Abstract
Background
While cardiotoxic chemotherapy is known to negatively impact cardiac function and hemoglobin levels, the impact on skeletal muscle has been understudied among patients. The purpose was to longitudinally characterize myosteatosis (muscle fat), skeletal muscle metabolism, and oxygen (O2) consumption during cardiotoxic chemotherapy for breast cancer.
Patients and Methods
Thirty-four patients with stage I-III breast cancer were enrolled before trastuzumab-containing and/or anthracycline-containing chemotherapy. We used magnetic resonance imaging to non-invasively quantify thigh myosteatosis (fat-water imaging), and lower leg metabolism (31P spectroscopy), O2 consumption (custom techniques), and peak power output during single-leg plantarflexion exercise at pre-, mid-, end-chemotherapy, and 1-year. We also measured pulmonary VO2peak and maximal leg press strength.
Results
During chemotherapy, VO2peak and leg press strength decreased while peak plantarflexion power output was maintained. At mid-chemotherapy, hemoglobin decreased (16%) and lower leg blood flow increased (37%) to maintain lower leg O2 delivery; exercise Pi:PCr and myosteatosis increased. Between mid- and end-chemotherapy, lower leg O2 extraction (28%) and O2 consumption (21%) increased, while plantarflexion exercise efficiency (watts/O2 consumed) decreased. At one year, VO2peak and leg press strength returned to pre-chemotherapy levels, but lower leg exercise O2 extraction, consumption and Pi:PCr, and myosteatosis remained elevated.
Conclusion
Lower leg skeletal muscle blood flow and O2 extraction adapt to compensate for chemotherapy-related hemoglobin reduction for small muscle mass exercise but are insufficient to maintain large muscle mass exercise (pulmonary VO2peak, leg press strength). The excess O2 required to perform work, increased Pi:PCr ratio and myosteatosis together suggest suppressed fat oxidation during chemotherapy.
Keywords: breast neoplasm, cardiotoxicity, muscle, skeletal, drug therapy, magnetic resonance imaging
Cardiotoxic chemotherapy is known to negatively affect cardiac function and hemoglobin levels, but its effect on skeletal muscle is less understood. This article examines the changes in skeletal muscle energy metabolism and oxygen consumption during cardiotoxic chemotherapy for early-stage breast cancer and evaluates chemotherapy-related changes in myosteatosis and related performance metrics.
Implications for Practice.
This study found skeletal muscle metabolic perturbations and persistent myosteatosis (muscle fat infiltration) during breast cancer chemotherapy treatment. These findings identify targetable mechanisms to address patient-reported symptoms (eg, fatigue, exercise intolerance) and long-term metabolic impairment (eg, suppressed fat oxidation leading to insulin resistance). The implications for practice are two-fold. First, physical activity that does not require large muscle mass may be more tolerable for patients while hemoglobin levels are suppressed by chemotherapy. Second, further clinical follow-up is indicated well beyond completion of breast cancer treatment to monitor and manage potential development of insulin resistance and metabolic diseases.
Introduction
A decline in cardiopulmonary fitness, measured as peak exercise pulmonary oxygen uptake (VO2peak), is a well-established adverse consequence of chemotherapy treatment. VO2peak is an independent predictor of cardiovascular morbidity and mortality1 that integrates cardiopulmonary, vascular, and skeletal muscle system function in the transport of oxygen (O2) from atmospheric air to the mitochondria to perform physical work.2 It is also well-established that anthracycline- and trastuzumab-based chemotherapy may promote cardiac and hematologic toxicity that results in reduced whole-body convective O2 delivery.3,4
In contrast, skeletal muscle metabolism and O2 delivery and utilization have received relatively little study in patients with breast cancer. Histologic analysis of thigh muscle tissue in 10 patients with breast cancer receiving anthracycline-containing chemotherapy showed significant decreases in citrate synthase activity, oxidative muscle fiber cross-sectional area, and capillary number per muscle fiber.5 Given the inherent risks and discomfort of invasive tissue sampling in chemotherapy recipients, non-invasive imaging may be more feasible for serial assessments and offers the ability for provocative real-time testing with exercise. In a recent cross-sectional study of breast cancer survivors one year after anthracycline treatment, we found no evidence of impaired skeletal muscle metabolism nor deficits in O2 delivery and utilization using magnetic resonance imaging (MRI), despite a 20% reduction in VO2peak compared to matched controls.6 Yet, we observed increased myosteatosis (ie, fatty infiltration of skeletal muscle) in the thigh, lower leg, and paraspinal regions among the breast cancer survivors.6 Further, thigh myosteatosis had similar predictive power for low pulmonary VO2peak as exercise cardiac function.7 We also found that greater myosteatosis in the lower leg was associated with decreased O2 consumption and extraction by the lower leg during submaximal plantarflexion (ie, toe-pointing) exercise.6 Similarly, while the functional implications are not yet clear, we found that intermuscular fat within the paraspinal (postural) muscles significantly increased within the first 3 months of trastuzumab-containing chemotherapy and persisted to 12 months.8
The primary aim of this exploratory study was to examine the changes in skeletal muscle energy metabolism, O2 consumption and its determinants, and composition over 12 months of cardiotoxic chemotherapy for early-stage breast cancer. A secondary aim was to evaluate the interrelationships among chemotherapy-related changes in myosteatosis, isolated skeletal muscle metabolism and O2 consumption, as well as performance metrics (eg, cycle ergometer pulmonary VO2peak, leg press maximal strength). We hypothesized that (1) chemotherapy would result in metabolic perturbations, and alterations in O2 delivery and utilization, and (2) leg myosteatosis that would be related to skeletal muscle performance.
Methods
Design
This study was a sub-study enrolling the last 34 of 80 participants in a parent study whose rationale and design have previously been reported.9 The sub-study was designed with the independent goal of a comprehensive characterization of longitudinal skeletal muscle changes across chemotherapy treatment. Participants enrolled in the sub-study performed longer MRI scans and one additional scan to quantify the changes in skeletal muscle O2 consumption and its determinants, metabolism, and composition across 4 time points. The parent study randomly assigned patients to a multi-disciplinary team care intervention for cardiovascular risk reduction (described in detail in Supplementary material) or to usual cancer care with a primary outcome of left ventricular ejection fraction (LVEF) (ClinicalTrials.gov: NCT01621659). The parent intervention was not targeted to impact the exploratory skeletal muscle analysis in the sub-study, nor was the sub-study powered to detect a group difference. Rather, we accounted for the potential influence of the random group assignment in our analyses. Here, we report the skeletal muscle evaluations collected exclusively for the sub-study as well as measures of pulmonary VO2peak and leg muscular strength completed for the parent study for the 34 participants in the sub-study. The Health Research Ethics Board of Alberta provided ethical approval for the study. All participants provided written informed consent.
Patients
Patients were recruited from the Cross Cancer Institute (Edmonton, Canada). Eligible patients were age >18 years, with a recent diagnosis of stage I-III breast cancer, scheduled to receive trastuzumab-containing and/or anthracycline-containing chemotherapy, and able to give consent in English. Patients were excluded if they had contraindications to MRI or exercise testing, previous heart failure, baseline LVEF <50%, or prior cardiotoxic treatment. Age-, sex-, and body mass index (BMI)-matched controls without a history of cancer were recruited by word of mouth for comparison data. Controls were excluded for MRI or exercise testing contraindications, and diagnoses of other major comorbid conditions (diabetes, cardiovascular disease, respiratory disease).
Outcome Measures
We performed MRI scans on the patients with breast cancer at pre-chemotherapy (baseline), the mid-point of chemotherapy (~2-3 weeks after cycle 3 or ~9-10 weeks after baseline), at the end of chemotherapy (~24 weeks after baseline), and at 1 year after baseline on a 3.0T system (Prisma, Siemens Healthcare, Erlangen, Germany). For the non-cancer controls, we performed a single scan using identical protocols; results were previously reported.6 Magnetic resonance imaging studies included assessment of leg skeletal muscle composition and functional responses to in-magnet exercise challenges (described briefly below, with further detail in Supplementary material). Cardiac MRI was not included in this sub-study.
Skeletal Muscle and Fat Composition
The fat and muscle compartments of the right lower leg and thigh were evaluated using the chemical-shift encoded MRI approach.10 For the thigh, 5 cm of consecutive slices were standardized to a mid-thigh location across all participants. For the lower leg, a matched number and location of consecutive slices with near full coverage below the knee were standardized within each participant over time. Volumes of subcutaneous and intermuscular fat, and muscle were summed across slices within each region. Myosteatosis was represented by the ratio between intermuscular fat and muscle and by muscle fat fraction (intermuscular fat/(intermuscular fat + skeletal muscle) × 100%).
Small Muscle Mass Exercise Test 1: Incremental-to-Maximum Workload with 31P Spectroscopy
To evaluate muscle energy metabolism, 31P spectra were acquired at rest, during an incremental-to-maximum workload (4 W with 2-W/min increments) unilateral (left leg), plantarflexion (toe-pointing) exercise test, and for 4 minutes of recovery. The exercise device was a commercially available MRI ergometer (Trispect Module, Ergospect, Austria). Single-leg plantarflexion exercise requires a small volume of muscle mass, which limits the role of the heart, thus isolating our evaluation primarily to skeletal muscle function. Relative concentrations of phosphocreatine (PCr) and inorganic phosphate (Pi), and the intracellular pH were evaluated using standard methods11 for rest, low intensity (~40% of peak), high intensity (~80% of peak), and peak exercise. The Pi:PCr ratio represents coupling between ATP use and resynthesis.11 The PCr recovery time constant, an indicator of muscle oxidative capacity, was calculated by fitting a mono-exponential curve to the recovery data.
Small Muscle Mass Exercise test 2: 60% of Peak Workload with Evaluation of O2 Consumption Determinants
Following 15-20 minutes of rest, participants performed a second plantarflexion test on the opposite (right) leg for 4 minutes at a constant workload corresponding to 60% of peak workload achieved on the first test. Within <1 second of exercise completion, lower leg blood flow and venous O2 saturation (SvO2) were measured in an image slice perpendicular to the superficial femoral vein just superior to the knee to measure blood flow, and together with hemoglobin (from complete blood count) and arterial oxygen saturation (from pulse oximetry), were used to calculate lower leg O2 delivery, consumption, and extraction.12,13
Large Muscle Mass Exercise Tests: Pulmonary VO2 Peak and Leg Muscular Strength
At pre-chemotherapy, end-chemotherapy, and 1 year (typically within 1-2 weeks of the MRI scans) all patients performed an incremental to-maximum workload cardiopulmonary exercise test (Encore229 Vmax; SensorMedics, Yorba Linda, USA) on a cycle ergometer (Ergoselect II 1200 Ergoline, Germany). Pulmonary VO2peak was measured as the highest 20-second average volume of O2 uptake. Further test details are available in the Supplementary material. Leg muscular strength was assessed as the leg press 1-repetition maximum (1-RM) estimated using the maximal weight lifted for 7-10 repetitions.14 Cycling and leg press require substantially greater muscle mass, and thus greater contribution from the heart for delivery of O2 relative to the plantarflexion tests. The controls completed the cardiopulmonary exercise test but not the leg press test.
Sample Size
We received ethics approval to begin enrolling participants into the sub-study after the 46th of 80 planned participants in the parent study. We performed a post hoc sensitivity analysis to estimate the detectable effect sizes for a repeated measures within factor design using the acquired sample size of 34 participants and acquired correlations among repeated measures for several variables. This sample size provided 95% power to detect effect sizes (Cohen’s f) ranging from 0.04 to 0.21, where 0.1 is considered small and 0.25 is considered medium.
Analyses
We compared all measures between the controls and the patients with breast cancer at pre-chemotherapy with independent t-tests to help isolate whether the changes over time in the patients were related to cancer treatment or the cancer/pre-diagnosis risk factors. To evaluate changes over time in the patients for each variable, time was used as a repeated and fixed factor in a generalized linear mixed model (GLMM). We included the intervention groups from the parent study as both a fixed and interaction (with time) effect in all GLMMs to account for potential effects of group assignment. In the case of a non-significant group × time interaction effect, the main effect of time was interpreted using pairwise contrasts between pre-chemotherapy and each later time point, and mid-chemotherapy and each later time point with significant differences interpreted with P ≤ 0.05. We also evaluated a potential influence of treatment type (anthracycline versus trastuzumab treatment protocols) on the changes over time by performing a second GLMM for each variable with intervention group replaced with treatment type. In all GLMMs, we included participant as a random effect to account for correlation over time and chose the distribution and link function that provided normally distributed residuals (determined by QQ plots) and/or provided the best model fit (determined by Akaike information criterion) for each variable. We performed Pearson correlations between changes in metrics of lower leg muscle metabolism or O2 consumption and its determinants with concurrent changes in exercise performance metrics (ie, cycle ergometer pulmonary VO2peak, leg press 1-RM, plantarflexion peak power) or myosteatosis. Change scores were calculated for mid-chemotherapy minus pre-chemotherapy and end-chemotherapy minus pre-chemotherapy. Analyses were performed using SPSS Version 26 with P ≤ .05 considered significant.
Results
Patients
Thirty-four patients were enrolled into this sub-study with 14 randomly assigned to the multi-disciplinary care intervention group and 20 to the usual care group; 24 received anthracyclines (epirubicin, combined with fluorouracil and cyclophosphamide, followed by docetaxel); 10 received trastuzumab combined with docetaxel and carboplatin. No relevant interaction effects were present for the parent study’s intervention group or for treatment protocol; thus all presented results are for the main effect of time. Sixteen controls were evaluated. All patients and controls were female; diagnosis and treatment and resting clinical data are shown in Table 1.
Table 1.
Descriptive and resting variables.
| Controls (n = 16) | Patients (n = 34) | ||||
|---|---|---|---|---|---|
| Age (years), mean ± SD | 56 ± 10 | 51 ± 10 | |||
| Breast cancer stage, n (%) | n/a | ||||
| I | 4 (12%) | ||||
| II | 25 (74%) | ||||
| III | 5 (15%) | ||||
| Chemotherapy regimen | n/a | ||||
| Anthracycline-containing, n (%) | 24 (71%) | ||||
| Anthracycline dose (mg/m2), median | 301 | ||||
| Trastuzumab-containing, n (%) | 9 (26%) | ||||
| Trastuzumab dose (mg/kg), median | 107 | ||||
| Radiation therapy, n (%) | n/a | ||||
| Left sided | 18 (53%) | ||||
| Dose (cGy), median | 4500 | ||||
| Right sided | 13 (38%) | ||||
| None | 3 (9%) | ||||
| Controls | Pre-chemotherapy | Mid-chemotherapy | End-chemotherapy | One year | |
|---|---|---|---|---|---|
| Mean ± SD | |||||
| Body mass (kg) | 74.9 ± 11.7 | 73.3 ± 16.6 | 74.1 ± 17.4 | 73.5 ± 15.9 | 73.9 ± 16.8 |
| Body mass index (kg/m2) | 27.9 ± 4.9 | 27.3 ± 5.4 | 27.6 ± 5.4 | 27.4 ± 5.1 | 27.6 ± 5.3 |
| Systolic blood pressure (mmHg) | 114 ± 11 | 112 ± 12 | 109 ± 10a | 109 ± 11a | 113 ± 17 |
| Diastolic blood pressure (mmHg) | 69 ± 6 | 69 ± 9 | 65 ± 8a | 66 ± 9a | 68 ± 13 |
| Arterial O2 saturation (%) | 98 ± 2 | 99 ± 1 | 99 ± 1 | 98 ± 2 | 98 ± 2 |
Different from pre-chemotherapy (P ≤ .05).
Patients with Breast Cancer vs Non-cancer Controls
Pre-chemotherapy age, BMI, blood pressure, hemoglobin, all muscle composition variables, and all lower leg O2 consumption and 31P spectroscopy variables did not differ in patients from controls (Tables 1-3), suggesting that changes over time in the patients were most likely attributable to the direct and indirect effects of cancer treatment rather than cancer itself or pre-diagnosis risk factors.
Table 3.
Skeletal muscle performance metrics.
| Spectroscopy variable | Control (n = 16) | Patients (n = 34) | |||
|---|---|---|---|---|---|
| Pre-chemotherapy | Mid-chemotherapy | End-chemotherapy | One year | ||
| Incremental maximal test plantar flexion test peak power output (W) | 15.7 (3.1) | 14.5 (3.5) | 14.7 (3.8) | 14.3 (3.8) | 14.6 (3.6) |
| Submaximal 4-minute steady-state plantarflexion test power output (W) | 10.0 (1.9) | 8.9 (2.0) | 9.1 (2.1) | 8.9 (2.3) | 9.2 (2.2) |
| Plantarflexion exercise efficiency (power output/VO2 reserve, W/mL/minute) | 0.25 (0.08) | 0.24 (0.07) | 0.22 (0.05) | 0.21 (0.07)ab | 0.23 (0.07) |
| Cycle ergometer pulmonary VO2peak (mL/kg/minute) | 29.5 (7.7) | 27.5 (7.1) | n/a | 25.1 (6.7)a | 26.8 (7.0) |
| Cycle ergometer pulmonary VO2peak (L/minute) | 2.13 (0.41) | 1.97 (0.49) | n/a | 1.82 (0.47)a | 1.94 (0.44) |
| Leg press 1-repetition maximum (kg) | n/a | 86.2 (38.4) | n/a | 77.7 (30.9)a | 86.0 (29.3) |
Different from pre-chemotherapy (P ≤ .05).
Different from mid-chemotherapy (P ≤ .05).
n/a = not collected.
Change in Lower Leg Skeletal Muscle Metabolism, O2 Consumption and its Determinants
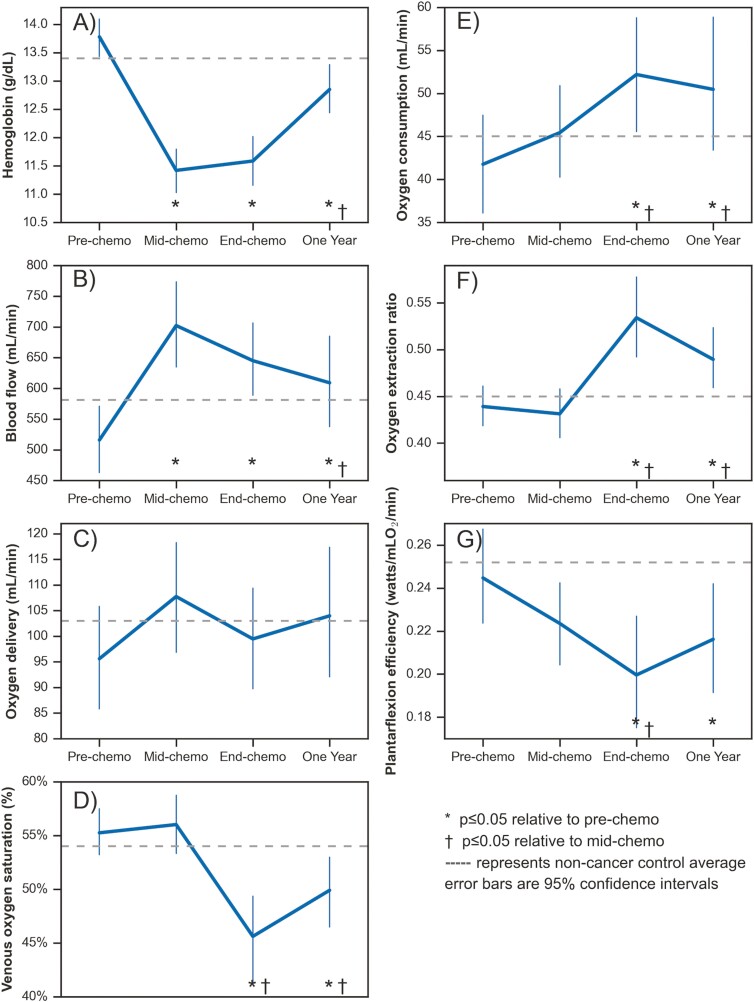
Resting arterial O2 saturation was normal (98%-99%) at all time points and did not change over time (Table 1). Hemoglobin (and hence arterial O2 content) decreased substantially by mid-chemotherapy, did not change further at end-chemotherapy, and remained lower than pre-chemotherapy at end-chemotherapy and 1 year; 1 year was higher than mid-chemotherapy (Fig. 1A). There were several corresponding changes in lower leg muscle O2 consumption and metabolism that compensated for the reduced hemoglobin (Fig. 1B-F). Specifically, lower leg exercise blood flow increased at mid-chemotherapy and remained elevated to 1 year resulting in no significant change in lower leg O2 delivery over time. At mid-chemotherapy, lower leg O2 consumption, O2 extraction, and SvO2, during exercise were not different from pre-chemotherapy. However, by end-chemotherapy, exercise SvO2 was lower, reflecting higher O2 extraction and O2 consumption relative to both pre- and mid-chemotherapy. In terms of persistent effects at 1 year, hemoglobin was increased from mid-chemotherapy, but remained lower than pre-chemotherapy with corresponding but inverse changes in lower leg exercise blood flow. At 1 year, exercise O2 extraction and consumption remained higher than pre-chemotherapy.
Figure 1.
Changes to lower leg oxygen delivery, extraction, consumption, and exercise efficiency with single leg plantarflexion exercise across chemotherapy treatment.
Table 2 shows lower leg 31P spectroscopy results. From pre to mid-chemotherapy, Pi:PCr increased at rest, 40% and 80% exercise intensity, and pH for the 80% intensity workload was reduced. Pi:PCr remained elevated relative to pre-chemotherapy at end-chemotherapy and one year for rest and 40% intensity workload. Peak pH and PCr recovery time constant did not change over time.
Table 2.
31P spectroscopy data for rest, low intensity (40% peak power), high intensity (80% peak power) and peak exercise, and PCr recovery.
| Spectroscopy variable | Control (n = 16) | Patients (n = 34) | |||
|---|---|---|---|---|---|
| Pre-chemotherapy | Mid-chemotherapy | End-chemotherapy | One year | ||
| Pi:PCr resting | 0.10 (0.02) | 0.11 (0.02) | 0.12 (0.03)a | 0.12 (0.02)a | 0.12 (0.02)a |
| Pi:PCr 40% intensity | 0.31 (0.08) | 0.29 (0.07) | 0.34 (0.08)a | 0.32 (0.07)a | 0.32 (0.06)ab |
| Pi:PCr 80% intensity | 0.61 (0.17) | 0.58 (0.22) | 0.62 (0.19)a | 0.56 (0.17) | 0.56 (0.22)b |
| Pi:PCr peak exercise | 0.84 (0.23) | 0.78 (0.29) | 0.84 (0.26) | 0.74 (0.22)‡ | 0.72 (0.28) |
| pH resting | 7.09 (0.11) | 7.07 (0.05) | 7.09 (0.07) | 7.07 (0.04) | 7.08 (0.04) |
| pH 40% intensity | 7.07 (0.08) | 7.08 (0.06) | 7.06 (0.07) | 7.09 (0.04) | 7.11 (0.06) |
| pH 80% intensity | 6.75 (0.3) | 6.86 (0.19) | 6.79 (0.22)a | 6.84 (0.20) | 6.90 (0.21)b |
| pH peak exercise | 6.62 (0.28) | 6.73 (0.21) | 6.67 (0.22) | 6.73 (0.29) | 6.77 (0.24) |
| PCr recovery time constant (s) | 33 (9) | 37 (13) | 40 (17) | 38 (11) | 33 (10)b |
Different from pre-chemotherapy (P ≤ .05).
Different from mid-chemotherapy (P ≤ .05).
Change in Skeletal Muscle Performance Metrics
Despite the multitude of changes in metabolism and O2 delivery and utilization in the lower leg skeletal muscle, there was no significant changes over time in plantarflexion peak power output (Table 3). However, because the lower leg O2 consumption increased at end-chemotherapy, there was a corresponding decrease in plantarflexion exercise efficiency (watts/O2 consumed, Table 3; Fig. 1G). Leg press 1-RM and cycle ergometer pulmonary VO2peak both decreased from pre to end-chemotherapy, then returned to pre-chemotherapy levels by 1 year (Table 3).
Associations with Skeletal Muscle Performance
We found evidence to suggest that changes to muscle metabolism, lower leg O2 consumption and its determinants could be underlying mechanisms in chemotherapy-related reductions in muscular performance. Regarding small muscle mass exercise, a reduction in peak plantarflexion power output during both the first half and full length of chemotherapy was associated with increased Pi:PCr at 40% intensity (r = −0.38, P = .04; r = −0.50, P = .004), decreased lower leg O2 delivery (r = .63, P = .0001; r = .58, P = .0005) and O2 consumption (r = .61, P = .0002; r = .55, P = .001).
In terms of large muscle mass exercise, a reduction in leg press 1-RM during chemotherapy (end-pre-chemotherapy) was associated with a concurrent worsening of markers of lower leg muscle metabolism (ie, slower PCr recovery, r = −0.41, P = .04; increased Pi:PCr at 80% intensity, r = −0.60, P = .001; increased Pi:PCr at 40% intensity, r = −0.39, P = .05), but not lower leg O2 consumption or O2 extraction. There was no association between change in pulmonary VO2peak (absolute or indexed to body mass) and changes in lower leg muscle metabolism or O2 consumption.
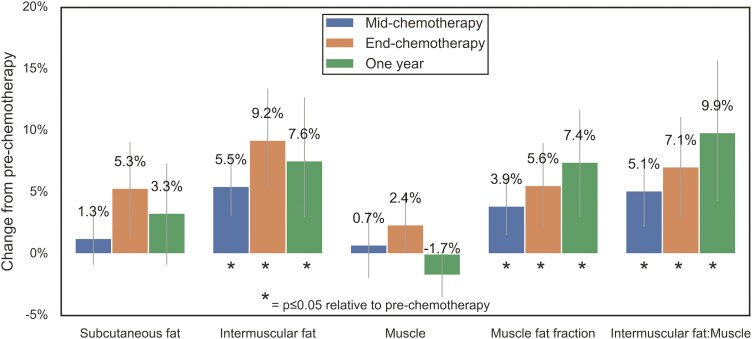
Change in Myosteatosis
Figure 2 shows the relative changes to muscle and fat compartments of the thigh as a percentage of pre-chemotherapy values at each time point. Muscle and subcutaneous fat volumes did not significantly change. Thigh intermuscular fat and the myosteatosis metrics all significantly increased by mid-chemotherapy and stayed elevated at end-chemotherapy and 1 year. The lower leg muscle and fat volumes did not change significantly (data not shown).
Figure 2.
Relative changes in thigh muscle and fat across chemotherapy treatment error bars are 95% confidence intervals.
Associations with Myosteatosis
We found associations between increased myosteatosis (muscle fat fraction) in the first half of chemotherapy with reduced lower leg O2 consumption (r = −0.36, P = .05), increased SvO2 (r = .37, P = .04), and O2 extraction (r = −0.37, P = .03). An increase in lower leg myosteatosis across the whole course of chemotherapy was associated with increased pH at 80% intensity and peak exercise (r = .43, P = .02; r = .44, P = .01). Change in cycle ergometer pulmonary VO2peak during chemotherapy was not related to change in thigh myosteatosis.
Discussion
To our knowledge, this study provides the most comprehensive longitudinal characterization of changes in both skeletal muscle composition (myosteatosis), real-time evaluation of exercise muscle metabolism, and O2 delivery and utilization during chemotherapy treatment. Among women receiving cardiotoxic chemotherapy treatment for early-stage breast cancer, we found increased thigh myosteatosis, pertubations in lower leg skeletal muscle energy metabolism, and adaptive changes in lower leg O2 delivery and extraction during plantarflexion exercise. No differences were present in any of these metrics between patients with breast cancer prior to chemotherapy and age- and BMI-matched non-cancer controls, suggesting that the direct and indirect (ie, lifestyle toxicity such as physical inactivity, stress, poor diet) effects of breast cancer treatment were likely primarily responsible for the noted changes over time.15 While receipt of a known cardiotoxic agent was a key component of our inclusion criteria, the noted changes could also be associated with the other chemotherapeutic agents and supportive therapies (eg, dexamethasone, granulocyte colony stimulating factor) received concurrently. The associations between changes in myosteatosis, lower leg muscle metabolism and O2 consumption with changes in physical performance measures during chemotherapy suggest that skeletal muscle toxicity has important functional consequences for patients. Further, our findings of skeletal muscle metabolic perturbations have implications as potential underlying mechanisms of patient-reported symptoms such as fatigue and reduced exercise capacity and deserve further study.
The well-known hemoglobin suppression effects of chemotherapy resulted in an −16% average decline by mid-chemotherapy, triggering several compensatory responses. At mid-chemotherapy relative to pre-chemotherapy, lower leg blood flow significantly increased by +37%, resulting in a +15% non-significant excess lower leg O2 delivery and a +12% non-significant increase in lower leg O2 consumption. Increased blood flow would be expected to reduce transit time in the capillaries and hence O2 extraction, which could perhaps explain the non-significant −2.4% decrease in lower leg O2 extraction, yet this small change should be interpreted cautiously. However, by end-chemotherapy, the adaptive mechanisms to perform the same plantarflexion workload with a sustained reduction in hemoglobin changed. Between mid- and end-chemotherapy, lower leg O2 extraction increased (+28%) and lower leg O2 delivery slightly decreased (−3%), suggesting that muscle O2 diffusive conductance (ie, transport of O2 from microvasculature to muscle mitochondria) also increased during this time.16 These significant changes resulted in a concomitant significant increase in O2 consumption (34% and 21% relative to pre- and mid-chemotherapy), but reduced exercise efficiency (ie, higher O2 consumption relative to workload, -16% relative to pre-chemotherapy). To our knowledge, this is the first study to suggest that changes in both convective O2 delivery and diffusive O2 transport throughout chemotherapy are important adaptive mechanisms to maintain or increase small muscle mass exercise O2 consumption and performance.
The lack of association between change in pulmonary VO2peak measured during cycling and lower leg muscle metabolism or O2 consumption during plantarflexion highlights an important distinction between compensatory patterns with relatively small muscle mass exercise (eg, lower leg plantarflexion) and large muscle mass exercise (eg, cycling, leg press). Similar to previous reports,17,18 we found that pulmonary VO2peak and leg press strength significantly decreased with receipt of chemotherapy, yet we found that plantarflexion power output was maintained. The hyperemic compensation for the chemotherapy-induced reduction in arterial O2 content that occurred with small muscle mass lower leg exercise would not be as substantial with large muscle mass exercise where the heart and vasculature play an important role in augmenting blood flow (ie, cardiac output). In fact, Howden et al showed that concurrent to a reduction in hemoglobin, exercise cardiac output was also reduced with cardiotoxic treatment,19 which would limit the reserve capacity for global blood flow to perform activities requiring large muscle mass such as running or cycling. Reduced global blood flow combined with reduced O2 carrying capacity of the blood would result in reduced O2 delivery and contribute to commonly observed reduction pulmonary VO2peak during chemotherapy. Our new data illustrates that physical activity types and intensities requiring lower amounts of muscle mass/O2 consumption may be a more tolerable alternative for patients experiencing chemotherapy-related hemoglobin suppression.19
Our 31P spectroscopy analysis provides further insight into chemotherapy-related adaptations in exercise metabolism. Pi:PCr at rest, 40% and 80% intensity workloads increased with chemotherapy, but without significant changes to the PCr recovery time constant. Increased Pi:PCr for the same workload reflects greater ADP accumulation which triggers greater PCr breakdown in compensation for inadequate ATP production. When compared over time for the same workload, increased Pi:PCr represents an uncoupling of mitochondrial ATP production and the stimulus of ADP accumulation to drive muscle metabolism.11 This uncoupling is further reflected by our finding of reduced exercise efficiency (watts/O2 consumed). Together these findings suggest a state of metabolic stress, which may relate to cancer-related fatigue, impairments in capacity to perform activities of daily living or exercise during and after chemotherapy.
Our finding of no change to the PCr recovery time constant offers insight into potential mechanisms of skeletal muscle metabolic perturbations. The PCr recovery time constant is a validated measure of general mitochondrial oxidative capacity but does not offer information about the types or patterns of substrates used by the mitochondrial respiratory chain.20 In animal models, chemotherapy downregulates the expression of genes regulating lipogenesis and proteins involved in fatty acid β-oxidation.21 Similar results of elevated exercise Pi:PCr but normal PCr recovery were reported among patients with a defect in the fatty acid oxidation pathway.22 It is possible that chemotherapy reduced fat oxidative capacity that was not detected by our postexercise PCr recovery analysis. The amount of ATP generated per unit of O2 consumption is 15% lower when oxidizing carbohydrate compared with fat.23 Thus, a chemotherapy-induced reduction in fat oxidation (and thus ATP production per unit O2) is in line with our finding of increased lower leg muscle exercise Pi:PCr and O2 consumption. Notably, at rest and low-intensity (40%) exercise, where fatty acid oxidation is the primary fuel source,24 Pi:PCr remained elevated until 1 year, suggesting persistent impairment in fat oxidative metabolism. Evans et al also found evidence of impaired whole-body utilization of fat as an exercise substrate post-chemotherapy among breast cancer survivors.25 Survivors had an elevated respiratory exchange ratio (preferential carbohydrate fuel use) during moderate-intensity cycling exercise compared with non-cancer controls.25
Our study results and prior research provide further evidence for upstream mechanisms and downstream effects of chemotherapy-related suppression of fat oxidation. A downstream effect of suppression of fatty acid oxidation is increased lipid storage in muscle,26 which predisposes individuals to weight gain,27 another common and persistent side effect of chemotherapy for breast cancer.28 To our knowledge, we are the first to report that thigh myosteatosis significantly increased within the first half of chemotherapy (~9 weeks) and stayed consistently elevated to 1 year. Several upstream effects of chemotherapy could be responsible. The excess lipid content in muscle cells that precedes intermuscular fat deposition is related to an imbalance between the rate of fatty acid uptake by the cells and the rate of mitochondrial fat oxidation,29 which can be suppressed by excess insulin levels.30 Dieli-Conwright et al recently reported that chemotherapy treatment resulted in an average 73% increase in fasting insulin and an 108% increase in insulin resistance among patients with early-stage breast cancer.31 Mitochondrial dysfunction is also linked to ectopic lipid accumulation and insulin resistance in skeletal muscle among aging populations.32,33 The anthracycline-related decrease in citrate synthase reported by Mijwel et al,5 suggests reduced mitochondrial activity and dysfunction. In addition to its known association with insulin resistance, our prior cross-sectional work suggested that myosteatosis may impede O2 extraction within that muscle during exercise. The current study’s longitudinal findings support this as there was a significant association between an increase in myosteatosis and reduced O2 consumption and extraction in the lower leg within the first half of chemotherapy.
Future Directions and Limitations
Future studies are needed to confirm these changes in skeletal muscle mitochondrial fatty acid oxidation during chemotherapy via myocyte biopsy in humans. Another potential area of future research to address these findings may include use of nutritional strategies shown to increase fat oxidation,34 such as intermittent fasting, performing exercise fasted, ingestion of fat prior to exercise, or following a high-fat (≥60% of calories from fat) low-carbohydrate diet during chemotherapy treatment.
A limitation of this study is small sample size that limited our ability to evaluate the effect of a multi-disciplinary team care intervention from the parent study. However, all analyses were adjusted for the influence of group assignment. Likewise, this sub-study was not powered to detect small potential differences between treatment protocols.
Conclusion
This study demonstrated that anthracycline or trastuzumab-containing chemotherapy results in skeletal muscle energy metabolism and O2 extraction/utilization perturbations in response to chemotherapy-related reduced hemoglobin. Blood flow and O2 extraction increase to maintain O2 delivery and consumption and power output during small muscle mass exercise, but cannot fully compensate for exercise utilizing large muscle mass, as evidenced by reductions in pulmonary VO2peak and leg press 1-RM with chemotherapy. Together, our findings of increased thigh myosteatosis, lower leg muscle resting and exercise Pi:PCr, and reduced plantarflexion exercise efficiency suggest chemotherapy-related suppression of fat oxidation rate. Future research is needed to identify potential defects in the fat oxidation pathway and to test nutritional and exercise strategies to upregulate fat oxidation during chemotherapy for breast cancer.
Supplementary Material
Contributor Information
Amy A Kirkham, University of Toronto, Toronto, Canada.
Edith Pituskin, University of Alberta, Alberta, Canada.
John R Mackey, University of Alberta, Alberta, Canada.
Justin G Grenier, University of Alberta, Alberta, Canada.
D Ian Paterson, University of Alberta, Alberta, Canada.
Mark J Haykowsky, University of Alberta, Alberta, Canada.
Richard B Thompson, University of Alberta, Alberta, Canada.
Funding
This work was supported by the University Hospital Foundation. During the study, A.K. was supported by Postdoctoral Fellowships from Susan G. Komen Foundation (PDF17483149) and the Canadian Institutes of Health Research. D.I.P. was supported by the Canadian Institutes of Health Research and/or the Heart and Stroke Foundation of Canada. M.H. is supported in part by a Faculty of Nursing Research Chair in Aging and Quality of Life at the University of Alberta.
Conflict of Interest
The authors indicated no financial relationships.
Author Contributions
Conception/design: A.A.K., E.P., J.G.G., D.I.P., M.J., R.B.T. Provision of study material/patients: J.R.M., D.I.P., R.B.T. Collection and/or assembly of data: A.K., J.G.G. Data analysis and interpretation: A.A.K., J.G.G., M.J.H., R.B.T. Manuscript writing: A.A.K., M.J.H., R.B.T. Final approval of manuscript: all authors.
Data Availability
The data underlying this article cannot be shared publicly due to research ethics restrictions. The data will be shared on reasonable request to the corresponding author.
References
- 1. Schmid D, Leitzmann MF.. Cardiorespiratory fitness as predictor of cancer mortality: a systematic review and meta-analysis. Ann Oncol. 2015;26:272-278. 10.1093/annonc/mdu250. [DOI] [PubMed] [Google Scholar]
- 2. Ross R, Blair SN, Arena R, et al. . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:1-47. [DOI] [PubMed] [Google Scholar]
- 3. Kirkham AA, Lloyd MG, Claydon VE, et al. . A longitudinal study of the association of clinical indices of cardiovascular autonomic function with breast cancer treatment and exercise training. Oncologist. 2019;24:273-284. 10.1634/theoncologist.2018-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chuy KL, Yu AF.. Cardiotoxicity of contemporary breast cancer treatments. Curr Treat Options Oncol. 2019;20:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mijwel S, Cardinale DA, Norrbom J, et al. . Exercise training during chemotherapy preserves skeletal muscle fiber area, capillarization, and mitochondrial content in patients with breast cancer. FASEB J. 2018;32:5495-5505. 10.1096/fj.201700968R. [DOI] [PubMed] [Google Scholar]
- 6. Beaudry RI, Kirkham AA, Thompson RB, et al. . Exercise intolerance in anthracycline-treated breast cancer survivors: the role of skeletal muscle bioenergetics, oxygenation, and composition. Oncologist. 2020;25:e852-e860. 10.1634/theoncologist.2019-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirkham AA, Haykowsky MJ, Beaudry RI, et al. . Cardiac and skeletal muscle predictors of impaired cardiorespiratory fitness post-anthracycline chemotherapy for breast cancer. Sci Rep. 2021;11:14005. 10.1038/s41598-021-93241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kirkham AA, Pituskin E, Thompson RB, et al. . Cardiac and cardiometabolic phenotyping of trastuzumab-mediated cardiotoxicity: a secondary analysis of the MANTICORE trial. Eur Heart J Cardiovasc Pharmacol. 2022;8:130-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pituskin E, Haykowsky M, McNeely M, et al. . Rationale and design of the multidisciplinary team IntervenTion in cArdio-oNcology study (TITAN). BMC Cancer. 2016;16:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hernando D, Kellman P, Haldar JP, et al. . Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn Reson Med. 2010;63:79-90. 10.1002/mrm.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chance B, Leigh JS, Clark BJ, et al. . Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Natl Acad Sci USA. 1985;82:8384-8388. 10.1073/pnas.82.24.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mathewson KW, Haykowsky MJ, Thompson RB.. Feasibility and reproducibility of measurement of whole muscle blood flow, oxygen extraction, and VO2 with dynamic exercise using MRI. Magn Reson Med. 2014;74:1640-1651. 10.1002/mrm.25564. [DOI] [PubMed] [Google Scholar]
- 13. Yang E, Kirkham AA, Grenier J, et al. . Measurement and correction of the bulk magnetic susceptibility effects of fat: application in venous oxygen saturation imaging. Magn Reson Med. 2018;68:863-814. [DOI] [PubMed] [Google Scholar]
- 14. Knutzen K, Brilla LR, Caine D.. Validity of 1RM prediction equations for older adults. J Strength Cond Res. 1999;13. [Google Scholar]
- 15. Kirkham AA, Beaudry RI, Paterson DI, et al. . Curing breast cancer and killing the heart: a novel model to explain elevated cardiovascular disease and mortality risk among women with early stage breast cancer. Prog Cardiovasc Dis. 2019;62:116-126. 10.1016/j.pcad.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 16. Poole DC, Richardson RS, Haykowsky MJ, et al. . Exercise limitations in heart failure with reduced and preserved ejection fraction. J Appl Physiol. 2018;124:208-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Courneya KS, Segal RJ, Mackey JR, et al. . Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396-4404. 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 18. Courneya K, McKenzie DC, Mackey JR, et al. . Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst. 2013;105:1821-1832. [DOI] [PubMed] [Google Scholar]
- 19. Howden EJ, Bigaran A, Beaudry R, et al. . Exercise as a diagnostic and therapeutic tool for the prevention of cardiovascular dysfunction in breast cancer patients. Eur J Prev Cardiol 2019;26:305-315. 10.1177/2047487318811181. [DOI] [PubMed] [Google Scholar]
- 20. Feyter HMD, Lenaers E, Houten SM, et al. . Increased intramyocellular lipid content but normal skeletal muscle mitochondrial oxidative capacity throughout the pathogenesis of type 2 diabetes. FASEB J. 2008;22:3947-3955. [DOI] [PubMed] [Google Scholar]
- 21. Ebadi M, Field CJ, Lehner R, et al. . Chemotherapy diminishes lipid storage capacity of adipose tissue in a preclinical model of colon cancer. Lipids Health Dis. 2017;16:247. 10.1186/s12944-017-0638-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diekman EF, Visser G, Schmitz JPJ, et al. . Altered energetics of exercise explain risk of rhabdomyolysis in very long-chain Acyl-CoA dehydrogenase deficiency. PLoS One. 2016;11:e0147818. 10.1371/journal.pone.0147818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salin K, Auer SK, Rey B, et al. . Variation in the link between oxygen consumption and ATP production, and its relevance for animal performance. Proc R Soc B Biological Sci 2015;282:20151028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baak MAV. Physical activity and energy balance. Public Health Nutr. 1999;2:335-339. [DOI] [PubMed] [Google Scholar]
- 25. Evans E, Hackney A, Pebole M, et al. . Adrenal hormone and metabolic biomarker responses to 30 min of intermittent cycling exercise in breast cancer survivors. Int J Sports Med. 2016;37:921-929. 10.1055/s-0042-110654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Houmard JA. Intramuscular lipid oxidation and obesity. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1111-R1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Consitt LA, Bell JA, Houmard JA.. Intramuscular lipid metabolism, insulin action, and obesity. IUBMB Life. 2009;61:47-55. 10.1002/iub.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Makari-Judson G. Weight gain following breast cancer diagnosis: Implication and proposed mechanisms. World J Clin Oncol. 2014;5:272-282. 10.5306/wjco.v5.i3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:1131-1141. 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- 30. Kelley DE. Skeletal muscle fat oxidation: timing and flexibility are everything. J Clin Invest. 2005;115:1699-1702. 10.1172/JCI25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dieli-Conwright CM, Wong L, Waliany S, et al. . An observational study to examine changes in metabolic syndrome components in patients with breast cancer receiving neoadjuvant or adjuvant chemotherapy. Cancer. 2016;122:2646-2653. 10.1002/cncr.30104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee H-Y, Choi CS, Birkenfeld AL, et al. . Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010;12:668-674. 10.1016/j.cmet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petersen KF, Befroy D, Dufour S, et al. . Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140-1142. 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Achten J, Jeukendrup AE.. Optimizing fat oxidation through exercise and diet. Nutrition. 2004;20:716-727. 10.1016/j.nut.2004.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to research ethics restrictions. The data will be shared on reasonable request to the corresponding author.