Abstract
Objective:
To compare the outcomes of subdural electrode (SDE) implantations versus stereoelectroencephalography (SEEG), the two predominant methods of intracranial EEG (iEEG) performed in difficult to localize drug-resistant focal epilepsy.
Methods:
The Surgical Therapies Commission of the International League Against Epilepsy created an international registry of iEEG patients implanted between 2005-2019 with ≥ 1 year follow-up. We used propensity score matching to control exposure selection bias and generate comparable cohorts. Study endpoints: 1) likelihood of resection after iEEG; 2) seizure-freedom at last follow-up; and 3) complications (composite of either post-operative infection, symptomatic intracranial hemorrhage, or permanent neurologic deficit).
Results:
Ten study sites from seven countries and three continents contributed 2,012 patients, including 1,468 (73%) eligible for analysis (526 SDE, 942 SEEG) of whom 988 (67%) underwent subsequent resection. Propensity score matching improved covariate balance between exposure groups for all analyses. Propensity-matched patients who underwent SDE had higher odds of subsequent resective surgery (odds ratio OR = 1.4, 95% CI 1.05 – 1.84), and higher odds of complications (OR=2.24, 95% CI 1.34-3.74; unadjusted: 9.6% after SDE vs. 3.3% after SEEG). Odds of seizure-freedom in propensity-matched resected patients were 1.66 times higher (95% CI 1.21, 2.26) for SEEG compared to SDE (unadjusted: 55% seizure-free after SEEG-guided resections vs. 41% after SDE)
Interpretation:
Compared to SEEG, SDE evaluations are more likely to lead to brain surgery in patients with drug-resistant epilepsy, but have more surgical complications and lower probability of seizure-freedom. This comparative-effectiveness study provides the highest feasible evidence level to guide decisions on iEEG.
Introduction
Resective brain surgery is the gold standard for treating drug-resistant focal epilepsy. When a surgical plan cannot be developed via non-invasive tests, intracranial EEG (iEEG) recordings are obtained for epilepsy localization. Subdural EEG (SDE) and stereotactic EEG (SEEG) are the two major iEEG tools used in practice. Both modalities require complex technical resources, significant costs, prolonged hospitalizations with significant risks, and multiple surgical procedures for intracranial electrode implantation and explantation. Yet, SDE and SEEG necessitate different neurosurgical expertise, ancillary personnel, and technical support. SDE requires a craniotomy but offers the chance of a resection in the same hospitalization. The less invasive SEEG, by comparison, is completed through a series of minimally invasive burr holes, but often requires a separate admission weeks later for the therapeutic brain resection. While many surgical programs could support both modalities, most exhibit a strong preference for one over another, with these preferences changing slowly over time. A recent shift in North American centers from SDE to SEEG has occurred1, 2 with little scientific data, and no Class 1 or 2 evidence comparing the two invasive-EEG practices.
The highest level of available evidence is a “consensus-based expert recommendation” from the Diagnostic Methods Commission of the International League Against Epilepsy (ILAE) and the Pediatric Epilepsy Surgery Taskforce describing the perceived strengths and weaknesses of SDE and SEEG3, and proposing specific clinical scenarios for which each technique is “believed to be best suited”. Few retrospective descriptive case series using traditional regression methodologies have tried to compare outcomes across the two modalities2, 4, 5, but demonstrated drastic baseline differences between patient cohorts who had SDE versus SEEG, preventing clear and meaningful comparisons. As with other significantly different neurosurgical procedures, randomized clinical trials (RCTs) comparing the two methods are virtually impossible6: only a handful of surgical programs have the versatility needed to offer both procedures3, and patients often have strong preferences given the significantly different approaches7. Over the past 20 years, the number of iEEG investigations without a subsequent therapeutic resection has tripled in the United States and Europe8. Clinical practice has become preference-sensitive, rather than evidence-based.
We generate strong observational evidence comparing the yield of SDE and SEEG in three specific outcomes: the likelihood of subsequent resection, achieving seizure-freedom after surgery, and neurosurgical morbidity/mortality. We leverage an international network of epilepsy surgery programs, a robust approach to data and outcome definition and collection, and the statistical methodology of propensity score matching to account for exposure selection bias between these two patient populations (SDE vs. SEEG) providing robust causal inferences beyond the current descriptive literature.
Methods:
Registry creation and study design:
We created an international registry governed by the Surgical Therapies Commission of the ILAE, to consolidate outcomes and complications information on patients with drug-resistant epilepsy who underwent iEEG in one centralized database. The ten participating sites included Cleveland Clinic (USA); University of Calgary (Canada); Northwestern University, (USA); University of California Irvine, (USA); University of Utah, (USA); SanBo Brain Hospital, Capital Medical University, Beijing (China); Aix Marseille University Hospital, (France); Ospedale Niguardia, Milan (Italy); University Medical Center, Utrecht, (Netherlands); and University College London (United Kingdom). Sites were selected to ensure uniformity in neurosurgical standards while providing diversity in geographical representation. The study team held a series of regular structured meetings over the course of three years, initially to finalize study design, define variables of interest, and develop a uniform data dictionary for variable collection, and then to periodically review data collection efforts, analysis, and results. All decisions were made by consensus.
Patient selection and data management:
Patients included in this study were 16 years or older at the time of SEEG or SDE implantation. At least 1 year of follow-up post-implantation was required. Patients who had both frame-based depth electrodes and craniotomy for SDE were designated as an SDE implantation. To ensure comparable levels of maturity in SEEG practice, and since sites started practicing SEEG at different dates in this international cohort, the sites were instructed to provide either a complete dataset of all their iEEG patients implanted over a 5-year timeframe between 2005 and 2019 inclusive, starting at least two years after their first SEEG case, or provide a random subset of at least 50 patients/site who fulfill these same inclusion criteria. For patients who underwent several iEEG procedures during the study timeframe, we only included the patients’ first iEEG and analyzed outcomes of that first implantation. We excluded patients with epilepsy and progressive neurodegenerative diseases. The data dictionary is provided in the supplementary material. Data sharing abided by the appropriate confidentiality and compliance standards, including the General Data Protection Regulation. All data were sent to the Data Coordinating Center (Cleveland Clinic) and were de-identified prior to pooling and statistical analysis.
Outcome measures and assessment:
Yield of invasive EEG was expressed as the proportion of patients who underwent resective epilepsy surgery as a result of invasive monitoring via SEEG or SDE.
Seizure outcome was assessed as the proportion of resected patients who achieved seizure-freedom at last follow-up after resection, allowing for isolated auras and for breakthrough seizures with anti-seizure medication withdrawal (Engel class 1). Patients who undergo epilepsy surgery are routinely followed up with an outpatient visit at 6 postoperative weeks, then at 6 months, and yearly thereafter. Patients with recurrent seizures have more frequent clinic visits, and those who are not seen in clinic are typically contacted yearly by telephone to update their clinical and drug treatment status.
Patient and surgery characteristics were obtained from clinical charts. For patients with acute postoperative seizures, time to recurrence was the time to the first seizure occurring after the acute postoperative period (>1 week). For patients who did not have an exact date of recurrence, the recurrence date was estimated to be halfway between the two dates of ascertained outcome neighboring it.
Surgical complications were assessed using three key morbidity outcomes: frequency of post-operative infection (including wound or intracranial infections occurring within 30 days of surgery), symptomatic intracranial hemorrhage (defined as radiologically proven intracranial hemorrhage within the brain parenchyma, attributable to surgery, and leading to altered mental status, neurological deficits, or requiring escalation of care or further surgery to treat the hemorrhage), and complication resulting in a permanent neurologic deficit lasting at least 6 months following the procedure. Given the small number of patients with the individual complications, we elected a composite outcome (defined as the incidence of any one of these three complications in a patient) as our analysis endpoint. Only two out of 2,012 patients died following iEEG across all centers; so, mortality outcomes were not amenable to robust statistical analysis.
Statistical Methods: Propensity score matching and choice of covariates
In RCTs, patients are randomly assigned to treatment arms, thereby minimizing the confounding effect of baseline differences in patient characteristics. In observational studies where randomization is not utilized, analyses must be carefully designed to minimize the potential for confounding. One way to accomplish this goal in an observational dataset is to generate comparable cohorts in such a way that cohorts are acceptably similar in all respects except for the intervention of interest. One of the most statistically robust methods to build such comparable cohorts is through propensity score matching 6, 9, 10. All available baseline patient characteristics potentially related to treatment choice or outcome are used to create a logistic regression model that yields a propensity score for each patient. This score reflects the probability that the patient would undergo a given treatment. A patient with a given propensity score who happened to receive treatment A is matched with a patient with a similar score who happened to receive treatment B. Thus, two comparable cohorts are created analogous to an RCT though a trial may not be feasible. The improved power and accuracy of causal inferences after generation of comparable cohorts using propensity scores were emphasized at a recent workshop in the National Institute of Neurological Disorders and Stroke on launching effectiveness research in neurosurgery6, and have been exemplified in several neurosurgical contexts such as the International Spine Study Group data set11, cognitive outcomes after invasive EEG12, and other fields in medicine13.
Our propensity score model estimates the probability of undergoing invasive monitoring with SDE. We identified 18 covariates believed to be important contributors to the likelihood of choosing SDE versus SEEG and/or to undergo subsequent resection, and to achieve seizure freedom (Table 1-2). To study complications, we also identified 8 key co-variates that influence choice of iEEG and surgical risk to include major medical co-morbidities (age, sex, prior surgery, unilateral/bilateral disease, presence of abnormal MRI findings, diabetes, hypertension, hyperlipidemia) (Table 3). All chosen covariates are measured prior to invasive EEG monitoring and/or resective epilepsy surgery. Evidence for the choice of these variables is found in multiple publications on surgical seizure outcomes from our group and others14. Specifically, we created separate propensity-matched pairs (each containing a unique SDE patient and a matching SEEG patient) for each studied outcome to balance the two study groups on the following covariates: basic demographic characteristics (age, gender)15, 16, clinical seizure outcome determinants such as epilepsy duration17, baseline seizure-frequency18-20, and presence of focal to bilateral tonic-clonic seizures20, with additional interaction terms to account for the differential prognostic impact of these variables in different types of epilepsy (such as a higher probability of poor seizure outcome in patients with frontal lobe epilepsy and long epilepsy duration17, or in patients with temporal lobe epilepsy and a history of focal to bilateral tonic clonic seizures20). Our covariates also included the MRI categorization21-23, the expected extent of the epileptogenic zone (lobar, multilobar; unilateral vs bilateral), and dominant hemisphere localization, as all of these variables are expected to influence the choice of invasive EEG modality and surgical outcome3, 21, 24-27. History of prior failed epilepsy surgery was also accounted for as it may lead one to favor SEEG28 to avoid the risks of a repeat craniotomy and has been linked to poorer seizure outcomes after resective surgery16, 29. Since we can only use covariates available PRIOR to intervention, epilepsy etiology was determined based on MRI findings and clinical history, rather than on histopathological findings.
Table 1:
Baseline Characteristics of the Resection Analysis Cohort before and after Propensity Score Matching. Despite obvious baseline differences across most variables, matching accomplished very good to excellent balance.
| The “Resection Analysis Cohort” | |||
|---|---|---|---|
| SEEG before Matching (n = 880) |
SDE (n = 513) |
SEEG after Matching to 513 SDE subjects1 |
|
| Demographic Variables | |||
| Mean age at implant (± SD), years | 31.5 (±10.8) | 33.0 (±11.6) | 33.8 (±11.7) |
| Male, n (%) | 474 (53.9) | 259 (50.5) | 256 (49.9) |
| Epilepsy-Related Variables | |||
| Mean epilepsy duration (± SD), years | 17.4 (±10.7) | 19.0 (±12.1) | 19.3 (±11.9) |
| Seizure frequency | |||
| High, n (%) | 237 (26.9) | 184 (35.9) | 190 (37.0) |
| Intermediate, n (%) | 482 (54.8) | 261 (50.9) | 246 (48.0) |
| Low, n (%) | 161 (18.3) | 68 (13.3) | 77 (15.0) |
| Prior focal to bilateral tonic-clonic ? Yes, n (%) | 467 (53.1) | 352 (68.6) | 341 (66.5) |
| MRI Abnormal? Yes, n (%) | 570 (64.8) | 313 (61.0) | 315 (61.4) |
| Unilateral invasive EEG? Yes, n (%) | 564 (64.1) | 444 (86.5) | 446 (86.9) |
| Prior neurosurgery for epilepsy? Yes, n (%) | 148 (16.8) | 108 (21.1) | 92 (17.9) |
Epilepsy duration = time between seizure onset and implantation. Low seizure frequency was defined as two or less seizures/month, high seizure frequency was 1 or more seizures per day, and n = number of cases.
In this match, to create 513 matched pairs, we use 317 SEEG subjects. 193 of those subjects are used just once, 301 of those subjects are used three times or less, and the remaining 16 more than three times. Summaries in the table describe the 513 matched pairs.
Table 2:
Baseline Characteristics of the Seizure Outcomes Cohort before and after Propensity Score Matching. Despite obvious baseline differences across most variables, matching accomplished very good to excellent balance.
| The “Seizure Outcomes Cohort” | |||
|---|---|---|---|
| SEEG before Matching (n = 531) |
SDE (n = 306) |
SEEG after Matching to 306 SDE subjects1 |
|
| Demographic Variables | |||
| Mean age at implant (± SD), years | 31.0 (±10.8) | 34.5 (±11.7) | 33.7 (±11.0) |
| Male, n (%) | 280 (52.7) | 150 (49.0) | 163 (53.3) |
| Epilepsy-Related Variables | |||
| Mean epilepsy duration (± SD), years | 17.3 (±10.8) | 19.1 (±12.6) | 19.6 (±13.2) |
| Seizure frequency | |||
| High, n (%) | 132 (24.9) | 74 (24.2) | 70 (22.9) |
| Intermediate, n (%) | 293 (55.2) | 183 (59.8) | 175 (57.2) |
| Low, n (%) | 106 (20.0) | 49 (16.0) | 61 (19.9) |
| Prior Convulsions? Yes, n (%) | 273 (51.4) | 206 (67.3) | 212 (69.3) |
| MRI Abnormal? Yes, n (%) | 343 (64.6) | 179 (58.5) | 182 (59.5) |
| Unilateral invasive EEG? Yes, n (%) | 371 (69.9) | 268 (87.6) | 268 (87.6) |
| Prior neurosurgery for epilepsy? Yes, n (%) | 96 (18.1) | 75 (24.5) | 71 (23.2) |
| Language Dominant hemisphere? Yes, n (%) | 236 (44.4) | 201 (65.7) | 196 (64.1) |
| Epilepsy localization | |||
| Frontal, n (%) | 113 (21.3) | 54 (17.6) | 70 (22.9) |
| Multilobar, n (%) | 128 (24.1) | 30 (9.8) | 20 (6.5) |
| Posterior Quadrant, n (%) | 30 (5.6) | 14 (4.6) | 14 (4.6) |
| Temporal, n (%) | 260 (49.0) | 208 (68.0) | 202 (66.0) |
| Etiology (%) | |||
| Cryptogenic, n (%) | 166 (31.3) | 97 (31.7) | 79 (25.8) |
| MCD, n (%) | 203 (38.2) | 64 (20.9) | 82 (26.8) |
| MTS, n (%) | 71 (13.4) | 57 (18.6) | 46 (15.0) |
| Other, n (%) | 91 (17.1) | 88 (28.8) | 99 (32.4) |
Epilepsy duration = time between seizure onset and implantation. Low seizure frequency was defined as two or less seizures/month, high seizure frequency was 1 or more seizures per day, and n = number of cases.
In this match, to create 306 matched pairs, we use 170 SEEG subjects. 112 of those subjects are used just once, 151 of those subjects are used three times or less, and the remaining 19 more than three times. Summaries in the table describe the 306 matched pairs.
Table 3:
Baseline Characteristics of the Complications Analysis Cohort before and after Propensity Score Matching. Despite obvious baseline differences across most variables, matching accomplished very good to excellent balance
| The “Complication Analysis Cohort” | |||
|---|---|---|---|
| SEEG before Matching (n = 903) |
SDE (n = 502) |
SEEG after Matching to 502 SDE subjects1 |
|
| Demographic Variables | |||
| Mean age at implant (± SD), years | 31.7 (±10.9) | 33.3 (±11.4) | 33.7 (±11.0) |
| Male, n (%) | 486 (53.8) | 254 (50.6) | 236 (47.0) |
| Epilepsy-Related Variables | |||
| MRI Abnormal? Yes, n (%) | 590 (65.3) | 302 (60.2) | 303 (60.4) |
| Unilateral invasive EEG? Yes, n (%) | 567 (62.8) | 435 (86.7) | 433 (86.3) |
| Prior Surgery? Yes, n (%) | 146 (16.2) | 106 (21.1) | 108 (21.5) |
| Comorbidities | |||
| Diabetes? Yes, n (%) | 22 (2.4) | 15 (3.0) | 12 (2.4) |
| Hypertension? Yes, n (%) | 43 (4.8) | 46 (9.2) | 39 (7.8) |
| Hyperlipidemia? Yes, n (%) | 31 (3.4) | 28 (5.6) | 29 (5.8) |
Epilepsy duration = time between seizure onset and implantation. Low seizure frequency was defined as two or less seizures/month, high seizure frequency was 1 or more seizures per day, and n = number of cases.
In the third match, to create 502 matched pairs, we use 343 SEEG subjects. 235 of those subjects are used just once, 332 of those subjects are used three times or less, and the remaining 11 more than three times. Summaries in the table describe the 502 matched pairs.
We then used logistic regression models to compare the two surgical groups relative to the categorical outcomes (resection, seizure freedom since surgery, complications), accounting for the propensity to be in the SDE group determined through matching.
We used R version 4.1.0 for all statistical analyses30. The key packages used for propensity score matching and evaluation of covariate balance were the "Matching"31 and "cobalt"32 packages.
Matching with vs without replacement:
Each of our propensity-matched samples was created by matching each of the available subjects in the relevant SDE sample to subjects in the SEEG sample. That matching was done with replacement, so that the same SEEG subject could be used to match to different SDE subjects in creating our matched pairs. In each case, we evaluated whether matching with replacement in this way provided a meaningful improvement in covariate balance over the alternative strategy, which would have been to still match all available SDE subjects to SEEG subjects, but not allow the same SEEG subject to match to multiple SDE subjects – which we call matching without replacement. This would have created the same number of matched pairs, but those individual pairs (in some cases) would have been less well-balanced. We made our decisions between whether to match with or without replacement on the basis of covariate balance, prior to examining the outcomes. The decision about whether the improved “balance” between SDE and SEEG subjects after matching with replacement is strong enough to use compared to matching without replacement needs to be made without reference to the outcome data, so as to avoid creating a bias.
In the “Resection analysis cohort” and the “Complications analysis cohort”, the balance is stronger after matching with replacement, but is still reasonable even if we had chosen to use matching without replacement. However, this was not true of the “Seizure outcomes cohort”, where the balance is too weak after matching without replacement to justify our inferences, and so matching with replacement was required and that is the method we followed.
Results:
Patient population:
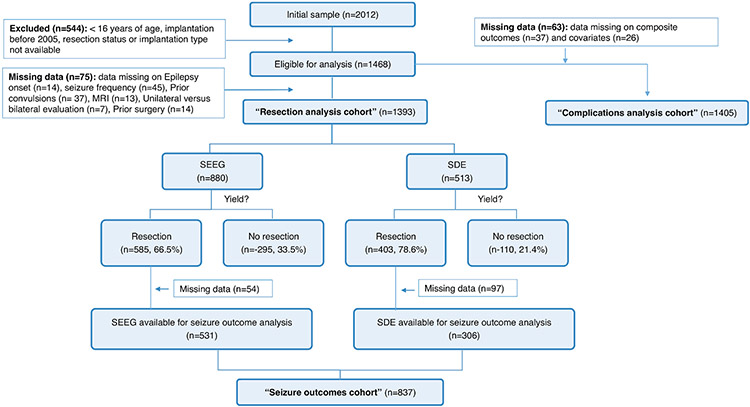
A summary of the patient distributions for the two study arms is provided in Figure 1. The ten participating sites provided 2,012 patients. After verifying the study criteria (age, surgery period), and known resection and implantation status, 1,468 were eligible for analysis (526 SDE and 942 SEEG), including 1,405 patients with complete data for complications analyses, (the “complications analysis cohort”), and 1,393 patients with complete data to analyze the likelihood of undergoing subsequent resection (the “resection analysis cohort”). Of those, 405 patients did not proceed to subsequent resection (295/942 (31%) in the SEEG group, and 110/526 (21%) in the SDE group), and 988 patients underwent subsequent resective surgery. After excluding 15 patients with missing seizure-outcome data, 130 patients with missing localization of resection, and six with missing epilepsy etiology, we were left with 837 study subjects (531 SEEG and 306 SDE) in the “seizure outcomes cohort” used for the corresponding analyses.
Figure 1:
Patient distribution across the different study arms. *some patients had multiple fields with missing data.
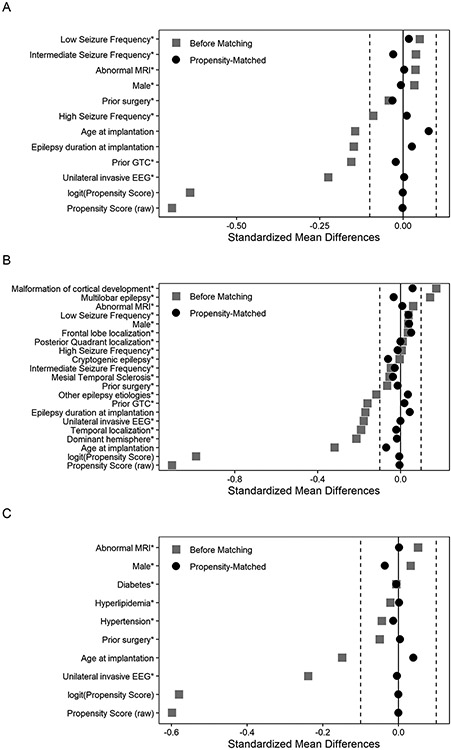
Propensity score matching improved covariate balance between the two exposure (iEEG) groups in all study cohorts. Characteristics of the “resection analysis cohort”, “seizure outcomes cohort”, and “complications analysis cohort” before and after matching are in Tables 1-3 and Figure 2. Despite clear baseline differences prior to matching, there are no substantial differences between the propensity score matched cohorts in terms of key baseline characteristics (Figure 2).
Figure 2.
Standardized differences for baseline covariates for patients who underwent invasive EEG (the “resection analysis cohort”, panel 2A), for patients who had resective surgery (the “seizure outcomes cohort”, panel 2B), and for the complications analysis cohort (panel 2C) before and after propensity score matching.
Likelihood of resection:
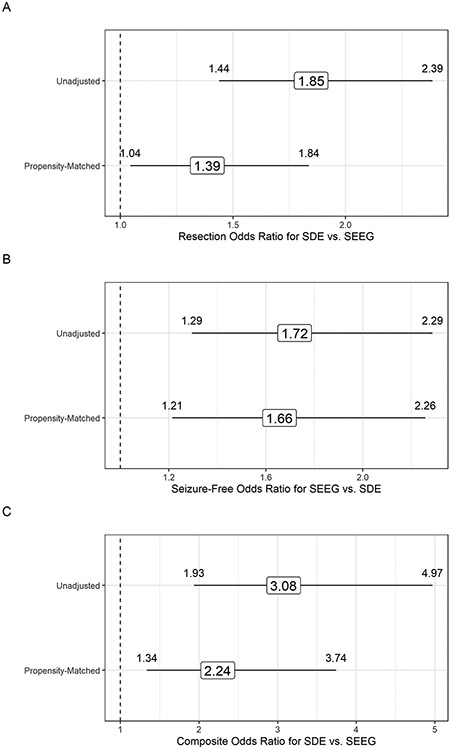
After SDE implantations, 78.6% (95% CI 74.8%-82.0%) underwent resective brain epilepsy surgery as opposed to 66.5% (95% CI 63.3%-69.5%) after SEEG. Propensity-matched patients who underwent SDE had higher odds of resective epilepsy surgery (odds ratio OR = 1.4, 95% CI: 1.05 – 1.84), matching the direction of the unadjusted estimate (OR = 1.85, 95% CI: 1.44-2.39).
Seizure outcomes:
In patients who underwent resective surgery after iEEG, 54.6% (95% CI 50.4%- 58.8%) were seizure-free at last follow-up after SEEG-guided resection as opposed to 41.1% (95% CI 35.8%- 46.8%) after SDE-guided resections. Odds of seizure-freedom following resection were 1.66 times higher (95% CI 1.21, 2.26) for propensity-matched patients undergoing SEEG as compared to SDE, mirroring the unadjusted effect estimate (OR = 1.72, 95% CI: 1.29, 2.29).
Surgical complications:
In the complications analysis cohort, 9.6% (95% CI 7.3%- 12.5%) had a major surgical complication after SDE implantation as opposed to 4.4% (95% CI 2.9%- 6.6%) after SEEG implantation. Odds of complications following SDE were 2.24 times higher (95% CI 1.34, 3.74) for propensity-matched patients undergoing SDE as compared to SEEG, mirroring the unadjusted effect estimate (OR = 3.08, 95% CI: 1.93, 4.97). One patient died following complications attributed to SDE, and one patient died after SEEG.
The estimates of the risks of the individual complications included in the composite outcome and adjusted Odds Ratios are shown in Table 4.
Table 4:
Raw baseline rates of specific neurosurgical complications in the total cohort, and adjusted odds ratios for each in the propensity matched cohorts.
| SEEG before Matching (n = 903) |
SDE (n = 502) |
Odds Ratio (95% CI) in SDE after Matching SEEG to 502 SDE subjects |
|
|---|---|---|---|
| Symptomatic intracranial hemorrhage? Yes, n (%; 95% CI) | 14 (1.6%; 0.9%-2.6%) | 9 (1.8%; 0.9%- 3.4%) | 1.29 (0.48-3.45) |
| Peri-operative infections? Yes, n (%; 95% CI) | 8 (0.9%; 0.4%- 1.8%) | 35 (7.0%; 5.1%-9.6%) | 5.83 (2.45-13.9) |
| Postoperative permanent neurological deficit? Yes, n (%; 95% CI) | 15 (1.7%; 1.0%- 2.7%) | 8 (1.6%; 0.8%- 3.2%) | 0.53 (0.23-1.26) |
| Composite outcome? Yes, n (%; 95% CI) | 30 (3.3%; 2.3%-4.7%) | 48 (9.6%; 7.3%- 12.5%) | 2.24 (1.34-3.74) |
The adjusted and unadjusted propensity score odds ratios are shown in Figure 3. In a post-hoc calculation of propensity-matched patients without replacement done per reviewer’s request, similar direction of the OR was observed [1.53 (1.16, 2.02) for likelihood of resection, 1.63 (1.18,2.25) for seizure-freedom albeit with a worse matching balance, and 2.32 (1.35, 3.97) for complications].To address a potential “site and time effect” resulting from varying levels of experience with SEEG across our centers over the study period, we repeated our analyses excluding three sites that only contributed SEEG data and one site that only contributed SDE data. The results and conclusions were unchanged.
Figure 3.
Panel A. Odds ratio estimates (with 95% confidence intervals) for resection in the resection analysis cohort before and after propensity score matching. Odds ratio here describes the odds of resection in SDE patients as compared to SEEG. Higher OR indicates higher probability of resection after SDE. The “unadjusted” estimate fails to account for the large separation in baseline covariate distributions across the SDE and SEEG groups within this cohort.
Panel B. Odds ratio estimates (with 95% confidence intervals) for seizure-free status in the seizure outcomes cohort before and after propensity score matching. Odds ratio here describes the odds of seizure-freedom in SEEG patients as compared to SDE. Higher OR indicates higher probability of seizure freedom after SEEG. The “unadjusted” fails to account for the large separation in baseline covariate distributions across the SDE and SEEG groups within patients who had resective surgery after iEEG in this cohort.
Panel C. Odds ratio estimates (with 95% confidence intervals) for incidence of surgical complications (defined by composite outcome of any one of post-operative infection, symptomatic intracranial hemorrhage, or complication resulting in a permanent neurologic deficit) before and after propensity score matching. Odds ratio here describes the odds of complications in SDE patients as compared to SEEG. Higher OR indicates higher probability of complications after SDE. The “unadjusted” fails to account for the large separation in baseline covariate distributions across the SDE and SEEG groups within the complications analysis cohort.
Discussion:
Currently, invasive EEG is the ultimate electrophysiological tool available to localize the epileptogenic zone (EZ) in challenging patients with drug-resistant focal epilepsy. Such patients face a 1% yearly risk of sudden death30. In this context, the surgical risks of invasive EEG, regardless of the modality (SDE or SEEG), often are justifiable to improve patient survival and quality of life7, 34. However, current data comparing the two modalities are limited to expert-opinion review articles3, 35-37, single center descriptive studies with insufficient sample size to compare outcomes7, 38, an exclusive focus on surgical complications and length of stay favoring SEEG4, with the two single center case series on the topic reporting unadjusted estimates of seizure-freedom (one with equivalent outcomes between SEEG and SDE5, and one with better outcomes of SEEG compared to SDE2). All studies reported important differences in the baseline characteristics of patients who had SDE vs. SEEG, limiting any meaningful conclusions on how the two modalities truly compare. Without hard data comparing these two modalities, one cannot validate the current patient care approach where the choice of SDE vs. SEEG is guided by “beliefs”, logistical considerations, and physician/patient preference.
The gold standard randomized controlled clinical trial cannot be successfully completed to obtain the sought-after, objective, comparative data for SDE and SEEG for multiple reasons. First, from a logistical perspective, very few surgeons are experienced in both techniques, so finding enough study sites for an adequately powered randomized controlled trial would be challenging. Second, even in hypothetically acceptable study sites, and despite an argument for equipoise at the level of the scientific community as a whole, strong “beliefs” for the superiority of one technique for any given patient would compromise equipoise at the individual physician level and delay recruitment6, 39, 40. Third, given significant pragmatic differences between the two modalities (required craniotomy in SDE vs. burr holes only for SEEG placement; immediate resection upon conclusion of SDE evaluation vs. a 6 week delay after SEEG, among other differences), patients are more likely to choose one over the other upfront, rather than be equally comfortable with both and thus open for randomization. Observational research is an excellent feasible option to investigate this topic, as supported by a recent workshop at the National Institute of Neurological Disorders and Stroke on effectiveness research in neurosurgical interventions6. Propensity modeling, in particular, provides a unique opportunity to statistically equate these disparate patient groups (SDE vs. SEEG) in order to approximate a randomized controlled trial using observational data9. Estimating a valid propensity score requires considerable reflection on the types of data that influence the decision makers, and might include inherent patient characteristics, prognostic factors, factors related to the surgery, and other patient characteristics. It is not unusual for around 20 variables to contribute to the propensity score10: in our model, we used 18 in the resection and seizure outcome analyses, and 8 in the complications analysis.
Before propensity matching was applied, patients in the observed SDE and SEEG groups did differ meaningfully across several key baseline characteristics. The power of propensity score matching is that it allows for the creation of pairs of similar patients that differ only in regard to treatment type, thereby reducing the effect of potentially confounding baseline differences. The propensity score adjustment used here resulted in excellent covariate balance between the two surgical groups, as demonstrated by the small differences in baseline characteristics between the matched SDE and SEEG cohorts (Table 1-3) and the markedly narrower spread around the zero standardized-difference line after matching (Figure 2). Having generated well matched cohorts, we were able to conduct statistically robust inferential analyses to find differences in our outcomes of interest that were attributable to treatment type alone.
Our findings (Figure 3) suggest that SDE evaluations are associated with a higher likelihood of being followed by a resective epilepsy surgery (adjusted O.R. of 1.39) and developing complications (adjusted O.R. of 2.24), while SEEG is associated with higher odds of seizure-freedom (adjusted O.R. of 1.66).
Resection yield:
The completion of a resective surgery after iEEG depends on measurable factors such as satisfactory localization of the EZ, and acceptable risk versus benefit equation following this localization. SDE implantations provide a more comprehensive cortical coverage allowing for a more straightforward mapping of functional eloquent cortex, facilitating the risk versus benefit assessment41. This extensive cortical coverage may also better enable the EZ delineation in a neocortical epilepsy. When coupled with free-hand depth electrode implantations in the insula, operculum, or cingulate, it can also provide reasonable confidence of localization in these more complex epilepsies37. However, proceeding to a resection after iEEG also depends on more impalpable factors such as patient willingness to proceed with surgery, and the logistical considerations of convenience and feasibility of the procedure. A higher likelihood of resection following SDE evaluation - even after adjusting for the epilepsy’s biology as we did in our propensity matched model - may mostly reflect a reality where these intangible influencers favor SDE over SEEG. The threshold to proceed with a morbid craniotomy for SDE may be higher than that of performing burr-holes for SEEG, and thus require a higher level of pre-procedural confidence in ability to localize the EZ and resect it. A patient who committed to the morbidity of SDE may be less risk-averse than one who only committed to a SEEG exploration. The SDE patient may also have a harder time foregoing a subsequent resection when the iEEG localization is suboptimal. Lastly, the second craniotomy to remove SDE electrodes provides a convenient and tempting opportunity to perform a resection, even if expected to be purely palliative rather than curative.
Seizure outcomes:
Postoperative seizure freedom largely depends on successful localization of the EZ and its complete resection. The localization of the EZ with any invasive EEG tool depends primarily on the strength and clarity of the implantation hypothesis, which in turn drives the design of the implantation and the interpretation of its result. In patients with a robust pre-implantation hypothesis, the findings of the iEEG are interpreted in the appropriate context and can lead to favorable results. Emphasis on this anatomo-electro-clinical correlation is high in SEEG, optimizing the design of the implantation to comprehensively define the epileptic network and to better understand its extent, enhancing the surgical strategy. In addition, while SDE recordings predominantly provide coverage of the cortical surface, SEEG allows targeting of deeper structures, often suspected to account for seizure recurrence after surgery. An obvious example is the exploration of the opercular, cingulate, and insular regions in challenging patients considered for a temporal lobe resection, the most common type of epilepsy surgery often thought to fail due to a “temporal plus” extent involving the peri-sylvian region. Lastly, the risk versus benefits balance in SEEG as opposed to SDE favors resections with a high likelihood of success.
Surgical complications:
A higher risk of peri-operative infection was the main driver of increased major complications after SDE as compared to SEEG. The two surgeries present significantly different levels of complexity (one requires a craniotomy; the other, burr holes), so it is not unexpected that complications, particularly peri-operative infections vary. Our findings are consistent with the conclusions of a recent systematic review on iEEG morbidity finding lower morbidity with SEEG compared to SDE 43.
Limitations:
Our list of covariates cannot be exhaustive of all specific clinical scenarios where one may speculate a superiority of one technique over the other: for example, a fronto-parietal opercular epilepsy involving also the depths of the insula may be more appropriately explored with SEEG, whereas a depth of the sulcus dysplasia near the perirolandic cortex may be best approached via a combination of SDE and SEEG42. A confounding by indication may occur where significant modality-related outcome differences in these very specific situations may be masked by a comparison of SDE and SEEG in the larger, more prevalent, patient groups. We do not have center-specific data to quantify what proportion of patients who were labeled “SDE” had in fact “SDE+depths”, but this is unlikely to significantly change our findings since this is a minority of patients across our study sites, and depths are typically used to supplement SDE in a combined approach, rather than the reverse. We did not specifically study durations of implantations, or other specific neurosurgical variables (e.g: type of electrodes) across the two iEEG modalities in this analysis, but this would also factor in patient and physician choice. Since surgical resection followed immediately after SDE electrode explantation, it is difficult to distinguish precisely if the complication was due to the resection or the SDE. Nonetheless, the fact remains that the decision to perform SDE carries a higher risk of complications than SEEG. The superiority of one iEEG modality over another should inform surgical decision making, but does not override other considerations such as local surgical expertise and special patient considerations. The “best” surgery for a patient with drug-resistant epilepsy is the surgery that can be accomplished most expeditiously and safely. In fact, a “center effect” with varying outcomes by site (better outcomes with a given procedure in centers who have longer experience in it) cannot be entirely ruled out. In our study, we tried to mitigate this potential confounding factor by limiting our analysis to patients operated at least two years after first SEEG case in sites that started their SEEG programs during the study duration to increase homogeneity of experience, and by reanalyzing the data excluding sites that exclusively perform one procedure. This analysis yielded the same results.
Conclusions:
Both SDE and SEEG are acceptable invasive EEG modalities for patients with focal drug-resistant epilepsy. While SDE evaluations are more likely to lead to a therapeutic resection, they are associated with higher surgical morbidity, and a lower likelihood of postoperative seizure freedom as compared to SEEG. Future work is necessary to continue the incremental progress of our approach to invasive EEG, better understand how to inform patient choices, and how to understand the implications of these choices.
Supplementary Material
Summary for Social Media If Published.
The corresponding author uses the following Twitter handle: @LaraJehiMD
Current knowledge: Both stereo-EEG and subdural electrode evaluations are used for epileptogenic zone localization when considering surgery for drug resistant epilepsy (DRE), but the choice of modality is largely preference based.
Question addressed: Using propensity score matching, this study investigated whether the two invasive EEG modalities differed in terms of outcomes, specifically with regards to resection likelihood, seizure freedom, and major neurosurgical complications after surgery.
What the study adds: This study provides the first evidence-based approach towards the selection of two commonly used invasive EEG modalities in epilepsy surgery.
Impact on the practice of neurology: The choice of modality when proceeding with invasive EEG should be subject to shared decision making and incorporate the key findings of this study: SDE is more likely to lead to resection, but sEEG is more likely to lead to seizure freedom, and less likely to lead to major neurosurgical complications.
Acknowledgments
This project received funding from the International League Against Epilepsy (funding of statistical analysis) and National Institute of Neurological Disorders and Stroke (number R01NS097719; effort for data collection in Cleveland Clinic). Some of the data reported in this paper were collected as part of a project undertaken by the International League against Epilepsy (ILAE) and some of the authors are experts selected by the ILAE. Opinions expressed by the authors, however, do not necessarily represent the policy or position of the ILAE.
Footnotes
Potential Conflicts of Interest
None
Data availability
The datasets are available from the corresponding author upon request and review by the Surgical Therapies Consortium Steering Committee.
References:
- 1.Abou-Al-Shaar H, Brock AA, Kundu B, Englot DJ, Rolston JD. Increased nationwide use of stereoencephalography for intracranial epilepsy electroencephalography recordings. J Clin Neurosci. 2018. Jul;53:132–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tandon N, Tong BA, Friedman ER, et al. Analysis of Morbidity and Outcomes Associated With Use of Subdural Grids vs Stereoelectroencephalography in Patients With Intractable Epilepsy. JAMA Neurol. 2019. Jun 1;76(6):672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jayakar P, Gotman J, Harvey AS, et al. Diagnostic utility of invasive EEG for epilepsy surgery: Indications, modalities, and techniques. Epilepsia. 2016. Nov;57(11):1735–47. [DOI] [PubMed] [Google Scholar]
- 4.Kim LH, Parker JJ, Ho AL, et al. Postoperative outcomes following pediatric intracranial electrode monitoring: A case for stereoelectroencephalography (SEEG). Epilepsy Behav. 2020. Mar;104(Pt A):106905. [DOI] [PubMed] [Google Scholar]
- 5.Joswig H, Lau JC, Abdallat M, et al. Stereoelectroencephalography Versus Subdural Strip Electrode Implantations: Feasibility, Complications, and Outcomes in 500 Intracranial Monitoring Cases for Drug-Resistant Epilepsy. Neurosurgery. 2020. Jul 1;87(1):E23–e30. [DOI] [PubMed] [Google Scholar]
- 6.Walicke P, Abosch A, Asher A, et al. Launching Effectiveness Research to Guide Practice in Neurosurgery: A National Institute Neurological Disorders and Stroke Workshop Report. Neurosurgery: Copyright © 2017 by the Congress of Neurological Surgeons.; 2017. p. 505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim LH, Parker JJ, Ho AL, et al. Contemporaneous evaluation of patient experience, surgical strategy, and seizure outcomes in patients undergoing stereoelectroencephalography or subdural electrode monitoring. Epilepsia. 2020. Nov 25. [DOI] [PubMed] [Google Scholar]
- 8.Jehi L, Friedman D, Carlson C, et al. The evolution of epilepsy surgery between 1991 and 2011 in nine major epilepsy centers across the United States, Germany, and Australia. Epilepsia. 2015. Oct;56(10):1526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin DB. 2 Statistical Inference for Causal Effects, With Emphasis on Applications in Epidemiology and Medical Statistics. In: Rao CR, Miller JP, Rao DC, editors. Handbook of Statistics: Elsevier; 2007. p. 28–63. [Google Scholar]
- 10.Rubin D For objective causal inference, design trumps analysis. Ann App Stat. 2008;2:32. [Google Scholar]
- 11.Smith JS, Lafage V, Shaffrey CI, et al. Outcomes of Operative and Nonoperative Treatment for Adult Spinal Deformity: A Prospective, Multicenter, Propensity-Matched Cohort Assessment With Minimum 2-Year Follow-up. Neurosurgery. 2016. Jun;78(6):851–61. [DOI] [PubMed] [Google Scholar]
- 12.Busch RM, Love TE, Jehi LE, et al. Effect of invasive EEG monitoring on cognitive outcome after left temporal lobe epilepsy surgery. Neurology. 2015. Oct 27;85(17):1475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S, Wu H, Yang T, Li B, Sun H. Early outcomes of perioperative statin therapy for elderly patients undergoing off-pump coronary artery bypass surgery: a propensity score-matched study. The Lancet. 2017. 2017/December/01/;390:S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jehi L, Yardi R, Chagin K, et al. Development and validation of nomograms to provide individualised predictions of seizure outcomes after epilepsy surgery: a retrospective analysis. Lancet Neurol. 2015. Mar;14(3):283–90. [DOI] [PubMed] [Google Scholar]
- 15.Aull-Watschinger S, Pataraia E, Czech T, Baumgartner C. Outcome predictors for surgical treatment of temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2008. Aug;49(8):1308- [DOI] [PubMed] [Google Scholar]
- 16.Yardi R, Morita-Sherman ME, Fitzgerald Z, et al. Long-term outcomes of reoperations in epilepsy surgery. Epilepsia. 2020. Mar;61(3):465–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simasathien T, Vadera S, Najm I, Gupta A, Bingaman W, Jehi L. Improved outcomes with earlier surgery for intractable frontal lobe epilepsy. Ann Neurol. 2013. May;73(5):646–54. [DOI] [PubMed] [Google Scholar]
- 18.Boesebeck F, Janszky J, Kellinghaus C, May T, Ebner A. Presurgical seizure frequency and tumoral etiology predict the outcome after extratemporal epilepsy surgery. Journal of Neurology. 2007. 2007/May/08;254(8):996. [DOI] [PubMed] [Google Scholar]
- 19.Foldvary N, Nashold B, Mascha E, et al. Seizure outcome after temporal lobectomy for temporal lobe epilepsy: a Kaplan-Meier survival analysis. Neurology. 2000. Feb 8;54(3):630–4. [DOI] [PubMed] [Google Scholar]
- 20.Jeha LE, Najm IM, Bingaman WE, et al. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology. 2006;66(12):1938. [DOI] [PubMed] [Google Scholar]
- 21.Englot DJ, Breshears JD, Sun PP, Chang EF, Auguste KI. Seizure outcomes after resective surgery for extra-temporal lobe epilepsy in pediatric patients. J Neurosurg Pediatr. 2013. Aug;12(2):126–33. [DOI] [PubMed] [Google Scholar]
- 22.Jeha LE, Najm I, Bingaman W, Dinner D, Widdess-Walsh P, Lüders H. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain. 2007. Feb;130(Pt 2):574–84. [DOI] [PubMed] [Google Scholar]
- 23.Cardinale F, Rizzi M, Vignati E, et al. Stereoelectroencephalography: retrospective analysis of 742 procedures in a single centre. Brain. 2019. Sep 1;142(9):2688–704. [DOI] [PubMed] [Google Scholar]
- 24.Harward SC, Chen WC, Rolston JD, Haglund MM, Englot DJ. Seizure Outcomes in Occipital Lobe and Posterior Quadrant Epilepsy Surgery: A Systematic Review and Meta-Analysis. Neurosurgery. 2018. Mar 1;82(3):350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krucoff MO, Chan AY, Harward SC, et al. Rates and predictors of success and failure in repeat epilepsy surgery: A meta-analysis and systematic review. Epilepsia. 2017. Dec;58(12):2133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez-Martinez J, Bulacio J, Alexopoulos A, Jehi L, Bingaman W, Najm I. Stereoelectroencephalography in the "difficult to localize" refractory focal epilepsy: early experience from a North American epilepsy center. Epilepsia. 2013. Feb;54(2):323–30. [DOI] [PubMed] [Google Scholar]
- 27.Blumcke I, Spreafico R, Haaker G, et al. Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery. N Engl J Med. 2017. Oct 26;377(17):1648–56. [DOI] [PubMed] [Google Scholar]
- 28.Vaugier L, Lagarde S, McGonigal A, et al. The role of stereoelectroencephalography (SEEG) in reevaluation of epilepsy surgery failures. Epilepsy Behav. 2018. Apr;81:86–93. [DOI] [PubMed] [Google Scholar]
- 29.McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GC, Briellmann RS, Berkovic SF. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain. 2004. Sep;127(Pt 9):2018–30. [DOI] [PubMed] [Google Scholar]
- 30.R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- 31.Sekhon Jasjeet S.. Multivariate and Propensity Score Matching Software with Automated Balance Optimization: The Matching Package for R. Journal of Statistical Software. 2011; 42 (7), 1–52. https://www.jstatsoft.org/v42/i07/. [Google Scholar]
- 32.Greifer Noah. cobalt: Covariate Balance Tables and Plots. R package version 4.3.1 (2021).https://CRAN.R-project.org/package=cobalt. [Google Scholar]
- 33.Shorvon S, Tomson T. Sudden unexpected death in epilepsy. Lancet. 2011. Dec 10;378(9808):2028–38. [DOI] [PubMed] [Google Scholar]
- 34.Hader WJ, Tellez-Zenteno J, Metcalfe A, et al. Complications of epilepsy surgery: a systematic review of focal surgical resections and invasive EEG monitoring. Epilepsia. 2013. May;54(5):840–7. [DOI] [PubMed] [Google Scholar]
- 35.Katz JS, Abel TJ. Stereoelectroencephalography Versus Subdural Electrodes for Localization of the Epileptogenic Zone: What Is the Evidence? Neurotherapeutics. 2019. Jan;16(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan H, Katz JS, Anderson M, et al. Method of invasive monitoring in epilepsy surgery and seizure freedom and morbidity: A systematic review. Epilepsia. 2019. Sep;60(9):1960–72. [DOI] [PubMed] [Google Scholar]
- 37.Chauvel P, Gonzalez-Martinez J, Bulacio J. Presurgical intracranial investigations in epilepsy surgery. Handb Clin Neurol. 2019;161:45–71. [DOI] [PubMed] [Google Scholar]
- 38.Punia V, Bulacio J, Gonzalez-Martinez J, et al. Extra operative intracranial EEG monitoring for epilepsy surgery in elderly patients. Epilepsy Behav Case Rep 2018. p. 92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin E, Muskens IS, Senders JT, et al. Randomized controlled trials comparing surgery to non-operative management in neurosurgery: a systematic review. Acta neurochirurgica. 2019;161(4):627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mansouri A, Cooper B, Shin SM, Kondziolka D. Randomized controlled trials and neurosurgery: the ideal fit or should alternative methodologies be considered? J Neurosurg. 2016. Feb;124(2):558–68. [DOI] [PubMed] [Google Scholar]
- 41.Bulacio JC, Jehi L, Wong C, et al. Long-term seizure outcome after resective surgery in patients evaluated with intracranial electrodes. Epilepsia. 2012. Oct;53(10):1722–30. [DOI] [PubMed] [Google Scholar]
- 42.Jobst BC, Bartolomei F, Diehl B, et al. Intracranial EEG in the 21st Century. Epilepsy Curr. 2020. Jul;20(4):180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan H, Katz JS, Anderson M, Mansouri A, Remick M, Ibrahim GM, Abel TJ. Method of invasive monitoring in epilepsy surgery and seizure freedom and morbidity: A systematic review. Epilepsia. 2019. Sep;60(9):1960–1972. doi: 10.1111/epi.16315. Epub 2019 Aug 19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets are available from the corresponding author upon request and review by the Surgical Therapies Consortium Steering Committee.