Abstract
Over the past two decades, small noncoding RNAs (sRNAs) that regulate mRNAs by short base pairing have gone from a curiosity to a major class of post-transcriptional regulators in bacteria. They are integral to many stress responses and regulatory circuits, affecting almost all aspects of bacterial life. Following pioneering sRNA searches in the early 2000s, the field quickly focused on conserved sRNA genes in the intergenic regions of bacterial chromosomes. Yet, it soon emerged that there might be another rich source of bacterial sRNAs—processed 3′ end fragments of mRNAs. Several such 3′ end-derived sRNAs have now been characterized, often revealing unexpected, conserved functions in diverse cellular processes. Here, we review our current knowledge of these 3′ end-derived sRNAs—their biogenesis through ribonucleases, their molecular mechanisms, their interactions with RNA-binding proteins such as Hfq or ProQ and their functional scope, which ranges from acting as specialized regulators of single metabolic genes to constituting entire noncoding arms in global stress responses. Recent global RNA interactome studies suggest that the importance of functional 3′ end-derived sRNAs has been vastly underestimated and that this type of cross-regulation between genes at the mRNA level is more pervasive in bacteria than currently appreciated.
Keywords: 3′ UTR, sRNA, post-transcriptional control, regulatory networks, bacteria
There are a growing number of examples of bacterial mRNAs that produce small RNAs cleaved from their own 3′ ends to post-transcriptionally control other genes.
Introduction
Bacteria regulate and fine-tune gene expression at all levels, including post-transcriptional control mechanisms acting at the mRNA level. Evidence for post-transcriptional control of bacterial genes dates back to the early days of molecular biology (Wagner and Simons 1994). However, unlike gene control at the DNA level, which one readily associates with transcription factors (TFs), an abundant class of molecular factors that selectively target mRNAs was long unknown. This began to change when systematic searches in the early 2000s discovered hitherto unexpected numbers of small regulatory RNAs (sRNAs) in Escherichia coli (Argaman et al. 2001,Rivas et al. 2001, Wassarman et al. 2001, Chen et al. 2002 , Vogel et al. 2003 ).
The design of these foundational screens has strongly influenced our view of sRNAs. Based on the few sRNAs known at that time (Wassarman et al. 1999), these screens made two general assumption: (i) that sRNAs were primary transcripts of 100–200 nucleotides in length, produced from independent noncoding genes with their own promoter and Rho-independent terminator; and (ii) that they were encoded in otherwise empty intergenic regions (IGRs), reasonably spaced from the next open reading frame (ORF). Sequence conservation in the few other available enterobacterial genomes was considered an additional hallmark of a true sRNA gene (Argaman et al. 2001, Rivas et al. 2001, Wassarman et al. 2001).
Numerous sRNAs from these screens have since been functionally characterized in both E. coli and Salmonella enterica, and assigned a cellular pathway, molecular mechanism and physiological function (Hör et al. 2020a). Mechanistically, almost every one of them has turned out to regulate multiple mRNAs by short, imperfect base pairing. Target recognition typically involves 8–10 strongly conserved bases in the sRNA, its so-called ‘seed region’ (Storz et al. 2011). The major mode of action of sRNAs is repression of protein synthesis through hindering access to an mRNA's ribosome binding site (RBS), which additionally often leads to degradation of the mRNA. Importantly, most well-characterized sRNAs also require an RNA-binding protein (RBP) such as Hfq or ProQ for both intracellular stability and mRNA targeting (Holmqvist and Vogel 2018)—a fact that can be utilized for the detection of new sRNAs, as discussed later.
The overall picture emerging from these studies is that among the ∼300 sRNAs annotated in E. coli and Salmonella, there is a conserved set of 20–30 sRNA genes with core functions (Hör et al. 2020a). Similar to core TFs acting on the promoters of multiple genes within conserved regulons, these core sRNAs often target large suites of mRNAs with related functions. Importantly, sRNAs often substantially expand the regulatory scope of the TFs they are regulated by. This is especially apparent in the case of sigma factors, which are intrinsically restricted to activating genes; by activating an sRNA, a sigma factor can then also repress gene expression, albeit indirectly, at the post-transcriptional level. As such, sRNAs endow these regulons with a ‘noncoding arm’ that complements the TF-driven ‘coding arm’ (Gogol et al. 2011).
Two decades after the initial screens, sRNA genes are now known to provide a noncoding arm in many major stress regulons and signaling pathways. In γ-proteobacteria, for example, sRNA functions run the gamut from control of iron availability, cell surface or envelope stress, and sugar fluctuations, to the regulation of virulence gene expression in response to population density (Storz and Papenfort 2019). However, the more evident the central role of these sRNAs became, the more puzzling it was that there were several fundamental pathways with no obvious associated sRNA. For example, the Cpx response to inner membrane stress was known to rely on Hfq, indicating the involvement of an sRNA, but there was no intergenic sRNA gene with a conserved binding site for the major response regulator, CpxR (Vogt et al. 2014, Chao and Vogel 2016). Could it be that there was another source of sRNAs?
As outlined in this review and related previous ones (Miyakoshi et al. 2015b ,Adams and Storz 2020), we now know that fragments derived from 3′ untranslated regions (UTRs) of bacterial mRNAs provide another abundant class of sRNAs whose full scope and importance we are only beginning to understand. These 3′ UTR-derived sRNAs constitute a previously unknown layer of gene control in which mRNAs influence each other's expression without changes at the level of transcription. We will briefly summarize how these sRNAs were discovered, how they are produced in the cell, highlight several well-characterized examples and discuss emerging principles in the functional relationship with their parental mRNAs before ending with an outlook on where this exciting area of bacterial RNA biology may lead us in the next few years.
Small RNAs from mRNA regions: a bit of history
Hints that noncoding genes may not be the sole origin of sRNAs were provided by some of the first studies that focused on IGRs. Performed in E. coli, these experimental screens recovered unusually abundant, defined small fragments derived not only from rRNAs and tRNAs, but also from different regions of mRNAs (Wassarman et al. 2001, Vogel et al. 2003, Kawano et al. 2005). 3′ UTR-derived sRNAs, in particular, showed expression patterns that differed from their corresponding mRNAs, suggesting independent functions (Kawano et al. 2005). Their intracellular half-lives were similar to those of known regulatory sRNAs (Vogel et al. 2003). Some of these fragments, such as SroC, were even conserved in the few other enterobacterial genomes available at the time. Although somewhat speculative, these observations were conceptualized as ‘parallel transcriptional output’ (Vogel et al. 2003) whereby the same transcriptional unit provides both a coding (mRNA) and a noncoding (sRNA) function (Fig. 1). However, the only promising candidate at the time was the long-known prophage-derived DicF sRNA, which, when overexpressed, blocks FtsZ protein synthesis leading to cell filamentation (Faubladier et al. 1990, Tétart and Bouché 1992).
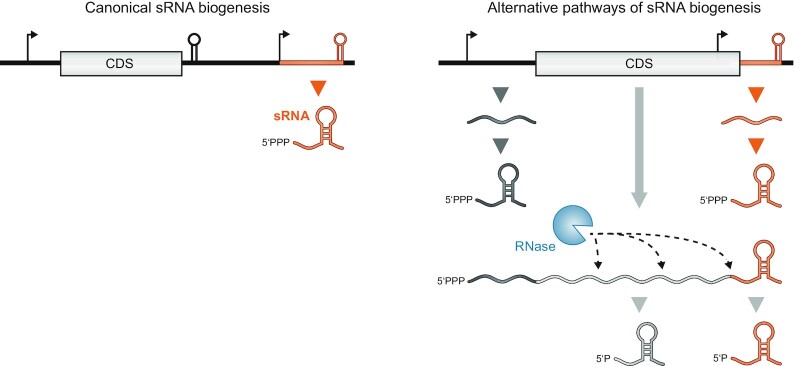
Figure 1.
The many different sources of sRNAs as ‘parallel transcription output’. The canonical sRNA biogenesis pathways (left column) refer to sRNA production by transcription of stand-alone noncoding genes (right) located next to protein-coding genes (left). Alternative pathways (right column) produce sRNAs from mRNA loci by premature transcription termination in the 5′ region, by transcription starting inside the coding sequence (CDS) but using the same terminator or by mRNA processing. The focus of this review is on sRNAs that accumulate as 3′ end processing products of mRNAs and thus carry a monophosphate at their 5′ end.
The subsequent years yielded more evidence for functional sRNAs originating from protein-coding genes. Work in Listeria monocytogenes showed that sRNAs from mRNA 5′ regions could repress the synthesis of virulence regulator PrfA in trans (Loh et al. 2012). These sRNAs are derived from 5′ UTR-borne S-adenosyl methionine riboswitches (Loh et al. 2009), echoing earlier observations in E. coli, where flavin and thiamine riboswitches were found to produce stable 5′ mRNA fragments (Vogel et al. 2003). Concerning the 3′ end, work in Vibrio cholerae described the MicX sRNA, which is transcribed from an ORF-internal promoter and accumulates as a stable ∼190-nt processed species that roughly corresponds to the 3′ UTR of the ORF (Davis and Waldor 2007).
With the introduction of RNA-seq, comprehensive profiling of cellular ligands of Hfq by a RIP-seq approach revealed mRNA 3′ ends as a large potential sRNA pool (Sittka et al. 2008, Chao et al. 2012). In addition to determining the exact boundaries of enriched transcripts for the first time, RNA-seq also revealed their relative abundance on Hfq. Indeed, stable 3′ UTR fragments were found to occupy a substantial fraction of cellular Hfq (Chao et al. 2012). By that time, it had become clear that Hfq in the cell was present in a limiting concentration, resulting in competition amongst RNAs for access to this central sRNA chaperone (Fender et al. 2010). Thus, these abundant Hfq-associated 3′ UTR fragments were likely to have a cellular function. Moreover, the Rho-independent terminator structure at the 3′ end of sRNAs had been shown to be important for Hfq binding (Zhang et al. 2003, Sauer and Weichenrieder2011, Morita et al. 2017). Obviously, such structure was also present at the 3′ end of many mRNAs. Together, this suggested a scenario in which final products of mRNA turnover or processing accumulated on Hfq to exert an independent function as sRNAs (Chao et al. 2012).
Yet, not every Hfq-enriched 3′ UTR was a product of mRNA processing. There were several cases like the aforementioned MicX where sRNA transcription starts within the upstream CDS. For example, Salmonella DapZ, which acts to regulate amino acid synthesis and transport genes, possesses a conserved promoter that lies just upstream of the stop codon of the protein-coding gene dapB (Chao et al. 2012). Similarly, the 80-nt MicL sRNA, a conserved σE-dependent repressor of Lpp synthesis in E. coli, is processed from a ∼300-nt precursor whose transcription starts in the middle of cutC (Guo et al. 2014, Updegrove et al. 2019). In the Gram-positive bacterium Lactococcus lactis, the ∼66-nt ArgX sRNA is transcribed from the 3′ end of argR and downregulates the arc operon involved in arginine metabolism (van der Meulen et al. 2019). For a very recent example, the cyanobacterium Synechocystis sp. PCC 6803 was found to express an sRNA called ApcZ from the 3′ end of a key operon involved in the collection of light energy, which acts to regulate the expression of a protein involved in energy dissipation (Zhan et al. 2021). Such sRNAs with ORF-embedded promoters will still accumulate when transcription of the overlapping protein-coding gene is inactivated, and unless they undergo further processing as do the MicL or MicX sRNAs (Davis and Waldor 2007, Guo et al. 2014), they will carry the characteristic 5′ triphosphate group of primary transcripts.
The Hfq ligand maps left plenty of strong sRNA candidates that were obviously cleaved from longer mRNAs, and their characterization in Salmonella and E. coli soon revealed Hfq-dependent functions as trans-acting regulators of other transcripts, both mRNAs and sRNAs (Miyakoshi et al. 2015a, Chao and Vogel 2016, Grabowicz et al. 2016). At the same time, work in Streptomyces coelicolor revealed Hfq-independent, 3′ UTR-mediated repression for two superoxide dismutase mRNAs (Kim et al. 2014). In addition, the newly discovered global RNA-binding properties of ProQ provided yet another set of potentially functional 3′ mRNA fragments (Smirnov et al. 2016, Holmqvist et al. 2018, Melamed et al. 2020). Together, these observations led to the notion that mRNA crosstalk, increasingly investigated in eukaryotes (Tay et al. 2014), might be quite common in bacteria, too. To date, about a dozen sRNAs from mRNA 3′ ends have been functionally characterized in phylogenetically diverse bacteria (Table 1).
Table 1.
An overview of discussed 3′ UTR-derived sRNAs.
| sRNA | Parental mRNA | Main ribonuclease(s) involved in biogenesis | Target(s) | Organism(s) | References |
|---|---|---|---|---|---|
| CpxQ | cpxP | RNase E | nhaB, skp, agp, ydjN, fimA | Enterobacteriales | (Bianco et al. 2019, Chao and Vogel 2016, Grabowicz et al. 2016, Melamed et al. 2016) |
| DicF | ydfABC-dicF-dicB-ydfD | RNase E & RNase III |
pchA
ftsZ, manX |
EHEC E. coli K12 |
(Azam and Vanderpool 2018, Balasubramanian et al. 2016, Bouché and Bouché 1989, Faubladier et al. 1990, Melson and Kendall 2019, Murashko and Lin-Chao 2017, Tétart and Bouché 1992) |
| SdhX | sdhCDAB-sucABCD | RNase E |
ackA
fumB, yfbV fdoG, katG |
Salmonella/E. coli K12 Salmonella E. coli K12 |
(Cronan and Laporte 2005, De Mets et al. 2019, Melamed et al. 2016, 2020, Miyakoshi et al. 2019, Zhang et al. 2003) |
| SroC | gltIJKL | RNase E | fliE, GcvB | Salmonella | (Fuentes et al. 2015, Miyakoshi et al.2015a ; Vogel et al. 2003) |
| NarS | narK | RNase E | nirC | Salmonella | (Wang et al. 2020) |
| MalH | malEFG | RNase E | ompC, ompA | Enterobacteriales | (Iosub et al. 2021) |
| FarS | fabB | RNase E | fadE | Vibrio cholerae | (Huber et al. 2020) |
| OppZ | oppABCDF | RNase E | oppBCDF | Salmonella | (Hoyos et al. 2020) |
| RaiZ | raiA | RNase E | hupA | Salmonella | (Chao et al. 2012, Holmqvist et al. 2018, Melamed et al. 2020, Smirnov et al. 2016, 2017b, Westermann et al. 2019) |
| s-SodF | sodF | Unknown; not RNase E or RNase III | sodN | Streptomyces coelicolor | (Kim et al. 2014) |
| RsaC | mntABC | RNase III | sodA | Staphylococcus aureus | (Lalaouna et al. 2019) |
| RsaG | uhpT | RNase J1/J2 | rex, ldh1, RsaI | Staphylococcus aureus | (Desgranges et al. 2021) |
| SorX | RSP_0847 | Unknown | potA | Rhodobacter sphaeroides | (Peng et al. 2016) |
| PcrX | pufQBALMX | RNase E | pufLMX | Rhodobacter sphaeroides | (Eisenhardt et al. 2018) |
Biogenesis
There is currently no evidence for specialized biogenesis factors for 3′ UTR-derived sRNAs, and they often appear to constitute a terminal fragment of normal mRNA decay. A global analysis of cellular RNA 5′ ends before and after inactivation of RNase E (so-called TIER-seq) in Salmonella indicated that most 3′ UTR-derived sRNAs are generated by this major endoribonuclease (Chao et al. 2017). TIER-seq analyses in the model proteobacteria V. cholerae and Rhodobacter sphaeroides came to similar conclusions (Förstner et al. 2018, Hoyos et al. 2020). Despite being an apparent product of typical RNA decay, the cleavage generating the final 5′ end tends to be well defined, and often occurs near the stop codon of the upstream ORF.
RNase E-mediated biogenesis has been particularly well studied for the Hfq-dependent CpxQ sRNA, which—as described in the next section—is generated from the mRNA of the stress response protein CpxP. Mutation of an internal conserved RNase E cleavage site near the 3′ end of the cpxP mRNA (Fig. 2A) abrogates CpxQ production in vivo . Importantly this has little effect on the parental cpxP mRNA (Chao and Vogel 2016). In vitro reconstitution experiments suggested that RNase E and Hfq are the two key factors for generating CpxQ from the processed 5′P-cpxP mRNA. Hfq may have a dual role in this process: it enhances the precision of the required RNase E cleavage while it also protects the final CpxQ species from further degradation (Chao and Vogel 2016).
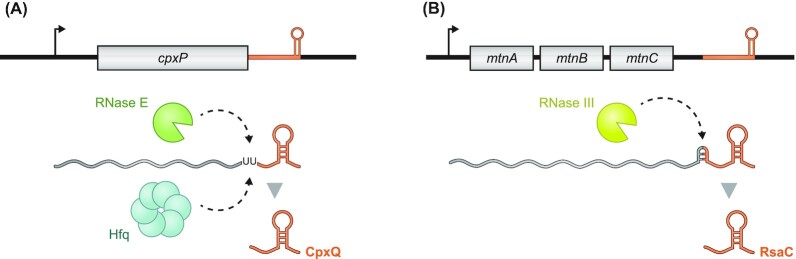
Figure 2.
Biogenesis of 3′ UTR-derived sRNAs in Gram-negative and -positive bacteria. (A) In Gram-negative bacteria, the major endonuclease RNase E is the primary nuclease to produce 3′ UTR-derived sRNAs from their parental mRNAs, as shown here for the CpxQ sRNA and the cpxP mRNA. In this case, the Hfq protein is also required for biogenesis. (B) While RNase E is lacking in Gram-positive bacteria, RNase III was shown to free the RsaC sRNA from the mntABC operon mRNA by recognizing a double-stranded RNA structure.
Gram-positive bacteria have a very different set of RNases than Gram-negative bacteria and do not encode RNase E (Durand and Condon 2018, Bechhofer and Deutscher 2019). Biogenesis of 3′ UTR-derived sRNAs must therefore occur via a different mechanism, as in the case of the Staphylococcus aureus RsaC sRNA that was shown to be generated by the double-strand specific endoribonuclease RNase III (Lioliou et al. 2012) (Fig. 2B). It has been argued in a recent review (Mediati et al. 2021) that the smaller number of processed 3′ UTR-derived sRNAs in Gram-positive bacteria might be due to the presence of 5′ → 3′ exoribonucleolytic activity. Specifically, Gram-positive bacteria possess RNase J1, which fully degrades mRNAs from the 5′ end in one go, resulting in fewer stable intermediates for evolution to act on and limiting the development of 3′ UTR-derived sRNAs. Nonetheless, there is a recent report in a Gram-positive bacterium of how blockage of 5′ → 3′ exoribonucleolytic activity by a stable hairpin structure in the 3′ region of a longer polycistronic mRNA generates an sRNA; this is an interesting analogy to the actions of RNase E in Gram-negative bacteria (Desgranges et al. 2021).
In the following, we will highlight well-studied examples of sRNAs that are generated by mRNA 3′ end processing and describe their function (Fig. 1). Moreover, recently developed global RNA interactome methods will be discussed to argue that there is still much to learn about mRNA crosstalk mediated by 3′ UTR-derived sRNAs.
CpxQ: the noncoding arm of the inner membrane stress response
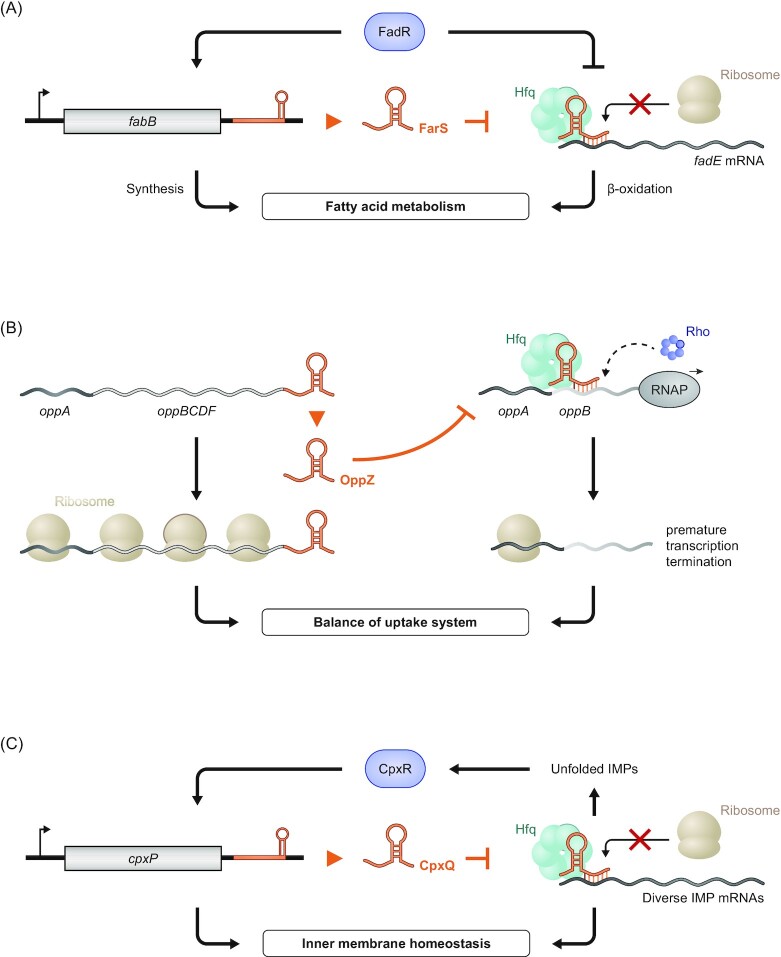
The ∼60-nt CpxQ sRNA is a poster child for 3′ UTR-derived sRNAs as its discovery exemplifies the importance of both looking beyond IGRs for a missing sRNA in a conserved stress response and paying attention to unusually high sequence conservation at the 3′ end of bacterial genes. As previously mentioned, CpxQ is cleaved off the mRNA of CpxP, a protein with an important role in the stress response to misfolded inner membrane proteins (IMPs) (Chao and Vogel 2016, Grabowicz et al. 2016). CpxQ spans almost the entire cpxP 3′ UTR. Not only is this region exceptionally conserved amongst enterobacteria (Fig. 3A), it is also more conserved than any other region of the cpxP gene. As to function, CpxQ acts by Hfq-dependent base pairing to repress the synthesis of several IMPs including the NhaB Na+/H+ antiporter, thus limiting the loss of membrane potential under stress (Chao and Vogel 2016).
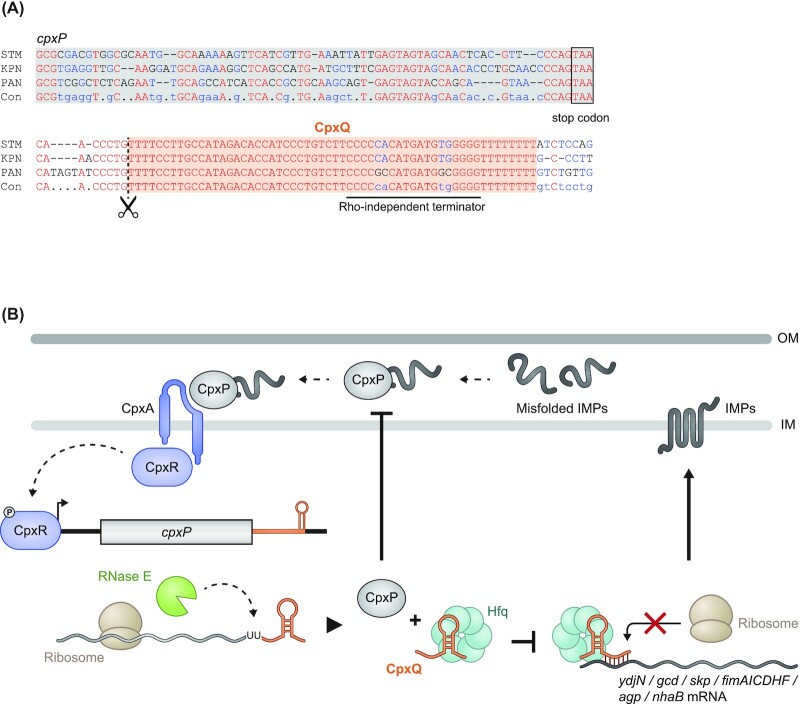
Figure 3.
CxpQ as a well-understood example of 3′ UTR-derived sRNAs. (A) Nucleotide sequence alignment highlighting the strong conservation of the CpxQ sRNA in comparison to the 3′ region of the cpxP mRNA (STM: S. Typhimurium; KPN: Klebsiella pneumoniae; PAN: Pantoea spp.; Con: consensus sequence). (B) Inner membrane (IM) stress leads to the phosphorylation of the transcription factor CpxR. Phosphorylated CpxR activates transcription of cpxP mRNA that is translated, yielding CpxP protein, and processed by RNase E to yield CpxQ sRNA. While CpxP is involved in the degradation of misfolded inner membrane proteins (IMPs), CpxQ acts as a post-transcriptional regulator by repressing the translation of several IMPs. Thus, both the coding and noncoding parts of the cpxP mRNA act cooperatively to maintain IM homeostasis in the Cpx pathway.
CpxQ solved a conundrum. It had been known that hfq mutants experience chronic stress at both the outer membrane (OM) and the inner membrane (IM), strongly suggesting that Hfq-dependent sRNAs were involved in maintaining homeostasis at both of these two membranes. However, while sRNAs had been found for the OM-related σE response (Johansen et al. 2006, Papenfort et al. 2006, Thompson et al. 2007), sRNA genes linked to the IM-related Cpx response remained unknown. The discovery of CpxQ explained this puzzling observation, showing that an mRNA 3′ fragment served as the elusive noncoding arm in the Cpx response to IM stress. Thus, both of these two major stress pathways employ Hfq-dependent sRNAs, yet of different nature, to counteract problematic overproduction of membrane proteins (Fig. 3B).
Interestingly, CpxQ also cross-connects these stress responses at the level of the σE-induced Skp protein, a periplasmic chaperone that binds unfolded OMPs. Skp is known to accidently mistarget OMPs into the IM, causing membrane depolarization (Grabowicz et al. 2016). CpxQ counteracts this potential toxicity by downregulating Skp production (Chao and Vogel 2016, Grabowicz et al. 2016). Recent studies have further expanded the targetome of CpxQ, showing that this sRNA also represses the cfa mRNA, which encodes cyclopropane fatty acid synthetase (Melamed et al. 2016, Bianco et al. 2019). If and how this regulation expands the function of CpxQ in protecting IM integrity needs to be explored further.
DicF: more than just a prophage function
Though not initially realized as such, DicF was the first 3′ UTR-derived sRNA to be characterized (Fig. 4A). It originates from the 3′ UTR of ydfC in the ydfABC-dicF-dicB-ydfD operon (historically named the ‘dicBF operon’), which is located in a defective lambdoid prophage in the E. coli K-12 genome (Bouché and Bouché 1989). The functional 53-nt DicF sRNA is generated via early Rho-independent transcription termination followed by RNase E-dependent processing at the 5′ end (Faubladier et al. 1990). An alternative 3′ end of DicF is generated by RNase III-mediated processing of the full-length dicBF RNA, releasing a 72-nt DicF species, which represents an intra-operonic sRNA rather than a true 3′ UTR-derived one (Faubladier et al. 1990, Balasubramanian et al. 2016).
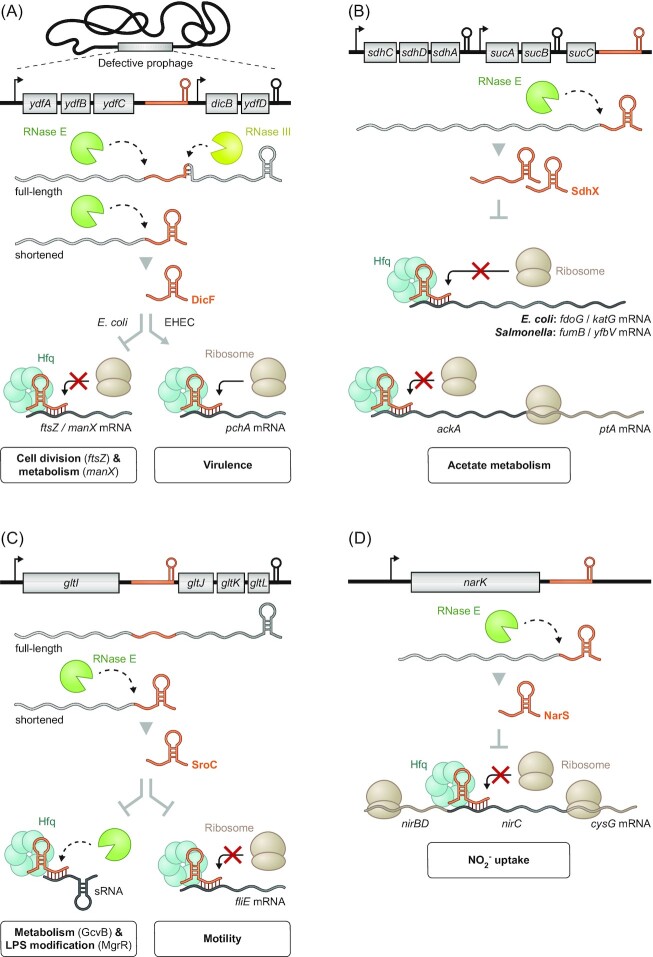
Figure 4.
Enterobacterial 3′ UTR-derived sRNAs are involved in diverse pathways. (A) The DicF sRNA stems from inside the E. coli ydfABC-dicF-dicB-ydfD operon mRNA that is transcribed from a defective prophage region. Processing of the mRNA by both RNase E and RNase III yields DicF, which in turn is involved in the regulation of cell division and metabolism. Additionally, in EHEC, DicF is also important for the regulation of the pathogen's virulence by upregulating the transcriptional activator PchA. (B) RNase E-dependent processing at the 3′ end of the sdhCDAB-sucABCD mRNA generates the sRNA SdhX, which selectively acts on ackA of the bicistronic ackA-ptA operon to regulate acetate metabolism. Additionally, SdhX exhibits divergent targetomes in E. coli and Salmonella. (C)Premature transcriptional termination of the gltIJKL operon and subsequent RNase E-mediated cleavage frees the sRNA SroC from its parental operon. While SroC is involved in regulating motility by repressing translation of fliE, SroC has an expanded regulatory capacity through sponging the sRNAs GcvB and MgrR to further affect metabolism and LPS modification, respectively. (D) NarS is processed off the 3′ end of narK mRNA by RNase E. By selectively inhibiting translation of nirC as part of the nirBDC-cysG operon, this sRNA works synergistically with the NarK protein to fine-tune nitrite uptake.
DicF was originally described as a genomic element that blocked cell division in E. coli, inhibiting the synthesis of the essential cell division protein FtsZ (Tétart et al. 1992). More recently, it was shown that DicF is an Hfq-dependent sRNA that inhibits not only the translation of the ftsZ mRNA but also of additional mRNAs (manX,pykA and xylR) with functions in metabolism (Balasubramanian et al. 2016). This also involves a noncanonical mechanism whereby DicF may repress manX translation indirectly, through loading Hfq onto the RBS of this mRNA (Azam and Vanderpool 2018).
Furthermore, DicF was shown to function as a specific translational activator in enterohemorrhagic E. coli. Here, the sRNA competes with the formation of an intrinsic inhibitory structure in the pchA mRNA under microaerobic conditions. By upregulating the transcriptional regulator PchA, DicF indirectly activates synthesis of the virulence-associated type 3 secretion system of this pathogen (Melson and Kendall 2019, Murashko and Lin-Chao 2017). Together, these examples illustrate that the physiological impact of the 3′ UTR-derived DicF sRNA extends well beyond the initially described inhibition of cell division.
SdhX: coordination with the TCA cycle
CpxQ, described further earlier, is the released 3′ UTR of a monocistronic mRNA. By contrast, the sRNA SdhX (originally known as RybD; Zhang et al. 2003) is generated from the very end of the large sdhCDAB-sucABCD operon mRNA (Zhang et al. 2003, Cronan and Laporte 2005) (Fig. 4B). Encoding key proteins of the tricarboxylic acid cycle (TCA cycle), the sdh-suc operon is subject to complex regulation by TFs and sRNAs (Park et al. 1997, Nam et al. 2005). Successive RNase E cleavages generate two variants of SdhX: one ∼101 nt and the other only ∼38 nt in length (Miyakoshi et al. 2019). Both of these variants contain the conserved seed region of SdhX through which this sRNA recognizes its major target, the mRNA of acetate kinase AckA (De Mets et al.2019, Miyakoshi et al. 2019). Intriguingly, the SdhX-mediated translational repression affects only ackA but not the other gene of the bicistronic ackA-pta operon. The pta gene is also involved in acetate metabolism, and the discordant regulation by SdhX may have evolved to selectively increase the accumulation of the signaling molecule acetyl phosphate (De Mets et al. 2019, Miyakoshi et al. 2019).
The targetome of SdhX is likely to be much larger, yet also species-specific. Additional mRNA targets in Salmonella are fumB and yfbV (Miyakoshi et al. 2019), whereas in E. coli SdhX also represses the fdoG and katG mRNAs (De Mets et al. 2019, Melamed et al. 2016, 2020). These differences are the result of single-nucleotide changes between the respective E. coli and Salmonella genes; whether they are just random mutations or reflect different physiological needs of these two species is unknown. The conserved regulation of ackA by SdhX, however, is a paramount example of how the transcript of a major metabolic operon (i.e. the TCA cycle) post-transcriptionally influences another metabolic operon (i.e. acetate metabolism) via the activity of a 3′ UTR-derived sRNA. In addition, the high variability of the SdhX sequence outside the short seed (Miyakoshi et al. 2019) further argues that mRNA 3′ UTRs are a playground for the evolution of regulatory sRNAs.
SroC: sponging another sRNA
While the hitherto described examples regulate mRNAs, the primary target of SroC is different: SroC acts as a regulatory sponge of another sRNA (Fig. 4C). Similar to the biogenesis of DicF, SroC is produced from within the gltIJKL operon such that early Rho-independent termination yields a monocistronic gltI (a.k.a. ybeJ) transcript, processing of which leaves the final 163-nt SroC species (Vogel et al. 2003, Miyakoshi et al. 2015a). SroC acts by Hfq-dependent base pairing to accelerate the decay of GcvB, the latter of which is a well-characterized sRNA that represses ∼1% of all E. coli and Salmonella genes (Urbanowski et al. 2000, Pulvermacher et al. 2009, Sharma et al. 2007, 2011, Busi et al. 2010, Vanderpool 2011, Stauffer and Stauffer 2012, Wright et al. 2013, Yang et al. 2014, Miyakoshi et al. 2022). Many of the mRNAs repressed by GcvB function in amino acid transport and metabolism. Intriguingly, since the gltIJKL mRNA itself is a target of GcvB, the SroC sponge seems to enable both a positive feedback loop to activate its parental mRNA in cis, while also activating many trans-encoded mRNAs in the same pathway. Physiologically, loss of SroC impairs bacterial growth when peptides are the sole carbon and nitrogen sources (Miyakoshi et al. 2015a).
Interestingly, SroC sponges more than one Hfq-dependent sRNA. In E. coli, SroC was also shown to downregulate the MgrR sRNA, thereby alleviating MgrB-mediated translational repression of the LPS modification enzyme EptB (Acuña et al. 2016). Furthermore, classic sRNA activity on mRNAs has also been reported, showing that SroC negatively regulates the flhBAE and fliE mRNAs through direct base pairing and thus flagella synthesis in Salmonella (Fuentes et al. 2015). Therefore, SroC—once considered ‘a putative processed fragment of the ybeJ–gltJ spacer’ (Vogel et al. 2003)—has emerged as a multi-facetted regulator of processes as diverse as metabolism, motility and surface modification.
NarS: avoiding self-poisoning
As mentioned earlier, our recognition of processed 3′ UTRs as potential sRNAs grew with their detection as stable fragments in northern blot or RNA-seq analyses. Such detection requires the parental mRNA to be expressed under the condition of the assay, but many bacterial genes are only transcribed under specific growth conditions. A case in point is the NarS sRNA (Wang et al. 2020), whose parental gene is only activated by FNR or NarL under anaerobic growth or high levels of nitrite, respectively, to produce the nitrate transport protein NarK (Kolesnikow et al. 1992, Kröger et al. 2013).
The mature 63-nt NarS sRNA (Fig. 4D) spans a little more than the 3′ UTR of narK, and is conserved in a subclade of the Enterobacteriaceae. NarS appears to have one main activity, which is to repress the nirC mRNA encoding a major nitrite importer. A simple working model postulates that the NarK protein will both import extracellular nitrate for nitrate reduction and export the product nitrite. Concomitantly, the narK-derived sRNA NarS functions to prevent expression of the nitrite importer NirC to limit uptake of excessive nitrite from the environment in order to avoid self-poisoning. Questions remain with respect to the molecular mechanism of target regulation, for example, why and how the NarS sRNA very selectively regulates only the nirC cistron of the much longer nirBDC-cysG operon mRNA (Wang et al. 2020).
MalH: helping to use the right carbon source through porin regulation
Continuing on expression under very specific conditions, the 104 nt long MalH sRNA from the 3′ end of the maltose uptake operon malEFG mRNA (Iosub et al. 2021) shows expression that is as selective as that of NarS (Wang et al. 2020). This mal operon is strongly suppressed in the presence of glucose, but when glucose becomes scarce or maltose is present, its expression is activated by the TF MalT to ensure maltose uptake. Under these latter conditions, MalH accumulates and negatively regulates the mRNAs of the abundant OmpA and OmpC porins. Additionally, high levels of MalH lead to a reduction of the σE-dependent sRNA MicA, which is expected to lift the known MicA-mediated repression of the mRNA of the maltoporin LamB (Bossi and Figueroa-Bossi 2007, Gogol et al. 2011, Wang et al. 1997). While the exact mechanisms of MalH, especially how it derepresses LamB synthesis, are yet to be resolved, the current working model suggests that this sRNA helps to shift carbon metabolism to maltose when E. coli bacteria run out of their favored carbon source, glucose. Interestingly, MalH shares the same genomic location as MicX found in V. cholerae. However, thus far no common targets are known for both sRNAs and MicX is transcribed from its own promoter, in contrast to the biogenesis of MalH (Davis and Waldor 2007). This makes the MalH–MicX pair an interesting example of the divergent evolution of an sRNA encoded in two bacteria living different lifestyles.
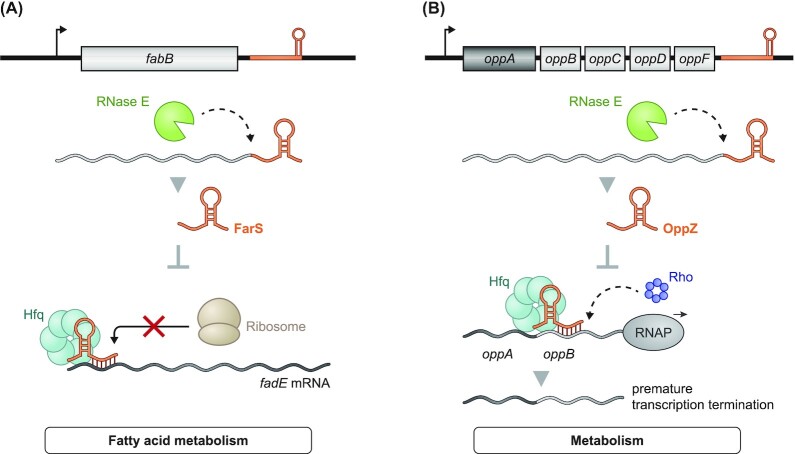
FarS: switching fatty acid metabolism
V. cholerae is a human pathogen in which 3′ UTR-derived sRNAs seem to be very prominent. A global Hfq coimmunoprecipitation study (Huber et al. 2020) found about half of all sRNAs that were enriched with this RNA chaperone to be processed mRNA 3′ fragments, of which FarS (Fig. 5A) was studied in detail. RNase E-mediated mRNA turnover generates FarS from fabB, which encodes the first enzyme in the fatty acid synthesis pathway. While FabB promotes the production of acyl-ACP from acyl-CoA, the associated FarS sRNA together with Hfq translationally represses the synthesis of FadE, which is the first enzyme in the opposing β-oxidation cycle that produces acetyl-CoA from long chain acyl-CoA. On top of that, the fabB and fadE genes are antagonistically regulated by the same major TF of fatty acid metabolism, FadR. Hence, the FarS sRNA and the FadR regulatory protein constitute a mixed feed-forward loop regulating the transition between fatty acid biosynthesis and degradation (Huber et al. 2020).
Figure 5.
3′ UTR-derived sRNAs in V. cholerae. (A) RNase E-mediated processing yields the sRNA FarS from its parental mRNA fabB. Through inhibition of fadE translation, FarS is involved in the regulation of the fatty acid metabolism of the pathogen. (B) The 3′ end of the oppABCDF operon contains the sRNA OppZ, which is generated through RNase E processing. OppZ acts self-regulating by initiating Rho-dependent transcription termination downstream of oppA. This leads to the fine-tuning of the protein stoichiometry of the OppABCDF system.
OppZ: enabling gene autoregulation
Staying with 3′ UTR-derived sRNAs of V. cholerae, functional characterization of OppZ has helped to identify a new sRNA-dependent mechanism: autoregulation of the parental gene (Hoyos et al. 2020). The 52-nt OppZ sRNA is generated from the 3′ end of the oppABCDF operon in an RNase E-dependent fashion, and its stability and function rely on Hfq (Fig. 5B). Surprisingly, OppZ was found to bind its own operon mRNA in the IGR between oppA and oppB, leading to downregulation of translation of all encoded proteins except OppA. This further caused downregulation of OppZ itself. However, stability of the oppB mRNA was not affected by OppZ, indicating that this sRNA acted by terminating transcription rather than inducing transcript decay. Mutational studies confirmed this hypothesis, revealing that translational inhibition of oppB by OppZ causes Rho-dependent transcription termination and with this downregulation of oppBCDF-oppZ without affecting oppA (Hoyos et al. 2020).
Negative autoregulation is common among TFs, which often control their own expression by blocking their own promoter (Rosenfeld et al. 2002). In contrast, OppZ and the V. cholerae CarZ sRNA (identified in the same study) represent the first examples of sRNAs that use this principle to control their own expression (Hoyos et al. 2020). Autoregulation via 3′ UTR-derived sRNAs seems to be particularly suited for polycistronic mRNAs such as oppABCDF since it requires the operon to be fully transcribed for the negative feedback mechanism to kick in, ensuring balanced protein synthesis and enabling discordant expression of the operon's individual genes.
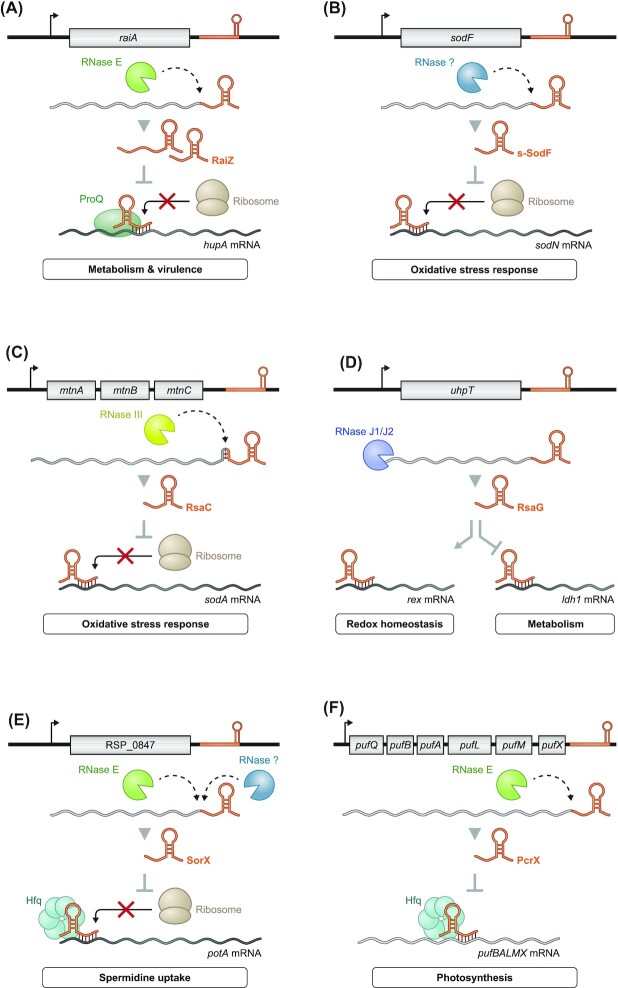
RaiZ: moving to ProQ
The protein ProQ has recently been established as the third globally acting sRNA-related RBP of Gram-negative bacteria, after CsrA and Hfq (Smirnov et al. 2016). While initially described in Salmonella as a target of Hfq (Chao et al. 2012), the RaiZ sRNA (Fig. 6A) has since been recovered in association with ProQ in several independent studies (Smirnov et al. 2016, Holmqvist et al. 2018, Melamed et al. 2020). Moreover, RaiZ requires ProQ rather than Hfq for its intracellular stability, and is therefore now considered a ProQ-dependent sRNA (Smirnov et al. 2016, Smirnov et al. 2017b). Its biogenesis, however, follows the same pattern as Hfq-dependent 3′ UTR-derived sRNAs: RNase E degrades the mRNA of the translational inhibitor and ribosome stability factor RaiA, resulting in the accumulation of two forms (∼160 and ∼122 nt) of the RaiZ sRNA (Kröger et al. 2013, Smirnov et al. 2017b).
Figure 6.
Further examples of 3′ UTR-derived sRNA in diverse organisms. (A) The sRNA RaiZ derived from the 3′ end of the raiA mRNA acts in ProQ-dependent manner by suppressing the globally acting DNA-binding protein hupA affecting metabolism and virulence of Salmonella and E. coli. (B) Processing of the sodF mRNA by an unknown ribonuclease gives rise to the s-SodF sRNA. The sRNA allows S. coelicolor to switch the superoxide dismutase (SOD) in response to oxidative stress under Ni-limited conditions. (C) In Staphylococcus, the sRNA RsaC is processed via RNase III from the mntABC operon at low Mn2+ levels. Under these conditions, RsaC represses the Mn-dependent SOD sodA allowing an efficient response against oxidative stress. (D) The sRNA RsaG is generated via 5′ → 3′ degradation by RNase J1/J2 of the full-length uhpT mRNA. Binding of RsaG to its target can lead to either stabilization or degradation of the bound mRNAs. In case of the former, RsaG binds the rex mRNA, which is a redox regulator. In case of the latter, binding of RsaG accelerates degradation of the lactate dehydrogenase (ldh1) mRNA and thus impacts metabolism. (E) The sRNA SorX is part of the 3′ end of RSP_0847 in R. sphaeroides and generated through an unknown RNase combined with RNase E. The released sRNA inhibits spermidine uptake through translational inhibition of the polyamine uptake transporter potA and thus supports fighting off oxidative stress. (F) The sRNA PcrX represents the 3′ end of the photosynthesis complex pufQBALMX operon and is generated via RNase E. PcrX acts self-regulating by destabilizing its parental mRNA through a yet unknown mechanism.
The first RaiZ target to be identified was the hupA mRNA encoding the α-subunit of histone-like protein HU. Mechanistically, RaiZ inhibits hupA translation, forming an imperfect RNA duplex long enough to attract RNase III for mRNA cleavage (Smirnov et al. 2017b). As such, knowledge of RaiZ enabled the first mechanistic investigation of mRNA repression in trans by a ProQ-dependent sRNA.
It is yet to be understood why the 3′ UTR of a translation-associated protein would repress the synthesis of a major DNA-binding protein with roles in controlling central metabolism and virulence. Interestingly, RaiZ is also upregulated during the early stages of Salmonella infection of HeLa cells (Westermann et al. 2019). Thus, RaiZ may help the bacteria to adjust global transcription to changing environments, while the protein encoded by the raiA mRNA acts similarly to globally adjust translation rates. These activities may be conserved as an independent screen in E. coli confirmed hupA mRNA as a top interactor of RaiZ (Melamed et al. 2020). This screen further identified additional putative targets of RaiZ such as the lpp mRNA and the abundant ProQ-associated sRNA RyfD. It may be through the investigation of these additional targets that we will fully understand the physiological implications of RaiZ activity.
s-SodF: switching to the better enzyme
The 90-nt s-SodF sRNA is a particularly clear example of how bacteria use released 3′ UTR fragments as regulatory mRNA switches (Kim et al. 2014) (Fig. 6B). Enzymes of the superoxide dismutase (SOD) family generally protect bacteria from oxygen-derived superoxides and resulting oxidative damage. Such is the case in S. coelicolor, which possesses two mutually exclusively expressed SOD proteins, a Ni-dependent SOD (sodN) and an Fe-dependent SOD (sodF) (Chung et al. 1999). Their mutually exclusive expression is mainly regulated by the TF Nur, which represses the sodF gene and activates the sodN gene in the presence of nickel (Chung et al.1999, Ahn et al. 2006).
This transcriptional switch is elegantly complemented by the s-SodF sRNA, which works at the post-transcriptional level in the opposite direction. When the sodF mRNA is expressed, a yet unknown nuclease releases its 3′ UTR in the form of s-SodF. The s-SodF sRNA forms a 19-bp RNA duplex with the 5′ region of the sodN mRNA, triggering rapid mRNA decay. The result is a mixed feed-forward loop composed of a TF and two cross-talking mRNAs that should facilitate rapid activation of the expression of the Fe-dependent SodF as nickel becomes scarce (Kim et al. 2014). Whether s-SodF requires other factors for function is unknown, but the clarity of the system begs for a genetic screen, which may eventually find a currently elusive sRNA-related RBP in the genus Streptomyces.
RsaC: keeping things running during manganese limitation
The human pathogen S. aureus has been a model species for sRNA screens in Gram-positive bacteria. An early screen described the RsaC sRNA (Geissmann et al. 2009), and a more recent one found RsaC to be upregulated in vivo in a mouse model of osteomyelitis (Szafranska et al. 2014). RsaC, which is conserved in S. aureus and Staphylococcus argenteus, is unusually long. It ranges in length from 584 nt to 1116 nt due to repeats in its 5′ region (Lalaouna et al. 2019) and is generated by RNase III-dependent cleavage from the mntABC operon mRNA (Lioliou et al. 2012) (Fig. 6C). This operon encodes a major importer of manganese. When manganese is plentiful, the TF MntR represses the mntABC genes, resulting in low levels of RsaC (Lieser et al. 2003).
All of this makes sense in light of the function of SodA, whose mRNA has been identified as the main target of RsaC. SodA is a Mn-dependent SOD, explaining why RsaC represses it under Mn-limiting conditions. Consistently, while RsaC represses SodA synthesis under Mn-limiting conditions, the alternative SOD SodM, which is active when loaded with either Mn2+ or Fe2+, shows increased expression under these conditions (Lalaouna et al. 2019). Of note, sequestration of metal ions such as Mn2+ represents an important host defense mechanism to limit pathogen growth. Thus, RsaC acts at the interface of the S. aureus oxidative stress response and host nutritional immunity.
RsaG: a jack of all trades
The global screen that identified RsaC in S. aureus also discovered RsaG, an sRNA derived from the mRNA 3′ end of hexose phosphate antiporter UhpT (Geissmann et al. 2009). The uhpT-rsaG operon is induced when coculturing this extracellular pathogen with mucin-producing eukaryotic cells, when internalized or—most simply—when glucose-6-phosphate (G6P) is present in the growth medium (Bronesky et al. 2019, Desgranges et al. 2021). Invariably, transcriptional activation of RsaG's parental mRNA depends on the G6P-sensing two-component system HptRS (Desgranges et al. 2021, Park et al. 2015). Remarkably, RsaG is generated via 5′ → 3′ degradation by the exoribonucleases J1/J2 and as such represents the first example for this route of sRNA biogenesis. This process requires the full-length uhpT-rseG RNA and possibly a hairpin structure at the 5′ end of RsaG, which is thought to block the exonucleolytic activities of the RNases to yield the mature sRNA (Desgranges et al. 2021).
With respect to function, RsaG is a great example of the diversity of molecular mechanisms utilized by sRNAs: it sequesters the RBS of some of its targets, accelerating their degradation, while also stabilizing other targets (Desgranges et al. 2021). Regarding the latter, binding of RsaG to the rex mRNA increases the transcripts half-life of this global redox regulator. Of note, Rex is a repressor of the lactate-dehydrogenase ldh1 gene, whose mRNA is downregulated by RsaG (Fig. 6D) (Pagels et al. 2010, Desgranges et al. 2021). Moreover, RsaG can interact with the sRNA RsaI, an additional negative regulator of ldh1 (Bronesky et al. 2019, Desgranges et al. 2021). The consequences of this sRNA–sRNA interaction are not fully understood. Still, this complex targetome suggests that RsaG is a versatile regulator, contributing to the modulation of redox homeostasis and metabolism upon changing environmental conditions in S. aureus. Other putative targets of RsaG include mRNAs involved in virulence and biofilm formation (Desgranges et al. 2021).
SorX: combatting oxidative stress
Similar to the earlier examples from S. coelicolor and S. aureus, the photoheterotrophic bacterium R. sphaeroides also employs a 3′ UTR-derived sRNA, called SorX (Fig. 6E), to combat oxidative stress. This Hfq-dependent sRNA responds to, and confers resistance to, stress by singlet oxygen and organic hydroperoxides (Peng et al. 2016). It is cleaved off the 3′ end of RSP_0847 (encoding an OmpR-type TF) by an unknown nuclease to generate a 116-nt pre-SorX RNA, which is subsequently processed by RNase E to release the mature 75-nt SorX sRNA. In contrast to E. coli, where polyamines such as spermidine are thought to be beneficial under oxidative stress conditions (Rhee et al. 2007), spermidine exacerbates the detrimental effects of reactive oxygen species in R. sphaeroides (Peng et al. 2016). SorX counteracts this toxicity by reducing spermidine uptake via translation inhibition of potA, which is part of the PotABCD polyamine transporter.
PcrX: balancing photosynthesis
R. sphaeroides is able to perform both aerobic respiration and anoxygenic photosynthesis, which need to be tightly controlled since bacteriochlorophyll can generate harmful singlet oxygen in presence of light and oxygen (Glaeser et al. 2011). Photosynthesis requires proteins encoded by the pufQBALMX operon. Intriguingly, this polycistronic mRNA also harbors a 3′ UTR-derived sRNA, termed PcrX (Fig. 6F), which is generated by RNase E-dependent cleavage (Eisenhardt et al. 2018). PcrX destabilizes the 3′ region (pufLMX) of its parental transcript in an Hfq-dependent manner, probably by targeting a region within pufX, which subsequently leads to a decrease in functional photosynthetic complex levels. This putative mechanism exemplifies how bacteria might use 3′ UTR-derived sRNAs to maintain steady levels of important complexes.
Common regulatory networks of 3′ UTR-derived sRNAs
Most of the well-studied examples of 3′ UTR-derived sRNAs described earlier are involved in the regulation of various metabolic pathways. They often seem to do so by acting on a single main target, in contrast to the common multi-target regulation by classic sRNAs (Hör et al. 2020a). Additionally, the preferred regulatory circuit of 3′ UTR-derived sRNAs involved in metabolism seems to be a mixed feed-forward loop, with a TF that regulates the levels of both the sRNA and its target. This type of regulation is especially well suited to buffer residual transcriptional noise through additional regulation at the post-transcriptional level, thereby leading to tighter control of expression (Nitzan et al. 2017). In the case of metabolic regulons, this tight control is of particular importance as many mRNAs act in opposing pathways to those regulated by the sRNAs they are carrying. FarS is an exceptionally clear example of this relationship: The TF FadR upregulates the fatty acid synthesis gene fabB, and concomitantly downregulates fadE, which is involved in the opposing β-oxidation pathway (Fig. 7A). The fabB-derived FarS sRNA completes the feed-forward loop through a post-transcriptional repression of fadE, thereby ensuring that fatty acids are not being synthesized and degraded at the same time (Quax et al. 2013, Huber et al. 2020).
Figure 7.
Common regulatory networks of 3′ UTR-derived sRNAs. (A) In V. cholerae, the transcription factor FadR activates the expression of FarS and its parental mRNA fabB, of which the latter is involved in fatty acid synthesis. Simultaneously, FadR and FarS repress fadE expression that is part of the opposing β-oxidation pathway for fatty acids. Thus, this mixed feed-forward loop enables an efficient fatty acid metabolism. (B)The autoregulatory sRNA OppZ ensures a proper balance of the different proteins of the Opp peptide uptake system in V. cholerae. Through causing premature transcription termination of its parental mRNA, for all but the peptide-binding protein OppA, OppZ limits expression of oppBCDF as well as itself. (C) Mixed feed-forward loops can also be involved in the regulation of stress. As exemplified by the CpxR-dependent protein CpxP and sRNA CpxQ of the Enterobacteriales. Through translational inhibtion of diverse IMPs, CpxQ reduces the CpxR-mediated transcription of CpxP as well as the sRNA itself. Both components of the Cpx pathway ensure a reduction of misfolded IMPs and thus alleviate IM stress.
Another important feature in the regulation of metabolic pathways is to retain optimal stoichiometry between different subunits of complexes or between enzymes of the same pathway. The structure and translational efficiency of polycistronic mRNAs in bacteria are known to be key to maintaining stoichiometry (Li et al. 2014, Burkhardt et al. 2017). Similarly, 3′ UTR-derived sRNAs can regulate stoichiometry by forming a negative feedback loop leading to repression of only a part of their parental polycistronic mRNAs, as shown for OppZ in V. cholerae (Fig. 7B). Here, the sRNA does not regulate oppA, the first cistron of the transcript, which encodes for a stand-alone periplasmic protein needed in high quantity. Instead, OppZ downregulates the downstream oppBCDF (and thereby itself), whose protein products form a transporter complex for oligopeptides. Given that the oppABCDF transcript is exclusively regulated upstream of oppA, regulation in cis via a 3′ UTR-derived sRNA ensures protein copy numbers independent of other regulators. The authors of this study argue that 3′ UTR-derived autoregulation is best for this purpose, as the OppZ sRNA being generated by ribonucleolytic cleavage comes at a 1:1 stoichiometry with its sole target, the oppBCDF mRNA (Hoyos et al. 2020).
In a mixed circuit, two regulators deriving from the same transcript are involved in the same or complementary pathways (Nitzan et al. 2017). This type of regulation is particularly well suited for stress response genes, as transcription of a single RNA leads to a fast, multi-pronged answer to the stress. There are two types of mixed circuits involving sRNAs: dual-function sRNAs, such as SgrS, encode a regulatory protein that functions in the same pathway as its parental sRNA (Wadler and Vanderpool 2007, Raina et al. 2018). The other type involves 3′ UTR-derived sRNAs, of which the best-studied example is CpxQ. This sRNA together with the CpxP protein from its parental mRNA forms a mixed circuit to alleviate IM stress (Chao and Vogel 2016, Grabowicz et al. 2016). While CpxP and CpxQ work coordinately, CpxP acts on IMPs at the post-translational level, whereas CpxQ downregulates IMP synthesis at the translational level (Fig. 7C).
Overall, 3′ UTR-derived sRNAs mainly seem to be specialized regulators involved in the maintenance of currently needed or favored metabolic processes, which typically depends on the availability of nutrients or trace elements. Yet, global methods such as RIL-seq (discussed later) are challenging this view by identifying a plethora of additional targets of known 3′ UTR-derived sRNAs (Melamed et al. 2016), questioning our current understanding of the regulatory roles of these sRNAs.
Using global methods to predict 3′ UTR-derived sRNAs and their targets
Going forward, it will be useful to employ global methods to comprehensively search for 3′ UTR-derived sRNAs. This will be of special importance for understudied species that are evolutionarily distant from model γ-proteobacteria such as E. coli,S. enterica and V. cholerae (Hör et al. 2018).
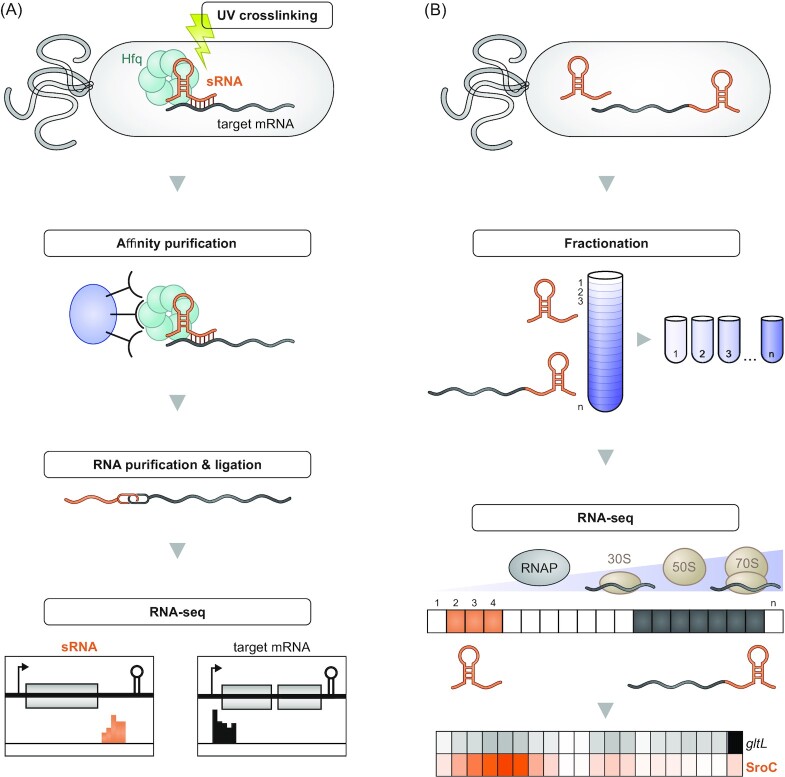
RIP-seq and the related CLIP-seq technique can be exploited to globally identify a pool of potential 3′ UTR-derived sRNAs associated with an RBP of interest. Yet, these methods cannot easily distinguish between the 3′ UTR of a CDS and a functionally independent sRNA derived from the same sequence. To circumvent this issue, RIL-seq and CLASH add a proximity ligation step to the CLIP-seq protocol, which enables the identification of functional RNA–RNA interactions in vivo (Hör and Vogel 2017, Waters et al. 2017, Iosub et al. 2020, Matera et al. 2022, Melamed et al. 2016, 2018, 2020) (Fig. 8A). RIL-seq in E. coli, for instance, revealed two hitherto unknown 3′ UTR-derived sRNAs, PspH and GadF, to be a sponge of the sRNA Spot 42 and a regulator of acid stress response genes, respectively—interactions that are easily overlooked by conventional target searches (Melamed et al. 2016). Most recently, the development of a new machine learning-based algorithm helped to discover several additional 3′ UTR-derived sRNAs and their targets in E. coli RIL-seq data (Bar et al. 2021).
Figure 8.
Global methods to uncover 3′ UTR-derived sRNAs and their targets. (A) A schematic overview for the workflow of methods relying on proximity ligation to identify sRNAs and their targets such as RIL-seq or CLASH. Split read mapping of the ligated RNA products allows the identification of the interacting transcripts and can thus globally uncover interactions between 3′ UTRs of genes and potential targets. (B) Grad-seq can enable the identification of novel 3′ UTR-derived sRNAs in a global manner. Fractionation of a cell lysate and subsequent sequencing can uncover differential migration patterns of a parental mRNA and its 3′ UTR and thus indicate an additional function of the latter as an sRNA, as shown for the example of SroC (data acquired from Smirnov et al. 2016).
Where a major sRNA-related RBP is unknown, as is the case with most Gram-positive species, GRIL-seq and its variant Hi-GRIL-seq can be used in a similar way as RIL-seq or CLASH (Han et al. 2016, Zhang et al. 2017). In GRIL-seq, an RNA ligase is expressed in vivo, causing proximity ligation between interacting RNAs. Following sequencing, the chimeric reads containing ligated transcripts can be searched for 3′ UTRs that interact with other mRNAs or sRNAs. In Pseudomonas aeruginosa, this led to the identification of SkatA, a 3′ UTR-derived sRNA that functions as a sponge of the PrrF1 sRNA involved in iron regulation (Han et al. 2016, Zhang et al. 2017). Intriguingly, expression of SkatA protected its parental mRNA katA from PrrF1-dependent downregulation, suggesting a mechanism that ensures basal expression of the catalase protein encoded by katA.
A recent modification of the GRIL-seq approach has focused on identifying novel sRNAs that interact with an mRNA of interest. Using this so-called ‘reverse GRIL-seq’ approach, analysis of the interaction partners of the rpoS mRNA in V. cholerae identified Vcr043 as a novel activator of this important mRNA (Han and Lory 2021).
Alternatively, a combined approach identifying processed RNA 5′ ends via dRNA-seq or Cappable-Seq (Sharma et al. 2010, Ettwiller et al. 2016) and RNA 3′ ends via term-seq (Dar et al. 2016) or similar methods (Shishkin et al. 2015, Yan et al. 2018, Ju et al. 2019, Fuchs et al. 2021) is able to directly predict 3′ UTR-derived sRNAs with single-nucleotide precision. Such a two-pronged approach was recently applied to map both 5′ UTRs and 3′ UTRs in the human gastrointestinal pathogen Clostridioides difficile and led to the identification of 18 hitherto unknown 3′ UTR-derived sRNAs (Fuchs et al. 2021), illustrating the strength of combining synergistic methods.
Finally, full-length single molecule sequencing methods such as PacBio and Oxford Nanopore sequencing—the latter of which even allows direct RNA sequencing—are expected to bolster functional transcript discovery without the need for special sample preparation. For all of the mentioned methods, downstream verification of the existence and targets of putative 3′ UTR-derived sRNAs should be performed via standard techniques such as northern blotting or extrachromosomal expression.
Using grad-seq to predict functional 3′ UTR fragments
Global transcriptomics via RNA-seq allows us to detect transcripts from any region of an organism's genome, i.e., noncoding as well as coding transcripts including transcribed 5′ and 3′ UTRs. However, the currently dominant method of short read sequencing used in standard RNA-seq cannot easily tell a functionally independent 3′ UTR-derived sRNA apart from the regular 3′ end of its much longer mRNA. This caveat necessitates the development of alternative transcriptomic methods that enable the identification of functional classes of transcripts rather than looking at the mere presence of transcripts.
In this regard, Grad-seq (Smirnov et al. 2016, 2017a, Hör et al. 2020b,c, Gerovac et al. 2021a,b, Hör and Vogel2021, Lamm-Schmidt et al. 2021, Riediger et al. 2021) is a promising technique for the identification of new 3′ UTR-derived sRNAs in both model and understudied or genetically intractable organisms. In Grad-seq, the soluble molecules of a bacterial lysate are separated on a glycerol gradient according to their weight and shape. Subsequent fractionation of the gradient followed by RNA-seq of each fraction then reflects the sedimentation of each transcript (Gerovac et al. 2021a). If a 3′ UTR-derived sRNA has an independent function, it can be expected to be involved in different interactions than its parental mRNA (i.e. it binds its target RNA and/or interacting RBP instead of the ribosome), meaning it will sediment in different parts of the gradient, thereby facilitating its identification (Fig. 8B). This functional relationship was recently exploited to identify a 3′ UTR-derived sRNA in the giant bacteriophage ΦKZ, highlighting the power of Grad-seq for sRNA discovery (Gerovac et al. 2021b).
Conclusion and outlook
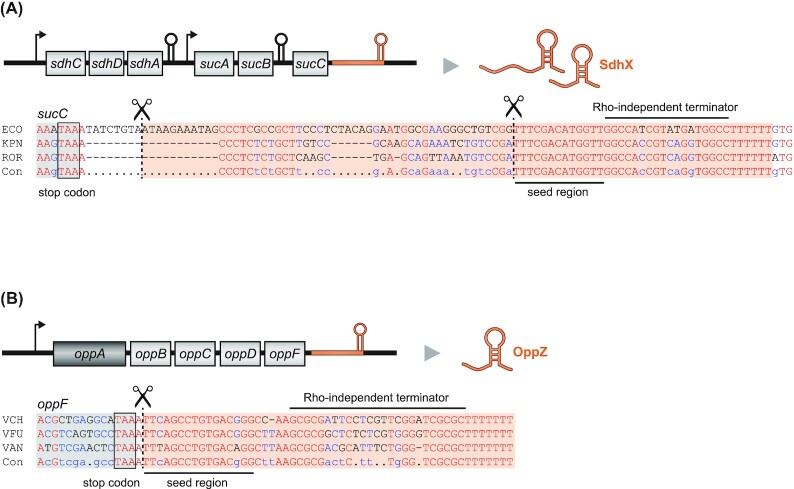
While the 5′ end was generally considered to be the more important one of the two noncoding ends of bacterial mRNAs in the context of gene regulation, the growing list of functional 3′ end-derived sRNAs suggests that it clearly deserves more attention. These mRNA-derived fragments constitute an underappreciated layer of lateral gene control whereby a gene influences the expression of another after transcription has taken place. Importantly, many of the earlier described cases impressively illustrate that it is worth looking for sequence conservation at the 3′ end of genes beyond the typical Rho-independent terminator structures because such conserved sequences downstream of the stop codons might indicate the conserved seed sequences of such sRNAs (Fig. 9).
Figure 9.
3′ UTR-derived sRNAs are a conserved feature of their parental mRNA. (A) Sequence alignment for sucC and the sRNA SdhX (ECO: E. coli; KPN: Klebsiella pneumoniae; ROR: Raoultella ornithinolytica; Con: consensus sequence).(B) Sequence alignment for oppF and the sRNA OppZ (VCH: V. cholerae; VFU: V. furnissii; VAN: V. anguillarum; Con: consensus sequence). The RNase E cleavage sites are indicated.
3′ UTR-derived sRNAs initially seemed to exhibit a narrow target spectrum and tended to impact their parental mRNAs or their functions—be it as a synergist as in the case of FarS (Huber et al. 2020) or, as shown for OppZ (Hoyos et al. 2020), as an antagonist. However, examples such as SroC, which sponges the GcvB sRNA (Miyakoshi et al. 2015a) and acts on other targets as well, have already blurred this simple view of 3′ UTR-derived sRNAs. Sponging as an alternative mode of action also appears more common than once thought, leading to questions as to whether sponging sRNAs should be considered a subclass of sRNAs rather than their own class (Denham 2020). Additionally, recent studies have predicted several sRNAs derived from 5′ UTRs or from within CDSs, even though it currently remains unknown whether these novel sRNAs have a similarly limited targetome to 3′ UTR-derived sRNAs or whether they act globally akin to intergenic sRNAs (Dar and Sorek 2018, Adams et al. 2021).
Regardless of the class of sRNA, the now established global techniques should be applied outside the commonly studied model organisms to yield a more comprehensive view of the abundance as well as the regulatory capacity of novel sRNAs. Herein lies yet another challenge, since most bacteria do not encode homologs of well-known RBPs or are not genetically tractable, rendering many approaches difficult if not impossible to apply. A point in case is the cancer-associated species Fusobacterium nucleatum. A recent RNA census of this and related fusobacterial species identified about two dozen sRNAs, of which four derived from 3′ UTRs (Ponath et al. 2021). Due to the lack of known RBPs and the limited genetic tools available for F. nucleatum and other bacteria, future studies analyzing their full sRNA repertoires will need to rely on the establishment of global methods independent of genetic manipulation or the presence of a global RBP. This will be important as the examples described earlier highlight the diverse regulatory roles of 3′ UTR-derived sRNAs in facilitating adaption to environmental changes or stresses. Thus, by studying this subset of sRNAs, we will further unravel and expand the complex regulatory networks crucial for model organisms and medically relevant pathogens alike.
Acknowledgments
We thank Lars Barquist, Svetlana Ðurica-Mitić, Youssef El Mouali and Kai Papenfort for helpful comments on the manuscript and Dr Sandy Westermann (www.scigraphix.com) for help with preparing the figures. We are grateful to the Vogel Stiftung Dr Eckernkamp for supporting FP with a Dr Eckernkamp Fellowship.
Funding
This work was funded by a DFG (Deutsche Forschungs Gesellschaft) Gottfried Wilhelm Leibniz Award to Jörg Vogel (DFG Vo875‐18).
Contributor Information
Falk Ponath, Helmholtz Institute for RNA-based Infection Research (HIRI), Helmholtz Centre for Infection Research (HZI), D-97080 Würzburg, Germany.
Jens Hör, Institute for Molecular Infection Biology, University of Würzburg, D-97080 Würzburg, Germany.
Jörg Vogel, Helmholtz Institute for RNA-based Infection Research (HIRI), Helmholtz Centre for Infection Research (HZI), D-97080 Würzburg, Germany; Institute for Molecular Infection Biology, University of Würzburg, D-97080 Würzburg, Germany.
Conflict of interest statement
None declared.
References
- Acuña LG, Barros MJ, Peñaloza Det al. . A feed-forward loop between SroC and MgrR small RNAs modulates the expression of eptB and the susceptibility to polymyxin B in Salmonella Typhimurium. Microbiology. 2016;162:1996–2004. [DOI] [PubMed] [Google Scholar]
- Adams PP, Baniulyte G, Esnault Cet al. . Regulatory roles of Escherichia coli 5′ UTR and ORF-internal RNAs detected by 3′ end mapping. eLife. 2021;10:e62438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PP, Storz G. Prevalence of small base-pairing RNAs derived from diverse genomic loci. Biochim Biophys Acta. 2020;1863:194524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn BE, Cha J, Lee EJet al. . Nur, a nickel-responsive regulator of the Fur family, regulates superoxide dismutases and nickel transport in Streptomyces coelicolor. Mol Microbiol. 2006;59:1848–58. [DOI] [PubMed] [Google Scholar]
- Argaman L, Hershberg R, Vogel Jet al. . Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr Biol. 2001;11:941–50. [DOI] [PubMed] [Google Scholar]
- Azam MS, Vanderpool CK. Translational regulation by bacterial small RNAs via an unusual Hfq-dependent mechanism. Nucleic Acids Res. 2018;46:2585–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian D, Ragunathan PT, Fei Jet al. . A prophage-encoded small RNA controls metabolism and cell division in Escherichia coli. mSystems. 2016;1:e00021–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar A, Argaman L, Altuvia Yet al. . Prediction of novel bacterial small RNAs from RIL-Seq RNA–RNA interaction data. Front Microbiol. 2021;12:635070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechhofer DH, Deutscher MP. Bacterial ribonucleases and their roles in RNA metabolism. Crit Rev Biochem Mol Biol. 2019;54:242–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco CM, Fröhlich KS, Vanderpool CK. Bacterial cyclopropane fatty acid synthase mRNA is targeted by activating and repressing small RNAs. J Bacteriol. 2019;201:e00461–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi L, Figueroa-Bossi N. A small RNA downregulates LamB maltoporin in Salmonella. Mol Microbiol. 2007;65:799–810. [DOI] [PubMed] [Google Scholar]
- Bouché F, Bouché JP. Genetic evidence that DicF, a second division inhibitor encoded by the Escherichia coli dicB operon, is probably RNA. Mol Microbiol. 1989;3:991–4. [DOI] [PubMed] [Google Scholar]
- Bronesky D, Desgranges E, Corvaglia Aet al. . A multifaceted small RNA modulates gene expression upon glucose limitation in Staphylococcus aureus. EMBO J. 2019;38:e99363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt DH, Rouskin S, Zhang Yet al. . Operon mRNAs are organized into ORF-centric structures that predict translation efficiency. eLife. 2017;6:e22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busi F, Le Derout J, Cerciat Met al. . Is the secondary putative RNA–RNA interaction site relevant to GcvB mediated regulation of oppA mRNA in Escherichia coli?. Biochimie. 2010;92:1458–461. [DOI] [PubMed] [Google Scholar]
- Chao Y, Li L, Girodat Det al. . In vivo cleavage map illuminates the central role of RNase E in coding and non-coding RNA pathways. Mol Cell. 2017;65:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y, Papenfort K, Reinhardt Ret al. . An atlas of Hfq-bound transcripts reveals 3′ UTRs as a genomic reservoir of regulatory small RNAs. EMBO J. 2012;31:4005–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y, Vogel J. A 3′ UTR-derived small RNA provides the regulatory noncoding arm of the inner membrane stress response. Mol Cell. 2016;61:352–63. [DOI] [PubMed] [Google Scholar]
- Chen S, Lesnik EA, Hall TAet al. . A bioinformatics based approach to discover small RNA genes in the Escherichia coli genome. Biosystems. 2002;65:157–77. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Choi JH, Kim EJet al. . Negative regulation of the gene for Fe-containing superoxide dismutase by an Ni-responsive factor in Streptomyces coelicolor. J Bacteriol. 1999;181:7381–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan JE Jr, Laporte D. Tricarboxylic acid cycle and glyoxylate bypass. EcoSal Plus. 2005;1:ecosalplus.3.5.2. [DOI] [PubMed] [Google Scholar]
- Dar D, Shamir M, Mellin JRet al. . Term-seq reveals abundant ribo-regulation of antibiotics resistance in bacteria. Science. 2016;352:aad9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar D, Sorek R. Bacterial noncoding RNAs excised from within protein-coding transcripts. mBio. 2018;9:e01730–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BM, Waldor MK. RNase E-dependent processing stabilizes MicX, a Vibrio cholerae sRNA. Mol Microbiol. 2007;65:373–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mets F, Van Melderen L, Gottesman S. Regulation of acetate metabolism and coordination with the TCA cycle via a processed small RNA. Proc Natl Acad Sci USA. 2019;116:1043–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham EL. The sponge RNAs of bacteria: how to find them and their role in regulating the post-transcriptional network. Biochim Biophys Acta. 2020;1863:194565. [DOI] [PubMed] [Google Scholar]
- Desgranges E, Barrientos L, Herrgott Let al. . The 3′ UTR-derived sRNA RsaG coordinates redox homeostasis and metabolism adaptation in response to glucose-6-phosphate uptake in Staphylococcus aureus. Mol Microbiol. 2021;117:193–214. [DOI] [PubMed] [Google Scholar]
- Durand S, Condon C. RNases and helicases in Gram-positive bacteria. Microbiol Spectr. 2018;6:RWR–0003-2017. [DOI] [PubMed] [Google Scholar]
- Eisenhardt KMH, Reuscher CM, Klug G. PcrX, an sRNA derived from the 3′-UTR of the Rhodobacter sphaeroides puf operon modulates expression of puf genes encoding proteins of the bacterial photosynthetic apparatus. BMC Genomics. 2018;17:325–34. [DOI] [PubMed] [Google Scholar]
- Ettwiller L, Buswell J, Yigit Eet al. . A novel enrichment strategy reveals unprecedented number of novel transcription start sites at single base resolution in a model prokaryote and the gut microbiome. BMC Genomics. 2016;17:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubladier M, Cam K, Bouché JP. Escherichia coli cell division inhibitor DicF-RNA of the dicB operon. Evidence for its generation in vivo by transcription termination and by RNase III and RNase E-dependent processing. J Mol Biol. 1990;212:461–71. [DOI] [PubMed] [Google Scholar]
- Fender A, Elf J, Hampel Ket al. . RNAs actively cycle on the Sm-like protein Hfq. Genes Dev. 2010;24:2621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förstner KU, Reuscher CM, Haberzettl Ket al. . RNase E cleavage shapes the transcriptome of Rhodobacter sphaeroides and strongly impacts phototrophic growth. Life Sci Alliance. 2018;1:e201800080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs M, Lamm-Schmidt V, Sulzer Jet al. . An RNA-centric global view of Clostridioides difficile reveals broad activity of Hfq in a clinically important gram-positive bacterium. Proc Natl Acad Sci USA. 2021;118:e2103579118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes DN, Calderón PF, Acuña LGet al. . Motility modulation by the small non-coding RNA SroC in Salmonella Typhimurium. FEMS Microbiol Lett. 2015;362:fnv135. [DOI] [PubMed] [Google Scholar]
- Geissmann T, Chevalier C, Cros MJet al. . A search for small noncoding RNAs in Staphylococcus aureus reveals a conserved sequence motif for regulation. Nucleic Acids Res. 2009;37:7239–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerovac M, Vogel J, Smirnov A. The world of stable ribonucleoproteins and its mapping with Grad-seq and related approaches. Front Mol Biosci. 2021a;8:661448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerovac M, Wicke L, Chihara Ket al. . A Grad-seq view of RNA and protein complexes in Pseudomonas aeruginosa under standard and bacteriophage predation conditions. mBio. 2021b;12:e03454–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser J, Nuss AM, Berghoff BAet al. . Singlet oxygen stress in microorganisms. Adv Microb Physiol. 2011;58:141–73. [DOI] [PubMed] [Google Scholar]
- Gogol EB, Rhodius VA, Papenfort Ket al. . Small RNAs endow a transcriptional activator with essential repressor functions for single-tier control of a global stress regulon. Proc Natl Acad Sci USA. 2011;108:12875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowicz M, Koren D, Silhavy TJ. The CpxQ sRNA negatively regulates Skp to prevent mistargeting of β-barrel outer membrane proteins into the cytoplasmic membrane. mBio. 2016;7:e00312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo MS, Updegrove TBet al. . MicL, a new σE-dependent sRNA, combats envelope stress by repressing synthesis of Lpp, the major outer membrane lipoprotein. Genes Dev. 2014;28:1620–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K, Lory S. Toward a comprehensive analysis of posttranscriptional regulatory networks: a new tool for the identification of small RNA regulators of specific mRNAs. mBio. 2021;12:e03608–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K, Tjaden B, Lory S. GRIL-seq provides a method for identifying direct targets of bacterial small regulatory RNA by in vivo proximity ligation. Nat Microbiol. 2016;2:16239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist E, Li L, Bischler Tet al. . Global maps of ProQ binding in vivo reveal target recognition via RNA structure and stability control at mRNA 3′ ends. Mol Cell. 2018;70:971–82. [DOI] [PubMed] [Google Scholar]
- Holmqvist E, Vogel J. RNA-binding proteins in bacteria. Nat Rev Microbiol. 2018;16:601–15. [DOI] [PubMed] [Google Scholar]
- Hör J, Di Giorgio S, Gerovac Met al. . Grad-seq shines light on unrecognized RNA and protein complexes in the model bacterium Escherichia coli. Nucleic Acids Res. 2020b;48:9301–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hör J, Garriss G, Di Giorgio Set al. . Grad-seq in a Gram-positive bacterium reveals exonucleolytic sRNA activation in competence control. EMBO J. 2020c;39:e103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hör J, Gorski SA, Vogel J. Bacterial RNA biology on a genome scale. Mol Cell. 2018;70:785–99. [DOI] [PubMed] [Google Scholar]
- Hör J, Matera G, Vogel Jet al. . Trans-acting small RNAs and their effects on gene expression in Escherichia coli and Salmonella enterica. EcoSal Plus. 2020a;9:ESP–0030-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hör J, Vogel J. Analysis of the RNA and protein complexome by Grad-seq. Methods Mol Biol. 2021;2300:183–201. [DOI] [PubMed] [Google Scholar]
- Hör J, Vogel J. Global snapshots of bacterial RNA networks. EMBO J. 2017;36:245–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyos M, Huber M, Förstner KUet al. . Gene autoregulation by 3′ UTR-derived bacterial small RNAs. eLife. 2020;9:e58836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M, Fröhlich KS, Radmer Jet al. . Switching fatty acid metabolism by an RNA-controlled feed forward loop. Proc Natl Acad Sci USA. 2020;117:8044–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosub IA, Marchioretto M, van Nues RWet al. . The mRNA derived MalH sRNA contributes to alternative carbon source utilization by tuning maltoporin expression in E. coli. RNA Biol. 2021;18:914–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosub IA, van Nues RW, McKellar SWet al. . Hfq CLASH uncovers sRNA–target interaction networks linked to nutrient availability adaptation. eLife. 2020;9:e54655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J, Rasmussen AA, Overgaard Met al. . Conserved small non-coding RNAs that belong to the sigmaE regulon: role in down-regulation of outer membrane proteins. J Mol Biol. 2006;364:1–8. [DOI] [PubMed] [Google Scholar]
- Ju X, Li D, Liu S. Full-length RNA profiling reveals pervasive bidirectional transcription terminators in bacteria. Nat Microbiol. 2019; 4:1907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano M, Reynolds AA, Miranda-Rios Jet al. . Detection of 5′- and 3′-UTR-derived small RNAs and cis-encoded antisense RNAs in Escherichia coli. Nucleic Acids Res. 2005;33:1040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Shin JH, Cho YBet al. . Inverse regulation of Fe- and Ni-containing SOD genes by a Fur family regulator Nur through small RNA processed from 3′ UTR of the sodF mRNA. Nucleic Acids Res. 2014;42:2003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikow T, Schröder I, Gunsalus RP. Regulation of narK gene expression in Escherichia coli in response to anaerobiosis, nitrate, iron, and molybdenum. J Bacteriol. 1992;174:7104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger C, Colgan A, Srikumar Set al. . An infection-relevant transcriptomic compendium for Salmonella enterica Serovar Typhimurium. Cell Host Microbe. 2013;14:683–95. [DOI] [PubMed] [Google Scholar]
- Lalaouna D, Baude J, Wu Zet al. . RsaC sRNA modulates the oxidative stress response of Staphylococcus aureus during manganese starvation. Nucleic Acids Res. 2019;47:9871–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm-Schmidt V, Fuchs M, Sulzer Jet al. . Grad-seq identifies KhpB as a global RNA-binding protein in Clostridioides difficile that regulates toxin production. microLife. 2021;2:uqab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GW, Burkhardt D, Gross Cet al. . Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell. 2014;157:624–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieser SA, Davis TC, Helmann JDet al. . DNA-binding and oligomerization studies of the manganese(II) metalloregulatory protein MntR from Bacillus subtilis. Biochemistry. 2003;42:12634–42. [DOI] [PubMed] [Google Scholar]
- Lioliou E, Sharma CM, Caldelari Iet al. . Global regulatory functions of the Staphylococcus aureus endoribonuclease III in gene expression. PLos Genet. 2012;8,e1002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh E, Dussurget O, Gripenland Jet al. . A trans-acting riboswitch controls expression of the virulence regulator PrfA in Listeria monocytogenes. Cell. 2009;139:770–9. [DOI] [PubMed] [Google Scholar]
- Loh E, Memarpour F, Vaitkevicius Ket al. . An unstructured 5′-coding region of the prfA mRNA is required for efficient translation. Nucleic Acids Res. 2012;40:1818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matera G, Altuvia Y, Gerovac Met al. . Global RNA interactome of Salmonella discovers a 5′UTR sponge for the MicF small RNA that connects membrane permeability to transport capacity. Mol Cell. 2022;82:629–44. [DOI] [PubMed] [Google Scholar]
- Mediati DG, Lalaouna D, Tree JJ. Burning the candle at both ends: have exoribonucleases driven divergence of regulatory RNA mechanisms in bacteria?. mBio. 2021;12:e0104121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed S, Adams PP, Zhang Aet al. . RNA–RNA interactomes of ProQ and Hfq reveal overlapping and competing roles. Mol Cell. 2020;77:411–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed S, Faigenbaum-Romm R, Peer Aet al. . Mapping the small RNA interactome in bacteria using RIL-seq. Nat Protoc. 2018;13:1–33. [DOI] [PubMed] [Google Scholar]
- Melamed S, Peer A, Faigenbaum-Romm Ret al. . Global mapping of small RNA–target interactions in bacteria. Mol Cell. 2016;63:884–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melson EM, Kendall MM. The sRNA DicF integrates oxygen sensing to enhance enterohemorrhagic Escherichia coli virulence via distinctive RNA control mechanisms. Proc Natl Acad Sci USA. 2019;116:14210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakoshi M, Chao Y, Vogel J. Cross talk between ABC transporter mRNAs via a target mRNA-derived sponge of the GcvB small RNA. EMBO J. 2015a;34:1478–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakoshi M, Chao Y, Vogel J. Regulatory small RNAs from the 3′ regions of bacterial mRNAs. Curr Opin Microbiol. 2015b;24:132–9. [DOI] [PubMed] [Google Scholar]
- Miyakoshi M, Matera G, Maki Ket al. . Functional expansion of a TCA cycle operon mRNA by a 3′ end-derived small RNA. Nucleic Acids Res. 2019;47:2075–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakoshi M, Okayama H, Lejars Met al. . Mining RNA-seq data reveals the massive regulon of GcvB small RNA and its physiological significance in maintaining amino acid homeostasis in Escherichia coli. Mol Microbiol. 2022;117:160–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Nishino R, Aiba H. Role of the terminator hairpin in the biogenesis of functional Hfq-binding sRNAs. RNA. 2017;23:1419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashko ON, Lin-Chao S. Escherichia coli responds to environmental changes using enolasic degradosomes and stabilized DicF sRNA to alter cellular morphology. Proc Natl Acad Sci USA. 2017;114:E8025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam TW, Park YH, Jeong HJet al. . Glucose repression of the Escherichia coli sdhCDAB operon, revisited: regulation by the CRP*cAMP complex. Nucleic Acids Res. 2005;33:6712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitzan M, Rehani R, Margalit H. Integration of bacterial small RNAs in regulatory networks. Annu Rev Biophys. 2017;46:131–48. [DOI] [PubMed] [Google Scholar]
- Pagels M, Fuchs S, Pané-Farré Jet al. . Redox sensing by a Rex-family repressor is involved in the regulation of anaerobic gene expression in Staphylococcus aureus. Mol Microbiol. 2010;76:1142–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenfort K, Pfeiffer V, Mika Fet al. . SigmaE-dependent small RNAs of Salmonella respond to membrane stress by accelerating global omp mRNA decay. Mol Microbiol. 2006;62:1674–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Kim JW, Moon BYet al. . Characterization of a novel two-component regulatory system, HptRS, the regulator for the hexose phosphate transport system in Staphylococcus aureus. Infect Immun. 2015;83:1620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Chao G, Gunsalus RP. Aerobic regulation of the sucABCD genes of Escherichia coli, which encode alpha-ketoglutarate dehydrogenase and succinyl coenzyme A synthetase: roles of ArcA, Fnr, and the upstream sdhCDAB promoter. J Bacteriol. 1997;179:4138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T, Berghoff BA, Oh JIet al. . Regulation of a polyamine transporter by the conserved 3′ UTR-derived sRNA SorX confers resistance to singlet oxygen and organic hydroperoxides in Rhodobacter sphaeroides. RNA Biol. 2016;13:988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponath F, Tawk C, Zhu Yet al. . RNA landscape of the emerging cancer-associated microbe Fusobacterium nucleatum. Nat Microbiol. 2021;6:1007–20. [DOI] [PubMed] [Google Scholar]
- Pulvermacher SC, Stauffer LT, Stauffer GV. The small RNA GcvB regulates sstT mRNA expression in Escherichia coli. J Bacteriol. 2009;191:238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quax TE, Wolf YI, Koehorst JJet al. . Differential translation tunes uneven production of operon-encoded proteins. Cell Rep. 2013;4:938–44. [DOI] [PubMed] [Google Scholar]
- Raina M, King A, Bianco Cet al. . Dual-function RNAs. Microbiol Spectr. 2018;6:RWR–0032-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee HJ, Kim EJ, Lee JK. Physiological polyamines: simple primordial stress molecules. J Cell Mol Med. 2007;11:685–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riediger M, Spät P, Bilger Ret al. . Analysis of a photosynthetic cyanobacterium rich in internal membrane systems via gradient profiling by sequencing (Grad-seq). Plant Cell. 2021;33:248–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas E, Klein RJ, Jones TAet al. . Computational identification of noncoding RNAs in E. coli by comparative genomics. Curr Biol. 2001;11:1369–73. [DOI] [PubMed] [Google Scholar]
- Rosenfeld N, Elowitz MB, Alon U. Negative autoregulation speeds the response times of transcription networks. J Mol Biol. 2002;323:785–93. [DOI] [PubMed] [Google Scholar]
- Sauer E, Weichenrieder O. Structural basis for RNA 3′-end recognition by Hfq. Proc Natl Acad Sci USA. 2011;108:13065–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma CM, Darfeuille F, Plantinga THet al. . A small RNA regulates multiple ABC transporter mRNAs by targeting C/A-rich elements inside and upstream of ribosome-binding sites. Genes Dev. 2007;21:2804–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma CM, Hoffmann S, Darfeuille Fet al. . The primary transcriptome of the major human pathogen Helicobacter pylori. Nature. 2010;464:250–5. [DOI] [PubMed] [Google Scholar]
- Sharma CM, Papenfort K, Pernitzsch SRet al. . Pervasive post-transcriptional control of genes involved in amino acid metabolism by the Hfq-dependent GcvB small RNA. Mol Microbiol. 2011;81:1144–65. [DOI] [PubMed] [Google Scholar]
- Shishkin AA, Giannoukos G, Kucukural Aet al. . Simultaneous generation of many RNA-seq libraries in a single reaction. Nat Methods. 2015;12:323–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittka A, Lucchini S, Papenfort Ket al. . Deep sequencing analysis of small noncoding RNA and mRNA targets of the global post-transcriptional regulator, PLoS Genet. 2008;4:e1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov A, Förstner KU, Holmqvist Eet al. . Grad-seq guides the discovery of ProQ as a major small RNA-binding protein. Proc Natl Acad Sci USA. 2016;113:11591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov A, Schneider C, Hör Jet al. . Discovery of new RNA classes and global RNA-binding proteins. Curr Opin Microbiol. 2017a;39:152–60. [DOI] [PubMed] [Google Scholar]
- Smirnov A, Wang C, Drewry LLet al. . Molecular mechanism of mRNA repression in trans by a ProQ-dependent small RNA. EMBO J. 2017b;36:1029–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer LT, Stauffer GV. Antagonistic roles for GcvA and GcvB in hdeAB expression in Escherichia coli. ISRN Microbiol. 2012;2012:697308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G, Papenfort K. Regulating with RNA in Bacteria and Archaea. Washington, DC: ASM Press, 2019. [Google Scholar]
- Storz G, Vogel J, Wassarman KM. Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell. 2011;43:880–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafranska AK, Oxley AP, Chaves-Moreno Det al. . High-resolution transcriptomic analysis of the adaptive response of Staphylococcus aureus during acute and chronic phases of osteomyelitis. mBio. 2014;5:e01775–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014; 505:344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tétart F, Albigot R, Conter Aet al. . Involvement of FtsZ in coupling of nucleoid separation with septation. Mol Microbiol. 1992;6:621–7. [DOI] [PubMed] [Google Scholar]
- Tétart F, Bouché JP. Regulation of the expression of the cell-cycle gene ftsZ by DicF antisense RNA. Division does not require a fixed number of FtsZ molecules. Mol Microbiol. 1992;6:615–20. [DOI] [PubMed] [Google Scholar]
- Thompson KM, Rhodius VA, Gottesman S. SigmaE regulates and is regulated by a small RNA in Escherichia coli. J Bacteriol. 2007;189:4243–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegrove TB, Kouse AB, Bandyra KJet al. . Stem-loops direct precise processing of 3′ UTR-derived small RNA MicL. Nucleic Acids Res. 2019;47:1482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowski ML, Stauffer LT, Stauffer GV. The gcvB gene encodes a small untranslated RNA involved in expression of the dipeptide and oligopeptide transport systems in Escherichia coli. Mol Microbiol. 2000;37:856–68. [DOI] [PubMed] [Google Scholar]
- van der Meulen SB, Hesseling-Meinders A, de Jong Aet al. . The protein regulator ArgR and the sRNA derived from the 3′-UTR region of its gene, ArgX, both regulate the arginine deiminase pathway in Lactococcus lactis. PLoS One. 2019;14:e0218508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderpool CK. Combined experimental and computational strategies define an expansive regulon for GcvB small RNA. Mol Microbiol. 2011;81:1129–32. [DOI] [PubMed] [Google Scholar]
- Vogel J, Bartels V, Tang THet al. . RNomics in Escherichia coli detects new sRNA species and indicates parallel transcriptional output in bacteria. Nucleic Acids Res. 2003;31:6435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt SL, Evans AD, Guest RLet al. . The Cpx envelope stress response regulates and is regulated by small noncoding RNAs. J Bacteriol. 2014;196:4229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadler CS, Vanderpool CK. A dual function for a bacterial small RNA: sgrS performs base pairing-dependent regulation and encodes a functional polypeptide. Proc Natl Acad Sci USA. 2007;104:20454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EG, Simons RW. Antisense RNA control in bacteria, phages, and plasmids. Annu Rev Microbiol. 1994;48:713–42. [DOI] [PubMed] [Google Scholar]
- Wang C, Chao Y, Matera Get al. . The conserved 3′ UTR-derived small RNA NarS mediates mRNA crossregulation during nitrate respiration. Nucleic Acids Res. 2020;48:2126–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Dutzler R, Rizkallah PJet al. . Channel specificity: structural basis for sugar discrimination and differential flux rates in maltoporin. J Mol Biol. 1997;272:56–63. [DOI] [PubMed] [Google Scholar]
- Wassarman KM, Repoila F, Rosenow Cet al. . Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev. 2001;15:1637–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman KM, Zhang A, Storz G. Small RNAs in Escherichia coli. Trends Microbiol. 1999;7:37–45. [DOI] [PubMed] [Google Scholar]
- Waters SA, McAteer SP, Kudla Get al. . Small RNA interactome of pathogenic E. coli revealed through crosslinking of RNase E. EMBO J. 2017;36:374–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann AJ, Venturini E, Sellin MEet al. . The major RNA-binding protein ProQ impacts virulence gene expression in Salmonella enterica Serovar Typhimurium. mBio. 2019;10:e02504–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PR, Richter AS, Papenfort Ket al. . Comparative genomics boosts target prediction for bacterial small RNAs. Proc Natl Acad Sci USA. 2013;110:E3487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B, Boitano M, Clark TAet al. . SMRT-Cappable-seq reveals complex operon variants in bacteria. Nat Commun. 2018;9:3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Figueroa-Bossi N, Bossi L. Translation enhancing ACA motifs and their silencing by a bacterial small regulatory RNA. PLoS Genet. 2014;10,e1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan J, Steglich C, Scholz Iet al. . Inverse regulation of light harvesting and photoprotection is mediated by a 3′-end-derived sRNA in cyanobacteria. Plant Cell. 2021;33,358–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Wassarman KM, Rosenow Cet al. . Global analysis of small RNA and mRNA targets of Hfq. Mol Microbiol. 2003;50:1111–24. [DOI] [PubMed] [Google Scholar]
- Zhang YF, Han K, Chandler CEet al. . Probing the sRNA regulatory landscape of P. aeruginosa: post-transcriptional control of determinants of pathogenicity and antibiotic susceptibility. Mol Microbiol. 2017;106:919–37. [DOI] [PMC free article] [PubMed] [Google Scholar]