Abstract
Background
For patients with melanoma, gastrointestinal immune-related adverse events are common after receipt of anti-CTLA4 therapy. These present difficult decision points regarding whether to discontinue therapy. Detailing the situations in which colitis might predict for improved survival and how this is affected by discontinuation or resumption of therapy can help guide clinical decision-making.
Materials and Methods
Patients with stage IV melanoma receiving anti-CTLA4 therapy from 2008 to 2019 were analyzed. Immune-related colitis treated with ≥50 mg prednisone or equivalent daily or secondary immunosuppression was included. Moderate colitis was defined as receipt of oral glucocorticoids only; severe colitis was defined as requiring intravenous glucocorticoids or secondary immunosuppression. The primary outcome was overall survival (OS).
Results
In total, 171 patients received monotherapy, and 91 received dual checkpoint therapy. In the monotherapy group, 25 patients developed colitis and a nonsignificant trend toward improved OS was observed in this group. Notably, when colitis was categorized as none, moderate or severe, OS was significantly improved for moderate colitis only. This survival difference was not present after dual checkpoint therapy. There were no differences in known prognostic variables between groups, and on multivariable analysis neither completion of all ipilimumab cycles nor resumption of immunotherapy correlated with OS, while the development of moderate colitis did significantly affect OS.
Conclusion
This single-institution retrospective series suggests moderate colitis correlates with improved OS for patients with stage IV melanoma treated with single-agent anti-CTLA4, but not dual agent, and that this is true regardless of whether the immune-checkpoint blockade is permanently discontinued.
Keywords: melanoma, immune-related adverse event, immune-related colitis, anti, CTLA4
Gastrointestinal immune-related adverse events are common in patients with melanoma who have received anti-CTLA4 therapy. This presents difficult decisions regarding whether to discontinue therapy. This study focused on situations in which colitis might predict for improved survival and how continuation or termination of therapy can help guide clinical decision-making.
Implications for Practice.
Colitis is one of the most common immune-related adverse events (irAEs) following the administration of anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) therapy in patients with advanced melanoma. It is unclear, however, whether colitis development is associated with improved outcomes as are some other irAEs. Currently published observations are conflicting and have not yet detailed how combination ipilimumab/nivolumab therapy, which has increased rates of immune-related colitis, might affect this relationship. The details of a potential association with prognosis are essential to understand in order to help guide clinical decision-making when these situations arise.
Introduction
The development of immune checkpoint inhibitors (ICI) has revolutionized the management of many malignancies. However, the high rates of immune-related adverse events (irAEs) are a large source of morbidity for many patients, and these occurrences can present difficult decision points regarding whether to discontinue ICI. As these off-target effects are immune-mediated, the incidence and/or severity of these adverse events may correlate with the therapeutic effect on tumor. Indeed, many studies have shown that the incidence of some irAEs can predict the efficacy of ICI in certain malignancies.1,2 This relationship is seen more consistently with anti-programmed cell death protein 1 (PD1)/anti-programmed cell death ligand 1 (PDL1) therapy, while it is less clear after anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) therapy, such as ipilimumab.1,3-6 In addition, the effect of glucocorticoid and secondary immunosuppressive treatment for irAEs on survival remains controversial.4,7,8
Ipilimumab monotherapy and ipilimumab/nivolumab dual therapy are US FDA-approved treatments for patients with advanced melanoma. The most frequently cited irAEs after treatment with anti-CTLA4 in this population are gastrointestinal, dermatologic, and endocrine.3,5,8-10 Vitiligo and hypophysitis have been correlated with improved response to ICI, however, less is known about the predictive value of gastrointestinal irAEs and currently published observations are conflicting.7,11-17 One single-institution study suggested that the development of colitis was associated with improved survival with a higher grade of colitis predicting for better overall survival (OS).18 There was no apparent effect of secondary immunosuppressive treatment on survival in this study; however, the type of immunosuppression was not analyzed and the impact of dual checkpoint therapy versus single-agent ICI was not considered. Another single-institution study looked at patients requiring admission for colitis after checkpoint blockade and found decreased progression-free survival (PFS) in patients who were treated with intravenous high-dose glucocorticoids within 64 days of ICI administration compared to those who were not.19 This study inherently focused on only severe cases of colitis that required hospital admission and there was no comparison to patients that did not develop colitis. A prospective study by Lang et al used evidence of pancolitis on positron emission tomography-computed tomography (PET-CT) and found no correlation with response to ipilimumab.20 In this study, 50% of patients had evidence of “PET-colitis” which was more frequent than those with clinically significant diarrhea (29%), and historically more frequent than rates of colitis in this population.1 Only 7% of patients were treated with systemic steroids, suggesting the focus of this study was on patients with lower-grade colitis or diarrhea.
An important consideration for clinicians is whether ICI should be permanently discontinued after gastrointestinal irAEs. A post hoc, retrospective analysis of CheckMate 067 and 069 found that 24% of patients had treatment terminated early during the induction phase of dual checkpoint blockade due to irAE development with the most frequently cited irAE being gastrointestinal. Early termination of therapy did not affect disease outcomes in this study; however, patients in this group may have had lower overall risk with a lower proportion of M1c disease and lower lactate dehydrogenase (LDH) levels compared to those who did not have ICI terminated.21 A recent meta-analysis of immune-related diarrhea/colitis after ICI found that ICI was terminated permanently in about 50% of patients, and that in those who resumed, about 20% had a recurrence of symptoms, but oncologic outcomes were not analyzed.22 Given the paucity of prognostic information available to help guide decision-making regarding ICI termination and resumption after irAE development, we analyzed patients with stage IV melanoma treated consecutively at our institution with dual checkpoint blockade or single-agent anti-CTLA4 to determine the impact of both moderate and severe colitis on survival.
Materials and Methods
Data Source and Patient Cohort
Patients with AJCC 8th edition stage IV melanoma treated with anti-CTLA4 monotherapy (ipilimumab) or combination therapy (ipilimumab/nivolumab) between 2008 and 2019 at the University of Pennsylvania Abramson Cancer Center were included. Ipilimumab was administered at a starting dose of 3 mg/kg, including for those patients receiving it on trial before FDA approval, and adjusted as tolerated on an individual patient basis. Patients were excluded if they did not receive their first infusion of anti-CTLA4 ICI at University of Pennsylvania-associated facilities. Patients were excluded if they had a prior diagnosis of autoimmune colitis. Patients were excluded if follow-up time was shorter than 90 days from first infusion. All study data were stored using REDCap electronic data capture tools hosted at the University of Pennsylvania. This study was conducted after approval by the Institutional Review Board of the University of Pennsylvania.
Identification of Patients with ICI-associated Colitis
Patients were screened for ICI-associated colitis by 4 methods: (1) listed diagnosis of colitis using ICD-9 codes 555-559 or ICD10 codes K50-K52,23 (2) gastroenterology consult order, (3) receipt of high-dose glucocorticoids, defined as ≥50 mg oral prednisone total daily dose, equivalent dose of other glucocorticoids, or infliximab, (4) physician note free text search for “colitis” using PennSeek, a custom tool that searches free text in the electronic health record (EHR). All candidate cases of colitis were cross-checked by manual chart review to confirm cases were a presumed gastrointestinal irAE by the treating medical oncologist, were after treatment with ICI and occurred within 90 days of an infusion. Only colitis cases treated with high-dose oral glucocorticoids as defined above, intravenous glucocorticoids and infliximab were included. Therefore, all cases of colitis were treated as grade 2 or higher per Common Terminology Criteria for Adverse Events (CTCAE) 5.0, according to the recommended management by American Society of Clinical Oncology (ASCO) Clinical Practice Guideline (National Cancer Institute: CTCAE 5.024). The oral glucocorticoid cutoff of 50 mg of prednisone or higher was chosen given ASCO guidelines to initiate glucocorticoid treatment with 1 mg/kg daily. Due to the variability and subjectivity of recording of CTCAE toxicity grade in the EHR, we adopted an objective grouping system to differentiate between those who developed severe colitis and those who developed moderate colitis. The development of moderate colitis in our cohort was defined as receipt of oral glucocorticoids only, while the development of severe colitis was defined as having required intravenous therapy in the form of infliximab or glucocorticoids. Patients who received both monotherapy and dual therapy during their course were only analyzed within the dual therapy group and excluded from the monotherapy group (n = 11); if identified as having had developed colitis due to monotherapy, they were excluded from the dual therapy analysis as well so as not to confound the outcome data (n = 2).
Outcomes and Covariates
The primary outcome of this study was OS. Follow-up was defined as the date of first infusion of monotherapy or date of first infusion of dual therapy to the date of death or last known follow-up. Deaths were confirmed through the institutional tumor registry, EHR, or internet obituaries. Pre-specified covariates, including stage at initiation of ICI, ICI drug name, resumption of ICI after the development of colitis, and completion of four cycles of anti-CTLA4 ICI were ascertained from the University of Pennsylvania melanoma research program registry, the institutional tumor registry, and manual EHR review. Time to colitis development from first ICI infusion was analyzed as a time-dependent covariate.
Statistical Analyses
Kaplan-Meier curves were plotted to compare OS with the log-rank test to calculate statistical significance, with P < .05 as the threshold for significance. Log rank with Bonferroni correction was used to perform the pairwise comparison between two groups within a three-group comparison. Time to colitis development from first ICI infusion was treated as a time-dependent covariate to account for immortal time bias in a Cox proportional hazards model. Categorical variables in Table 1 were compared by Fisher’s exact and continuous variables using t test. The chi-square and Fisher’s exact tests were performed for severity of colitis with the pre-specified variables of clinical interest including resumption of ICI and completion of all four cycles of ipilimumab. Cox proportional hazards models were used to estimate the effect of completing all four cycles of ipilimumab and the resumption of ICI therapy after colitis on survival. All analyses were performed using R 3.6.1 (R Foundation), Stata version 12.0 (College Station, TX, USA), and SAS Version 9.4 (Cary, NC, USA).
Table 1.
Patient characteristics. General patient characteristics and risk factors within the final analytic populations.
| Patients receiving only ipilimumab monotherapy (n = 171) | Patients receiving ipilimumab-nivolumab dual therapy (n = 91) | P value | |
|---|---|---|---|
| n (%), median [range] | n (%), median [range] | ||
| Sex | .056 | ||
| Female | 66 (38.6) | 24 (26.4) | |
| Male | 105 (61.4) | 67 (73.6) | |
| Age at first infusion | 63 [23-85] | 58 [18-83] | .002 |
| Race | .019 | ||
| Black | 6 (3.5) | 0 (0) | |
| White | 152 (88.9) | 80 (87.9) | |
| Other/unknown | 13 (7.6) | 11 (12) | |
| Received prior anti-PD1/PDL1a | 19 (11) | 38 (41.8) | <.001 |
| ECOG PSb | .470 | ||
| 0/1 | 153 (89.5) | 84 (92.3) | |
| 2 | 11 (6.4) | 6 (6.6) | |
| 3 | 2 (1.2) | 1 (1.1) | |
| Unknown | 5 (2.9) | 0 (0) | |
| M stage | .110 | ||
| M1a | 14 (8.2) | 7 (7.7) | |
| M1b | 42 (24.6) | 12 (13.2) | |
| M1c | 69 (40.4) | 38 (41.8) | |
| M1d | 46 (26.9) | 34 (37.4) | |
| LDHc | .021 | ||
| Within normal limits | 70 (40.9) | 28 (30.8) | |
| Above upper limit of normal | 63 (36.8) | 28 (30.8) | |
| Unknown | 38 (22.2) | 35 (38.5) | |
| BRAF mutation | 67 (39.2) | 31 (34.1) | .562 |
Anti-programmed cell death protein 1/anti-programmed cell death ligand 1; bEastern Cooperative Oncology Group performance score; cLactate dehydrogenase.
Results
Patient Characteristics
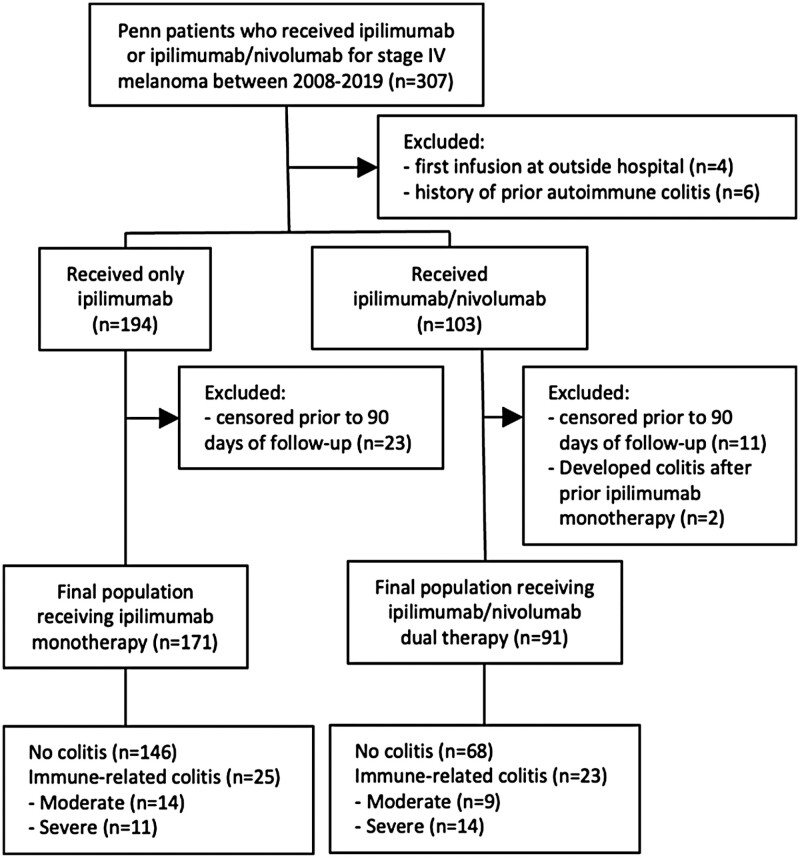
The final analytic population, as described in Methods and depicted in the CONSORT diagram, comprised 171 patients who received ipilimumab monotherapy and 91 patients who received ipilimumab/nivolumab dual therapy (Figure 1). Patient characteristics are included in Table 1. Of note, patients receiving dual therapy were more likely to have prior treatment with anti-PD1/PDL1 (P < .001) and be younger (P = .002). While LDH levels differed, there were more patients with known levels above the upper limit of normal in the monotherapy group. M stage, ECOG (Eastern Cooperative Oncology Group) performance status, and BRAF mutation status were not significantly different between groups.
Figure 1.
CONSORT diagram. Flow chart describing how the final analytic populations were reached.
Development of Colitis
The median time to the development of ICI-associated colitis from the first infusion of ICI among all patients was 49 days. The median time to colitis was 54 days after monotherapy and 35 days after dual therapy. Within the monotherapy group, 25 patients developed ICI-associated colitis (14.6%) within 90 days of any infusion (Table 2). Among these, 14 (56%) required oral glucocorticoid treatment (moderate colitis), and 11 (44%) required intravenous glucocorticoid and/or infliximab treatment (severe colitis). Seven patients (28% of all colitis patients in this group) required secondary immunosuppression with infliximab. Five patients resumed ipilimumab after developing colitis and three of these patients developed recurrent symptoms. Of note, all of the patients who developed recurrent symptoms had all initially developed moderate colitis. Five patients resumed anti-PD1/PDL1 therapy and one of these patients, initially with moderate colitis, developed recurrent colitis (Table 2). For those treated with dual therapy, 23 patients developed ICI-associated colitis (25.2%), of which 9 (39.1%) were moderate and 14 (60.9%) were severe. Eight patients (35% of all colitis patients in this group) required secondary immunosuppression with infliximab. Five patients resumed ipilimumab after developing colitis and four of these patients developed recurrent symptoms. Three out of four of these patients initially had developed severe colitis. Eleven patients resumed anti-PD1/PDL1 therapy and two of these patients developed recurrent colitis. One out of two of these patients initially had developed severe colitis (Table 2). ICI-associated colitis occurred more frequently in patients receiving ipilimumab/nivolumab (P = .044), but there was no significant difference in the severity of colitis between those receiving ipilimumab/nivolumab compared to ipilimumab alone (P = .265).
Table 2.
Incidence of colitis by single or dual agent therapy. Development of immune-related colitis in patients with stage IV melanoma after treatment with anti-CTLA4 therapy.
| Ipilimumab monotherapy | Ipilimumab/nivolumab dual therapy | Fisher’s exact test | |
|---|---|---|---|
| n/total (%) | n/total (%) | P value | |
| Developed colitis within 90 days of anti-CTLA4a | 25/171 (14.6) | 23/91 (25.2) | .044 |
| Moderate (oral steroids) | 14/25 (56.0) | 9/23 (39.1) | .265 |
| Severe (IVb steroids or infliximab) | 11/25 (44.0) | 14/23 (60.9) | |
| Developed recurrent colitis if resumed anti-CTLA4 | 3/5 (60.0) | 4/5 (80.0) | |
| Developed recurrent colitis if resumed anti-PDL1 | 1/5 (12.5) | 2/11 (18.2%) |
Anti-cytotoxic T-lymphocyte-associated protein 4; bIntravenous.
Survival in Patients Who Developed Immune-related Colitis
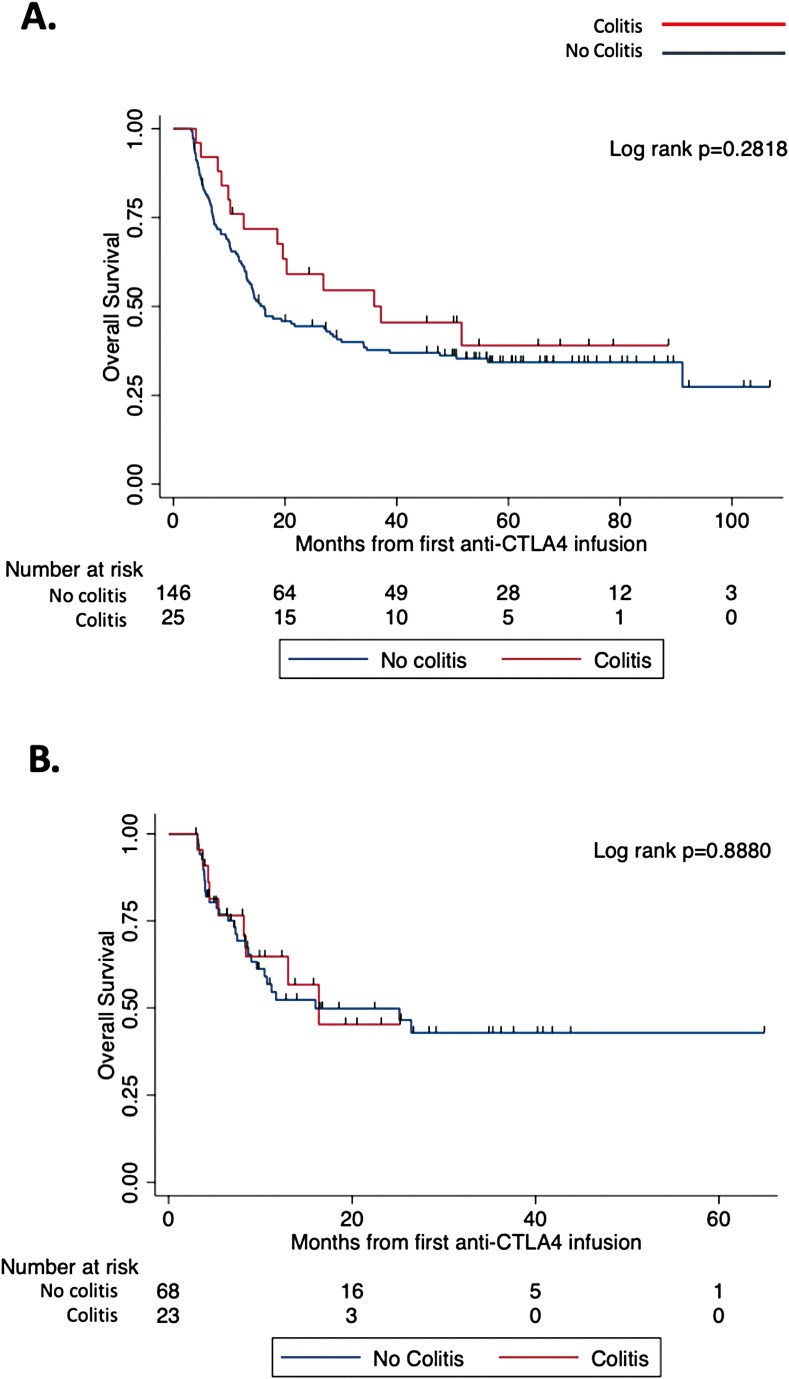
Among patients who received ipilimumab monotherapy, there was a nonsignificant increase in OS in patients who developed colitis; a median survival of 37.2 versus 16.3 months was found for those who developed colitis versus those who did not, respectively (Figure 2A, log-rank P = .282). Among patients receiving ipilimumab/nivolumab, there was no difference in OS between the two groups (Figure 2B, log-rank P = .888).
Figure 2.
Overall survival in patients receiving anti-CTLA4 by the development of colitis. Follow-up time was defined from first anti-CTLA4 infusion (dual or monotherapy) to last known follow-up or death. (A) A non-significantly trend in improved overall survival was observed in patients who developed colitis after monotherapy anti-CTLA4 therapy. Kaplan-Meier log-rank P = .2818; colitis n = 25, no colitis n = 146. (B) No difference in overall survival was observed in patients who developed colitis after dual therapy. Kaplan-Meier log-rank P = .8880; colitis n = 23, no colitis n = 68.
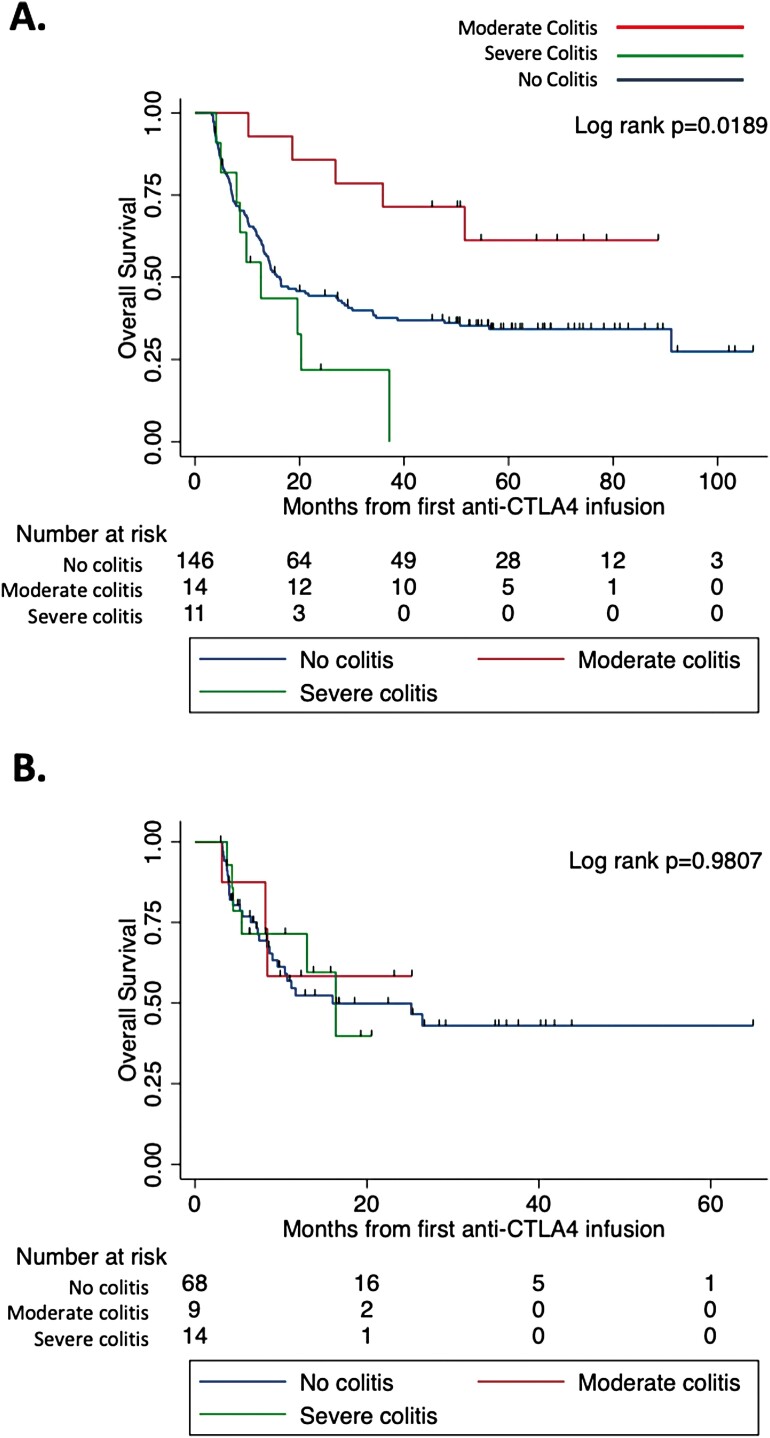
When the development of colitis was categorized as none, moderate or severe for those receiving ipilimumab monotherapy, there was a significant OS difference (Figure 3A, log-rank P = .019). The difference was driven by an increase in OS for those who developed moderate colitis (median survival not met) compared to those with severe colitis (median survival of 12.6 months) and those who never developed colitis (median survival of 16.3 months). Patients developing moderate colitis had OS times ranging from 10 to 88 months. Only two patients had OS less than 2 years and nine patients has OS greater than 4 years. When colitis was categorized into moderate or severe for those receiving combination therapy, there was no OS difference (Figure 3B, log-rank P = .981).
Figure 3.
Overall survival in patients receiving anti-CTLA4 by the development of moderate or severe colitis. Follow-up time was defined from first anti-CTLA4 infusion (dual or monotherapy) to last known follow-up or death. (A) A significantly improved overall survival was observed in patients who developed moderate colitis after monotherapy anti-CTLA4. Kaplan-Meier log-rank P = .0189. Log rank with Bonferroni correction for moderate versus severe colitis P = .0145 and moderate versus no colitis P = .2376; moderate colitis n = 14, severe colitis n = 11, no colitis n = 146. (B) No difference in overall survival was observed in patients who developed moderate or severe colitis after dual therapy. Kaplan-Meier log-rank P = .9807; moderate colitis n = 9, severe colitis n = 14, no colitis n = 68.
To confirm the above findings while accounting for immortal time bias, time to colitis development from first ICI infusion was treated as a time-dependent covariate in a Cox proportional hazards model with colitis severity. In the monotherapy group, moderate colitis was associated with a lower risk of death compared to all other groups (HR 0.367, P = .0288) and severe colitis trended toward a higher risk of death (HR 1.804, P = .0926) compared to all other groups. In the dual therapy group, neither moderate colitis nor severe colitis was significantly associated with OS when treated as a time-dependent covariate (HR 1.151, P = .815; HR 0.993, P = .9879).
Within the group receiving monotherapy, there were no significant differences in known prognostic indicators for this population, including age, ECOG performance status, M stage, LDH elevation, or BRAF mutation status between the three groups (Table 3). Among those who developed colitis, the median time to colitis diagnosis from first infusion of immunotherapy was 54 days. The proportion of those who developed early colitis (≤54 days) did not significantly differ between moderate colitis and severe colitis groups (35.7% vs 72.7%; P = .111, Table 3). There was also no difference in the development of multisystem irAEs (64.3% in moderate vs 45.5% in severe, P = .435) or in the timing of glucocorticoid initiation after colitis diagnosis. The median time to glucocorticoid initiation after colitis diagnosis was 0 days (initiated on the same day as diagnosis). 42.9% with moderate colitis and 63.6% with severe colitis initiated glucocorticoids on day 0 (P = .428). The mean total number of cycles of any ICI received was four cycles. There was no difference between those developing moderate or severe colitis in patients who had >4 cycles (42.9% vs 45.5%; P = 1.0, Table 3).
Table 3.
Patient characteristics by the development of colitis among those receiving ipilimumab monotherapy (n = 146). Comparison of patient risk factors among those who developed moderate or severe colitis and those who did not.
| n (%) | Moderate colitis (n = 14) | Severe colitis (n = 11) | P value | |
|---|---|---|---|---|
| Age at first infusion, years, median [range] | 64 [23-85] | 68 [40-84] | 62 [28-80] | .410 |
| ECOGa performance status | .257 | |||
| 0/1 | 132 (49.3) | 11 (79) | 10 (90.9) | |
| 2 | 9 (6.2) | 1 (7.1) | 1 (9.09) | |
| 3 | 1 (0.7) | 1 (7.1) | 0 (0) | |
| Unknown | 4 (2.7) | 1 (7.1) | 0 (0) | |
| M stage | .289 | |||
| M1a | 10 (6.8) | 3 (21.4) | 1 (9.09) | |
| M1b | 36 (24.7) | 5 (35.7) | 1 (9.09) | |
| M1c | 59 (40.4) | 4 (28.6) | 6 (54.4) | |
| M1d | 41 (28.0) | 2 (14.3) | 3 (27.3) | |
| LDHb | .881 | |||
| Normal | 59 (40.4) | 5 (35.7) | 6 (54.4) | |
| Above upper limit of normal | 55 (37.7) | 5 (35.7) | 3 (27.3) | |
| Unknown | 32 (21.9) | 5 (35.7) | 2 (18.18) | |
| BRAF mutation | 57 (39.0) | 6 (42.9) | 4 (36.36) | .954 |
| Completed all 4 cycles of anti-CTLA4c | N/A | 8 (57.1) | 3 (27.3) | .227 |
| Resumed ICI after colitis | N/A | 8 (57.1) | 4 (36.4) | .428 |
| Received >4 cycles of ICI | N/A | 6 (42.9) | 5 (45.5) | 1.000 |
| Colitis leading to early high-dose steroids (≤54 days) | N/A | 5 (35.7) | 8 (72.7) | .111 |
Eastern Cooperative Oncology Group performance score; bLactate dehydrogenase; cAnti-cytotoxic T-lymphocyte-associated protein 4.
We also looked at whether the observed difference in OS could be explained by differences in completion of all four cycles of ipilimumab or in whether any ICI was resumed after colitis and found no significant difference between moderate and severe colitis (Table 3); however, given that there was a trend toward significance for both of these factors we conducted a multivariable Cox proportional hazards analysis (MVA) with completion of all four cycles of ipilimumab, resumption of ICI after colitis and severity of colitis, and only colitis severity was shown to have a significant effect on OS (HR 7.58, P = .002). However, after the development of colitis on dual therapy, resumption of ICI did have a significant effect on OS on MVA (HR 0.22, P = .033), whereas completion of all four cycles of ipilimumab and severity of colitis did not.
Discussion
This single-institution retrospective analysis suggests that the development of moderate colitis, defined as requiring treatment with oral glucocorticoids at an equivalent of at least 50 mg prednisone daily, is correlated with improved OS for patients with stage IV melanoma treated with monotherapy anti-CTLA4. It is noteworthy that the development of severe colitis, defined as receipt of IV glucocorticoids and/or infliximab, was not similarly correlated with improved OS but instead trended toward inferior survival. It is also noteworthy that an improvement in OS as a function of colitis was not observed in patients treated with a combination of ipilimumab/nivolumab. Within the group treated with ipilimumab monotherapy, patient M stage, LDH, ECOG, and BRAF mutation status were similar between those who developed severe and moderate colitis, and the difference in prognosis between these groups was not explained by factors such as whether patients completed all cycles of ipilimumab or resumed ICI after the development of colitis. Taken together, these findings suggest that moderate colitis not requiring IV glucocorticoids is associated with improved survival after ipilimumab monotherapy for patients with advanced melanoma. Additionally, this study suggests that resumption of ICI, which is associated with a risk of recurrent colitis, may not be necessary for this population as permanent discontinuation did not seem to affect survival.
Our finding that patients with moderate, but not severe, colitis have improved survival may at first glance seem to contradict the recent analysis by Abu-Sbieh et al, who reported increasing grades of colitis correlated with improved survival.18 Some key differences between the two studies explain the apparent discrepancy: Abu-Sbieh et al studied any grade of diarrhea (including grade 1) and only a minority had grade 3 or 4 (severe) colitis, while our analysis focused exclusively on patients requiring at least a 50 mg equivalent of prednisone for the treatment of colitis (moderate/severe). Our analysis does not capture patients with “mild” colitis or diarrhea, and the Abu-Sbieh study had few patients with severe colitis. Second, while the Abu-Sbieh analysis combined all immunosuppressive therapy into one group, we separated colitis patients into those requiring oral steroids versus intravenous steroids/infliximab and found that it was those patients treated with oral steroids that drove the improvement in survival. As such, the two studies are complementary and suggest that there is a “sweet spot” of severity where moderate colitis not requiring IV glucocorticoids may be associated with improved survival.
In our analysis, the reason that moderate but not severe colitis was associated with improved survival is not readily apparent. After accounting for immortal time bias with a cox proportional hazards model using time to colitis as a time-dependent covariate, we still observed a significant improvement in OS among patients with moderate colitis. It is reasonable to consider the possibility that providers caring for patients with severe colitis were more hesitant to complete all four cycles of ipilimumab or resume any ICI afterward. In our cohort, however, these factors did not appear to affect survival. This finding is concordant with a retrospective study that reported no difference in PFS or objective response rates in patients with advanced melanoma who discontinued dual checkpoint blockade early due to irAEs.21 There was also no difference in time on treatment between patients developing moderate or severe colitis which was evaluated by looking at a total number of overall ICI cycles received. In addition, others have shown that multisystem irAEs have been associated with improved survival in non-small cell lung cancer, but we did not find a difference in patients developing multisystem irAEs between colitis groups.25
Another potential explanation for this observation is that treatment with early glucocorticoids or infliximab may downregulate the systemic immune response, negating the effect of a robust response to immunotherapy. Although not significant, more patients developing severe colitis were diagnosed and treated for colitis before the median time to development of 54 days compared to those with moderate colitis. While time to colitis development did not affect the association of moderate colitis with improved OS when added as a time-dependent covariate on multivariable cox regression, this is worth noting given the study by Hughes et al that reported decreased PFS among patients admitted for colitis who were treated with intravenous glucocorticoids within 64 days of ICI administration, as well as the study by Bai et al who reported decreased PFS among patients who developed irAEs (the majority of which were gastrointestinal) and were treated with high-dose glucocorticoids within 8 weeks of ICI initiation.19,26 It is also noteworthy that in the Bai et al publication, treatment with IV glucocorticoids specifically was strongly associated with worse PFS. While we cannot separate the effect of colitis severity from intensity or route of treatment for colitis in our study, in the context of the Bai et al publication, these findings may suggest that colitis treated with intravenous (IV) steroids and/or infliximab may abrogate an otherwise improved anti-tumor immune response that is observed in patients with colitis not requiring intravenous immunosuppression.
Lastly, it has also been suggested that time to glucocorticoid initiation after irAE diagnosis could impact severity of toxicity, and potentially therefore impact morbidity and survival.27 Most patients in the monotherapy group initiated steroids on the same day of colitis diagnosis. This did not significantly differ between moderate and severe colitis groups and so does not explain the difference in OS observed.
Another notable finding of this analysis is that the difference in survival as a function of colitis was observed in patients receiving single-agent ipilimumab but not dual checkpoint blockade. The reason for this remains unclear. One potential explanation is that this study was underpowered to detect a difference. In addition, 28% of the patients developing colitis after monotherapy and 35% of patients developing colitis after combination therapy in our cohort required secondary immunosuppression with infliximab which is within the large range reported in other published series (22%-93%) but also may reflect an important difference which allows observation of our findings in one cohort but not the other.19,28,29 It is also likely that patients receiving dual checkpoint blockade were at higher risk for disease progression and death given the increased frequency of patients having prior single-agent anti-PD-1 therapy and younger age; whether colitis is a biomarker for or somehow causative of an increased response to ICI, it is possible that the effect size is simply not large enough to observe a survival difference in a higher-risk population with this sample size. We found that resumption of ICI after developing colitis on dual checkpoint therapy significantly affected OS on MVA. While this subset of patients may truly benefit from resumption of ICI after colitis, an alternative explanation could be appropriate patient selection to continue therapy among those with a better performance status compared to hospice or best supportive care for others. Whether resumption of ICI truly benefits patients developing colitis on dual checkpoint therapy should be validated in larger studies.
With this in mind, reviewing the rate of recurrent colitis development among patients in this study informs the overall risk/benefit discussion of resuming ICI. In our cohort, high rates of recurrent colitis were seen after restarting ipilimumab (80% in the dual therapy group and 60% in the monotherapy group). Rates were lower after restarting anti-PD1/PDL1 therapy (18.2% in the dual therapy group and 12.5% in the monotherapy group). Therefore, this study suggests that in those developing moderate colitis after ICI, there is a high risk of recurrent colitis if restarting anti-CTLA4 and no observed benefit in resumption of ICI on survival. Understanding the prognostic value of moderate and severe colitis development after ICI as well as the risk of recurrent colitis after various agents are resumed can help guide decision-making for clinicians and patients.
Limitations of our study include the non-randomized, retrospective design at a single institution, which limits the generalizability of the findings. Type of immunosuppression in our study was used as an objective way to characterize the severity of colitis with retrospective chart review where documentation of grade was not always present. Therefore, it is impossible to separate the effect of colitis severity from type of immunosuppression in our study. We were also unable to adequately assess this relationship among patients receiving single-agent PD1 inhibition due to the lower incidence rate of colitis in this population and an insufficient sample size among our data to draw a meaningful conclusion. A larger, perhaps multi-institutional, cohort would be very useful for this purpose as this could additionally provide information for the many other groups of cancer patients receiving anti-PD1 therapy. In addition, we did not study low-grade colitis/diarrhea (ie, mild diarrhea not treated with glucocorticoids) due to the challenges of identifying nonspecific symptoms in the manual chart review. Vitiligo, specifically, has been consistently observed to be associated with improved OS; however, documentation of vitiligo was not consistent in the EHR, and because it does not require treatment there was no objective way to capture these patients reliably. In addition, vitiligo can take months to years to develop, which makes any association with survival particularly susceptible to survivorship bias in this population of advanced melanoma.30,31 A prospective study involving careful documentation of gastrointestinal symptoms and other irAEs after treatment with ICI would address these limitations. Including correlative stool samples collected longitudinally in such a prospective study would show changes in the microbiome associated with colitis and subsequent treatment with glucocorticoids, which could lead to the development of a mechanistic hypothesis for our clinical findings.
Conclusion
Given the high rates of irAEs experienced by patients on ICI, the dilemma of re-initiation of immunotherapy after recovery from colitis can be a challenging one. In our overall population, about one-sixth of patients developed colitis as a result of anti-CTLA4, with cases almost evenly split between moderate colitis and severe colitis. We found that the development of moderate colitis, defined as colitis requiring treatment with at least a 50 mg prednisone equivalent of oral glucocorticoids, was associated with improved survival in metastatic melanoma patients receiving monotherapy anti-CTLA4. Severe colitis requiring intravenous steroids or infliximab was not associated with improved survival and this relationship was also not observed for those who received dual checkpoint blockade, regardless of colitis severity. Whether all four cycles of ipilimumab were completed or any ICI was resumed afterward did not affect survival. These findings add to the growing body of literature demonstrating the correlation of irAEs with prognosis, and suggest that the development of moderate colitis after monotherapy is associated with improved survival regardless of whether ICI is permanently discontinued.
Contributor Information
Emily J Anstadt, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA, USA.
Brian Chu, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Nikhil Yegya-Raman, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA, USA.
Xiaoyan Han, Department of Biostatistics, Epidemiology, and Informatics, University of Pennsylvania, Philadelphia, PA, USA.
Abigail Doucette, Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA, USA.
Kendra Poirier, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA, USA.
Jahan J Mohiuddin, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA, USA.
Amit Maity, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA, USA.
Andrea Facciabene, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA, USA.
Ravi K Amaravadi, Division of Hematology and Oncology, Department of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Giorgos C Karakousis, Division of Endocrine and Oncologic Surgery, Department of Surgery, University of Pennsylvania, Philadelphia, PA, USA.
Justine V Cohen, Division of Hematology and Oncology, Department of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Tara C Mitchell, Division of Hematology and Oncology, Department of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Lynn M Schuchter, Division of Hematology and Oncology, Department of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
John N Lukens, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA, USA.
Funding
This work was supported in part by The Tara Miller Foundation.
Conflict of Interest
Amit Maity: Merck (RF); Ravi K. Amaravadi: Pinpoint Therapeutics (OI, IP—patents, royalties, or other intellectual property), Deciphera (H), Immunaccel, Sprint Bioscience (C/A), Novartis, Bristol-Myers Squibb (RF); Lynn M. Schuchter: GlaxoSmithKline, Merck, Bristol-Myers Squibb (RF); Tara C. Mitchell: Bristol-Myers Squibb, Merck, Oncosec (C/A), Merck, Bristol-Myers Squibb (Other—travel and accommodations), Merck, Incyte, Bristol-Myers Squibb, Roche (RF); Justine V. Cohen: Sanofi-Genzyme, Bristol-Myers Squibb (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Author Contributions
Conception/design: E.J.A., B.C., J.J.M., J.N.L. Provision of study material/patients: E.J.A., B.C., A.D., K.P., J.J.M., J.N.L. Collection and/or assembly of data: E.J.A., B.C., N.Y.-R., A.D., K.P., J.J.M., J.N.L. Data analysis and interpretation: E.J.A., B.C., N.Y.-R., X.H., J.N.L. Manuscript writing: E.J.A., B.C., X.H., J.N.L. Final approval of manuscript: All authors.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Das S, Johnson DB.. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J ImmunoTher Cancer. 2019;7:306. 10.1186/s40425-019-0805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Owen DH, Wei L, Bertino EM, et al. Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer. Clin Lung Cancer. 2018;19:e893-e900. 10.1016/j.cllc.2018.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681-6688. 10.1158/1078-0432.ccr-07-0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mian I, Yang M, Zhao H, et al. Immune-related adverse events and survival in elderly patients with melanoma treated with ipilimumab. J Clin Oncol. 2016;34:3047. 10.1200/jco.2016.34.15_suppl.304727432924 [DOI] [Google Scholar]
- 5. Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968-8977. 10.1200/jco.2005.01.109 [DOI] [PubMed] [Google Scholar]
- 6. Weber JS, O’Day S, Urba W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950-5956. 10.1200/jco.2008.16.1927 [DOI] [PubMed] [Google Scholar]
- 7. Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018;124:3706-3714. 10.1002/cncr.31629 [DOI] [PubMed] [Google Scholar]
- 8. Horvat TZ, Adel NG, Dang T-O, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33:3193-3198. 10.1200/jco.2015.60.8448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043-6053. 10.1200/jco.2005.06.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283-2289. 10.1200/jco.2005.04.5716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51. 10.1001/jamadermatol.2015.2707 [DOI] [PubMed] [Google Scholar]
- 13. Indini A, Di Guardo L, Cimminiello C, et al. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol. 2019;145:511-521. 10.1007/s00432-018-2819-x [DOI] [PubMed] [Google Scholar]
- 14. Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122. 10.1111/1346-8138.13520 [DOI] [PubMed] [Google Scholar]
- 15. Quach HT, Dewan AK, Davis EJ, et al. Association of anti-programmed cell death 1 cutaneous toxic effects with outcomes in patients with advanced melanoma. JAMA Oncol. 2019;5:906-908. 10.1001/jamaoncol.2019.0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206-1212. 10.1001/jamadermatol.2015.1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teulings H-E, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773-781. 10.1200/JCO.2014.57.4756 [DOI] [PubMed] [Google Scholar]
- 18. Abu-Sbeih H, Ali FS, Qiao W, et al. Immune checkpoint inhibitor-induced colitis as a predictor of survival in metastatic melanoma. Cancer Immunol Immunother. 2019;68:553-561. 10.1007/s00262-019-02303-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hughes MS, Zheng H, Zubiri L, et al. Colitis after checkpoint blockade: a retrospective cohort study of melanoma patients requiring admission for symptom control. Cancer Med. 2019;8:4986-4999. 10.1002/cam4.2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lang N, Dick J, Slynko A, et al. Clinical significance of signs of autoimmune colitis in 18F-fluorodeoxyglucose positron emission tomography-computed tomography of 100 stage-iv melanoma patients. Immunotherapy 2019;11:667-676. 10.2217/imt-2018-0146 [DOI] [PubMed] [Google Scholar]
- 21. Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol. 2017;35:3807-3814. 10.1200/jco.2017.73.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tran AN, Wang M, Hundt M, et al. Immune checkpoint inhibitor-associated diarrhea and colitis: a systematic review and meta-analysis of observational studies. J Immunother. 2021;44:325-334. 10.1097/CJI.0000000000000383 [DOI] [PubMed] [Google Scholar]
- 23. Nashed A, Zhang S, Chiang C-W, et al. Comparative assessment of manual chart review and ICD claims data in evaluating immunotherapy-related adverse events. Cancer Immunol Immunother. 2021;70:2761-2769. 10.1007/s00262-021-02880-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714-1768. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shankar B, Zhang J, Naqash AR, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 2020;6:1952-1956. 10.1001/jamaoncol.2020.5012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bai X, Hu J, Betof Warner A, et al. Early use of high-dose glucocorticoid for the management of irAE is associated with poorer survival in patients with advanced melanoma treated with anti-PD-1 monotherapy. Clin Cancer Res. 2021;27:5993-6000. 10.1158/1078-0432.CCR-21-1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang L, Zlotoff DA, Awadalla M, et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation. 2020;141:2031-2034. 10.1161/CIRCULATIONAHA.119.044703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheung VTF, Gupta T, Olsson-Brown A, et al. Immune checkpoint inhibitor-related colitis assessment and prognosis: can IBD scoring point the way? Br J Cancer. 2020;123:207-215. 10.1038/s41416-020-0882-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nahar KJ, Rawson RV, Ahmed T, et al. Clinicopathological characteristics and management of colitis with anti-PD1 immunotherapy alone or in combination with ipilimumab. J ImmunoTher Cancer. 2020;8:e001488. 10.1136/jitc-2020-001488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan L, Hwang S, Byth K, et al. Survival and prognosis of individuals receiving programmed cell death 1 inhibitor with and without immunologic cutaneous adverse events. J Am Acad Dermatol. 2020;82:311-316. 10.1016/j.jaad.2019.06.035 [DOI] [PubMed] [Google Scholar]
- 31. Larsabal M, Marti A, Jacquemin C, et al. Vitiligo-like lesions occurring in patients receiving anti-programmed cell death-1 therapies are clinically and biologically distinct from vitiligo. J Am Acad Dermatol. 2017;76:863-870. 10.1016/j.jaad.2016.10.044 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.