Abstract
Conjugal transfer of the Ti plasmids from Agrobacterium tumefaciens is controlled by autoinduction via the transcriptional activator TraR and the acyl-homoserine lactone ligand, Agrobacterium autoinducer (AAI). This control process is itself regulated by opines, which are small carbon compounds produced by the crown gall tumors that are induced by the bacteria. Opines control autoinduction by regulating the expression of traR. Transfer of pTiC58 from donors grown with agrocinopines A and B, the conjugal opines for this Ti plasmid, was detected only after the donors had reached a population level of 107 cells per cm2. Donors incubated with the opines and AAI transferred their Ti plasmids at population levels about 10-fold lower than those incubated with opines only. Transcription of the tra regulon, as assessed by monitoring a traA::lacZ reporter, showed a similar dependence on the density of the donor population. However, even in cultures at low population densities that were induced with opines and AAI, there was a temporal lag of between 15 and 20 h in the development of conjugal competence. Moreover, even after this latent period, maximal transfer frequencies required several hours to develop. This lag period was independent of the population density of the donors but could be reduced somewhat by addition of exogenous AAI. Quorum-dependent development of conjugal competence required control by the opine regulon; donors harboring a mutant of pTiC58 deleted for the master opine responsive repressor accR transferred the Ti plasmid at maximum frequencies at very low population densities. Similarly, an otherwise wild-type derivative of pTiC58 lacking traM, which codes for an antiactivator that inhibits TraR activity, transferred at high frequency in a population-independent manner in the absence of the conjugal opines. Thus, while quorum sensing is dependent upon autoinduction, the two phenomena are not synonymous. We conclude that conjugal transfer of pTiC58 is regulated in a quorum-dependent fashion but that supercontrol of the TraR-AAI system by opines and by TraM results in a complex control process that requires not only the accumulation of AAI but also the expression of TraR and the synthesis of this protein at levels that overcome the inhibitory activity of TraM.
Conjugal transfer of the Ti plasmids from Agrobacterium tumefaciens is regulated directly by the transcriptional activator TraR and its acyl-homoserine lactone (acyl-HSL) ligand, Agrobacterium autoinducer [AAI; N-(3-oxo-octanoyl)-l-homoserine lactone] (16, 34, 40; reviewed in reference 12). TraR, in its interaction with AAI, controls conjugation by autoinduction, a process by which the bacteria induce gene sets in response to signals they themselves produce. This regulatory strategy is believed to tie plasmid transfer to the population density of the donor in what has come to be called the quorum-sensing effect (reviewed in reference 18). The acyl-HSL autoinducers, which are produced by the bacteria themselves, are released into the environment, where they accumulate to ever higher concentrations. Moreover, because these molecules apparently can exchange between the intracellular and extracellular compartments, they transduce the signal among the individual members of the population. The quorum-sensing phenomenon results from the need for the accumulation of the autoinducer to some threshold concentration within the habitat. Not until it reaches this critical level does the autoinducer productively interact with the transcriptional activator, thereby initiating expression of the target genes. Thus, the bacteria gauge their population size by sensing the amount of the autoinducer present in the environment.
Expression of bioluminescence in Vibrio fischeri, the paradigm quorum-sensing system, is controlled by the transcriptional activator LuxR and the acyl-HSL signal molecule, Vibrio autoinducer [VAI; N-(3-oxo-hexanoyl)-l-homoserine lactone] (reviewed in reference 7). At low population densities, the lux operon is not expressed. However, luminescence is strongly induced when, due to population growth, VAI accumulates to its threshold level. Experimentally, the quorum dependence of lux gene activation can be circumvented by adding the acyl-HSL signal to cultures of cells at low population density (8, 10). LuxR is produced at a relatively high basal level during growth (reviewed in reference 36); under such conditions, the activator is not limiting and the lux operon is almost immediately induced.
Autoinduction of Ti plasmid transfer is somewhat more complex than that of lux-mediated bioluminescence. Expression of the tra regulon also is controlled by a second set of exogenous signals, highly specific compounds called the conjugal opines that are produced by the crown gall tumors induced by the phytopathogen (reviewed in reference 12). These compounds, the production of which is coded for by genes inherited from the bacterium by the transformed plant cells, control conjugation by regulating the expression of traR (15, 35). Thus, unlike lux, autoinduction of tra is tightly controlled at the transcriptional level by regulating the expression of traR. Furthermore, the activity of TraR itself is modulated by the antiactivator, TraM (17, 21). This small protein interacts with TraR to form a complex that no longer can bind to promoters of the tra regulon (22, 26a). Like the lux system, the expression of traI, the gene responsible for the production of AAI, requires activated TraR (23). However, given that the expression of traR requires the opine signal, only very small amounts of AAI are produced when donor populations are growing in the absence of crown gall tumors. Thus, autoinduction of the tra regulon first requires the induction of the expression of traR. Then, the activator must accumulate to levels sufficient to overcome the inhibitory activity of TraM. In the meantime, autoinduction and its attendant quorum dependence require that AAI accumulate to the levels necessary to activate the newly synthesized TraR.
The requirements for TraR and AAI indicate that conjugal transfer is controlled by autoinduction and predict that transfer is regulated in a quorum-dependent manner. This appears to be the case; transfer of the octopine-type Ti plasmid pTiR10 occurs only when the donor population has reached a critical size (14). Moreover, density dependence could be circumvented by addition of exogenous AAI to the culture. However, how the hierarchical control exerted by the opine regulon influences the TraR-dependent quorum-sensing system has not been critically examined, nor has the role of TraM, if any, in quorum dependence been determined. In this study, we analyzed the influence of regulation by the conjugal opine on the expression of the quorum-sensing system of the nopaline-type Ti plasmid, pTiC58. We also examined the roles of the opine-responsive regulatory protein AccR and the antiactivator TraM in controlling Ti plasmid transfer. Our results indicate that these regulatory components, while not required for TraR-AAI-mediated autoinduction, are essential for the quorum-dependent character of Ti plasmid transfer.
MATERIALS AND METHODS
Bacterial strains and plasmids.
All strains of A. tumefaciens used in this study are derivatives of the nopaline-agrocinopine-type pathogen C58 (Table 1). The Ti plasmids used in this study are derivatives of pTiC58 and are described in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| NT1 | pTiC58-cured derivative of C58, contains pAtC58 | Our collection |
| C58C1RS | pTiC58-cured derivative of C58, Rifr Strr, contains pAtC58 | 37 |
| Plasmids | ||
| pTiC58 | Wild-type Ti plasmid, Noc+ Acc+ Trai | Our collection |
| pTiC58ΔaccR | accR deletion derivative of pTiC58, Noc+ Accc Trac | 1 |
| pCMA1 | traM::nptII mutant of pTiC58, Noc+ Acc+ Trac Kanr | 21 |
| pJM749 | pTHB58 traA::lacZ749 | 1 |
Noc+, regulated catabolism of nopaline; Acc+, regulated catabolism of agrocinopines A and B; Accc, constitutive catabolism of agrocinopines A and B; Trai, inducible for conjugal transfer; Trac, constitutive for conjugal transfer; Rifr, rifampin resistant; Strr, streptomycin resistant; Kanr, kanamycin resistant.
Media and growth conditions.
Bacteria were grown in Luria-Bertani broth (Gibco-BRL, Gaithersburg, Md.), on nutrient agar plates (Difco Laboratories, Detroit, Mich.), or in AB minimal medium containing 0.2% mannitol (ABM) (5). AB medium solidified with 1.5% agar and containing 1 mM nopaline (Sigma Chemical Co., St. Louis, Mo.) and 9 mM arginine, along with rifampin (50 μg per ml) and streptomycin (200 μg per ml), was used as the selection medium for conjugation assays (2). In this medium, nopaline allows Ti plasmid-dependent utilization of arginine as the sole source of carbon (2). A preparation containing a mixture of agrocinopines A and B was partially purified from extracts of crown gall tumors induced on tomato plants by A. tumefaciens C58 as described by Hayman et al. (19). The concentration of the opines in the mixture, expressed as arabinose equivalents, was determined by the phloroglucinol assay as previously described (19). All cultures were incubated at 28°C. Cultures in liquid medium were incubated with shaking to ensure aeration. Growth of liquid cultures was followed by Klett colorimetry (red filter) or by turbidity measurements at 600 nm with a Spectronic 20 spectrophotometer.
Preparation of AAI.
Crude preparations of AAI were prepared by growing cells of strain NT1(pTiC58ΔaccR) to saturation in ABM medium. The cells were removed by centrifugation, and the culture supernatant was sterilized by filtration. The preparation was stored at 4°C. Pure synthetic AAI was the gift of David Lynn, University of Chicago.
Induction of conjugation with agrocinopines.
Donors were grown on sterile filters (diameter, 13 mm; Millipore Corp., Bedford, Mass.) on small towers (5 mm [diameter] by 8 mm [height]) of ABM agar medium impregnated with a mixture of agrocinopines A and B (2 mM). When required, the towers also were impregnated with an aqueous solution of AAI. At the appropriate time intervals, cells were removed from the filters by vortexing them in 500-μl volumes of 0.9% NaCl.
Conjugation assays.
Conjugal transfer was assessed by the spot plate mating method, which measures only initial transfer events (2). The recipient was spread as a confluent lawn over the surfaces of the selection plates. Five-microliter volumes of donor cells at decreasing cell concentrations were spotted onto the surface of the recipient lawn, and the cultures were incubated at 28°C for 48 h. Transconjugant colonies appearing within the donor inoculum spots were enumerated with the aid of a dissecting microscope. In all cases, A. tumefaciens C58C1RS was used as the recipient. Titers of donor cultures were determined by dilution plating in triplicate on NA plates at the time of mating.
Alternatively, when it was necessary to control the numbers of both donors and recipients, matings were performed on nitrocellulose filters essentially as described by Cook and Farrand (6). Donor and recipient bacteria grown in liquid ABM medium were adjusted to the desired population densities. Volumes were mixed, the cells were collected onto sterile nitrocellulose filters (diameter, 25 mm; Millipore Corp.), and the filters were incubated on ABM plates for 2 h at 28°C. The cells were resuspended from the filters in 1-ml volumes of 0.9% NaCl and diluted, and 10-μl volumes of appropriate dilutions were spotted in triplicate onto the surface of the selection medium. Following incubation for 48 h, transconjugant colonies appearing within the spots were enumerated as described above. Titers of donor and recipient cultures were determined by dilution plating in triplicate on NA plates at the time of mating.
To assess the effects of Ti plasmid mutations on quorum sensing, a series of 1:2 dilutions of an early-exponential-phase culture of the appropriate donor was prepared in 1-ml volumes of ABM medium and the cultures were incubated overnight at 28°C with shaking. The next morning, the culture showing the faintest turbidity (ca. 106 to 107 CFU per ml) was chosen, and the cells were collected by centrifugation, washed twice with AB medium lacking mannitol, and resuspended in a 1-ml volume of ABM medium. A 750-μl volume of this preparation was used to inoculate 200 ml of ABM medium, and the culture was incubated at 28°C with shaking. At intervals of time, appropriate volumes were removed and the cells were concentrated by filtration onto nitrocellulose membranes if needed and mated on filters with C58C1RS as described above. Titers of the donor were determined by dilution plating in triplicate on NA plates at the time of mating.
β-Galactosidase assays.
β-Galactosidase activity, expressed as units per 109 cells, was quantified as described previously (13).
RESULTS
Conjugal transfer of pTiC58 is dependent upon donor population size.
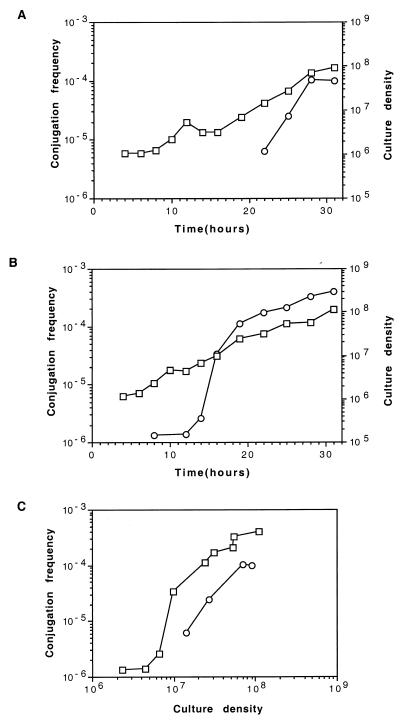
The transfer of wild-type pTiC58 (Fig. 1) requires induction by the sugar phosphodiester opines agrocinopines A and B (1, 9) and also activation of TraR by AAI (35). Since TraR and AAI comprise the two components of a typical quorum-sensing system, we determined whether the frequency of transfer of wild-type pTiC58 is influenced by the size of the donor population. Filters inoculated with cultures of NT1(pTiC58) at low density were placed on agar towers impregnated with agrocinopines A and B with or without AAI, and the filter-bound cultures were incubated at 28°C. At intervals of time, a filter of each culture set was removed, the cells were resuspended in buffer and diluted, and volumes of appropriate dilutions were spot-mated with a confluent culture of the recipient strain, C58C1RS, as described in Materials and Methods. As shown in Fig. 2, the two culture sets, one with AAI and the other without, grew exponentially at similar rates from beginning titers of about 106 CFU per cm2. In the set induced with agrocinopines alone, transfer was first detected after 22 h of growth, when the donors had reached a density of 1.6 × 107 CFU per cm2 (Fig. 2A). Transfer frequencies increased steadily as the density of the donor population increased and plateaued when the culture reached a population density of close to 108 CFU per cm2. Adding AAI at the time of inoculation onto opine induction medium resulted in detectable transfer after 8 h of incubation at a donor population density of 2 × 106 CFU per cm2 (Fig. 2B). Transfer frequencies increased rapidly over the next 10 h as the density of the donor population increased to about 3 × 107 CFU per cm2. From that point, transfer frequencies increased more slowly as the donor population reached a density of 1.1 × 108 CFU per cm2. When examined as a function of population density, the donor set incubated with opines and AAI initiated conjugal transfer at a significantly lower density compared to the culture set incubated with the conjugal opine only (Fig. 2C). Thus, a threshold donor population density is required to initiate conjugation, and this density can be lowered by addition of exogenous AAI.
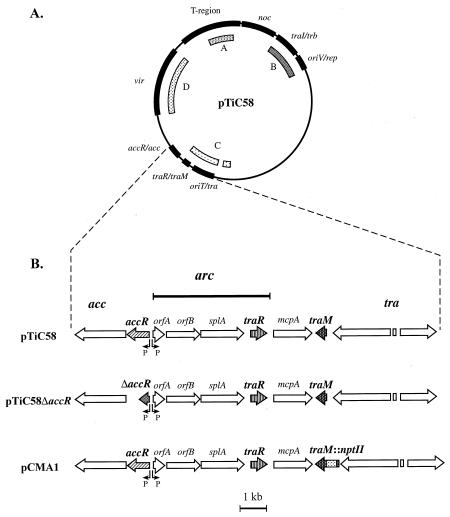
FIG. 1.
Physico-genetic map of pTiC58 and the gene structure of the conjugation control region. (A) Location of gene systems on pTiC58 (20). T-region, region of the plasmid transferred to plants; noc, catabolism of nopaline; traI/trb, conjugation mating bridge and synthesis of AAI; oriV/rep, plasmid replication; oriT/tra, conjugation functions and oriT; traR/traM, conjugation control region; accR/acc, catabolism of agrocinopines A and B; vir, T-region processing and transfer to plants. The shaded arcs labelled A to D denote those regions of pTiC58 strongly related to corresponding regions of the octopine-mannityl opine-type Ti plasmids (11). (B) Gene structure of the conjugation control region and locations of mutations affecting regulation used in this study. The structure and functions of the acc, tra, and arc operons have been described (6, 13, 24, 26, 35). pTiC58 is wild type and conjugal transfer requires induction by agrocinopines A and B. pTiC58ΔaccR contains a deletion mutation in accR resulting in the complete loss of repressor activity (1) and in the constitutive expression of acc and arc. This Ti plasmid is constitutive for transfer. pCMA1 is derived from pTiC58 and contains an insertion of an nptII cassette into a deletion allele of traM (21). The Ti plasmid, although wild type for regulation of acc and arc by opines, is constitutive for conjugal transfer.
FIG. 2.
Conjugal transfer of pTiC58 is dependent upon the size of the donor population. Cultures of NT1(pTiC58) were induced for transfer with agrocinopines (A) or with agrocinopines and AAI (B) as described in the text. Transfer frequencies (○) are expressed as transconjugants per input donor and donor population densities (□) are expressed as CFU per square centimeter. Each mating was quantified in triplicate, and the experiment was repeated three times. The results of a representative experiment are presented. (C) The results of the two experiments in which transfer frequency is expressed as a function of the donor population density. ○, donors induced with agrocinopines A and B; □, donors induced with agrocinopines A and B and AAI.
Induction of tra genes is dependent upon donor population density.
We determined whether induction of transcription of the tra regulon is itself dependent upon donor population density and the accumulation of AAI. Strain NT1(pTiC58; pJM749), which harbors a wild-type Ti plasmid and a clone that contains a TraR-dependent traA::lacZ reporter fusion (Table 1), was induced with agrocinopines on solid medium in the presence and absence of exogenous AAI as described above. Expression of the reporter fusion was monitored at different incubation times by assessing levels of β-galactosidase activity. In the absence of AAI, the reporter was not expressed at detectable levels until the population had reached a density of between 106 and 107 CFU per cm2 (Fig. 3). Expression levels increased almost 10-fold as the population increased to about 2 × 108 CFU per cm2. Adding AAI at the beginning of growth resulted in expression of the traA reporter at a population density of between 105 and 106 CFU per cm2, a donor density at least 10-fold lower than that observed for the culture incubated with the opines alone (Fig. 3). Moreover, expression of the reporter increased to almost 1,500 U per 109 cells as the culture density rose to around 107 CFU per cm2. This level of expression is almost 100 times that seen in cells of the culture not exposed to exogenous AAI that were at a similar population density.
FIG. 3.
The traAFB operon is expressed in a density-dependent manner. Expression of a traA::lacZ reporter fusion present in NT1(pTiC58, pJM749) was measured in strains induced on solid medium supplemented with either agrocinopines A and B (○) or agrocinopines A and B and AAI (□). β-Galactosidase activity is expressed as units per 109 CFU and population densities are expressed as CFU per square centimeter. The experiment was repeated twice, with similar results, and the results from a representative experiment are presented.
Induction of conjugation is dependent upon population density and induction time.
Transfer of the Ti plasmid from a culture of NT1(pTiC58) induced with agrocinopines only was undetectable (<10−6 transconjugants per input donor) for the first 15 h of incubation (Fig. 2A). Like other quorum-sensing systems, this could reflect the time required for the autoinducer to accumulate to levels sufficient to activate TraR. However, transfer from a culture of NT1(pTiC58) induced with the opines and AAI simultaneously also was undetectable for a period of almost 8 h and then steadily increased over a period beginning about 15 h after inoculation (Fig. 2B). This lag is not characteristic of other quorum-sensing systems (8, 10, 38). We suspected that even with sufficient AAI, the donor population requires time to fully express and assemble a functional transfer system. To differentiate between the time required to express the transfer system and population density effects, a set of 10-fold serially diluted donor cultures was prepared from an early-exponential-phase culture of NT1(pTiC58). Two sets of such diluted cultures were incubated for 15 h on solid ABM medium supplemented with agrocinopines, one with and the other without AAI. Two additional sets were incubated for 20 h, on the same two types of medium. This resulted in sets of donor cultures exposed to opines or to opines and AAI, one for 15 h, the other for 20 h, each with increasing initial donor population densities within the set.
Among the cultures grown for 15 h with agrocinopines, transfer occurred at a very low but detectable level only from donors that had attained the highest population level (Table 2). Addition of AAI lowered the critical density by about 1 order of magnitude, but transfer frequencies remained low. However, among donors incubated for 20 h with opines only, all but the lowest-density culture transferred the Ti plasmid at a reasonable frequency (Table 2). Addition of AAI resulted in transfer at similar frequencies among donors at all population levels.
TABLE 2.
Induction time influences development of conjugation competency
| Induction medium supplement | Donor titers and transfer frequencies following induction for:
|
|||
|---|---|---|---|---|
| 15 h
|
20 h
|
|||
| Donor density (CFU/cm2) | Transfer frequencya | Donor density (CFU/cm2) | Transfer frequencya | |
| Agrocinopines | 1 × 106 | <10−6 | 1 × 106 | <10−6 |
| 2 × 107 | <10−7 | 3 × 107 | 3 × 10−5 | |
| 4 × 108 | 7 × 10−6 | 2 × 108 | 1 × 10−4 | |
| Agrocinopines plus AAI | 1 × 106 | <10−6 | 3 × 106 | 1 × 10−4 |
| 2 × 107 | 2 × 10−5 | 4 × 107 | 3 × 10−4 | |
| 3 × 108 | 4 × 10−5 | 3 × 108 | 1 × 10−4 | |
Expressed as the number of transconjugants recovered per input donor. The experiment was repeated once, yielding a similar patterns of results.
Opine control and TraM are required for quorum-dependent Ti plasmid transfer.
The quorum-sensing system of pTiC58 is itself controlled by the opine regulon (35). In the absence of the conjugal opine, expression of traR is strongly repressed. Opines induce transfer in part because these signals induce the expression of traR. Thus, failure to transfer at low densities, even in the presence of the conjugal opine, may result from the need to produce adequate levels of TraR. We tested this possibility by examining the conjugal transfer properties of pTiC58ΔaccR at increasing donor densities in the absence and presence of AAI. This derivative of pTiC58 contains a deletion mutation in accR, the gene coding for the opine-responsive repressor that regulates expression of traR (Fig. 1) (1). As a consequence, strains harboring this Ti plasmid express traR constitutively, produce large amounts of AAI, and transfer the plasmid at high frequency in the absence of the conjugal opine (1, 23, 34).
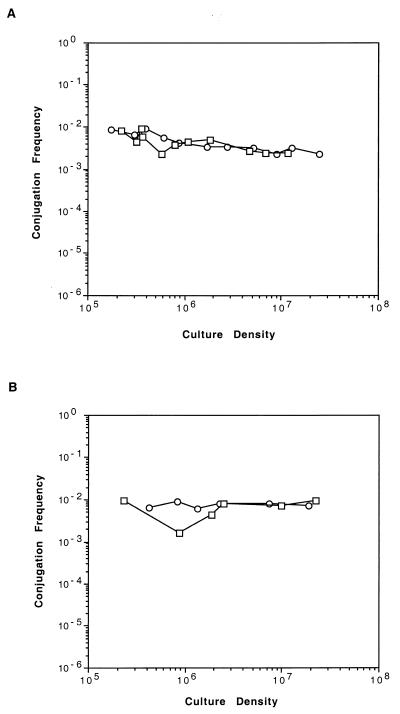
A culture of NT1(pTiC58ΔaccR) with an initial population density of about 104 CFU per ml was prepared from a low-density (ca. 106 CFU/ml) exponential-phase preculture as described in Materials and Methods. The culture was split in two, AAI was added to one subculture, and the two cultures were incubated in parallel. Samples were removed at intervals, the donor titers were determined by dilution plating, and the donors were mated with C58C1RS on filters. As shown in Fig. 4A, the Ti plasmid transferred at high frequency from all samples, even from those in which the donor population density was about 105 CFU per cm2. Furthermore, transfer frequency did not increase as the donor population density increased. Such high frequency transfer was not influenced by the addition of exogenous AAI at the beginning of the culture period.
FIG. 4.
Quorum dependence of conjugal transfer requires a functional opine-control system and traM. The transfer frequency of pTiC58ΔaccR (A) and of pCMA1 (B) was assessed at various densities of the donor population grown in the absence (○) or the presence (□) of AAI. Transfer frequencies are expressed as transconjugants per input donor and population densities are expressed as CFU per square centimeter. Each mating was quantified in triplicate, and the two experiments were repeated twice each. The results from a representative experiment for each donor are presented.
The activity of the Ti plasmid quorum-sensing system also is modulated by TraM, a small protein that binds to and inactivates TraR (17, 21, 26a). Strains harboring otherwise wild-type derivatives of pTiC58 with null mutations in traM produce large amounts of AAI and transfer the Ti plasmid at high frequency even in the absence of the conjugal opine (21). Thus, we assessed the influence of TraM on the quorum dependence of Ti plasmid transfer.
Two parallel cultures of NT1(pCMA1), one with and the other without AAI, were prepared, grown in opine-free medium, sampled, and mated with C58C1RS essentially as described above. pCMA1 is a derivative of pTiC58 that contains a kanamycin resistance cassette inserted into a deletion allele of traM (Fig. 1) (21). As assessed by the filter mating technique, the traM mutant Ti plasmid transferred at a high and constant frequency from all donor samples independent of their population densities (Fig. 4B). Additions of exogenous AAI had no influence on the frequency of transfer at any population density.
Ti plasmid-less A. tumefaciens is an inefficient conjugal recipient.
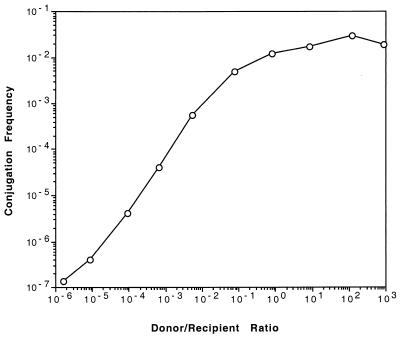
We have proposed that autoinduction and TraM cooperate to prevent the induction of the Ti plasmid tra system at low donor population levels (21, 22, 35). However, it is not clear why activation of the transfer system should be avoided under such conditions. It is possible that the agrobacteria that comprise the recipient pool are not efficient acceptors of Ti plasmids via conjugation. Delaying expression of the tra apparatus until donor population levels are high may represent a mechanism that has evolved to overcome such a deficiency. We tested this hypothesis by determining the efficiency by which a Ti plasmid-less A. tumefaciens strain inherits pTiC58. Late-exponential-phase cultures of NT1(pTiC58ΔaccR) were mated at input densities of 102 to 108 CFU per cm2 with C58C1RS at densities between 108 and 102 CFU per cm2 (33). This yielded ratios of donors to recipients ranging from 10−6 to 106. As expected, transfer frequencies, expressed as the number of transconjugants per input recipient, were highest when donors were present in numbers equal to or greater than those of the recipients (Fig. 5). However, even when donors were in 1,000-fold excess, transfer frequencies never exceeded 10−2 per input recipient.
FIG. 5.
A. tumefaciens C58C1RS is an inefficient conjugal recipient. Donors harboring pTiC58ΔaccR were mated with C58C1RS on nitrocellulose filters at various ratios as described in the text. The total cell density in each mating was constant and was greater than 107 CFU per square centimeter. Frequencies of transfer are expressed as the number of transconjugants recovered per input recipient. Shown is a new analysis of data collected for a previous publication (33).
DISCUSSION
Although TraR serves to regulate conjugation in a density-dependent manner, our results indicate that control of plasmid transfer is a complex process involving other environmental inputs. Clearly, the availability of opines constitutes the primary determinant for initiating the induction of the conjugal transfer process. Opines trigger conjugation by controlling the expression of traR (15, 30, 35). Furthermore, the active traR alleles of all Ti plasmids examined to date are expressed as components of opine-regulated operons, suggesting that regulation by these nutrient sources is important to the biology of Ti plasmid transfer (15, 30, 35; reviewed in reference 12).
In the lux system, LuxR is produced at a relatively high level in the absence of VAI (reviewed in reference 36), making the induction of bioluminescence dependent primarily on the accumulation of the autoinducer. Thus, the addition of exogenous signal results in an almost immediate induction of the lux operon. However, with the availability of TraR itself controlled, the Ti plasmid quorum-sensing system is insensitive to exogenous autoinducer in the absence of the conjugal opine. This hierarchical control may represent a protective measure to ensure that, when not influenced by crown gall tumors, the tra regulon will not respond inadvertently to acyl-HSLs produced by other microorganisms present in the soil (32). Furthermore, even upon opine induction, there is a substantial lag period before the tra regulon is induced. Some of this lag can be accounted for by the necessity to accumulate AAI to sufficient levels. Thus, in the presence of opines, the addition of AAI to the cultures results in tra gene induction and a corresponding increase in conjugal transfer frequencies at population levels lower than those in cultures induced with the opines alone (Fig. 2 and 3). However, there remained a temporal lag even when AAI was added early in the culture cycle, and this lag was independent of the population density of the donor culture (Table 2). Thus, in addition to a dependence on the accumulation of AAI for quorum sensing, there is a temporal component to the induction of conjugal transfer. Under these conditions, between 15 and 20 h is required for the development of conjugation competence following addition of the conjugal opine.
We propose that the observed lag in the induction of conjugation following the addition of opines is multivariate. Clearly, TraR first must be expressed to levels sufficient to activate transcription. Concomitantly, AAI must accumulate to its threshold concentration. The kinetics of autoinducer production and accumulation no doubt contribute to the lag period although, since growth is occurring, this requirement establishes the quorum-sensing nature of the system. This conclusion is supported by our observations that, under conditions in which AAI is not limiting, high levels of transfer can occur at low donor population densities as long as sufficient time is allowed, presumably for gene induction and construction of the conjugal apparatus (Table 2). Alternatively, the lag may result from changes in culture conditions, such as oxygen availability or pH, attendant on the growth of the donor on the filter surface. However, this hypothesis is highly unlikely, as conditions of anaerobiosis and acidic pH are strongly inhibitory to Ti plasmid conjugation (39). Similarly, the low transfer frequencies at early times cannot reflect the need to overcome an inhibitor present in the medium; conjugal transfer of the ΔaccR and traM mutants of pTiC58 is not inhibited by fresh medium, even at very low population densities (Fig. 4).
The induction of TraR to levels sufficient to activate tra gene expression is itself not simple. In the wild-type case, the activity of the transcription factor is inhibited by TraM, also coded for by the Ti plasmid (21). This antiactivator binds to TraR, thereby preventing activation of expression of the tra regulon (22, 26a). Thus, induction of traR expression is not sufficient to immediately activate the tra regulon, even when AAI is present in nonlimiting amounts. Rather, TraR first must accumulate to a level in excess of that of TraM. Finally, once TraR has been activated by AAI, the tra regulon must be expressed and the components of the transfer machinery must be assembled.
Actual expression of the tra regulon, as assessed by a traA::lacZ reporter fusion, mirrored the kinetics of development of conjugation competence in the donor population (Fig. 3). Thus, transcription is dependent on induction by the conjugal opines and also on the accumulation of the donors to a critical density. This threshold population level can be lowered by addition of the acyl-HSL signal (Fig. 3), indicating that quorum sensing operates at the level of transcription. Addition of AAI at the time of opine induction led to levels of traA expression substantially higher than those finally obtained by cultures induced with the opine only (Fig. 3). Yet, at comparable threshold population levels between 106 and 107 CFU per cm2, the two donor cultures transferred the Ti plasmid at similar frequencies (Fig. 2 and data not shown). Thus, the extraordinarily high level of traA expression observed in the donor culture incubated with opines and AAI did not translate to higher levels of conjugation. Similarly, increased levels of tra gene expression from the octopine-type Ti plasmid pTiR10 do not result in higher frequencies of plasmid transfer (14). Ti plasmid copy number is up-regulated five- to sevenfold by TraR and AAI (25). We suggest that the very high levels of β-galactosidase present in donors cultured with opines and AAI are due to this increase in plasmid copy number rather than to increased rates of transcription of the tra regulon itself. The effect is cumulative; cells incubated with opines only do not exhibit this high level of activity until late in the cycle because the increase in Ti plasmid copy number is itself controlled by quorum sensing and does not become a factor until after the threshold population level is reached.
Remarkably, the quorum dependency of conjugal transfer is reliant upon supercontrol of TraR. Releasing TraR from the control of opines or of TraM results in donors that become competent for conjugal transfer at population densities several orders of magnitude lower than that required by the wild-type system (Fig. 4). While it could be argued that these donors still may show a density dependence for transfer, the threshold is well below that intended by the system. Transfer by these two mutant donor types still requires TraR and AAI. Thus, abolishing supercontrol separates autoinduction, which is dependent on the activator and its acyl-HSL signal, from quorum sensing. Given this differentiation, it is clear that autoinduction and quorum sensing are not synonymous. We propose that the former term be used as first defined by Nealson et al. (29): the self-induced expression of a gene system in response to a signal produced by the population of bacteria itself. On the other hand, the population density-dependent character of quorum sensing is an outcome of autoinduction and, depending on the system, may or may not require additional regulatory components.
Clearly, Ti plasmid transfer is regulated in a population-dependent manner. However, quorum sensing requires not only TraR and AAI but also control of traR expression by the opine regulon and TraR activity by TraM. From our results, we propose an integrated model in which, in the absence of the conjugal opines, expression of traR on pTiC58 is repressed by AccR (Fig. 6A). However, under such conditions, the arc operon is expressed at a basal level sufficiently high to produce enough TraR to activate the tra regulon. TraM serves to inactivate this small amount of TraR, thus preventing premature conjugation (Fig. 6A). When opines are present, AccR is inactivated, expression of the arc operon is derepressed, and TraR is produced in amounts sufficient to overcome the available TraM (Fig. 6B). At the same time, AAI is accumulating in the environment, and when the signal has reached its threshold level corresponding to some critical population size, TraR is activated and induces expression of the tra regulon. Following gene expression, the conjugal apparatus is assembled and the donors become competent to transfer the Ti plasmid.
FIG. 6.
Integrated model for the control of Ti plasmid conjugal transfer by opines, TraM, and the TraR-mediated quorum-sensing system. In the absence of the conjugal opines (A), AccR represses expression of the arc operon, of which traR is a member. TraM serves to inactivate the small amounts of the activator expressed from the arc promoter and perhaps from a weak promoter located immediately upstream of traR (21, 35). In the presence of the conjugal opines (B), AccR is inactivated, arc is expressed, and TraR accumulates to levels in excess of those of TraM. During growth of the donors, AAI is produced by TraI and eventually accumulates to a threshold concentration at which it can interact with and activate TraR. This level of signal corresponds to some critical density of the donor population. Activated TraR then initiates transcription of the tra and trb operons, the mating apparatus is assembled, and the Ti plasmid can then transfer on contact between donors and suitable recipients.
While the model is consistent with the available information, it is not at all clear why conjugation should be dependent upon the size of the donor population. Transfer of the Ti plasmids is important in two respects. First, such transfer provides a mechanism by which this virulence element can test new chromosomal backgrounds for greater fitness in a given environment (12). Second, Ti plasmids evolve in part by recombination with other, dissimilar Ti and opine-catabolism plasmids (31). We have proposed that the quorum-sensing system evolved in response to the need of the Ti plasmid to transfer from tumorigenic donors to such Agrobacterium recipients (12). Consistent with this hypothesis, nonpathogenic agrobacteria, some with large opine-catabolic plasmids, commonly are isolated from crown gall tumors and surrounding soils (3, 4, 27, 28). Thus, a large population of primed donors would maximize the probability that these recipients are efficiently mated. Our results showing that such recipients may not be fully competent to receive a Ti plasmid (Fig. 5) suggest that there exist genetic barriers to the transfer of these elements and provide an additional impetus for the induction of conjugation among all members of the donor population.
ACKNOWLEDGMENTS
We thank Zhao-Qing Luo, Pei-Li Li, Philippe Oger, and Clay Fuqua for helpful discussions and David Cook and Pei-Li Li for excellent graphics assistance.
This work was supported in part by grant no. R01 GM52465 from the NIH to S.K.F.
REFERENCES
- 1.Beck von Bodman S, Hayman G T, Farrand S K. Opine catabolism and conjugal transfer of the nopaline Ti plasmid pTIC58 are coordinately regulated by a single repressor. Proc Natl Acad Sci USA. 1992;89:643–647. doi: 10.1073/pnas.89.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck von Bodman S, McCutchan J E, Farrand S K. Characterization of conjugal transfer functions of Agrobacterium tumefaciens Ti plasmid pTiC58. J Bacteriol. 1989;171:5281–5289. doi: 10.1128/jb.171.10.5281-5289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouzar H, Moore L W. Isolation of different Agrobacterium biovars from a natural oak savanna and tallgrass prairie. Appl Environ Microbiol. 1987;53:717–721. doi: 10.1128/aem.53.4.717-721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canfield M L, Moore L W. Isolation and characterization of opine-utilizing strains of Agrobacterium tumefaciens and fluorescent strains of Pseudomonas spp. from rootstocks of Malus. Phytopathology. 1991;81:440–443. [Google Scholar]
- 5.Chilton M-D, Currier T C, Farrand S K, Bendich A J, Gordon M P, Nester E W. Agrobacterium tumefaciens and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci USA. 1974;71:3672–3676. doi: 10.1073/pnas.71.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook D M, Farrand S K. The oriT region of the Agrobacterium tumefaciens Ti plasmid pTiC58 shares DNA sequence identity with the transfer origins of RSF1010 and RK2/RP4 and with T-region borders. J Bacteriol. 1992;174:6238–6246. doi: 10.1128/jb.174.19.6238-6246.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunlap P V, Greenberg E P. Role of intracellular chemical communication in the Vibrio fischeri-monocentrid fish symbiosis. In: Dworkin M, editor. Microbial cell-cell interactions. Washington, D.C.: American Society for Microbiology; 1991. pp. 219–253. [Google Scholar]
- 8.Dunlap P V, Kuo A. Cell density-dependent modulation of the Vibrio fischeri luminescence system in the absence of autoinducer and LuxR protein. J Bacteriol. 1992;174:2440–2448. doi: 10.1128/jb.174.8.2440-2448.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis J G, Kerr A, Petit A, Tempé J. Conjugal transfer of nopaline and agropine Ti-plasmids—the role of agrocinopines. Mol Gen Genet. 1982;186:269–273. [Google Scholar]
- 10.Engebrecht J, Nealson K H, Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983;32:773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- 11.Engler G, Depicker A, Maenhaut R, Villarroel R, van Montagu M, Schell J. Physical mapping of DNA base sequence homologies between an octopine and a nopaline Ti plasmid of Agrobacterium tumefaciens. J Mol Biol. 1981;152:183–208. doi: 10.1016/0022-2836(81)90239-4. [DOI] [PubMed] [Google Scholar]
- 12.Farrand S K. Conjugal plasmids and their transfer. In: Spaink H P, Kondorosi A, Hooykaas P J J, editors. The Rhizobiaceae: molecular biology of model plant-associated bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishing; 1998. pp. 199–233. [Google Scholar]
- 13.Farrand S K, Hwang I, Cook D M. The tra region of the nopaline-type Ti plasmid is a chimera with elements related to the transfer systems of RSF1010, RP4, and F. J Bacteriol. 1996;178:4233–4247. doi: 10.1128/jb.178.14.4233-4247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuqua C, Winans S C. Conserved cis-acting promoter elements are required for density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J Bacteriol. 1996;178:435–440. doi: 10.1128/jb.178.2.435-440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuqua C, Winans S C. Localization of OccR-activated and TraR-activated promoters that express two ABC-type permeases and the traR gene of Ti plasmid pTiR10. Mol Microbiol. 1996;20:1199–1210. doi: 10.1111/j.1365-2958.1996.tb02640.x. [DOI] [PubMed] [Google Scholar]
- 16.Fuqua W C, Winans S C. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol. 1994;176:2796–2806. doi: 10.1128/jb.176.10.2796-2806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuqua W C, Burbea M, Winans S C. Activity of the Agrobacterium Ti plasmid conjugal transfer regulator TraR is inhibited by the product of the traM gene. J Bacteriol. 1995;177:1367–1373. doi: 10.1128/jb.177.5.1367-1373.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuqua W C, Winans S C, Greenberg E P. Quorum-sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayman G T, Beck von Bodman S, Kim H, Jiang P, Farrand S K. Genetic analysis of the agrocinopine catabolic region of Agrobacterium tumefaciens Ti plasmid pTiC58, which encodes genes required for opine and agrocin 84 transport. J Bacteriol. 1993;175:5575–5584. doi: 10.1128/jb.175.17.5575-5584.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holsters M, Silva B, van Vliet F, Genetello C, De Block M, Dhaese P, Depicker A, Inzé D, Engler G, Villarroel R, van Montagu M, Schell J. The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid. 1980;3:212–230. doi: 10.1016/0147-619x(80)90110-9. [DOI] [PubMed] [Google Scholar]
- 21.Hwang I, Cook D M, Farrand S K. A new regulatory element modulates homoserine lactone-mediated autoinduction of Ti plasmid conjugal transfer. J Bacteriol. 1995;177:449–458. doi: 10.1128/jb.177.2.449-458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang I, Smyth A, Luo Z-Q, Farrand S K. Modulating quorum-sensing by antiactivation: TraM interacts with TraR to inhibit activation of Ti plasmid conjugal transfer genes. Mol Microbiol. 1999;34:282–294. doi: 10.1046/j.1365-2958.1999.01595.x. [DOI] [PubMed] [Google Scholar]
- 23.Hwang I, Li P-L, Zhang L, Piper K R, Cook D M, Tate M E, Farrand S K. TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc Natl Acad Sci USA. 1994;91:4639–4643. doi: 10.1073/pnas.91.11.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H, Farrand S K. Characterization of the acc operon from the nopaline-type Ti plasmid pTiC58, which encodes utilization of agrocinopines A and B and susceptibility to agrocin 84. J Bacteriol. 1997;179:7559–7572. doi: 10.1128/jb.179.23.7559-7572.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li P-L, Farrand S K. The replicator of the nopaline-type Ti plasmid pTiC58 is a member of the repABC family and is influenced by the TraR-dependent quorum-sensing regulatory system. J Bacteriol. 2000;182:179–188. doi: 10.1128/jb.182.1.179-188.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P-L, Everhart D M, Farrand S K. Genetic and sequence analysis of the trb locus on pTiC58, a mating-pair formation system related to members of the type IV secretion family. J Bacteriol. 1998;180:6164–6172. doi: 10.1128/jb.180.23.6164-6172.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Luo, Z.-Q., Y. Qin, and S. K. Farrand. The antiactivator TraM interferes with the autoinducer-dependent binding of TraR to DNA by interacting with the C-terminal region of the quorum-sensing activator. J. Biol. Chem., in press. [DOI] [PubMed]
- 27.Moore L W, Chilton W S, Canfield M L. Diversity of opines and opine-catabolizing bacteria isolated from naturally occurring crown gall tumors. Appl Environ Microbiol. 1997;63:201–207. doi: 10.1128/aem.63.1.201-207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nautiyal C S, Dion P. Characterization of the opine-utilizing microflora associated with samples of soil and plants. Appl Environ Microbiol. 1990;56:2576–2579. doi: 10.1128/aem.56.8.2576-2579.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nealson K H, Platt T, Hastings J W. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970;104:313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oger P, Kim K-S, Sackett R L, Piper K R, Farrand S K. Octopine-type Ti plasmids code for a mannopine-inducible dominant-negative allele of traR, the quorum-sensing activator that regulates Ti plasmid conjugal transfer. Mol Microbiol. 1998;27:277–288. doi: 10.1046/j.1365-2958.1998.00671.x. [DOI] [PubMed] [Google Scholar]
- 31.Otten L, Canaday J, Gérard J-C, Fournier P, Crouzet P, Paulus F. Evolution of agrobacteria and their plasmids—a review. Mol Plant-Microbe Interact. 1992;5:279–287. doi: 10.1094/mpmi-5-279. [DOI] [PubMed] [Google Scholar]
- 32.Pierson E A, Wood D W, Cannon J A, Blachere F M, Pierson L S., III Interpopulation signaling via N-acyl-homoserine lactones among bacteria in the wheat rhizosphere. Mol Plant-Microbe Interact. 1998;11:1078–1084. [Google Scholar]
- 33.Piper K R, Farrand S K. Conjugal transfer but not quorum-dependent tra gene induction of pTiC58 requires a solid surface. Appl Environ Microbiol. 1999;65:2798–2801. doi: 10.1128/aem.65.6.2798-2801.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piper K R, Beck von Bodman S, Farrand S K. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature (London) 1993;362:448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- 35.Piper K R, Beck von Bodman S, Hwang I, Farrand S K. Hierarchical gene regulatory systems arising from fortuitous gene associations: regulating quorum-sensing by the opine regulon of Agrobacterium. Mol Microbiol. 1999;32:1077–1089. doi: 10.1046/j.1365-2958.1999.01422.x. [DOI] [PubMed] [Google Scholar]
- 36.Sitnikov D M, Schineller J B, Baldwin T O. Transcriptional regulation of bioluminescence genes from Vibrio fischeri. Mol Microbiol. 1995;17:801–812. doi: 10.1111/j.1365-2958.1995.mmi_17050801.x. [DOI] [PubMed] [Google Scholar]
- 37.Van Larebeke, N., C. Genetello, J. Schell, R. A. Schilperoort, A. K. Hermans, J. P. Hernalsteens, and M. van Montagu. Acquisition of tumor-inducing ability by non-oncogenic agrobacteria as a result of plasmid transfer. Nature (London) 255:742–743. [DOI] [PubMed]
- 38.Wood D W, Pierson L S., III The phzI gene of Pseudomonas aureofaciens 30-84 is responsible for production of a diffusible signal required for phenazine antibiotic production. Gene. 1996;168:49–53. doi: 10.1016/0378-1119(95)00754-7. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L. Molecular biology and biochemistry of a novel conjugation factor in Agrobacterium. Ph.D. dissertation. Adelaide, Australia: University of Adelaide; 1993. [Google Scholar]
- 40.Zhang L, Murphy P J, Kerr A, Tate M E. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature (London) 1993;362:446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]