moments. J Bus Econ Stat 20: 518–529, 2002. 10.1198/073500102288618658 [DOI] [Google Scholar]
- 18.Burgess S, Thompson SG; CRP CHD Genetics Collaboration : Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol 40: 755–764, 2011. 10.1093/ije/dyr036 [DOI] [PubMed] [Google Scholar]
- 19.Rangaswamy D, Guddattu V, Webster AC, Borlace M, Boudville N, Clayton P, Badve S, Johnson DW, Sud K: Icodextrin use for peritoneal dialysis in Australia: A cohort study using Australia and New Zealand Dialysis and Transplant Registry. Perit Dial Int 40: 209–219, 2020. 10.1177/0896860819894058 [DOI] [PubMed] [Google Scholar]
- 20.Wang AY, Zhao J, Bieber B, Kanjanabuch T, Wilkie M, Marshall MR, Kawanishi H, Perl J, Davies S; PDOPPS Dialysis Prescription and Fluid Management Working Group : International comparison of peritoneal dialysis prescriptions from the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS). Perit Dial Int 40: 310–319, 2020. 10.1177/0896860819895356 [DOI] [PubMed] [Google Scholar]
- 21.Wong MM, McCullough KP, Bieber BA, Bommer J, Hecking M, Levin NW, McClellan WM, Pisoni RL, Saran R, Tentori F, Tomo T, Port FK, Robinson BM: Interdialytic weight gain: Trends, predictors, and associated outcomes in the international Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 69: 367–379, 2017. 10.1053/j.ajkd.2016.08.030 [DOI] [PubMed] [Google Scholar]
- 22.Saran R, Bragg-Gresham JL, Levin NW, Twardowski ZJ, Wizemann V, Saito A, Kimata N, Gillespie BW, Combe C, Bommer J, Akiba T, Mapes DL, Young EW, Port FK: Longer treatment time and slower ultrafiltration in hemodialysis: Associations with reduced mortality in the DOPPS. Kidney Int 69: 1222–1228, 2006. 10.1038/sj.ki.5000186 [DOI] [PubMed] [Google Scholar]
- 23.Li PK, Dorval M, Johnson DW, Rutherford P, Shutov E, Story K, Bargman JM: The benefit of a glucose-sparing PD therapy on glycemic control measured by serum fructosamine in diabetic patients in a randomized, controlled trial (IMPENDIA). Nephron 129: 233–240, 2015. 10.1159/000371554 [DOI] [PubMed] [Google Scholar]
- 24.Sniderman AD, Sloand JA, Li PK, Story K, Bargman JM: Influence of low-glucose peritoneal dialysis on serum lipids and apolipoproteins in the IMPENDIA/EDEN trials. J Clin Lipidol 8: 441–447, 2014. 10.1016/j.jacl.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 25.de Moraes TP, Andreoli MC, Canziani ME, da Silva DR, Caramori JC, Ponce D, Cassi HV, de Andrade Bastos K, Rio DR, Pinto SW, Filho SR, de Campos LG, Olandoski M, Divino-Filho JC, Pecoits-Filho R: Icodextrin reduces insulin resistance in non-diabetic patients undergoing automated peritoneal dialysis: Results of a randomized controlled trial (STARCH). Nephrol Dial Transplant 30: 1905–1911, 2015. 10.1093/ndt/gfv247 [DOI] [PubMed] [Google Scholar]
- 26.Davies SJ, Brown EA, Frandsen NE, Rodrigues AS, Rodriguez-Carmona A, Vychytil A, Macnamara E, Ekstrand A, Tranaeus A, Filho JC; EAPOS Group : Longitudinal membrane function in functionally anuric patients treated with APD: Data from EAPOS on the effects of glucose and icodextrin prescription. Kidney Int 67: 1609–1615, 2005. 10.1111/j.1523-1755.2005.00243.x [DOI] [PubMed] [Google Scholar]
- 27.Al Sahlawi M, Zhao J, McCullough K, Fuller DS, Boudville N, Ito Y, Kanjanabuch T, Nessim SJ, Piraino BM, Pisoni RL, Teitelbaum I: Variation in peritoneal dialysis-related peritonitis outcomes in the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS). Am J Kidney Dis 79: 45–55.e1, 2022. 10.1053/j.ajkd.2021.03.022 [DOI] [PubMed] [Google Scholar]
- 28.Vychytil A, Remón C, Michel C, Williams P, Rodríguez-Carmona A, Marrón B, Vonesh E, van der Heyden S, Divino Filho JC; Extraneal Peritonitis Study Group : Icodextrin does not impact infectious and culture-negative peritonitis rates in peritoneal dialysis patients: A 2-year multicentre, comparative, prospective cohort study. Nephrol Dial Transplant 23: 3711–3719, 2008. 10.1093/ndt/gfn322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampaio J, Machado D, Gomes AM, Machado I, Santos C, Lima N, Carvalho MJ, Cabrita A, Rodrigues A, Martins M: Deciphering the contribution of biofilm to the pathogenesis of peritoneal dialysis infections: Characterization and microbial behaviour on dialysis fluids. PLoS One 11: e0157870, 2016. 10.1371/journal.pone.0157870 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Icodextrin use by peritoneal dialysis vintage and by country. Download Supplemental Figure 1, DOCX file, 256 KB (256KB, docx)
Distribution of the center-level proportion of patients using icodextrin by country, excluding automated peritoneal dialysis dry-day patients. Download Supplemental Figure 2, DOCX file, 256 KB (256KB, docx)
Percent of facilities routinely performing peritoneal equilibration test (PET) by country. Download Supplemental Figure 3, DOCX file, 256 KB (256KB, docx)
Twenty-four-hour peritoneal ultrafiltration by urine volume, icodextrin, and glucose, excluding dry-day patients. Download Supplemental Figure 4, DOCX file, 256 KB (256KB, docx)
Reasons for permanent switch to hemodialysis/hybrid by icodextrin use at baseline. Download Supplemental Figure 5, DOCX file, 256 KB (256KB, docx)
Patient characteristics by country and by icodextrin use. Download Supplemental Table 1, DOCX file, 256 KB (256KB, docx)
Proportion of patients started icodextrin by country and by months since start of peritoneal dialysis. Download Supplemental Table 2, DOCX file, 256 KB (256KB, docx)
Icodextrin use and clinical outcomes, excluding automated peritoneal dialysis dry-day patients. Download Supplemental Table 3, DOCX file, 256 KB (256KB, docx)
P values for interactions by model type and outcome, including all patients. Download Supplemental Table 4, DOCX file, 256 KB (256KB, docx)
P values for interactions by model type and outcomes, excluding automated peritoneal dialysis dry-day patients. Download Supplemental Table 5, DOCX file, 256 KB (256KB, docx)