Abstract
The devastating COVID-19 pandemic has caused more than six million deaths worldwide during the last 2 years. Effective therapeutic agents are greatly needed, yet promising magic bullets still do not exist. Numerous natural products (cordycepin, gallinamide A, plitidepsin, telocinobufagin, and tylophorine) have been widely studied and play a potential function in treating COVID-19. In this paper, we reviewed published studies (from May 2021 to April 2022) relating closely to bioactive natural products (isolated from medicinal plants, animals products, and marine organisms) in COVID-19 therapy in vitro to provide some essential guidance for anti-SARS-CoV-2 drug research and development.
Keywords: natural products, COVID-19, SARS-CoV-2, cordycepin, gallinamide A, plitidepsin, telocinobufagin, tylophorine
1 Introduction
The ongoing coronavirus disease 2019 (COVID-19) pandemic, the sixth public health emergency of international concern, has resulted in 505,035,185 cases and 6,210,719 deaths worldwide during the last 2 years (at the time of writing). (World Health Organization, 2022). The Alpha, Beta, Gamma, and Delta variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) responsible for COVID-19 have created recurrent pandemic alerts. (Nasreen et al., 2022). Alarmingly, the novel Omicron (South Africa) variant was firstly confirmed on 24 November 2021. Still, it became the most predominant strain internationally within months because of its increased transmissibility and extensive immune evasion ability. (Scott et al., 2021; Del Rio et al., 2022). Up to now, the devastating Omicron variant has spread to almost all countries. Effective measures, such as vaccines, (Andrews et al., 2022; Chandrashekar et al., 2022) traditional medicine, (Liu et al., 2020; Alam et al., 2021) and small-molecule inhibitors, (Wang and Yang, 2020a; Reis et al., 2022; Sourimant et al., 2022) are greatly needed to reduce human-to-human transmission.
However, promising magic bullets still do not exist. (Kozlov, 2022). As an indispensable resource for promising compounds, natural products have attracted significant attention in countering SARS-CoV-2 infection via targeting its main protease (Mpro, also called 3CLpro), (Jin et al., 2020; Mengist et al., 2020) RNA-dependent RNA polymerase (RdRp), (Hillen et al., 2020; Wang et al., 2021a) papain-like protease (PLpro), (Yin et al., 2020; Gao et al., 2021) and spike (S) glycoprotein. (Toelzer et al., 2020; Walls et al., 2020). Building on our previously published work, (Wang and Yang, 2020b; Yang and Wang, 2021) we systematically discuss the landmark studies (published between May 2021 and April 2022) relating to bioactive natural products in COVID-19 therapy in vitro to support anti-SARS-CoV-2 drugs research and development.
2 Promising bioactive natural products in COVID-19 therapy
Bioactive natural products, isolated from medicinal plants, animal products, and marine organisms, are widely studied (in in vitro, animal models, and clinical trials) and play an important role in COVID-19 therapy. (Wei et al., 2020; Sahoo et al., 2021; Alqathama et al., 2022). Natural products are still considered one of the most positive and practical approaches to defeating the ongoing pandemic.
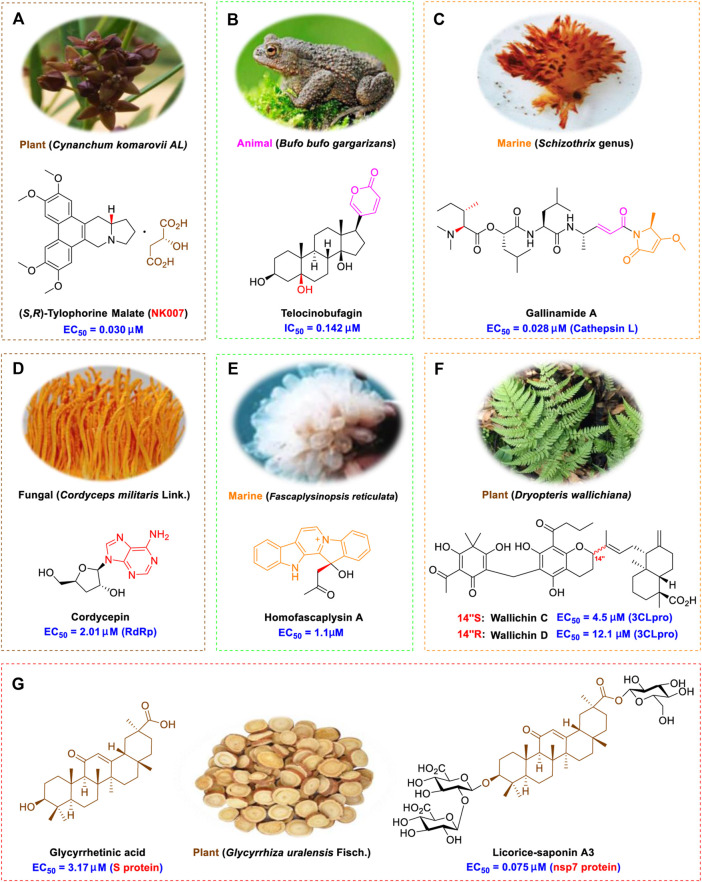
Tylophorine, a remarkable tylophora alkaloid, is an active pharmaceutical ingredient of the medicinal plant Cynanchum komarovii AL (Figure 1A) (An et al., 2001). NK007(S,R), a racemate of tylophorine malate, was prepared from S-tylophorine to improve its poor solubility. (Wang et al., 2010). NK007(S,R) displays significant inhibitory activity against SARS-CoV-2 at a half maximal effective concentration (EC50) of 0.030 μM in Vero cells, with an excellent selectivity profile (selectivity index, [SI] = 868). (Wang et al., 2021b). Hossain et al. (2022) found that tylophorine showed binding affinity (−8.5 kcal/mol) against abelson murine leukemia viral oncogene homolog one protein. Additionally, NK007(S,R) exhibits excellent in vivo antiviral efficacy in the COVID-19 golden hamster rat model by significantly reducing viral loads in the lungs. NK007(S,R) could protect against lung injury by decreasing lung inflammation with a dose of 5 mg/kg. (Wang et al., 2021b). Briefly, the abovementioned evidence has highlighted the superior activity of NK007(S,R) against SARS-CoV-2 infection in in vitro and in the rat model. (Wang et al., 2021b). Numerous natural product-based nanomedicines have been sprung up during the past several decades in the field of medicinal chemistry, providing a valuable reference for anti-COVID-19 therapeutics. (Sharma et al., 2021). To evaluate the potential of the candidate NK007(S,R), Wang et al. (Wang et al., 2021b) prepared self-assembled poly (ethylene glycol)–poly (lactide-co-glycolide) nanoparticles, NP-NK007 and LP-NK007. The optimized NP-NK007 exhibited small particle size (145.8 nm), high NK007(S,R) loading (13.10%), maximized encapsulation efficiency (87.47%), and sustained release (66.51% in 48 h). The optimal lung-targeted liposome LP-NK007 exhibited smaller particle size (75 nm), higher drug loading (36.7%), and excellent encapsulation efficiency (62.4%). Subsequent experiments implied that the nanoparticles NP-NK007 and LP-NK007 are effective SARS-CoV-2 inhibitors with higher EC50 values of 0.007 and 0.014 μM, respectively, because they improve the accumulation and delivered efficiency of NK007(S,R) in the lung. (Wang et al., 2021b). Collectively, NK007(S,R) NPs could provide a workable strategy for overcoming the lack of COVID-19-targeting treatment. Theoretically, more validation studies in vivo are needed to systematically assess the anti-SARS-CoV-2 potential of NK007(S,R)-based nanoparticles.
FIGURE 1.
Promising natural products in COVID-19 therapy. (A) Tylophorine can be isolated from the medicinal plant Cynanchum komarovii AL. (B) Telocinobufagin can be isolated from the traditional medicinal animal toad Bufo gargarizans. (C) Gallinamide A can be isolated from the marine cyanobacteria Schizothrix genus. (D) Cordycepin can be isolated from the traditional medicine Cordyceps militaris Link. (E) Homofascaplysin A can be isolated from the marine sponge Fascaplysinopsis reticulata. (F) Wallichins C and D can be isolated from the medicinal fern Dryopteris wallichiana. (G) Licorice-saponin A3 and glycyrrhetinic acid can be isolated from the medicinal plant Glycyrrhiza uralensis Fisch.
Venenum Bufonis (Chinese name: ChanSu), a well-known secretion of a traditional medicine animal (toad Bufo bufo gargarizans), is commonly used in China to treat various diseases, including heart failure, infections, toothaches, and cancers. (Tian et al., 2017; Shen et al., 2022). For example, Huachansu injection, a valuable anticancer agent, has been used in tumour treatment in China for more than 30 years. (Wu et al., 2022a). ChanSu’s main active constituents are bufadienolides that have an unusual 2-pyrone ring, which contributes to their pharmacological activities via inhibiting Na+/K+ ATPase. (Prassas and Diamandis, 2008). Recently, Jin et al. (2021) demonstrated that six bufadienolides (bufalin, bufotalin, cinobufagin, cinobufotalin, resibufogenin, and telocinobufagin) have potent broad-spectrum antiviral activities in vitro (Figure 1B). Experiments showed that bufalin could inhibit virus replication in the nanomolar range, including MERS-CoV at a half-maximal inhibitory concentration (IC50) of 0.018 μM, SARS-CoV at an IC50 of 0.016 μM, and SARS-CoV-2 at an IC50 of 0.019 μM; cinobufagin can inhibit MERS-CoV, SARS-CoV, and SARS-CoV-2 replication at IC50 values of 0.017, 0.060, and 0.072 μM; telocinobufagin can inhibit MERS-CoV, SARS-CoV and SARS-CoV-2 replication with IC50 values of 0.027, 0.071, and 0.142 μM; bufotalin, cinobufotalin and resibufogenin can inhibit the MERS-CoV, SARS-CoV and SARS-CoV-2 replication in vitro with high IC50 values (0.027–1.612 µM). (Jin et al., 2021). This study showed that the unusual 2-pyrone ring in bufadienolides plays an essential role in inhibiting SARS-CoV-2 replication. Subsequent dose toxicity studies (10 mg/kg/day, 5 days) revealed that bufalin and cinobufagin have strong toxicity in the mouse model, while the pharmacokinetic model predicts that telocinobufagin has lower toxicity, better metabolic stability, excellent oral bioavailability, and proper anti-SARS-CoV-2 activity. (Jin et al., 2021). Taken together, telocinobufagin might be a more promising broad‐spectrum inhibitor among the bufadienolides, and thus worthy of multifaceted properties investigation from in vitro studies to clinical practice.
Gallinamide A, possessing an α,β-unsaturated imide moiety, is a novel linear depsipeptide first isolated in 2008 from the marine cyanobacteria Schizothrix genus and Symploca sp. with critical pharmacological effects (Figure 1C) (Linington et al., 2009; Taori et al., 2009). Gallinamide A is a highly selective covalent inhibitor targeting human cathepsin L-like cysteine proteases, which is a promising drug target. (Barbosa Da Silva et al., 2022). Gerwick’s group showed that gallinamide A had a 28- to 320-fold higher affinity and selectivity towards cathepsin L than cathepsin V or B. (Miller et al., 2014). In vitro, gallinamide A demonstrates significant bioactivity against Trypanosoma cruzi at an IC50 of 0.005 μM by irreversible Michael addition. (Miller et al., 2014). It has been reported that gallinamide A can decrease viral load in VeroE6 cells with an IC90 of 0.088 µM and inhibit SARS-CoV-2 cathepsin L-mediated endosomal entry with an EC50 value of 0.028 µM in a dose-dependent manner. (Ashhurst et al., 2022). Angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) are two essential host determinants for SARS-CoV-2 infection and pathogenesis in vivo. (Hoffmann et al., 2020). Specifically, the S glycoprotein helps the virus enter inside the host cell via cellular receptor ACE2 binding; then TMPRSS2 helps SARS-CoV-2 contents fuse and release into the host cell cytosol via enzymatical activation of the S glycoprotein. (Liu et al., 2022). Based on combination drug therapies, Payne et al. (Ashhurst et al., 2022) recently demonstrated that the combined use of the cathepsin L inhibitor gallinamide A and the TMPRSS2 protease inhibitor nafamostat mesylate exerts a synergistic inhibitory effect in HEK-ACE2-TMPRSS2 cells via inhibiting multiple routes of SARS-CoV-2 entry. Taking gallinamide A as the lead, Payne et al. (Ashhurst et al., 2022) further explored and synthesized 32 analogues for the assessment of SARS-CoV-2 cathepsin L inhibitory activities; the study revealed two lead analogues of gallinamide A with EC50 values in the nanomolar range. Taken together, gallinamide A is a highly selective SARS-CoV-2 cathepsin L inhibitor, thus worthy of further investigation via combination therapies and lead optimization.
Natural products with broad‐spectrum bioactivities and multi-organ protection are an essential class of anti‐SARS‐CoV‐2 agents that play vital roles in COVID-19 therapy. (Wang and Yang, 2021). RdRp could regulate viral replication through catalyzing the RNA template–dependent development of phosphodiester bonds. (Wang et al., 2021a). The adenosine analogue cordycepin (3′-deoxyadenosine) is a unique fungal product isolated from the traditional medicine fungi Cordyceps militaris (Cunningham et al., 1950) and Ophiocordyceps sinensis (Figure 1D) (Zhou et al., 2008). Interestingly, cordycepin is known to have broad‐spectrum pharmacological properties against several diseases (e.g., virulent RNA viruses) and multi-organ protective effects (e.g. acute lung injury). Specifically, cordycepin is a promising therapeutic against several viruses in vitro, including dengue virus, (Panya et al., 2021) Epstein-Barr virus, (Choi et al., 2019) and hepatitis C virus. (Ueda et al., 2014). Because of its close structural similarity to the cellular nucleoside adenosine (except for the absence of a hydroxyl group at the 3′-position of the five-membered ring), cordycepin is a possible potent anti-SARS-CoV-2 agent. Rabie et al. (Rabie, 2022) showed that cordycepin could inhibit SARS-CoV-2 replication in Vero E6 cells with an EC50 value of 2.01 µM and without observable cytotoxicity (SI > 49.8) in a time-dependent manner. It is worth noting that cordycepin is a long-acting antiviral for SARS-CoV-2 prevention with high metabolic stability, reaching maximal anti-SARS-CoV-2 potency within 1.5–2.0 days of treatment. (Rabie, 2022). With respect to the activation mechanism, cordycepin is rapidly converted in vivo to its mono-, di-, and triphosphate forms; then, the active form cordycepin triphosphate can serve as a substrate for the RNA-dependent RNA polymerase (RdRp) to terminate the synthesis of viral RNA sequences. (Rabie, 2022). Bibi et al. (2022) revealed that two pivotal amino acid residues (Asp760 and Asp761) play critical roles in the binding of cordycepin with RdRp. Notably, SARS-CoV-2 infection—even in mild cases—can increase the long-term risk of a broad range of cardiovascular and cerebrovascular complications in COVID-19 patients. (Wang and Yang, 2022c). In terms of organ protection, cordycepin has unique advantages. For example, cordycepin plays a key role in long-term neuroprotection for traumatic brain injury (through inhibiting neutrophil infiltration and preserving neuroinflammation), (Wei et al., 2021) protecting diabetic hearts from ischemia/reperfusion injury (via up-regulating AMPK/Mfn2-dependent mitochondrial fusion and expression), (Yu et al., 2021) and ameliorating cerebral ischemic damage (via improving the memory ability, up-regulating the level of adenosine A1 receptors, and reducing dendritic morphology scathing). (Chen et al., 2021). Thus, cordycepin has its advantages in organ protection and broad-spectrum antiviral activities. Further study is still needed, however, to evaluate its antiviral potency in vitro.
The marine environment is a valuable source of structurally unique natural products with diverse bioactivity targeted at life-threatening diseases, including the emerging COVID-19. (Panggabean et al., 2022; Pokharkar et al., 2022; Zhang et al., 2022). Homofascaplysin A, isolated from the marine sponge Fascaplysinopsis reticulata (Figure 1E), is a well-established β-carboline alkaloid reported to exhibit promising activity against many viruses, including hepatitis C virus, (Ishida et al., 2001) human coronavirus NL63, (Tsai et al., 2020) and dengue virus. (Quintana et al., 2016). Kubanek et al. (Chhetri et al., 2022) revealed that homofascaplysin A can inhibit SARS-CoV-2 replication in Calu-3 cells at an EC50 value of 1.1 µM with relatively slight cytotoxicity (SI ∼4.55). Additionally, Kubanek et al. (Chhetri et al., 2022) found that the viral load was substantially reduced (by >90%) for infections in harvested SARS-CoV-2 RNA after administration of 2.8 μM of homofascaplysin A. Therefore, homofascaplysin A could be used as a unique lead compound for the rapid screening of novel analogues with promising anti-SARS-CoV-2 activity and minimal cytotoxicity.
Chirality is a critical attribute of natural products. (Wang, 2019). Wallichin C and wallichin D, isolated from the medicinal fern Dryopteris wallichiana (Figure 1F), exhibit potent anti-SARS-CoV-2 activities in Vero-E6 cells at EC50 values of 4.5 and 12.1 μM, respectively. (Socolsky et al., 2012; Hou et al., 2022). The corresponding SI values of wallichins C and D were >35 and >11. (Hou et al., 2022). Furthermore, phloroglucinol-terpenoids wallichins C and D exhibit potent inhibitory activities in SARS-CoV-2-infected Calu-3 cells at EC50 values of 20.2 and 30.0 μM, with moderate cytotoxicity (SI values were 4.88 and 2.14 μM, respectively). (Hou et al., 2022). Notably, both wallichins C and D have the same core structure except for the chirality at C-14 position. The study has demonstrated that the slight differences in the chirality at C-14″ S (wallichin C) or C-14″ R (wallichin D) account for differences in their antiviral activities. (Hou et al., 2022). As for the activation mechanism, Zhou et al. (Hou et al., 2022) unambiguously showed that wallichins C and D have higher selectivity and stronger interaction toward the 3CLpro with Kd values of 12.0–16.6 μM, while not active against the TMPRSS2, spike glycoprotein, and ACE2 proteins. Taken together, wallichin C might be the more promising 3CLpro inhibitor, thus worthy of further investigation.
Among pharmacological interventions, traditional medicine plays a positive role in the prevention and treatment of the COVID-19 pandemic. (Lyu et al., 2021; Zhan et al., 2022). For example, the Qingfei Paidu decoction has shown amazing clinical efficacy in treating COVID-19 patients. (Li Y. et al., 2021). It is crucial to support scientific foundations for the clinical use of Chinese herbal medicine by exploring the underlying molecular mechanisms. (Cui et al., 2021; De Jin et al., 2021). Ye and co-workers (Yi et al., 2022) recently indicated that licorice-saponin A3 and its genine aglycone glycyrrhetinic acid, famous triterpenoids that could be isolated from the most frequently used medicinal plant Glycyrrhiza uralensis Fisch. (Figure 1G), show a remarkably different inhibitory potency against SARS-CoV-2 infection in Vero E6 cells at EC50 values of 0.075 μM (targeting SARS-CoV-2 nsp7 protein) and 3.17 μM (targeting the S protein receptor-binding domain [RBD]), respectively in a dose-dependent manner. Interestingly, licorice-saponin A3 and glycyrrhetinic acid were effective in inhibiting the SARS-CoV-2 spike RBD activities, with similar IC50 values of 8.3 and 10.9 μM, respectively. (Yi et al., 2022). To elucidate the remarkable difference between S-RBD inhibitory effects and their antiviral activities, the underlying molecular mechanisms were further explored by Ye and co-workers. Based on molecular docking analysis of licorice-saponin A3 with nsp7 (PDB ID:7JIT), Yi et al. (2022) propose that nsp7 is another vital target for licorice-saponin A3 via seven hydrogen bond interactions (binding energy −8.7 kcal/mol). Qingfei Paidu decoction extracted from 21 types of traditional Chinese medicines (including Glycyrrhiza uralensis Fisch.) could effectively treat COVID-19, highlighting an important contributor to the active components (such as licorice-saponin A3, glycyrrhetinic acid, and so on) in herbal medicine treatment. (Wu et al., 2022b). Importantly, the results provided valuable data on the “multi-components, multiple-pathways, and multi-targets” feature of traditional herbal medicine.
Glycosylation is an important structural modification that increases water solubility, enhances pharmacological activity, and improves the bioavailability of natural products. 11) In fact, GA mainly exists in the form of functional glycosides in licorice. At present, more than 43 saponins have been identified in licorice, many of which are glycosylated derivatives of GA. 12) These glycosylated derivatives have different sugar numbers and types and display various pharmacological activities.
3 Other promising natural products for treating SARS-CoV-2 infection
Innovative drug development is an arduous process; bioactive natural products greatly expedite the development of antiviral drugs. (Abdelmohsen et al., 2017). In addition to the abovementioned agents, numerous other natural products (Table 1) have exhibited highly efficacious anti-SARS-CoV-2 activities in vitro and clinical practice. For example, plitidepsin (Aplidin®), a eukaryotic translation elongation factor 1A (eEF1A) inhibitor of marine origin, was initially approved to treat multiple myeloma. (Rodon et al., 2021). Sachse et al. (2022) showed that plitidepsin is highly effective at inhibiting SARS‐CoV‐2 replication in a dose-dependent manner in Vero E6 cells at IC50 values of 0.0052 μM for D614G variants, 0.0039 μM for Delta variants, and 0.0043 μM for Omicron variants. Furthermore, White et al. (2021) showed that plitidepsin can inhibit SARS-CoV-2 replication in Vero E6 cells, hACE2-293T cells and pneumocyte-like cells at IC50 values of 0.00070, 0.00073, and 0.0016 μM, respectively, via targeting the host protein eEF1A. Notably, Guisado-Vasco et al. (2022) showed that plitidepsin is well-tolerated in humans and can lower viral load in SARS-CoV-2-infected chronic lymphocytic leukemia patients. Clinical trials of plitidepsin have been registered (NCT04382066 and NCT05121740) and will be reported shortly. Further study is still needed to evaluate its anti-SARS-CoV-2 potency in vivo and in vitro.
TABLE 1.
Other promising natural products for treating SARS-CoV-2 infection in vitro.
| No. | Name | Structure | EC50 or IC50 (μM) | Strain | Refs |
|---|---|---|---|---|---|
| 1 | Aloin A |
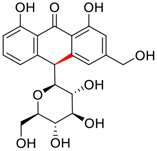
|
15.68 | Vero E6 cells | Lewis et al. (2022) |
| 2 | Aloin B |

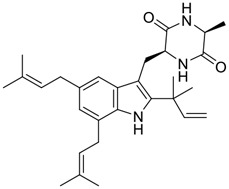
|
17.51 | Vero E6 cells | Lewis et al. (2022) |
| 3 | Andrographolide |
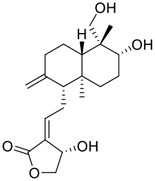
|
0.034 | Calu-3 cells | Sa-Ngiamsuntorn et al. (2021), Schulte et al. (2022) |
| 4 | Aspulvinone D |
|
10.3 | J774A.1 cells | Liang et al. (2022) |
| 5 | Aspulvinone M |
|
9.4 | J774A.1 cells | Liang et al. (2022) |
| 6 | Aspulvinone R |
|
7.7 | J774A.1 cells | Liang et al. (2022) |
| 7 | (+)-Aureol |
|
4.00 | Calu-3 cells | Chhetri et al. (2022) |
| 8 | Baicalein |
|
1.11 | E. coli BL21 cells | Wu et al., 2022a, Xiao et al. (2021) |
| 9 | Baicalin |
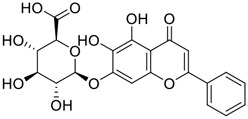
|
8.8 | Vero E6 cells | Ngwe Tun et al. (2022) |
| 10 | Bromophycolide A |
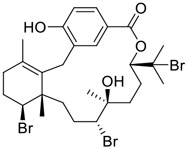
|
6.90 | Calu-3 cells | Chhetri et al. (2022) |
| 11 | Bufotalin |
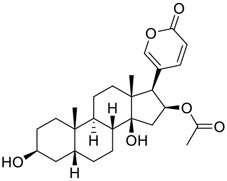
|
0.072 | Vero E6 cells | Jin et al. (2021) |
| 12 | Cannabidiol |
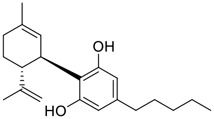
|
1.24 | A549-ACE2 cells | Corpetti et al. (2021), Nguyen et al. (2022) |
| 13 | Cannabidiolic acid |

|
24 μg/mL | Vero E6 cells | van Breemen et al. (2022) |
| 14 | Cannabigerolic acid |
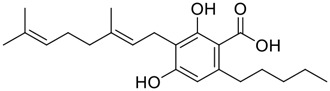
|
37 μg/mL | Vero E6 cells | van Breemen et al. (2022) |
| 15 | Chebulagic acid |
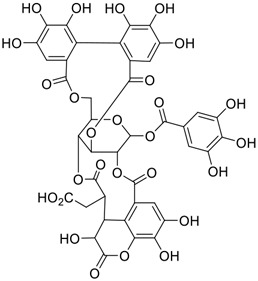
|
9.76 | Vero E6 cells | Du et al. (2021) |
| 16 | Cinobufagin |
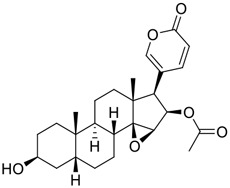
|
0.072 | Vero E6 cells | Corpetti et al. (2021), Nguyen et al. (2022) |
| 17 | Cinobufotalin |
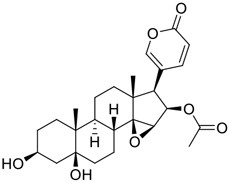
|
0.399 | Vero E6 cells | Jin et al. (2021) |
| 18 | Corilagin |
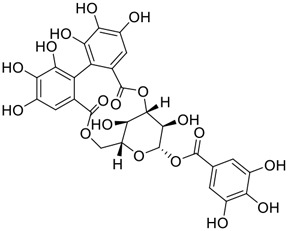
|
24.9 | HEK293 cells | Yang et al. (2021a) |
| 19 | Curcumin |
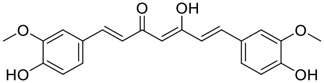
|
11.9 | Vero E6 cells | Bahun et al. (2022) |
| 20 | Cyclopeniol |

|
0.39 | RAW264.7 cells | Thissera et al. (2021) |
| 21 | Cyclopeptin |
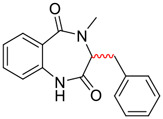
|
0.40 | RAW264.7 cells | Thissera et al. (2021) |
| 22 | Dehydrocyclopeptin |
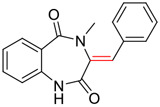
|
0.89 | RAW264.7 cells | Thissera et al. (2021) |
| 23 | Dieckol |
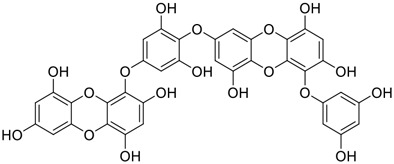
|
4.50 | Vero E6 cells | Yan et al. (2021) |
| 24 | Digitoxin |
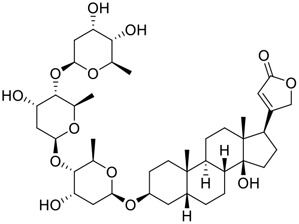
|
0.059 | Vero E6 cells | Caohuy et al. (2021), Jin et al. (2021), Caohuy et al. (2022) |
| 25 | Dihydromyricetin |
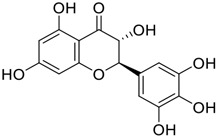
|
1.14 | Vero E6 cells | Su et al. (2021), Xiao et al. (2021) |
| 26 | Dihydrotanshinone I |

|
8.14 | Vero E6 cells | Ma and Wang, (2022) |
| 27 | Dithymoquinone |
|
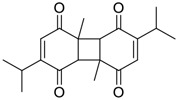
0.275 μg/mL | Vero E6 cells | Esharkawy et al. (2022) |
| 28 | Echinulin |
|
3.90 | Vero E6 cells | Alhadrami et al. (2022) |
| 29 | EGCG |
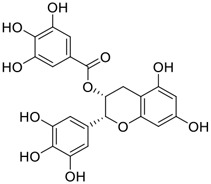
|
4.24 | Vero E6 cells | Chiou et al. (2022) |
| 30 | Ellagic acid |

|
11.8 | Vero E6 cells | Bahun et al. (2022) |
| 31 | (-)-Gallocatechin gallate |
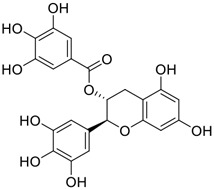
|
5.77 | Vero E6 Cells | Xiao et al. (2021) |
| 32 | Glabridin |
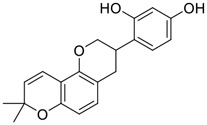
|
2.5 | Vero E6 cells | Ngwe Tun et al. (2022) |
| 33 | Hesperidin |
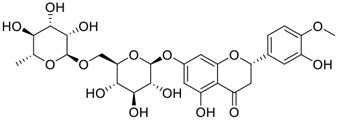
|
51.5 | Vero E6 cells | Huang et al. (2022) |
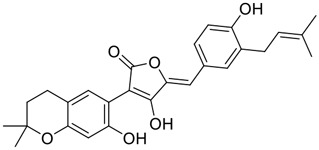
| 34 | Isopetasin |
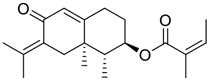
|
0.37 | Vero E6 cells | Urda et al. (2022) |
| 35 | Ivermectin |

|
0.55 | Vero E6 cells | Chable-Bessia et al. (2022) |
| 36 | Licoflavone C |
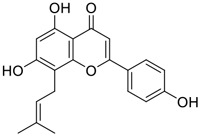
|
1.34 | Vero E6 cells | Corona et al. (2022) |
| 37 | LPC (14:0/0:0) |
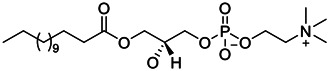
|
0.92 | Vero E6 cells | Du et al. (2022) |
| 38 | LPC (16:0/0:0) |
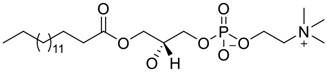
|
1.48 | Vero E6 cells | Du et al. (2022) |
| 39 | LPC (16:0/18:1) |
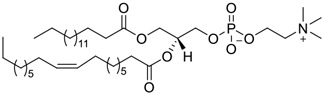
|
0.14 | Vero E6 cells | Du et al. (2022) |
| 40 | Myricetin |

|
0.63 | Vero E6 cells | Kato et al. (2021), Su et al. (2021) |
| 41 | Neferine |
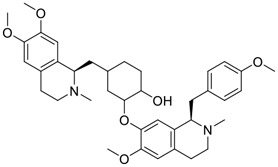
|
0.36 | HEK293/hACE2 cells | Yang et al. (2021b) |
| 42 | (+)-Neoechinulin A |

|
0.47 | Vero E6 cells | Alhadrami et al. (2022) |
| 43 | Neoechinulin B |
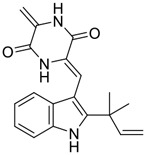
|
32.9 | Vero E6 cells | Nishiuchi et al. (2022) |
| 44 | Neopetasin |

|
1.26 | Vero E6 cells | Urda et al. (2022) |
| 45 | Oridonin |

|
2.16 | Vero E6 cells | Zhong et al. (2022) |
| 46 | Petasin |
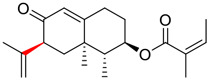
|
10.79 | Vero E6 cells | Urda et al. (2022) |
| 47 | PGG |
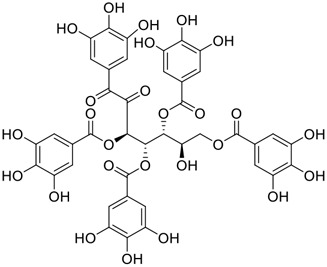
|
3.66 | Vero E6 cells | Chiou et al. (2022) |
| 48 | Piniterpenoid A |

|
64.5 | Vero E6 cells | Li et al. (2021b) |
| 49 | Piniterpenoid C |

|
76.1 | Vero E6 cells | Li et al. (2021b) |
| 50 | Plitidepsin |
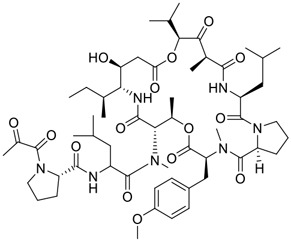
|
0.0043 | Vero E6 cells | Guisado-Vasco et al. (2022), Sachse et al. (2022) |
| 51 | Punicalagin |
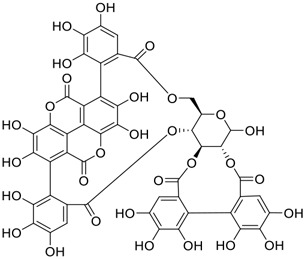
|
6.19 | Vero E6 cells | Saadh et al. (2021) Suručić et al. (2021) |
| 52 | Resibufogenin |

|
1.606 | Vero E6 cells | Jin et al. (2021) |
| 53 | Salvianolic acid A |

|
2.49 | Vero E6 cells | Zhong et al. (2022) |
| 54 | Sangivamycin |

|
0.015 | Vero E6 cells | Bennett et al. (2022) |
| 55 | Scutellarein |

|
5.68 | E. coli BL21 cells | Wu et al. (2022c) |
| 56 | (+)-Shikonin |
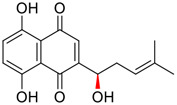
|
4.38 | Vero E6 cells | Zhao et al. (2021), Ma et al. (2022) |
| 57 | Shikonin |
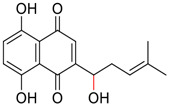
|
4.50 | Vero E6 cells | Cui and Jia (2021), Zhao et al. (2021) |
| 58 | Sulforaphane |
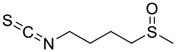
|
2.40 | Caco-2 cells | Ordonez et al. (2022) |
| 59 | Tanshinone IIA |
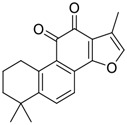
|
7.82 μg/mL | Vero E6 cells | Elebeedy et al. (2022) |
| 60 | Telocinobufagin |

|
0.142 | Vero E6 cells | Jin et al. (2021) |
| 61 | Thymohydroquinone |
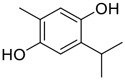
|
0.023 μg/mL | Vero E6 cells | Esharkawy et al. (2022) |
| 62 | Tubercidin |
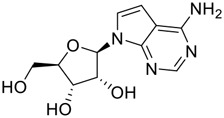
|
0.05 | Calu-3 cells | Schultz et al. (2022) |
| 63 | Ugonin J |
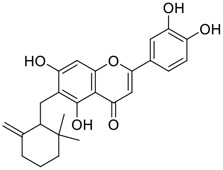
|
2.38 | Vero E6 Cells | Chiou et al. (2021) |
| 64 | 5,3′,4′-trihydroxyflavone |
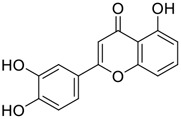
|
8.22 | Vero E6 cells | Zhao et al. (2021) |
| 65 | 7-OH-Cannabidiol |
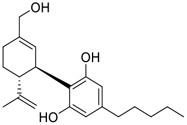
|
3.60 | A549-ACE2 cells | Nguyen et al. (2022) |
4 Conclusion and outlook
The devastating SARS-CoV-2 variants have caused over six million deaths worldwide. Natural products and small-molecule inhibitors have been widely studied (in in vitro studies, animal models, and clinical trials) and play an essential function in treating COVID-19. Drug research and development is a highly time‐consuming process. To date, Gilead’s controversial Veklury® (Remdesivir, RdRp inhibitor) was conditionally approved to combat the outbreak. (Kalil et al., 2021; Wang and Yang, 2022a). Pfizer’s oral broad‐spectrum candidate Paxlovid® (PF‐07321332, Mpro inhibitor) and Merck’s oral prodrug Lagevrio® (Molnupiravir, RdRp inhibitor) raise new hope for a COVID‐19 cure. (Cully, 2022; Wang and Yang, 2022b). Promising clinical results have occurred, while small-molecule inhibitors still have a long way to go.
The substantial progress in treating COVID‐19 patients is not sufficient. Multiple factors must be considered. The first feasible factor, optimized drug combination therapy (such as gallinamide A + remdesivir, licorice-saponin A3 + PF‐07321332, telocinobufagin + molnupiravir, and cordycepin + tylophorine), targeting multiple targets, could not only enhance synergistic efficacy but also reduce drug resistance and toxicity. However, any potential combination would need to be tested in vitro and in vivo to verify the anticipated synergistic or additive effect. The second workable approach is natural product-based nanomedicines therapy. For example, the tylophorine-based lung-targeted liposome LP-NK007 could inhibit SARS-CoV-2 replication with a higher EC50 value via improving the accumulation and efficient delivery in the lung. Third, natural product-based lead optimization offers a valuable reference for enhancing anti‐SARS‐CoV‐2 potency and pharmacokinetic parameters. For example, taking gallinamide A as the lead, Payne et al. (Ashhurst et al., 2022) synthesized two highly selective SARS-CoV-2 cathepsin L inhibitors with nanomolar EC50 values. Taken together, we hope natural products (with the help of natural product-based nanomedicines therapy, lead optimization, and drug combination) prove to be a compelling direction in COVID-19 therapy.
Author contributions
ZW conceived the review. NW, LY, and XS collected the literatures. ZW and LY wrote the manuscript. ZW and XS edited the manuscript. All authors read and approved the final version of the manuscript.
Funding
This work was supported by the project of the PhD research start-up fund of Qufu Normal University, China (Grant No. 614901 and 615201).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdelmohsen U. R., Balasubramanian S., Oelschlaeger T. A., Grkovic T., Pham N. B., Quinn R. J., et al. (2017). Potential of marine natural products against drug-resistant fungal, viral, and parasitic infections. Lancet. Infect. Dis. 17 (2), e30–e41. 10.1016/S1473-3099(16)30323-1 [DOI] [PubMed] [Google Scholar]
- Alam S., Sarker M., Rahman M., Afrin S., Richi F. T., Zhao C., et al. (2021). Traditional herbal medicines, bioactive metabolites, and plant products against COVID-19: Update on clinical trials and mechanism of actions. Front. Pharmacol. 12, 671498. 10.3389/fphar.2021.671498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadrami H. A., Burgio G., Thissera B., Orfali R., Jiffri S. E., Yaseen M., et al. (2022). Neoechinulin A as a promising SARS-CoV-2 Mpro inhibitor: In vitro and in silico study showing the ability of simulations in discerning active from inactive enzyme inhibitors. Mar. Drugs 20 (3), 163. 10.3390/md20030163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqathama A. A., Ahmad R., Alsaedi R. B., Alghamdi R. A., Abkar E. H., Alrehaly R. H., et al. (2022). The vital role of animal, marine, and microbial natural products against COVID-19. Pharm. Biol. 60 (1), 509–524. 10.1080/13880209.2022.2039215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An T. Y., Huang R. Q., Yang Z., Zhang D. K., Li G. R., Yao Y. C., et al. (2001). Alkaloids from Cynanchum komarovii with inhibitory activity against the tobacco mosaic virus. Phytochemistry 58 (8), 1267–1269. 10.1016/s0031-9422(01)00382-x [DOI] [PubMed] [Google Scholar]
- Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., et al. (2022). Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N. Engl. J. Med. 386, 1532–1546. 10.1056/NEJMoa2119451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashhurst A. S., Tang A. H., Fajtová P., Yoon M. C., Aggarwal A., Bedding M. J., et al. (2022). Potent anti-SARS-CoV-2 activity by the natural product gallinamide A and analogues via inhibition of cathepsin L. J. Med. Chem. 65 (4), 2956–2970. 10.1021/acs.jmedchem.1c01494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahun M., Jukić M., Oblak D., Kranjc L., Bajc G., Butala M., et al. (2022). Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols. Food Chem. 373, 131594. 10.1016/j.foodchem.2021.131594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa Da Silva E., Sharma V., Hernandez-Alvarez L., Tang A. H., Stoye A., O’Donoghue A. J., et al. (2022). Intramolecular interactions enhance the potency of gallinamide A analogues against Trypanosoma cruzi . J. Med. Chem. 65 (5), 4255–4269. 10.1021/acs.jmedchem.1c02063 [DOI] [PubMed] [Google Scholar]
- Bennett R. P., Postnikova E. N., Eaton B. P., Cai Y., Yu S., Smith C. O., et al. (2022). Sangivamycin is highly effective against SARS-CoV-2 in vitro and has favorable drug properties. JCI insight 7 (1), e153165. 10.1172/jci.insight.153165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibi S., Hasan M. M., Wang Y. B., Papadakos S. P., Yu H. (2022). Cordycepin as a promising inhibitor of SARS-CoV-2 RNA dependent RNA polymerase (RdRp). Curr. Med. Chem. 29 (1), 152–162. 10.2174/0929867328666210820114025 [DOI] [PubMed] [Google Scholar]
- Cui J., Jia J. (2021). Discovery of juglone and its derivatives as potent SARS-CoV-2 main proteinase inhibitors. Eur. J. Med. Chem. 225, 113789. 10.1016/j.ejmech.2021.113789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caohuy H., Eidelman O., Chen T., Liu S., Yang Q., Bera A., et al. (2021). Common cardiac medications potently inhibit ACE2 binding to the SARS-CoV-2 Spike, and block virus penetration and infectivity in human lung cells. Sci. Rep. 11, 22195. 10.1038/s41598-021-01690-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caohuy H., Eidelman O., Chen T., Yang Q., Walton N. I., Pollard H. B., et al. (2022). Inflammation in the COVID-19 airway is due to inhibition of CFTR signaling by the SARS-CoV-2 Spike protein. bioRxiv [Preprint]. 10.1101/2022.01.18.476803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chable-Bessia C., Boullé C., Neyret A., Swain J., Hénaut M., Merida P., et al. (2022). Low selectivity indices of ivermectin and macrocyclic lactones on SARS-CoV-2 replication in vitro . COVID 2, 60–75. 10.3390/covid2010005 [DOI] [Google Scholar]
- Chandrashekar A., Yu J., McMahan K., Jacob-Dolan C., Liu J., He X., et al. (2022). Vaccine protection against the SARS-CoV-2 Omicron variant in macaques. Cell. 185 (9), 1549–1555.e11. e11. 10.1016/j.cell.2022.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. H., Han Y. Y., Shang Y. J., Zhuang S. Y., Huang J. N., Wu B. Y., et al. (2021). Cordycepin ameliorates synaptic dysfunction and dendrite morphology damage of hippocampal CA1 via A1R in cerebral ischemia. Front. Cell. Neurosci. 15, 783478. 10.3389/fncel.2021.783478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhetri B. K., Tedbury P. R., Sweeney-Jones A. M., Mani L., Soapi K., Manfredi C., et al. (2022). Marine natural products as leads against SARS-CoV-2 infection. J. Nat. Prod. 85, 657–665. 10.1021/acs.jnatprod.2c00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou W. C., Chen J. C., Chen Y. T., Yang J. M., Hwang L. H., Lyu Y. S., et al. (2022). The inhibitory effects of PGG and EGCG against the SARS-CoV-2 3C-like protease. Biochem. Biophys. Res. Commun. 591, 130–136. 10.1016/j.bbrc.2020.12.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou W. C., Lu H. F., Hsu N. Y., Chang T. Y., Chin Y. F., Liu P. C., et al. (2021). Ugonin J acts as a SARS-CoV-2 3C-like protease inhibitor and exhibits anti-inflammatory properties. Front. Pharmacol. 12, 720018. 10.3389/fphar.2021.720018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. J., Ryu E., Lee S., Huh S., Shin Y. S., Kang B. W., et al. (2019). Adenosine induces EBV lytic reactivation through ADORA1 in EBV-associated gastric carcinoma. Int. J. Mol. Sci. 20 (6), 1286. 10.3390/ijms20061286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona A., Wycisk K., Talarico C., Manelfi C., Milia J., Cannalire R., et al. (2022). Natural compounds inhibit SARS-CoV-2 nsp13 unwinding and ATPase enzyme activities. ACS Pharmacol. Transl. Sci. 5, 226–239. 10.1021/acsptsci.1c00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpetti C., Del Re A., Seguella L., Palenca I., Rurgo S., De Conno B., et al. (2021). Cannabidiol inhibits SARS‐Cov‐2 spike (S) protein‐induced cytotoxicity and inflammation through a PPARγ‐dependent TLR4/NLRP3/Caspase‐1 signaling suppression in Caco‐2 cell line. Phytother. Res. 35 (12), 6893–6903. 10.1002/ptr.7302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H. R., Chen K. D., Zhang X. Y., Shang H. C. (2021). Network pharmacology-based analysis on bioactive compounds and mechanisms in Yiqifumai formula in the treatment of heart failure. TMR Mod. Herb. Med. 4 (4), 27. 10.53388/mhm2021a1017001 [DOI] [Google Scholar]
- Cully M. (2022). A tale of two antiviral targets—And the COVID-19 drugs that bind them. Nat. Rev. Drug Discov. 21 (1), 3–5. 10.1038/d41573-021-00202-8 [DOI] [PubMed] [Google Scholar]
- Cunningham K. G., Manson W. I. L. L. I. A. M., Spring F. S., Hutchinson S. A. (1950). Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link. Nature 166 (4231), 949. 10.1038/166949a0 [DOI] [PubMed] [Google Scholar]
- De Jin X. A., Zhang Y., Zhao S., Duan L., Duan Y., Lian F., et al. (2021). Potential mechanism prediction of herbal medicine for pulmonary fibrosis associated with SARS-CoV-2 infection based on network analysis and molecular docking. Front. Pharmacol. 12, 602218. 10.3389/fphar.2021.602218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio C., Omer S. B., Malani P. N. (2022). Winter of omicron-the evolving COVID-19 pandemic. JAMA 327 (4), 319–320. 10.1001/jama.2021.24315 [DOI] [PubMed] [Google Scholar]
- Du R., Cooper L., Chen Z., Lee H., Rong L., Cui Q., et al. (2021). Discovery of chebulagic acid and punicalagin as novel allosteric inhibitors of SARS-CoV-2 3CLpro. Antivir. Res. 190, 105075. 10.1016/j.antiviral.2021.105075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Xinyi, Xu Longxin, Ma Yiming, Lu Shuaiyao, Tang Kegong, Qiao Xiangyu, et al. (2022). Herbal inhibitors of SARS-CoV-2 Mpro effectively ameliorate acute lung injury in mice. IUBMB Life 74, 532–542. 10.1002/iub.2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elebeedy D., Badawy I., Elmaaty A. A., Saleh M. M., Kandeil A., Ghanem A., et al. (2022). In vitro and computational insights revealing the potential inhibitory effect of Tanshinone IIA against influenza A virus. Comput. Biol. Med. 141, 105149. 10.1016/j.compbiomed.2021.105149 [DOI] [PubMed] [Google Scholar]
- Esharkawy E. R., Almalki F., Hadda T. B. (2022). In vitro potential antiviral SARS-CoV-19-activity of natural product thymohydroquinone and dithymoquinone from Nigela sativia . Bioorg. Chem. 120, 105587. 10.1016/j.bioorg.2021.105587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Qin B., Chen P., Zhu K., Hou P., Wojdyla J. A., et al. (2021). Crystal structure of SARS-CoV-2 papain-like protease. Acta Pharm. Sin. B 11 (1), 237–245. 10.1016/j.apsb.2020.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisado-Vasco P., Carralón-González M. M., Aguareles-Gorines J., Martí-Ballesteros E. M., Sánchez-Manzano M. D., Carnevali-Ruiz D., et al. (2022). Plitidepsin as a successful rescue treatment for prolonged viral SARS-CoV-2 replication in a patient with previous anti-CD20 monoclonal antibody-mediated B cell depletion and chronic lymphocytic leukemia. J. Hematol. Oncol. 15, 4. 10.1186/s13045-021-01220-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen H. S., Kokic G., Farnung L., Dienemann C., Tegunov D., Cramer P., et al. (2020). Structure of replicating SARS-CoV-2 polymerase. Nature 584 (7819), 154–156. 10.1038/s41586-020-2368-8 [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 181 (2), 271–280. e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain R., Sarkar C., Hassan S. M. H., Khan R. A., Arman M., Ray P., et al. (2022). In silico screening of natural products as potential inhibitors of SARS-CoV-2 using molecular docking simulation. Chin. J. Integr. Med. 28 (3), 249–256. 10.1007/s11655-021-3504-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou B., Zhang Y. M., Liao H. Y., Fu L. F., Li D. D., Zhao X., et al. (2022). Target-based virtual screening and LC/MS-guided isolation procedure for identifying phloroglucinol-terpenoid inhibitors of SARS-CoV-2. J. Nat. Prod. 85 (2), 327–336. 10.1021/acs.jnatprod.1c00805 [DOI] [PubMed] [Google Scholar]
- Huang Y., Zhou W., Sun J., Ou G., Zhong N. S., Liu Z., et al. (2022). Exploring the potential pharmacological mechanism of hesperidin and glucosyl hesperidin against COVID-19 based on bioinformatics analyses and antiviral assays. Am. J. Chin. Med. 50 (2), 351–369. 10.1142/S0192415X22500148 [DOI] [PubMed] [Google Scholar]
- Ishida J., Wang H. K., Oyama M., Cosentino M. L., Hu C. Q., Lee K. H., et al. (2001). Anti-AIDS agents. 46.1 Anti-HIV activity of harman, an anti-HIV principle from Symplocos setchuensis, and its derivatives. J. Nat. Prod. 64 (7), 958–960. 10.1021/np0101189 [DOI] [PubMed] [Google Scholar]
- Jin Y. H., Jeon S., Lee J., Kim S., Jang M. S., Park C. M., et al. (2021). Broad spectrum antiviral properties of cardiotonic steroids used as potential therapeutics for emerging coronavirus infections. Pharmaceutics 13 (11), 1839. 10.3390/pharmaceutics13111839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., et al. (2020). Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 582 (7811), 289–293. 10.1038/s41586-020-2223-y [DOI] [PubMed] [Google Scholar]
- Kalil A. C., Mehta A. K., Patterson T. F., Erdmann N., Gomez C. A., Jain M. K., et al. (2021). Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: A double-bind, randomised, placebo-controlled, phase 3 trial. Lancet. Respir. Med. 9 (12), 1365–1376. 10.1016/S2213-2600(21)00384-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Higashiyama A., Takaoka E., Nishikawa M., Ikushiro S. (2021). Food phytochemicals, epigallocatechin gallate and myricetin, covalently bind to the active site of the coronavirus main protease in vitro . Adv. Redox Res. 3, 100021. 10.1016/j.arres.2021.100021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov M. (2022). Merck's COVID pill loses its lustre: What that means for the pandemic. Nature [Epub ahead of print]. 10.1038/d41586-021-03667-0 [DOI] [PubMed] [Google Scholar]
- Lewis D. S., Ho J., Wills S., Kawall A., Sharma A., Chavada K., et al. (2022). Aloin isoforms (A and B) selectively inhibits proteolytic and deubiquitinating activity of papain like protease (PLpro) of SARS-CoV-2 in vitro . Sci. Rep. 12, 2145. 10.1038/s41598-022-06104-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Gao J., Li M., Cui H., Jiang W., Tu Z. C., et al. (2021. a). Aromatic cadinane sesquiterpenoids from the fruiting bodies of Phellinus pini block SARS-CoV-2 Spike–ACE2 interaction. J. Nat. Prod. 84 (8), 2385–2389. 10.1021/acs.jnatprod.1c00426 [DOI] [PubMed] [Google Scholar]
- Li Y., Li B., Wang P., Wang Q. (2021. b). Traditional Chinese medicine, Qingfei Paidu decoction and xuanfei baidu decoction, inhibited cytokine production via NF-κB signaling pathway in macrophages: Implications for coronavirus disease 2019 (COVID-19) therapy. Front. Pharmacol. 12, 722126. 10.3389/fphar.2021.722126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. X., Zhang X. J., Zhao Y. X., Feng J., Zeng J. C., Shi Q. Q., et al. (2022). Aspulvins A–H, aspulvinone analogues with SARS-CoV-2 Mpro inhibitory and anti-inflammatory activities from an endophytic Cladosporium sp . J. Nat. Prod. 85 (4), 878–887. 10.1021/acs.jnatprod.1c01003 [DOI] [PubMed] [Google Scholar]
- Linington R. G., Clark B. R., Trimble E. E., Almanza A., Ureña L. D., Kyle D. E., et al. (2009). Antimalarial peptides from marine cyanobacteria: Isolation and structural elucidation of gallinamide A. J. Nat. Prod. 72 (1), 14–17. 10.1021/np8003529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Du W., Sang X., Tong Q., Wang Y., Chen G., et al. (2022). RNA G-quadruplex in TMPRSS2 reduces SARS-CoV-2 infection. Nat. Commun. 13, 1444. 10.1038/s41467-022-29135-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Li S., Fan K., Lu T., Li T. (2020). The prevention and treatment of COVID-19 with Qingfei Paidu decoction in Shanxi China. TMR Mod. Herb. Med. 3 (3), 1–5. [Google Scholar]
- Lyu M., Fan G., Xiao G., Wang T., Xu D., Gao J., et al. (2021). Traditional Chinese medicine in COVID-19. Acta Pharm. Sin. B 11 (11), 3337–3363. 10.1016/j.apsb.2021.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Tan H., Choza J., Wang Y., Wang J. (2022). Validation and invalidation of SARS-CoV-2 main protease inhibitors using the Flip-GFP and Protease-Glo luciferase assays. Acta Pharm. Sin. B 12 (4), 1636–1651. 10.1016/j.apsb.2021.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Wang J. (2022). Validation and invalidation of SARS-CoV-2 papain-like protease inhibitors. ACS Pharmacol. Transl. Sci. 5 (2), 102–109. 10.1021/acsptsci.1c00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengist H. M., Fan X., Jin T. (2020). Designing of improved drugs for COVID-19: Crystal structure of SARS-CoV-2 main protease Mpro. Signal Transduct. Target. Ther. 5, 67. 10.1038/s41392-020-0178-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B., Friedman A. J., Choi H., Hogan J., McCammon J. A., Hook V., et al. (2014). The marine cyanobacterial metabolite gallinamide A is a potent and selective inhibitor of human cathepsin L. J. Nat. Prod. 77 (1), 92–99. 10.1021/np400727r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreen S., Chung H., He S., Brown K. A., Gubbay J. B., Buchan S. A., et al. (2022). Effectiveness of COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario. Nat. Microbiol. 7 (3), 379–385. 10.1038/s41564-021-01053-0 [DOI] [PubMed] [Google Scholar]
- Nguyen L. C., Yang D., Nicolaescu V., Best T. J., Gula H., Saxena D., et al. (2022). Cannabidiol inhibits SARS-CoV-2 replication through induction of the host ER stress and innate immune responses. Sci. Adv. 8, eabi6110. 10.1126/sciadv.abi6110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwe Tun M. M., Toume K., Luvai E., Nwe K. M., Mizukami S., Hirayama K., et al. (2022). The discovery of herbal drugs and natural compounds as inhibitors of SARS-CoV-2 infection in vitro . J. Nat. Med. 76, 402–409. 10.1007/s11418-021-01596-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiuchi K., Ohashi H., Nishioka K., Yamasaki M., Furuta M., Mashiko T., et al. (2022). Synthesis and antiviral activities of neoechinulin B and its derivatives. J. Nat. Prod. 85 (1), 284–291. 10.1021/acs.jnatprod.1c01120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordonez A. A., Bullen C. K., Villabona-Rueda A. F., Thompson E. A., Turner M. L., Merino V. F., et al. (2022). Sulforaphane exhibits antiviral activity against pandemic SARS-CoV-2 and seasonal HCoV-OC43 coronaviruses in vitro and in mice. Commun. Biol. 5, 242. 10.1038/s42003-022-03189-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panggabean J. A., Adiguna S. B. P., Rahmawati S. I., Ahmadi P., Zainuddin E. N., Bayu A., et al. (2022). Antiviral activities of algal-based sulfated polysaccharides. Molecules 27 (4), 1178. 10.3390/molecules27041178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panya A., Songprakhon P., Panwong S., Jantakee K., Kaewkod T., Tragoolpua Y., et al. (2021). Cordycepin inhibits virus replication in dengue virus-infected Vero cells. Molecules 26 (11), 3118. 10.3390/molecules26113118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokharkar O., Lakshmanan H., Zyryanov G., Tsurkan M. (2022). In silico evaluation of antifungal compounds from marine sponges against COVID-19-associated mucormycosis. Mar. Drugs 20 (3), 215. 10.3390/md20030215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prassas I., Diamandis E. P. (2008). Novel therapeutic applications of cardiac glycosides. Nat. Rev. Drug Discov. 7 (11), 926–935. 10.1038/nrd2682 [DOI] [PubMed] [Google Scholar]
- Quintana V. M., Piccini L. E., Zénere J. D. P., Damonte E. B., Ponce M. A., Castilla V., et al. (2016). Antiviral activity of natural and synthetic β-carbolines against dengue virus. Antivir. Res. 134, 26–33. 10.1016/j.antiviral.2016.08.018 [DOI] [PubMed] [Google Scholar]
- Rabie A. M. (2022). Potent inhibitory activities of the adenosine analogue cordycepin on SARS-CoV-2 replication. ACS Omega 7, 2960–2969. 10.1021/acsomega.1c05998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis G., Silva E. A., Silva D. C., Thabane L., Milagres A. C., Ferreira T. S., et al. (2022). Effect of early treatment with ivermectin among patients with Covid-19. N. Engl. J. Med. 386, 1721–1731. 10.1056/NEJMoa2115869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodon J., Muñoz-Basagoiti J., Perez-Zsolt D., Noguera-Julian M., Paredes R., Mateu L., et al. (2021). Identification of plitidepsin as potent inhibitor of SARS-CoV-2-induced cytopathic effect after a drug repurposing screen. Front. Pharmacol. 12, 646676. 10.3389/fphar.2021.646676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa-Ngiamsuntorn K., Suksatu A., Pewkliang Y., Thongsri P., Kanjanasirirat P., Manopwisedjaroen S., et al. (2021). Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component Andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. J. Nat. Prod. 84, 1261–1270. 10.1021/acs.jnatprod.0c01324 [DOI] [PubMed] [Google Scholar]
- Saadh M. J., Almaaytah A. M., Alaraj M., Dababneh M. F., Sa'adeh I., Aldalaen S. M., et al. (2021). Punicalagin and zinc (II) ions inhibit the activity of SARS-CoV-2 3CL-protease in vitro . Eur. Rev. Med. Pharmacol. Sci. 25 (10), 3908–3913. 10.26355/eurrev_202105_25958 [DOI] [PubMed] [Google Scholar]
- Sachse M., Tenorio R., de Castro I. F., Muñoz-Basagoiti J., Perez-Zsolt D., Raïch-Regué D., et al. (2022). Unraveling the antiviral activity of plitidepsin against SARS-CoV-2 by subcellular and morphological analysis. Antivir. Res. 200, 105270. 10.1016/j.antiviral.2022.105270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo A., Fuloria S., Swain S. S., Panda S. K., Sekar M., Subramaniyan V., et al. (2021). Potential of marine terpenoids against SARS-CoV-2: An in silico drug development approach. Biomedicines 9 (11), 1505. 10.3390/biomedicines9111505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte B., König M., Escher B. I., Wittenburg S., Proj M., Wolf V., et al. (2022). Andrographolide derivatives target the KEAP1/NRF2 axis and possess potent anti‐SARS‐CoV‐2 activity. ChemMedChem 17 (5), e202100732. 10.1002/cmdc.202100732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz D. C., Johnson R. M., Ayyanathan K., Miller J., Whig K., Kamalia B., et al. (2022). Pyrimidine inhibitors synergize with nucleoside analogues to block SARS-CoV-2. Nature 604, 134–140. 10.1038/s41586-022-04482-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L., Hsiao N. Y., Moyo S., Singh L., Tegally H., Dor G., et al. (2021). Track Omicron’s spread with molecular data. Science 374 (6574), 1454–1455. 10.1126/science.abn4543 [DOI] [PubMed] [Google Scholar]
- Sharma V., Sharma A., Bharate S. B. (2021). Natural products in mitigation of SARS-CoV infections. Curr. Med. Chem. 28 (22), 4454–4483. 10.2174/0929867327666201027153940 [DOI] [PubMed] [Google Scholar]
- Shen Y., Cai H., Ma S., Zhu W., Zhao H., Li J., et al. (2022). Telocinobufagin has antitumor effects in non-small-cell lung cancer by inhibiting STAT3 signaling. J. Nat. Prod. 85 (4), 765–775. 10.1021/acs.jnatprod.1c00761 [DOI] [PubMed] [Google Scholar]
- Socolsky C., Domínguez L., Asakawa Y., Bardón A. (2012). Unusual terpenylated acylphloroglucinols from Dryopteris wallichiana. Phytochemistry 80, 115–122. 10.1016/j.phytochem.2012.04.017 [DOI] [PubMed] [Google Scholar]
- Sourimant J., Lieber C. M., Aggarwal M., Cox R. M., Wolf J. D., Yoon J. J., et al. (2022). 4′-Fluorouridine is an oral antiviral that blocks respiratory syncytial virus and SARS-CoV-2 replication. Science 375 (6577), 161–167. 10.1126/science.abj5508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Yao S., Zhao W., Zhang Y., Liu J., Shao Q., et al. (2021). Identification of pyrogallol as a warhead in design of covalent inhibitors for the SARS-CoV-2 3CL protease. Nat. Commun. 12, 3623. 10.1038/s41467-021-23751-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suručić R., Travar M., Petković M., Tubić B., Stojiljković M. P., Grabež M., et al. (2021). Pomegranate peel extract polyphenols attenuate the SARS-CoV-2 S-glycoprotein binding ability to ACE2 receptor: In silico and in vitro studies. Bioorg. Chem. 114, 105145. 10.1016/j.bioorg.2021.105145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taori K., Liu Y., Paul V. J., Luesch H. (2009). Combinatorial strategies by marine cyanobacteria: Symplostatin 4, an antimitotic natural dolastatin 10/15 hybrid that synergizes with the coproduced HDAC inhibitor largazole. ChemBioChem 10 (10), 1634–1639. 10.1002/cbic.200900192 [DOI] [PubMed] [Google Scholar]
- Thissera B., Sayed A. M., Hassan M. H., Abdelwahab S. F., Amaeze N., Semler V. T., et al. (2021). Bioguided isolation of cyclopenin analogues as potential SARS-CoV-2 Mpro inhibitors from Penicillium citrinum TDPEF34. Biomolecules 11 (9), 1366. 10.3390/biom11091366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H. Y., Ruan L. J., Yu T., Zheng Q. F., Chen N. H., Wu R. B., et al. (2017). Bufospirostenin A and Bufogargarizin C, steroids with rearranged skeletons from the toad Bufo bufo gargarizans . J. Nat. Prod. 80 (4), 1182–1186. 10.1021/acs.jnatprod.6b01018 [DOI] [PubMed] [Google Scholar]
- Toelzer C., Gupta K., Yadav S. K., Borucu U., Davidson A. D., Kavanagh Williamson M., et al. (2020). Free fatty acid binding pocket in the locked structure of SARS-CoV-2 spike protein. Science 370 (6517), 725–730. 10.1126/science.abd3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. C., Lee C. L., Yen H. R., Chang Y. S., Lin Y. P., Huang S. H., et al. (2020). Antiviral action of tryptanthrin isolated from Strobilanthes cusia leaf against human coronavirus NL63. Biomolecules 10 (3), 366. 10.3390/biom10030366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y., Mori K., Satoh S., Dansako H., Ikeda M., Kato N., et al. (2014). Anti-HCV activity of the Chinese medicinal fungus Cordyceps militaris. Biochem. Biophys. Res. Commun. 447 (2), 341–345. 10.1016/j.bbrc.2014.03.150 [DOI] [PubMed] [Google Scholar]
- Urda L., Kreuter M. H., Drewe J., Boonen G., Butterweck V., Klimkait T., et al. (2022). The petasites hybridus CO2 extract (Ze 339) blocks SARS-CoV-2 replication in vitro . Viruses 14, 106. 10.3390/v14010106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen R. B., Muchiri R. N., Bates T. A., Weinstein J. B., Leier H. C., Farley S., et al. (2022). Cannabinoids block cellular entry of SARS-CoV-2 and the emerging variants. J. Nat. Prod. 85, 176–184. 10.1021/acs.jnatprod.1c00946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A. C., Park Y. J., Tortorici M. A., Wall A., McGuire A. T., Veesler D., et al. (2020). Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 181 (2), 281–292. 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Su B. O., Wang Z., Wu M., Li Z., Hu Y., et al. (2010). Synthesis and antiviral activities of phenanthroindolizidine alkaloids and their derivatives. J. Agric. Food Chem. 58 (5), 2703–2709. 10.1021/jf902543r [DOI] [PubMed] [Google Scholar]
- Wang Z. (2019). Advances in the asymmetric total synthesis of natural products using chiral secondary amine catalyzed reactions of α, β-unsaturated aldehydes. Molecules 24 (18), 3412. 10.3390/molecules24183412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yang L. (2022a). Broad‐spectrum prodrugs with anti‐SARS‐CoV‐2 activities: Strategies, benefits, and challenges. J. Med. Virol. 94 (4), 1373–1390. 10.1002/jmv.27517 [DOI] [PubMed] [Google Scholar]
- Wang Z., Yang L. (2021). Chinese herbal medicine: Fighting SARS-CoV-2 infection on all fronts. J. Ethnopharmacol. 270, 113869. 10.1016/j.jep.2021.113869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yang L. (2020a). GS-5734: A potentially approved drug by FDA against SARS-cov-2. New J. Chem. 44 (29), 12417–12429. 10.1039/d0nj02656e [DOI] [Google Scholar]
- Wang Z., Yang L. (2022b). In the age of Omicron variant: Paxlovid raises new hopes of COVID‐19 recovery. J. Med. Virol. 94 (5), 1766–1767. 10.1002/jmv.27540 [DOI] [PubMed] [Google Scholar]
- Wang Z., Yang L. (2022c). Post-acute sequelae of SARS-CoV-2 infection: A neglected public health issue. Front. Public Health 10, 908757. 10.3389/fpubh.2022.908757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yang L. (2020b). Turning the tide: Natural products and natural-product-inspired chemicals as potential counters to SARS-CoV-2 infection. Front. Pharmacol. 11, 1013. 10.3389/fphar.2020.01013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yang L., Zhao X. E. (2021a). Co-crystallization and structure determination: An effective direction for anti-SARS-CoV-2 drug discovery. Comput. Struct. Biotechnol. J. 19, 4684–4701. 10.1016/j.csbj.2021.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Ye F., Feng Y., Xiao W., Song H., Zhao L., et al. (2021b). Discovery and nanosized preparations of (S, R)-tylophorine malate as novel anti-SARS-CoV-2 agents. ACS Med. Chem. Lett. 12 (11), 1840–1846. 10.1021/acsmedchemlett.1c00481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P., Wang K., Luo C., Huang Y., Misilimu D., Wen H., et al. (2021). Cordycepin confers long-term neuroprotection via inhibiting neutrophil infiltration and neuroinflammation after traumatic brain injury. J. Neuroinflammation 18 (1), 137. 10.1186/s12974-021-02188-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X. X., Zhao M. Z., Zhao C., Zhang X., Qiu R., Lin Y., et al. (2020). The global registry of COVID-19 clinical trials: Indicating the design of traditional Chinese medicine clinical trials. TMR Mod. Herb. Med. 3 (3), 140–146. [Google Scholar]
- White K. M., Rosales R., Yildiz S., Kehrer T., Miorin L., Moreno E., et al. (2021). Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science 371 (6532), 926–931. 10.1126/science.abf4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization WHO coronavirus (COVID-19) dashboard, 2022. Available at: https://covid19.who.int/(Assessed April 22, 2022).
- Wu H., Cheng H., Luo S., Peng C., Zhou A., Chen Z., et al. (2022a). Use of cellular metabolomics and lipidomics to decipher the mechanism of Huachansu injection-based intervention against human hepatocellular carcinoma cells. J. Pharm. Biomed. Anal. 212, 114654. 10.1016/j.jpba.2022.114654 [DOI] [PubMed] [Google Scholar]
- Wu Q., Yan S., Wang Y., Li M., Xiao Y., Li Y., et al. (2022b). Discovery of 4′-O-methylscutellarein as a potent SARS-CoV-2 main protease inhibitor. Biochem. Biophys. Res. Commun. 604, 76–82. 10.1016/j.bbrc.2022.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu L., Cao G., Min L., Dong T. (2022c). Effect and mechanism of Qingfei Paidu decoction in the management of pulmonary fibrosis and COVID-19. Am. J. Chin. Med. 50 (1), 33–51. 10.1142/S0192415X22500021 [DOI] [PubMed] [Google Scholar]
- Xiao T., Cui M., Zheng C., Zhang P., Ren S., Bao J., et al. (2022). Both baicalein and gallocatechin gallate effectively inhibit SARS-CoV-2 replication by targeting Mpro and Sepsis in Mice. Inflammation 45, 1076–1088. 10.1007/s10753-021-01602-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T., Wei Y., Cui M., Li X., Ruan H., Zhang L., et al. (2021). Effect of dihydromyricetin on SARS-CoV-2 viral replication and pulmonary inflammation and fibrosis. Phytomedicine. 91, 153704. 10.1016/j.phymed.2021.153704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G., Li D., Lin Y., Fu Z., Qi H., Liu X., et al. (2021). Development of a simple and miniaturized sandwich-like fluorescence polarization assay for rapid screening of SARS-CoV-2 main protease inhibitors. Cell. Biosci. 11, 199. 10.1186/s13578-021-00720-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. J., Chen R. H., Hamdoun S., Coghi P., Ng J. P., Zhang D. W., et al. (2021a). Corilagin prevents SARS-CoV-2 infection by targeting RBD-ACE2 binding. Phytomedicine. 87, 153591. 10.1016/j.phymed.2021.153591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Wang Z. (2021). Natural products, alone or in combination with FDA-approved drugs, to treat COVID-19 and lung cancer. Biomedicines 9 (6), 689. 10.3390/biomedicines9060689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Yang P., Huang C., Wu Y., Zhou Z., Wang X., et al. (2021b). Inhibitory effect on SARS‐CoV‐2 infection of neferine by blocking Ca2+‐dependent membrane fusion. J. Med. Virol. 93 (10), 5825–5832. 10.1002/jmv.27117 [DOI] [PubMed] [Google Scholar]
- Yi Y., Li J., Lai X., Zhang M., Kuang Y., Bao Y. O., et al. (2022). Natural triterpenoids from licorice potently inhibit SARS-CoV-2 infection. J. Adv. Res. 36, 201–210. 10.1016/j.jare.2021.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W., Mao C., Luan X., Shen D. D., Shen Q., Su H., et al. (2020). Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science 368 (6498), 1499–1504. 10.1126/science.abc1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Hong X., Liu L., Wu Y., Xie X., Fang G., et al. (2021). Cordycepin decreases ischemia/reperfusion injury in diabetic hearts via upregulating AMPK/Mfn2-dependent mitochondrial fusion. Front. Pharmacol. 12, 754005. 10.3389/fphar.2021.754005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y. Q., Chen R. F., Ma Q. H., Zheng J., Deng X., Yang W., et al. (2022). Efficacy and safety of phillyrin (KD-1) capsule in the treatment of moderate COVID-19: Protocol for a randomized controlled trial. TMR Mod. Herb. Med. 5 (1), 5. 10.53388/mhm2021p1204001 [DOI] [Google Scholar]
- Zhang S., Pei R., Li M., Su H., Sun H., Ding Y., et al. (2022). Cocktail polysaccharides isolated from Ecklonia kurome against the SARS-CoV-2 infection. Carbohydr. Polym. 275, 118779. 10.1016/j.carbpol.2021.118779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Ma Q., Zhang B., Guo P., Wang Z., Liu Y., et al. (2021). Exploration of SARS-CoV-2 3CLpro inhibitors by virtual screening methods, FRET detection, and CPE assay. J. Chem. Inf. Model. 61 (12), 5763–5773. 10.1021/acs.jcim.1c01089 [DOI] [PubMed] [Google Scholar]
- Zhong B., Peng W., Du S., Chen B., Feng Y., Hu X., et al. (2022). Oridonin inhibits SARS‐CoV‐2 by targeting its 3C‐Like protease. Small Sci. 2, 2100124. 10.1002/smsc.202100124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Luo L., Dressel W., Shadier G., Krumbiegel D., Schmidtke P., et al. (2008). Cordycepin is an immunoregulatory active ingredient of Cordyceps sinensis. Am. J. Chin. Med. 36 (05), 967–980. 10.1142/S0192415X08006387 [DOI] [PubMed] [Google Scholar]