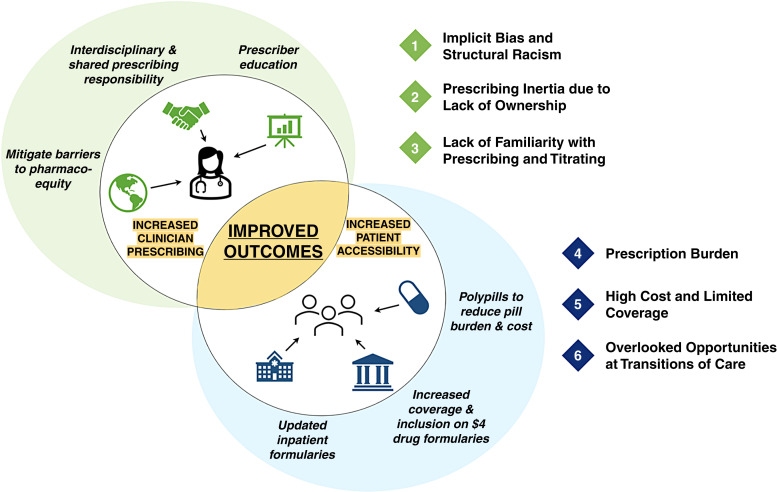
Diabetes is a leading worldwide medical comorbidity affecting approximately 537 million adults, nearly three-quarters of whom live in low- and middle-income countries (1). Nearly 30% of adults with diabetes have kidney disease, and most are unaware of their risk for morbidity associated with CKD and incident kidney failure (1,2). Sodium-glucose co-transporter 2 inhibitors (SGLT2i) are groundbreaking medications with the potential to slow disease progression and improve clinical outcomes in conditions ranging from diabetic kidney disease to heart failure (3). The delayed adoption of SGLT2i compared to angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is disappointing. Across the United States, <10% of eligible patients were prescribed an SGLT2i between 2015 and 2019 (4). Multifactorial, systematic barriers are the tacit undercurrent of these findings, and innovation is needed for successful implementation of SGLT2i for appropriate clinical indications. Within health care systems, multifactorial barriers limit effective quality improvement, and ascribing a single one-size-fits-all solution is unrealistic. However, several unifying themes may guide health care innovators in identifying and tailoring solutions to achieve equitable SGLT2i access (Figure 1).
Figure 1.
Barriers and solutions to equitable prescribing and access to sodium-glucose co-transporter 2 inhibitors. Numbered elements represent barriers, whereas potential solutions are represented within the shaded diagram. Increasing both clinician prescribing and patient access may result in improved health outcomes for diabetic kidney disease, heart failure, and type 2 diabetes.
Adoption of a Collaborative Prescribing Framework
Worldwide, clinicians associate SGLT2i primarily with hypoglycemic effects, and this may inhibit prescribing for cardiovascular and kidney outcomes. Patients with diabetes, CKD, heart failure, or prior myocardial infarction have lower odds of being prescribed an SGLT2i, despite being clinically indicated. Rates of SGLT2i initiation are much higher among internists and endocrinologists compared with cardiologists (5). Among cardiologists, primary barriers to initiating SGLT2i in heart failure were concern for precipitating hypoglycemia, the need to adjust diabetic medications, and perceived lack of prescribing responsibility (versus cost for internists and endocrinologists) (5). Existing studies poorly characterize nephrology prescribing practices; however, they do highlight the need for structured, interdisciplinary management.
Initiation of SGLT2i often requires concurrent titration of diuretics, hypoglycemic agents, and antihypertensives, and clinicians may be less familiar with this stepwise deprescribing. Algorithms are widely available to assist with these decisions; however, they may conflict across subspecialties. Due to the constantly evolving data and increasingly complex patient populations, interprofessional communication is strongly recommended (3,6). Indeed, interdisciplinary care teams with clinical pharmacists are the preferred modality for SGLT2i prescribing and monitoring (6), which can be paired with updated patient-personalized tools such as the START (Screening Tool to Alert to Right Treatment) criteria and FORTA (Fit for the Aged) scores (7). Incorporation of clinical pharmacists improves adherence to clinical guidelines in CKD and may alleviate prescribing inertia and concerns regarding adverse effects. However, economic incentives and reimbursement structures will likely need to be recalibrated to truly enable unimpeded interdisciplinary care.
Improved Primary-Care Screening and Referrals for Diabetic Kidney Disease
Primary-care physicians are at the forefront of diabetes management and screening for diabetic kidney disease. Unfortunately, albuminuria screening rates remain low, despite well-established guidelines. Expanding screening enhances earlier diagnosis of kidney disease and offers the opportunity for prompt intervention before a patient develops complications of advanced disease. Timely referrals to subspecialists are also key to ensuring patients receive appropriate care before progression to end stage disease. However, in the case of diabetic kidney disease, identifying those patients in highest need for nephrology referral remains nebulous, even in the presence of risk-prediction tools (8). There are, unfortunately, more patients with CKD than can be cared for directly by the current nephrology workforce. Innovations in co-management strategies to support primary-care clinicians should be pursued, such as increased access to electronic consults and decision-support tools (8).
Reduced Insurance-Derived Prescribing Barriers
In the United States, a country with patchwork insurance coverage that is primarily dependent on employers and inconsistent across states, patient out-of-pocket costs are highly variable and may affect both prescribing and adherence. For example, there are higher SGLT2i prescribing rates in states that expanded Medicaid. Although there is high prescription drug coverage for SGLT2i through Medicare Part D (i.e., 95% for empagliflozin), out-of-pocket costs remain prohibitively high ($300–$520 median retail price for a 30-day supply) (9). Clinicians are mindful of the cost on behalf of their patients and are also attuned to their own workflow burden with regard to prior authorizations (5). Finally, SGLT2i are often not included in inpatient formularies, which limit implementation before discharge, despite evidence for safe in-hospital initiation. These issues compound the lack of pharmaco-equity and treatment disparities among Black and lowest-income patients (4). Developing a wider range of low-cost polypills may reduce out-of-pocket costs and improve adherence for patients at a steady-state dose. A coverage commitment from insurance providers could alleviate some cost barriers but would need to account for fragmented payment models in the United States.
Commitment to Equity in Implementation and Research
Medications exist to slow the progression of kidney disease, and now more than ever, leaders in health care need to ensure that improved initiatives increase implementation among systematically disadvantaged patients to reverse widening health disparities (10). Addressing a topic as complex as ensuring medications reach the right patients at the right time will require sustained, intentional strategies to mitigate social determinants of health. For example, clustering prescriptions to eliminate multiple monthly pharmacy trips, automating prescriptions as 3-month supplies, and direct mailing can reduce the burden on patients working atypical hours or without reliable transportation. Community-based participatory research further allows key decision makers to understand the patient’s perspective directly and solicit ideas for innovation that will have the highest likelihood of positively affecting the community. Collaboration outside of traditional university-based academia to include a focus on community clinics caring for the most underserved populations will also likely engender more meaningful outcomes.
Conclusion
SGLT2i represents a key pharmacotherapy for improving patient outcomes and yet remains suboptimally implemented in the United States. Implementation and systems science innovations with specific attention to promoting health equity, forming multidisciplinary care teams, and combatting financial barriers are urgently needed to improve access to this life-saving therapy worldwide.
Disclosures
A. Verma reports being on the editorial board for Therapeutic Advances in Endocrinology and Metabolism, Frontiers in Medicine, and Therapeutic Advances in Chronic Disease and is a contributor to BMJ Best Practices. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
The content of this article reflects the personal experience and views of the authors and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the authors.
Author Contributions
S. Claudel was responsible for visualization; S. Claudel and A. Verma were responsible for conceptualization and wrote the original draft of the manuscript; I. Schmidt and A. Verma were responsible for supervision; and all authors were responsible for reviewing and editing the manuscript.
References
- 1.Koye DN, Magliano DJ, Nelson RG, Pavkov ME: The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis 25: 121–132, 2018. 10.1053/j.ackd.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu CD, McCulloch CE, Banerjee T, Pavkov ME, Burrows NR, Gillespie BW, Saran R, Shlipak MG, Powe NR, Tuot DS; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team : CKD awareness among US adults by future risk of kidney failure. Am J Kidney Dis 76: 174–183, 2020. 10.1053/j.ajkd.2020.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam D, Shaikh A: Real-life prescribing of SGLT2 inhibitors: How to handle the other medications, including glucose-lowering drugs and diuretics. Kidney360 2: 742–746, 2021. 10.34067/KID.0000412021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eberly LA, Yang L, Eneanya ND, Essien U, Julien H, Nathan AS, Khatana SAM, Dayoub EJ, Fanaroff AC, Giri J, Groeneveld PW, Adusumalli S: Association of race/ethnicity, gender, and socioeconomic status with sodium-glucose cotransporter 2 inhibitor use among patients with diabetes in the US. JAMA Netw Open 4: e216139, 2021. 10.1001/jamanetworkopen.2021.6139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Y, Peterson E, Pagidipati N: Barriers to prescribing glucose-lowering therapies with cardiometabolic benefits. Am Heart J 224: 47–53, 2020. 10.1016/j.ahj.2020.03.017 [DOI] [PubMed] [Google Scholar]
- 6.Triantafylidis LK, Hawley CE, Fagbote C, Li J, Genovese N, Paik JM: A pilot study embedding clinical pharmacists within an interprofessional nephrology clinic for the initiation and monitoring of empagliflozin in diabetic kidney disease. J Pharm Pract 34: 428–437, 2021. 10.1177/0897190019876499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker K, Bull-Engelstad I, Benth JS, Aasebø W, von der Lippe N, Reier-Nilsen M, Os I, Stavem K: Effectiveness of using STOPP/START criteria to identify potentially inappropriate medication in people aged ≥65 years with chronic kidney disease: A randomized clinical trial. Eur J Clin Pharmacol 75: 1503–1511, 2019. 10.1007/s00228-019-02727-9 [DOI] [PubMed] [Google Scholar]
- 8.Chu CD, Lamprea-montealegre JA, Estrella MM: Too many for too few: Finding appropriate nephrology referrals for patients with CKD that optimize outcomes. Am J Kidney Dis 79: 330–332, 2022. 10.1053/j.ajkd.2021.09.020 [DOI] [PubMed] [Google Scholar]
- 9.Luo J, Feldman R, Rothenberger SD, Hernandez I, Gellad WF: Coverage, formulary restrictions, and out-of-pocket costs for sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists in the Medicare Part D Program. JAMA Netw Open 3: e2020969, 2020. 10.1001/jamanetworkopen.2020.20969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nundy S, Cooper LA, Mate KS: The quintuple aim for health care improvement: A new imperative to advance health equity. JAMA 327: 521–522, 2022. 10.1001/jama.2021.25181 [DOI] [PubMed] [Google Scholar]