Abstract
Pseudomonas aeruginosa is a human pathogen associated with both acute and chronic infections. While intensively studied, the basic mechanisms enabling the long-term survival of P. aeruginosa in the host, despite massive immune system attack and heavy antimicrobial treatment, remain to be identified. We argue that such infections may represent niche invasions by P. aeruginosa that influence the microenvironment by depleting host-derived substrate and activating the immune response. Bacteria embedded in cell aggregates establish a microenvironmental niche, where they endure the initial host response by slowing down their metabolism. This provides stable, lasting growth conditions with a constant, albeit slow supply of substrate and electron acceptors. Under such stable conditions, P. aeruginosa exhibits distinct adaptive traits, where its gene expression pattern reflects a life exposed to continuous attack by the host immune system and antimicrobials. Here, we review fundamental microenvironmental aspects of chronic P. aeruginosa infections and examine how their structural organization influences their in vivo microenvironment, which in turn affects the interaction of P. aeruginosa biofilm aggregates with the host immune system. We discuss how improving our knowledge about the microenvironmental ecology of P. aeruginosa in chronic infections can be used to combat persistent, hard-to-treat bacterial infections.
Keywords: biofilm, chronic infections, host–pathogen interactions, immune response, microenvironment, quorum sensing
We review microbe–microbe and host–microbe interactions and their influence on the bacterial microenvironment alongside alternative interventions based on the physiology of P. aeruginosa in infections, where the bacteria are often found as small aggregates embedded in host material and surrounded by immune cells.
Introduction
Pseudomonas aeruginosa is a prominent opportunistic pathogen involved in chronic bacterial infections of, e.g. wounds and the respiratory tract, or associated with implants. Numerous studies have demonstrated the large genetic versatility and phenotypic plasticity of P. aeruginosa (Shen et al. 2006, Turner et al. 2015). There is also abundant literature on specific biochemical pathways or molecular characteristics of P. aeruginosa or the host immune response [recently reviewed in La Rosa et al. (2019) and Moser et al. (2021)]. However, the mechanisms governing the persistence of P. aeruginosa in chronic infections remain elusive. In this review, we assess fundamental knowledge about growth patterns of P. aeruginosa in chronic infections and their microenvironment, and discuss how these are affected by the host immune response. The latter is a surprisingly underexplored topic that may reveal essential insights into the long-term persistence mechanisms of chronic P. aeruginosa infections despite a strong immune response and antibiotic treatment. Increased understanding of the ecological niche that P. aeruginosa inhabits after successful colonization and consecutive infection of the human body may also identify important new targets for both diagnosis and treatment of chronic infections. While we focus on the well-studied species of P. aeruginosa, we also draw parallels to other important pathogens where appropriate.
The conditions leading up to a chronic infection are not caused by the bacteria themselves but a dysfunction in the host that creates conditions promoting subsequent bacterial invasion and infection (Bjarnsholt et al. 2021). For example, in patients suffering from the hereditary condition cystic fibrosis (CF), a malfunction in the chloride channels leads to dehydrated mucus in the lower respiratory tract, causing an impaired mucociliary clearance of inhaled microbes (Høiby et al. 2010). For chronic wounds, a lowered or impaired vascularization and other impairments followed by a breach in the skin lead to abnormal healing and opportunities for persistent infection (Singer and Clark 2008). Additionally, the insertion of a foreign body and the subsequent destabilization of tissue can create niches for infection development (Jakobsen et al. 2018). While none of these conditions necessarily are the direct cause of infection, they involve formation of a matrix of abnormal host material, such as thickened mucus in the CF lung or slough in chronic wounds, wherein intruding bacteria may then gain a foothold and cause infection. Bacterial colonization can arise from exogenous sources or from the existing microbiome of the hosts, and can involve single cells or small aggregates (Jelsbak et al. 2007, Hansen et al. 2012).
Most of our knowledge about the initial events leading up to a chronic infection is derived from patient samples. These are obtained either after the establishment of a chronic infection, typically in its late stages, or from acute infections that will not progress into the chronic state. The precise conditions that lead to chronic infections, whether bacteria- or host-specific, are therefore, still debated.
For bacteria, numerous in vitro studies have concluded that bacterial aggregation leads to increased tolerance toward antibiotics and the host immune defense response (Jensen et al. 2010, Goltermann and Tolker-Nielsen 2017, Ciofu and Tolker-Nielsen 2019, Moser et al. 2021). In vivo animal studies have demonstrated similar mechanisms (Pedersen et al. 1990, Lebeaux et al. 2013, Reizner et al. 2014, Jensen et al. 2019a). However, most animal models fail to mimic a native chronic infection, since the animal has to be manipulated into infection. Such models are also poor at emulating a persisting chronic infection, as the bacteria are usually either eradicated by the host or the animal succumbs to the infection over the experimental time interval. Besides attaining an increased tolerance toward antibiotics and host immune evasion, we know that: (i) bacteria gather in small colonies, or biofilms (Rudkjøbing et al. 2012, Bjarnsholt et al. 2013, Bay et al. 2018); (ii) the bacteria display much slower growth rates within the patients than subsequent in vitro growth rates (Yang et al. 2008, Kragh et al. 2014); (iii) the conditions within the host material are anoxic or hypoxic (Worlitzsch et al. 2002, Kolpen et al. 2010, James et al. 2016, Jensen et al. 2017); and (iv) the genetic diversity of bacteria is large and differs from the reference or environmental strains of the same species (Smith et al. 2006; Yang et al. 2011a,b, Jiricny et al. 2014, Vanderwoude et al. 2020, Armbruster et al. 2021; Fig. 1).
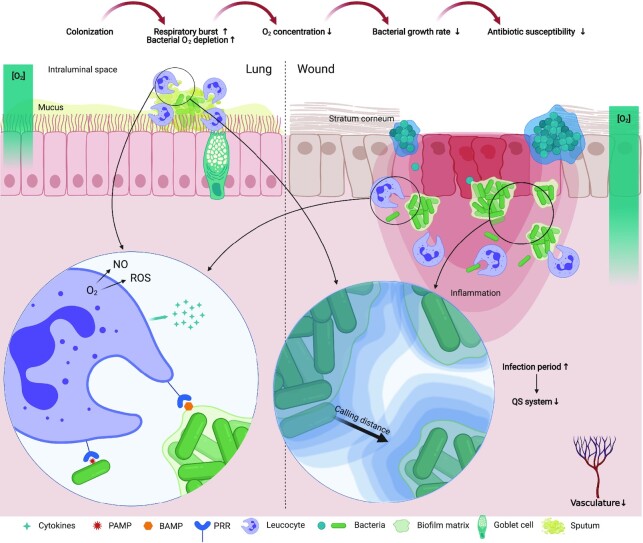
Figure 1.
Conceptual drawing of the microenvironment of infections in the lung (left) and wound (right). Colonization by bacteria leads to innate immune activation by recognition of pathogen-associated molecular patterns (PAMP) and biofilm-associated molecular patterns (BAMP) by pattern recognition receptors (PRR) and the release of proinflammatory cytokines. Immune cell activation leads to increased O2 consumption for the respiratory burst which, along with bacterial respiration, leads to lowered O2 tension. In wounds, bacteria are found as monospecies aggregates separated from each other where different species appear to inhabit different zones of the wound. In lungs of CF patients, bacteria are found intraluminally embedded in thickened sputum. Bacterial interactions occur if signaling molecules reach high enough concentrations to elicit a response. The quorum sensing (QS) system has been shown to be lost or inactive in late infection stages.
The structural organization of bacteria in infections
In a range of laboratory biofilm models (flow cells, drip-flow reactors, and alike), bacteria are grown in a manner that allows for development of complex structures and many studies have shown that bacteria are capable of organizing themselves in 3D biofilm landscapes (Hall-Stoodley et al. 2004). This concept is not novel or controversial in any way, and the fossil record shows that some of the oldest known biotic structures, i.e. stromatolites, were organized as microbial biofilms communities (Garwood 2012). Such structural organization has been explained as a response to the physicochemical microenvironment surrounding the structures. For example, researchers have shown that architecture was governed by an optimal diffusive exchange of solutes in a hot-spring microbial mat, where the biomass was structured as stromatolite-like pillars (Petroff et al. 2010). However, in many cases bacterial growth is characterized by flat slabs or simple aggregates (Bridier et al. 2010).
While there is a good understanding of such structure–function relationships in many natural biofilm communities (e.g. Depetris et al., 2021, 2022), the question remains how bacteria are organized in chronic infections and whether a complex 3D structural organization and derived changes of their microenvironment confer any advantage for their persistence and resilience to the immune response or antibiotic treatment.
Surface-attached biofilms remain relevant to numerous systems such as fouling of industrial equipment (Flemming 2011) and aquatic plants (Noisette et al. 2020), stream biofilms (Besemer et al. 2012, Depetris et al. 2021), oral biofilms (Bowen et al. 2018), and implant-associated infections (Arciola et al. 2018). However, in most types of bacterial infections it is now becoming widely accepted that biofilms are not necessarily attached directly to a surface but rather suspended in an extracellular matrix (Bjarnsholt et al. 2013, Kragh et al. 2016). In the CF lung, aggregates are, thus located endobronchially, with one report showing that ∼95% of the bacteria are located more than 5 µm away from the epithelial surface (Worlitzsch et al. 2002). In wounds, bacteria aggregate in a host- or self-produced matrix (Kirketerp-Møller et al. 2008), whereby different species appear to inhabit different niches in the wound (Fazli et al. 2009). How bacteria come to be distributed in chronic wounds remains unclear, but their nonrandom distribution (Fazli et al. 2009) could be linked to differences in the microenvironment and the availability of electron acceptors for respiration between the surface and deeper parts of the wound (James et al. 2016). In acute wounds, it has been shown that bacterial aggregates form at the wound edges and in the crevices of the stratum corneum, whereas no bacteria were found in the acute wound bed (Bay et al. 2018).
It has been proposed that even multispecies infections are primarily composed of small monospecies aggregates spatially separated from each other by the host material (Burmølle et al. 2010, Rudkjøbing et al. 2012, Kvich et al. 2020). In most types of human biofilm infections, the dominating aggregate diameters are found to be 5–200 µm (Bjarnsholt et al. 2013). Here, catheter-associated biofilm patches are an exception reaching up to 1200 µm, possibly due to the large abiotic surface presenting a distinct niche for microbial colonization (Jakobsen et al. 2018). It, thus appears that there is an upper size limit of biofilms in human infections, which is significantly lower than seen for laboratory-grown surface-bound biofilms that can easily cover several square centimeters of surface. The factors that govern this apparent size limit are still not understood but may arise as a balance between phagocytosis by leucocytes and resource depletion decreasing the bacterial growth rate (Stewart 2003, Aristotelous et al. 2015, 2018).
The dynamics of phagocytosis by leucocytes has mainly been studied using single particles, where an increase in target size has been shown to prolong engulfment time and interestingly, nonspherical shapes also resulted in much slower engulfment than spherical particles (Paul et al. 2013). In contrast, the dynamics of phagocytosis of bacterial aggregates remain almost unexplored. A recent study demonstrated a negative correlation between the probability of phagocytosis by single polymorphonuclear neutrophils (PMNs) and the biofilm aggregate diameter (Alhede et al. 2020a), while another study showed that aggregates > 50 µm2 resisted killing by human neutrophils (Pettygrove et al. 2021). It, thus appears that attaining a certain bacterial aggregate size can present a selective advantage. The main determinant for the switch from acute to chronic infections has been assumed to be correlated with bacterial aggregation (Bjarnsholt et al. 2012), but this paradigm was recently challenged. It was, thus shown that the biomass proportion of individual bacterial cells and those in biofilm aggregates were equal between acute- and chronic pulmonary infections (Kolpen et al. 2022). Rather than aggregation being the distinguishing factor of acute versus chronic infection, it was argued that metabolic activity might play a more central role, where acute infections are characterized by higher bacterial growth rates.
Factors shaping the microenvironment within infections
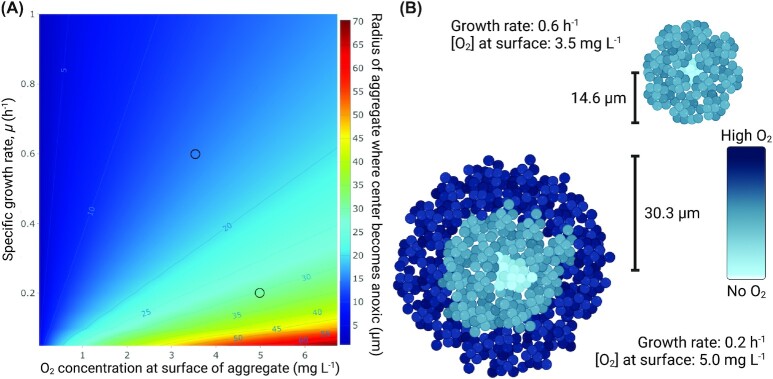
The growth limitation imposed by insufficient electron acceptor availability is influenced by the metabolic activity of the bacteria themselves, as well as human immune cells that consume O2 for their respiratory burst (Jensen et al. 2017). Bacteria, thus influence their own microenvironment and larger aggregates will have less O2 toward their center (Ploug et al. 1997, Kühl et al. 2007, Sønderholm et al. 2018). The minimum aggregate size necessary to deplete O2 in the center can be calculated by simple diffusion–reaction models (Ploug et al. 1997, Stewart 2003). Here, we used the formulation of Stewart (2003) to explore how bacterial aggregate size varies according to the bacterial growth rate and the O2 availability at the surface of the aggregate. The strong influence of O2 concentration at the surface of aggregates on oxygen penetration and the subsequent growth rate of bacteria is illustrated in Fig. 2.
Figure 2.
(A) Modeling of the radius of aggregates at which the O2 concentration in the aggregate center goes to zero depending on the growth rate of bacteria (divisions hour–1) and the O2 concentration at the surface of the aggregate using the expressions from Stewart (2003). We used a yield coefficient of biomass on O2, 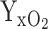 = 0.85 mg mg–1, a biomass density of bacteria in aggregates of 2.0⋅105 mg l–1, a diffusion coefficient of O2 in water, Daq = 2.0⋅10–5 cm2 s–1, and an effective diffusion coefficient in the biofilm, De/Daq = 0.2. (B) The two examples of the influence of the growth rate and surface O2 concentration on the aggregate size where the center exactly becomes anoxic.
= 0.85 mg mg–1, a biomass density of bacteria in aggregates of 2.0⋅105 mg l–1, a diffusion coefficient of O2 in water, Daq = 2.0⋅10–5 cm2 s–1, and an effective diffusion coefficient in the biofilm, De/Daq = 0.2. (B) The two examples of the influence of the growth rate and surface O2 concentration on the aggregate size where the center exactly becomes anoxic.
Even at low growth rates observed in vivo in the lungs of CF patients (0.217 divisions hour–1; range: −0.10 to 0.67; Kragh et al. 2014), only aggregates with a very small radius (0–35 µm) are fully aerobic. This modeling assumes steady-state O2 concentration at the surface as well as an equal growth rate of all bacteria in the aggregate. This of course is not the case in vivo, where other types of electron acceptors are also present. Electron acceptors are used in succession based on their bioenergetic potential. At low O2 tension, P. aeruginosa is known to switch to denitrification if nitrate or nitrite is available (Hasset et al. 2009, Kolpen et al. 2014b), and long-term survival of P. aeruginosa on pyruvate and arginine fermentation has previously been documented (Schreiber et al. 2006). However, the precise regulation of respiratory pathways is complex and is dependent on multiple factors such as substrate availability, affinities of terminal oxidases, and inhibitor molecules (Kawakami et al. 2010, Trunk et al. 2010, Lichtenberg et al. 2021). This complicates attempts to extend this type of modeling to chronic infections in vivo. Yet it seems, at least to some extent, that electron acceptor availability could explain the upper size limit of bacterial aggregates. At the same time, such aggregates may gain an advantage from attaining a certain size because the risk of being eliminated by phagocytosis is decreased (Alhede et al. 2020a).
The susceptibility to antibiotics is determined by the bacterial growth rate (Tuomanen et al. 1986, Evans et al. 1991), their metabolic state (Meylan et al. 2017, Lopatkin et al. 2019, Stokes et al. 2019), and the availability of O2 (Brochmann et al. 2014, Dwyer et al. 2014). Thus, the uptake of O2 by inflammatory cells has the potential to significantly affect the outcome of treating biofilm infections with antibiotics. It is difficult to distinguish between the O2 consumption of inflammatory cells and bacterial cells in the infectious biofilm, and the resulting O2 profiles in infected tissue, thus depend on the concerted action of both host cells and the bacterial biofilm (Wu et al. 2018). It should be noted, however, that in infected anaerobic endobronchial secretions from CF patients with chronic P. aeruginosa lung infection (Worlitzsch et al. 2002), the overall O2 consumption is dominated by the host response, while the contribution of bacterial aerobic respiration to the total amount of O2 consumed is apparently minimal (Kolpen et al. 2010).
The host response to biofilm infections has been thoroughly investigated in CF patients with chronic P. aeruginosa lung infections and from P. aeruginosa-specific infection models (Lorenz et al. 2016). Pathogen-associated molecular patterns (PAMPs) expressed on P. aeruginosa are recognized by PMNs and macrophages through pattern recognition receptors (PRRs). Further, biofilm-associated molecular patterns (BAMPs) constitute a subpopulation of PAMPs that when expressed in biofilm, induces a distinct innate immune response (Moser et al. 2021). Accordingly, components of the extracellular polysaccharide matrix components in P. aeruginosa biofilm may qualify as BAMPs by inducing distinct responses by PMNs. In contrast, flagella failed to qualify as BAMPs due to the absence of an increased PMN response to P. aeruginosa biofilm with expression of flagella, even though the expression of flagella by planktonic cells increased the PMN response (Rybtke et al. 2020, Moser et al. 2021). Binding of molecular patterns to PRRs activate the innate immune response, leading to the attraction of macrophages and a multitude of PMNs (Moser et al. 2021). Further activation steps involve stimulation of the respiratory burst by the PMNs, leading to intense consumption of O2 for the production of reactive oxygen species (ROS; Kolpen et al. 2010) and nitric oxide (NO) (Kolpen et al. 2014a). Additional innate responses include the PMN-mediated secretion of proteases that cause proteolytic tissue lesions (Wilgus et al. 2013) and the release of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) interleukins (IL)-1, IL-6, IL-8, and IL-12 by macrophages, which may further enhance the inflammatory response (Lavoie et al. 2011, Sweere et al. 2020).
As the adaptive immune response matures, the T-cells and the B-cells reside distantly, such as in the secondary lymphoid organs, while the plasma cells are located in the bone marrow. Activated T-cells release cytokines that reinforce the inflammation by stimulating the accumulation and activation of PMNs and production of IgG, causing further immune complex-mediated stimulation of the PMNs and activation of the classical complement pathway (Moser et al. 2017). Thus, the chronicity of biofilm infections provides the time span needed for the adaptive immune response to develop and contribute to the host response by further increasing the accumulation and activation of PMNs. This leads to the acceleration of local inflammation, resulting in collateral tissue damage but without eradicating the infectious biofilm (Jensen et al. 2010).
The ability of activated PMNs to deplete O2 limits bacterial aerobic respiration, which may determine bacterial growth. Accordingly, the growth of P. aeruginosa is inversely correlated to the amount of PMNs surrounding the biofilms in CF patients with chronic lung infection (Kragh et al. 2014). The O2 consumption by the host response may also slow down the bacterial growth of pathogens other than P. aeruginosa (Jensen et al. 2017). Diverse methodologies, such as rRNA fluorescence in situ hybridization and trace incorporation of heavy water, have indicated the slow growth of Stenotrophomonas maltophilia (Kolpen et al. 2015), Achromobacter xylosoxidans (DePas et al. 2016), and Staphylococcus aureus (Kopf et al. 2016) in expectorated sputum from CF patients with chronic lung infections. Because of the association of slow bacterial metabolism with low susceptibility to antibiotics, the O2 consumption by the PMNs may play a significant role in the recalcitrance of chronic biofilm infections to intense antibiotic treatment in CF patients (Lopatkin et al. 2019). Besides the increased tolerance imposed by low substrate-availability, antibiotic treatment may also cause low level mutations in metabolic genes conferring increased resistance by lowering basal respiration (Lopatkin et al. 2021).
The contribution of bacterial biofilms to the poor healing of chronic wounds is increasingly recognized (Bjarnsholt et al. 2008, James et al. 2008) and has been confirmed in experimental wounds infected with P. aeruginosa biofilms (Seth et al. 2012, Watters et al. 2013). The incidence of bacterial biofilms in chronic wounds may exceed 80% (Malone et al. 2017). The microenvironment of chronic wounds with biofilm infections may also be hypoxic as evidenced by the presence of steep O2 gradients (Schreml et al. 2014), transcriptomic profiling (James et al. 2016), and the occurrence of many anaerobic bacteria (Dowd et al. 2008) and metabolites (Debats et al. 2006).
The key mechanisms of O2 depletion in infected wounds remain elusive, but the accumulation of PMNs is increased in wounds with biofilm infection (Fazli et al. 2011, Trøstrup et al. 2013). The concerted activity of biofilms and the summoned PMNs may, thus cause the steep O2 gradients found in chronic wounds (Wu et al. 2018). The consumption of O2 by PMNs is evidenced from the relation between the extent of the respiratory burst and the bacterial load in infected wounds (Belotsky et al. 1990), but the influence of the adaptive immune response remains largely unknown (Moser et al. 2021). While the resulting lack of O2 may contribute significantly to delayed wound healing (Hunt et al. 1969, Gottrup et al. 2017, Frykberg et al. 2020), the influence of hypoxia on the outcome of antibiotic treatment in chronic wounds is largely unknown. Apart from the concerted O2 consumption of inflammatory cells and bacteria in wounds, the hypoxic conditions may be further exacerbated by impaired vascularization in patients suffering from conditions such as atherosclerosis and diabetes. This impairment may also lead to inadequate delivery of systemically administered antibiotics possibly resulting in sub-MIC concentrations of therapeutic drugs being delivered at the infection site (Bue et al. 2017, Jensen et al. 2019a).
Bacterial interactions in infections
Bacterial interactions in infections are most likely important both within aggregates and between aggregates in close proximity (Azimi et al. 2020). However, a study of the distribution and diversity of bacteria in chronic venous leg ulcers showed that the diversity of bacteria in the wound could not be captured if only one biopsy was investigated (Thomsen et al. 2010), which is indicating a heterogeneous distribution of different bacteria into biofilm aggregates that are spatially separated (Burmølle et al. 2010, Kvich et al. 2020). Other studies have shown that P. aeruginosa and S. aureus colonize different depths within wounds (Fazli et al. 2009), and the overall species diversity in wounds is low and only comprises a handful of species (Thomsen et al. 2010). The majority of aggregated bacteria in infections are surrounded by PMNs (Høiby et al. 2010, Kragh et al. 2014), and thus the interaction with the host is more likely to be the predominating form of direct cell–cell interaction at the level of single aggregates.
Inter and intraspecies signaling
Due to the obvious complications of measuring calling distances in vivo in humans, the scale and importance of inter and intraspecific cell–cell signaling in infections remains unknown. However, interspecies interactions and calling distances have been frequently studied in the laboratory in well-shaken cultures or in dense biofilms of both P. aeruginosa and other microbes (Egland et al. 2004, Weigert and Kümmerli 2017, Darch and Koley 2018). The scale of calling distance may be dependent on the surrounding environment and the specific microbe. For rhizobacteria one study e.g. reports that calling distances frequently approach 4–5 µm, while extending up to 78 µm in some cases (Gantner et al. 2006). Others studies argue that diffusible signals for interspecies interactions only function over very short distances of ∼1 µm in open systems, which means that they effectively only reach neighboring cells (Egland et al. 2004).
The redox-active phenazine pyocyanin, which is controlled by the quorum sensing (QS) system and secreted extracellularly by P. aeruginosa, can be a good indicator of sharing distances between cells. By using P. aeruginosa colonies attached to a surface, the sharing distances of the phenazine pyoverdine was reported to be at least 100 µm when the surface was soft, although they were reduced on a hard surface (Weigert and Kümmerli 2017). In a CF lung infection model, it was estimated that aggregates of ∼2000 pyocyanin-producing P. aeruginosa cells were unable to interact with neighboring aggregates, while clusters containing > 5000 cells could interact with others over longer distances of up to 176 µm (Darch et al. 2018). Even though impressively large calling distances relative to the size of individual bacteria have been recorded (e.g. Gantner et al. 2006), these distances are still short compared with the distribution of bacteria in infections (Thomsen et al. 2010). Furthermore, we note that even aggregates containing only 2000 bacteria are still twice as large as the aggregates observed in the CF lung (Darch et al., 2017, 2018), suggesting that the small clusters observed in vivo (Bjarnsholt et al. 2013) have a limited capacity for interaggregate interactions.
We still know very little of the potential interactions between cells over micrometer-scales in chronic infections and how different microenvironments can affect the calling distance of different molecules. Along with the complex task of untangling signal-response networks, the signaling molecules themselves are characterized by different diffusion coefficients and chemical stabilities (Yates et al. 2002), which makes the calling distance of individual molecules unique.
Interkingdom host–bacteria signaling
Several indications of a hormonal interaction between microorganisms and their hosts exist (Singh et al. 2000). The first signs of interkingdom signaling were shown when N-acyl homoserine lactone (AHL) signaling molecules were found capable of modulating mammalian cell signal transduction (Telford et al. 1998), and hormones from the host were observed to modulate bacterial gene expression (Sperandio et al. 2003). Purified AHLs have been reported to increase IL-8 in respiratory epithelial cells (DiMango et al. 1995), to inhibit lymphocyte proliferation, and to downregulate the production of TNF-α and IL-12 in lipopolysaccharide-stimulated macrophages (Telford et al. 1998). Phenazines from P. aeruginosa have been shown to bind to the aryl hydrocarbon receptor (AhR), a highly conserved ligand-dependent transcription factor in mammalian cells, affecting the expression of several host genes e.g. for production of chemokines, cytokines, and detoxifying enzymes (Moura-Alves et al. 2014). A recent study by the same group demonstrated a qualitative and quantitative interaction of QS molecules and phenazines with AhR in zebrafish, mice, and humans (Moura-Alves et al. 2019). While numerous studies have shown a multitude of indications for interkingdom signaling between bacteria and host, it remains uncertain whether such interactions are important in infections, where only a small number of pathogenic bacteria are present. Most studies use cell lines and purified test compounds such as AHL signaling molecules to show a response, which makes it difficult to extrapolate the findings directly to P. aeruginosa infections.
Genetic changes in P. aeruginosa signaling systems during infection
For P. aeruginosa, it has been observed that certain genes mutate over infection periods (Diaz Caballero et al. 2015), which affect the functionality of the QS system in particular, as well as the secondary signaling system cyclic diguanylate (c-di-GMP; Jiricny et al. 2014). In CF sputum samples especially, mutations seem to develop over long infection periods (Jelsbak et al. 2007, Bjarnsholt et al. 2010, Folkesson et al. 2012, Armbruster et al. 2021). For the QS system, mutations in the lasR gene (Smith et al. 2006, Ciofu et al. 2010, Folkesson et al. 2012) as well as mutations in mucA also leading to QS repression (Ryall et al. 2014) have been reported in P. aeruginosa infections. In addition, transcription of the las QS system has been shown to be significantly lower in patient samples from different infections, compared to in vitro P. aeruginosa biofilms (Cornforth et al. 2018).
The observed increase in lasR mutants during infection has been the subject of speculation in recent decades (Feltner et al. 2016, Kostylev et al. 2019). It has been suggested that lasR mutants are selected for by an apparent increased metabolic advantages by upregulation of catabolic metabolism (D'Argenio et al. 2007) and a lowered probability of lytic death in stationary growth phase (Heurlier et al. 2006). Another suggestion is that lasR mutants can spread in populations with QS-proficient bacteria, where the QS mutants might behave as social cheaters, avoiding the costs of producing messenger molecules, which leads to a mixed population (Diggle et al. 2007). Alternatively, bacteria adjust to a mixed population over time followed by a complete loss of a functional QS system in the entire population later in the infection period. Such dynamics have previously been observed in clinical settings (Köhler et al. 2010). It has also been suggested that LasR deficient P. aeruginosa prevent robust neutrophil extracellular trap (NET) formation in neutrophils via transcriptional regulation of LasA protease and LasB elastase (Skopelja-Gardner et al. 2019), while another study suggested that most host-derived eDNA, in vivo, is not a result of NETosis (Alhede et al. 2020b) in accordance with the increased proportion of lasR mutants observed in infections.
A total of two well-known examples of QS-regulated compounds produced by P. aeruginosa are rhamnolipid, the rhamnose-containing glycolipid biosurfactant, and phenazines, which are extracellular redox-active compounds. Rhamnolipid can cause lysis of PMNs (Jensen et al. 2007) and macrophages (McClure and Schiller 1992), while pyocyanin can impose oxidative stress in human airway cells, by generating superoxide leading to the depletion of intracellular NADPH stores (Rada et al. 2008).
Functional mutations in above-mentioned systems can thus potentially change the local microenvironment surrounding the bacteria in the infection sites.
The nucleotide-based intracellular signaling molecule c-di-GMP works as a switch between a motile bacterial state and a sessile, biofilm mode of growth (Boyd and O'Toole 2012). Low intracellular concentrations of c-di-GMP favor cell motility, whereas a high concentration increases the expression of adhesion factors and extracellular matrix components, leading to cell aggregation. Pseudomonas aeruginosa isolated from CF patients displaying the rugose small-colony variant (RSCV) phenotype exhibit an elevated level of c-di-GMP caused by mutations in the wsp and yfi loci causing a hyperinflammatory phenotype (Starkey et al. 2009, Malone et al. 2010, Pestrak et al. 2018). This leads to high levels of c-di-GMP, which suggests a selection for the biofilm phenotype in prolonged infections (Smith et al. 2006, Blanka et al. 2015). However, it has recently been reported that aggregates and single cells can be found in equal proportions in a range of acute and chronic pulmonary diseases (Kolpen et al. 2022). C-di-GMP regulates many other cellular functions besides aggregation, so it remains unresolved whether the same mutations are found across both aggregates and single cells in long-term infections.
Treatment of biofilms based on microenvironmental characteristics
Tolerance toward antibiotics in biofilms is recognized as a major cause of therapeutic failure during chronic infection, but the mechanisms of antimicrobial tolerance in vivo are not completely understood (Walters et al. 2003). As part of the respiratory burst of PMNs attempting to eradicate bacteria, O2 is consumed in the formation of ROS and reactive nitrogen species (RNS; via the inducible NO synthase; Kolpen et al., 2010, 2014a). Decreased O2 tension in the biofilm environment induces reduced, hibernation-like metabolism characterized by anaerobic respiration (Kolpen et al. 2015). Consequently, the efficacy of antibiotics targeting metabolically active bacteria is reduced (Sønderholm et al. 2017, Van Acker and Coenye 2017, Crabbé et al. 2019, Jensen et al. 2019b).
Limited O2-supply in bacterial biofilms has been demonstrated in several infections, such as necrotizing soft-tissue infections (NSTI; Siemens et al. 2016), cerebral abscesses, certain implant-related cerebral infections, refractory osteomyelitis, chronic ischemic ulcers, and pulmonary lung infections (Bartek et al. 2018, Moon 2019). Therefore, bacteria are subject to a hypoxic or even anoxic microenvironment affecting their sensitivity to certain types of antibiotics intended for infection control (Sønderholm et al. 2017, Jensen et al. 2019b).
Stratification of O2 in biofilm aggregates grown in vitro confers tolerance to several commonly used antibiotics due to limited O2 availability toward the center of the aggregates (Walters et al. 2003, Pamp et al. 2008). Common types of antibiotics, such as aminoglycosides, beta-lactams, and quinolones, target processes linked to the tricarboxylic acid (TCA) cycle in metabolically active bacteria, leading to formation of toxic ROS that contribute to the bactericidal activity of the antibiotic during aerobic respiration (Pakman 1971, Van Acker et al. 2013, Brochmann et al. 2014, Dwyer et al. 2014, Jensen et al. 2014, Haj et al. 2021). The bactericidal activity of quinolones and aminoglycosides decreases when the availability of O2 is reduced (Borriello et al. 2004, Brochmann et al. 2014). The slow bacterial growth associated with low levels of O2 (Schreiber et al. 2007) may, therefore, contribute to tolerance against both quinolones and aminoglycosides in biofilms as well as in planktonic cultures (Cozens et al. 1986, Tuomanen et al. 1986, Evans et al. 1991).
Hyperbaric oxygen treatment
To overcome antibiotic tolerance in biofilms, introducing more O2 may activate aerobic respiration and, thus increase the susceptibility of pathogens to several antibiotics that target metabolically active bacteria (Fig. 3). The addition of extra O2 by hyperbaric oxygen treatment (HBOT) can significantly enhance the efficacy of antibiotic treatment in vitro (Mader et al. 1980, Lima et al. 2015, Kolpen et al. 2016) and has been shown to enhance antibiotic activity during experimental in vivo biofilm infections (Stewart et al. 1999, Kolpen et al. 2016, Özkan et al. 2016). Biofilm infections that may become susceptible to antibiotics through the use of oxygenation include endocarditis (Özkan et al. 2016, Lerche et al. 2017), osteomyelitis (Yu et al. 2011), brain abscesses (Bartek et al. 2016, Kutlay et al.2005), and device-related infections (Bartek et al. 2018). However, the clinical effects of HBOT treatment on infections are mainly available from pro and retrospective case-control studies (Thom 2011), whereas randomized, controlled trials are still lacking. Traditionally, the rationale for the use of HBOT, especially for necrotizing soft tissue infections, is based on retro and prospective clinical and preclinical data showing a bacteriostatic effect on anaerobic bacterial growth and reduction in the production of bacterial toxins (Moon 2019). However, recent preclinical data suggest that it is the combination of HBOT with certain types of antibiotics that contributes to infection control, as bacteria are subject to metabolic adaptations to the biofilm environment in which O2 is involved (Sønderholm et al. 2017, Jensen et al. 2019b).
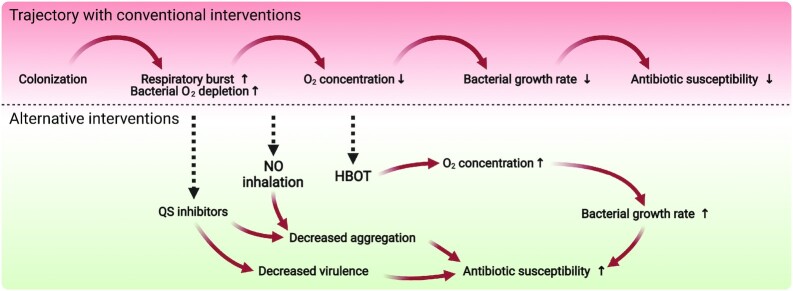
Figure 3.
Bacterial colonization can lead to acute infections, which in healthy individuals are usually cleared by the immune response and in some cases with the aid of antibiotics. In immunocompromised patients, the infection can progress into a chronic state characterized by a continuous inflammatory response with collateral tissue damage, hypoxic conditions, and low bacterial growth rates, resulting in low antibiotic susceptibility. Alternative antipathogenic strategies include the use of QS inhibitors or quorum quenching enzymes to decrease bacterial expression of virulence factors and biofilm formation. The QS system has been shown be lost or inactive in late infection stages so the efficacy of using QS inhibitors is most likely restricted to a certain time window. The low growth rates and high antibiotic susceptibility of bacteria in chronic infections can be reversed by treating with supplemental O2 by breathing pure oxygen in either normo or hyperbaric conditions. The associated higher tissue concentrations of O2 will lead to increased bacterial growth rates and higher susceptibility toward antibiotics targeting metabolically active bacteria. Alternatively, inhalation of NO can lead to upregulation of phosphodiesterases that break down the biofilm promoting molecule cyclic-di-GMP resulting in disaggregation.
The amount of dissolved O2 is proportional to its partial pressure at a specific temperature, according to Henry's law (Trayhurn 2019). Therefore, the standard therapy of HBOT exploits this phenomenon by increasing the pressure and reducing the volume of gas-filled spaces according to Boyle's law (Thom 2011). The state of hyperoxia obtained using HBOT is a treatment modality, in which patients breathe 100% O2 at increased atmospheric pressure (ATA) of up to 2.0–2.8 bar to enhance the amount of O2 dissolved in the body tissues. During HBOT, arterial O2 tension typically exceeds 2000 mmHg, and levels of 200–400 mmHg occur in tissues (Thom 1989, Choudhury 2018).
Reoxygenation by HBOT in an agarose P. aeruginosa biofilm model with slow-growing bacterial subpopulations in O2-free zones leads to increased susceptibility to antibiotics (Kolpen et al., 2016, 2017, Møller et al. 2019). In combination with tobramycin treatment, reoxygenation with HBOT enhanced the killing of clinical P. aeruginosa isolates from CF patients grown as biofilm more than a million times (Møller et al. 2019), while a combination HBOT and ciprofloxacin treatment enhanced the eradication of P. aeruginosa biofilm more than 100 times (Kolpen et al., 2016, 2017). HBOT also reduced the amount of tobramycin needed to achieve the clinically relevant biofilm bactericidal concentration (BBC) by more than 50% (Møller et al. 2019).
NO treatment
Another potential treatment of infections involves NO, which is an effective dispersal agent of bacterial biofilms that can lead to increased susceptibility to antimicrobials (Barraud et al. 2006). Here, NO acts as a signaling molecule leading to upregulation of phosphodiesterases that break down the biofilm promoting molecule cyclic-di-GMP (Barraud et al. 2009). In a randomized clinical trial, adjunctive NO was shown to decrease P. aeruginosa aggregate sizes in lungs of CF patients and to induce biofilm dispersal and decreased tolerance toward tobramycin and ceftazidime ex vivo (Howlin et al. 2017). Furthermore, NO is a potential CF therapeutic due to its mucolytic and bactericidal properties (Reighard et al. 2017, Ahonen et al. 2019), where NO can e.g. be released by polymers or nanoparticles with superior bactericidal and mucolytic action (Yepuri et al. 2013, Barraud et al. 2015, Rouillard et al. 2020).
QS inhibition
Novel antipathogenic strategies beyond the use of antibiotics have gained considerable attention over the past few decades as alternative methods alleviating the increasing challenge from antibiotic resistance and tolerance in bacterial infections. Degradation of signal molecules to change the functionality of the QS system using enzymes (quorum quenching) and chemical compounds for inhibiting the functionality of the system (QS inhibitors, or QSIs) are two ways of targeting bacterial virulence (Fig. 3). Several studies have identified potent QSIs with highly diverse molecular structures originating both from natural sources (Jakobsen et al. 2012a,b, Chatterjee et al. 2017, Cheng et al. 2020) and synthetic compound libraries (Borlee et al. 2010, de Lima Pimenta et al. 2013, Starkey et al. 2014). The change from QS-proficient to QS-deficient P. aeruginosa isolates due to increasing lasR mutants during infection (Jiricny et al. 2014, Cornforth et al. 2018) raises questions about targeting the QS system for the treatment of chronic infections in particular. However, the loss of a functional Las system supports the Rhl and Pseudomonas quinolone signal (PQS) parts of the QS system as a focus for treatment, maybe especially in the early infection state. A range of other possible limitations in the use of QSIs have been identified. For example, low selectivity of quorum quenching substances could possibly lead to disturbance of the commensal microbiome and opposing effects on virulence have been reported, where some species showed increased aggregation (see Krzyżek 2019 for a recent review).
Conclusion
In summary, the structural organization of bacteria in chronic infections and derived microenvironmental consequences for the pathogens are still not completely resolved, and the involved bacteria are not necessarily organized solely as aggregates but also as single cells (Kolpen et al. 2022). Bacterial biofilm aggregates are typically small and surrounded by host immune cells (Bjarnsholt et al., 2009, 2013, Jensen et al. 2017), and individual aggregates in multispecies infections are mainly composed of single species (Burmølle et al. 2010, Kvich et al. 2020). The growth rates of bacteria in infections are slow due to substrate limitation (Kragh et al. 2014), hypoxic zones are often present (Worlitzsch et al. 2002, James et al. 2016), and high doses of antibiotics are not able to eradicate all bacteria in such cases (Jensen et al. 2019a).
It is of paramount importance to improve our understanding of the infectious microenvironment, which is highly dynamic as the infection progresses and exhibits distinct changes in both physico-chemical properties as well as the gene expression profiles of both host and microbe. We argue that such information should be put into context, depending on the scientific question asked, and adapted for relevant in vitro models. New tools are being developed to validate in vitro models against the transcriptome of both bacteria and host cells in infections (Cornforth et al. 2020). The use of alternative interventions for biofilm eradication is still in its infancy compared to conventional antibacterial therapies and clinical trials are missing to get a better understanding of their efficacy. Further, we suggest that a better simulation of the infectious microenvironment, combined with relevant in vitro testing of clinical isolates, is needed for the development of optimized treatment strategies.
Authors' contributions
M.L. and T.B. conceived and outlined the review. M.L. initiated the first draft and T.H.J., M.I.K., M.K., P.Ø.J., and T.B. all added significantly to the review. All authors have edited and approved the review.
ACKNOWLEDGEMENTS
We thank Phil Stewart for initial help with the calculations for Fig. 1. Figures were prepared in Biorender (biorender.com).
Contributor Information
Mads Lichtenberg, Costerton Biofilm Center, Department of Immunology and Microbiology, University of Copenhagen, Blegdamsvej 3B, 2200, København, Denmark.
Tim Holm Jakobsen, Costerton Biofilm Center, Department of Immunology and Microbiology, University of Copenhagen, Blegdamsvej 3B, 2200, København, Denmark.
Michael Kühl, Marine Biological Section, Department of Biology, University of Copenhagen, Strandpromenaden 5, 3000 Helsingør, Denmark.
Mette Kolpen, Department of Clinical Microbiology, Copenhagen University Hospital, Ole Maaløes vej 26, 2200, København, Denmark.
Peter Østrup Jensen, Costerton Biofilm Center, Department of Immunology and Microbiology, University of Copenhagen, Blegdamsvej 3B, 2200, København, Denmark; Department of Clinical Microbiology, Copenhagen University Hospital, Ole Maaløes vej 26, 2200, København, Denmark.
Thomas Bjarnsholt, Costerton Biofilm Center, Department of Immunology and Microbiology, University of Copenhagen, Blegdamsvej 3B, 2200, København, Denmark; Department of Clinical Microbiology, Copenhagen University Hospital, Ole Maaløes vej 26, 2200, København, Denmark.
Conflicts of interest statement
The authors declare no conflicts of interest.
Funding
This work was supported by grants from the Lundbeck Foundation (grant number R250-2017-633 to M.L. and R105-A9791 to T.B.), the Novo Nordisk Foundation (Challenge programme #0056411 to T.B. and Tandem programme NNF16OC0023482 to M.K.), and the Independent Research Fund Denmark (grant number DFF-8022–00301B and DFF-8021–00308B to M.I.K.).
References
- Ahonen MJR, Hill DB, Schoenfisch MH. Nitric oxide-releasing alginates as mucolytic agents. ACS Biomater Sci Eng. 2019;5:3409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhede M, Alhede M, Qvortrup Ket al. . The origin of extracellular DNA in bacterial biofilm infections in vivo. Pathog Dis. 2020b;78: ftaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhede M, Lorenz M, Fritz BGet al. . Bacterial aggregate size determines phagocytosis efficiency of polymorphonuclear leukocytes. Med Microbiol Immunol. 2020a;209:669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciola CR, Campoccia D, Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol. 2018;16:397–409. [DOI] [PubMed] [Google Scholar]
- Aristoteleus AC, Grabovsky Y, Klapper I. Heterogeneity formation within biofilm systems. Eur J Appl Math. 2018;62:1–15. [Google Scholar]
- Aristotelous AC, Klapper I, Grabovsky Yet al. . Diffusive transport through a model host-biofilm system. Phys Rev E. 2015;92:2517–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster CR, Marshall CW, Garber AIet al. . Adaptation and genomic erosion in fragmented Pseudomonas aeruginosa populations in the sinuses of people with cystic fibrosis. Cell Rep. 2021;37:109829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimi S, Klementiev AD, Whiteley Met al. . Bacterial quorum sensing during infection. Annu Rev Microbiol. 2020;74: 201–19. [DOI] [PubMed] [Google Scholar]
- Barraud N, Hassett DJ, Hwang SHet al. . Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J Bacteriol. 2006;188:7344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud N, Kelso MJ, Rice SAet al. . Nitric oxide: a key mediator of biofilm dispersal with applications in infectious diseases. Curr Pharm Des. 2015;21:31–42. [DOI] [PubMed] [Google Scholar]
- Barraud N, Schleheck D, Klebensberger Jet al. . Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J Bacteriol. 2009;191:7333–42.. DOI: 10.1128/JB.00975-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartek J, Jakola AS, Skyrman Set al. . Hyperbaric oxygen therapy in spontaneous brain abscess patients: a population-based comparative cohort study. Acta Neurochir. 2016;158:1259–67. [DOI] [PubMed] [Google Scholar]
- Bartek JJ, Skyrman S, Nekludov Met al. . Hyperbaric oxygen therapy as adjuvant treatment for hardware-related infections in neuromodulation. Stereotact Funct Neurosurg. 2018;96:100–7. [DOI] [PubMed] [Google Scholar]
- Bay L, Kragh KN, Eickhardt SRet al. . Bacterial aggregates establish at the edges of acute epidermal wounds. Adv Wound Care. 2018;7:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belotsky SM, Guzu EV, Karlov VAet al. . Wound tissue respiratory burst and local microbial inflammation. Inflammation. 1990;14:663–8. [DOI] [PubMed] [Google Scholar]
- Besemer K, Peter H, Logue JBet al. . Unraveling assembly of stream biofilm communities. ISME J. 2012;6:1459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnsholt T, Alhede M, Alhede Met al. . The in vivo biofilm. Trends Microbiol. 2013;21:466–74. [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T, Høiby N, Donelli Get al. . Understanding biofilms—are we there yet?. FEMS Immunol Med Microbiol. 2012;65:125–6. [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T, Jensen PØ, Fiandaca MJet al. . Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol. 2009;44:547–58. [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T, Kirketerp-Møller K, Jensen PØet al. . Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen. 2008;16:2–10. [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T, PØ Jensen, Jakobsen THet al. . Quorum sensing and virulence of Pseudomonas aeruginosa during lung infection of cystic fibrosis patients. PLoS ONE. 2010;5:e10115–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnsholt T, Whiteley M, Rumbaugh KPet al. . The importance of understanding the infectious microenvironment. Lancet Infect Dis. 2021;22:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanka A, Düvel J, Dötsch Aet al. . Constitutive production of c-di-GMP is associated with mutations in a variant of Pseudomonas aeruginosa with altered membrane composition. Sci Signal. 2015;8:ra36. [DOI] [PubMed] [Google Scholar]
- Borlee BR, Geske GD, Blackwell HEet al. . Identification of synthetic inducers and inhibitors of the quorum-sensing regulator LasR in Pseudomonas aeruginosa by high-throughput screening. Appl Environ Microbiol. 2010;76:8255–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borriello G, Werner E, Roe Fet al. . Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob Agents Chemother. 2004;48:2659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen WH, Burne RA, Wu Het al. . Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018;26:229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd CD, O'Toole GA. Second messenger regulation of biofilm formation: breakthroughs in understanding c-di-GMP effector systems. Annu Rev Cell Dev Biol. 2012;28:439–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridier A, Dubois-Brissonnet F, Boubetra Aet al. . The biofilm architecture of sixty opportunistic pathogens deciphered using a high throughput CLSM method. J Microbiol Methods. 2010;82:64–70. [DOI] [PubMed] [Google Scholar]
- Brochmann RP, Toft A, Ciofu Oet al. . Bactericidal effect of colistin on planktonic Pseudomonas aeruginosa is independent of hydroxyl radical formation. Int J Antimicrob Agents. 2014;43:140–7. [DOI] [PubMed] [Google Scholar]
- Bue M, Hanberg P, Koch Jet al. . Single-dose bone pharmacokinetics of vancomycin in a porcine implant-associated osteomyelitis model. J Orthop Res. 2017;36:68–6. [DOI] [PubMed] [Google Scholar]
- Burmølle M, Thomsen TR, Fazli Met al. . Biofilms in chronic infections - a matter of opportunity - monospecies biofilms in multispecies infections. FEMS Immunol Med Microbiol. 2010;59:324–36. [DOI] [PubMed] [Google Scholar]
- Caballero JD, Clark ST, Coburn Bet al. . Selective sweeps and parallel pathoadaptation drive Pseudomonas aeruginosa evolution in the cystic fibrosis lung. mBio. 2015;6:e00981–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, D'Morris S, Paul Vet al. . Mechanistic understanding of phenyllactic acid mediated inhibition of quorum sensing and biofilm development in Pseudomonas aeruginosa. Appl Microbiol Biotechnol. 2017;101:8223–36. [DOI] [PubMed] [Google Scholar]
- Cheng W-J, Zhou J-W, Zhang P-Pet al. . Quorum sensing inhibition and tobramycin acceleration in Chromobacterium violaceum by two natural cinnamic acid derivatives. Appl Microbiol Biotechnol. 2020;104:5025–37. [DOI] [PubMed] [Google Scholar]
- Choudhury R. Hypoxia and hyperbaric oxygen therapy: a review. Int J Gen Med. 2018;11:431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofu O, Mandsberg LF, Bjarnsholt Tet al. . Genetic adaptation of Pseudomonas aeruginosa during chronic lung infection of patients with cystic fibrosis: strong and weak mutators with heterogeneous genetic backgrounds emerge in mucA and/or lasR mutants. Microbiology. 2010;156:1108–19. [DOI] [PubMed] [Google Scholar]
- Ciofu O, Tolker-Nielsen T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents - how P. aeruginosa can escape antibiotics. Front Microbiol. 2019;10:114–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornforth DM, Dees JL, Ibberson CBet al. . Pseudomonas aeruginosa transcriptome during human infection. Proc Natl Acad Sci USA. 2018;115:E5125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornforth DM, Diggle FL, Melvin JAet al. . Quantitative framework for model evaluation in microbiology research using Pseudomonas aeruginosa and cystic fibrosis infection as a test case. mBio. 2020;11:e03042–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens RM, Tuomanen E, Tosch Wet al. . Evaluation of the bactericidal activity of beta-lactam antibiotics on slowly growing bacteria cultured in the chemostat. Antimicrob Agents Chemother. 1986;29:797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbé A, Jensen PØ, Bjarnsholt Tet al. . Antimicrobial tolerance and metabolic adaptations in microbial biofilms. Trends Microbiol. 2019;27:850–63. [DOI] [PubMed] [Google Scholar]
- D'Argenio DA, Wu M, Hoffman LRet al. . Growth phenotypes of Pseudomonas aeruginosa lasR mutants adapted to the airways of cystic fibrosis patients. Mol Microbiol. 2007;64:512–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darch SE, Koley D. Quantifying microbial chatter: scanning electrochemical microscopy as a tool to study interactions in biofilms. Proc R Soc A. 2018;474:20180405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darch SE, Kragh KN, Abbott EAet al. . Phage inhibit pathogen dissemination by targeting bacterial migrants in a chronic infection model. mBio. 2017;8:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darch SE, Simoska O, Fitzpatrick Met al. . Spatial determinants of quorum signaling in a Pseudomonas aeruginosa infection model. Proc Natl Acad Sci USA. 2018;115:4779–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima Pimenta A, Chiaradia-Delatorre LD, Mascarello Aet al. . Synthetic organic compounds with potential for bacterial biofilm inhibition, a path for the identification of compounds interfering with quorum sensing. Int J Antimicrob Agents. 2013;42:519–23. [DOI] [PubMed] [Google Scholar]
- Debats IBJG, Booi D, Deutz NEPet al. . Infected chronic wounds show different local and systemic arginine conversion compared with acute wounds. J Surg Res. 2006;134:205–14. [DOI] [PubMed] [Google Scholar]
- DePas WH, Starwalt-Lee R, Van Sambeek Let al. . Exposing the three-dimensional biogeography and metabolic states of pathogens in cystic fibrosis sputum via hydrogel embedding, clearing, and rRNA labeling. mBio. 2016;7:e00796–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depetris A, Peter H, Bordoloi ADet al. . Morphogenesis and oxygen dynamics in phototrophic biofilms growing across a gradient of hydraulic conditions. iScience. 2021;24:102067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depetris A, Tagliavini G, Peter Het al. . Biophysical properties at patch scale shape the metabolism of biofilm landscapes. npj Biofilms Microbiomes. 2022;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle SP, Griffin AS, Campbell GSet al. . Cooperation and conflict in quorum-sensing bacterial populations. Nature. 2007;450:411–4. [DOI] [PubMed] [Google Scholar]
- DiMango E, Zar HJ, Bryan Ret al. . Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Invest. 1995;96:2204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd SE, Sun Y, Secor PRet al. . Survey of bacterial diversity in chronic wounds using pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiol. 2008;8:43–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer DJ, Belenky PA, Yang JHet al. . Antibiotics induce redox-related physiological alterations as part of their lethality. Proc Natl Acad Sci USA. 2014;111:E2100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egland PG, Palmer RJ, Kolenbrander PE. Interspecies communication in Streptococcus gordonii–Veillonella atypica biofilms: signaling in flow conditions requires juxtaposition. Proc Natl Acad Sci USA. 2004;101:16917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DJ, Allison DG, Brown MRWet al. . Susceptibility of Pseudomonas aeruginosa and Escherichia coli biofilms towards ciprofloxacin: effect of specific growth rate. J Antimicrob Chemother. 1991;27:177–84. [DOI] [PubMed] [Google Scholar]
- Fazli M, Bjarnsholt T, Kirketerp-Møller Ket al. . Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J Clin Microbiol. 2009;47:4084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazli M, Bjarnsholt T, Kirketerp-Møller Ket al. . Quantitative analysis of the cellular inflammatory response against biofilm bacteria in chronic wounds. Wound Repair Regen. 2011;19:387–91. [DOI] [PubMed] [Google Scholar]
- Feltner JB, Wolter DJ, Pope CEet al. . LasR variant cystic fibrosis isolates reveal an adaptable quorum-sensing hierarchy in Pseudomonas aeruginosa. mBio. 2016;7:e01513–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming H-C. Microbial biofouling: unsolved problems, insufficient approaches, and possible solutions. In: Biofilm Highlights. Vol 5. Berlin, Heidelberg: Springer, 2011, 81–109. [Google Scholar]
- Folkesson A, Jelsbak L, Yang Let al. . Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol. 2012;10:841–51. [DOI] [PubMed] [Google Scholar]
- Frykberg RG, Franks PJ, Edmonds Met al. . A multinational, multicenter, randomized, double-blinded, placebo-controlled trial to evaluate the efficacy of cyclical topical wound oxygen (TWO2) therapy in the treatment of chronic diabetic foot ulcers: the TWO2 study. Diabetes Care. 2020;43:616–24. [DOI] [PubMed] [Google Scholar]
- Gantner S, Schmid M, Dürr Cet al. . In situ quantitation of the spatial scale of calling distances and population density-independent N-acylhomoserine lactone-mediated communication by rhizobacteria colonized on plant roots. FEMS Microbiol Ecol. 2006;56:188–94. [DOI] [PubMed] [Google Scholar]
- Garwood R. Patterns in palaeontology: the first 3 billion years of evolution. Palaeontology. 2012;2:1–22. [Google Scholar]
- Goltermann L, Tolker-Nielsen T. Importance of the exopolysaccharide matrix in antimicrobial tolerance of Pseudomonas aeruginosa aggregates. Antimicrob Agents Chemother. 2017;61:e02696–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottrup F, Dissemond J, Baines Cet al. . Use of oxygen therapies in wound healing. J Wound Care. 2017;26:S1–S43. [DOI] [PubMed] [Google Scholar]
- Haj El C, Lichtenberg M, Nielsen KLet al. . Catalase protects biofilm of Staphylococcus aureus against daptomycin activity. Antibiotics. 2021;10:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. [DOI] [PubMed] [Google Scholar]
- Hansen SK, Rau MH, Johansen HKet al. . Evolution and diversification of Pseudomonas aeruginosa in the paranasal sinuses of cystic fibrosis children have implications for chronic lung infection. ISME J. 2012;6:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett DJ, Sutton MD, Schurr MJet al. . Pseudomonas aeruginosa hypoxic or anaerobic biofilm infections within cystic fibrosis airways. Trends Microbiol. 2009;17:130–8. [DOI] [PubMed] [Google Scholar]
- Heurlier K, Dénervaud V, Haas D. Impact of quorum sensing on fitness of Pseudomonas aeruginosa. Int J Med Microbiol. 2006;296:93–102. [DOI] [PubMed] [Google Scholar]
- Høiby N, Ciofu O, Bjarnsholt T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010;5:1663–74. [DOI] [PubMed] [Google Scholar]
- Howlin RP, Cathie K, Hall-Stoodley Let al. . Low-dose nitric oxide as targeted anti-biofilm adjunctive therapy to treat chronic Pseudomonas aeruginosa infection in cystic fibrosis. Mol Ther. 2017;25:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt TK, Zederfeldt B, Goldstick TK. Oxygen and healing. Am J Surg. 1969;118:521–5. [DOI] [PubMed] [Google Scholar]
- Jakobsen TH, Bragason SK, Phipps RKet al. . Food as a source for quorum sensing inhibitors: iberin from horseradish revealed as a quorum sensing inhibitor of Pseudomonas aeruginosa. Appl Environ Microbiol. 2012a;78:2410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen TH, Eickhardt SR, Gheorghe AGet al. . Implants induce a new niche for microbiomes. APMIS. 2018;126:685–92. [DOI] [PubMed] [Google Scholar]
- Jakobsen TH, van Gennip M, Phipps RKet al. . Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob Agents Chemother. 2012b;56:2314–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James GA, Ge Zhao A, Usui Met al. . Microsensor and transcriptomic signatures of oxygen depletion in biofilms associated with chronic wounds. Wound Repair Regen. 2016;24:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James GA, Swogger E, Wolcott Ret al. . Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37–44. [DOI] [PubMed] [Google Scholar]
- Jelsbak L, Johansen HK, Frost ALet al. . Molecular epidemiology and dynamics of Pseudomonas aeruginosa populations in lungs of cystic fibrosis patients. Infect Immun. 2007;75:2214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen LK, Bjarnsholt T, Kragh KNet al. . In vivo gentamicin susceptibility test for prevention of bacterial biofilms in bone tissue and on implants. Antimicrob Agents Ch. 2019a;63:e01889–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PØ, Bjarnsholt T, Phipps Ret al. . Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology. 2007;153:1329–38. [DOI] [PubMed] [Google Scholar]
- Jensen PØ, Briales A, Brochmann RPet al. . Formation of hydroxyl radicals contributes to the bactericidal activity of ciprofloxacin against Pseudomonas aeruginosa biofilms. Pathog Dis. 2014;70:440–3. [DOI] [PubMed] [Google Scholar]
- Jensen PØ, Givskov M, Bjarnsholt Tet al. . The immune system vs. Pseudomonas aeruginosa biofilms. FEMS Immunol Med Microbiol. 2010;59:292–305. [DOI] [PubMed] [Google Scholar]
- Jensen PØ, Kolpen M, Kragh KNet al. . Microenvironmental characteristics and physiology of biofilms in chronic infections of CF patients are strongly affected by the host immune response. APMIS. 2017;125:276–88. [DOI] [PubMed] [Google Scholar]
- Jensen PØ, Møller SA, Lerche CJet al. . Improving antibiotic treatment of bacterial biofilm by hyperbaric oxygen therapy: not just hot air. Biofilm. 2019b;1:100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiricny N, Molin S, Foster Ket al. . Loss of social behaviours in populations of Pseudomonas aeruginosa infecting lungs of patients with cystic fibrosis. PLoS ONE. 2014;9:e83124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T, Kuroki M, Ishii Met al. . Differential expression of multiple terminal oxidases for aerobic respiration in Pseudomonas aeruginosa. Environ Microbiol. 2010;12:1399–412. [DOI] [PubMed] [Google Scholar]
- Kirketerp-Møller K, Jensen PØ, Fazli Met al. . Distribution, organization, and ecology of bacteria in chronic wounds. J Clin Microbiol. 2008;46:2717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler T, Guanella R, Carlet Jet al. . Quorum sensing-dependent virulence during Pseudomonas aeruginosa colonisation and pneumonia in mechanically ventilated patients. Thorax. 2010;65:703–10. [DOI] [PubMed] [Google Scholar]
- Kolpen M, Bjarnsholt T, Moser Cet al. . Nitric oxide production by polymorphonuclear leucocytes in infected cystic fibrosis sputum consumes oxygen. Clin Exp Immunol. 2014a;177:310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpen M, Hansen CR, Bjarnsholt Tet al. . Polymorphonuclear leucocytes consume oxygen in sputum from chronic Pseudomonas aeruginosa pneumonia in cystic fibrosis. Thorax. 2010;65:57–62. [DOI] [PubMed] [Google Scholar]
- Kolpen M, Kragh KN, Bjarnsholt Tet al. . Denitrification by cystic fibrosis pathogens - Stenotrophomonas maltophilia is dormant in sputum. Int J Med Microbiol. 2015;305:1–10. [DOI] [PubMed] [Google Scholar]
- Kolpen M, Kragh KN, Enciso JBet al. . Bacterial biofilms predominate in both acute and chronic human lung infections. Thorax. 2022;35017313. DOI: 10.1136/thoraxjnl-2021-217576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpen M, Kühl M, Bjarnsholt Tet al. . Nitrous oxide production in sputum from cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. PLoS ONE. 2014b;9:e84353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpen M, Lerche CJ, Kragh KNet al. . Hyperbaric oxygen sensitizes anoxic Pseudomonas aeruginosa biofilm to ciprofloxacin. Antimicrob Agents Ch. 2017;61:e01024–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpen M, Mousavi N, Sams Tet al. . Reinforcement of the bactericidal effect of ciprofloxacin on Pseudomonas aeruginosa biofilm by hyperbaric oxygen treatment. Int J Antimicrob Agents. 2016;47:163–7. [DOI] [PubMed] [Google Scholar]
- Kopf SH, Sessions AL, Cowley ESet al. . Trace incorporation of heavy water reveals slow and heterogeneous pathogen growth rates in cystic fibrosis sputum. Proc Natl Acad Sci USA. 2016;113:E110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostylev M, Kim DY, Smalley NEet al. . Evolution of the Pseudomonas aeruginosa quorum-sensing hierarchy. Proc Natl Acad Sci USA. 2019;116:7027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh KN, Alhede M, Jensen PØet al. . Polymorphonuclear leukocytes restrict growth of Pseudomonas aeruginosa in the lungs of cystic fibrosis patients. Infect Immun. 2014;82:4477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh KN, Hutchison JB, Melaugh Get al. . Role of multicellular aggregates in biofilm formation. mBio. 2016;7:e00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyżek P. Challenges and limitations of anti-quorum sensing therapies. Front Microbiol. 2019;10:2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühl M, Rickelt LF, Thar R. Combined imaging of bacteria and oxygen in biofilms. Appl Environ Microbiol. 2007;73:6289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlay M, Colak A, Yıldız Şet al. . Stereotactic aspiration and antibiotic treatment combined with hyperbaric oxygen therapy in the management of bacterial brain abscesses. FEMS Microbiol Lett. 2005;57:1140–6. [PubMed] [Google Scholar]
- Kvich L, Burmølle M, Bjarnsholt Tet al. . Do mixed-species biofilms dominate in chronic infections? Need for in situ visualization of bacterial organization. Front Cell Infect Microbiol. 2020;10:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa R, Johansen HK, Molin S. Adapting to the airways: metabolic requirements of Pseudomonas aeruginosa during the infection of cystic fibrosis patients. Metabolites. 2019;9:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie EG, Wangdi T, Kazmierczak BI. Innate immune responses to Pseudomonas aeruginosa infection. Microbes Infect. 2011;13: 1133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeaux D, Chauhan A, Rendueles Oet al. . From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens. 2013;2:288–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerche CJ, Christophersen LJ, Kolpen Met al. . Hyperbaric oxygen therapy augments tobramycin efficacy in experimental Staphylococcus aureus endocarditis. Int J Antimicrob Agents. 2017;50:406–12. [DOI] [PubMed] [Google Scholar]
- Lichtenberg M, Line L, Schrameyer Vet al. . Nitric-oxide-driven oxygen release in anoxic Pseudomonas aeruginosa. iScience. 2021;24:103404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima FL, Joazeiro PP, Lancellotti Met al. . Effects of hyperbaric oxygen on Pseudomonas aeruginosa susceptibility to imipenem and macrophages. Future Microbiol. 2015;10:179–89. [DOI] [PubMed] [Google Scholar]
- Lopatkin AJ, Bening SC, Manson ALet al. . Clinically relevant mutations in core metabolic genes confer antibiotic resistance. Science. 2021;371:eaba0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatkin AJ, Stokes JM, Zheng EJet al. . Bacterial metabolic state more accurately predicts antibiotic lethality than growth rate. Nat Microbiol. 2019;4:2109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz A, Pawar V, Haussler S, Weiss S. Insights into host-pathogen interactions from state-of-the-art animal models of respiratory Pseudomonas aeruginosa infections. FEBS Lett. 2016;590:3941–59. [DOI] [PubMed] [Google Scholar]
- Mader JT, Brown GL, Guckian JCet al. . A mechanism for the amelioration by hyperbaric oxygen of experimental Staphylococcal osteomyelitis in rabbits. J Infect Dis. 1980;142:915–22. [DOI] [PubMed] [Google Scholar]
- Malone JG, Jaeger T, Spangler Cet al. . YfiBNR mediates cyclic di-GMP dependent small colony variant formation and persistence in Pseudomonas aeruginosa. PLoS Pathog. 2010;6:e1000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone M, Bjarnsholt T, McBain AJet al. . The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J Wound Care. 2017;26:20–5. [DOI] [PubMed] [Google Scholar]
- McClure CD, Schiller NL. Effects of Pseudomonas aeruginosa rhamnolipids on human monocyte-derived macrophages. J Leukocyte Biol. 1992;51:97–102. [DOI] [PubMed] [Google Scholar]
- Meylan S, Porter CBM, Yang JHet al. . Carbon sources tune antibiotic susceptibility in Pseudomonas aeruginosa via tricarboxylic acid cycle control. Cell Chem Biol. 2017;24:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller SA, Jensen PØ, Høiby Net al. . Hyperbaric oxygen treatment increases killing of aggregating Pseudomonas aeruginosa isolates from cystic fibrosis patients. J Cyst Fibros. 2019;18:657–64. [DOI] [PubMed] [Google Scholar]
- Moon RE. Hyperbaric Oxygen Therapy. 14 edn. Moon RE (ed.), North Palm Beach, FL: Best Publishing Company, 2019. [Google Scholar]
- Moser C, Jensen PØ, Thomsen Ket al. . Immune responses to Pseudomonas aeruginosa biofilm infections. Front Immunol. 2021;12:625597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser C, Pedersen HT, Lerche CJet al. . Biofilms and host response – helpful or harmful. APMIS. 2017;125:320–38. [DOI] [PubMed] [Google Scholar]
- Moura-Alves P, Faé K, Houthuys Eet al. . AhR sensing of bacterial pigments regulates antibacterial defence. Nature. 2014;512:387–92. [DOI] [PubMed] [Google Scholar]
- Moura-Alves P, Puyskens A, Stinn Aet al. . Host monitoring of quorum sensing during Pseudomonas aeruginosa infection. Science. 2019;366:eaaw1629. [DOI] [PubMed] [Google Scholar]
- Noisette F, Depetris A, Kühl Met al. . Flow and epiphyte growth effects on the thermal, optical and chemical microenvironment in the leaf phyllosphere of seagrass (Zostera marina). J Cyst Fibros. 2020;18:20200485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özkan MTA, Vural A, Çiçek ÖFet al. . Is hyperbaric oxygen or ozone effective in experimental endocarditis?. J Surg Res. 2016;202:66–70. [DOI] [PubMed] [Google Scholar]
- Pakman LM. Inhibition of Pseudomonas aeruginosa by hyperbaric oxygen. I. Sulfonamide activity enhancement and reversal. Infect Immun. 1971;4:479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamp SJ, Gjermansen M, Johansen HKet al. . Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexAB-oprM genes. Mol Microbiol. 2008;68:223–40. [DOI] [PubMed] [Google Scholar]
- Paul D, Achouri S, Yoon Y-Zet al. . Phagocytosis dynamics depends on target shape. Biophys J. 2013;105:1143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SS, Shand GH, Hansen BLet al. . Induction of experimental chronic Pseudomonas aeruginosa lung infection with P. aeruginosa entrapped in alginate microspheres. APMIS. 1990;98:203–11. [PubMed] [Google Scholar]
- Pestrak MJ, Chaney SB, Eggleston HCet al. . Pseudomonas aeruginosa rugose small-colony variants evade host clearance, are hyper- inflammatory, and persist in multiple host environments. PLoS Pathog. 2018;14:e1006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff AP, Sim MS, Maslov Aet al. . Biophysical basis for the geometry of conical stromatolites. Proc Natl Acad Sci USA. 2010;107:9956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettygrove BA, Kratofil RM, Alhede Met al. . Delayed neutrophil recruitment allows nascent Staphylococcus aureus biofilm formation and immune evasion. Biomaterials. 2021;275:120775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploug H, Kühl M, Buchholz-Cleven Bet al. . Anoxic aggregates - an ephemeral phenomenon in the pelagic environment?. Aquat Microb Ecol. 1997;13:285–94. [Google Scholar]
- Rada B, Lekstrom K, Damian Set al. . The Pseudomonas toxin pyocyanin inhibits the dual oxidase-based antimicrobial system as it imposes oxidative stress on airway epithelial cells. J Immunol. 2008;181:4883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reighard KP, Ehre C, Rushton ZLet al. . Role of nitric oxide-releasing chitosan oligosaccharides on mucus viscoelasticity. ACS Biomater Sci Eng. 2017;3:1017–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizner W, Hunter JG, O'Malley NTet al. . A systematic review of animal models for Staphylococcus aureus osteomyelitis. Eur Cell Mater. 2014;27:196–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouillard KR, Hill DB, Schoenfisch MH. Antibiofilm and mucolytic action of nitric oxide delivered via gas or macromolecular donor using in vitro and ex vivo models. J Cyst Fibros. 2020;19:1004–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudkjøbing VB, Thomsen TR, Alhede Met al. . The microorganisms in chronically infected end-stage and non-end-stage cystic fibrosis patients. FEMS Immunol Med Microbiol. 2012;65:236–44. [DOI] [PubMed] [Google Scholar]
- Ryall B, Carrara M, Zlosnik JEAet al. . The mucoid switch in Pseudomonas aeruginosa represses quorum sensing systems and leads to complex changes to stationary phase virulence factor regulation. PLoS ONE. 2014;9:e96166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybtke M, Jensen PØ, Nielsen CHet al. . The extracellular polysaccharide matrix of Pseudomonas aeruginosa biofilms is a determinant of polymorphonuclear leukocyte responses. Infect Immun. 2020;89:e00631–20.. DOI: 10.1128/IAI.00631-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber K, Boes N, Eschbach Met al. . Anaerobic survival of Pseudomonas aeruginosa by pyruvate fermentation requires an Usp-type stress protein. J Bacteriol. 2006;188:659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber K, Krieger R, Benkert Bet al. . The anaerobic regulatory network required for Pseudomonas aeruginosa nitrate respiration. J Bacteriol. 2007;189:4310–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreml S, Meier RJ, Kirschbaum Met al. . Luminescent dual sensors reveal extracellular pH-gradients and hypoxia on chronic wounds that disrupt epidermal repair. Theranostics. 2014;4:721–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK, Geringer MR, Galiano RDet al. . Quantitative comparison and analysis of species-specific wound biofilm virulence using an in vivo, rabbit-ear model. J Am Coll Surg. 2012;215:388–99. [DOI] [PubMed] [Google Scholar]
- Shen K, Sayeed S, Antalis Pet al. . Extensive genomic plasticity in Pseudomonas aeruginosa revealed by identification and distribution studies of novel genes among clinical isolates. Infect Immun. 2006;74:5272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemens N, Chakrakodi B, Shambat SMet al. . Biofilm in group A streptococcal necrotizing soft tissue infections. JCI Insight. 2016;1:e87882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med. 2008;341:738–46. [DOI] [PubMed] [Google Scholar]
- Singh PK, Schaefer AL, Parsek MRet al. . Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature Med. 2000;407:762–4. [DOI] [PubMed] [Google Scholar]
- Skopelja-Gardner S, Theprungsirikul J, Lewis KAet al. . Regulation of Pseudomonas aeruginosa-mediated neutrophil extracellular traps. Front Immunol. 2019;10:1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Buckley DG, Wu Zet al. . Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA. 2006;103:8487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sønderholm M, Bjarnsholt T, Alhede Met al. . The consequences of being in an infectious biofilm: microenvironmental conditions governing antibiotic tolerance. Int J Mol Sci. 2017;18:2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sønderholm M, Koren K, Wangpraseurt Det al. . Tools for studying growth patterns and chemical dynamics of aggregated Pseudomonas aeruginosa exposed to different electron acceptors in an alginate bead model. NPJ Biofilms Microbiomes. 2018;4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio V, Torres AG, Jarvis Bet al. . Bacteria-host communication: the language of hormones. Proc Natl Acad Sci USA. 2003;100:8951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey M, Hickman JH, Ma Let al. . Pseudomonas aeruginosa rugose small-colony variants have adaptations that likely promote persistence in the cystic fibrosis lung. J Bacteriol. 2009;191:3492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey M, Lepine F, Maura Det al. . Identification of anti-virulence compounds that disrupt quorum-sensing regulated acute and persistent pathogenicity. PLOS Pathogens. 2014;10:e1004321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PS, Wattanakaroon W, Goodrum Let al. . Electrolytic generation of oxygen partially explains electrical enhancement of tobramycin efficacy against Pseudomonas aeruginosa biofilm. Antimicrob Agents Ch. 1999;43:292–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PS. Diffusion in biofilms. J Bacteriol. 2003;185:1485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes JM, Lopatkin AJ, Lobritz MAet al. . Bacterial metabolism and antibiotic efficacy. Cell Metabolism. 2019;30:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweere JM, Ishak H, Sunkari Vet al. . The immune response to chronic Pseudomonas aeruginosa wound infection in immunocompetent mice. Adv Wound Care. 2020;9:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford G, Wheeler D, Williams Pet al. . The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone Has immunomodulatory activity. Infect Immun. 1998;66:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom SR. Hyperbaric oxygen – its mechanisms and efficacy. Plast Reconstr Surg. 2011;127:131S–41S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom SR. Hyperbaric oxygen therapy. J Intensive Care Med. 1989;4:58–74. [Google Scholar]
- Thomsen TR, Aasholm MS, Rudkjøbing VBet al. . The bacteriology of chronic venous leg ulcer examined by culture-independent molecular methods. Wound Repair Regen. 2010;18:38–49. [DOI] [PubMed] [Google Scholar]
- Trayhurn P. Oxygen—a critical, but overlooked, nutrient. Front Nutr. 2019;6:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trøstrup H, Thomsen K, Christophersen LJet al. . Pseudomonas aeruginosa biofilm aggravates skin inflammatory response in BALB/c mice in a novel chronic wound model. Wound Repair Regen. 2013;21:292–9. [DOI] [PubMed] [Google Scholar]
- Trunk K, Benkert B, Quaeck Net al. . Anaerobic adaptation in Pseudomonas aeruginosa: definition of the Anr and Dnr regulons. Environ Microbiol. 2010;12:1719–33. [DOI] [PubMed] [Google Scholar]
- Tuomanen E, Cozens R, Tosch Wet al. . The rate of killing of Escherichia coli by β-lactam antibiotics is strictly proportional to the rate of bacterial growth. J Gen Microbiol. 1986;132:1297–304. [DOI] [PubMed] [Google Scholar]
- Turner KH, Wessel AK, Palmer GCet al. . Essential genome of Pseudomonas aeruginosa in cystic fibrosis sputum. Proc Natl Acad Sci USA. 2015;112:4110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Acker H, Coenye T. The role of reactive oxygen species in antibiotic-mediated killing of bacteria. Trends Microbiol. 2017;25:456–66. [DOI] [PubMed] [Google Scholar]
- Van Acker H, Sass A, Bazzini Set al. . Biofilm-grown Burkholderia cepacia complex cells survive antibiotic treatment by avoiding production of reactive oxygen species. Plos ONE. 2013;8:e58943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwoude J, Fleming D, Azimi Set al. . The evolution of virulence in Pseudomonas aeruginosa during chronic wound infection. Proc Biol Sci R Soc. 2020;287:20202272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters MC, Roe F, Bugnicourt Aet al. . Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Ch. 2003;47:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters C, DeLeon K, Trivedi Uet al. . Pseudomonas aeruginosa biofilms perturb wound resolution and antibiotic tolerance in diabetic mice. Med Microbiol Immunol. 2013;202:131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert M, Kümmerli R. The physical boundaries of public goods cooperation between surface-attached bacterial cells. Proc R Soc B Med Microbiol Immunol. 2017;284:20170631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilgus TA, Roy S, McDaniel JC. Neutrophils and wound repair: positive actions and negative reactions. Adv Wound Care. 2013;2:379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worlitzsch D, Tarran R, Ulrich Met al. . Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Klapper I, Stewart PS. Hypoxia arising from concerted oxygen consumption by neutrophils and microorganisms in biofilms. Pathog Dis. 2018;76:fty043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Haagensen JAJ, Jelsbak Let al. . In situ growth rates and biofilm development of Pseudomonas aeruginosa populations in chronic lung infections. J Bacteriol. 2008;190:2767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Jelsbak L, Marvig RLet al. . Evolutionary dynamics of bacteria in a human host environment. Proc Natl Acad Sci USA. 2011a;108:7481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Rau MH, Yang Let al. . Bacterial adaptation during chronic infection revealed by independent component analysis of transcriptomic data. BMC Microbiol. 2011b;11:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates EA, Philipp B, Buckley Cet al. . N-acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect Immun. 2002;70:5635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepuri NR, Barraud N, Mohammadi NSet al. . Synthesis of cephalosporin-3-diazeniumdiolates: biofilm dispersing NO donor prodrugs activated by beta-lactamase. Chem Commun. 2013;49:4791–3. [DOI] [PubMed] [Google Scholar]
- Yu W-K, Chen Y-W, Shie H-Get al. . Hyperbaric oxygen therapy as an adjunctive treatment for sternal infection and osteomyelitis after sternotomy and cardiothoracic surgery. J Cardiothorac Surg. 2011;6:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]