Summary
Background
Vaccines offer people with multiple sclerosis (PwMS) an effective protection against severe COVID-19 disease courses. However, representative real-world data on the tolerability of SARS-CoV-2 vaccines in PwMS are limited. We aimed at analysing vaccination reactions (VRs) and MS deterioration following SARS-CoV-2 vaccinations in German and United Kingdom (UK) PwMS, especially regarding gender-specific differences.
Methods
The German Multiple Sclerosis Society and the UK MS Registry acquired health data via an online system following the first (X1) and second SARS-CoV-2 vaccination (X2), respectively: sociodemographic and clinical data, vaccines used, VRs, MS deterioration (worsened or new MS symptoms, Germany only) and relapses (Germany only). The frequencies of VRs and MS deterioration were analysed stratified by gender.
Findings
Following X1 (X2), 2346 (1835) German PwMS and 3796 (683) UK PwMS participated in the study. The most frequent vaccination scheme was two-dose tozinameran for Germany (77·1%, 1424/1847) and two-dose AZD1222 for the UK (61·3%, 419/683). The most common VRs were fatigue, headache and pain (at the injection site) and occurred more often in women compared with men. German PwMS reported VRs more frequently after X2 vs. X1 (65·4% [1201/1835] vs. 61·2% [1435/2346]), while for UK patients it was the opposite (X1 vs. X2: 48·7% [1849/3796] vs. 30·0% [205/683]). MS deterioration occurred in 19·0% (445/2346) of the German PwMS without resulting in gender-specific differences. Fatigue and gait impairment were the most frequent deteriorated MS symptoms.
Interpretation
Female PwMS reported experiencing VRs more often than men. Longitudinal data are needed to enable valid statements regarding long-term MS deterioration and long-lasting VRs.
Funding
German Multiple Sclerosis Society (DMSG Bundesverband e.V.), Biogen, Bristol Myers Squibb, Merck Serono, Mylan, Novartis, Roche and Sanofi.
Keywords: SARS-CoV-2, COVID-19, Vaccination, Vaccination reaction, Multiple sclerosis, Gender, Adverse events
Research in context.
Evidence before this study
Previous studies on the use of SARS-CoV-2 vaccine in PwMS provided limited data on the tolerability, vaccine reactions and side effects. Those studies included fewer than 600 patients and focused on a few or single SARS-Cov-2 vaccines. For example, the studies by Achiron et al. and Lotan et al. only provided data on tozinameran. What is currently completely lacking in the literature is representative real-world data on vaccination reactions and MS deterioration following SARS-CoV-2 vaccination in PwMS, particularly considering gender-specific differences. Due to the design of the pivotal SARS-CoV-2 vaccination trials that exclude patients with chronic diseases such as MS, results from large multicentre studies in real world settings are urgently needed for the clinical practice.
Added value of this study
This prospective, non-interventional, observational study provides real-world data of 6142 PwMS from Germany and the UK and represents the largest investigation of SARS-CoV-2 vaccine tolerability in MS to date. In addition to the vaccine distribution among German and UK PwMS, the study analysed the occurrence of vaccination reactions and MS deterioration following vaccination with tozinameran, elasomeran, AZD1222 or Ad26.COV2.S, with special regard to differences between male and female PwMS. The gender analysis revealed that women with MS reported vaccination reactions considerably more often than men. Regarding the worsening and new onset of MS symptoms in total, no gender differences occurred. Age, gender, disability level and DMD treatment status were analysed in terms of an association with the worsening or new onset of single MS symptoms following SARS-CoV-2 vaccination. Furthermore, the short-term relapse activity following vaccination was considered.
Implications of all the available evidence
Gender seems to play a role in the short-term occurrence of vaccination reactions. Data with a minimum observation period of one year are necessary to conclude solid statements on long-lasting vaccination reactions and long-term MS deterioration following SARS-CoV-2 vaccination.
Alt-text: Unlabelled box
Introduction
Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) is responsible for the infectious disease known as COVID-19, which has spread rapidly throughout the world.1 As of July 2022, over 554 million cases of SARS-CoV-2 infections and over 6·4 million deaths associated with SARS-CoV-2 were confirmed by the World Health Organization.2
For the estimated 2·8 million people with multiple sclerosis (PwMS) worldwide, infectious episodes, for example with SARS-CoV-2, can noticeably increase the risk of a worsened MS disease course as well as relapses, especially in patients with comorbidities other than MS, certain (B cell depleting) disease-modifying drugs (DMDs) and neurological disability.1,3,4 One of the most important preventive measures against infectious diseases is vaccination. There is no evidence of a link between inactivated vaccines and a worsening of MS disease progression or relapses.5 However, there is evidence for disease exacerbations after the application of live-attenuated vaccines such as yellow fever in immunocompromised patients.6 Regarding SARS-CoV-2, vaccines based on new modes of action have been approved in the European Union and the United Kingdom (UK): the mRNA vaccines tozinameran (BNT162b2, Comirnaty® from BioNTech/Pfizer) as well as elasomeran (mRNA-1273, Spikevax® from Moderna) and the vector-based vaccines AZD1222 (Vaxzevria® from AstraZeneca) and Ad26.COV2.S (COVID-19 Vaccine Janssen from Janssen/Johnson&Johnson).1 Until July 2022, over 12·0 billion vaccine doses globally had already been administered against SARS-CoV-2, of which over 183 million doses had been administered in Germany and over 149 million doses in the UK.2 Internationally and nationally, there are recommendations for PwMS to get vaccinated against SARS-CoV-2.7,8 However, the timing and choice of DMDs to treat PwMS play an important role in vaccine efficacy.1 The willingness of PwMS to get vaccinated is about 70% according to different surveys.9,10 The main concerns of SARS-CoV-2 vaccination hesitants included the possible adverse long-term effects of the vaccine, safety aspects, insufficient information and concerns due to accelerated approval procedures.9,11 Further concerns are the fear of MS worsening, especially the occurrence of MS relapses following vaccination and adverse events, such as (life-threatening) thromboembolic events associated with vector-based vaccines, which led to a short-term or permanent suspension of the vaccine in several countries.12 The willingness to get vaccinated with vector-based vaccines was much lower in Germany than in the UK at the time after these side effects were reported.12
In Germany and the UK, the most populous countries in Europe, comprehensive data on vaccination reactions and associations of SARS-CoV-2 vaccinations with disease worsening as well as new-onset symptoms among PwMS were not yet available. The known studies on the efficacy and safety of the available vaccines were related to relatively compact study populations and were monocentric in design as well as limited to only one of the new SARS-CoV-2 vaccines.13, 14, 15 Although gender plays an important role in the epidemiology and therapy of MS,16 no data on gender-specific differences in the post-vaccinal disease status are currently available either. However, data acquisition on the tolerability of the SARS-CoV-2 vaccines in PwMS was comparable in Germany and the UK. Therefore, the German MS Society and the UK MS Registry decided to cooperate and compare their results. The aim of this observational study was to present data regarding the frequency of vaccination reactions, worsened MS symptoms and new-onset MS symptoms of a broad study population in Germany and the UK considering gender-specific differences. Furthermore, associations of worsened or new MS symptoms after SARS-CoV-2 vaccinations with age at vaccination, gender, disability level and DMD use were analysed.
Materials and methods
Data collection in Germany
This prospective non-interventional observational study is based on a longitudinal nationwide online survey. The first data acquisition (start date 3rd May 2021) was performed using a (patient-reported) online questionnaire after voluntary registration of MS patients with at least one SARS-CoV-2 vaccination on the website of the German Multiple Sclerosis Society (baseline survey). Participants of the baseline survey were contacted by email at a time when they should have received their second SARS-CoV-2 vaccination (for two-dose vaccines) following the recommendations of the German Standing Committee on Vaccination (STIKO), depending on the vaccine used and asked to complete a first follow-up survey. At approximately three months following the second vaccination, these patients were contacted again for a second follow-up survey. A third follow-up survey is planned for one year after the patient's first vaccination. An additional survey based on questions developed during the study (e.g., on specific patient subgroups) within the 1-year period is optional. Thus, four surveys will be conducted per patient within the year following the first SARS-CoV-2 vaccination: a baseline survey and three follow-up surveys, with the option of an additional survey of patient subgroups.
Following patient-reported sociodemographic and clinical baseline data were recorded: age (date of birth), gender (female, male, gender-diverse), date of first MS symptom, date of MS diagnosis, disease course (relapsing remitting MS [RRMS]; primary progressive MS [PPMS], secondary progressive MS [SPMS], undefined MS course), degree of disability (patient-determined disease steps [PDDS]), current and past DMDs, concomitant other autoimmune diseases and date of last relapse prior to the first SARS-CoV-2 vaccination. In addition, data on the respective vaccines (tozinameran, elasomeran, AZD1222 and Ad26.COV2.S), immediate vaccination reactions, MS deterioration (defined here as the onset of new or the worsening of already present MS symptoms), the disability level (PDDS) and the occurring relapses following the SARS-CoV-2 vaccinations were collected on a patient-reported base.
Data collection in the United Kingdom
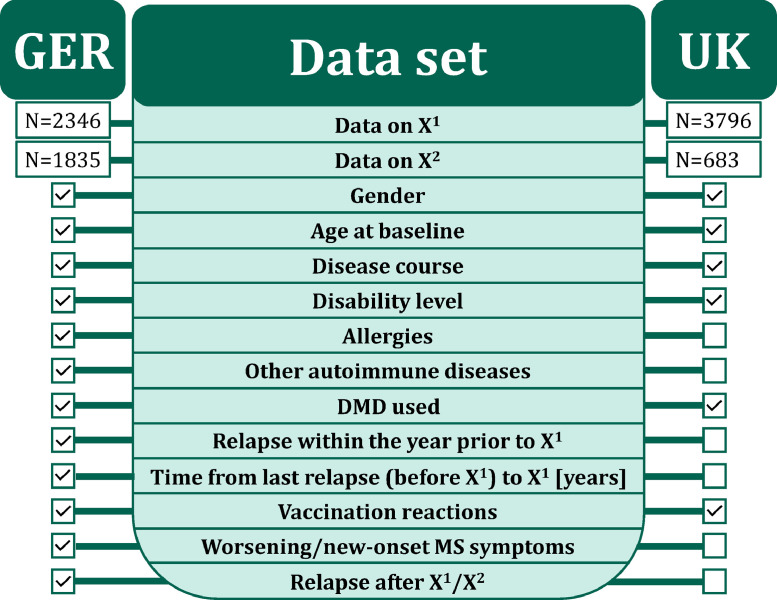
A comparable online survey regarding the SARS-CoV-2 vaccination distribution and vaccination reactions among PwMS was conducted by the UK MS Registry. Patient-reported data from the UK MS Registry comparable to the German survey were age at the baseline survey, gender, disease course, DMD treatment status, disability level, vaccine distribution and vaccination reactions following the first as well as the second SARS-CoV-2 vaccination. Unification of PDDS (Germany) and MS Impact Scale–29 (MSIS-29; UK) to the disability level categories mild, moderate and severe was performed according to Salter et al.17 The data set of the German as well as the UK study population is shown in Figure 1.
Figure 1.
Data set of the study populations. The green middle area reveals the variables of interest of the study. The boxes on the left and right indicate the availability of the corresponding data in the German and UK study populations.
DMD – disease-modifying drug
GER – Germany
N – number of patients
SARS-CoV-2 – severe acute respiratory syndrome coronavirus 2
UK – United Kingdom
X1 – first SARS-CoV-2 vaccination
X2 – second SARS-CoV-2 vaccination.
Inclusion criteria and ethics
This study included people from Germany and the UK with a diagnosis of MS who were at least 18 years of age for Germany and 20 years of age for the UK at the time of the survey. The participants should have received at least one SARS-CoV-2 vaccination and electronically consented to participate in the survey voluntarily.
The study was approved by the institutional review board of the University Medical Center at the University of Rostock (registration number: A 2021-0079) and prospectively registered under the number: DRKS00025221 in the German Registry for Clinical Trials. The UK MS Register has research ethics approval from South West Central Bristol Research Ethics Committee 16/SW/0194.
Statistics
The data export for the first analysis of this prospective non-interventional observational study was 19th August 2021. For this analysis, data of the baseline survey (following the first SARS-CoV-2 vaccination) and the first follow-up (following the second SARS-CoV-2 vaccination) were considered. Means, standard deviations, medians, percentages and confidence intervals (CIs) were calculated to characterize the study population. Each individual analysis included patients for whom data were available. Patients with incomplete data were excluded for the respective analyses. Thus, different numbers of patients are available for some analyses. The frequency of vaccination reactions, the worsening of MS symptoms and the onset of new MS symptoms after the first as well as the second vaccination were calculated. Fisher's exact tests, chi-square tests, Mann-Whitney U tests were used to compare patient subgroups, such as men vs. women or patients with relapses following vaccinations (from the first vaccination to the first follow-up) vs. patients without relapses during this period. The frequencies of vaccination reactions and new as well as worsened MS symptoms following the first and second SARS-CoV-2 vaccination were compared using the McNemar test. The significance level was set at α=0·05. The p-value adjustment according to the false discovery rate (FDR) of 5% was conducted to take into account the alpha error accumulation in multiple testing. To investigate associations of the worsening of present MS symptoms or the onset of new symptoms with age, gender, disability level and DMD treatment status, univariable as well as multivariable logistic regressions were conducted. Statistical analyses, data transformation and generation of figures were performed using R 4.0 (The R Foundation for Statistical Computing, Vienna, Austria). R packages used for the analysis were a part of R base installation available from the stats package v4.3.0.18 Additional compareGroups19 and gtsummary20 packages were retrieved from the Comprehensive R Archive Network (CRAN) repository. Tidyverse21 packages were also retrieved from CRAN for data management, manipulation and graphical representation.
Data statement
Anonymised data will be made available on request by any qualified investigator under the terms of the registries’ usage and access guidelines and subject to informed consent of the patients.
Role of funding source
The conduct of this study was supported by multistakeholder sponsors organized by the DMSG. The list of sponsors included (in alphabetical order) Biogen, Bristol Myers Squibb, Merck Serono, Mylan, Novartis, Roche and Sanofi. Industry funding did not result in restrictions to publishing data, nor did the funders have access to the raw data or exert any influence over the scientific conduct of the study.
Results
Study population
So far, 2346 German PwMS completed the baseline survey (after the first SARS-CoV-2 vaccination) and 1835 also participated in the first follow-up survey (after the second SARS-CoV-2 vaccination in the case of two-dose vaccines). Furthermore, 3796 PwMS from the UK participated in a comparable survey (3796 PwMS with data on the first vaccination, 683 PwMS with data on the second vaccination). The median period from the first vaccination to the first follow-up survey was 2·0 months [25% quantile: 1·9 months, 75% quantile: 2·9 months] in the German PwMS and 6·4 months [25% quantile: 5·3 months, 75% quantile: 7·3 months] in the UK PwMS. Overall, 6142 subjects were included in this study. German and UK patients differed considering some variables: the German PwMS were on average 9·6 years younger as well as more often DMD-treated (72·6% vs. 45·7%) and reported an RRMS course more frequently (74·6% vs. 56·7%) compared with the UK patients (Table 1). Considering gender-specific differences among the German PwMS analysed, women were considerably younger (44·6 vs. 49·5 years, two-sample two-tailed Student's t-test: p<0·0001) and reported more frequently an RRMS disease course (78·1% vs. 61·4%, Fisher's exact test: p<0·0001), mild disability (54·0% vs. 41·2%, Fisher's exact test: p<0·0001), allergies (47·1% vs. 33·3%, Fisher's exact test: p<0·0001), autoimmune diseases other than MS (24·9% vs. 9·7%, Fisher's exact test: p<0·0001), relapses within the year prior to the first SARS-CoV-2 vaccination (16·2% vs. 9·7%, Fisher's exact test: p<0·01) and a shorter median time between the last relapse (before the first vaccination) to the first vaccination (3·0 vs. 3·8 years, Mann-Whitney U test: p<0·01) compared with men (Supplementary Table S1). Among the UK patients, female PwMS were also younger in mean (54·2 vs. 59·0 years, two-sample two-tailed Student's t-test: p<0·0001) and reported RRMS (63·1% vs. 41·1%, Fisher's exact test: p<0·0001), mild disability (44·6% vs. 35·7%, Fisher's exact test: p<0·0001) as well as a DMD treatment (47·5% vs. 39·4%, Fisher's exact test: p<0·0001) more often than male patients.
Table 1.
Sociodemographic, clinical, and therapeutic characterization of people with multiple sclerosis participated at baseline.
| Germany | United Kingdom | |
|---|---|---|
| N | 2346 | 3796 |
| Gender, N (%) [CP CI] | ||
| Female | 1819 (78·1) [76·3–79·7] | 2918 (76·9) [75·4–78·2] |
| Male | 503 (21·6) [19·9–23·3] | 875 (23·1) [21·7–24·4] |
| Diverse (GER)/not indicated (UK) | 7 (0·3) [0·1–0·6] | 3 (0·1) [<0·1–0·2] |
| Age at baseline [years], mean (±SD) [95% CI] | 45·7 (±11·4) [45·2–46·1] | 55·3 (±11·5) [54·9–55·6] |
| Disease course, N (%) [CP CI] | ||
| RRMS | 1749 (74·6) [72·7–76·2] | 2151 (56·7) [55·0–58·2] |
| SPMS | 403 (17·2) [15·7–18·7] | 949 (25·0) [23·6–26·4] |
| PPMS | 92 (3·9) [3·2–4·7] | 445 (11·7) [10·7–12·7] |
| Undefined | 102 (4·3) [3·5–5·2] | 96 (2·5) [2·1–3·1] |
| Benigna | n.a. | 155 (4·1) [3·4–4·7] |
| Disability level, N (%) [CP CI] | ||
| Mild | 1206 (51·4) [49·3–53·4] | 1494 (42·5) [40·8–44·1] |
| Moderate | 862 (36·7) [34·8–38·7] | 1261 (35·9) [34·3–37·5] |
| Severe | 278 (11·8) [10·6–13·2] | 757 (21·6) [20·2–22·9] |
| Allergies, N (%) [CP CI] | 994 (44·3) [42·0–46·1] | n.a. |
| Other autoimmune diseases, N (%) [CP CI] | 509 (21·7) [20·0–23·4] | n.a. |
| DMD-treated (yes), N (%) [CP CI] | 1698 (72·6) [70·7–74·3] | 1733 (45·7) [44·0–47·2] |
| IFNβ/GLAT | 501 (30·2) [28·0–32·4] | 430 (24·8) [22·8–26·9] |
| CLAD/DMF/TER | 476 (28·7) [26·6–30·9] | 566 (32·7) [30·4–34·9] |
| S1P RM | 293 (17·7) [15·9–19·5] | 206 (11·9) [10·4–13·5] |
| Anti-CD20 MAB | 244 (14·7) [13·1–16·5] | 207 (11·9) [10·5–13·5] |
| Natalizumab | 114 (6·9) [5·7–8·2] | 205 (11·8) [10·3–13·4] |
| Other DMDs | 29 (1·8) [1·2–2·5] | 119 (6·9) [5·7–8·1] |
| Relapse within the year prior to X1, N (%) [CP CI] | 347 (14·8) [13·4–16·2] | n.a. |
| Time from last relapse (before X1) to X1 [years], median [95% CI] | 3·1 [2.9–3.3] | n.a. |
Anti-CD20 MAB – anti-CD 20 monoclonal antibodies: ocrelizumab/ ofatumumab/ rituximab.
CLAD/DMF/TER – cladribine/ dimethyl fumarate/ teriflunomide.
CP CI – 95% Clopper and Pearson confidence interval.
DMD, disease-modifying drug.
GER – Germany.
IFNβ/GLAT – interferon beta-1a/ interferon beta-1b/ peginterferon beta-1a/ glatiramer acetate.
MS – multiple sclerosis.
N – number of patients.
n.a. – not available.
PPMS – primary progressive MS.
RRMS – relapsing remitting MS.
S1P RM – sphingosine-1-phosphate receptor modulators: fingolimod/ ozanimod/ ponesimod/ siponimod.
SARS-CoV-2 – severe acute respiratory syndrome coronavirus 2.
SD – standard deviation.
SPMS – secondary progressive MS.
UK – United Kingdom.
X1 – first SARS-CoV-2 vaccination.
95% CI – 95% confidence interval.
– defined by the UK MS Registry as a version of RRMS with mild or no attacks separated by long periods with no symptoms over a minimum disease duration of 15 years with mild or no disability.
SARS-CoV-2 vaccine distribution among people with multiple sclerosis
The vaccination coverage in PwMS compared with the general German and UK population, including approval dates and national vaccination recommendations, is shown in Table 2.
Table 2.
Vaccine distribution in people with multiple sclerosis in Germany and the United Kingdom.
| Vaccine | Vaccine | Tozinameran | Elasomeran | AZD1222 | Ad26.COV2.S |
|---|---|---|---|---|---|
| Preparation | Comirnaty® | Spikevax® | Vaxzevria® | COVID-19 Vaccine Janssen | |
| Company | BioNTech/ Pfizer | Moderna | AstraZeneca | Janssen/ Johnson&Johnson | |
| Vaccination frequency in MS N (%)I | |||||
| GER | |||||
| Solely (NI=1682) | 1424 (84·7) | 177 (10·5) | 64 (3·8) | 17 (1·0) | |
| Heterologous after X1 (NI=165) | 3 (1·8) | 4 (2·4) | 158 (95·8) | 0 (0·0) | |
| UK | |||||
| Solely (NI=675) | 256 (37·9) | 0 (0·0) | 419 (62·1) | 0 (0·0) | |
| Heterologous after X1 (NI=8) | 2 (25·0) | 1 (12·5) | 5 (62·5) | 0 (0·0) | |
| Applied vaccine doses in the general population (GER), N (%)II× | Total: 74,896,395 (75·4) |
Total: 9,167,210 (9·2) |
Total: 12,631,735 (12·7) |
Total: 2,690,046 (2·7) |
|
| X1 (NII=53,276,005) | 37,004,574 (69·5) | 4,359,449 (8·2) | 9,221,989 (17·3) | 2,689,993 (5·0) | |
| X2 (NII=46,104,401) | 37,887,668 (82·2) | 4,807,022 (10·4) | 3,409,711 (7·4) | 0 (0·0) | |
| X3 (NII=4980) | 4153 (83·4) | 739 (14·8) | 35 (0·7) | 53 (1·1) | |
| Applied vaccine doses in the general population (UK), N (%)IIa | Total: 25,613,328 (40·9) |
Total: 442,837 (0·7) |
Total: 36,602,474 (58·4) |
Total: 0 (0·0) |
|
| X1 (NII=38,614,683) | 15,424,349 (39·9) | 405,814 (1·1) | 22,784,520 (59·0) | 0 (0·0) | |
| X2 (NII=24,043,956) | 10,284,910 (42·8) | 1,645 (<0·01) | 13,757,400 (57·2) | 0 (0·0) | |
| Approval date | |||||
| EMA (GER) | 21/12/2020 | 06/01/2021 | 29/01/2021 | 11/03/2021 | |
| MHRA (UK) | 21/12/2020 | 08/01/2021 | 30/12/2020 | 28/05/2021 | |
EMA – European Medicines Agency.
GER – Germany.
MHRA (UK) – Medicines & Healthcare products Regulatory Agency (United Kingdom).
MS – multiple sclerosis.
N (%)I – number of patients (proportion of patients).
N (%)II – number of applied vaccine doses (proportion of applied doses).
SARS-CoV-2 – severe acute respiratory syndrome coronavirus 2.
UK – United Kingdom.
X1 – first SARS-CoV-2 vaccination.
X2 – second SARS-CoV-2 vaccination.
X3 – booster vaccination.
× – according to reports of the Robert Koch Institute(Germany) and referred to a total of 99,385,386 applied vaccine doses in Germany (status 19/08/2021).18
– according to reports of the UK COVID-19 dashboard and referred to a total of 62,658,639 applied vaccine doses in the UK, extrapolated from Welsh trends (status 26/05/2021).19
In Germany, the administration of twice tozinameran was the most common vaccine combination (77·1%) in PwMS with complete vaccination data (N=1847). This was followed by twice elasomeran in 9·6% of PwMS, twice AZD1222 in 3·5% and one dose of Ad26.COV2.S in 0·9%. A heterologous vaccination scheme (use of two different vaccines) was reported by 8·9% of patients. There was no significant difference between men and women regarding the vaccines used for the first and second SARS-CoV-2 vaccination (chi-square test: p=0·09). The median vaccination interval between the first and the second SARS-CoV-2 vaccination was 5·5 weeks (25%/75% quantiles: 4·5/5·5 weeks) for patients receiving twice tozinameran, 5·5 weeks (25%/75% quantiles: 5·3/5·5 weeks) for two doses of elasomeran, 9·5 weeks (25%/75% quantiles: 8·3/11·0 weeks) for two doses of AZD1222 and 10·1 weeks (25%/75% quantiles: 8·7/11·0 weeks) for a heterologous vaccination scheme. Half of the German patients analysed received their vaccinations in vaccination centres (51·8%), followed by practices or clinics of general practitioners or specialists (40·8%), medical facilities (as an employee [5·6%] or patient [1·0%]) and nursing homes (as a resident [0·9%]).
In the UK, two doses of AZD1222 (61·3%) were administered most frequently among PwMS with complete vaccination data (N=683), while 37·5% reported the injection of two doses of tozinameran. Elasomeran was not used in a homologous vaccination scheme and Ad26.COV2.S was not used in either a homologous or heterologous scheme. A heterologous vaccination scheme appeared in eight patients (1·2%). There were no significant differences between male and female PwMS regarding the frequencies of vaccines used for the first (p=0.08) and second vaccinations (p=0.15). For patients vaccinated solely with AZD1222, the period between both vaccinations was 11·1 weeks in median (25%/75% quantiles: 10·2/35·3 weeks) while it was 11·4 weeks (25%/75% quantiles: 10·6/36·9 weeks) for patients solely using tozinameran.22,23
Vaccination reactions following SARS-CoV-2 vaccinations in people with multiple sclerosis
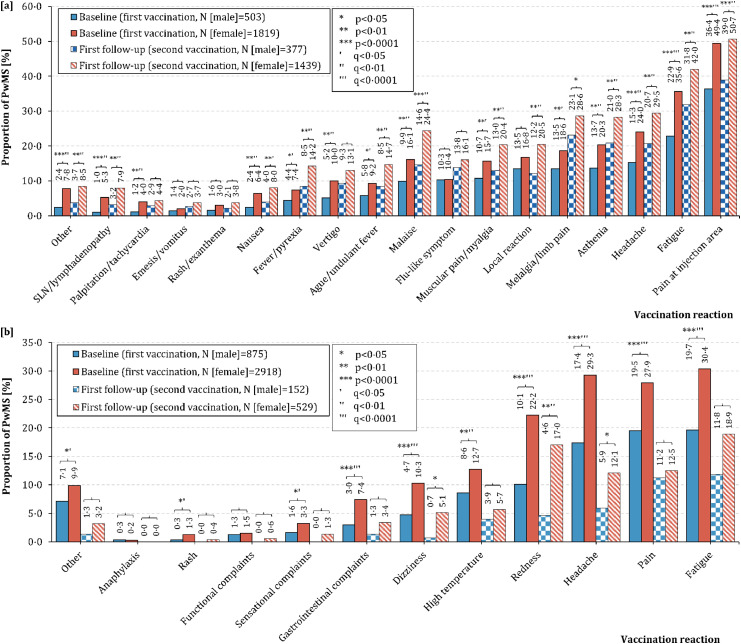
Following the first vaccination, 61·2% of the 2346 German patients reported at least one vaccination reaction and 19·6% experienced at least five vaccination reactions (range: 0–18 reported reactions). After the second vaccination (N=1835), vaccination reactions occurred more frequently: at least one reaction in 65·4% (McNemar test: p=0·02) and at least five reactions in 28·8% of patients (p<0·0001) with a range of zero to 18 reported reactions. In total, women reported vaccination reactions significantly more often than men following both the first (≥1: 64·2% vs. 50·3%, p<0·0001 [Fisher's exact test]; ≥5: 21·4% vs. 13·3%, p<0·0001) and the second vaccination (≥1: 67·9% vs. 56·0%, p<0·0001; ≥5: 31·4% vs. 19·1%, p=0·02) (Figure 2).Following both vaccinations, pain at the injection area (46·6% vs. 48·3%; McNemar test: p=0·55), fatigue (32·9% vs. 39·8%; p<0·0001) and headache (22·0% vs. 27·7%; p<0·0001) represented the most common vaccination reactions among German PwMS (Table 3). Vaccination reactions were observed more frequently following the second SARS-CoV-2 vaccination than following the first one among patients vaccinated with elasomeran (86·4% vs. 70·5%; McNemar test: p<0·01). Among patients vaccinated with AZD1222, vaccination reactions occurred more often after the first than after the second vaccination (74·4% vs. 43·1%; p<0·01), while there was no significant difference among patients who received tozinameran (60·8% vs. 63·8%; p=0·10). Whereas vaccination reactions occurred considerably more frequently in women compared with men following the first vaccination with tozinameran (63·5% vs. 50·3%; Fisher's exact test: p<0·0001), elasomeran (74·1% vs. 57·1%; p=0·03) or AZD1222 (78·5% vs. 62·5%, p=0·02), there was only one significant gender difference following the second vaccination: in patients vaccinated with tozinameran (men: 53·8% vs. women: 66·3%, p<0·0001).
Figure 2.
Frequency of vaccination reactions following SARS-CoV-2 vaccinations in male and female people with multiple sclerosis in [a] Germany and [b] the United Kingdom. [a] In Germany, the most common vaccination reaction was pain at the injection area, both for male (first vaccination: 36·4%, second vaccination: 39·0%) and female PwMS (first vaccination: 49·4%, second vaccination: 50·7%). German females reported significantly more often vaccination reactions compared with males: pain at the injection area, fatigue, headache, asthenia, limb pain, malaise, myalgia, ague, vertigo, fever, nausea, tachycardia, swollen lymph nodes and others for the first vaccination (chi-square test: p≤0·02). There were the same significant differences in the second vaccination, except for local reactions (only significant following the second vaccination: p<0·01), vertigo (p=0·06) and tachycardia (p=0·26). [b] In the United Kingdom (UK), fatigue was the most frequent vaccination reaction among male (first vaccination: 19·7%, second vaccination: 11·8%) and female PwMS (first vaccination: 30·4%, second vaccination: 18·9%). Particularly following the first vaccination, women suffered from vaccination reactions significantly more frequently than men: fatigue, pain (muscle/joint/other), headache, redness, high temperature, dizziness, gastrointestinal complaints, sensational complaints, rash and others (chi-square test: p≤0·03). Following the second vaccination, female patients reported headache, redness and dizziness significantly more often than male patients (p≤0·04).
MS – multiple sclerosis
p – p-value
q – adjusted p-values using the false discovery rate
PwMS – people with multiple sclerosis
SARS-CoV-2 – severe acute respiratory syndrome coronavirus 2
SLN – Swollen lymph nodes.
Table 3.
Frequency of vaccination reactions following SARS-CoV-2 vaccinations in people with multiple sclerosis in Germany and the United Kingdom.
| First vaccination |
Second vaccination |
pMc | q | |||||
|---|---|---|---|---|---|---|---|---|
| Germany | ||||||||
| N | 2346 |
1835 |
||||||
| N | % | CP CI | N | % | CP CI | |||
| Pain at injection area | 1094 | 46·6 | 44·5–48·6 | 887 | 48·3 | 46·0–50·6 | 0·55 | 0·55 |
| Fatigue | 771 | 32·9 | 30·9–34·8 | 731 | 39·8 | 37·5–42·1 | <0·0001 | <0·0001 |
| Headache | 517 | 22·0 | 20·3–23·7 | 509 | 27·7 | 25·6–29·8 | <0·0001 | <0·0001 |
| Asthenia | 441 | 18·8 | 17·2–20·4 | 490 | 26·7 | 24·6–28·7 | <0·0001 | <0·0001 |
| Melalgia/limb pain | 412 | 17·6 | 16·0–19·1 | 506 | 27·6 | 25·5–29·6 | <0·0001 | <0·0001 |
| Local reaction | 377 | 16·1 | 14·6–17·6 | 345 | 18·8 | 17·0–20·6 | <0·01 | <0·01 |
| Malaise | 347 | 14·8 | 13·3–16·1 | 411 | 22·4 | 20·5–24·3 | <0·0001 | <0·0001 |
| Muscular pain/myalgia | 344 | 14·7 | 13·2–16·1 | 346 | 18·9 | 17·0–20·0 | <0·0001 | <0·0001 |
| Flu-like symptom | 244 | 10·4 | 9·1–11·7 | 285 | 15·5 | 13·9–17·2 | <0·0001 | <0·0001 |
| Vertigo | 208 | 8·9 | 7·7–10·0 | 225 | 12·3 | 10·7–13·8 | <0·0001 | <0·0001 |
| Ague/undulant fever | 197 | 8·4 | 7·3–9·5 | 247 | 13·5 | 11·9–15·1 | <0·0001 | <0·0001 |
| Fever/pyrexia | 157 | 6·7 | 5·7–7·7 | 240 | 13·1 | 11·5–14·7 | <0·0001 | <0·0001 |
| Nausea | 128 | 5·5 | 4·5–6·4 | 132 | 7·2 | 6·1–8·4 | <0·01 | <0·01 |
| Swollen lymph nodes/lymphadenopathy | 101 | 4·3 | 3·5–5·2 | 129 | 7·0 | 5·9–8·2 | <0·0001 | <0·0001 |
| Palpitation/tachycardia | 80 | 3·4 | 2·7–4·2 | 75 | 4·1 | 3·2–5·0 | 0·03 | 0·04 |
| Rash/exanthema | 63 | 2·7 | 2·0–3·4 | 63 | 3·4 | 2·6–4·3 | 0·10 | 0·11 |
| Emesis/vomitus | 44 | 1·9 | 1·3–2·5 | 64 | 3·5 | 2·6–4·4 | <0·01 | <0·01 |
| Other | 154 | 6·6 | 5·5–7·6 | 139 | 7·6 | 6·4–8·8 | 0·17 | 0·19 |
| United Kingdom | pMc | q | ||||||
|---|---|---|---|---|---|---|---|---|
| N | 3796 |
683 |
||||||
| N | % | CP CI | N | % | CP CI | |||
| Fatigue | 1060 | 27·9 | 26·5–29·3 | 118 | 17·2 | 14·5–20·3 | <0·0001 | <0·0001 |
| Headache | 1007 | 26·5 | 25·1–27·9 | 73 | 10·6 | 8·4–13·2 | <0·0001 | <0·0001 |
| Pain (muscle/joint/other) | 986 | 26·0 | 24·5–27·4 | 83 | 12·1 | 9·7–14·8 | <0·0001 | <0·0001 |
| Redness | 737 | 19·4 | 18·1–20·7 | 97 | 14·2 | 11·6–17·0 | <0·0001 | <0·0001 |
| High temperature | 448 | 11·8 | 10·7–12·8 | 36 | 5·2 | 3·7–7·2 | <0·0001 | <0·0001 |
| Dizziness | 341 | 9·0 | 8·0–9·9 | 28 | 4·0 | 2·7–5·8 | <0·0001 | <0·0001 |
| Gastrointestinal complaints | 242 | 6·4 | 5·6–7·1 | 20 | 2·9 | 1·7–4·4 | <0·0001 | <0·0001 |
| Sensational complaints | 109 | 2·9 | 2·3–3·4 | 7 | 1·0 | 0·4–2·1 | <0·0001 | <0·0001 |
| Functional complaints | 55 | 1·4 | 1·0–1·8 | 3 | 0·4 | <0·1–1·3 | <0·0001 | <0·0001 |
| Rash | 40 | 1·1 | 0·8–1·4 | 2 | 0·3 | <0·1–1·0 | <0·0001 | <0·0001 |
| Anaphylaxis | 10 | 0·3 | 0·1–0·5 | 0 | 0·0 | n.a. | <0·0001 | <0·0001 |
| Other | 351 | 9·2 | 8·3–10·2 | 19 | 2·7 | 1·6–4·3 | <0·0001 | <0·0001 |
CP CI – 95% Clopper and Pearson confidence interval.
Mc – McNemar test.
N – number of patients.
p – p-value.
q – p-value adjusted according to false discovery rate.
SARS-CoV-2 – severe acute respiratory syndrome coronavirus 2.
About half of the UK PwMS (48·7%) reported vaccination reactions after the first SARS-CoV-2 vaccination, while less than one third (30·0%) did so after the second vaccination. Fatigue (first: 27·9%, second: 3·1%; McNemar test: p<0·0001), headache (first: 26·5%, second: 1·9%; p<0·0001) and pain (muscle/joint/other; first: 26·0%, second: 2·2%; p<0·0001) occurred also among the most common vaccination reactions following SARS-CoV-2 vaccination in UK patients (Table 3). PwMS vaccinated with AZD1222 reported more frequently vaccination reactions following the first vaccination than those vaccinated with tozinameran (57·8% vs. 35·7%; chi-square test: p<0·0001). After the second vaccination, there was no significant difference between these vaccines regarding the occurrence of vaccination reactions (28·7% vs. 32·2%; p=0·39). Among the UK patients, women also experienced vaccination reactions more often than men, both following the first (52·1% vs. 37·6%; p<0·0001) and second SARS-CoV-2 vaccination (32·7% vs. 21·1%; p<0·01). The frequencies of the single vaccination reactions stratified by vaccine type as well as gender are presented in Supplementary Tables S2 and S3.
Disease deterioration following SARS-CoV-2 vaccinations among people with multiple sclerosis
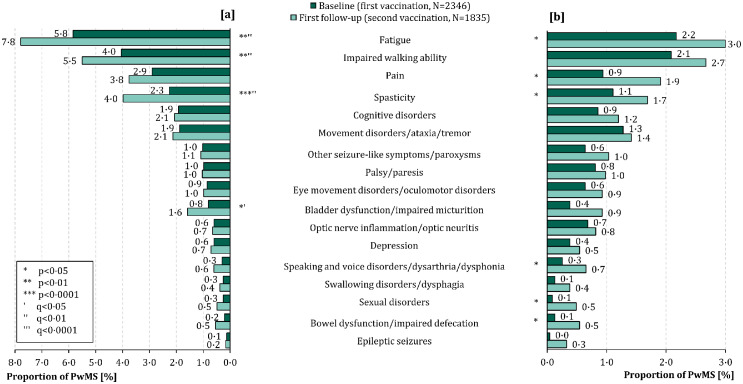
Of the German PwMS, 19·0% reported MS deterioration (worsened or new MS symptoms) following any SARS-CoV-2 vaccination: 11·6% (N=273) following the first vaccination and 14·6% (N=267) after the second vaccination (including patients who reported adverse events following both vaccinations). Considering solely the worsened symptoms, 9·3% PwMS were affected following the first SARS-CoV-2 vaccination. This proportion was significantly higher after the second vaccination (11·8%; McNemar test: p<0·01). The most common worsened MS symptoms were fatigue (first vaccination: 5·8% vs. second vaccination: 7·8%; McNemar test: p<0·01), impaired walking ability (4·0% vs. 5·5%; p<0·01) and pain (2·9% vs. 3·8%; p=0·14) (Figure 3). In addition to fatigue and impaired walking ability, worsened spasticity (4·0% vs. 2·3%; p<0·0001) as well as bladder dysfunction (1·6% vs. 0·8%; p=0·02) occurred significantly more often after the second vaccination than after the first one. These differences between the two vaccinations remained significant after p-value correction according to FDR (q≤0·04). There were no significant gender differences concerning the MS symptom worsening. Among PwMS vaccinated with tozinameran, the worsening of MS symptoms was reported more often following the second vaccination than following the first one (12·8% vs. 9·8%; McNemar test: p<0·01), while for patients who received AZD1222, symptom worsening was more frequent following the first vaccination (14·3% vs. 6·2%; p<0·01). In this regard, no significant difference was observed for elasomeran (p=0·23).
Figure 3.
Frequency of [a] worsened and [b] new MS symptoms following SARS-CoV-2 vaccinations in German people with multiple sclerosis. Fatigue ([a]: 5·8% vs. 7·8%; [b]: 2·2% vs. 3·0%) and impaired walking ability ([a]: 4·0% vs. 5·5%; [b]: 2·1% vs. 2·7%) were on the one hand the most common [a] worsened MS symptoms and on the other hand the most prevalent [b] new MS symptoms after both the first (N=2346) and second SARS-CoV-2 vaccination (N=1835).
FDR – false discovery rate
MS – multiple sclerosis
p – p-value
PwMS – people with MS
q – adjusted p-values using the false discovery rate
SARS-CoV-2 – severe acute respiratory syndrome coronavirus 2.
The onset of new MS symptoms among German PwMS was also found more frequently after the second than after the first SARS-CoV-2 vaccination (7·5% vs. 4·9%; McNemar test: p<0·01). Fatigue (2·2% vs. 3·0%, McNemar test: p=0·02) and impaired walking ability (2·1% vs. 2·7%; p=0·09) were the most common new-onset MS symptoms, both following the first and the second vaccination (Figure 3). No significant differences in the frequencies of the single new-onset symptoms between the first and the second vaccination persisted after the adjustment of the p-values according to the FDR (q≥0·11). Only one difference regarding the onset of new MS symptoms following SARS-CoV-2 vaccinations between men and women remained significant after p-value adjustment according to the FDR: men reported new-onset sexual disorders more frequently than women following the second vaccination (1·6% vs. 0·2%; chi-square test: p<0·01, q=0·05). Patients vaccinated with AZD1222 reported the onset of new MS symptoms more often after the first than after the second vaccination (9·8% vs. 6·2%; McNemar test: p<0·01) while patients vaccinated with tozinameran or elasomeran showed no significant differences (Fisher's exact test: p≥0·22).
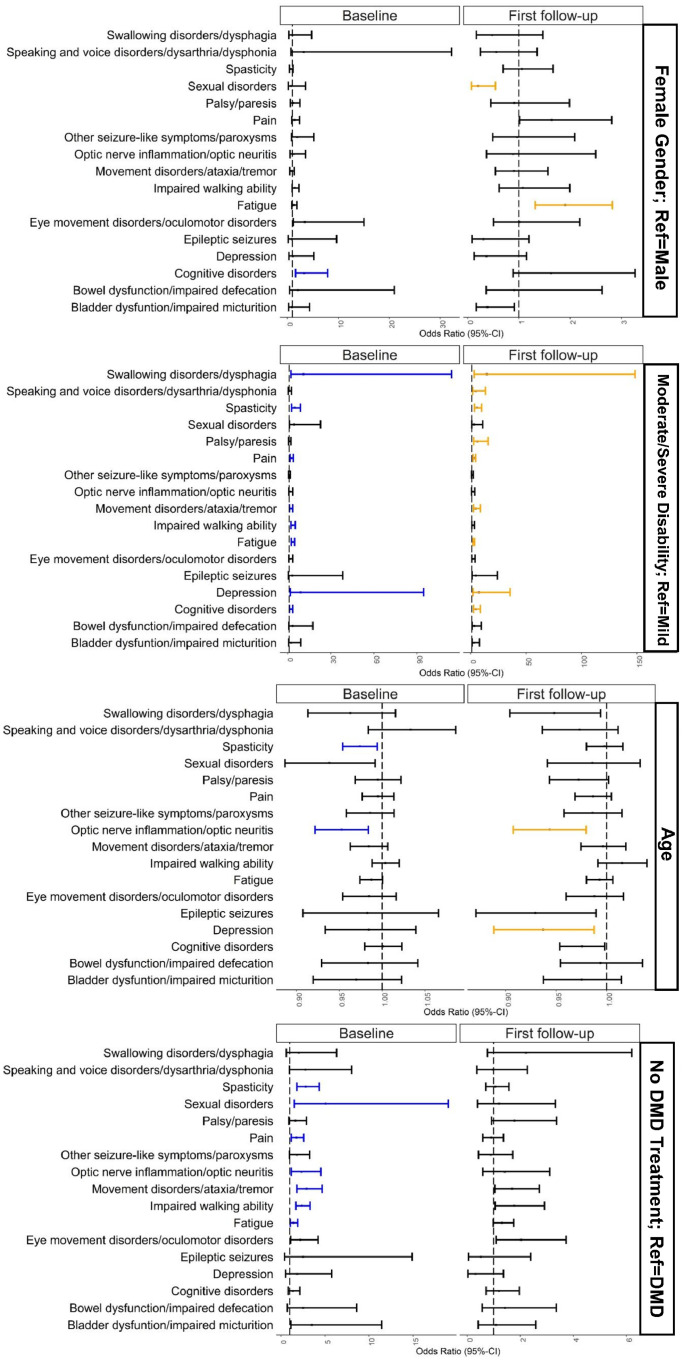
According to a multivariable logistic regression model, disease deterioration following SARS-CoV-2 vaccinations, defined as the worsening or the onset of single MS symptoms within 42 days after the respective vaccination, was significantly associated with age as well as female gender in three of 17 MS symptoms (age: depression, optic nerve inflammation/optic neuritis, spasticity; female gender: cognitive disorders, fatigue, sexual disorders), respectively, moderate or severe disability in ten of 17 symptoms (cognitive disorders, depression, fatigue, impaired walking ability, movement disorders/ataxia/tremor, pain, palsy/paresis, spasticity, speaking and voice disorders/dysarthria/dysphonia, swallowing disorders/dysphagia) and the absence of DMD treatment in seven of 17 symptoms (fatigue, impaired walking ability, movement disorders/ataxia/tremor, optic nerve inflammation/optic neuritis, pain, sexual disorders, spasticity) among German PwMS (Figure 4). Considering the most common two worsened or new-onset MS symptoms, the multivariable logistic regression model revealed significant associations of fatigue with moderate/severe disability (first vaccination: OR=3·52, p<0·0001; second vaccination: OR=2·65, p<0·0001), the absence of a DMD treatment (first vaccination: OR=1·42, p=0·05) and female gender (second vaccination: OR=1·90, p<0·01) and of impaired walking ability with moderate/severe disability (first vaccination: OR=3·52, p<0·0001) and the absence of a DMD treatment (first vaccination: OR=2·38, p<0·0001). The detailed results of the univariable and multivariable models are shown in Supplementary Table S4. No collinearity existed in the individual multivariable models.
Figure 4.
Associations between disease exacerbation following SARS-CoV-2 vaccinations and age, gender, immunomodulating treatment and disability level among German people with multiple sclerosis.
This figure shows the results of a multivariable logistic regression model regarding the associations between worsened/new-onset MS symptoms and sociodemographic, clinical as well as therapeutic patient characteristics. The small, black-framed boxes show the values of the odds ratios on the x-axis. The whiskers emanating from the boxes enclose the 95% confidence interval of the odds ratios. Significant associations were represented by blue (following the first vaccination) and yellow markers (following the second vaccination).
CI – confidence interval
DMD – disease-modifying drug
MS – multiple sclerosis
Ref – reference
SARS-CoV-2 – severe acute respiratory syndrome coronavirus 2.
Relapse activity following SARS-CoV-2 vaccinations in people with multiple sclerosis
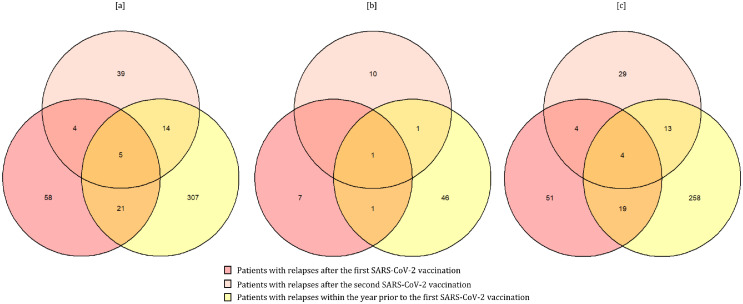
MS relapses were reported by 141 German PwMS in total: by 79 patients solely following the first SARS-CoV-2 vaccination (56·0%), by 53 patients solely after the second vaccination (37·6%) and by nine patients following both vaccinations (6·4%). Furthermore, 347 of the 2346 analysed PwMS (14·8%) reported relapses within the year prior to the first vaccination. Women showed patient-reported relapses following the vaccinations significantly more often compared with men (6·6% vs. 3·8%; Fisher's exact test: p=0·02), especially solely after the first vaccination (3·8% vs. 1·6%; p=0·01) (Figure 5). This is also reflected in the frequencies of relapses before the first vaccination (women vs. men: 16·2% vs. 9·7%; Fisher's exact test: p<0·01). Patients with at least one relapse following the SARS-CoV-2 vaccinations were on average 2·5 years younger (43·3±10·9 years vs. 45·8±11·4 years; two-sample two-tailed Student's t-test: p<0·01), more frequently diagnosed with RRMS (87·9% vs. 73·7%; Fisher's exact test: p<0·01), less often treated with DMDs (57·4% vs. 73·5%; Fisher's exact test: p<0·01), had less frequently a severe disability level (5·0% vs. 12·3%; Fisher's exact test: p<0·01), reported relapses within the year prior to the first vaccination more often (28·4% vs. 13·9%; Fisher's exact test: p<0·0001) and had their last relapse shorter before the first SARS-CoV-2 vaccination (Median: 1·2 years vs. 3·2 years; Mann-Whitney U test: p<0·0001) compared with patients without relapses following the vaccinations, see Supplementary Table S5. Patients vaccinated with AZD1222 reported MS relapses following the first vaccination more often than those vaccinated with tozinameran (6·8% vs. 3·3%; Fisher's exact test: p<0·01). This difference remained not significant after the p-value adjustment according to the FDR (q=0·05). No other significant differences regarding the post-vaccinal relapses occurred between the four vaccines, respectively (p≥0·06). There were no significant differences in terms of relapse frequency between the first or second vaccination among PwMS vaccinated with tozinameran (3·3% vs. 3·3%; two proportions Z test: p>0·99), elasomeran (5·0% vs. 4·5%; p>0·99) or AZD1222 (6·8% vs. 1·5%; p=0·17) (Supplementary Table 6).
Figure 5.
Relapse frequency following SARS-CoV-2 vaccinations [a] in total and among [b] male and [c] female people with multiple sclerosis. The Venn diagrams show the numbers of PwMS (total, men, women) with relapses following the first SARS-CoV-2 vaccination (deep pink circles, N=88), following the second vaccination (light pink circle, N=62) and within the year prior to the first vaccination (yellow circle, N=347). Overlaid areas show intersections between patient groups, e.g., in total, there were nine patients that reported relapses after both vaccinations.
MS – multiple sclerosis
N – number of patients
p – p-value
PwMS – people with MS
SARS-CoV-2 – severe acute respiratory syndrome coronavirus 2.
Discussion
The pandemic has been gripping the world since 2020. This manifested in over 554 million COVID-19 cases and over 6·4 million deaths worldwide.2 Solely in Europe, more than 233 million people have been fallen ill with COVID-19 and about 2·0 million people have died from or with SARS-CoV-2.2 Due to the international efforts to develop vaccines against SARS-CoV-2, numerous vaccines based on different modes of action are available worldwide: mRNA-based, vector-based, protein-based and based on the inactivated virus.1 Unfortunately, representative real-world data concerning the tolerability of SARS-CoV-2 vaccines are still rare among PwMS, especially with regard to gender-specific differences. The limitations of the previous studies concerning the tolerability of SARS-CoV-2 vaccines involved the study design (often mono-centric), the relatively limited size of the study cohort and the focus on only single vaccines.13, 14, 15,24 Our study investigated the safety of both mRNA- and vector-based vaccines in PwMS in two of the most populous countries in Europe: Germany and the United Kingdom. More than 6000 PwMS were analysed with regard to the reported vaccination reactions as well as MS progression after the first two SARS-CoV-2 vaccinations. In addition, we considered the tolerability of the applied vaccines in PwMS in relation to gender, on which, to the best of our knowledge, no data are yet available.
The most common vaccination reactions among the analysed PwMS of Germany and the UK were pain (at the injection site), fatigue and headache. This result is reflected in a meta-analysis of 87 articles providing safety data of SARS-CoV-2 vaccines from clinical trials and post-authorization studies not specified on MS.25 In the aforementioned study, vaccination reactions were additionally divided into systemic and local reactions. The most common local reaction was pain at the injection site and both fatigue as well as headache were the most common systemic reactions.25 Related to our study, the occurrence of fatigue (first vaccination: 32·9% vs. 25·8%, second vaccination: 39·8% vs. 26·4%) and pain (at the injection area) (first vaccination: 46·6% vs. 23·9%, second vaccination: 48·3% vs. 22·2%) was higher among German PwMS than in UK patients, both for the first as well as the second vaccination. This could be due to the different frequencies of the vaccines used in the two settings. In the UK, AZD1222 was the most commonly used vaccine (first vaccination: 58·7%, second vaccination: 61·3%), whereas in Germany, tozinameran was applied most frequently (first vaccination: 77·9%, second vaccination: 84·3%). One reason for the difference considering the occurrence of vaccination reactions especially in pain could be the different variables in data collection: German patients were explicitly asked about pain at the injection area, while UK patients were asked about muscle, joint or other pain together. Comparing our results internationally, two Israeli single-centre studies revealed similar results. The study by Achiron et al. with 555 tozinameran-vaccinated PwMS showed pain at the injection site (16·0%, 14·2%) and fatigue (9·2%, 15·9%) as the most common vaccination reactions following the first and second SARS-CoV-2 vaccinations.13 The low rates of vaccination reactions can be attributed to a relatively short follow-up period. In the study by Lotan et al. with 239 tozinameran-vaccinated PwMS, local pain/redness/swelling at the injection site (46·4%) and fatigue (38·1%) were also the most common adverse events following the vaccinations.15 The results of these two pioneer studies, with relatively small study populations of MS patients and a focus on only one SARS-CoV-2 vaccine, were consistently extended and finally confirmed by our study with more than 6000 PwMS. To extend the discussion of our results with international studies to further SARS-CoV-2 vaccine doses, we should also take a look at an Israeli study that examined 211 MS patients who had already received a third dose of tozinameran (first booster vaccination).26 In those patients, vaccination reactions occurred in 54·5% during a median follow-up of 66 days following the third vaccination, with fatigue, pain at the injection area, fever, muscle pain and joint pain as the most frequent adverse events. Thus, a significant proportion of MS patients are still affected by vaccination reactions after the first booster vaccination. Shifting the focus from MS to further neurological diseases, similar observations can be made regarding vaccination reactions after the first two SARS-CoV-2 vaccinations. The study by Boekel et al. with 505 patients with autoimmune diseases and 204 healthy controls revealed that the occurrence of vaccination reactions between patients with autoimmune diseases and healthy controls were comparable (≥1 mild: 51% vs. 52%, ≥1 moderate: 21% vs. 19%, ≥1 severe: 1% vs. 0%), with pain at the injection area as the most frequent adverse event (39% vs. 40%). With reference to this, the lack of a matched control group was a limitation of our study. A healthy and vaccinated control group would allow further conclusions on the occurrence and persistence of vaccination reactions in PwMS. An unvaccinated control group of MS patients could provide further information on the influence of vaccination on relapse frequency and worsening of MS symptoms.
In both the German and the UK study population, vaccination reactions were reported considerably more frequently by women than by men. A similar result was obtained in a South Korean study by Bae et al. in which 5589 healthcare workers were vaccinated with AZD1222 and 277 with tozinameran for the first time.27 They documented the vaccination reactions using a mobile self-report questionnaire within three days following the SARS-CoV-2 vaccination. Among participants vaccinated with AZD1222, women also had significantly more vaccination reactions than men. This could not be observed for tozinameran.27 It should be noted that the tozinameran group in the study was much smaller than in our analysis. In addition, only the first vaccination was examined and the vaccination reactions were only recorded over the relatively short period of three days following the vaccination. Numerous studies on the immune response to established vaccines for the protection against other infectious diseases, e.g. dengue fever, hepatitis A and B, diphtheria and measles, revealed that women (in general) also show a higher immune response as well as more frequent adverse events following vaccination than men.28,29 This could be an explanation for the more frequent vaccination reactions in women in our study. Similar results are also found in a study from the Netherlands, which examined 2081 patients with immune-mediated inflammatory diseases (for example MS, rheumatoid arthritis or Crohn's disease) and 178 healthy controls for the occurrence of vaccination reactions after SARS-CoV-2 vaccinations.30 One of the results was an association between the occurrence of relevant vaccination reactions (persistence >2 days) and female sex (adjusted relative risk: 1·43, 95% CI: 1·32–1·56). This observation could also be found in the general population. In a study analysing the occurrence of adverse events following vaccination with tozinameran in men and women, the female-to-male risk ratios of reported adverse events following the first and second dose were 1·89 and 1·82.31
In UK PwMS, vaccination reactions occurred more frequently after the first vaccination, whereas in German PwMS, reactions tended to occur more often after the second vaccination. This is related to the different vaccine distribution in both countries. In the German cohort, mRNA-based vaccines (tozinameran, elasomeran) were used in the majority of cases: in 87·7% of the analysed patients as the first vaccine, in 96·3% as the second vaccine. This also corresponds to the vaccine distribution in the German general population: here, too, mRNA-based vaccines were used in the majority of cases (first vaccination: 77·6%, second vaccination: 92·6%), although not quite as frequently as in the German MS population of our observational vaccination study.22 The situation is different in the UK MS patients; the majority (61·3%) received the vector-based vaccine AZD1222. This is consistent with the UK general population, where about 60% of the vaccine doses administered were AZD1222 (extrapolated from the Welsh vaccination data).23 We know that the timing of tangible vaccination reactions might depend on the type of the SARS-CoV-2 vaccine. When using mRNA-based vaccines, noticeable vaccination reactions occur more often following the second vaccination,13,15 whereas in the vector-based vaccine AZD1222, these reactions already occur more frequently following the first vaccination.27
Comparing the study populations from Germany and the UK with their national MS cohorts from previous studies revealed overwhelming similarities, respectively.17 In addition, a study on data harmonisation between the MS registries of Germany, the UK and Northern America, in which the socio-demographic basic data of these three national MS cohorts have already been matched, should serve as a reference.17 German participants of the observational study on SARS-CoV-2 vaccination showed a slightly higher proportion of women than the German MS cohort from the data harmonisation study (78·1% vs. 71·7%) and UK participants of the vaccination study were on average six years older than the UK MS cohort of the data harmonisation study (55·3 years vs. 47·8 years). However, these differences should not be over-interpreted. They are probably due to the design of our observational study on vaccination (voluntary online survey). The comparison of the baseline data between the German and UK participants of the vaccination study revealed differences in the mean age of about ten years (Germany vs. UK: 45·7 years vs. 55·3 years) and in the proportions of RRMS patients (74·6% vs. 56·7%) as well as DMD treated patients (72·6% vs. 45·7%) (Table 1). These differences schematically match the composition of the respective national MS cohorts and are due to the differences in data collection between European MS registries on the one hand and the differentiated health care systems between Germany and the UK on the other.32
In general, 19·0% of the analysed German PwMS reported the worsening of already existing symptoms or the onset of completely new MS symptoms (first vaccination: 11·6%, second vaccination: 14·6%). Previous studies on the tolerability of the administered SARS-CoV-2 vaccines revealed considerable differences in the frequencies of the worsened or new-onset MS symptoms.13,15 In the study by Lotan et al., 15·1% of the analysed PwMS showed worsened or new MS symptoms after the vaccinations,15 while the rates were much lower in the study by Achiron et al. (2% following the first and 4·8% following the second SARS-CoV-2 vaccination).13 A study by Boekel et al. on the tolerability of vaccines in patients with autoimmune diseases in general (e.g. MS and rheumatoid arthritis) also revealed a deterioration of the disease over two months following SARS-CoV-2 vaccination in 5% of patients.33 Extending the analysis with regard to the first booster vaccination (third dose), Dreyer-Alster et al. revealed that 3·8% of tozinameran-vaccinated PwMS experienced a transient deterioration of MS symptoms.26 These differences in patient proportions experiencing disease deterioration following the first two doses of SARS-CoC-2 vaccines might arise from the different study designs. The previous studies examined relatively small patient populations (N≤555). The MS studies by Achiron et al. and Lotan et al. gathered data from one MS centre, respectively, and were limited to tozinameran as the administered vaccine. In contrast, data collection in the study with further autoimmune diseases was conducted via an online survey and the vaccines used were tozinameran, elasomeran and AZD1222. Moreover, the recording of changes in disease symptoms differed between these studies: Lotan et al. and Boekel et al. provided anonymised questionnaires (in case of Boekel et al.: online) to the patients (patient-reported),15,33 whereas in the study by Achiron et al., data were collected by the physician via face-to-face meetings (physician-reported).13 The most common worsened and new-onset MS symptoms among German participants in our study were fatigue (worsened [first vs. second vaccination]: 5·8% vs. 7·8%; new-onset: 2·2% vs. 3·0%) and gait impairment (worsened: 4·0% vs. 5·5%; new-onset: 2·1% vs. 2·7%), respectively. In the articles by Achiron et al. as well as Boekel et al., the single symptoms were not listed.13 In the study by Lotan et al., although worsening or new onset of fatigue was not assessed, the occurrence of gait impairment (the worsening or new onset of walking difficulty and gait instability was summarised) was within the range of our finding (first vs. second vaccination: seven of 239 PwMS [2·9%] vs. 15 of 221 PwMS [6·8%]).15 Our analysis revealed that disease deterioration (worsening or new onset) of many MS symptoms was associated with moderate/severe disability (in ten of 17 symptoms) and a lack of DMD treatment (in seven of 17 symptoms). More disabled patients often have a more severe disease progression, which, as a hypothesis, may correlate with the perception of worsened or new-onset MS symptoms. Without a DMD treatment, a stronger immune response is expected following the vaccinations.34 This may have a more significant impact on the occurrence of worsened or new MS symptoms. On the other hand, the worsening or onset of new MS symptoms may directly be explained by the absence of an immunomodulatory treatment and not related to the administered SARS-CoV-2 vaccines.
The frequency of patient-reported relapses after vaccination in our study was 7·7% among German MS patients. However, these data do not allow us to draw conclusions about a causal relationship between the SARS-CoV-2 vaccinations and a subsequent increase in disease activity. Rather, the clinical and demographic composition of the study population and increased disease activity before the vaccination could be related to the occurrence of post-vaccination relapses. As our data revealed, patients with relapses were characterized by a younger age, a relapsing MS course, the absence of an immunomodulatory therapy and relapses in the recent past. Longitudinal 1-year data expected this year could contribute to clarify the question of causality. Other studies also showed no evidence of disease exacerbation following generally recommended vaccinations, for example against poliomyelitis, hepatitis B and influenza.35 The SARS-CoV-2 vaccine tolerability study among PwMS by Achiron et al. also did not find considerably increased short-term relapse activity following the vaccinations compared to an unvaccinated control group.13 Another study on the tolerability of SARS-CoV-2 vaccines in MS patients, which was multicentre and prospectively designed, found relapses in 2·2% of the 178 MS patients examined over eight weeks after the first vaccination.36 In contrast, relapses within eight weeks before the initial vaccination occurred in 6·2% of patients (p=0·06). These data therefore reinforce the clear expert recommendation for MS patients to get vaccinated against SARS-CoV-2.37 Studies on other autoimmune diseases also showed no unusually increased relapse rates following the vaccinations. Dinoto et al. analyzed 66 patients with antibody-mediated disorders affecting the central nervous system. Post-vaccination disease relapses occurred in only 8% of patients and were associated with an increased disability level at vaccination (p=0·025).38 A larger study of 300 patients with autoimmune neurological conditions and 347 healthy controls found that the relapse incidence prior and posterior to the vaccination did not differ (incidence rate ratio: 0·72, 95% CI: 0·29–1·83). The decision against the vaccination could lead to severe COVID-19 courses in the case of an infection,3 especially in PwMS with particular DMDs and disease features. Moreover, data from a longitudinal study by the UK MS registry suggest long-lasting COVID-19 symptoms after overcoming COVID-19 in at least 30% of the PwMS studied.39
There are some limitations in this study. The outcomes were not assessed against a control group of non-vaccinated PwMS. For this reason, there is no information concerning the number of patients who might have developed disease exacerbation/progression without vaccination. Additionally, patients who experienced vaccination reactions or MS deterioration following the vaccinations could be more likely to continue participating in the follow-up surveys. In this case, when the lack of follow-up is not missing (completely) at random, methods to address for sample selection biases ought to be used in future research. This requires first the identification of a set of relevant covariates to be used when adjusting through imputation or selection and pattern-mixture models. Another limitation could be the patient-reported data acquisition. One the one hand, the data were directly obtained from the participants and represent up-to-date and comprehensive real-world data. On the other hand, we do not have clinically confirmed data on vaccinations, vaccination reactions, disease deterioration and relapses but only patient-reported data. This could have led to over- or underestimation of the occurrence of vaccination reactions and clinical changes in MS progression. In addition, the temporal delay from the time of vaccination to the occurrence of the relapses afterwards as well as changes in results of magnetic resonance imaging (MRI) could not be assessed, as these data were not available. However, questions on clinically confirmed, so physician-diagnosed, relapses (including the date of the relapse diagnosis) were included in another follow-up survey collecting 1-year data following initial SARS-CoV-2 vaccination, which are expected this year. There might also be a reporting bias with people experiencing possible side effects being more interested in taking part in this study. Furthermore, we lost patients from the first to the second vaccination documentation. This may indicate that only those with adverse events continued the study. However, this is the largest investigation of post-vaccine adverse events and disease worsening with over 6000 involved PwMS in two of the biggest countries in Europe and revealed important safety data of the new SARS-CoV-2 vaccines.
In conclusion, we presented the largest dataset concerning adverse events and disease deterioration following SARS-CoV-2 vaccination among PwMS from Germany and the UK, with a special regard to gender-specific differences. Vaccination reactions occurred in nearly the half of the German and UK PwMS examined, with fatigue, headache and pain (at the injection site) as the most common adverse events. Generally, women reported vaccination reactions more frequently than men. One fifth of the German PwMS suffered from MS deterioration, with fatigue and gait impairment as the most frequent worsened or new MS symptoms. The worsening or onset of these two MS symptoms were associated with the absence of a DMD treatment and moderate or severe disability. Considering the disease deterioration, male and female patients showed no significant differences. Valid statements regarding long-term MS deterioration and long-lasting vaccination reactions following SARS-CoV-2 vaccination might be possible when data with a minimum observation period of one year are available, which are expected this year. MS disease deterioration, relapse activity or MRI changes after one or more booster vaccinations against SARS-CoV-2 are other important research topics for the future.
Contributors
Niklas Frahm, Firas Fneish, David Ellenberger, Uwe K. Zettl, Alexander Stahmann, Jeff Rodgers and Rodden M. Middleton conceptualised and designed the study. Firas Fneish, Niklas Frahm, David Ellenberger, Alexander Stahmann, Jeff Rodgers and Rodden M. Middleton analysed and interpreted the data gathered. Niklas Frahm drafted the manuscript. David Ellenberger, Firas Fneish, Sarah Schilling, Judith Haas, Micha Loebermann, Melanie Peters, Dieter Pöhlau, Anna-Lena Röper, Alexander Stahmann, Herbert Temmes, Uwe K. Zettl, Tina Parciak, Jeff Rodgers and Rodden M. Middleton critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Data sharing statement
Anonymized data will be made available on request by any qualified investigator under the terms of the registries’ usage and access guidelines and subject to informed consent of the patients.
Declaration of interests
Niklas Frahm is an employee of the MSFP. Moreover, he is an employee of Rostock's University Medical Center and received travel funds for research meetings from Novartis; none resulted in a conflict of interest.
Firas Fneish, Melanie Peters, David Ellenberger and Sarah Schilling had no personal financial interests to disclose other than being employees of the German MS Registry; none resulted in a conflict of interest.
Tina Parciak had no personal financial interests to disclose.
Anna-Lena Röper is employee of the MSFP and Germany MS society, which is funded by many public and corporate sponsors. She received travel funds from Novartis. None resulted in a conflict of interest.
Micha Löbermann received speaker honoraria from Sanofi, AbbVie and Pfizer, he served as investigator in vaccine studies sponsored by Janssen, GSK and Novartis; none resulted in a conflict of interest.
Herbert Temmes has no personal pecuniary interests to disclose, other than being the Secretary General of the German MS Society, federal association, which receives funding from a range of public and corporate sponsors, recently including Bundesgesundheitsministerium (BMG), The German Innovation Fund (G-BA), The German MS Trust, Biogen, Bristol Myers Squibb, Merck Serono, Novartis, Roche, Sanofi, Viatris (former Mylan); none resulted in a conflict of interest.
Judith Haas has no personal pecuniary interests to disclose, other than being the President of the German MS Society, federal association, which receives funding from a range of public and corporate sponsors, recently including BMG, G-BA, The German MS Trust, Biogen, Bristol Myers Squibb, Merck Serono, Novartis, Roche, Sanofi, Viatris (former Mylan); none resulted in a conflict of interest.
Dieter Pöhlau received speaking fees, travel support and financial support for research projects from: Allmirall, Bayer, Biogen-Idec, Merck-Serono, Octapharm, Novartis, Roche, Sanofi-Aventis and Teva; none resulted in a conflict of interest.
Jeff Rodgers has no personal pecuniary interests to disclose, other than being an employee of the UK MS Register which is funded by the MS Society, travel research and conference costs are also funded by them; none resulted in a conflict of interest.
Uwe K. Zettl has received speaking fees, travel support and /or financial support for research activities from Alexion, Almirall, Bayer, Biogen, Bristol-Myers-Squibb, Janssen, Merck Serono, Novartis, Octapharm, Roche, Sanofi Genzyme, Teva as well as EU, BMBF, BMWi and DFG; none resulted in a conflict of interest.
Alexander Stahmann has no personal financial interests to disclose, other than being the leader of the German MS Registry, which receives funding from a range of public and corporate sponsors, recently including G-BA, The German MS Trust, German MS Society, Biogen, Bristol Myers Squibb, Merck, Novartis, Roche and Sanofi; none resulted in a conflict of interest.
Rod Middleton has no personal pecuniary interests to disclose, other than being the lead of the UK MS Register which is funded by the MS Society, travel research and conference costs are also funded by them; none resulted in a conflict of interest.
Acknowledgements
We would like to thank all patients that have given their informed consent. This study would not have been possible without the efforts of the participating patients.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2022.100502.
Appendix. Supplementary materials
References
- 1.Monschein T, Hartung HP, Zrzavy T, et al. Vaccination and multiple sclerosis in the era of the COVID-19 pandemic. J Neurol Neurosurg Psychiatry. 2021;92(10):1033–1043. doi: 10.1136/jnnp-2021-326839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int. Accessed 13 July 2022
- 3.Sormani MP, Schiavetti I, Carmisciano L, et al. COVID-19 severity in multiple sclerosis: putting data into context. Neurol Neuroimmunol Neuroinflammation. 2022;9(1):e1105. doi: 10.1212/NXI.0000000000001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson-Yap S, Brouwer ED, Kalincik T, et al. Associations of DMT therapies with COVID-19 severity in multiple sclerosis. Neurology. 2021;97(19):e1870–e1885. doi: 10.1101/2021.02.08.21251316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centonze D, Rocca MA, Gasperini C, et al. Disease-modifying therapies and SARS-CoV-2 vaccination in multiple sclerosis: an expert consensus. J Neurol. 2021;268(11):3961–3968. doi: 10.1007/s00415-021-10545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farez MF. Yellow fever vaccination and increased relapse rate in travelers with multiple sclerosis. Arch Neurol. 2011;68(10):1267. doi: 10.1001/archneurol.2011.131. [DOI] [PubMed] [Google Scholar]
- 7.Gold R, Fätkenheuer G, Hartung HP, et al. Vaccination in multiple sclerosis patients treated with highly effective disease-modifying drugs: an overview with consideration of cladribine tablets. Ther Adv Neurol Disord. 2021;14 doi: 10.1177/17562864211019598. 175628642110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Filippo M, Cordioli C, Malucchi S, et al. mRNA COVID-19 vaccines do not increase the short-term risk of clinical relapses in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2022;93(4):448–450. doi: 10.1136/jnnp-2021-327200. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Rodgers WJ, Middleton RM, et al. Willingness to receive a COVID-19 vaccine in people with multiple sclerosis – UK MS Register survey. Mult Scler Relat Disord. 2021;55 doi: 10.1016/j.msard.2021.103175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heidler F, Baldt J, Frahm N, et al. Vaccination setting of patients with autoimmune diseases in times of SARS CoV-2 pandemic using the example of multiple sclerosis patients: a longitudinal multi-center study. Eur Neurol. 2022;85(2):104–111. doi: 10.1159/000519582. [DOI] [PubMed] [Google Scholar]
- 11.Ehde DM, Roberts MK, Humbert AT, Herring TE, Alschuler KN. COVID-19 vaccine hesitancy in adults with multiple sclerosis in the United States: a follow up survey during the initial vaccine rollout in 2021. Mult Scler Relat Disord. 2021;54 doi: 10.1016/j.msard.2021.103163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Statista. Willingness for taking AstraZeneca COVID-19 vaccine in Europe 2021. https://www.statista.com/statistics/1220729/willingness-for-taking-astrazeneca-covid-19-vaccine-in-europe/. Accessed 13 July 2022
- 13.Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021. Mult Scler. 2021;27(6):864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lotan I, Romanow G, Levy M. Patient-reported safety and tolerability of the COVID-19 vaccines in persons with rare neuroimmunological diseases. Mult Scler Relat Disord. 2021;55 doi: 10.1016/j.msard.2021.103189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lotan I, Wilf-Yarkoni A, Friedman Y, Stiebel-Kalish H, Steiner I, Hellmann MA. Safety of the BNT162b2 COVID-19 vaccine in multiple sclerosis (MS): early experience from a tertiary MS center in Israel. Eur J Neurol. 2021;28(11):3742–3748. doi: 10.1111/ene.15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilli F, DiSano KD, Pachner AR. SeXX matters in multiple sclerosis. Front Neurol. 2020;11:616. doi: 10.3389/fneur.2020.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salter A, Stahmann A, Ellenberger D, et al. Data harmonization for collaborative research among MS registries: a case study in employment. Mult Scler. 2021;27(2):281–289. doi: 10.1177/1352458520910499. [DOI] [PubMed] [Google Scholar]
- 18.R Core Team. R: The R Stats Package. https://stat.ethz.ch/R-manual/R-devel/library/stats/html/00Index.html. Accessed 13 July 2022
- 19.Subirana I, Sanz H, Vila J. Building bivariate tables: the comparegroups package for R. J Stat Softw. 2014;57:1–16. doi: 10.18637/jss.v057.i12. [DOI] [Google Scholar]
- 20.Sjoberg DD, Whiting K, Curry M, Lavery JA, Larmarange J. Reproducible summary tables with the gtsummary package. R J. 2021;13(1):570–580. [Google Scholar]
- 21.Wickham H, Averick M, Bryan J, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4(43):1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- 22.Robert Koch Institute. COVID-19-impfungen in Deutschland. https://github.com/robert-koch-institut/COVID-19-Impfungen_in_Deutschland/blob/4bcef2add8a9df79471f56a74693ed2485391987/Aktuell_Deutschland_Bundeslaender_COVID-19-Impfungen.csv. Accessed July 13, 2022
- 23.UK Government Digital Service. Vaccinations in the UK | Coronavirus in the UK. https://coronavirus.data.gov.uk/details/vaccinations. Accessed July 13, 2022
- 24.König M, Torgauten HM, Tran TT, et al. Immunogenicity and safety of a third SARS-CoV-2 vaccine dose in patients with multiple sclerosis and weak immune response after COVID-19 vaccination. JAMA Neurol. 2022;79(3):307–309. doi: 10.1001/jamaneurol.2021.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Q, Dudley MZ, Chen X, et al. Evaluation of the safety profile of COVID-19 vaccines: a rapid review. BMC Med. 2021;19(1):173. doi: 10.1186/s12916-021-02059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dreyer-Alster S, Menascu S, Mandel M, et al. COVID-19 vaccination in patients with multiple sclerosis: safety and humoral efficacy of the third booster dose. J Neurol Sci. 2022;434 doi: 10.1016/j.jns.2022.120155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bae S, Lee YW, Lim SY, et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021;36(17):e115. doi: 10.3346/jkms.2021.36.e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32(2):e00084. doi: 10.1128/CMR.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33:577–599. doi: 10.1146/annurev-cellbio-100616-060718. [DOI] [PubMed] [Google Scholar]
- 30.Wieske L, Kummer LYL, van Dam KPJ, et al. Risk factors associated with short-term adverse events after SARS-CoV-2 vaccination in patients with immune-mediated inflammatory diseases. BMC Med. 2022;20(1):100. doi: 10.1186/s12916-022-02310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green MS, Peer V, Magid A, Hagani N, Anis E, Nitzan D. Gender differences in adverse events following the Pfizer-BioNTech COVID-19 vaccine. Vaccines. 2022;10(2):233. doi: 10.3390/vaccines10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karampampa K, Gustavsson A, Miltenburger C, Eckert B. Treatment experience, burden and unmet needs (TRIBUNE) in MS study: results from five European countries. Mult Scler. 2012;18(2_suppl):7–15. doi: 10.1177/1352458512441566. [DOI] [PubMed] [Google Scholar]
- 33.Boekel L, Kummer LY, van Dam KPJ, et al. Adverse events after first COVID-19 vaccination in patients with autoimmune diseases. Lancet Rheumatol. 2021;3(8):e542–e545. doi: 10.1016/S2665-9913(21)00181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: a review. Mult Scler Relat Disord. 2020;45 doi: 10.1016/j.msard.2020.102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loebermann M, Winkelmann A, Hartung HP, Hengel H, Reisinger EC, Zettl UK. Vaccination against infection in patients with multiple sclerosis. Nat Rev Neurol. 2012;8(3):143–151. doi: 10.1038/nrneurol.2012.8. [DOI] [PubMed] [Google Scholar]
- 36.Ciampi E, Uribe-San-Martin R, Soler B, et al. Safety and humoral response rate of inactivated and mRNA vaccines against SARS-CoV-2 in patients with multiple sclerosis. Mult Scler Relat Disord. 2022;59 doi: 10.1016/j.msard.2022.103690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reyes S, Cunningham AL, Kalincik T, et al. Update on the management of multiple sclerosis during the COVID-19 pandemic and post pandemic: an international consensus statement. J Neuroimmunol. 2021;357 doi: 10.1016/j.jneuroim.2021.577627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinoto A, Gastaldi M, Iorio R, et al. Safety profile of SARS-CoV-2 vaccination in patients with antibody-mediated CNS disorders. Mult Scler Relat Disord. 2022;63 doi: 10.1016/j.msard.2022.103827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garjani A, Middleton RM, Nicholas R, Evangelou N. Recovery from COVID-19 in multiple sclerosis: a prospective and longitudinal cohort study of the united kingdom multiple sclerosis register. Neurol Neuroimmunol Neuroinflammation. 2022;9(1):e1118. doi: 10.1212/NXI.0000000000001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.