Introduction
Immune checkpoint inhibitors (ICIs) are a promising new therapeutic class of drugs that has achieved remarkable progress among patients with hematologic and solid organ malignancies. They block the inhibitors of T lymphocytes, impair the survival of regulatory T cells, and thereby potentiate the immune system to fight against cancer cells. With increased use of ICIs, patients developing immune-related adverse events (irAEs) are proportionately rising. The overall incidence of ICI-related irAEs ranges from 60% to 85%; the skin, gastrointestinal tract, lungs, and liver are the most commonly affected organs (1). The incidence of kidney injury from irAEs is estimated to be 2%–5%, where the risk with monotherapy is around 2%, and combination therapy accounts for <5% (2,3). The true incidence is likely higher, given the lack of kidney biopsies in patients with mild AKI and the potential masking effects of steroids when used for treatment of other irAEs. Acute interstitial nephritis and acute tubular necrosis are the most common injury patterns associated with irAEs.
In the study by Cortazar et al. (4) including 138 patients with ICI-AKI, at a median of 14 weeks after ICI initiation, 43% experienced stage 2 AKI, 57% sustained stage 3 AKI, and 9% were dependent on RRT. Although complete recovery of ICI-AKI is noted among 40% of the patients and partial recovery among 45%, 15% had no recovery of kidney function. In the subgroup analysis of patients with ICI-AKI who required RRT, seven out of 13 (54%) had no recovery of renal function, indicating patients with ICI-AKI sustain a complicated clinical course, especially if they have RRT dependency. Additionally, steroids are the mainstay of treatment for ICI-associated irAEs and are frequently associated with multiple adverse effects, including fluid retention, weight gain, cushingoid features, and glucose intolerance (5,6).
On the basis of the grade and severity of irAEs, ICI therapies are typically held while battling with adverse effects. Rechallenging ICIs after a period of temporary discontinuation could potentially improve overall survival of patients with resistant cancers, although putting them at risk of recurrent irAEs. Among patients with severe grade 3 or higher kidney toxicity (SCr >3× baseline, or >4 mg/dl or need for RRT), the American Society of Clinical Oncology and the National Comprehensive Cancer Network recommended permanent discontinuation of ICIs (7–9). Among patients with lower-grade kidney toxicities, there is no clear consensus on appropriate timing of rechallenge, although some studies suggest holding ICI until the AKI episode is almost completely resolved (9,10).
Although no randomized controlled studies have explored this area, a few shed some light on the clinical course of patients who were rechallenged with ICI therapies. In a study by Isik et al. (11) including 37 patients, rechallenge of ICI was attempted in 16 patients who sustained ICI-AKI at a median of 2 months after the initial AKI episode. Recurrence of AKI was noted in three of 16 patients who were rechallenged (19%). One remained on RRT and none achieved complete kidney recovery after a recurrent AKI episode. The authors also reported a favorable survival trend among patients who were not rechallenged with ICI compared with rechallenged patients. However, this observation could be biased because patients who were rechallenged likely necessitated chemotherapy to combat active cancer, although that might not have always been the case for the nonrechallenged group. A significant number of patients (81%) were on lower doses of corticosteroids at the time of rechallenge and the majority received the same ICI regimen. In a study by Manohar (10), rechallenge was attempted among four out of 14 patients, and one sustained AKI recurrence (25%). Rechallenge was attempted after the serum creatinine neared the baseline. In the multicenter study by Cortazar et al. (4), 31 patients were rechallenged at a median of 1.8 months after the initial ICI-AKI episode. Although most of them were rechallenged with the same ICI agent, the recurrence of AKI was observed in 23%. A shorter latency between the initial AKI episode and rechallenge was identified as one of the major risk factors associated with recurrent AKI. Furthermore, a recent study by Allouchery et al. (12) demonstrated that among patients with ICI rechallenge, 39% experienced at least one grade ≥2 irAE. and ICI rechallenge should be considered with caution among patients with grade 4 irAE. Moreover, in study by Simonaggio et al. (13), out of 40 patients who were rechallenged with the same ICI, 55% encountered same or a different irAE. Subsequently, a trend towards a higher recurrence rate was noted on rechallenging the patient after more severe initial irAE. Lastly, in a multicenter study by Abu-Sbeih et al. (14), the recurrence of immune-mediated colitis was reported to be higher in patients with anti–cytotoxic T-lymphocyte antigen 4 (CTLA4) (44%) as compared with those receiving anti–programmed cell death 1 or ligand 1 (PD1/PDL1) (32%).
Summarizing the above-mentioned studies, the overall risk of irAE after rechallenge with ICI is high and ranges from 28% to 55% (12–14), whereas the risk of ICI-AKI after rechallenge is reported to vary between 19% and 25% (4,10,11). Further, in most available case series, it is left to the treating physician to decide whether the AKI episode is ICI induced without the need for a biopsy. Therefore, it is possible that in patients without recurrence after ICI reintroduction, the initial AKI episode was not ICI induced (because most AKI episodes in patients who are ICI treated are of prerenal origin), emphasizing the importance of kidney biopsies in patients experiencing AKI under ICI treatment without another obvious explanation. Hence, we propose that ICI rechallenge should not be attempted among patients with biopsy-proven ICI-AKI.
Major risk factors are associated with AKI after rechallenge, although not consistent across the reported studies, include shorter latency to rechallenge, combination therapies, and persistence of underlying AKI. Rechallenging increases risk of recurrent AKI, hospitalizations, RRT dependency, severe CKD, and recurrence of irAEs in other organs. Therefore, ICI-AKI should not be seen as a simply treatable condition (Table 1).
Table 1.
CONS of rechallenging with immune checkpoint inhibitors
| 1 | Recurrence of AKI |
| 2 | Potential RRT dependency |
| 3 | Increased hospitalizations |
| 4 | Accelerated progression to severe CKD |
| 5 | Increased health care utilization |
| 6 | Risk of irAE in other organs |
irAE, immune-related adverse effects; RRT, renal replacement therapy.
The precise mechanism of AKI after ICI rechallenge remains unclear, but is likely similar to the mechanisms that precipitated the initial bout of AKI. We hypothesize that on rechallenging, there could be an increased immunologic activation of effector T cells that were primed during initial ICI exposure, intensifying autoimmune response. The tubular and interstitial injury after initial AKI episode could be indolent even after cessation of ICI therapy and on reinitiation, the underlying disease process could be revamped with more severe injury. Additionally, long-lived memory T cells that were activated during previous AKI episode could trigger turbulent inflammatory response with release of cytokines and inflammatory markers, accelerating the tubular injury (15). Recurrent AKI episodes may result in eventual loss of kidney function, with a subsequent negative effect on clinical outcomes.
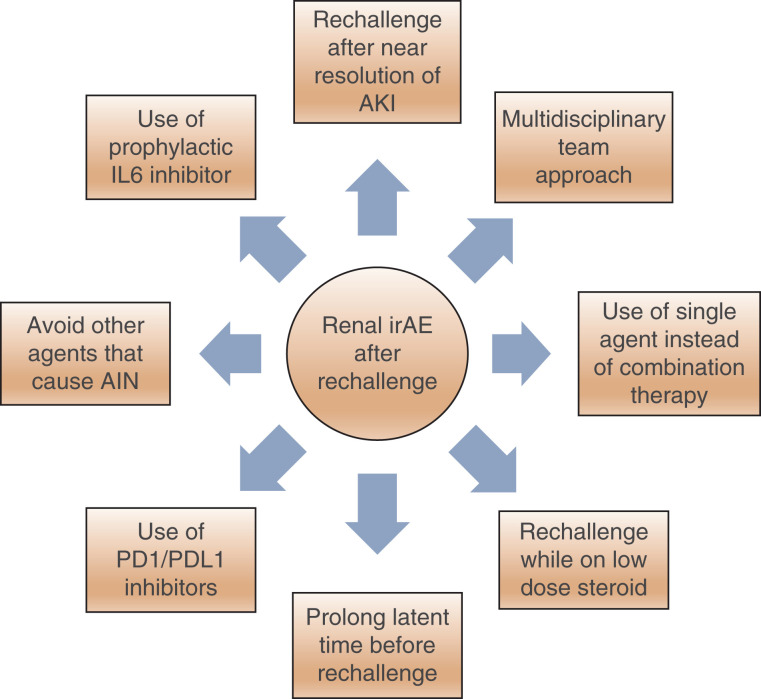
Considering the high recurrence rates of irAEs and ICI-AKI on rechallenge and the lack of data on long-term effects of rechallenging ICIs, rechallenging should be discouraged and considered as a last resort, especially among patients who sustained severe grade kidney irAE on initial administration. Among the subset of patients who are at risk of developing severe irAE and who must receive ICI as essential component of anticancer therapy, the following measures should be considered while reinitiating ICIs to reduce AKI from irAE: (1) rechallenge after near resolution of initial AKI episode, (2) use of single ICI instead of combination therapy, (3) rechallenge while patients are still on low-dose corticosteroids, (4) increase the latency between the initial AKI episode and rechallenge, (5) use of PD1/PDL1 inhibitors if possible, as compared with CTLA4 inhibitors, (6) avoidance of concomitant agents that could cause acute interstitial nephritis, (7) use of Interleukin (IL)-6 inhibitor, tocilizumab for patients with severe grade >3 kidney toxicity in combination with ICI use (16), and (8) a multidisciplinary team approach (Figure 1).
Figure 1.
Recommended measures to potentially decrease the risk of recurrent AKI from immune-related adverse events on rechallenge with immune checkpoint inhibitors. irAE, immune-related adverse events; PD1, programmed cell death protein 1; PD-L1, programmed death ligand 1; AIN, acute interstitial nephritis.
In conclusion, multiple factors play a part in the decision to rechallenge a patient with ICIs and we believe this decision should be individualized considering the availability of alternative therapies, severity, and grade of initial irAE, tumor response, and coexisting comorbid conditions. Further, it is essential to identify risk factors associated with severe irAE and consider necessary preventive strategies while reintroducing ICI therapies in an attempt to reduce the risk of kidney injury and severity of CKD.
Disclosures
J. Velez reports consulting for Bayer and Mallinckrodt Pharmaceuticals; reports being on the advisory board for Mallinckrodt Pharmaceuticals and Travere Therapeutics; and reports speakers bureau for Otsuka Pharmaceuticals. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the author(s).
Footnotes
Author Contributions
J. Velez provided supervision, reviewed and edited the manuscript; S. Kanduri conceptualized the study, was responsible for the visualization and wrote the original draft.
References
- 1.Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K; ESMO Guidelines Committee : Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28(S4): iv119–iv142, 2017. 10.1093/annonc/mdx225 [DOI] [PubMed] [Google Scholar]
- 2.Sznol M, Ferrucci PF, Hogg D, Atkins MB, Wolter P, Guidoboni M, Lebbé C, Kirkwood JM, Schachter J, Daniels GA, Hassel J, Cebon J, Gerritsen W, Atkinson V, Thomas L, McCaffrey J, Power D, Walker D, Bhore R, Jiang J, Hodi FS, Wolchok JD: Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol 35: 3815–3822, 2017. 10.1200/JCO.2016.72.1167 [DOI] [PubMed] [Google Scholar]
- 3.Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, Le DT, Lipson EJ, Glezerman IG, Wolchok J, Cornell LD, Feldman P, Stokes MB, Zapata SA, Hodi FS, Ott PA, Yamashita M, Leaf DE: Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90: 638–647, 2016. 10.1016/j.kint.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortazar FB, Kibbelaar ZA, Glezerman IG, Abudayyeh A, Mamlouk O, Motwani SS, Murakami N, Herrmann SM, Manohar S, Shirali AC, Kitchlu A, Shirazian S, Assal A, Vijayan A, Renaghan AD, Ortiz-Melo DI, Rangarajan S, Malik AB, Hogan JJ, Dinh AR, Shin DS, Marrone KA, Mithani Z, Johnson DB, Hosseini A, Uprety D, Sharma S, Gupta S, Reynolds KL, Sise ME, Leaf DE: Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: A multicenter study. J Am Soc Nephrol 31: 435–446, 2020. 10.1681/ASN.2019070676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waljee AK, Rogers MAM, Lin P, Singal AG, Stein JD, Marks RM, Ayanian JZ, Nallamothu BK: Short term use of oral corticosteroids and related harms among adults in the United States: Population based cohort study. BMJ 357: j1415, 2017. 10.1136/bmj.j1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers MAM, Lin P, Nallamothu BK, Kim C, Waljee AK: Longitudinal study of short-term corticosteroid use by working-age adults with diabetes mellitus: Risks and mitigating factors. J Diabetes 10: 546–555, 2018. 10.1111/1753-0407.12631 [DOI] [PubMed] [Google Scholar]
- 7.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network: Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 36: 1714–1768, 2018. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, Budde LE, Costa L, Davies M, Dunnington D, Ernstoff MS, Frigault M, Kaffenberger BH, Lunning M, McGettigan S, McPherson J, Mohindra NA, Naidoo J, Olszanski AJ, Oluwole O, Patel SP, Pennell N, Reddy S, Ryder M, Santomasso B, Shofer S, Sosman JA, Wang Y, Weight RM, Johnson-Chilla A, Zuccarino-Catania G, Engh A: NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020. J Natl Compr Canc Netw 18: 230–241, 2020. 10.6004/jnccn.2020.0012 [DOI] [PubMed] [Google Scholar]
- 9.Perazella MA, Sprangers B: Checkpoint inhibitor therapy-associated acute kidney injury: Time to move on to evidence-based recommendations. Clin Kidney J 14: 1301–1306, 2021. 10.1093/ckj/sfab052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manohar S, Ghamrawi R, Chengappa M, Goksu BNB, Kottschade L, Finnes H, Dronca R, Leventakos K, Herrmann J, Herrmann SM: Acute interstitial nephritis and checkpoint inhibitor therapy. Kidney360 1: 16–24, 2020. 10.34067/KID.0000152019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isik B, Alexander MP, Manohar S, Vaughan L, Kottschade L, Markovic S, Lieske J, Kukla A, Leung N, Herrmann SM: Biomarkers, clinical features, and rechallenge for immune checkpoint inhibitor renal immune-related adverse events. Kidney Int Rep 6: 1022–1031, 2021. 10.1016/j.ekir.2021.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allouchery M, Lombard T, Martin M, Rouby F, Sassier M, Bertin C, Atzenhoffer M, Miremont-Salame G, Perault-Pochat MC, Puyade M; French Network of Regional Pharmacovigilance Centers : Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade ≥2 immune-related adverse events in patients with cancer. J Immunother Cancer 8: e001622, 2020. 10.1136/jitc-2020-001622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A, Cengizalp G, Vozy A, Laparra A, Varga A, Hollebecque A, Champiat S, Marabelle A, Massard C, Lambotte O: Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol 5: 1310–1317, 2019. 10.1001/jamaoncol.2019.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abu-Sbeih H, Ali FS, Naqash AR, Owen DH, Patel S, Otterson GA, Kendra K, Ricciuti B, Chiari R, De Giglio A, Sleiman J, Funchain P, Wills B, Zhang J, Naidoo J, Philpott J, Gao J, Subudhi SK, Wang Y: Resumption of immune checkpoint inhibitor therapy after immune-mediated colitis. J Clin Oncol 37: 2738–2745, 2019. 10.1200/JCO.19.00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, Gaber L, Lahoti A, Workeneh B, Chen S, Lin J, Abdel-Wahab N, Tayar J, Lu H, Suarez-Almazor M, Tannir N, Yee C, Diab A, Abudayyeh A: Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: Single-center experience. J Immunother Cancer 7: 2, 2019. 10.1186/s40425-018-0478-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haanen J, Ernstoff M, Wang Y, Menzies A, Puzanov I, Grivas P, Larkin J, Peters S, Thompson J, Obeid M: Rechallenge patients with immune checkpoint inhibitors following severe immune-related adverse events: Review of the literature and suggested prophylactic strategy. J Immunother Cancer 8: e000604, 2020. 10.1136/jitc-2020-000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.