Key Points
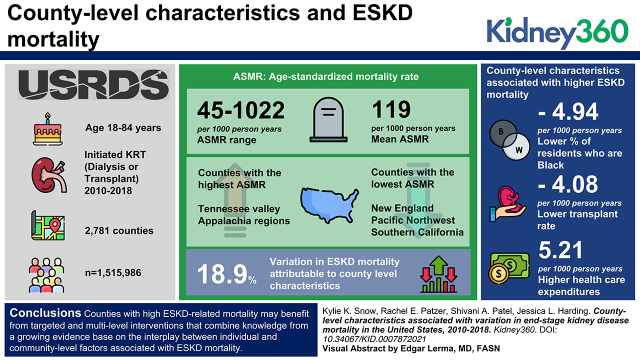
There is substantial variation in county-level ESKD mortality across the United States, with highest rates seen in the Southeastern United States.
County characteristics explain approximately 19% of variation in ESKD mortality.
Counties with high ESKD-related mortality may benefit from targeted and multilevel interventions.
Keywords: clinical nephrology, disparity, end stage kidney disease, epidemiology and outcomes, kidney disease, mortality, neighborhood, risk factors, socioeconomic, United States
Visual Abstract
Abstract
Background
Geographic and neighborhood-level factors, such as poverty and education, have been associated with an increased risk for incident ESKD, likelihood of receiving pre-ESKD care, and likelihood of receiving a transplant. However, few studies have examined whether these same factors are associated with ESKD mortality. In this study, we examined county-level variation in ESKD mortality and identified county-level characteristics associated with this variation.
Methods
We identified 1,515,986 individuals (aged 18–84 years) initiating RRT (dialysis or transplant) between 2010 and 2018 using the United States Renal Data System. Among 2781 counties, we estimated county-level, all-cause, age-standardized mortality rates (ASMR) among patients with ESKD. We then identified county-level demographic (e.g., percent female), socioeconomic (e.g., percent unemployed), healthcare (e.g., percent without health insurance), and health behavior (e.g., percent current smokers) characteristics associated with ASMR using multivariable hierarchic linear mixed models and quantified the percentage of ASMR variation explained by county-level characteristics.
Results
County-level ESKD ASMR ranged from 45 to 1022 per 1000 person-years (PY) (mean, 119 per 1000 PY). ASMRs were highest in counties located in the Tennessee Valley and Appalachia regions, and lowest in counties located in New England, the Pacific Northwest, and Southern California. In fully adjusted models, county-level characteristics significantly associated with higher ESKD mortality included a lower percentage of Black residents (−4.94 per 1000 PY), lower transplant rate (−4.08 per 1000 PY), and higher healthcare expenditures (5.21 per 1000 PY). Overall, county-level characteristics explained 19% of variation in ESKD mortality.
Conclusions
Counties with high ESKD-related mortality may benefit from targeted and multilevel interventions that combine knowledge from a growing evidence base on the interplay between individual and community-level factors associated with ESKD mortality.
Introduction
In 2018, >785,000 people in the United States were receiving RRT for the treatment of ESKD (1). Once diagnosed with ESKD, the mortality risk is high. For example, among patients on hemodialysis, 5-year survival is only 41%, but this increases to 85% for those who receive a kidney transplant (1). ESKD mortality risk is not uniform across the population. A large body of literature has identified several individual-level sociodemographic risk factors for ESKD mortality, with older (versus younger) age (1), female (versus male) sex (2,3), White (versus Black) race (1,4), and lower (versus higher) socioeconomic (5) groups having higher mortality rates. Patients with multiple comorbidities (versus few comorbidities) (6), and those with diabetes-related ESKD (versus other causes) also have higher mortality (7). In contrast, far fewer studies have examined the association between community-level factors and ESKD mortality.
Community- or neighborhood-level factors have previously been associated with an increased risk for several chronic diseases. For example, living in a neighborhood with health-promoting resources (i.e., infrastructure or built environment) is associated with a reduced risk of cardiovascular disease (CVD) (8). In the context of ESKD, where a person lives has previously been linked to the risk of ESKD incidence (9–11), likelihood of receiving pre-ESKD care (12,13), and likelihood of receiving a transplant (14–17). Neighborhood-level factors, such as poverty and education, have also been associated with ESKD incidence and kidney transplant access (11,16,17). Whether these same patterns are seen for ESKD mortality remains unknown. Given the complex interplay between individuals and the neighborhoods in which we live, learn, and play, identifying community-level risk factors at each stage of disease progression (e.g., pre-ESKD, ESKD incidence, and ESKD mortality) are needed to identify areas of highest risk for aggressive intervention and may also inform the development of multilevel interventions to reduce the ESKD burden.
Using a national registry of patients with ESKD receiving RRT, we (1) examined county- and state-level variation in all-cause mortality among people with ESKD, (2) identified county-level factors associated with this mortality risk, and (3) estimated the proportion of variance in ESKD mortality explained by these factors.
Materials and Methods
Study Design and Data Sources
This is an ecologic, cross-sectional study examining the association between county-level characteristics and county-level all-cause mortality among people with ESKD (herein referred to as ESKD mortality). Counties were defined using Federal Information Processing System (FIPS) codes. County-level ESKD mortality was estimated from the United States Renal Data System (USRDS), a national registry of all US patients with ESKD initiating RRT (1), detailed below. Data on several county-level characteristics were ascertained from several sources, including the Census Population Estimates, American Community Survey, Small Area Income and Poverty Estimates, Bureau of Labor Statistics, American Medical Association, Small Area Health Insurance Estimates, Centers for Medicare and Medicaid Services (CMS), Environmental Public Health Tracking Network, Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry, Uniform Crime Reporting Program, Federal Bureau of Investigation, National Center for Health Statistics, Dialysis Facility Compare, USRDS, Business Analyst, DeLorme map data, Esri, US Census TIGER/Line File, US Department of Agriculture Food Environment Atlas and Map the Meal Gap from Feeding America, and Behavioral Risk Factor and Surveillance System (Supplemental Table 1). Our study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for cross-sectional studies (Supplementary Table 2). Use of USRDS data was approved by the Emory University Institutional Review Board (IRB00063645). All other data are publicly available and thus exempt from institutional review board approval.
ESKD Population
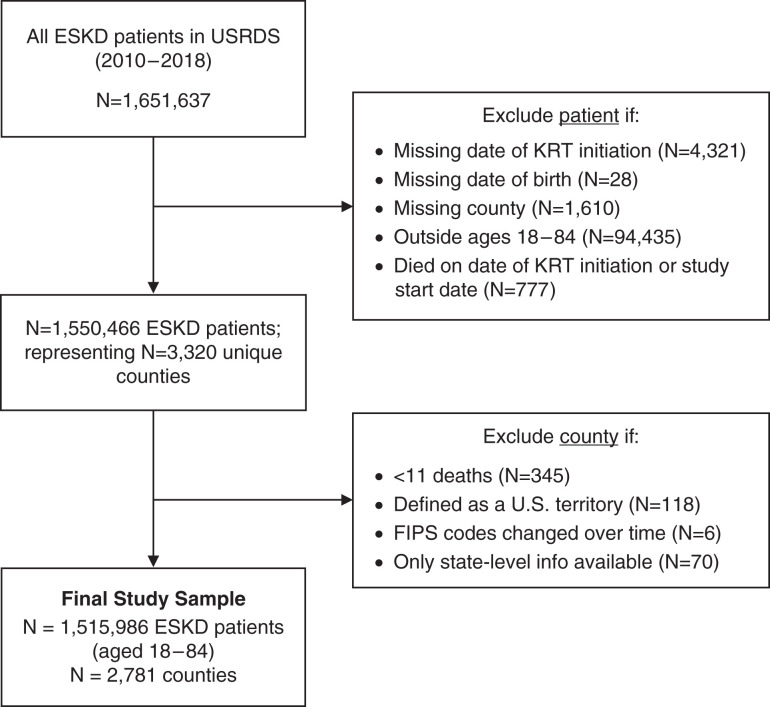
To determine county-level ESKD mortality, we identified all prevalent patients with ESKD between January 1, 2010, and August 23, 2018 from the USRDS. We excluded patients with missing information on date of RRT initiation (n=4321), date of birth (n=28), county (n=1610), and those who died on the same day as RRT initiation or study start (n=777). We also excluded counties with <11 deaths (n=345), counties defined as US territories, and counties whose FIPS code changed over time or those with noncounty-specific FIPS codes (n=194). The final ESKD-related mortality estimates were determined on the basis of 1,515,986 patients, aged 18–84 years, residing across 2781 counties (Figure 1).
Figure 1.
Flowchart of final ESKD study cohort (n=1,515,986 patients and n=2781 counties). FIPS, Federal Information Processing System; KRT, kidney replacement therapy; USRDS, United States Renal Data System.
ESKD Mortality
The county-level, all-cause ESKD, age-standardized mortality rate (ASMR) was defined as the number of deaths per 1000 person-years (PY) per county among patients with ESKD; the ASMR was age standardized to the 2000 US population (18) using age groups (18–44, 45–64, 65–74, and 75–84 years) and the direct method of standardization. Mortality data in the USRDS were augmented by Social Security Death Master File data to the extent allowed by regulation. Further, universal reporting of the death of patients with ESKD, and date of death, is required via CMS form 2746 as a condition of coverage for dialysis units and transplant centers.
County-Level Characteristics
County-level characteristics are defined in Supplemental Table 1. In brief, we considered four sets of characteristics broadly characterized as demographic, socioeconomic, healthcare, and health behavior characteristics. County-level characteristics included in models were chosen on the basis of a priori knowledge of factors associated with ESKD (2,4,5,9,11) and other similar studies of CVD (19) and diabetes (20). For each variable, we included the most contemporaneously available data.
Statistical Analyses
In descriptive analyses, county-level characteristics were described using mean±SD. County-level characteristics were standardized to have a mean of zero and SD of one to compare associations of county characteristics with ASMR and quantify the correlation between each county characteristic and ASMR. Because all independent variables were standardized, reported coefficients represent the average change in ESKD deaths per 1000 PY for each 1-SD increase in a county characteristic. To avoid collinearity, when two similar characteristics had a correlation r of >0.8 and variance inflation factors of more than five, the variable with the smaller variance inflation factor was selected to include in regression models to avoid collinearity (21). Given this, in final models, percent not proficient in English was not included in the final model because it correlated highly with percent foreign born and percent Hispanic.
We used hierarchic linear mixed models to estimate the association between county-level characteristics and ASMR, in which counties were the level-1 unit and states were the level-2 unit with a random intercept to eliminate the effect of state-level features that influence mortality and to account for clustering within states.
We modeled county-level ESKD ASMRs using four sequential linear regression models with county-level characteristics, so that model 1 includes demographic characteristics, model 2 includes model 1 characteristics plus socioeconomic characteristics, model 3 includes model 2 characteristics plus healthcare characteristics, and model 4 includes model 3 characteristics plus health behavior characteristics. Owing to the small number of missing data, multivariable models include counties with complete data only (i.e., 136 counties were missing violent crime data, one county was missing social vulnerability index data, two counties were missing injury death data, and 19 counties were missing food environment index data). All other counties had complete data. Coefficients in the final model are interpreted as the association between each county-level characteristic and ASMR, adjusting for all other county-level characteristics. We applied a Bonferroni correction to adjust for multiple testing and calculated the proportion of variance in county-level ASMR explained by each set of county characteristics. The final marginal variance modeled is the percentage of variation in ESKD mortality explained by the contribution of the county-level characteristics, including only the fixed effects.
To examine state-level variation in ASMR, we estimated the random effect for each state in both a null and fully adjusted model, including all noncolinear county-level characteristics. By comparing the random intercepts, we explored the extent to which county-level characteristics accounted for state-level variation in county-level ASMR. In the null model, the random intercepts represent state deviation from the national mean county-level ASMR. In fully adjusted models, the random intercepts represent state deviation from the national mean county-level ESKD-related ASMR that is not explained by other county-level characteristics. All analyses were conducted using R software (version 4.0.2).
Results
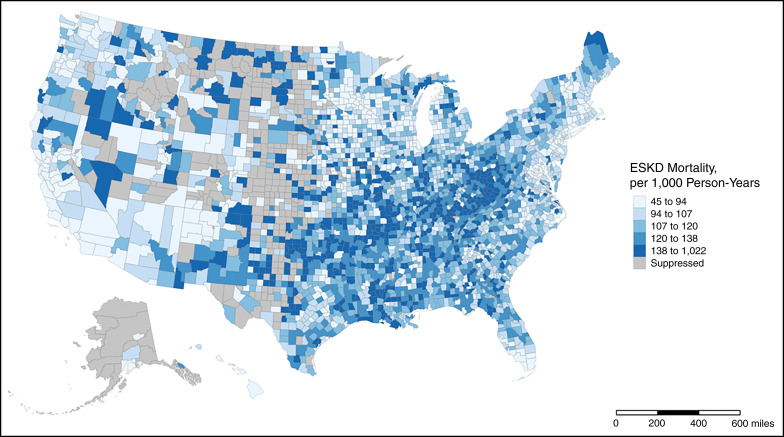
Among 2781 counties (representing 1,515,986 patients with ESKD), the average (SD) ESKD ASMR was 119 (38.4) per 1000 PY, and ranged from 45 to 1022 per 1000 PY (Figure 2). In general, higher ASMRs were seen among counties located in the Appalachia region, the Tennessee Valley, and the South (including Arkansas, Oklahoma, and Louisiana), whereas lower ASMRs were seen in counties located in New England, the Upper Midwest, and along the West Coast (in Washington state and Southern California).
Figure 2.
Age-standardized ESKD mortality rates across 2781 counties in the United States, 2010–2018. Higher ASMRs were seen among counties located in the Appalachia region, the Tennessee Valley, and the South. Mapped rates were obtained from the USRDS. Rates for counties with <11 deaths were suppressed. Alaska and Hawaii have been shifted and are not to scale.
Table 1 shows the distribution of the demographic, socioeconomic, healthcare, and health behavior characteristics among 2781 counties. On average, the county-level proportions of people aged >65 years, Black residents, and people living in a rural area were 19%, 10%, and 55%, respectively. The average county-level proportions of people unemployed, of low education, and without health insurance were 4%, 14%, and 11%, respectively. In addition, in the average county, 64% and 18% of residents did not have access to exercise opportunities and smoked, respectively.
Table 1.
Summary of United States county-level characteristics among 2781 counties included in this analysis, 2010–2018
| County Characteristic | Mean (SD)a | Range |
|---|---|---|
| Demographic | ||
| Population size, N (in thousands) | 117.0 (352.4) | 1.6–10,105.5 |
| Female, % | 50 (2) | 32–57 |
| Aged ≥65 yr, % | 19 (4) | 5–58 |
| Hispanic, % | 10 (14) | 0.6–96 |
| Asian American, % | 2 (3) | 0–43 |
| Black, % | 10 (15) | 0.1–85 |
| Foreign born, % | 5 (6) | 0–53 |
| Not proficient in English, % | 2 (3) | 0.0–30 |
| Rural, % | 55 (30) | 0–100 |
| Environmental and social | ||
| Income level, median ($ in thousands) | 52.8 (14.2) | 25.4–140.4 |
| Unemployed, % | 4 (1) | 2–18 |
| Without high school education, % | 14 (6) | 1–49 |
| In poverty, % | 15 (6) | 3–48 |
| Air pollution, µg/m3b | 9.3 (1.8) | 3.0–19.7 |
| Social vulnerability indexc | 0.53 (0.28) | 0.0–1.0 |
| Violent crime, (per 100,000)d | 263.9 (193.6) | 0.0–1,819.5 |
| Injury deaths, N (per 100,000)e | 85.4 (23.7) | 26.8–224.4 |
| Healthcare | ||
| Primary care physicians, N (per 100,000) | 52.6 (33.8) | 0.0–559.2 |
| Internal medicine subspecialists, N (per 100,000) | 6.6 (14.9) | 0.0–350.9 |
| Without health insurance, % | 11 (5) | 2–34 |
| Healthcare expenditures, $ in thousandsf | 10.4 (1.4) | 5.5–18.4 |
| Dialysis facilities, N (per 100,000) | 2.5 (3.0) | 0.0–28.1 |
| Transplant recipients, N (per 1000 person years)g | 34.1 (20.7) | 0.0–191.7 |
| Health behaviors | ||
| Access to exercise opportunities, %h | 64 (22) | 0–100 |
| Food environment indexi | 7.5 (1.1) | 0.0–10.0 |
| Current smoking, % | 18 (4) | 6–42 |
| Excessive drinking, % | 17 (3) | 8–29 |
Mean and SD of counts, proportions, medians, or indices, as appropriate.
Average daily density of fine particulate matter in micrograms per cubic meter; nine counties were missing data.
Percentile ranking of relative vulnerability, ranging from zero to one with higher values indicating greater vulnerability; one county was missing data.
Data were missing for 136 counties.
Two counties were missing data.
County-level price-, age-, sex-, race-, and ethnicity-adjusted Medicare expenditures per enrollee, in thousands of dollars.
Transplant was defined as receiving at least one transplant at any time during follow-up, and determined as N transplants per 1000 person-years of follow-up, censored for death.
Percent of the county population residing within 0.5 mile (0.8 km) of a park, or within 1 mile (1.6 km) (urban) or 3 miles (4.8 km) (rural) of a recreational facility.
Food environment index, a composite score (ranging from zero [worst] to 10 [best]) describing limits on access to healthy foods and food insecurity; 19 counties were missing data.
In unadjusted models, all county-level characteristics were significantly associated with ESKD-related mortality. Of note, the county-level characteristics most strongly and positively associated with ASMR were a higher percentage living in a rural area (10.46 per 1000 PY) and a higher percentage of current smokers (9.89 per 1000 PY), whereas the factors most strongly and negatively associated with ASMR included a higher median income level (−10.50 per 1000 PY) and higher percentage of excessive alcohol usage (−9.47 per 1000 PY) (Table 2).
Table 2.
Associations of United States county-level characteristics with ESKD mortality, 2010–2018
| County Characteristic | Coefficient (95% Confidence Interval)a | ||||
|---|---|---|---|---|---|
| Unadjusted Associationsb | Multivariable Adjusted Associationsc | ||||
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| Demographic | |||||
| Population size, N (in thousands) | −5.19 (−6.61 to −3.78)d | 0.08 (−2.18 to 2.33) | 0.24 (−2.26 to 2.71) | −0.63 (−3.21 to 1.92) | −0.51 (−3.15 to 2.09) |
| Female, % | −2.03 (−3.43 to −0.63)d | −0.31 (−2.31 to 1.69) | 0.21 (−2.06 to 2.48) | 0.72 (−1.63 to 3.08) | 0.32 (−2.17 to 2.79) |
| Aged ≥65 yr, % | 7.02 (5.62 to 8.41)d | 1.56 (−0.78 to 3.87) | −0.84 (−3.72 to 2.03) | −0.10 (−3.08 to 2.84) | −0.13 (−3.54 to 3.24) |
| Hispanic, % | −5.76 (−7.66 to −3.86)d | 0.69 (−3.03 to 4.41) | −3.51 (−7.94 to 0.91) | −3.37 (−7.77 to 1.05) | −2.73 (−7.43 to 2.00) |
| Asian American, % | −7.63 (−9.13 to −6.13)d | −1.24 (−4.25 to 1.75) | 1.07 (−2.85 to 4.99) | 1.22 (−2.82 to 5.29) | 1.09 (−3.06 to 5.32) |
| Black, % | −4.61 (−6.49 to −2.70)d | −2.33 (−4.89 to 0.24) | −5.21 (−8.73 to −1.67)d | −4.63 (−8.24 to −1.05)d | −4.94 (−8.97 to −0.93)d |
| Foreign born, % | −9.27 (−10.85 to −7.70)d | −4.18 (−8.26 to −0.10)d | −2.84 (−7.67 to 1.97) | −2.46 (−7.61 to 2.63) | −2.17 (−7.51 to 3.09) |
| Rural, % | 10.46 (9.07 to 11.86)d | 7.17 (4.63 to 9.73)d | 4.29 (1.08 to 7.51)d | 2.92 (−0.58 to 6.39) | 3.00 (−1.04 to 6.98) |
| Economic/social | |||||
| Income level, median ($ in thousands) | −10.50 (−12.00 to −9.01)d | −6.07 (−11.13 to −0.99)d | −5.19 (−10.35 to 0.00) | −5.02 (−10.39 to 0.42) | |
| Unemployed, % | 4.71 (3.08 to 6.33)d | −0.74 (−3.72 to 2.24) | −1.12 (−4.18 to 1.95) | −1.44 (−4.65 to 1.79) | |
| Without high school education, % | 6.09 (4.45 to 7.75)d | 3.98 (−0.36 to 8.38) | 2.62 (−1.90 to 7.16) | 2.23 (−2.59 to 7.02) | |
| Poverty, % | 6.65 (5.08 to 8.24)d | 0.93 (−4.07 to 5.96) | 0.97 (−4.17 to 6.18) | −0.15 (−5.73 to 5.52) | |
| Air pollution, µg/m3e | −6.30 (−8.59 to −3.95)d | −0.34 (−3.65 to 2.98) | −2.37 (−5.66 to 0.94) | −2.54 (−5.87 to 0.83) | |
| Social vulnerability indexf | 4.07 (2.41 to 5.74)d | −1.01 (−5.70 to 3.67) | −1.52 (−6.41 to 3.28) | −2.07 (−7.12 to 2.87) | |
| Violent crime, N (per 100,000)g | −3.54 (−5.12 to −1.96)d | −0.70 (−3.53 to 2.13) | −0.54 (−3.41 to 2.35) | −0.45 (−3.38 to 2.52) | |
| Injury deaths, N (per 100,000)h | 9.10 (7.64 to 10.57)d | 3.57 (0.68 to 6.48)d | 2.86 (−0.09 to 5.85) | 2.58 (−0.51 to 5.67) | |
| Healthcare | |||||
| Primary care physicians, N (per 100,000) | −6.93 (−8.35 to −5.53)d | −2.15 (−5.52 to 1.19) | −1.94 (−5.42 to 1.51) | ||
| Internal medicine subspecialists, N (per 100,000) | −5.37 (−6.76 to −4.00)d | 0.77 (−2.23 to 3.76) | 0.74 (−2.32 to 3.79) | ||
| Without health insurance, % | 7.57 (4.98 to 10.14)d | 0.05 (−4.74 to 4.75) | −0.50 (−5.40 to 4.37) | ||
| Healthcare expenditures, $ in thousandsi | 7.08 (5.31 to 8.88)d | 5.31 (2.46 to 8.22)d | 5.21 (2.30 to 8.19)d | ||
| Dialysis facilities, N (per 100,000) | −2.47 (−3.85 to −1.09)d | −2.19 (−4.51 to 0.12) | −2.23 (−4.59 to 0.13) | ||
| Transplant recipients, N (per 1000 PY)j | −6.92 (−8.49 to −5.35)d | −4.17 (−6.80 to −1.59)d | −4.08 (−6.77 to −1.45)d | ||
| Health behaviors | |||||
| Access to exercise opportunities, % | −7.80 (−9.29 to −6.33)d | 0.12 (−3.11 to 3.30) | |||
| Food environment indexk | −3.96 (−5.57 to −2.36)d | −0.68 (−4.89 to 3.56) | |||
| Current smoking, % | 9.89 (8.06 to 11.74)d | 2.01 (−2.97 to 7.00) | |||
| Excessive drinking, % | −9.47 (−11.65 to −7.29)d | −1.81 (−6.09 to 2.48) | |||
| Marginal variance modeled, % l | 9 | 15 | 18 | 19 | |
Bonferroni correction was used to adjust for multiple testing. PY, person-years.
All independent variables were standardized, thus reported coefficients from linear regression models indicate the average change in county-level ESKD deaths per 1000 PY for each 1-SD increase in each county-level characteristic.
We modeled age-standardized, county-level ESKD mortality rates per 1000 PY using four sequential linear regression models with county-level characteristics, so that model 1 includes demographic characteristics, model 2 includes model 1 characteristics plus economic and social characteristics, model 3 includes model 2 characteristics plus healthcare characteristics, and model 4 includes model 3 characteristics plus health behavior characteristics.
Coefficients and 95% CIs with P<0.05.
Average daily density of fine particulate matter in micrograms per cubic meter; nine counties were missing data.
Percentile ranking of relative vulnerability, ranging from zero to one, with higher values indicating greater vulnerability; one county was missing data.
Data were missing for 136 counties.
Two counties were missing data.
County-level price-, age-, sex-, race-, and ethnicity-adjusted Medicare expenditures per enrollee, in thousands of dollars.
Transplant was defined as receiving at least one transplant at any time during follow-up, and determined as N transplants per 1000 PY of follow-up, censored for death.
Food environment index, a composite score (ranging from zero [worst] to ten [best]) describing limits on access to healthy foods and food insecurity; 19 counties were missing data.
The marginal variance modeled is the percentage of variation in ESKD mortality explained by the contribution of the county-level characteristics, including only the fixed effects.
In the fully adjusted model (i.e., model 4), a higher percentage of Black residents (−4.94 per 1000 PY) and a higher transplant rate (−4.08 per 1000 PY) was associated with lower ASMR, whereas higher healthcare expenditure (5.21 per 1000 PY), defined as county-level price-, age-, sex-, race-, and ethnicity-adjusted Medicare expenditures per enrollee, was associated with a higher ASMR (Table 2).
Overall, county-level characteristics explained 19% of the variation in county-level ESKD-related ASMR (Table 2). Model 1 (including demographic characteristics) explained 9% of the variation, model 2 (model 1 plus socioeconomic characteristics) explained 15%, model 3 (model 2 plus healthcare characteristics) explained 18%, and model 4 (model 3 plus health behavior characteristics) explained 19%.
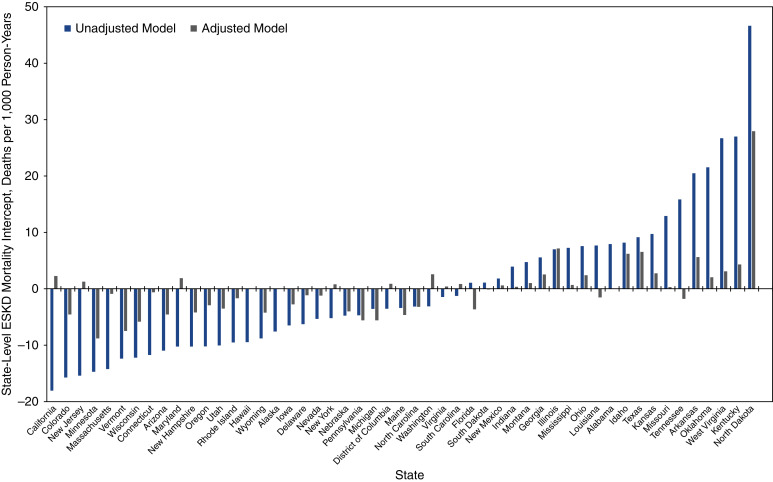
Figure 3 shows state-level variation in ESKD-related ASMRs and illustrates changes in state deviation from the national mean county-level ASMR in unadjusted and multivariable adjusted models. In unadjusted models, state-level deviations ranged from −18.1 per 1000 PY in California to 46.6 per 1000 PY in North Dakota. In fully adjusted models, state-level deviations ranged from −8.8 per 1000 PY in Minnesota to 27.9 per 1000 PY in North Dakota.
Figure 3.
State-level intercepts for age-standardized ESKD mortality rates before and after adjustment for county-level characteristics in 2781 counties in the United States, 2010–2018. The plot shows model intercepts in the unadjusted model (gray) and in the adjusted model (black).
Discussion
This is the first study to examine county-level variation in ESKD mortality, defined as all-cause mortality among people with ESKD, and has several key findings. First, there is substantial variation in county-level ESKD mortality across the United States, with a 20-fold difference in the county with the highest versus lowest ASMR. ESKD mortality rates were highest in counties in the Southern and Appalachian regions of the United States, and lowest in counties along the Northeast, West Coast, and the Upper Midwest. Second, in fully adjusted models, counties with a lower proportion of Black residents, lower transplant rate, and higher healthcare expenditures experienced higher ESKD mortality. Third, we found that county-level characteristics explained 19% of the variation in ESKD mortality. Finally, at the state level, adjusting for county-level characteristics reduced or eliminated state deviation from the national mean county-level ESKD ASMR, suggesting county-level characteristics, rather than state-level factors, accounted for much of the variation in ESKD mortality.
Our results are similar to previous studies of geographic variation in ESKD incidence, insofar as regions with high ESKD incidence also have high ESKD mortality. For example, a study using USRDS data between 1983 and 1988 identified clusters of high ESKD incidence in counties located in the Southwest and Southeast, whereas clusters of low ESKD incidence were found in counties located in the West and Northwest (9). A more recent study, using 2000–2008 data from CMS and the Organ Procurement and Transplantation Network, also showed that ESKD incidence was highest in the South and lowest in the West and Northwest regions (10). These findings, in general, reflect geographic patterns of key risk factors for ESKD, such as diabetes (22), chronic kidney disease (23), hypertension (24), and other chronic diseases, such as CVD, where the burden falls disproportionally in the Southern belt (25,26).
In this study, three factors were significantly associated with higher ESKD mortality rates in fully adjusted models. First, counties with a lower percentage of Black residents had higher ESKD mortality. This is in contrast to studies of ESKD incidence, in which regions with a higher proportion of Black residents had higher ESKD incidence (10), and several individual-level studies that demonstrate Black (versus White) people are more likely to develop ESKD and at a faster rate (27). In individual-level studies of ESKD mortality, however, Black patients with ESKD have a lower mortality rate relative to White patients with ESKD (28,29), consistent with county-level findings in this study. This so-called survival paradox, observed primarily in adults aged >50 years (30), has been previously described, and reasons postulated include the younger age at which Black patients develop ESKD compared with White patients (31); a survival bias among Black patients with ESKD who make it to RRT and are thus captured in registries like the USRDS (28); lower transplant rates in Black patients that artificially inflate the survival advantage of those on dialysis and transplant recipients, because they are a highly select population (1,32); and higher body mass index in Black adults (versus white adults), mirroring the well-known obesity paradox whereby higher body mass indexes tend to have lower mortality rates (31). Although previous literature has examined this survival paradox at the individual level, our results imply it may also hold true at the county-level.
Second, we show that higher healthcare expenditure was associated with higher ESKD mortality. In this study, healthcare expenditure was defined as the average Medicare spending per enrollee in each county. All patients with ESKD are eligible for Medicare coverage and thus a higher county-level Medicare expenditure may simply reflect a higher disease burden for individuals in the county. Further, increased healthcare expenditure does not necessarily indicate higher quality care or better access to care, but may rather indicate regional differences in patterns of practice, such as more frequent physician visits, greater use of medical subspecialists, and more discretionary care (33,34). This pattern has been demonstrated in other studies in which increased Medicare spending was associated with increased mortality for patients with diabetes who had foot ulcers and amputations (35), and for patients with colorectal cancer (36).
Finally, a lower rate of transplants per 100,000 PY in each county was associated with higher ESKD mortality. Interestingly, the number of internal medicine subspecialists per 100,000 population, a proxy for concentration of nephrologists, was not associated with ESKD mortality, suggesting that access (via total transplants), rather than quality of care (as per number of specialists), may be a more important factor for ESKD mortality. Healthcare access likely serves as a proxy for several other structural factors, such as racism, socioeconomic status, access to healthy foods, air quality, safety, and social connections (37), despite our best attempts to adjust for several of these factors. Reducing ESKD mortality disparities across counties will likely require additional policies aimed at improving the socioeconomic circumstances of disadvantaged counties, including reducing barriers in access to kidney care. For example, accumulating evidence has shown that multicomponent education and quality improvement interventions at the transplant program– and dialysis facility–level can improve transplant access (38–41). Prioritizing counties with high ESKD mortality may be a future priority for these programs.
In our study, we show that approximately 19% of the county-level variation in ESKD mortality is explained by county-level characteristics. In contrast to studies of other chronic diseases, this is a relatively smaller contribution. For example, approximately 75% (19) and approximately 42% (20) of the variation in CVD mortality and diabetes incidence, respectively, has previously been shown to be explained by county-level factors. In these studies, the largest contributors to variation in CVD mortality were county-level median income and education (19), whereas variation in diabetes incidence was driven predominately by unemployment, poverty, and access to exercise opportunities and healthy foods (20). The relatively smaller contribution of county-level characteristics to the variation in ESKD mortality may be explained by the fact that ESKD, compared with CVD and diabetes, is relatively rare, rendering prediction of variation difficult. For example, 48% (42) and 11% (43) of the US population has CVD and diabetes, respectively, as compared with just 0.2% of the population with ESKD (1). It is also possible that county-level factors play a more important role in upstream causes of chronic disease. Diabetes, for example, is an important risk factor for ESKD incidence (44), and county-level interventions to reduce diabetes incidence, such as improved access to exercise and healthy foods, may have downstream implications for ESKD incidence and mortality. Regardless, data on county-level ESKD mortality are useful to identify counties with high ESKD mortality that may benefit most from targeted multilevel interventions (i.e., those that address both individual- and county-level risk factors).
The key strengths of this study include the use of a large national registry of patients with ESKD receiving RRT to estimate county-level ESKD mortality rates in the United States, and the use of aggregated data from multiple sources to describe the effect of several county-level characteristics. However, there are several limitations that should also be considered. First, this is an ecologic study and results cannot be extrapolated to the individual level. Second, the use of county averages, as was done in this study, does not account for variations within counties. Data at levels lower than the county (i.e., census tracts) are not available in USRDS. Third, to ensure adequate power to examine county-level ESKD mortality, a rare outcome, we pooled ESKD mortality rates between 2010 and 2018, which may not reflect the most contemporary ESKD mortality rates. Regardless, we expect relative differences between counties to have remained relatively stable in this 9-year period. Last, there is likely to be residual confounding by county-level characteristics not included in this analysis.
In this national United States study, we demonstrate substantial variation in ESKD mortality across counties, and report that approximately 19% of this variation is explained by county-level characteristics. Future studies are needed to elucidate the mechanisms in which county-level factors affect variation in ESKD mortality. In the interim, interventions to reduce ESKD mortality could be targeted to counties with high ESKD mortality. Such interventions should combine knowledge from a growing evidence base on the individual- and county-level factors associated with ESKD mortality and its upstream causes.
Disclosures
J.L. Harding reports having an advisory or leadership role with Diabetes Care and JASN, and receiving honoraria from The Lancet Diabetes & Endocrinology and the University of Western Australia. S.A. Patel reports receiving research funding from Johnson and Johnson. R.E. Patzer reports having an advisory or leadership role on the editorial boards of the American Journal of Transplantation and CJASN, and as chair of the United Network for Organ Sharing Data Advisory Board. K.K. Snow reports being currently employed by Booz Allen Hamilton.
Funding
This project and The Reducing Disparities in Access to Kidney Transplantation Regional Study was funded, in part, by the National Institute on Minority Health and Health Disparities award U01MD010611. Support for the preparation of this document was funded by CMS (an agency of the US Department of Health and Human Services) ESRD Network 6 contract HHSM-500-2013-NW006C.
Acknowledgments
The authors would like to thank Zhensheng Wang, for his statistical support.
The data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Author Contributions
J.L. Harding conceptualized the study; J.L. Harding, R.E. Patzer, and K.K. Snow were responsible for methodology; S.A. Patel was responsible for investigation; K.K. Snow wrote the original draft and was responsible for data curation and formal analysis; and all authors reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0007872021/-/DCSupplemental.
Definitions of county-level characteristics and data sources. Download Supplemental Table 1, PDF file, 201 KB (200.1KB, pdf)
STROBE Statement—checklist of items that should be included in reports of observational studies. Download Supplemental Table 2, PDF file, 201 KB (200.1KB, pdf)
References
- 1.United States Renal Data System : 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2020 [Google Scholar]
- 2.Villar E, Remontet L, Labeeuw M, Ecochard R: Effect of age, gender, and diabetes on excess death in end-stage renal failure. J Am Soc Nephrol 18: 2125–2134, 2007. 10.1681/ASN.2006091048 [DOI] [PubMed] [Google Scholar]
- 3.Jindal RM, Ryan JJ, Sajjad I, Murthy MH, Baines LS: Kidney transplantation and gender disparity. Am J Nephrol 25: 474–483, 2005. 10.1159/000087920 [DOI] [PubMed] [Google Scholar]
- 4.Rostand SG, Kirk KA, Rutsky EA, Pate BA: Racial differences in the incidence of treatment for end-stage renal disease. N Engl J Med 306: 1276–1279, 1982. 10.1056/NEJM198205273062106 [DOI] [PubMed] [Google Scholar]
- 5.Tao S, Zeng X, Liu J, Fu P: Socioeconomic status and mortality among dialysis patients: a systematic review and meta-analysis. Int Urol Nephrol 51: 509–518, 2019. 10.1007/s11255-019-02078-5 [DOI] [PubMed] [Google Scholar]
- 6.Beddhu S, Zeidel ML, Saul M, Seddon P, Samore MH, Stoddard GJ, Bruns FJ: The effects of comorbid conditions on the outcomes of patients undergoing peritoneal dialysis. Am J Med 112: 696–701, 2002. 10.1016/S0002-9343(02)01097-5 [DOI] [PubMed] [Google Scholar]
- 7.Batista PB, Lopes AA, Costa FA: Association between attributed cause of end-stage renal disease and risk of death in Brazilian patients receiving renal replacement therapy. Ren Fail 27: 651–656, 2005. 10.1080/08860220500234832 [DOI] [PubMed] [Google Scholar]
- 8.Unger E, Diez-Roux AV, Lloyd-Jones DM, Mujahid MS, Nettleton JA, Bertoni A, Badon SE, Ning H, Allen NB: Association of neighborhood characteristics with cardiovascular health in the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes 7: 524–531, 2014. 10.1161/CIRCOUTCOMES.113.000698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foxman B, Moulton LH, Wolfe RA, Guire KE, Port FK, Hawthorne VM: Geographic variation in the incidence of treated end-stage renal disease. J Am Soc Nephrol 2: 1144–1152, 1991. 10.1681/ASN.V261144 [DOI] [PubMed] [Google Scholar]
- 10.Mathur AK, Ashby VB, Sands RL, Wolfe RA: Geographic variation in end-stage renal disease incidence and access to deceased donor kidney transplantation. Am J Transplant 10: 1069–1080, 2010. 10.1111/j.1600-6143.2010.03043.x [DOI] [PubMed] [Google Scholar]
- 11.Volkova N, McClellan W, Klein M, Flanders D, Kleinbaum D, Soucie JM, Presley R: Neighborhood poverty and racial differences in ESRD incidence. J Am Soc Nephrol 19: 356–364, 2008. 10.1681/ASN.2006080934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClellan WM, Wasse H, McClellan AC, Kipp A, Waller LA, Rocco MV: Treatment center and geographic variability in pre-ESRD care associate with increased mortality. J Am Soc Nephrol 20: 1078–1085, 2009. 10.1681/ASN.2008060624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao H, Lovasik BP, Pastan SO, Chang HH, Chowdhury R, Patzer RE: Geographic variation and neighborhood factors are associated with low rates of pre-end-stage renal disease nephrology care. Kidney Int 88: 614–621, 2015. 10.1038/ki.2015.118 [DOI] [PubMed] [Google Scholar]
- 14.Ashby VB, Kalbfleisch JD, Wolfe RA, Lin MJ, Port FK, Leichtman AB: Geographic variability in access to primary kidney transplantation in the United States, 1996-2005. Am J Transplant 7: 1412–1423, 2007. 10.1111/j.1600-6143.2007.01785.x [DOI] [PubMed] [Google Scholar]
- 15.Axelrod DA, Guidinger MK, Finlayson S, Schaubel DE, Goodman DC, Chobanian M, Merion RM: Rates of solid-organ wait-listing, transplantation, and survival among residents of rural and urban areas. JAMA 299: 202–207, 2008. 10.1001/jama.2007.50 [DOI] [PubMed] [Google Scholar]
- 16.Schold JD, Heaphy ELG, Buccini LD, Poggio ED, Srinivas TR, Goldfarb DA, Flechner SM, Rodrigue JR, Thornton JD, Sehgal AR: Prominent impact of community risk factors on kidney transplant candidate processes and outcomes. Am J Transplant 13: 2374–2383, 2013. 10.1111/ajt.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plantinga L, Pastan S, Kramer M, McClellan A, Krisher J, Patzer RE: Association of U.S. Dialysis facility neighborhood characteristics with facility-level kidney transplantation. Am J Nephrol 40: 164–173, 2014. 10.1159/000365596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein RJ, Schoenborn CA: Age adjustment using the 2000 projected U.S. population. Healthy People 2010 Stat Notes (20): 1–10, 2001 [PubMed] [Google Scholar]
- 19.Patel SA, Ali MK, Narayan KMV, Mehta NK: County-level variation in cardiovascular disease mortality in the United States in 2009–2013: Comparative assessment of contributing factors. Am J Epidemiol 184: 933–942, 2016. 10.1093/aje/kww081 [DOI] [PubMed] [Google Scholar]
- 20.Cunningham SA, Patel SA, Beckles GL, Geiss LS, Mehta N, Xie H, Imperatore G: County-level contextual factors associated with diabetes incidence in the United States. Ann Epidemiol 28: 20–25.e2, 2018. 10.1016/j.annepidem.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vatcheva KP, Lee M, McCormick JB, Rahbar MH: Multicollinearity in regression analyses conducted in epidemiologic studies. Epidemiology (Sunnyvale) 6: 227, 2016. 10.4172/2161-1165.1000227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barker LE, Kirtland KA, Gregg EW, Geiss LS, Thompson TJ: Geographic distribution of diagnosed diabetes in the U.S.: A diabetes belt. Am J Prev Med 40: 434–439, 2011. 10.1016/j.amepre.2010.12.019 [DOI] [PubMed] [Google Scholar]
- 23.Tanner RM, Gutiérrez OM, Judd S, McClellan W, Bowling CB, Bradbury BD, Safford MM, Cushman M, Warnock D, Muntner P: Geographic variation in CKD prevalence and ESRD incidence in the United States: Results from the reasons for geographic and racial differences in stroke (REGARDS) study. Am J Kidney Dis 61: 395–403, 2013. 10.1053/j.ajkd.2012.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olives C, Myerson R, Mokdad AH, Murray CJL, Lim SS: Prevalence, awareness, treatment, and control of hypertension in United States counties, 2001–2009. PLoS One 8: e60308, 2013. 10.1371/journal.pone.0060308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howard VJ, Woolson RF, Egan BM, Nicholas JS, Adams RJ, Howard G, Lackland DT: Prevalence of hypertension by duration and age at exposure to the stroke belt. J Am Soc Hypertens 4: 32–41, 2010. 10.1016/j.jash.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howard G, Howard VJ: Twenty years of progress toward understanding the stroke belt. Stroke 51: 742–750, 2020. 10.1161/STROKEAHA.119.024155 [DOI] [PubMed] [Google Scholar]
- 27.Xue JL, Eggers PW, Agodoa LY, Foley RN, Collins AJ: Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged Medicare beneficiaries. J Am Soc Nephrol 18: 1299–1306, 2007. 10.1681/ASN.2006050524 [DOI] [PubMed] [Google Scholar]
- 28.Kalantar-Zadeh K, Kovesdy CP, Derose SF, Horwich TB, Fonarow GC: Racial and survival paradoxes in chronic kidney disease. Nat Clin Pract Nephrol 3: 493–506, 2007. 10.1038/ncpneph0570 [DOI] [PubMed] [Google Scholar]
- 29.Kovesdy CP, Quarles LD, Lott EH, Lu JL, Ma JZ, Molnar MZ, Kalantar-Zadeh K: Survival advantage in black versus white men with CKD: Effect of estimated GFR and case mix. Am J Kidney Dis 62: 228–235, 2013. 10.1053/j.ajkd.2012.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kucirka LM, Grams ME, Lessler J, Hall EC, James N, Massie AB, Montgomery RA, Segev DL: Age and racial disparities in dialysis survival. JAMA 306: 620–626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agodoa L, Eggers P: Racial and ethnic disparities in end-stage kidney failure-survival paradoxes in African-Americans. Semin Dial 20: 577–585, 2007. 10.1111/j.1525-139X.2007.00350.x [DOI] [PubMed] [Google Scholar]
- 32.Saunders MR, Lee H, Alexander GC, Tak HJ, Thistlethwaite JR Jr, Ross LF: Racial disparities in reaching the renal transplant waitlist: Is geography as important as race? Clin Transplant 29: 531–538, 2015. 10.1111/ctr.12547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baicker K, Chandra A: Medicare spending, the physician workforce, and beneficiaries’ quality of care. Health Aff (Millwood) 23: W4-184–97, 2004. 10.1377/hlthaff.W4.184 [DOI] [PubMed] [Google Scholar]
- 34.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL: The implications of regional variations in Medicare spending. Part 1: The content, quality, and accessibility of care. Ann Intern Med 138: 273–287, 2003. 10.7326/0003-4819-138-4-200302180-00006 [DOI] [PubMed] [Google Scholar]
- 35.Sargen MR, Hoffstad O, Margolis DJ: Geographic variation in Medicare spending and mortality for diabetic patients with foot ulcers and amputations. J Diabetes Complications 27: 128–133, 2013. 10.1016/j.jdiacomp.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landrum MB, Meara ER, Chandra A, Guadagnoli E, Keating NL: Is spending more always wasteful? The appropriateness of care and outcomes among colorectal cancer patients. Health Aff (Millwood) 27: 159–168, 2008. 10.1377/hlthaff.27.1.159 [DOI] [PubMed] [Google Scholar]
- 37.Diez Roux AV, Mair C: Neighborhoods and health. Ann N Y Acad Sci 1186: 125–145, 2010. 10.1111/j.1749-6632.2009.05333.x [DOI] [PubMed] [Google Scholar]
- 38.Hamoda RE, Gander JC, McPherson LJ, Arriola KJ, Cobb L, Pastan SO, Plantinga L, Browne T, Hartmann E, Mulloy L, Zayas C, Krisher J, Patzer RE: Process evaluation of the RaDIANT community study: A dialysis facility-level intervention to increase referral for kidney transplantation. BMC Nephrol 19: 13, 2018. 10.1186/s12882-017-0807-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patzer RE, Paul S, Plantinga L, Gander J, Sauls L, Krisher J, Mulloy LL, Gibney EM, Browne T, Zayas CF, McClellan WM, Arriola KJ, Pastan SO; Southeastern Kidney Transplant Coalition : A randomized trial to reduce disparities in referral for transplant evaluation. J Am Soc Nephrol 28: 935–942, 2017. 10.1681/ASN.2016030320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waterman AD, Peipert JD, Cui Y, Beaumont JL, Paiva A, Lipsey AF, Anderson CS, Robbins ML: Your Path to Transplant: A randomized controlled trial of a tailored expert system intervention to increase knowledge, attitudes, and pursuit of kidney transplant. Am J Transplant 21: 1186–1196, 2021. 10.1111/ajt.16262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weng FL, Davis LA, Ohman-Strickland PA, Waterman AD: Destination transplant: Protocol for a parallel-group randomized trial of an educational intervention to increase kidney transplant among black people on the transplant waiting list. Transplant Direct 7: e683, 2021. 10.1097/TXD.0000000000001136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee : Heart disease and stroke statistics—2019 update: A report from the American Heart Association [published correction appears in Circulation 141: e33, 2020 10.1161/CIR.0000000000000746]. Circulation 139: e56–e528, 2019 [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention : National Diabetes Statistics Report. Estimates of diabetes and its burden in the United States, 2020. Available at https://www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed April 26, 2021
- 44.Shen Y, Cai R, Sun J, Dong X, Huang R, Tian S, Wang S: Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: A systematic review and meta-analysis. Endocrine 55: 66–76, 2017. 10.1007/s12020-016-1014-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Definitions of county-level characteristics and data sources. Download Supplemental Table 1, PDF file, 201 KB (200.1KB, pdf)
STROBE Statement—checklist of items that should be included in reports of observational studies. Download Supplemental Table 2, PDF file, 201 KB (200.1KB, pdf)