Abstract
It has been suggested that the benefit of adjuvant chemotherapy (CT) in premenopausal women with hormone receptor-positive (HR+), human epidermal growth factor receptor 2 negative (HER2−) early breast cancer may be related, at least in part, to CT-induced ovarian function suppression (OFS) in this subgroup of patients. Although this hypothesis has not been directly tested in large randomized clinical trials, the observations from prospective studies have been remarkably consistent in showing a late benefit of CT among the subgroup of patients who benefit (ie, women who were close to menopause). The hypothesis has important clinical implications, as it may be possible to spare the associated adverse effects of adjuvant CT in a select group of women with early breast cancer, in favor of optimizing OFS and endocrine therapy (ET), without compromising clinical outcomes. Such an approach has the added benefit of preserving the key quality of life outcomes in premenopausal women, particularly by preventing the irreversible loss of ovarian function that may result from CT use. For this reason, we convened an international panel of clinical experts in breast cancer treatment to discuss the key aspects of the available data in this area, as well as the potential clinical implications for patients. This article summarizes the results of these discussions and presents the consensus opinion of the panel regarding optimizing the use of OFS for premenopausal women with HR+, HER2− early breast cancer.
Keywords: ovarian function suppression, adjuvant chemotherapy, genomic testing, premenopausal, early breast cancer, MINDACT, TAILORx
This article summarizes the key results of an expert panel discussion regarding optimizing the use of ovarian function suppression for premenopausal women with HR+, HER2− early breast cancer.
Implications for Practice.
Studies including MINDACT and TAILORx have suggested a benefit of chemotherapy (CT) for premenopausal patients (<50 years) with hormone receptor-positive, human epidermal growth factor receptor 2 negative early breast cancer. Specifically, a late-occurring benefit of CT in the premenopausal subgroup was observed, suggesting that this could be due, at least in part, to CT-induced ovarian function suppression. This article reports the findings of an international expert panel discussion considering the data supporting the potential avoidance of CT in this subgroup of patients, a prospect that has important clinical and quality of life implications, including the preservation of fertility.
Purpose of the Panel and Role of the Funding Source
We assembled an international group of experts in breast cancer treatment, representing countries across North America, Latin America, and Europe. We believe the diversity achieved in the multidisciplinary panel is reflective of the differences in clinical practice, approved therapies, diagnostic methodologies (including genomic testing), and patient’s goals of care across different countries and clinical settings as it relates to the treatment of early breast cancer. The purpose of these discussions was to answer the central question: Should the role of ovarian function suppression (OFS) with endocrine therapy (ET) be discussed as an alternative to chemotherapy (CT) as effective adjuvant therapy in selected premenopausal women with early breast cancer?
Some of the principal findings that led to convening this panel discussion were presented at the American Society of Clinical Oncology (ASCO) Virtual Meeting in 2020. At that time, long-term findings from MINDACT were reported.1 An age-dependent benefit of CT, previously observed in the TAILORx study in 2018, was confirmed in the MINDACT trial results presented in 2020, leading to the hypothesis that indirect OFS resulting from CT could explain the difference in distant disease-free survival (DDFS) observed after 5 years.1,2 In particular, a major finding of both trials was that a late benefit of CT could be observed among the subgroup of women under 50 years of age (ie, premenopausal women) with clinical high risk for recurrence (as assessed by clinicopathologic factors) but genomic low-risk early breast cancer (based on the 21- or the 70-gene assay assessments). At the 2020 San Antonio Breast Cancer Symposium (SABCS), findings from the RxPonder study were presented which further corroborated the hypothesis, and the relevant findings from all these trials are detailed further below.3
Agendia, Inc. provided whole-genome analysis, without cost, to the European Organization for Research and Treatment of Cancer (EORTC) for the MINDACT trial, and also provided an unrestricted educational grant for the expert panel discussions and development of this publication. Total Health Conferencing, an independent medical education company, arranged the expert panel and had complete control over the content, topic, and presentation of the discussions. Review, editing, and final approval of the manuscript content was the sole responsibility of the expert panel. Participants received a nominal honorarium for their participation in the discussions and review of the manuscript, per fair market guidelines. It is understood that the discussion participants may have corporate relationships, both related and unrelated to the topic in question, and the discussion was not intended to endorse or recommend any treatment or diagnostic testing methodology over another, nor to serve as a complete or exhaustive review of the topic, or to replace any existing clinical guidance in any country. Rather, the content presented herein is intended to reflect the overall opinions of the expert panel, as gleaned from their own experiences, and to serve as a basis for shared decision-making with patients.
The Prospective Trials: TAILORx, MINDACT, and RxPonder
Most clinicians are now familiar with the available genomic tests with value as prognostic assays in early breast cancer, including EndoPredict, MammaPrint, Oncotype Dx, and Prosigna (PAM50). Prospective data, however, are only available for 2 of these tests, the 70-gene assay (MammaPrint) and the 21-gene assay (Oncotype Dx), and the focus of the current discussion regarding the impact of OFS with ET is based on the updated data presented in TAILORx (2019), MINDACT (2020), and RxPonder (2020).1-9 While the trial designs, treatments, and patient populations in these trials have been previously described and are not extensively discussed here, the primary results of these trials are summarized in Table 1.3-5 Briefly, both TAILORx and MINDACT were designed to assess whether women with hormone-receptor-positive (HR+), human epidermal growth factor receptor-negative (HER2−), early breast cancer who were candidates to receive CT (based on clinicopathologic features) could be safely spared adjuvant CT if their genomic risk was found to be intermediate (using the 21-gene assay, Oncotype Dx) or low (using the 70-gene assay, MammaPrint). RxPonder (SWOG S1007) also assessed a similar endpoint as TAILORx using the 21-gene assay but was specifically focused on patients with HR+/HER2− disease with 1–3 positive lymph nodes (LN+). Especially important for the reader to note are the results for long-term follow-up from MINDACT and TAILORx, which are summarized in Table 2.
Table 1.
Prospective trials: study design and primary results1-4
| Trial | TAILORx | MINDACT | RxPonder |
|---|---|---|---|
| NCT Number | NCT00310180 | NCT00433589 | NCT01272037 |
| Years of accrual | 2006–2010 | 2007–2011 | 2011–2017 |
| Genomic test | Oncotype Dx (21-gene assay) | MammaPrint (70-gene assay) | Oncotype Dx (21-gene assay) |
| Primary endpoint | Invasive disease-free survival (IDFS) | Distant metastasis-free survival (DMFS) | Invasive disease-free survival (IDFS) |
| Primary patient population | • HR+/HER2−/Node-negative (N0) with RS = 11–25; N = 6711 | • Patients at high clinical risk (C-High) and low genomic risk (G-Low); N = 1550 • 48% N+ disease; 90% with Luminal HER2− subtype |
• HR+/HER2−/Node positive (1–3 nodes), with RS <25; N = 5083 |
| Follow up for primary analysis | Median 7.5 years | Median 5.0 years | Median 5.1 years |
| Percentage premenopausal | 36% | 34.5% (<50 years) | 33% |
| Percentage postmenopausal | 64% | 65.5% (>50 years) | 67% |
| Primary results | • ET was non-inferior to chemo ET for IDFS (HR = 1.08; 95% CI = 0.94 – 1.24; P=.26) • IDFS = 83.3% vs. 84.3% • OS = 93.9% vs. 93.8% |
• 5-year DMFS was 94.7% for patients with C-high/G-low disease who did not receive CT; this exceeded the lower boundary (95% CI) of at least 92% DMFS in this population. | • Significant interaction observed for CT and menopausal status (P=.004) • In postmenopausal: HR for CT + ET vs. ET alone = 0.97 (P = .82); 5 year IDFS = 91.6% vs. 91.9% [no benefit of CT] • In premenopausal: HR for CT + ET vs. ET alone = 0.54 (P=.0004); 5 year IDFS = 94.2% vs. 89.0% [benefit of CT] |
| Clinical implications | • A similar benefit of ET versus chemo ET was found in women with an intermediate recurrence score (RS = 11–25) • Some benefits of chemo ET found in women 50 years or younger |
• Primary hypothesis was confirmed in this positive de-escalation study; women with C-high/G-low disease not treated with CT had excellent outcomes at 5 years. | • The primary endpoint, to show an interaction between RS and CT benefit was not significant (P = .3). • The study did suggest however that therapy can be de-escalated for patients with RS <25 and 1–3 positive nodes • Strong IDFS benefit observed with CT + ET among premenopausal women |
Table 2.
Enhanced OFS as an alternative to chemotherapy: What is the evidence?1-9
| Primary analysis population | ||||||
|---|---|---|---|---|---|---|
| 5-year analysis | Difference | 8-year analysis | Difference | |||
| CT | 95.7% | 0.9% | 92.0% | 2.6% | ||
| No CT | 94.8% | 89.4% | ||||
| Analysis by age group | ||||||
| Age <50 years: Benefit | ||||||
| CT | 96.2% | 2.6% | 93.6% | 5.0%← | ||
| No CT | 93.6% | 88.6% | ||||
| Age >50 years: No benefit | ||||||
| CT | 95.0% | −0.9% | 90.2% | 0.2%← | ||
| No CT | 95.8% | 90.0% | ||||
| Long-term results from MINDACT, at 8.7 years median follow-up, continued to show excellent distant metastasis-free survival (DMFS) for clinical high risk, genomic low-risk patients who received no CT (95.1%), confirming the primary analysis for non-inferiority between patients treated or not treated with adjuvant CT. | ||||||
| RS = 16–20 (n= 886) The absolute difference in distant recurrence |
RS = 21–25 (n= 476) The absolute difference in distant recurrence |
|||||
|---|---|---|---|---|---|---|
| Unstratified: +1.6% | Clinical low | -0.2% | Unstratified: +6.4% | Clinical Low | + 6.4% | |
| Clinical high | + 6.5%← | Clinical High | + 8.7%← | |||
Long-term results from TAILORx, at 9 years’ follow up, also showed an age-dependent impact of chemotherapy on distant recurrence in women <50 with clinical high-risk features and intermediate recurrence score (RS) as determined by Oncotype Dx, ranging from 6.5% for RS 16–20, to 8.7% for RS 21–25.
The Case for Enhanced Ovarian Function Suppression: What is the Evidence?
As shown in Table 1, the initial results from both TAILORx and MINDACT demonstrated that the primary analysis population, ie, women who were otherwise candidates for CT but had intermediate or low genomic risk (herein termed C-High/G-Low), could be safely spared the use of adjuvant CT, while still achieving excellent clinical outcomes; as such, both trials were positive de-escalation trials supporting the utility of either genomic assay as a means to potentially spare the use of CT in selected patients. In TAILORx, however, a significant impact of age (P = .004) was found, such that some benefit of CT was found in women 50 years of age or younger with a 21-gene recurrence score (RS) of 16–25.2 Long-term findings from MINDACT also showed an increasing absolute benefit of CT over time, from 0.9% at the initial analysis (5 years) to 2.6% after a median 8.7 years follow-up (Tables 1 and 2). Notably, additional analyses showed that the long-term benefit of CT occurring in MINDACT could be entirely attributed to the premenopausal group, for whom the absolute benefit was 5.0%, as compared to the postmenopausal group, which did not benefit (absolute benefit of 0.2%).6
The findings of a late occurring survival benefit in the chemotherapy-treated group of the MINDACT and TAILORx trials differ from that seen in major observational studies such as the Oxford overview (Early Breast Cancer Trialist’s Collaborative Group; EBCTCG) which demonstrate that the principal benefit of cytotoxic chemotherapy occurs early in the course of follow up (ie, within the first 5 years after diagnosis).10,11 Results from Swain et al (2010) have also shown that patients on CT who had amenorrhea for 6 or more months during 24 months of follow up had significantly better disease-free (P < .001) and overall (P = .04) survival as compared to those who did not; importantly, this was true for all subgroups, regardless of CT regimen, estrogen receptor status, and across all age groups (<40, 40–44, and >44 years), suggesting a prognostic effect of amenorrhea.12 Results such as these, in addition to those of TAILORx and MINDACT, have led Sparano et al (2019), Cardoso et al (2020), and Wolff (2020) to independently suggest that the benefit of chemotherapy in the subgroup of patients aged <50 years could, in fact, be occurring due to an indirect effect of chemotherapy causing potentially irreversible OFS, and as such, a similar incremental benefit as CT in preventing recurrence might be achieved for selected patients using OFS and ET alone.1,5,7,8 While not directly compared, these data imply that enhanced OFS in this particular subgroup of patients could lead to excellent long-term survival outcomes while avoiding the use of chemotherapy and its associated side effects. Such an alternative could prove highly desirable to those patients with underlying comorbid conditions that limit CT use, and/or those seeking to maintain fertility or avoid CT for other reasons.
Also important to note are the results of subgroup analyses from TAILORx, which demonstrated that, among those with an RS of 16–25, the benefit of adding CT to ET was most evident in the 41–45 age group, and among those in the 46–50 age range who were premenopausal, but not for those in this group who were postmenopausal. Notably, there was no benefit of adding CT in younger (premenopausal) women (≤40 years), or in the 51–55 age group.7 A similar analysis by age group of the MINDACT results for luminal subtype cancers (ie, HR+/HER2−) in the C-High/G-Low group is shown in Table 3.9 It should be noted that, although the groups were well matched for clinicopathologic features, the postmenopausal patients received mostly AIs, whereas tamoxifen only was used in over 90% of the younger patients. Although the analysis was limited by small numbers of events, the approximately 3.6% improvement in DFS with CT seen in the 40–50 age group, but not in the >50 age group (−0.2%) is consistent with the earlier suggestion of CT-induced OFS in TAILORx, and suggests that patients in this subgroup may be undertreated with tamoxifen alone in lieu of more optimized endocrine treatment (ie, OFS with tamoxifen or AI).9
Table 3.
MINDACT: Analysis by age.
| Luminal—C-high/G-low 40–50 years (n= 399) |
Luminal—C-high/G-low >50 years (n = 865) |
|||
|---|---|---|---|---|
| 5-year DMFS (%) | Difference (%) | 5-year DMFS (%) | Difference (%) | |
| CT | 96.2 | + 3.6← | 95.2 | -0.2 |
| No CT | 92.6 | 95.4 | ||
A total of 1358 patients with Luminal (HR+/HER2−) patients from MINDACT had C-high/G-low risk for recurrence. There were too few patients (n = 53) and events in the <40 age group for analysis. Results for the 40–50, and >50 age groups are shown in the table; the groups were well matched for tumor size (2.2 cm, both groups), nodal status (N0, >50% both groups), and grade (~65% Grade 2 both groups).9
Findings from these major prospective studies are also supported by the results of other large trials such as SOFT and TEXT, which show an approximate absolute distant metastasis-free survival benefit of 5% with OFS in premenopausal women using a gonadotropin-releasing hormone (GnRH) agonist in combination with oral anti-estrogen therapy, typically consisting of tamoxifen (TAM) or an aromatase inhibitor (AI).13 Furthermore, findings from the Zoladex Early Breast Cancer Research Association (ZEBRA) trial of OFS with the GnRH agonist goserelin versus cyclophosphamide, methotrexate, and fluorouracil (CMF)-based CT have also shown non-inferiority of goserelin to CMF in HR+ premenopausal patients with node-positive disease.14,15 In addition, although the trial results were reported subsequently and not available for discussion at the initial meetings of our expert panel, the initial findings from RxPonder also support an impact of CT in premenopausal, but not postmenopausal women with 1–3 positive nodes and with RS <25 (Table 1).3
In view of these historical findings, as well as the more recent corroborative findings from RxPonder, the primary purpose of the panel was to consider whether the observed benefit of CT as observed in the MINDACT and TAILORx trials might be due to treatment-induced OFS in the subgroup of premenopausal women with early breast cancer, and if so, assess whether enhanced OFS in this group (particularly those with C-High/G-Low early breast cancer) can be a feasible treatment option, after shared-decision discussions with patients.
Role of Genomic Testing
In accordance with the findings from the MINDACT and TAILORx trials, the panel recognizes the importance of identifying premenopausal patients with low genomic risk, but an otherwise high clinical risk for recurrence, who might safely forgo adjuvant chemotherapy in favor of considering the option of optimizing ET plus OFS. The specific population of women considered for the present discussion of OFS is those patients 50 years of age or younger, with hormone receptor-positive (HR+), human epidermal growth factor receptor 2 (HER2) negative disease with clinical high/genomic low risk (as determined by MammaPrint), or for those with node-negative disease and a recurrence score (RS) of between 16 and 25 (as determined by Oncotype Dx). The panel agrees that genomic testing, if available, can be included in the decision-making process noting, for example, that RS has been shown to be a continuous variable in terms of recurrence prognosis. Differences in the approval, reimbursement, and accessibility of these genomic tests across the different countries represented in this panel are also recognized which can lead to significant differences in patient preference and accessibility to care, depending on country and type of practice. Importantly, the group does not recognize or recommend the use of either genomic test over the other for this purpose in this population.
Challenges to Establishing a Definitive Statement
While it would be desirable for the panel to put forth a uniform recommendation or guidance statement on the potential use of OFS with ET as an alternative to CT in premenopausal women, there are a number of reasons why such a statement is not possible. For example, the panel noted the lack of direct (Level 1) evidence suggesting that ET can serve as a replacement for CT, citing trials such as Austrian Breast and Colorectal Cancer Study Group (ABCSG) Trial 5 which compared 5 years of tamoxifen/3 years of goserelin versus 6 cycles of cyclophosphamide, methotrexate, and fluorouracil (CMF)-based CT.16 While the results showed a benefit of endocrine therapy over CMF CT in this older trial, the same cannot be assumed for newer CT regimens which are now considered as adjuvant treatment for breast cancer.16 Indeed, the panelists note that the higher efficacy of CMF in this study may also have been due in part to the higher rate of therapy-induced amenorrhea with the CMF regimen.16 Also noted by some on the panel was the premature termination of the Premenopausal Endocrine Responsive Chemotherapy (PERCHE) trial, conducted by the International Breast Cancer Study Group (IBCSG) which aimed to compare ET alone (OFS with tamoxifen or exemestane for 5 years) versus CT + ET. Although viewed by many as a pivotal trial to definitively establish the role of CT in premenopausal patients with early breast cancer, this trial was closed due to poor accrual.17 Some panelists also noted the long-term findings of the ABCSG-12 study, which showed excellent survival outcomes without CT, even for premenopausal patients with N1 disease using OFS in combination with ET (albeit with the understanding that endpoints were addressed with zoledronic acid as a component of the regimen).18
In the course of the discussions, it was also suggested that, in the premenopausal group with low genomic risk, the prognosis with combined endocrine therapy (ie, TAM or AI with GnRH agonists) might be sufficiently good that the addition of CT to endocrine therapy may not provide sufficient additional benefit to justify the risks of CT. Indeed, it may be possible to identify specific subgroups of patients with a greater or lesser likelihood of response to endocrine therapies; for example, patients with Luminal A subtype may be more responsive to ET alone as compared to those with the Luminal B subtype.19 In this regard, the panel also recognizes the utility of freely available risk indexes arising from the SOFT and TEXT data, such as those developed by Regan and coworkers, as a means to identify patients more likely to benefit from enhanced ET + OFS.13,20 It should be noted, however, that these were developed in the context of older chemotherapy regimens, relate to absolute risk for recurrence, and are not necessarily reflective of CT benefit. Although many on the panel saw the need for a trial assessing OFS with ET with or without CT to be undertaken, it was agreed that such a trial would take many years to accrue sufficient patients, and many years of follow up to yield meaningful results; in this regard, we believe our consensus may be a useful means to navigate such a conversation with the individual patient in the interim, while also considering benefits and risks of the approach in the absence of such data.
At the time of the summit, prior to SABCS 2020, the panel noted that the results of the discussion might also be influenced by the forthcoming data from other trials such as WSG ADAPT; in this study, patients with HR+/HER2− early breast cancer with N0–N1 disease having a low or intermediate recurrence risk as assessed by the 21-gene assay (RS <11 or RS = 11–25) would receive a 3-week neoadjuvant treatment induction with ET; with subsequent assessment of the proliferation marker Ki67.21 Those having an early response to ET (defined as Ki67 ≤10% after induction) would then receive ET only, whereas those with >10% Ki67 after induction would be randomized to one of the CT arms in the study (as would patients with a higher risk, N2-3 and/or N0-1 with RS ≥26). Results from the ADAPT trial subsequently showed that a Ki67 drop in response to neoadjuvant endocrine therapy was more informative than pre-treatment RS for prediction of benefit and that RS could not be interpreted outside of the context of Ki67 response.22
It is important to emphasize that neither TAILORx nor MINDACT had sufficient numbers of patients in the target population in question (see Table 1). As such, there was significant dilution among the populations examined, particularly if one considers not only the number of premenopausal patients but also the percentage of these patients who also received OFS with ET and/or remained adherent to the regimen. While it is unclear whether confirmatory studies will ever be conducted, the panel believes that further exploratory analyses should certainly be considered in these trials. While the panel acknowledged the similar results observed in the MINDACT and TAILORx trials are indeed supportive of the hypothesis that indirect OFS could explain the survival benefit provided by CT in select premenopausal patients, it was emphasized that neither of these studies was designed nor powered to answer the question of whether OFS could safely replace CT in this group. As such, in the absence of an adequately powered trial to address the issue, the panel is left with the question of how to counsel the patient in situations where it is likely we may be overtreating a population of patients; whether to offer CT when it is felt to provide benefit, while also striving to spare the patient harmful adverse effects.
Practical Considerations of Enhanced OFS in Lieu of CT
There are several other considerations that prevent composing a uniform group recommendation on this issue, one of which is the regional differences in the global access to, reimbursement of, and routine use of genomic tests, including the 21-gene and 70-gene assays, which may be used to stratify early breast cancer patients for adjuvant therapies. As such, this limits the generalizability of any recommendation for selecting premenopausal patients who might safely forgo CT in favor of enhanced ET + OFS based on the use of a genomic assay.
Tolerability and Adherence
Tolerability of ET was one of the primary issues cited by the group as extremely important to consider if patients were to forgo CT in favor of OFS and endocrine therapy, particularly as these patients are often considered for extended adjuvant therapy approaches. While it was acknowledged that the long-term side effects of therapies such as TAM or an AI in combination with OFS have not been well studied, the general experience of the expert group was that patient acceptance and tolerability of ET have been uniformly poor. In clinical practice, some studies show that non-adherence could be as high as 50%, with some of the most important reasons being adverse events, medication costs, and lack of effective provider-patient communication.13,23-30 Increased menopausal symptoms, sexual dysfunction, diabetes, and osteoporosis have been more commonly reported, for example, in patients receiving OFS.24 Partridge et al (2003) previously noted up to 50% nonadherence overall for patients on TAM adjuvant therapy, and acknowledged the significant potential for underreporting non-adherence using measures such as patient surveys/self-reporting, as opposed to more objective means.31
Problems with long-term adherence and compliance with ET, particularly the enhanced OFS with ET considered here, are especially important in younger, premenopausal patients; these patients are already at increased risk for recurrence and will likely have to endure many years of therapy and its associated side effects; moreover, the optimal duration of ET is not known, and it may be difficult to justify continuing treatment for the patient.32 Indeed, in a more recent study, of 384 patients under age 40 on ET, at least 1 non-adherent behavior (eg, forgotten pills) was reported in 51% of the patients, with moderate to high non-adherent behaviors noted in 18%.33 Some of the factors identified as related to non-adherence in the study included lower educational level, a lack of social support, and lower confidence in the decision to undergo ET. In another recent retrospective study examining adherence and discontinuations in a large cohort of early breast cancer patients under 50 years, an increase in the use of OFS was observed over time, reaching 11.3% by 2016.34 These authors found a rate of discontinuation of 40.2% and 48.8% for ET+OFS and TAM alone users, respectively, although in adjusted analyses there was a similar likelihood of discontinuing either treatment (HR = 0.92). Over the first year of ET, approximately 1/3 of the women were reported to have had low adherence in this study. Although requiring prospective validation, the findings suggest that while the overall use of OS was low it did not lead to lower adherence to ET.34 Most of the panel were in agreement regarding the significant detrimental impact of enhanced endocrine therapy on the quality of life of breast cancer patients and emphasized the need to improve acceptance and management strategies. One of the suggestions to increase compliance and adherence to this treatment would be to use regular estradiol assessments as part of the treatment plan.
Potential for Estradiol Breakthrough
Another point noted by the panel was that the OFS agents, including GnRH agonists, are not 100% effective, and, as noted above, there may be a need for regular estradiol monitoring to prevent a breakthrough resumption of ovarian function. This risk is particularly important to consider as physicians may be more commonly using a 3-month depot dosing of GnRH as a means to reduce office visits, especially during the COVID-19 pandemic (although this strategy is usually not recommended); it was noted that such a strategy might be more conducive to estradiol breakthrough, although limited data suggest no difference between the dosing schedules.35,36 Results from the estrogen substudy from SOFT also showed that, while a majority of premenopausal patients receiving exemestane with triptorelin had reductions in estrogen levels comparable to postmenopausal patients receiving AIs, approximately one-third of patients had E2 levels greater than the predefined suboptimal threshold of 2.72 pg/mL.37 With regard to the use of GnRH agonists, some panelists also expressed concern over the limited data regarding 3-monthly depot dosing as compared with monthly dosing; in particular, from the patient perspective, monthly dosing may be taxing for younger, working-age patients and could result in excessive co-pays.
Also relevant to the long-term tolerability and efficacy of enhanced ET with OFS is the issue of obesity, which could render OFS less effective in patients with elevated body mass index (BMI).38 As reviewed by Goodwin (2013), for example, there has been observational evidence of less complete aromatase inhibition in heavier women across some of the major trials in the postmenopausal setting.38 The SOFT estrogen substudy also showed a marginal impact of increased BMI on E2 increases above the 2.72 pg/mL threshold. The impact of these elevations on treatment outcomes in premenopausal patients, however, could not be assessed in trials such as SOFT due to the low number of patients studied.37 A review by Jiralerspong and Goodwin has also shown a 35%–40% increase in breast cancer recurrence and death for obese women as compared to those with normal BMI, and these authors correctly suggest that such a decrement in outcomes could easily erase the benefit of many of the most effective breast cancer therapies.39 In this regard, assessing the impact of obesity on the efficacy of enhanced OFS, particularly if being considered as an alternative to CT for the patient population in question, will be an important consideration for further studies.
Patient Perspectives
In view of the significant QoL impact that avoidance of CT or the potential permanent loss of ovarian function in premenopausal women can have, the panel recognizes the importance of including the patient voice in this discussion, while also recognizing that regional and cultural differences may exist in overall patient input and/or interest in deciding on their treatment plan. One important consideration was exactly how much risk reduction was necessary for patients to justify the added risks of CT. It was recognized that patient input on issues such as the use or non-use of CT may depend, in part, on what the patient is told to expect by physicians, and/or the experiences of family and friends. The panel agreed that HR+ patients rarely, if ever, actually recognize that CT puts them into early menopause, and that since the patient is also on endocrine treatment, menopausal side effects would often be blamed on the endocrine therapy as opposed to CT. As such, it was thought that, if physicians were to offer a choice between enhanced OFS and CT, it would need to be made clear that the end result of menopause symptoms will be the same regardless, but the option is to perhaps avoid the more harmful CT-specific side effects. Some of these well documented side effects include acutely occurring issues such as alopecia, nail changes and nail loss, and fatigue, as well as possible late side effects of CT such as long-term neuropathy, impaired executive functioning (“chemobrain”), rare but serious cardiac toxicity, and risk for acute leukemias. The panel acknowledges that the full breadth of potential CT-associated toxicities is rarely discussed with patients, and the impact may often be understated, considering that the expected benefit of preventing cancer recurrence is emphasized as the greater priority. Nonetheless, the group recognizes these are women with a high chance of cure regardless of therapy selection, and likely have many life years ahead as survivors.
Central to the issue of avoiding CT in premenopausal patients with early breast cancer is the potential for maintaining fertility in very young patients. It was agreed that premenopausal women with early breast cancer would be likely to have many years of life after their cancer treatment, during which they could desire for future pregnancy.32 In this regard, the group noted that CT-induced OFS is more likely to become permanent in premenopausal women, as compared with OFS induced by treatment with GnRH analogs. In the ZEBRA trial, for example, menses returned for a majority of goserelin-treated patients, whereas amenorrhea was permanent in approximately 77% of CMF-treated patients after 3 years. Women in the 40–50 age range were also more likely to become amenorrheic with CT versus OFS. As such, if CT is to be used in these women, the possibility of permanent loss of fertility should be emphasized, and some panelists noted a lack of adequate information regarding the risk of cessation of ET with OFS to achieve pregnancy. Conversely, if a decision to forgo CT is made, the panel agreed that the patient would need to understand the importance of adherence and compliance with the more intensive OFS regimen over the long term, to optimize risk reduction. The panel did note, however, that approximately 40% of patients in SOFT/TEXT did not complete the 5 years of therapy but achieved the risk reduction.25Overall, the panel recognizes the importance of the patient voice and shared decision-making when deciding on the treatment strategy to be used, including the use or non-use of CT for premenopausal patients.
Conclusion and Consensus Opinion of the Group
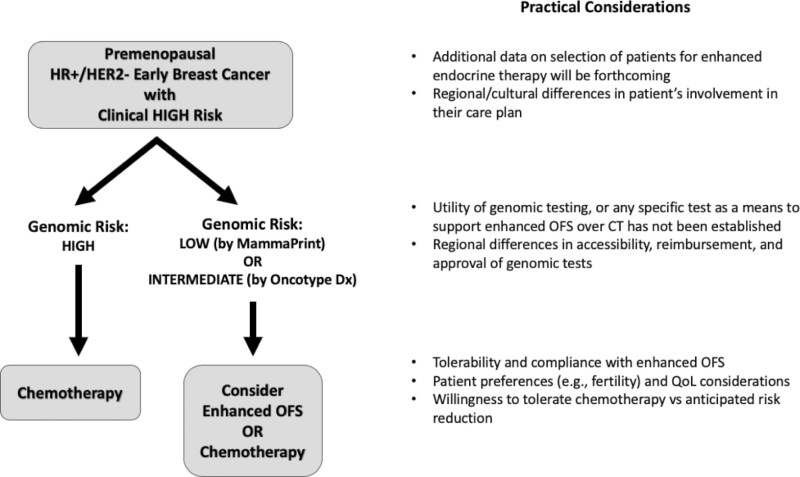
At present, no sufficient data is available to enable a definitive recommendation for the avoidance of CT in favor of optimizing ET with OFS in premenopausal breast cancer patients under 50 years of age who have high clinical risk but low genomic risk for disease recurrence. The panel does, however, believe that there are satisfactory data to enter into a discussion with the patient regarding reasonable choices for therapy. Algorithms, similar to that originally proposed by Cardoso et. al. at ASCO 2020 (Fig. 1) for the use of MammaPrint or OncotypeDx results in the context of clinical risk are worthy of consideration and could allow for a more informed shared decision-making process with the patient. In lieu of providing such a recommendation at this time, we hope that the present discussion would serve as a “call to action” on this topic whereby clinicians could further explore this option for their patients who would otherwise be considered candidates for CT and engage them in a discussion that incorporates the risks and benefits of each option as well as individual patient preference. We believe it is when the data are not definitive, and conclusive recommendations cannot be made, that an informed discussion with the patient and shared decision-making conversations are most needed.
Figure 1.
An algorithm for shared decision making: enhanced OFS or CT?1
An important point also raised in the discussions was that no single trial or data set fits every patient; as such, extrapolation of available data is essential as additional therapies continue to emerge and treatment plans become more and more individualized. We believe clinical trials such as those considered herein should not be interpreted in a vacuum and are best understood in the context of the data and observations which came before them; indeed, a possible OFS effect was considered in the earliest adjuvant CT trial ever conducted in breast cancer patients which showed a highly significant impact on disease-free interval for premenopausal patients.40 This is an especially important consideration for premenopausal patients seeking to retain fertility, as CT-induced OFS is more likely to be permanent. Despite the compelling results in favor of endocrine therapy from MINDACT and TAILORx, and now RxPonder, in a select group of premenopausal patients, the panel agreed that clearly there will remain patients with sufficiently high recurrence risk to warrant the use of both CT and endocrine therapy.
In view of the evolving data sets that seek to more precisely identify which patients are more likely to benefit from enhanced OFS, it was suggested that perhaps a better question to ask is, can a subset of premenopausal women be identified in whom it is possible to maximize endocrine therapy benefit such that the addition of CT would not afford sufficient additional benefit in risk reduction to justify the risk of CT. It is likely that further insight on the optimal use of enhanced OFS, and further refinements in risk stratification, will be forthcoming in the near future.
Future Directions
While acknowledging that trials such as PERCHE have proven difficult to accrue, the panel sees this discussion as signaling the need for a prospective, randomized clinical trial. The consistency in observations from the now 3 landmark studies for early breast cancer leaves us with the sentiment that the questions around the effect on ovarian function suppression in premenopausal patients, independent of risk, are too important to leave without pursuing definitive data. In the meantime, informed and shared discussions with patients around this topic are essential.
Acknowledgments
Medical writing/editorial assistance was provided by SciavoTECH Research and Consultancy Services, Inc. and was funded by Agendia, Inc.
Contributor Information
Bolivar Arboleda, Puerto Rican Society of Mastology, HIMA San Pablo Oncology Hospital, Caguas, Puerto Rico.
Rupert Bartsch, Department of Medicine I, Division of Oncology, Medical University of Vienna, Vienna, Austria.
Evandro de Azambuja, Institut Jules Bordet – Université Libre de Bruxelles, Brussels, Belgium.
Erika Hamilton, Breast and Gynecologic Research Program, Sarah Cannon Research Institute, Tennessee Oncology PLLC, Nashville, TN, USA.
Nadia Harbeck, LMU Munich, University Hospital, Department of Obstetrics and Gynecology, Breast Center and Comprehensive Cancer Center (CCLMU), Munich, Germany.
Jennifer Klemp, University of Kansas Cancer Center, Kansas City, KS, USA.
Michael Knauer, Department of Pathology and Laboratory Medicine, Western University and London Health Sciences Centre, London, ON, Canada.
Sherko Kuemmel, Department of Gynecology with Breast Center, Charité-Universitätsmedizin Berlin, Germany.
Reshma Mahtani, Baptist Health South Florida, Miami, FL, USA.
Lee Schwartzberg, West Cancer Center, Memphis, TN, USA.
Cynthia Villarreal-Garza, Breast Cancer Center, Hospital Zambrano Hellion, Tecnologico de Monterrey, San Pedro Garza Garcia, Mexico.
Antonio Wolff, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD, USA.
Final Note
The authors wish to recognize the updated findings from WSG-ADAPT as reported by Gluz and coworkers [Gluz O, Nitz U, Christgen M, et al J Clin Oncol. 39, 2021 (suppl 15; abstr 504)]. These results, presented at ASCO 2021, showed that patients who were 50 years or younger with 0–3 positive lymph nodes and an RS of 12–25 had an excellent 5-year invasive disease-free survival of 97% with endocrine therapy alone, provided there was a drop in Ki-67 to <10% after 3 weeks of neoadjuvant endocrine therapy (thus indicative of endocrine sensitivity). It should be noted that the proportion of endocrine sensitive patients was lower with tamoxifen alone, as compared to aromatase inhibitor induction in post-menopause. With the addition of GnRH in the premenopausal setting, the proportion of endocrine-sensitive patients also increases. In view of these results, it, therefore, remains questionable whether a study (for example, with a non-inferiority design) would be feasible to compare outcomes with chemotherapy versus combined ET with OFS, as such a study would require thousands of patients.
Conflict of Interest
Rupert Bartsch: AstraZeneca, Daiichi, Eisai, Eli Lilly, MSD, Novartis, Pfizer, Pierre-Fabre, Puma, Roche, Seagen (C/A), AstraZeneca, Celgene, Eli Lilly, Novartis, Pfizer, Pierre-Fabre, Roche, Seagen (H), Daiichi, MSD, Novartis, Roche (RF); Evandro de Azambuja: Roche/GNE, Novartis, Seattle Genetics, Libbs, Zodiac, Lilly, Pierre Fabre (H, C/A), Roche/GNE, GSK/Novartis (Other—travel grant), Roche/GNE, AstraZeneca, GSK/Novartis, Servier (RF—inst.); Erika Hamilton: OncoMed, Genentech/Roche, Zymeworks, Rgenix, ArQule, Clovis, Silverback Therapeutics, Millenium, Acerta Pharma, Sermonix Pharmaceuticals, Torque, Black Diamond, Karyopharm, Infinity Pharmaceuticals, Curis, Syndax, Novartis, Boehringer Ingelheim, Immunomedics, FujiFilm, Taiho, Deciphera, Fochon, Molecular Templates, Onconova Therapeutics, Dana-Farber Cancer Hospital, Hutchinson MediPharma, MedImmune, SeaGen, Puma Biotechnology, Compugen, TapImmune, Lilly, Pfizer, H3 Biomedicine, Takeda, Merus, Regeneron, Arvinas, StemCentRx, Verastem, eFFECTOR Therapeutics, CytomX, InventisBio, Lycera, Mersana, Radius Health, Abbvie, Nucana, Leap Therapeutics, Zenith Epigenetics, Harpoon, Orinove, AstraZeneca, Tesaro, Macrogenics, EMD Serono, Daiichi Sankyo, Syros, Sutro, G1 Therapeutics, Merck, PharmaMar, Olema, Polyphor, Immunogen, Plexxicon, Amgen, Akesobio Australia, Shattuck Labs (RF), Genentech/Roche, Boehringer Ingelheim, Novartis, Dantari, Lilly, Merck, Puma Biotechnology, Silverback Therapeutics, CytomX, Pfizer, Mersana, Black Diamond, H3 Biomedicine, Daiichi Sankyo, AstraZeneca, Arvinas, Deciphera Pharmaceuticals, Eisai, Seagen (C/A); Jennifer Klemp: Pfizer and AstraZeneca (H, C/A); Sherko Kuemmel: Amgen, AstraZeneca, Celgene, Daiichi-Sankyo, ExactScience, Gilead, Lilly, MSD, Novartis, Seagen (C/A), Pfizer, pfm Medical, Roche, Somatex (RF—inst.), Roche, Novartis (H), West German Study Group (OI); Reshma L. Mahtani: Agendia, Amgen, AstraZeneca, Biotheranostics, Daiichi, Eisia, Genentech, Gilead, Lilly, Merck, Novartis, Pfizer, Puma, Sanofi, SeaGen (C/A); Lee Schwartzberg: Helsinn Healthcare, Tesaro, Merck, Heron (C/A), Helsinn Healthcare, Tesaro (RF); Cynthia Villarreal-Garza: AstraZeneca, Roche (RF), Roche, Myriad Genetics, Novartis, Pfizer, Eli Lilly (H), Roche, Novartis, Pfizer, Eli Lilly (C/A), Roche, MSD Oncology, Pfizer (Other). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Author Contributions
Conception/design: All authors. Collection and/or assembly of data: All authors. Manuscript writing: All authors. Final approval of manuscript: All authors.
Data Availability
Data sharing not applicable as no new data were generated in the writing of this review article.
References
- 1. Cardoso F, van ‘t Veer L, Poncet C, et al. MINDACT: Long-term results of the large prospective trial testing the 70-gene signature MammaPrint as guidance for adjuvant chemotherapy in breast cancer patients. J Clin Oncol. 38:2020(suppl 15):506-–506.. 10.1200/JCO.2020.38.15_suppl.506. [DOI] [Google Scholar]
- 2. Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111-121. 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalinsky K, Barlow WE, Meric-Bernstam F, et al. First results from a phase III randomized clinical trial of standard adjuvant endocrine therapy (ET) +/− chemotherapy (CT) in patients (pts) with 1–3 positive nodes, hormone receptor-positive (HR+) and HER2− negative (HER2−) breast cancer (BC) with recurrence score (RS) < 25: SWOG S1007 (RxPonder). San Antonio Breast Cancer Symposium December 8–11, 2020. [Abstract GS3-00]
- 4. Sparano JAA. 21-Gene expression assay in breast cancer. N Engl J Med. 2016;374:1387. 10.1056/NEJMc1515988. [DOI] [PubMed] [Google Scholar]
- 5. Cardoso F, van’t Veer LJ, Bogaerts J, et al. MINDACT Investigators. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375:717-729. 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 6. Piccart M, van ‘t Veer LJ, Poncet C, et al. 70-gene signature as an aid for treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. 2021; 22:476-488. 10.1016/S1470-2045(21)00007-3 [DOI] [PubMed] [Google Scholar]
- 7. Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019;380(25):2395-2405. 10.1056/NEJMoa1904819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wolff AC. To Everything There Is a Season: A Time to De-Escalate? Oral Presentation. 2020. ASCO Virtual Scientific Program. [Google Scholar]
- 9. Piccart M, Poncet C, Cardoso F, Should age be integrated together with clinical and genomic risk for adjuvant chemotherapy decision in early luminal breast cancer? MINDACT results compared to those of TAILOR-X. Presented at: San Antonio Breast Cancer Symposium. December 12, 2019. [Abstract GS4-05]
- 10. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687-1717. 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 11. Peto R, Davies C, Godwin J, et al. Early Breast Cancer Trialists' Collaborative Group (EBCTCG)’ Collaborative Group (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432-444. 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swain SM, Jeong JH, Geyer CE Jr, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362(12):2053-2065. 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pagani O, Francis PA, Fleming GF, et al. SOFT and TEXT Investigators and International Breast Cancer Study Group. Absolute improvements in freedom from distant recurrence to tailor adjuvant endocrine therapies for premenopausal women: results from TEXT and SOFT. J Clin Oncol. 2020;38(12):1293-1303. 10.1200/JCO.18.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jonat W, Kaufmann M, Sauerbrei W, et al. Zoladex Early Breast Cancer Research Association Study. Goserelin versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: The Zoladex Early Breast Cancer Research Association Study. J Clin Oncol. 2002;20(24):4628-4635. 10.1200/JCO.2002.05.042. [DOI] [PubMed] [Google Scholar]
- 15. Kaufmann M, Jonat W, Blamey R, et al. Zoladex Early Breast Cancer Research Association (ZEBRA) Trialists’ Group. Survival analyses from the ZEBRA study. goserelin (Zoladex) versus CMF in premenopausal women with node-positive breast cancer. Eur J Cancer. 2003;39(12):1711-1717. 10.1016/s0959-8049(03)00392-7. [DOI] [PubMed] [Google Scholar]
- 16. Jakesz R, Hausmaninger H, Kubista E, et al. Austrian Breast and Colorectal Cancer Study Group Trial 5. Randomized adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer—Austrian Breast and Colorectal Cancer Study Group Trial 5. J Clin Oncol. 2002;20(24):4621-4627. 10.1200/JCO.2002.09.112. [DOI] [PubMed] [Google Scholar]
- 17. Regan MM, Pagani O, Walley B, et al. SOFT/TEXT/PERCHE Steering Committee and the International Breast Cancer Study Group. Premenopausal endocrine-responsive early breast cancer: who receives chemotherapy? Ann Oncol. 2008;19(7):1231-1241. 10.1093/annonc/mdn037. [DOI] [PubMed] [Google Scholar]
- 18. Gnant M, Mlineritsch B, et al. Austrian Breast and Colorectal Cancer Study Group, Vienna, Austria. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann Oncol. 2015;26(2):313-320. 10.1093/annonc/mdu544. [DOI] [PubMed] [Google Scholar]
- 19. Burstein HJ. Systemic Therapy for estrogen receptor-positive, HER2−negative breast cancer. N Engl J Med. 2020;383(26):2557-2570. 10.1056/NEJMra1307118. [DOI] [PubMed] [Google Scholar]
- 20. Tailoring Adjuvant Endocrine Therapy for Premenopausal Women. Accessed: April 27, 2021.https://rconnect.dfci.harvard.edu/CompositeRiskSTEPP/
- 21. Hofmann D, Nitz U, Gluz O, et al. WSG ADAPT—adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early breast cancer: study protocol for a prospective, multi-center, controlled, non-blinded, randomized, investigator initiated phase II/III trial. Trials. 2013;14:261. 10.1186/1745-6215-14-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gluz O, Degenhardt T, Marschner N, et al. Adaptlate—a randomized, controlled, open-label, phase-iii trial on adjuvant dynamic marker—adjusted personalized therapy comparing abemaciclib combined with standard adjuvant endocrine therapy versus standard adjuvant endocrine therapy in (clinical or genomic) high risk, hr+/her2− early breast cancer. In: Proceedings of the 2020 San Antonio Breast Cancer Virtual Symposium; Dec 8-11, 2020. San Antonio, TX. Philadelphia (PA): AACR. Cancer Res 2021;81(Suppl 4):Abstract OT-01-02 [Google Scholar]
- 23. Chlebowski RT, Kim J, Haque R.. Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prev Res (Phila). 2014;7(4):378-387. 10.1158/1940-6207.CAPR-13-0389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jain S, Santa-Maria CA, Gradishar WJ.. The role of ovarian suppression in premenopausal women with hormone receptor-positive early-stage breast cancer. Oncology (Williston Park). 2015; 29(7): 473-8, 481. [PubMed] [Google Scholar]
- 25. Pagani O, Regan MM, Walley BA, et al. TEXT and SOFT Investigators; International Breast Cancer Study Group. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107-118. 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park WC. Role of ovarian function suppression in premenopausal women with early breast cancer. J Breast Cancer. 2016;19(4):341-348. 10.4048/jbc.2016.19.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saha P, Regan MM, Pagani O, et al. SOFT; TEXT Investigators; International Breast Cancer Study Group. Treatment efficacy, adherence, and quality of life among women younger than 35 years in the international breast cancer study group TEXT and SOFT adjuvant endocrine therapy trials. J Clin Oncol. 2017;35(27):3113-3122. 10.1200/JCO.2016.72.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Francis PA, Pagani O, Fleming GF, et al. SOFT and TEXT Investigators and the International Breast Cancer Study Group. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379(2):122-137. 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Durrani S, Heena H.. Controversies regarding ovarian suppression and infertility in early stage breast cancer. Cancer Manag Res. 2020;12:813-817. 10.2147/CMAR.S231524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meng J, Wang X, Guan Y, Zhang D.. Aromatase inhibitors plus ovarian function suppression versus tamoxifen plus ovarian function suppression for premenopausal women with early stage breast cancer: a systematic review and meta-analysis. Ann Palliat Med. 2020;9(4):2294-2302. 10.21037/apm-20-488A. [DOI] [PubMed] [Google Scholar]
- 31. Partridge AH. Non-adherence to endocrine therapy for breast cancer. Ann Oncol. 2006;17(2):183-184. 10.1093/annonc/mdj141. [DOI] [PubMed] [Google Scholar]
- 32. Rosenberg SM, Partridge AH.. New insights into nonadherence with adjuvant endocrine therapy among young women with breast cancer. J Natl Cancer Inst. 2015;107(10):djv245. 10.1093/jnci/djv245. [DOI] [PubMed] [Google Scholar]
- 33. Wassermann J, Gelber SI, Rosenberg SM, et al. Nonadherent behaviors among young women on adjuvant endocrine therapy for breast cancer. Cancer. 2019;125(18):3266-3274. 10.1002/cncr.32192. [DOI] [PubMed] [Google Scholar]
- 34. Reeder-Hayes KE, Mayer SE, Lund JL.. Adherence to endocrine therapy including ovarian suppression: a large observational cohort study of US women with early breast cancer. Cancer. 2021;127(8):1220-1227. doi: 10.1002/cncr.33367. [DOI] [PubMed] [Google Scholar]
- 35. Masuda N, Iwata H, Rai Y, et al. Monthly versus 3-monthly goserelin acetate treatment in pre-menopausal patients with estrogen receptor-positive early breast cancer. Breast Cancer Res Treat. 2011;126(2):443-451. 10.1007/s10549-010-1332-y. [DOI] [PubMed] [Google Scholar]
- 36. Noguchi S, Kim HJ, Jesena A, et al. Phase 3, open-label, randomized study comparing 3-monthly with monthly goserelin in pre-menopausal women with estrogen receptor-positive advanced breast cancer. Breast Cancer. 2016;23(5):771-779. 10.1007/s12282-015-0637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bellet M, Gray KP, Francis PA, et al. Twelve-month estrogen levels in premenopausal women with hormone receptor-positive breast cancer receiving adjuvant triptorelin plus exemestane or tamoxifen in the suppression of ovarian function trial (SOFT): The SOFT-EST Substudy. J Clin Oncol. 2016;34(14):1584-1593. 10.1200/JCO.2015.61.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goodwin PJ. Obesity and endocrine therapy: host factors and breast cancer outcome. Breast. 2013;22(Suppl 2):S44-S47. 10.1016/j.breast.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 39. Jiralerspong S, Goodwin PJ.. Obesity and breast cancer prognosis: evidence, challenges, and opportunities. J Clin Oncol. 2016;34(35):4203-4216. 10.1200/JCO.2016.68.4480. [DOI] [PubMed] [Google Scholar]
- 40. Fisher B, Carbone P, Economou SG, et al. 1-Phenylalanine mustard (L-PAM) in the management of primary breast cancer. A report of early findings. N Engl J Med. 1975;292(3):117-122. 10.1056/NEJM197501162920301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable as no new data were generated in the writing of this review article.