Abstract
Purpose:
A systematic review of relevant studies that determined the dose response relationship (DRR) for the hematopoietic (H) ARS in the canine relative to radiation quality of mixed neutron:gamma radiations, dose rate, and exposure uniformity relative to selected reference radiation exposure has not been performed. The datasets for NHP exposure to mixed neutron:gamma radiation are utilized herein as a species comparative reference to the canine database.
Methods:
The selection of data cohorts was made from the following sources: Ovid Medline (1957-present), PubMed (1954-present), AGRICOLA (1976-present), Web of Science (1954-present and US HHS RePORT (2002 to present). The total number of hits across all search sites was 3077. Several referenced, unpublished, non-peer reviewed government reports were unavailable for review.
Results:
Primary published studies using canines, beagles and mongrels, were evaluated to provide an informative and consistent review of mixed neutron:gamma radiation effects to establish the DRRs for the H-ARS. Secondary and tertiary studies provided additional information on the hematologic response or the effects on hematopoietic progenitor cells, radiation dosimetry, absorbed dose and organ dose. The LD50/30 values varied with neutron quality, exposure aspect and mixed neutron:gamma ratio. The reference radiation quality varied from 250 kVp or 1 – 2 Mev x-radiation and Co-60 gamma radiation. A summary of a published review of a data set describing the DRR in rhesus macaques for mixed neutron:gamma radiation exposure in the H-ARS is included for a comparative reference to the canine dataset.
Conclusions:
The available evidence provided a reliable and extensive data base that characterized the DRR for the H-ARS in canines and young rhesus macaques exposed to mixed neutron:gamma radiations of variable energy relative to 250 kVp, 1–2 Mev x-radiation and Co-60 gamma, uniform and non-uniform total-body irradiation without the benefit of medical management. The mixed neutron:gamma radiation showed an energy-dependent RBE of ~ 1.0 to 2.0 relative to reference radiation exposure within both species. A marginal database described the DRR for the GI-ARS. Medical management showed benefit in both species relative to the mixed neutron:gamma as well as exposure to reference radiation. The DRR for the H-ARS was characterized by steep slopes and relative LD50/30 values that reflected the radiation quality, exposure aspect and dose rate over a range in time from 1956–2012.
Keywords: Radiation damage, neutrons, x-rays, gamma rays, canine, nonhuman primate
INTRODUCTION
The hematopoietic acute radiation syndrome (H-ARS) consequent to mixed neutron:gamma radiation has been described in large animal models over the last six decades. Reference radiation exposures included 250 kVp x- and 1–2 Mev x-radiation and Co-60 gamma radiation. Experimental, large animal species, included swine, mini-pigs, goats, sheep, canines and nonhuman primates (Tullis et al. 1954; George et al. 1968; Wise and Turbyfill 1968; Wise and Turbyfill 1970; Earle et al. 1971; Edmondson and Batchelor 1971; Jones et al. 1972; Ainsworth et al. 1984; Wang et al. 1991; MacVittie et al. 2015b). The variables relative to the experimental data sets were numerous, to include a) animal age, size, health, species and strain, b) radiation quality, dose, dose rate and exposure aspect/uniformity, c) study duration and d) consistency and extent of medical management. The primary endpoints were focused on determining the dose response relationship (DRR) for the H ARS and the relative biological effectiveness (RBE) of mixed neutron:gamma radiations against reference radiation exposures, e.g., 250 kVp x-, 1 Mev x-, 2 Mev x-radiation, and Co-60 gamma radiation. A marginal database showed evidence of radiation-induced gastrointestinal (GI) ARS following mixed neutron:gamma exposure and the projected, marked RBE, to reference radiation exposure (Conard 1956; Conard et al. 1956; Alpen and Baum 1959; Ainsworth et al. 1965; Wang et al. 1991; Yu et al. 2011; MacVittie et al. 2015b; MacVittie et al. 2019b; MacVittie and Jackson III 2020). The predominant database describing the occurrence of the GI ARS consequent to mixed neutron:gamma exposure was found in the canine models. Unfortunately, there was no information on the incidence of the delayed effects of acute radiation exposure (DEARE) characterized by multiple organ injury (MOI) to the lung, kidney, heart and prolonged injury to the GI system in either species, the canine or NHP relative to mixed neutron:gamma exposure. Consequently, there is a lack of validated biomarkers to predict clinical outcome after acute radiation exposure. This initial review of the mixed neutron:gamma radiation-induced effects in the canine and NHP, rhesus macaque, was summarized relative to the current knowledge base and identification of the “gaps in knowledge”. Note that recent systematic reviews characterized the DRR for NHP exposed to mixed neutron:gamma radiation and reference radiations relative to the H ARS and GI ARS (MacVittie et al. 2015b; MacVittie et al. 2019b). Alpen provided a summary-type review of the historical evidence of large animal studies exposed to neutron and mixed neutron:gamma radiation from 1950 through 1965 (Alpen 1991).
Radiobiology.
The radiobiology database is consistent relative to the variables noted above and their effect on induction of the H and GI ARS sub-syndromes. Respective dose response relationships, LD50/30 and LD50/6–7 values (respective authors used d6 or d7 survival times for GI-ARS in the canine) or LD50/15 values for NHP, survival times, RBE values and organ pathology were reported. Data was available for steady-state, moderate dose rate reactor-derived neutron:gamma exposure vs pulse exposure in canines, to include the response of prompt, nuclear weapon exposure to NHP. The database emphasized the consequences of uniform vs non-uniform exposure, dose rate and dose distribution-dependent radiobiology.
Medical management.
Subject-based, moderate medical management can mitigate the effects of acute radiation-induced H and GI ARS. It has increased survival time and survival. The benefit of medical management, while dose- and time-dependent, was independent of radiation quality (Furth et al. 1953; Bagdasarov et al. 1959; Sorensen et al. 1960; Perman et al. 1962; Taketa 1962; Byron et al. 1964; Broerse et al. 1978; MacVittie et al. 1991; Farese et al. 2012; Thrall et al. 2015; Yu et al. 2015).
Partial-body shielding (marrow-sparing).
A general assumption is that the radiation exposure consequent to a nuclear terrorist or accidental event will be ill-defined consequent to body position (exposure aspect), random shielding, differential dose rate, distance from the source and radiation intensity in the early, potentially lethal fallout field. These factors predict a non-uniform and heterogeneous exposure to victims. Additionally, the interval between exposure and treatment will be less than optimal. However, the circumstances described above, e.g., that exposure is non-uniform, predicts a differential dose distribution over the body, thus predicting a more favorable and optimistic outcome of survival due to the consequent sparing of a critical volume of BM, GI-tissue and that of other organs (Bond et al. 1957; Hansen et al. 1961; Maillie et al. 1966; Bond and Robinson 1967; Cole et al. 1967; Wingate et al. 1967; Monroy et al. 1988; Terry and Travis 1989; Bond et al. 1991; Bertho et al. 2005; MacVittie et al. 2012b; Farese et al. 2019). Thus, given time, spontaneous recovery can occur especially in the context of medical management which provided an extended time component and more efficient use of MCM (Furth et al. 1953; Bagdasarov et al. 1959; Sorensen et al. 1960; Byron et al. 1964; Gafter-Gvili et al. 2005; MacVittie et al. 2012a; MacVittie et al. 2015a; Yu et al. 2015; Farese et al. 2019).
Radiation dosimetry; differential tissue depth dose, organ-specific dose.
The radiation dosimetry is well described for respective studies although many authors refer to other, more focused reports for thorough descriptions. Several studies provided evidence of significant differential depth dose due to unilateral exposure. There are no studies that provided retrospective analysis to determine dose delivered to selected organ volume (Bond et al. 1957; Bond and Robinson 1967; Wingate et al. 1967; Bond et al. 1991; MacVittie et al. 1991; Wang et al. 1991; Yu et al. 2011; Prado et al. 2015; Prado et al. 2017).
Relative biologic effect (RBE).
RBE values for the neutron, high linear energy transfer (LET) radiation relative to low LET radiation exposure as well as mixed high and low LET radiation are dependent on defined endpoints and exposure conditions. The database in canines and NHP provided values for the H ARS relative to radiation quality, dose rate and exposure geometry. There is a marginal data set that clearly supported an RBE for GI ARS. Yet, there are no definitive studies using mixed neutron:gamma radiation that supported a clear DRR for mortality vs dose for the GI-ARS in either the NHP with a single exception in the canine (MacVittie and Jackson III 2020). Several variables must be considered in the current context of animal model development, these are noted below. The historical database for the NHP and canine using a predominant component of fission neutrons (range < 1 Mev to 9 Mev) provided an RBE in the range of 0.9 to 2.0 relative to the respective reference radiation quality (Furth et al. 1953; Bond et al. 1956; Alpen et al. 1960; Ainsworth et al. 1965; George et al. 1968; Wise and Turbyfill 1968; Earle et al. 1971; Broerse et al. 1978; MacVittie et al. 1984; MacVittie et al. 1991; Wang et al. 1991; MacVittie et al. 2015b; MacVittie and Jackson III 2020).
Knowledge gaps.
There are clear gaps in knowledge relative to the in vivo effects of acute mixed neutron:gamma radiation in preclinical models of the ARS and delayed effects of acute radiation exposure (DEARE). Several of these are: a) a clear representation of the effective neutron energy range in the prompt exposure from an improvised nuclear device, b) a retrospective analysis of dose distribution is lacking in assessment of organ-specific dose after unilateral prompt or pulse-rate exposure relative to standard, uniform TBI, c) assessment of a sex differential in radiation sensitivity, d) definition of delayed MOI, in the lung, kidney, heart and prolonged GI injury, relative to dose, neutron energy and exposure aspect, e) the RBE for mixed neutron:gamma radiation for the GI ARS and delayed MOI, f) established biomarkers for MOI consequent to unilateral, non-uniform, pulse-rate exposure and TBI, uniform exposure and g) lack of contemporary, well-characterized and validated large animal models.
Radiation effect scenario.
If prompt exposure is relevant to the nuclear radiation effect scenario, then, additional research (acute and delayed effects, MOI, biodosimetry, biomarkers, organ dose, MCM efficacy) is required relative to non-uniform, unilateral or partial-body exposure consequent to pulsed, mixed neutron:gamma dose rate and ratio of mixed neutron:gamma and neutron energy (Kramer et al. 2016).
Future studies should define selected relevant radiation exposure scenarios and develop models capable of defining the DRR for the H and GI ARS, as well as delayed MOI characteristic of the DEARE, especially in the context of medical management relevant to the post exposure scenario. Strategic and tactical questions that focus on assessing the link between acute and delayed MOI, should be investigated.
METHODS
Review Question
What is the DRR for mortality across the H ARS for canines and nonhuman primate (NHP), rhesus macaques, Macaca mulatta exposed to mixed neutron:gamma TBI relative to reference x-radiation and Co-60 gamma TBI without the benefit of medical management? The question focused on performing a systematic review of studies published in the open literature and those studies published in institutional or government reports that established the lethal DRR for the H ARS in normal beagle or mongrel canines and rhesus macaques not provided medical management after TBI with either reactor-derived mixed neutron:gamma radiation, nuclear weapon (mixed neutron:gamma radiation), Co-60 gamma-, or x-radiation. TBI was delivered in uniform, bilateral or rotational exposure or from a total-body unilateral, non-uniform steady-state or pulse exposure. The dose was assessed in roentgen (r), rad or cGy. To define the DRR in terms of approximate prescribed dose delivered to the tissue, the dose in r was converted to rad using a conversion factor of 0.96 unless a specific conversion factor was provided within the publication. The dose rates for mixed neutron:gamma or reference radiations varied from low to moderate range, 3 r min−1 to ~110 rad min−1 to pulse exposure (50msec) from a TRIGA nuclear reactor or prompt exposure from detonated nuclear weapons.
Eligibility study search criteria.
The authors assessed eligibility of the studies recovered from the published literature and published data (non-peer reviewed) in institutional or government report formats (Fig. 1).
Fig. 1.
Systematic review. The method for systematic review of published studies to determine the dose response relationship (DRR) for the lethal H-ARS in young Rhesus macaques and canines exposed to TBI conducted without medical management. Published studies included those in the open, peer-reviewed literature and published government reports. A total of 3,077 hits were obtained via the search criteria. Studies accepted by the reviewers were divided into primary and secondary cohorts. The primary studies included those that determined the full DRR for mortality due to the H-ARS; secondary studies provided added values for dose-related mortality (equivalent to the primary studies in all criteria: radiation quality, absence of medical management, rhesus macaques, canines, etc.) but did not conduct a complete DRR for the H-ARS.
Animals: Healthy Canine Beagle and mongrel dogs and Rhesus macaque, subspecies Macaca mulatta. Male and or female, young, quarantined and adherent to veterinary standards at the time of the study.
Radiation exposure: TBI, bilateral or rotational homogeneous uniform exposure or non-uniform unilateral exposure. Dose rate: variable rates from low dose rate [r, rad minute−1 (min)] to pulse-rate exposure. Exposure rates were dependent upon the various sources in the exposure facilities. Conversion from r to rad in tissue used a 0.96 conversion factor.
Radiation source: X ray machines, reactor-derived mixed neutron:gamma radiation, Co-60 gamma radiation or nuclear weapon-derived mixed neutron:gamma radiation.
Primary studies: Those focused on the assessment of the complete DRR for mortality/morbidity over the H ARS dose range.
Radiation Dosimetry: selected studies provided adequate dosimetry to determine neutron exposure, “r or rad” dose exposure in air, absorbed dose or to midline tissue of the animal.
Statistical analysis of the data provided probit analyses and estimation of slope, y-intercept and LD50/30 for the respective lethal DRR for the H ARS.
Search Strategy
The authors searched several databases for selection of data cohorts for published nonhuman primate and canine datasets including Ovid Medline (1956-present), PubMed (1954-present), AGRICOLA (1976-present), Web of Science (1954-present) and US HHS RePORT (2002-present). The following keyword terms were used: Canine, dog, rhesus, beagles, total-body irradiation, total-body x-irradiation, TBI, irradiation, mixed neutron:gamma radiation, gamma radiation, neutron, hematopoiesis, LD50/30, Macaca mulatta, whole-body irradiation, nonhuman primate, NHP, monkey, primates, hematopoietic radiation syndrome, mortality, and nuclear radiation. The reference lists of all recovered studies published in the open literature and published in institutional or government reports, were reviewed for additional studies. The total number of hits across all search sites was 3,077. Several referenced, unpublished, non-peer reviewed government reports were unavailable for review and were therefore not included in the review herein.
Search Results.
Canines: A total of 21 studies were evaluated; nine primary studies of both beagle and mongrels; three secondary and nine tertiary studies were evaluated. Nonhuman primate: Twenty-two total studies were evaluated, 11 primary, four secondary and seven additional studies were evaluated to provide an informative and consistent review.
Characteristics of Data Abstraction and Initial Search Results.
Radiation source, quality and exposure parameters in rhesus macaques.
Mixed gamma:neutron radiation
Mixed gamma:neutron radiation was delivered by three methods of variable energy, dose rate and exposure geometry.
TRIGA Reactor-derived mixed gamma:neutron radiation (60:40) delivered at steady state, rotational exposure with dose rate of 20 rad min−1 (Stanley et al. 1966; George et al. 1968).
TRIGA Reactor mixed gamma:neutron radiation exposure delivered unilaterally by a single (50 msec) pulse exposure with class b non-uniformity (Turbyfill et al. 1968; Wise and Turbyfill 1968).
Low Flux Reactor (LFR) of the Netherlands Energy Research Foundation ECN at Petten. It was a thermal research reactor of Argonaut type that could be operated at 10 kW (Broerse et al. 1978).
TRIGA reactor exposure geometry for steady state or pulse rate irradiation.
The steady state, rotational exposure to 250 kVp x-radiation used a dose rate of 20 rad min−1. The TRIGA-derived mixed gamma:neutron exposure used a dose rate of 16 rad min−1. The mechanical arrangements for animal restraint and rotation were the same as for x-irradiation. Depth dose measurements indicated that all exposures were considered uniform. The pulse rate, posterior to anterior exposure, was accomplished with each cohort of NHP positioned in an arc within the exposure room. The distribution of dose from nose to tail along the midline of the phantom varied less than 5% from the mean.
LFR reactor exposure.
The LFR reactor exposure design used a continuously rotating cylindrical cage that was mounted vertically in the exposure compartment. The exposures (total dose of neutron and gamma) were delivered at a mean dose rate of ~ 8 rad min−1. The exposure design and dosimetry are well described in the text.
Nuclear weapon detonation:
Two nuclear test weapon detonations at Mercury, NV (Operation Plumbbob); the mean energy of the “prompt” bomb-spectra gamma radiation closely approximated that of Co-60 gamma radiation (1.2 Mev). The calculated bomb-spectrum values for RBE were estimated to be 1.33 and 1.27 for the Wilson (10 kT) and Fiseau (11 kT) “shots” respectively (Zellmer and Pickering 1960). The exposure geometry is less defined for the nuclear weapon detonation. The “prompt” designation indicates the dose rate from the nuclear detonation.
X-radiation:
Sources delivered 250–300 kVp and 2 Mev quality x-rays. 1) 250 kVp therapeutic x-ray machines. All studies used 1.7–1.9–3.0 mm Cu HVL, 10–15 ma and dose rates varied from 3–23.5 r min−1 or 20–28 rad min−1 (Eldred and Trowbridge 1954; Schlumberger and Vazquez 1954; Haigh and Paterson 1956; Henschke and Morton 1957; Stanley et al. 1966; Broerse et al. 1978). 2) 2 Mev, accelerator-based x-radiation with a 7 mm Pb HVL and a dose rate of 10.7 rad min−1 (Dalrymple et al. 1965).
Co-60 gamma exposure:
Total-body, uniform Co-60 gamma radiation was delivered via a rotational exposure (3 rpm) in a Plexiglas cylinder at a dose rate of 54.6 rad min−1 (Eltringham 1967; MacVittie et al. 2015b).
The effect of radiation quality, dose rate and exposure parameters on the lethality dose response relationship (DRR) in rhesus macaques without medical management.
Search Results: NHP Summary Statement.
The authors chose a total of 4 of 11 studies in NHP models focused on assessing the H-ARS relative to exposure by mixed gamma:neutron radiation. These studies contributed to the systematic review of the DRR for the acute H ARS in NHP without medical management (Table 1). The seven additional studies were focused on using x-radiation and Co-60 gamma-radiation that served as reference radiation effects for the H-ARS DRR and estimation of RBE (Table 1). The seven additional primary studies of reference radiation were previously described by MacVittie et al., in a systematic review of the H-ARS in NHP (Table 1) (MacVittie et al. 2015b). Four additional secondary studies are included herein to provide a descriptive hematologic response and mortality to multiple or single doses of mixed neutron:gamma radiation in the NHP (Table 2) (Broerse et al. 1978; Broerse and Zoetelief 1984; Farese et al. 1993; Farese et al. 1994).
Table 1.
Characteristics of primary and secondary studies for nonhuman primate models included in the systematic review.
| Source/authors | Sample size | Radiation source | Exposure geometry | Dose rate |
|---|---|---|---|---|
| Stanley et al. 1966 | 80 | gamma:neutron | Rotational | 16 rads min−1 |
| Wise and Turbyfill 1986 | 66 | gamma:neutron | Unilateral pulse | < 50 msec |
| Turbyfill et al. 1968 | 45 | gamma:neutron | Unilateral pulse | < 50 msec |
| Zellmer and Pickering 1960 | 160 | gamma:neutron | Unilateral prompt | ------- |
| Eldred and Trowbridge 1954 | 37 | 250 kVp x-ray | Rotational | 13.7 r min−1 |
| Schlumberger and Vazquez 1954 | 92 | 250 kVp x-ray | Rotational | 23 r min−1 |
| Haigh and Paterson 1956 | 44 | 250 kVp x-ray | Rotational | 3 r min−1 |
| Henschke and Morton1957 | 110 | 250 kVp x-ray | Rotational | 22 r min−1 |
| Stanley, et al.1966 | 60 | 250 kVp x-ray | Rotational | 20 rads min−1 |
| Dalrymple et al. 1965 | 84 | 2 Mev x-ray | Rotational | 10.7 rads min−1 |
| Eltringham 1965 | 90 | Co-60 gamma | Rotational | 54.6 rads min−1 |
All studies for 250 kVp x-radiation, Co-60 gamma radiation, 2 Mev x-radiation and reactor- or nuclear weapon-derived mixed gamma/neutron radiation were published in the open literature or in government publications. The studies provided complete data sets for establishing the dose response relationship (DRR) for mortality versus radiation dose. Data published in abstracts was not included in the review. The radiation source and energy varied from 250 kVp x-radiation to 2 Mev x radiation, Co-60 gamma radiation by moderate or high dose rate and mixed gamma/neutron radiation from steady-state dose rate, pulse exposure and prompt exposure from nuclear weapon detonation.
Table 2.
Characteristics of secondary studies for nonhuman primates exposed to mixed neutron:gamma radiation included in the systematic review.
| Source/Authors | Sample size | Radiation Source | Exposure Geometry | Dose Rate |
|---|---|---|---|---|
| Broerse et al. 1978 | 15 | Mixed neutron:gamma; 300 kVp x-ray | Rotational | 28 rad min−1 8 rad min−1 |
| Broerse and Zoetelief 1984 | 15 | Mixed neutron:gamma; 300 kVp x-ray | Rotational | 28 rad min−1 8 rad min−1 |
| Farese et al. 1993 | 5 | Mixed neutron:gamma | Posterior anterior | pulse |
| Farese et al. 1994 | 5 | Mixed neutron:gamma | Posterior anterior | pulse |
All studies were published in the open literature or in government publications. Data published in abstracts was not included in the review. The secondary studies did not provide a complete dose response relationship (DRR) but did provide added value of multiple data points in support of the primary DRRs and dose range for high lethal, hematopoietic-acute radiation syndrome.
The primary studies assessing the DRR for total-body irradiation with mixed neutron:gamma radiations.
Stanley et al. (1996).
The authors provided a database to establish the DRR for reactor-derived mixed gamma:neutron radiations in rhesus macaques delivered via steady-state dose rate relative to 250 kVp x-rays (Stanley et al. 1966).
Animals.
Rhesus macaque (wild caught), n = 160 total, 2–5 y age and 3.1–5.5 kg, equal male/female, (all tuberculin-free tested 5 times, the country of origin was not stated); were used in a single study.
Radiation source.
The Armed Forces Radiobiology Research Institute (AFRRI) TRIGA Reactor delivered TBI to a mid-line tissue dose (MTLD). Approximately 75% of the neutron dose was attributed to fast neutrons (> 10 kev), the remaining 25% was of lower energies. The effective energy of the gamma radiation was between 1 and 2 Mev. Thirty control NHP were used for the mixed gamma:neutron exposure. The x-ray generator was 250 kVp, 30 ma with a HVL of 1.9 mm Cu.
Radiation exposure.
Rotational mixed gamma:neutron radiation, was delivered at a steady-state dose rate of 16 rads min−1 to midline tissue over a range of eight doses, n = 80, 304–567 rad, n = 10 each, of mixed gamma:neutron radiation (60:40). Rotational x-radiation, 250 kVp, was delivered to n = 60 NHP over a range of 380–665 rads at 20 rads min−1. Both exposures were Class A uniform. Twenty non-irradiated control NHP were used for the x-radiation exposure. All animals were rotated 180 degrees at half dose to achieve uniform exposure.
Results.
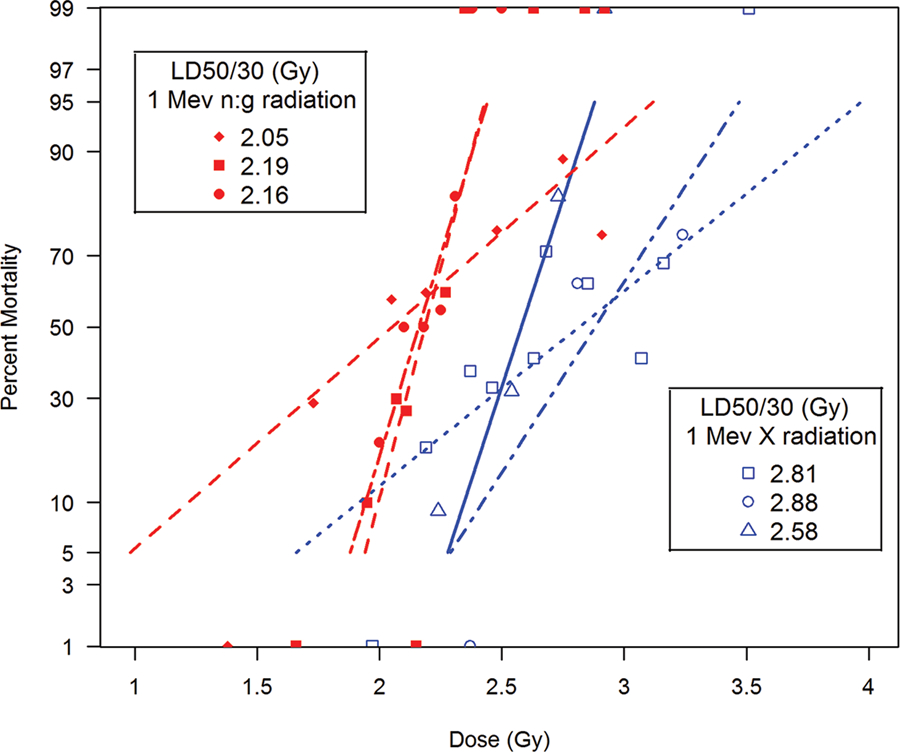
Total-body exposure with mixed gamma:neutron radiation yielded a median LD50/60 of 381 rads [365, 408]. Thirty-six of the 37 mortalities occurred within a range of 10–19 days, with an approximate mean survival time (MST) of 15.0 d for decedents. The LD50/60 estimated from the probit plot, provided herein was 385 rads [357, 413] (Fig. 2). The slope of the DRR was estimated to be 1.316 [0.719, 1.913]. The morbidity and mortality characteristics suggested a higher relative biological effect (RBE) for LD50/30 values of 1.31 for mixed gamma:neutron fission radiation for the H-ARS DRR relative to that noted for the 250 kVp x-radiation studies.
Fig. 2.
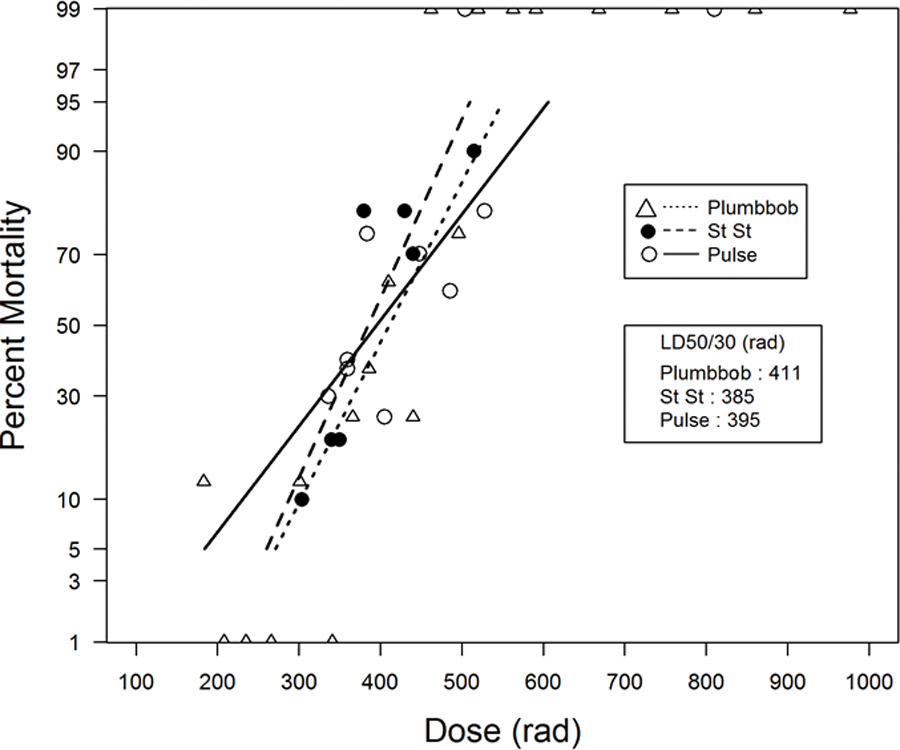
The DRR for nonhuman primates exposed to reactor-derived mixed gamma/neutron radiation and nuclear weapon-derived mixed gamma/neutron radiation. The dose response relationships (DRR) are shown for five primary studies of total-body irradiation (TBI, bilateral, unilateral) of rhesus macaques (MacVittie et al. 2015b). AFRRI TRIGA reactor-derived studies, n = 3 studies, (n = 191 total) with a.) mixed γ/n radiation delivered at steady state (20 rad min−1, n = 80) and b.) two combined studies that used pulse-mode exposure (< 50 msec) and performed sequentially at the same site and TRIGA reactor (n = 111), anterior to posterior exposure (Stanley et al. 1966; Wise and Turbyfill 1968). The DRR for two primary studies of mixed gamma/neutron TBI of rhesus macaques exposed at a “prompt” dose rate from two nuclear weapon detonations, “Operation Plumbbob”. Two detonations were conducted, the “Wilson shot” and the “Fiseau shot” at the nuclear test site (n = 160 total animals). The DRR is dose (rem, rads) vs mortality.
The median LD50/30 for TBI with 250 kVp x irradiation was 503 rads [448, 546]. Within the 35 mortalities from x-radiation, 31 occurred in the 10 to 19d interval. The LD50/30 values from historical studies ranged from 472 to 518 rads (Eldred and Trowbridge 1954; Schlumberger and Vazquez 1954; Haigh and Paterson 1956; Henschke and Morton 1957; Stanley et al. 1966).
The additional historical LD50/30 database for TBI with 2Mev x-radiation or Co-60 γ radiation was 670 rad and 644 rad, respectively (Dalrymple et al. 1965; MacVittie et al. 2015b). The respective RBE values were 1.74 and 1.67. Furthermore, the MST for decedents exposed to the high-dose radiation range indicated a concurrent acute GI ARS and H ARS contributed to the morbidity and mortality. GI pathology was also noted at the higher doses.
Wise and Turbyfill (1968); Turbyfill et al. (1968).
Two sequential studies were designed to establish the DRR for the H ARS from mixed gamma:neutron exposure administered at a (50 msec) pulse rate, unilateral exposure.
Animals.
Wise and Turbyfill, used n = 66 (wild-caught, male or female, rhesus macaques, 2–4 y of age, 3.0–6.0 kg, all tuberculin-free) and Turbyfill et al., used n = 50 (note: n = 45 NHP were used for the DRR) wild-caught male or female rhesus macaque, 2–4 y of age and 2.2–5.3 kg (Turbyfill et al. 1968; Wise and Turbyfill 1968).
Radiation source.
Both studies used a TRIGA reactor in a non-uniform, unilateral, posterior to anterior (PA) direction, pulse-mode (< 50 msec at half height) (mixed gamma:neutron radiation of 60:40) exposure across seven, ± [NHP number (cohort)] determined to be 336 (10), 360 (10), 360 (8), 384 (8), 448 (10), 504 (10) and 528 rad (10) (expressed as kerma, free-in-air, at the center of the exposure volume) and five, MTLDs as 405 (8), 486 (10), 810 (10), 1,215 (9) and 1,540 rad (8), respectively. The ratio of entrance dose to exit dose was 1.5 and considered Class B, non-uniform. The gamma radiation had an effective energy between 1–2 Mev. Of the 40% neutron dose, approximately 10% was from neutrons of > 3 Mev, 10% between 1.5 and 3.0 Mev, 10% between 0.01 and 1.5 Mev, and 10% slower neutrons.
Results.
The Wise and Turbyfill study resulted in a probit analysis to determine the DRR. The radiation dose, mortality (%) and MST (d) for each dose cohort was 336 rad, 30%, 17.3 d; 360 rad, 40.5%, 16.4 d; 360 rad, 37%, 14.9 d; 384 rad, 75%, 13.7 d; 448 rad, 70%, 14.0 d; 594 rad, 100%, 12.2 d and 528 rad, 80%, 13.2 d. This protocol yielded an LD50/30 of 377 rad [315,414] (Wise and Turbyfill 1968). The slope of the DRR was estimated to be 0.899 [0.393, 1.404] (Fig. 2, Table 3). Animals succumbed over a range of 7.3–20.7 days with the MST of all decedents at 13.9 d. The high dose rate and unilateral exposure resulted in an LD50/60 value of 377 rad; comparable to that of the steady-state bi-lateral LD50/60 of 385 rad exposure at 16 rad min−1 conducted at the same facility (Table 3) (Stanley et al. 1966).
Table 3.
Comparative LD50/30 values for nonhuman primates (NHP), rhesus macaques.
| Rhesus | ||||
|---|---|---|---|---|
| Radiation Quality | Energy (n, x-ray, photon) | LD50/30 rads/cGy | Exposure geometry, TBI, PBI# | Source/Authors |
| Neutron:gamma steady state | <1Mev mixed neutron:gamma | 385 | rotational | Stanley et al. 1966 |
| Neutron:gamma pulse | <1Mev | 395 | unilateral | Wise and Turbyfill 1968 |
| Neutron:gamma steady state | 1 Mev/ n 3:1 | 260a | rotational | Broerse et al. 1978; Broerse and Zoetelief 1984 |
| Neutron:gamma prompt | --- | 393, 433 | unilateral | Zellmer and Pickering 1960 |
| X-ray | 250 kVp | 521 | rotational | b5 primary studies |
| X-ray | 300 kVp | 525 a | rotational | Broerse et al. 1978; Broerse and Zoetelief 1984 |
| Co-60 gamma | 1.2 Mev | 644 | Bilateral | Eltringham 1967; MacVittie et al. 2015 |
| X-rays | 2 Mev | 670 | bilateral | Dalrymple et al. 1965 |
| LINAC | 2 Mev | 754 a | bilateral | Farese et al. 2012 |
| LINAC | 2 Mev | 1,088 a, c | Bilateral # | MacVittie et al. 2012 |
Dose response relationships (DRR) were established for animals exposed to Co-60 gamma radiation, 250 kVp x- and 2 Mev x-irradiation, mixed neutron:gamma radiation and LINAC-derived photons to include literature values for LD50/30 or LD50/60, the exposure aspect and literature source.
supportive care
Eldred and Trowbridge 1954, Schlumberger and Vazquez 1954, Haigh and Paterson 1956, Henschke and Morton 1957, Stanley et al. 1966
partial-body irradiation
The Turbyfill study was focused on the dose range at the high-lethal end of the H ARS and precluded an accurate probit analysis over the H ARS (Turbyfill et al. 1968). Therefore, the doses for both studies were combined since all exposure parameters and animal care were equivalent at the same site. All NHP in each dose exposure cohort (number of NHP) were irradiated to deliver the respective exposure doses to a MTLD of 405 rad (8), 486 rad (10), 810 rad (10), 1,215 rad (9) and 1,540 rad (8). Five of the 50 total NHP succumbed during the exposure procedure and are not included in the study. It was stated that these animals likely succumbed to the “stress associated with being restrained” during the exposure. The authors indicated that the dose was too low for a CNS effect. Therefore only 45 NHP as noted above, are included in the DRR analysis. The respective mortality and MST within 30 d post TBI, was 25% (2/8), 405 rad, 16.8 d; 60% (6/10) 486 rad, 12.7 d; 100%, (10/10), 7.7 d, 810 rad; 100% (9/9), 6.7 d, 1,215 rad; 100% (8/8), 6.9 d, 1,540 rad. The average MST over all doses was 14.8 d. The lowest MST ranged from 6.9 d to 7.7 d and is indicative of the acute GI ARS at the respective high exposure range 810 rad to 1,540 rad. The average MST was 7.1 d (Fig. 2, Table 3).
Unfortunately, a unilateral exposure at steady-state mode was not conducted making it difficult to compare dose rate effects relative to radiation quality and confirm the suggested increase in RBE of high dose rate (pulse-mode) TBI. The lowest MSTs within the total dose range for the combined studies were 6.7–7.7d. These values suggested the concomitant involvement of the acute GI ARS (MacVittie et al. 2012b).
The DRR for combined data sets for the H ARS due to pulse exposure with mixed gamma:neutron radiation resulted in an LD50/30 and slope of 395 rad [337, 432] and 0.779 relative to that of 377 rad and 0.899 from the single data set from Wise and Turbyfill noted above (Table 3) (Stanley et al. 1966).
Zellmer and Pickering (1960).
The authors reported on a nuclear test series named “Operation Plumbbob” that consisted of two cohorts of NHP exposed to two nuclear weapon detonations, the “Wilson” and “Fiseau shots” (Zellmer and Pickering 1960). It is an interesting comparative data set for NHP exposed to “prompt”, non-uniform, mixed fission neutron:gamma radiations in the absence of medical management (MacVittie et al. 2015b).
Animals.
Rhesus macaques, male/female, n = 80, total n = 160; two nuclear weapon detonations, prompt exposure: Wilson Shot and Fiseau Shot.
Radiation exposure.
The blast and thermal-resistant containers (aluminum restraining cages) were placed at 1mile distance from ground zero. Exposure was non-uniform. The reference radiation exposure mortality was Co-60 gamma radiation at 800 rad min−1 as established by Allen et al. (Allen et al. 1960). LD50/30: Wilson ~393 rad, Fiseau ~433 rad. Each Shot had a different dose range with respective MST of 13.9d (Wilson) and 17.4d (Fiseau). RBE ~ 1.33 and 1.27 for Wilson and Fiseau shots.
Radiation Dosimetry.
neutron measurements were made using a fission foil system including gold foils for neutrons in the thermal energy range and sulfur foils for the high energy neutrons. The gamma-ray measurements were made using a United States Air Force chemical dosimeter. It was stated that the bomb-spectrum gamma-ray energy closely approximated that of Co-60 gamma radiation. Every other animal was monitored for gamma radiation dose and each animal was measured for neutron exposure dose. Note the exposure was likely total-body, unilateral and thus non-uniform at a high dose rate. The authors used exposure to Co-60 gamma radiation delivered isotopically, via a “spherical cage-like arrangement” at a dose rate of 800 r min−1 to evaluate the RBE for survival time and survival, relative to the bomb-spectrum neutron flux (Allen et al. 1960). Note: A probit, mortality vs linear dose analysis of the Allen et al data set provided an LD50/30 of 438 rad. This value was significantly lower that the established values of 644 and 671 rad for Co-60 gamma and 2 MeV x-radiation delivered bilateral at 54.6 and 10.7 rad min−1 respectively. However, the current analysis of the data set for the high dose rate Co-60 exposure did not consider the data set rigorous enough to support the value of 438 rad.
Results.
Dose response relationship for mortality:
The RBE estimated for the weapons neutron component relative to gamma radiation alone were estimated to be 1.33 ± 0.09 for the Wilson shot and 1.27 ± 0.33 for the Fiseau shot. The author’s estimated LD50/30 values determined by probit analysis were 473 rem (393 rad) and 522 rem (433 rad) respectively for the Wilson and Fiseau shots. The combined exposures resulted in an LD50/30 of 412 [358, 466] rad and 496 [432, 562] rem (Fig. 2, Table 4) (Zellmer and Pickering 1960).
Table 4.
Effect of nuclear weapon radiation in rhesus macaques.
| LD 50/30 [95% CI] |
Slope [95% CI] |
n | Dose rate | aMST (d) | Reference |
|---|---|---|---|---|---|
| 393 rad, 473 rem | ----- | 80 | Prompt | 13.9 | Zellmer and Pickering 1960 |
| 433 rad, 522 rem | ----- | 80 | Prompt | 13.9 | |
| Combined | |||||
| a412 rad [394, 441], 496 rem | 1.26 [0.85, 1.67] |
160 | Prompt | ----- | Zellmer and Pickering 1960 |
Nuclear weapon radiation (Operation Plumbbob), mixed gamma:neutron, total-body, unilateral exposure of rhesus macaques: LD50/30, slope, sample size, dose rate, and mean survival time (MST) of decedents. Literature values for LD50/30 [95%CI] determined by probit analysis of mortality versus linear dose, slope (probit fits of linear dose, rem, with [95% CI]), dose rate and MST (d) of decedents were compiled. Rhesus macaques in two cohorts were exposed to two nuclear weapons detonations under “Operation Plumbbob”, the “Wilson shot” and the “Fiseau shot”.
combined exposures, LD50 estimate from probit plot of both data sets
Mean survival time.
The estimated range for nine exposure doses in the Wilson Shot was 441 rem (366 rad) to 1,177 rem (977 rad) and for the Fiseau shot, the 10 exposure doses ranged from 221 rem (183 rad) to 678 rem (563 rad). The lower MST for the Wilson shot suggested that the higher doses resulted in acute GI ARS. Four doses, 805 rem (668 rad), 918 rem (758 rad), 1,036 rem (860 rad) and 1,177 rem (977 rad) (n = 8 NHP dose−1) resulted in respective MST for decedents of 11.0 d, 8.5 d, 8.4 d and 7.8 d. Whereas the lower doses of 441 rem (366 rad), 497 rem (410 rad), 559 rem (462 rad), 629 rem (520 rad) and 712 rem (591 rad) (n = 8 NHP dose−1) resulted in respective MST for decedents of 19.0 d, 16.9 d, 17.6 d, 14.6 d and 13.3 d to 19.0 d representative of the H ARS. The MST for all decedents in the Wilson shot was 13.9 d. The respective MST of decedents for the Fiseau shot ranged from 13.4 d to 26.3 d relative to the high dose of 678 rem to the lowest dose of 221 rem. The MST for all decedents in the Fiseau shot was 17.4 d. Our analysis of the combined Wilson and Fiseau data estimated the LD50/30 to be 412 rad [394, 441] and a slope of 1.26 [0.85, 1.67] (Fig 2, Table 4).
Comparative DRRs for nuclear weapon- and reactor-derived steady state and pulse mixed gamma:neutron radiation.
The DRRs for the probit fits of nuclear weapon- and reactor-derived steady state and pulse mixed gamma:neutron radiation have slopes that are not significantly different from each other (F, p = 0.51). There is also no difference in the probit fits or intercepts (F, p = 0.57) (Fig. 2). It follows that the LDs and LD50/30 values do not differ.
MacVittie et al. (2015).
A recent review of the published literature on the acute radiation-induced H ARS in rhesus macaques defined mortality response relationships relative to dose, dose rate, exposure geometry and use of medical management. NHP were exposed to x-radiation (250 kVp, 2 Mev), Co-60 gamma radiation, reactor derived mixed neutron:gamma radiation, steady state and pulse, and nuclear weapon detonation. DRRs were established for each exposure protocol (Fig. 3) (MacVittie et al. 2015b).
Fig. 3.
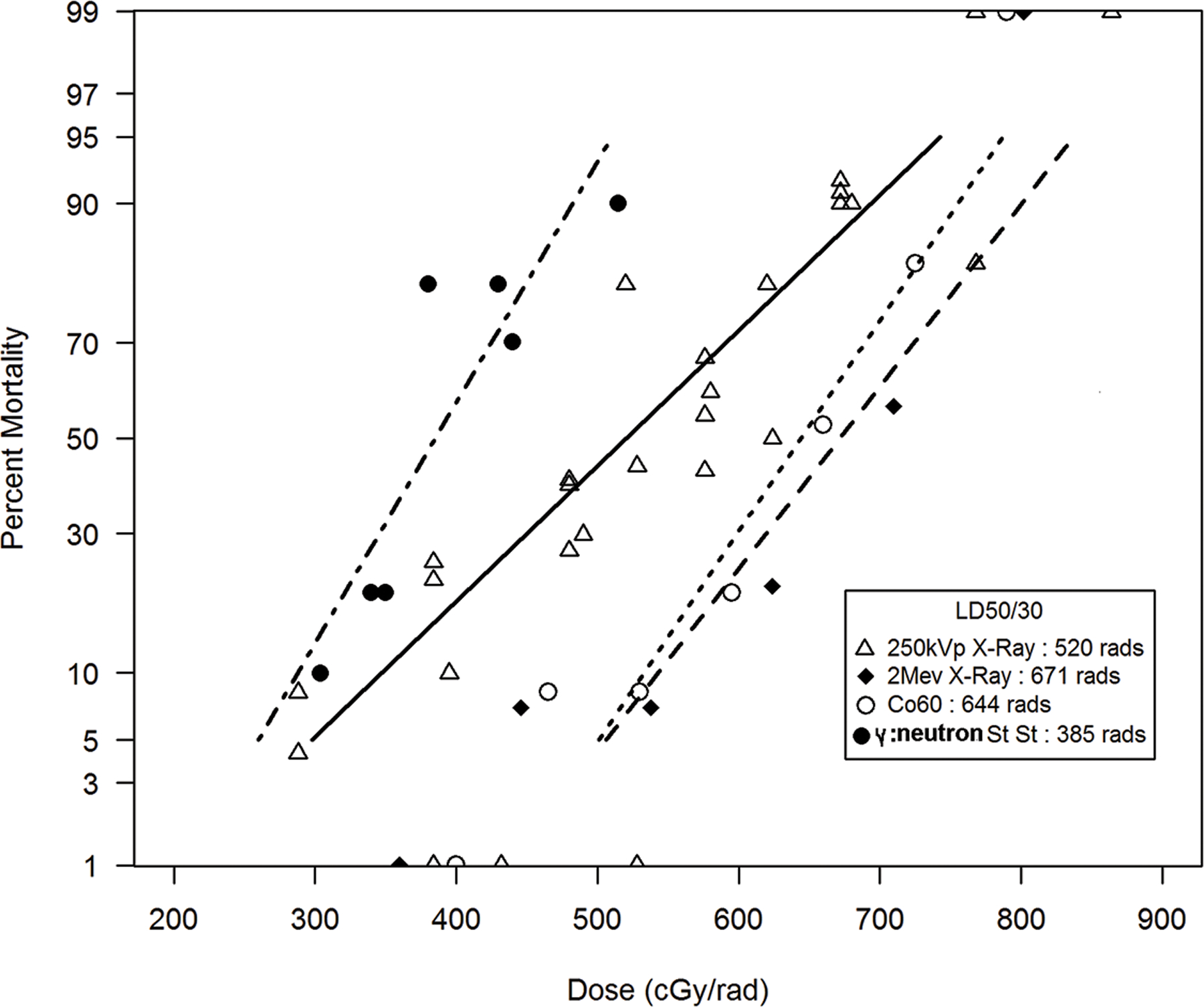
The comparative DRRs for nonhuman primates exposed to reactor-derived mixed gamma/neutron radiation at steady-state dose rate relative to reference radiation quality of 250 kVp, Co-60 gamma-radiation and 2 Mev x-radiation. The dose response relationships (DRR) for the: 1) primary 250 kVp x-radiation combined data set (n = 338) for dose in rads, dose rate range of 3–23 r min−1, 2) two primary studies of TBI of rhesus macaques (n = 174 total) with 2 Mev x-radiation, 10.7 rad min−1 (84), and Co-60 gamma-radiation, 54.6 rad min−1 (90) and a single primary studies of TBI with mixed gamma/neutron radiation delivered at steady state (20 rad min−1, n = 80) (MacVittie et al. 2015b). The database provided estimated RBE values for the LD50/30 NHP exposed to steady-state mixed gamma/neutron radiation relative to reference radiation exposure to 250 kVp, Co-60 γ-radiation and 2 Mev x-radiation of 1.35, 1.67 and 1.74, respectively.
Nonhuman Primates. The dose response relationships (DRR) and Kaplan-Meier analysis selected from the historical database for mixed neutron:gamma radiation:
Reactor-derived, steady state moderate dose rate, pulse rate or nuclear weapon-derived prompt rate bilateral or unilateral total-body exposure.
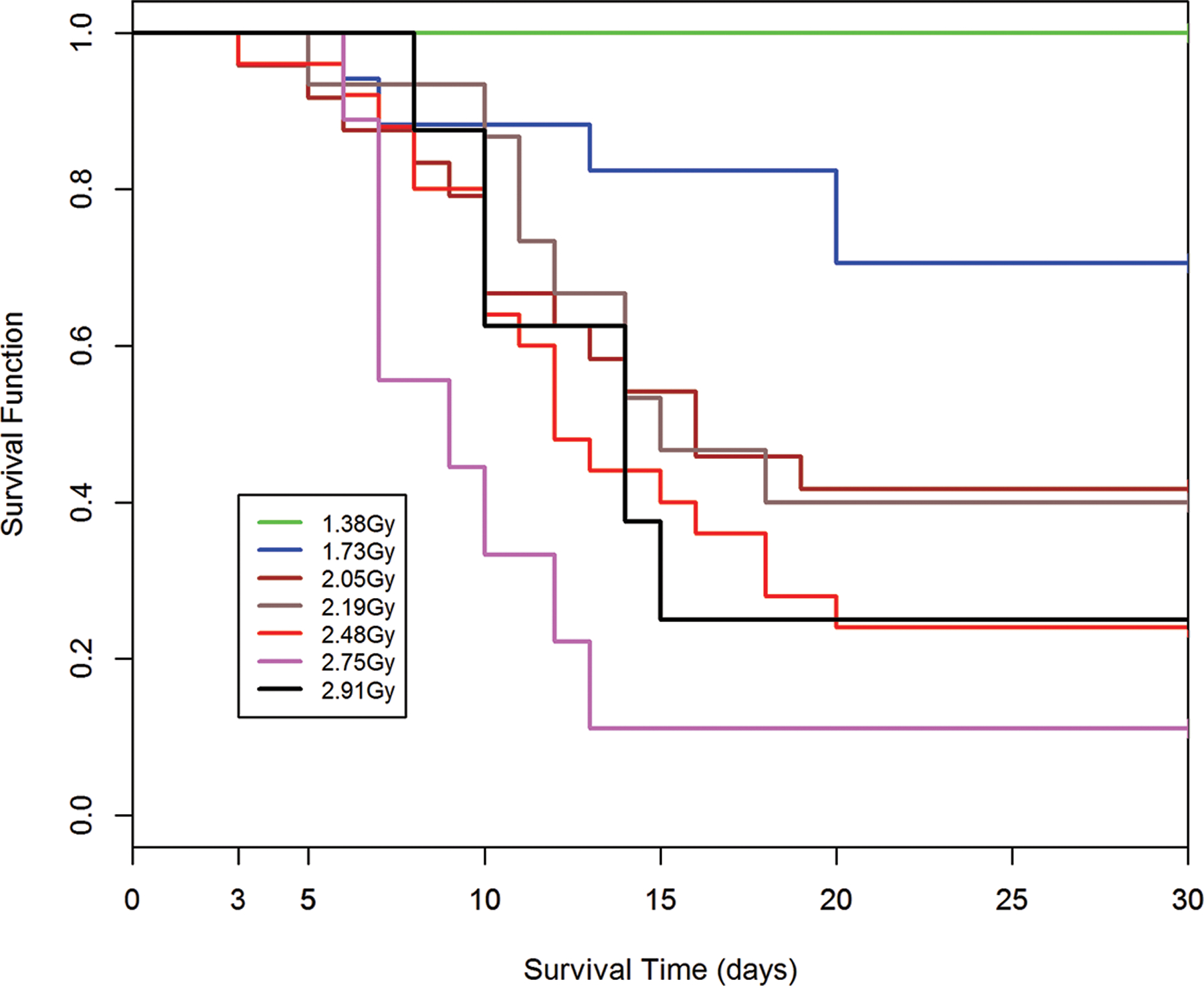
The DRRs for H ARS in NHP provided the database for a comparative analysis of radiation quality, dose, dose rate, exposure geometry, relative estimated LD50/30 values and slopes of the respective DRR (MacVittie et al. 2015b). The dose response relationships are shown in Fig. 2, 3. The respective LD50/60 values are noted within the figures. A Kaplan-Meier plot showed the time- and dose-dependent change in survival probability over time in response to pulse-rate exposure of NHP to mixed gamma:neutron radiation (Fig. 4). The time course of mortality relative to dose, emphasized the early GI component of the mixed gamma:neutron radiation.
Fig. 4.
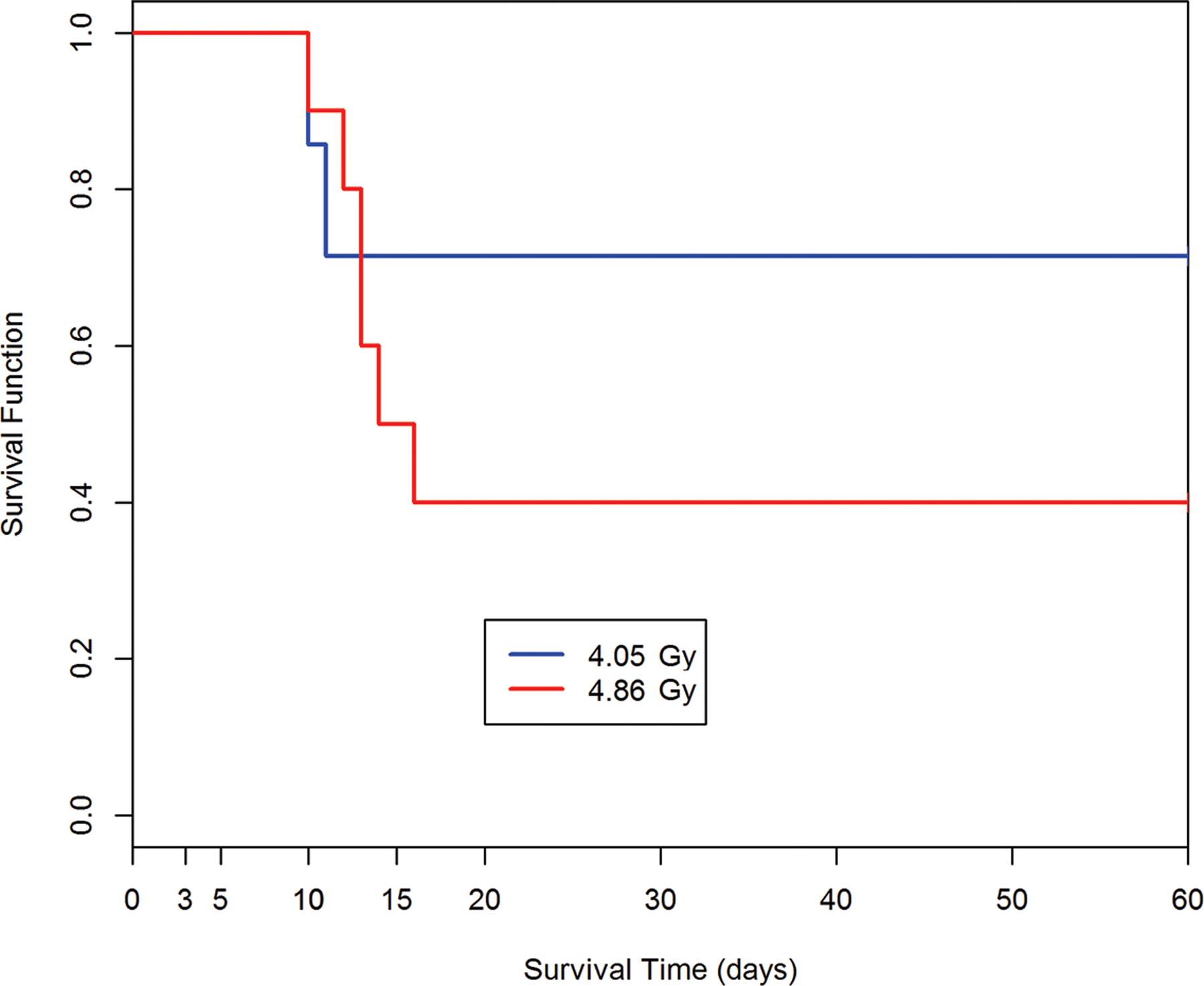
Kaplan-Meier survival curves for nonhuman primates: Demonstrating dose- and time-dependent survival probability relative to the dose cohorts exposed to reactor-derived mixed gamma/neutron radiation using unilateral exposure aspect at pulse dose rate. A dose-response relationship is suggested between the dose of total-body mixed gamma/neutron-radiation and loss of survival probability for nonhuman primates within the acute GI-ARS observed through 15d post exposure. The 60d time course provides perspective relative to the time course of lethality within the GI- and H-ARS. The survival plot for the AFRRI study cohorts (n = 116) exposed to 4.05 Gy, 4.86 Gy from the TRIGA, reactor-derived mixed gamma/neutron radiation unilateral at pulse-rate (Turbyfill et al. 1968; Wise and Turbyfill 1968).
Secondary studies that provided a database on NHP exposed to mixed fission neutron:gamma radiation as a control cohort of radiation-induced mortality and myelosuppression.
Broerse et al. (1978); Broerse and Zoetelief (1984).
The authors conducted studies assessing the effect of autologous BM transplants in NHP exposed to conditioning doses of either mixed neutron:gamma radiation or 300 kVp x-radiation plus administration of medical management (Broerse et al. 1978; Broerse and Zoetelief 1984). A modest number of NHP were used but the data suggested a marked RBE for the mixed neutron:gamma radiation relative to the 300 KvP x-radiation and established a threshold dose for GI-ARS involvement.
Animals.
Rhesus macaque were ~ 3 y of age, 2.3–3.0 kg bw; from a stock colony (India source); quarantined for 3 mo prior to experimentation. Animals were fed commercial food pellets and a diet of fresh fruits, vegetables and cooked rice which was supplemented with various vitamins.
Radiation sources. x-radiation:
Conscious animals were exposed to rotational whole-body x-irradiation (Phillips generator) with 300 kVp, 10 ma, (HVL 3 mm Cu) x-ray at a dose rate of 28 rad min −1. Neutron exposure: Mean energy 1 MeV (uranium-235 converter plate in Low Flux thermal research reactor of the Argonaut type, operated at maximum 10 kW (Netherlands Energy Research Foundation in Petten, NL). Boron-plastic sheets and lead shields were used to reduce thermal neutron and gamma-ray contamination. Animals were exposed in a rotating cylindrical cage at a dose rate of 8 rad min −1 to the prescribed total dose of neutrons and gamma radiation. The dose distribution was assessed in a phantom filled with tissue-equivalent liquid. The doses received by the animals were expressed as the absorbed dose in soft tissue averaged over the animal. The gamma radiation dose was ~ 24% of total dose, the neutron:gamma was ~ 3:1.
Autologous BM transplant (AuBMT).
BM was obtained from the femoral shaft (infused ~ 4–8 ×108 BMC kg bw−1) and administered by slow IV infusion. Animals, n = 15, were irradiated with fission neutron:gamma with total-body doses that ranged from 290–530 rad followed by AuBMT.
Control cohorts.
Animals, n = 15, were exposed to mixed fission neutron:gamma radiations with doses that ranged from 230–410 rad.
Medical management.
The animals were weighed and inspected daily, received barrier nursing; sterile materials and thrombocyte concentrates when thrombocyte counts decreased below 40,000 uL−1 or when signs of hemorrhagic diathesis were observed; whole blood or packed erythrocytes, were infused when hematocrit levels were below 20%. Balanced fluids (Ringer’s+ 5% glucose in equal volumes) were administered IV or SC in case of dehydration. Antibiotics (Nystatin, gentamicin (5 mg kg−1), cephaloridine (50 mg kg−1) based on sensitivity of fecal flora were administered at signs of infection or when leucocytes < 500 uL−1.
Results.
The authors provided figures of depth dose distribution of mixed neutron:gamma radiations and x-radiation doses from unilateral and bilateral exposure.
Control cohort:
Irradiated NHP, n = 15, were exposed to fission neutron:gamma radiation in the range 230–410 rad. It was established that doses greater than 260 rad were 100% lethal. The mean survival time was 11 d which suggested GI involvement in concert with classic H-ARS. Animals showed marrow and lymphoid aplasia, multiple hemorrhages, ulceration of the large bowel and bacterial invasion with sepsis. They noted that the mean survival time of lethal x-irradiation was 15 d.
Autologous (Au) BMT cohort.
The AuBMT cohort was exposed to a range from 290–530 rad. Hematological recovery was noted within 8–10 d post AuBMT.
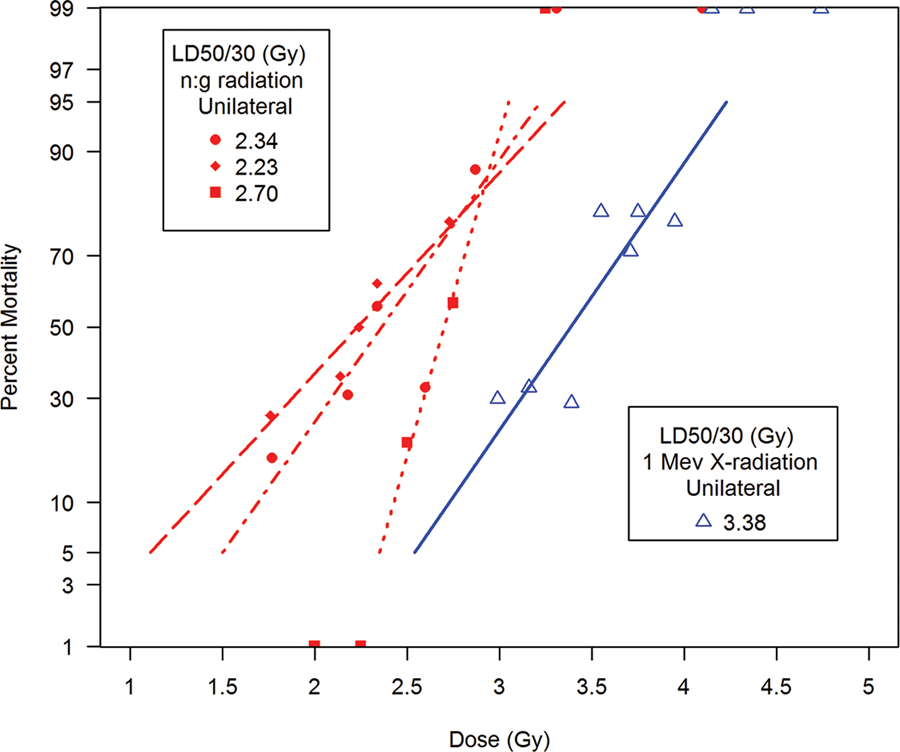
The LD50/30 values for cohorts exposed to x-radiation or mixed neutron (1 Mev):gamma radiation were 525 rad and 260 rad respectively. The RBE was 2.0 for the H-ARS. AuBMT was effective for survival to 440 rad mixed neutron (1 Mev):gamma radiation and 860 rad, 300 kVp x-irradiation, respectively. These doses established a threshold dose for the acute GI-ARS. The authors estimated the LD50/7 for GI-ARS at ~ 470 rad for mixed neutron (1 Mev):gamma radiation and x-irradiated animals to be ~ 860 rad.
Farese A, et al (1993); Farese A, et al (1994).
The control cohorts from each study provided a radiation-induced myelosuppression profile over time post a low-lethal (estimated LD10/60), unilateral, pulse-rate exposure of mixed fission neutron:gamma radiation plus administration of medical management. The studies were focused on assessing the efficacy of single and combined cytokines IL-3 and GM-CSF and leukemia inhibitory factor (LIF) to mitigate myelosuppression (Farese et al. 1993; Farese et al. 1994).
Animals.
Domestic-born, male rhesus macaques (n = 5 for each control cohort), Macaca mulatta (mean bw 2.9 ± 0.3 kg and 4.3 kg ± 0.59) were housed in individual stainless-steel cages in conventional holding rooms at the AFRRI, Bethesda, MD. Animals were provided commercial primate chow, supplemented with fresh fruit and tap water ad libitum. Research was conducted under the Guide for Care and Use of Laboratory Animals. All protocols were approved by the Institutional Animal Care and Use Committee.
Radiation source.
The AFRRI TRIGA reactor was used to total-body irradiate animals in a posterior-anterior direction to a pulse-rate of mixed fission neutron:gamma radiations (1:1) to a total free-in-air (skin surface dose) of 450 cGy. All irradiation procedures were monitored using ionization chambers, sulfur activation foils and radio-luminescent glass silicon diodes.
After a pre-habituation period, each monkey was placed in an aluminum restraining chair and subjected to posterior-to-anterior, total-body irradiation from the AFRRI Mark-F “TRIGA nuclear reactor”. The torso of each monkey was shielded from the intense gamma radiation emitted by placing a 2.5 cm lead shield between the core of the reactor and the exposure position. Each monkey received a pulse of 450 cGy (< 500 milliseconds) free-in-air, total mixed neutrons and gamma rays with a precision of 22.5% (% Std Dev, n = 16) and a statistical accuracy of 10%. The neutron dose relative to the total dose (neutron + gamma) was 0.50% ± 2.3% (% SD, n = 17) with a statistical accuracy of 14%. All exposures were actively monitored by ionization chambers and passively monitored by sulfur activation tablets.
Medical management.
Animals were administered an antibiotic regimen (Gentamicin, 1.5 mg kg d and rocephin, 100 mg kg d) prophylactically when the WBC was ≤ 1,000 µL−1 and continued daily until the WBC was > 1,000 µL−1 for 3 consecutive days. Fresh irradiated platelets (1,500 cGy) from a random donor pool (NHP > 10 kg bw) were administered every other day when the platelet count was < 30,000 µL−1.
Hematologic analysis.
Hematopoietic recovery was assessed by analysis of peripheral blood neutrophils and platelets as well the activation of neutrophils.
Results.
The unilateral, TBI with 450 cGy at pulse-rate with mixed (1:1) fission neutron:gamma radiations required clinical support with antibiotics and platelet transfusions to ensure 100% survival from the lethal, acute H ARS. The 450 exposure is an approximate LD70/60 in the absence of medical management and an approximate LD10/60 (1/10) with medical management (unpublished results, T. MacVittie). The myelosuppression consequent to 450 cGy was severe relative to recovery kinetics for neutrophils and platelets. The duration of neutropenia (ANC < 1,000 µL−1) was noted as 16 d and 17 d for each study whereas the duration of thrombocytopenia (platelet count < 30,000 µL−1) was 10 d and 9 d for each study respectively. Antibiotic support was required for an average 16–17 d post exposure.
Summary statement relative to NHP exposure.
The database for NHP exposed to mixed gamma:neutron radiation provided a consistent data set that underscored the RBE relative to predominant reference radiation qualities of 250 kVp and 2 MeV x-radiation and Co-60 gamma radiation. The database provided estimated RBE values for the H ARS, LD50/30 endpoint for NHP exposed to steady-state, pulse-rate and prompt nuclear weapon exposure with mixed gamma:neutron radiation relative to reference radiation delivered via moderate dose rate, bilateral, uniform TBI. The estimated RBE values, dependent on radiation quality and percentage of the neutron component in the mixed radiation exposure ranged from approximately 1.3 to 2.00 (1.35, 1.67 and 1.74, and 2.00) for 250 kVp, Co-60 gamma radiation and 2 MeV x-radiation, respectively. The database is marginal relative to the absence of contemporary studies with significant gaps in knowledge relative to, a) multiple organ injury characteristic of the ARS, GI and kidney, b) delayed effects of acute radiation exposure, e.g., prolonged GI injury, lung, kidney and heart, c) a defined exposure scenario(s) relevant to prompt exposure consequent to a nuclear event or space environment, d) the corresponding neutron energy range and e) depth-dose distribution relative to the prescribed dose and critical specific organ exposure and f) a published database relative to male vs female differential radiation sensitivity. A single, historical study, published the dose- and time-dependent mortality of male and female NHP to TBI with 250 kVp x-radiation (Schlumberger and Vazquez 1954). An unpublished analysis of the Schlumberger and Vasquez data showed no significant difference between the male and female dose response relationship (T. J. MacVittie, unpublished).
The effect of radiation quality, dose rate and exposure parameters on the lethality dose response relationship (DRR) in canines with and without medical management.
Search Results: Summary Statement.
The authors chose a total of nine complete studies that contributed to the systematic review of the DRR for the acute H ARS in canines exposed to mixed gamma:neutron radiation with or without medical management. A 1993 study dataset is in press (Table 5) (MacVittie and Jackson III 2020). Twelve additional studies were focused on defining the time course and severity of the H ARS consequent to effects of mixed gamma:neutron radiation of various dose rates, exposure geometry and depth dose assessment (Table 6).
Table 5.
Characteristics of primary studies for canine models included in the systematic review.
| Source/Authors | Samplesize | Radiation Source | Exposure Geometry | Dose Rate | |
|---|---|---|---|---|---|
| Bond et al. 1956 | 115 | 9 Mev neutron; 250 kVp x-ray | Bilateral | 20 rep min−1; 15.0 r min−1 | |
| Alpen et al. 1960 | 37 | 9 Mev neutron; 250 kVp x-ray | Bilateral | 20 rep min−1; 15.0 r min−1 | |
| Earle et al. 1971 | 82 | 14.6 Mev; 1 Mvp x-ray | Bilateral | 3 rads min−1; 35 R min−1 | |
| Ainsworth et al. 1965 | 457 | 1 Mev neutron; | Unilateral and bilateral | 40 rad min−1 | |
| 1 Mvp x-ray; | 110 cGy min−1 | ||||
| 1 Mev neutron | Unilateral | pulse rate | |||
| George et al. 1968 | 165 | 1 Mev neutron; 250 kVp x-ray | Rotational | 16.8 rad min−1 | |
| MacVittie et al. 1984 | 51 | 1 Mev neutron; Co-60 gamma-ray | Bilateral, steady state | 60 cGy min−1; 10.0 cGy min−1 | |
| MacVittie et al. 1991 | 144 | 1 Mev neutron; Co-60 gamma-ray | Bilateral, steady state | 60 cGy min−1; 10.0 cGy min−1 | |
| Wang et al. 1991 | 128 | 1.33 Mev neutron | Unilateral | 9.7–59.4 cGy min−1 | |
| a MacVittie and Jackson 2020 | 60 | 1 Mev neutron | Bilateral, steady state | 60 cGy min−1 | |
All primary studies were published in the open literature or in government publications. The primary studies provided complete data sets for establishing the dose response relationship (DRR) for mortality versus mixed gamma/neutron radiation dose. The radiation source, energy, gamma:neutron ratio, dose rate and exposure aspect varied.
Restrospective analysis of 1993 data.
Table 6.
Comparative LD50/30 values for canines.
| Canine | ||||
|---|---|---|---|---|
| Radiation Quality | Energy (n, x-ray, photon) |
LD50/30 rads/cGy | Exposure geometry, TBI, PBI# | Source/Authors |
| X-ray | 250 kVp | 206 | bilateral | George et al. 1968 |
| X-ray | 250 kVp | 252 | bilateral | Bond and Carter 1956 |
| X-ray | 250 kVp | 212 | bilateral | Alpen and Shill 1960 |
| X-ray | 1 Mev | 280 | bilateral | Ainsworth and Leong 1965 |
| X-ray | 1 Mev | 288 | bilateral | Earle 1971 |
| X-ray | 1 Mev | 337 | unilateral | Ainsworth and Leong 1965 |
| Co-60 gamma | 1.2 Mev | 260 | bilateral | MacVittie el al. 1991 |
| Co-60 gamma | 1.2 Mev | 258 | bilateral | Norris 1968 |
| neutron:gamma | 14.6 Mev | 281 | bilateral | Earle 1971 |
| neutron:gamma | 9 Mev | 289 | bilateral | Bond and Carter 1956 |
| neutron:gamma | 9 Mev | 239 | bilateral | Alpen and Shill 1960 |
| neutron:gamma | 1 Mev | 203 | bilateral | Ainsworth and Leong 1965 |
| neutron:gamma | 0.85 Mev | 153 | bilateral | MacVittie el al. 1991 |
| neutron:gamma | 0.85 Mev | 216 | bilateral | a MacVittie and Jackson 2020 |
| gamma:neutron | 1 Mev | 218 | bilateral | George et al. 1968 |
| gamma:neutron | 1 Mev | 230 | unilateral, steady state | Ainsworth and Leong 1965 |
| gamma:neutron pulse | 1 Mev | 221 | unilateral, pulse | Ainsworth and Leong 1965 |
| gamma:neutron pulse | 1 Mev | 210 | unilateral | Pitchford and Thorp 1968 |
Dose response relationships (DRR) were established for canines exposed to Co-60 gamma radiation, 250 kVp x-, 1Mev x-radiation, mixed neutron:gamma radiation to include literature values for LD50/30 or LD50/60, the differential exposure aspect (unilateral, bilateral) and literature source.
Retrospective unpublished 1993 dataset.
Radiation source, quality and exposure parameters in canines.
Mixed gamma:neutron radiation
Mixed gamma:neutron radiation was delivered by five methods that produced variable energy, dose rate and exposure geometry. All are briefly described in text with full description in respective references. (1) AFRRI TRIGA Mark-F pool-type thermal research reactor delivered bilateral, left/right side exposure (George et al. 1968; Pitchford and Thorp 1968; MacVittie et al. 1984; MacVittie et al. 1991; MacVittie and Jackson III 2020). (2) An insulated core transformer that produced a 2.5 mA beam of 200 keV deuterons directed at a tritiated titanium target that yielded a nearly homogeneous field of 14.6 MeV neutrons (Earle et al. 1971). (3) A thick beryllium target was bombarded with 20 Mev deuterons that delivered a mean neutron energy of ~9 Mev (Bond et al. 1956). (4) A thick beryllium target was bombarded with 12 Mev protons to produce neutrons of ~ 9 Mev (Alpen et al. 1960). (5) A “shielding experimental reactor” (light water-cooled and moderated pool type) that produced 1.33 Mev neutron of various neutron:gamma ratios (Wang et al. 1991).
X- and Co-60 gamma radiation.
X-radiation was of 250 kVp and 1 Mvp x-radiation, Co-60 gamma radiation was delivered by the AFRRI Cobalt source via opposing lateral exposure.
Canine Primary studies: Selected studies that used the canine, mongrel and beagle (≥ 10 kg), to establish the dose response relationship for exposure to mixed neutron-gamma radiation relative to comparator, reference studies of cobalt-60 gamma and 250 kVp or 1 Mvp x-radiation.
Bond et al. (1956).
The authors noted that the biological effects of single, fast neutron exposure to large animals had not been possible due to lack of a suitable radiation source. The flux density of the Crocker Laboratory cyclotron permitted lethal exposures delivered within minutes. They reported on the relative mortality consequent to total-body fast neutron versus 250 kVp x-radiation (Bond et al. 1956).
Animals.
Mongrel canines, 20–30 pounds (9.0–15.0 kg bw) were quarantined and immunized against hepatitis, distemper and rabies and treated for intestinal parasites. Animals (n = 115) were caged individually in temperature regulated rooms. They were observed daily within the pre- and post-exposure, 30 d study duration. Body weight, temperature (rectal), peripheral blood counts were taken 3 times a wk prior to exposure and at 2–3 d intervals post TBI. No medical management was provided to the animals.
Radiation source and exposure.
Both neutron (n = 50 canines) and x-ray (n = 65 canines) doses were delivered to the sagittal plane of the animal as calculated from free-in-air or surface doses and knowledge of the attenuation pattern from phantom studies. The dose rate was 20 rep min−1. Dose was reported in rep. The dosimetry employed to estimate dose and the phantom are well described by Bond and colleagues (Bond et al. 1957).
Neutron exposure.
A thick beryllium target was bombarded with 20 Mev deuterons. The study indicated a mean neutron energy of ~ 9 Mev with 98% of the neutrons above the sulfur threshold. The maximum neutron energy was 24 Mev.
X-radiation.
X-irradiation, 250 kVp (General Electric Maxitron unit), was performed bilateral with a radial beam, 15 ma, HVL of 1.5 mm Cu (filters 0.5 mm Cu plus 1.0 mm Al) and a dose rate of 15 r min−1.
Hematology parameters.
Hematology assays were standardized and included total white cell differential and platelet counts. The average pre-exposure value served as each animal’s normal baseline.
Results.
The mortality over 30d was calculated for neutron and 250 kVp x-irradiated canines. Probit regression lines were used to establish the respective DRR. Note that the neutron dose could not be determined until after the exposure when the sulfur detectors were counted. Thus, doses were combined as suggested by Finney (Finney 1952). Extreme and aberrant points were omitted.
The empirical probit of the mortality rate for the dose-groups was plotted against the mean dose for each group. The respective LD50/30 values were 289 ± std error of 3 rep and 252 ± 6 rep for fast neutron and 250 kVp x-radiation (Fig. 5, Table 7). The slopes of the regression lines were markedly different at 59 and 22, respectively. The average survival times for neutron and x-irradiated canines were not different at 14.2 d and 14.6 d respectively, suggesting that mortality in each exposure cohort was due to the H ARS. Several dogs within each neutron and x-radiation cohorts succumbed within 7–12 d suggesting that high doses encroached on the GI ARS.
Fig. 5.
The comparative DRRs for exposure of canines to bilateral, uniform mixed neutron:gamma radiation relative to bilateral, uniform 1 Mev x-irradiation and Co-60 gamma irradiation. Canines, male/female, beagles or mongrels, were exposed to mixed neutron:gamma radiation at steady state dose rate for a bilateral, uniform exposure relative to the reference total-body irradiation at 9–40 rad min−1 of Co-60 or 1 Mev x-radiation (Ainsworthet al. 1965; Earle et al. 1971; Norris et al. 1968; George et al. 1968; Macvittie and Jackson 2020).
Table 7.
Differential dose (cGy) distribution from unilateral radiation exposure.
| Radiation Source | Exposure aspect | Entrance dose (cGy) | Exit dose (cGy) | Mid dose (cGy) | Source/Authors |
|---|---|---|---|---|---|
| fission neutron | bilateral | 263 | 263 | 263 | Ainsworth et al. 1965 |
| fission neutron | unilateral | 455 | 121 | 299 | Ainsworth et al. 1965 |
| 1.9 Mev neutron | unilateral | 420 | 280 | --- | Wang et al. 1991 |
| 1 Mvp x-ray | unilateral | 631 | 237 | 453 | Ainsworth et al. 1965 |
All studies were published in the open literature or in government publications. The studies were conducted to provide a dose response relationship for the H-ARS and threshold GI-ARS mortality. All dose rates were steady state. Depth dose dosimetry provided air dose (cGy) at surface, midline and exit (surface).
The LD50/30 values were noted for neutrons and 250 kVp x-, 1000 kVp x- (HVL 2.0 mm Pb) and 2000 kVp x-radiation (HVL 4.3 mm Pb) measured free-in-air, at skin surface and at the median sagittal plane of the animals. The midline doses for bilateral exposure ranged from 289, 252, 255 and 268 cGy for 9 Mev neutrons, 250, 1000 and 2000 kVp respectively. The authors suggested, that the absorbed dose and correction of the x-ray dose associated with the neutron dose, the RBE values for 9 Mev neutrons compared to 250-, 1000-, and 2000-kVp x-rays were 0.81, 0.82 and 0.86, respectively. The authors state that the RBE was ~0.8 vs reference radiation of 1 Mev x-radiation.
Alpen et al. (1960).
The authors focused on establishing the RBE for the H-ARS relative to bilateral, relatively uniform, TBI from 250 kVp x-radiation by using a neutron source of lower energy than previous contemporary experiments (Alpen et al. 1960).
Animals.
Healthy mongrel, canines (6.9–13.9 kg bw) received bilateral TBI. The dogs were anesthetized with thialbarbitone sodium, a short-acting barbiturate that maintained the animals in light anesthesia throughout the exposure period. No medical management was provided to the animals
Radiation sources, exposure: Neutron source.
University of California Crocker laboratory, 60-in cyclotron. Physical description presented by Tochilin and Kohler (Tochilin and Kohler 1958). Bond and colleagues also used this source at 9 Mev neutrons (Bond et al. 1956). The authors used 12 Mev protons on a Beryllium target to produce neutrons of ~9 Mev. The reaction produced an exponential neutron spectrum which matched the fission spectrum. The fast neutron flux was measured with sulfur threshold detectors. Depth-dose measurements for neutrons and gamma radiation were made in a tissue-equivalent phantom formulated to be tissue-equivalent for both neutrons and gamma-rays. There was an approximate, primary cyclotron-derived 14% gamma radiation dose. The article provided a thorough description of neutron dosimetry. The midline dose was 84% of the first collision surface dose. At the midline, the animal received the largest contribution of gamma radiation amounting to 31% of the total midline dose. This represents a mix of cyclotron produced gamma-rays and those resulting from thermal neutron capture within the phantom. Animals (light anesthesia) received bilateral TBI with mixed neutron:gamma radiations.
X-radiation source.
X-radiation exposures were accomplished with the radial beam from a General Electric Maxitron unit, the radiation factors being 250 kVp, 15 ma, HVL of 1.5 mm Cu, filter 0.5 mm Cu plus 1.0 mm Al. The skin-to-target distance was 100 cm, and the dose rate was approximately 15 r min−1 as measured in air with a 100-r Victoreen ionization chamber. The canines to be x-irradiated were placed in a canvas sling, anesthetized with sodium pentobarbital, and one-half of the total dose was delivered to each side of the animal (Bond et al. 1956).
Results.
Neutron LD50/30 first collision surface dose was 285 rads relative to the MLTD ~ 239 rads with an average mean survival time of 13.5 d. No deaths occurred before 6 d, although several animals succumbed within 6–10 d range indicative of GI-ARS. The LD50/30 for total-body x-irradiation was ~ 212 rads with an average survival time of 16.4 d as performed in the authors laboratory (Fig. 5, Table 7) (Alpen et al. 1958). The RBE was approximately 0.9 for the mixed neutron:gamma radiation versus 250 kVp x-radiation. It was noted that midline dose of 239 rads was composed of 74 rads of gamma radiation and 165 rads from the ~ 9 Mev neutron component.
H ARS: The animals succumbed to signs of the H ARS, notably bleeding and infection. The authors noted that serial hematological parameters were in close agreement with the data from prior studies of irradiation of canines (Bond et al. 1956).
Ainsworth et al. (1965).
Earlier studies in mice suggested there was no dose-rate effect of neutron:gamma or gamma-irradiation on acute mortality when dose rates of 40–100 rads min−1 and 105 to 106 rads min−1 were compared. The authors extended the neutron:gamma radiation dose-rate effect studies to canines where they investigated the influence of exposure aspect (unilateral vs bilateral) relative to exposure to 1 Mvp x-radiation (Ainsworth et al. 1965).
Animals:
healthy, mongrel canines, 7–10 kg, (n = 457 total) 2 wk quarantine, during which they were dewormed, immunized against distemper and hepatitis were single housed in cages and fed standard lab chow supplemented w scrap beef. Water was ad libitum. Canine measurements were a mean chest diameter of 14 cm and a body length of 58 cm. The animals did not receive medical management.
Radiation source, exposure: Mixed neutron:gamma radiation.
TRIGA Mark F reactor (Ainsworth et al. 1964); (Simpson et al. 1963). Animals were irradiated under “light” anesthesia (pentobarbital sodium). TBI via bilateral exposure or unilateral exposure to the right side. Animals were transported to the reactor site (La Jolla, CA) in an air-conditioned van; transit time was 12–15 h, animals were watered but not fed during transit. A total period of 24–48 h was required for transit to and from the reactor site to include irradiation.
TBI, steady-state and pulse-rate.
Bilateral, steady state exposure, ~ 40 rads min−1; (dose rates measured as first collision doses in rads at midpoint of empty exposure volume). X-ray contribution was ~ 16%; Pulse-rate exposure: dose rate at pulse exposure was ~ 3.7–12.7 × 105 rads min−1, neutron:gamma 6:1; gamma:neutron 49:1. MTLD: MLTD estimates for neutron:gamma irradiated canine were based on radiation depth-dose, measurements made in cylindrical tissue-equivalent phantom (17.8 cm diameter, 50.8 cm height)
1 Mvp x-radiation.
1Mvp resonant transformer machine at ~ 1.0 Mev 110 cGy min−1; canines irradiated either bilaterally or unilaterally in individual plywood boxes.
Results.
Neutron:gamma radiation, steady state.
Bilateral (n = 105) vs unilateral (n = 76) exposure to mixed neutron:gamma at steady state dose rate of ~ 40 rads min−1 resulted in LD50/30 values of ~ 203 rads [183, 219], MST 11.4 d and ~ 230 rads [212, 253], MST 10.2 d. The LD50/30 values were not significantly different (Figs. 6, 7; Table 7).
Fig. 6.
The comparative DRRs for exposure of canines to unilateral, non-uniform, mixed neutron:gamma radiation relative to reference exposure to unilateral, non-uniform 1 Mev x-radiation. Canines, beagles or mongrels, were exposed to mixed neutron:gamma radiation at steady state or pulse rate exposure (Ainsworth et al. 1965).
Fig. 7.
The comparative DRRs for exposure of canines to bilateral, uniform versus unilateral, non-uniform mixed neutron:gamma radiation relative to unilateral, non-uniform, 1 Mev x-radiation. Canines, beagles or mongrels, were exposed to bilateral steady state uniform or unilateral, non-uniform mixed neutron:gamma radiation at steady state or pulse rate exposure relative to unilateral, non-uniform 1 Mev x-radiation (Ainsworth et al. 1965; George et al. 1968; MacVittie and Jackson III 2020).
Pulse rate. The mixed neutron:gamma pulse rate, unilateral exposure (n = 57) resulted in an LD50/30 ~ 221 rads [195, 268], MST 11.5 d. The LD50/30 values are not different with respect to exposure aspect or dose rate in the range used. The early MST values, < 12 d, suggested the presence of a GI component after neutron exposure.
1 MVp x-ray.
The bilateral (n = 134) vs unilateral (n = 85) exposure to 1Mvp x-radiation resulted in LD50/30 ~ 280 rads [265, 299], MST 16.9 d and 337 rads [318, 354], MST 15.5 d, respectively. The increased MST values were characteristic of the H-ARS and suggested the lack of a significant GI component after 1 Mvp exposure relative to that noted consequent to mixed neutron:gamma exposure (Figs. 6, 7; Table 5).
Comparative values for neutron:gamma and 1 Mvp x-ray relative to exposure aspect and dose rate.
The respective LD50/30 values for canines exposed to mixed neutron:gamma radiation at bilateral, steady state vs unilateral, steady state or pulse were 203 rads vs 230 rads and 221 rads. The 1 Mvp x-radiation LD50/30 values for bilateral vs unilateral exposure were 280 rads vs 337 rads.
The respective MST for canines exposed to mixed neutron:gamma radiation relative to x-radiation were 10.2 to 11.5 d and 15.5 to 17.0 d, respectively. A review of the median survival times for higher doses within each cohort suggested that mixed neutron:gamma radiation induced the acute GI-ARS (median ST of 5 to 12 d) relative to the 1 MVp x-radiation (median ST of 13 to16 d).
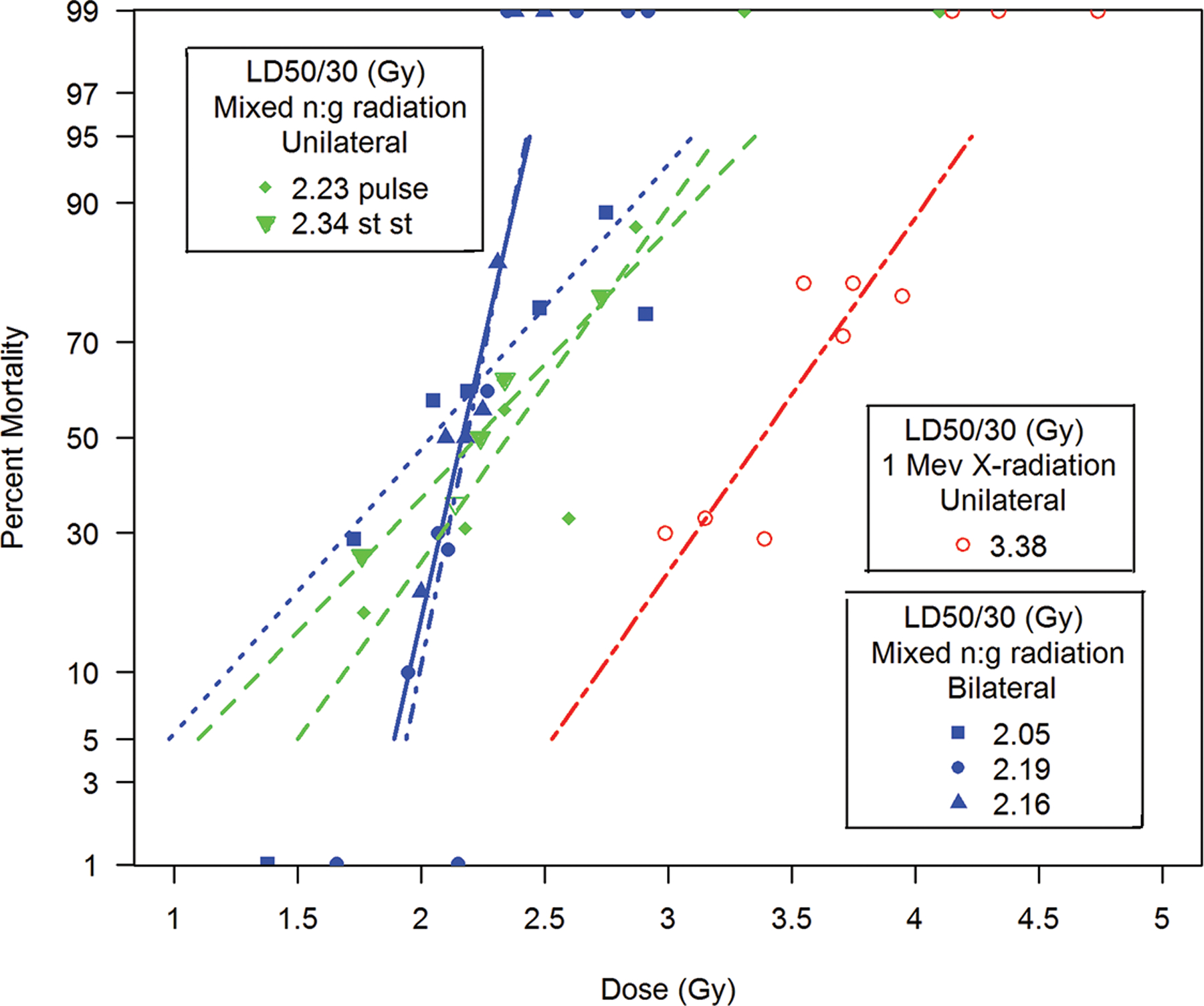
Ainsworth et al., provided a representative canine dataset that emphasized the: a) dose-dependent RBE, b) the influence of dose rate and c) exposure aspect of mixed neutron:gamma radiations relative to 1 Mev x-radiation effects on GI and hematopoietic systems (Figs. 8 a-e).
Fig. 8.
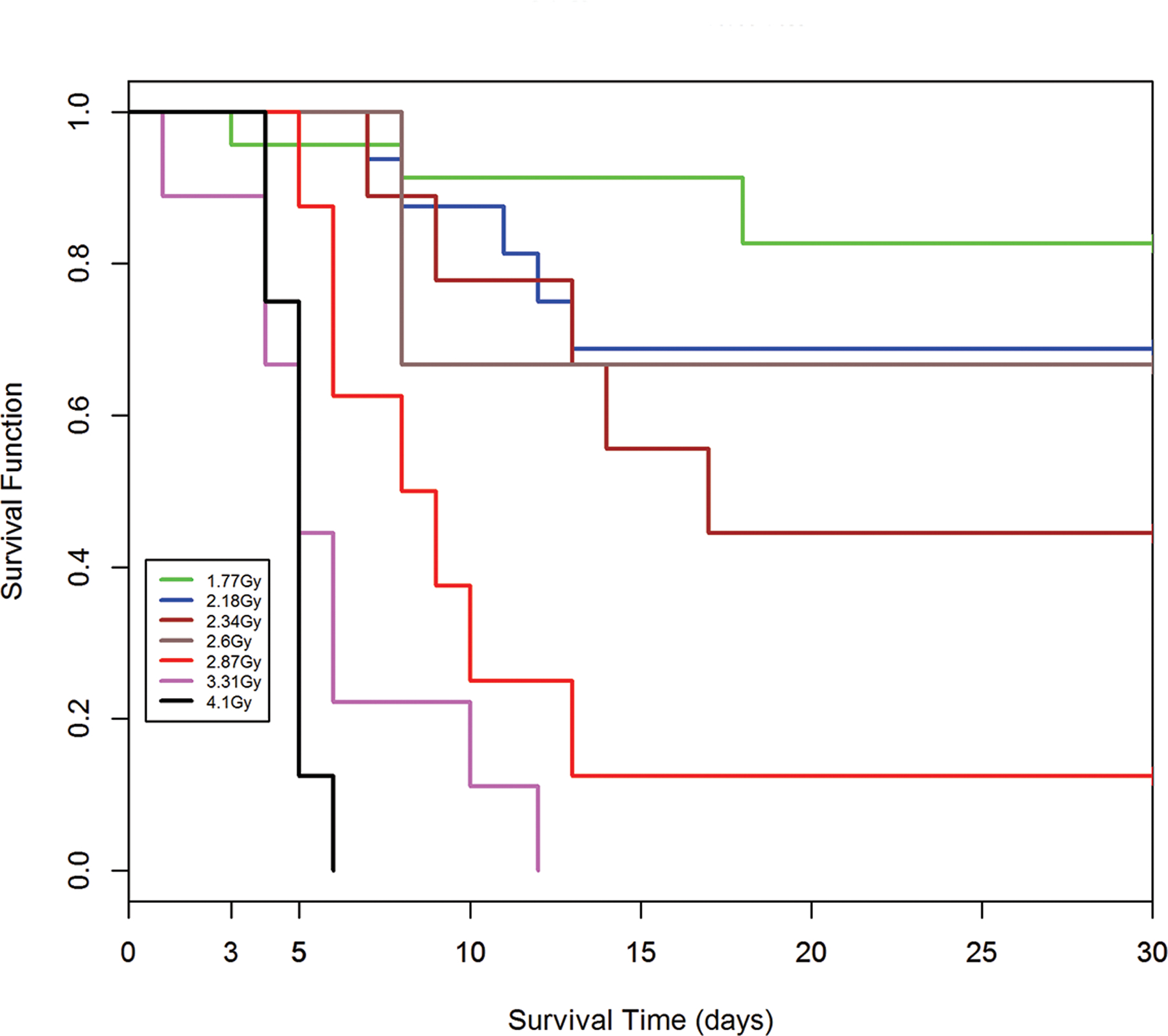
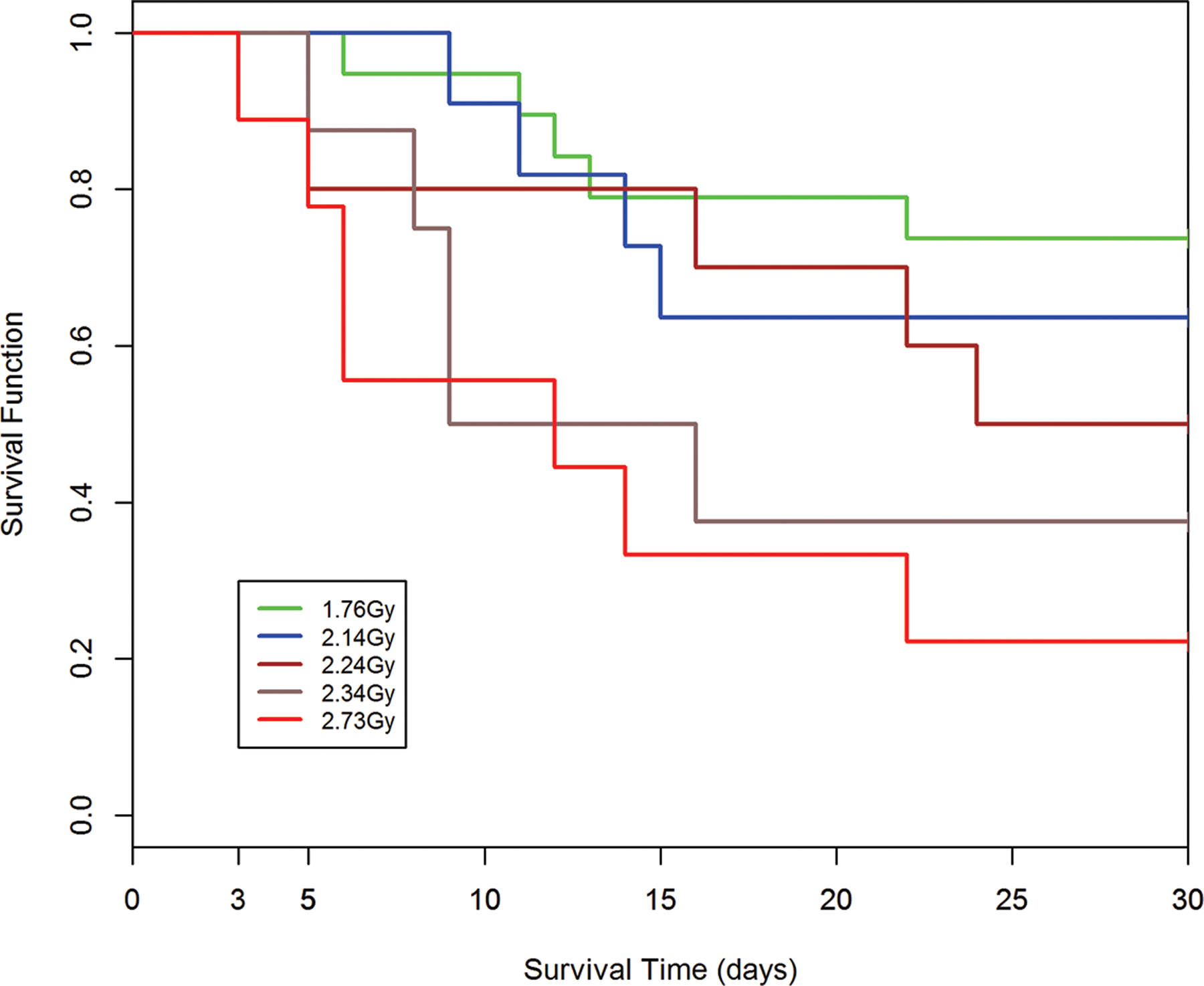
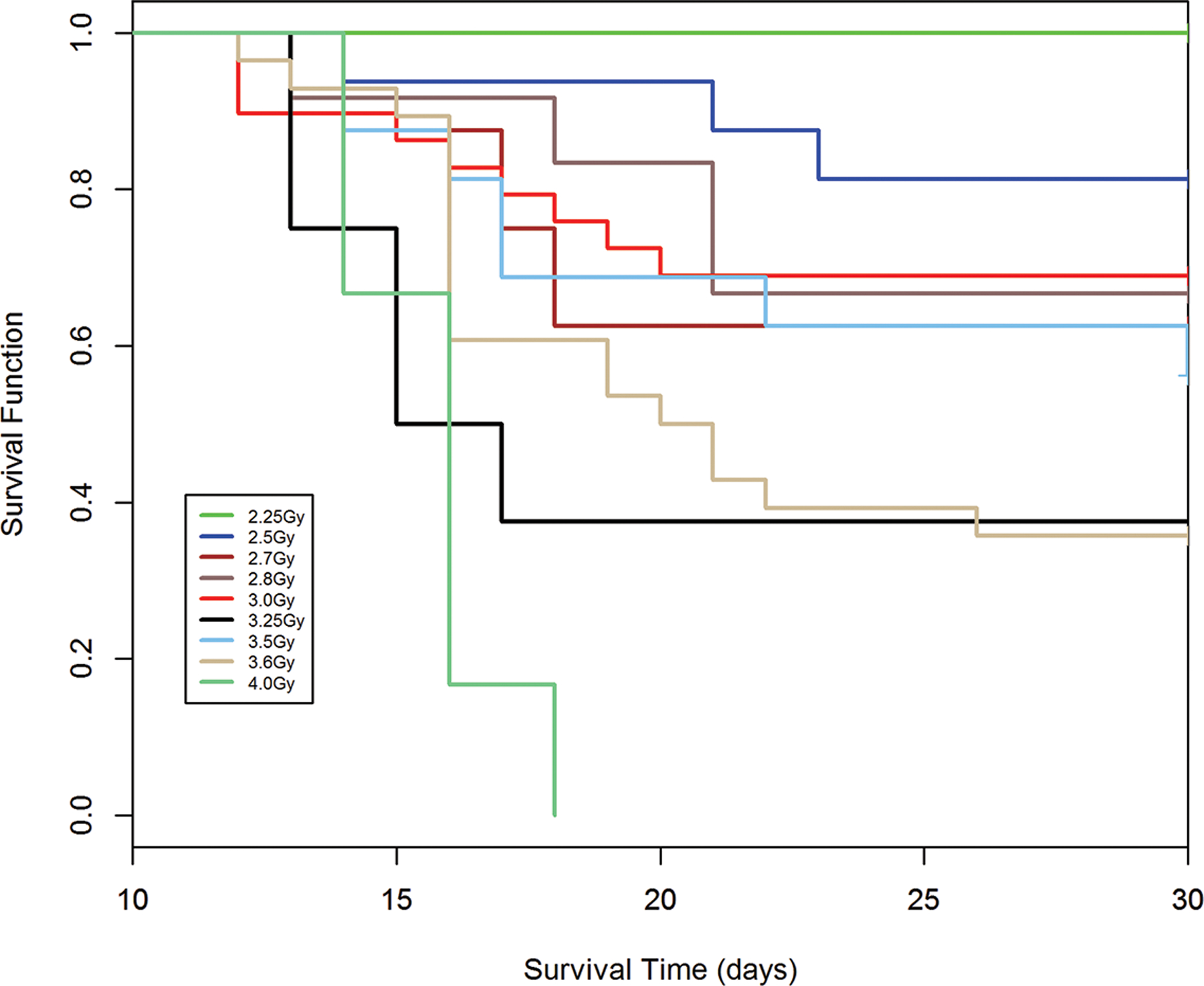
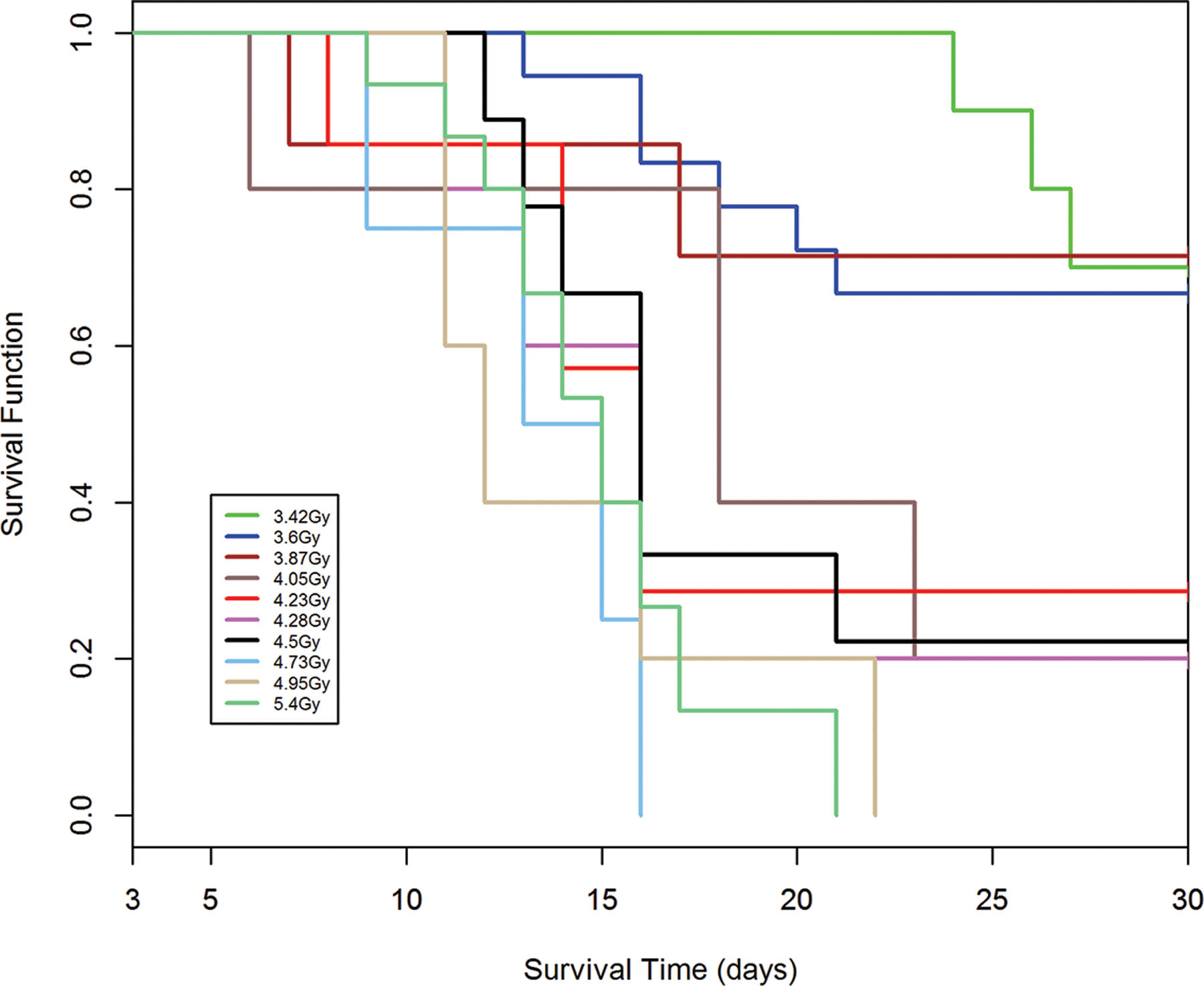
a-e. Kaplan-Meier analysis of survival probability after exposure of canines to mixed gamma/neutron- and 1 Mev x-radiations. The dose- and time-dependent survival over 30d post exposure showed survival probability through the acute GI- and H-ARS: a) *Table I: bilateral exposure at steady state dose rate ~ 40 rads min−1; b) Table II: Unilateral exposure at ~ 40 rads min−1; c) Table III: Unilateral, pulse exposure; d) Table IV 1 Mvp x-ray bilateral, dose rate ~ 8 – 10 R min−1, e) Table V 1 Mvp x-rays unilateral, dose rate ~ 8 – 10 R min−1. [*datasets are taken from respective Tables (Ainsworth et al. 1965)].
Kaplan-Meier survival curves demonstrated dose- and time-dependent survival probability relative to the dose cohorts exposed to reactor-derived mixed neutron:gamma radiation, bilateral, unilateral steady state and unilateral at pulse dose rate and reference bilateral and unilateral 1 Mev x-radiation exposure. Note the early mortality within an approximate 12d period post mixed neutron:gamma exposure relative to that for the 1 Mev x-radiation dataset. The respective MST for mixed neutron:gamma exposure were 11.4 d, 10.2 d and 11.5 d for the bilateral, unilateral steady state (40 rads min−1) and unilateral pulse-rate exposures (Figs. 8 a-c). The respective MST for the reference, 1 Mev x-radiation were 16.9 d and 15.5 d for bilateral and unilateral exposure (Figs. 8 d, e). The respective Kaplan-Meier plots and the noted MST values suggested a clear effect of the mixed neutron:gamma radiations on the GI system relative to that of the 1 Mvp x-radiation.
George et al. (1968).
The authors focused on determining the H-ARS DRR for mortality of canines exposed to total-body-irradiation (TBI) with mixed gamma:neutron or 250 kVp uniform radiation via rotational exposure (George et al. 1968).
Animals.
Healthy, n = 165, purebred beagles, 2–3 y age, male/female, Animals were immunized against distemper and rabies and examined for parasitic infections and after 4 wks of quarantine were transferred to temperature-controlled rooms and individual stainless-steel cages at the AFRRI; fed kibbled laboratory chow supplemented once a wk with high protein canned meat ration and water ad libitum. The animals did not receive medical management.
Radiation sources.
AFRRI radial beam generator for x-radiation: The generator operated at 250 kVp, current of 30 mA with filtration of 0.95 mm Cu and 1.2 mm Be resulted in an HVL of 1.9 mm Cu. Canines were TBI in a plexiglass box positioned in a circular arc about the x-ray generator. Distance from the x-ray source to the canine center line was minimized. The restraint box was placed on a turntable and rotated midway through the exposure to achieve bilateral exposure. The published text has figures showing the exposure design. Exposure rate was measured in a heterogeneous tissue equivalent phantom. The absorbed dose rate at the center of the animal was 16.8 rads min−1. The depth-dose measurements indicated that the exposures were Class A uniform. A total of 82 animals were exposed to 8 MTLDs that ranged from 175 to 271 rads. Ten controls were sham-irradiated.
AFRRI TRIGA reactor.
Animals were positioned in an arc about the core center line. Plexiglass boxes, turntables and rotational exposure protocol noted for the x-radiation studies were used for reactor TBI. Approximately 60% of the tissue kerma measured free-in-air was due to gamma radiation, 30% was neutrons with energies greater than 10 kev and 10% to slower neutrons. The effective gamma radiation energy was 1–2 Mev. The depth-dose measurements indicated that the exposures were Class A uniform. A total of 83 animals were exposed to 10 MTLDs that ranged from 166 to 292 rads. Twenty controls were sham-irradiated.
Clinical observations.
The study durations were 60 d post exposure, Animals were examined daily at least once every 6 h. Blood samples were procured from approximately one-third of the animals (two males and two females from each dose cohort of 10 canines) for hematological values. Rectal temperature was measured at that time.
Results: Survival.
The respective LD50/30 values consequent to mixed gamma:neutron (60:30) exposure was ~ 206 rads [198, 214] and ~ 218 rads [212, 225] for 250 kVp x-radiation. There was no difference noted in survival time between the mixed gamma:neutron- and 250 kVp-exposed cohorts. The RBE for the LD50/30 was 0.94 for mixed gamma:neutron (60/30) relative to 250 kVp x-radiation; the overall MST was ~ 13.5 – 15.5) (Fig. 5; Table 7).
Hematological recovery.
Lymphocytes decreased within d1–2 post exposure and increased slowly with values “much less” than baseline at 60d post TBI. Neutrophils were found at nadir levels within the 2 wk period post TBI and started recovery within 3 wk. However, it was noted that neutrophils had not recovered to within baseline through 60 d post TBI. Neutrophil ablation and slow recovery kinetics were more severe in the non-surviving animals. Platelet counts decreased within 1 wk with nadirs within 2 wk and return to baseline values within the 60 d study duration.
Earle et al. (1971).
This report presented data from bilateral, 14.6 Mev neutrons and 1 Mvp X-irradiation exposure of canines to assess respective LD50/30 values, the RBE and hematological response to exposures approximately 50% of the respective LD50/30 (Earle et al. 1971).
Animals.
Canine: beagles, male and female, n = 82, colony bred, 11.3 kg, 15.0 ± 2.2 mo of age and 14.1 ± 1.7 cm thick at mid-chest. Animals were transferred to the holding facility several months prior to experiments and then to individual cages 2 wk before irradiation. Dog chow and water provided ad libitum; food was removed 24 h prior to exposure. Animals were transported to the Lawrence Radiation Laboratory for neutron or 1 Mvp x-irradiation exposure. The animals received Sparine, im, 3 mg lb−1 (promazine hydrochloride, an anti-emetic, mild tranquilizer) 30–60 min prior to irradiation. No supportive care was administered to the irradiated animals.
Radiation sources: X-radiation exposure.
A 1 MVp resonant transformer machine that produced 35 R min−1 with HVL of 9.5 mm Cu at midpoint of the exposure volume. Animals were bilaterally irradiated with one-half of dose from each side. The maximum dose was less than 5% above the surface dose; for unilateral dose at 7 cm depth, the dose was 71% of the surface dose.
Neutron exposure.
The neutron source was the insulated core transformer at the University of California Lawrence Radiation Laboratory, Livermore, CA. The insulated core transformer produced a 2 mA beam of 200 keV deuterons directed at a water-cooled tritiated titanium target and yielded nearly isotropic fields of neutrons with a nearly homogeneous energy of 14.57 MeV at 45⁰ from the deuteron beam axis that were delivered at ~3 rads min−1.
LD50/30 values.
The data for neutron exposure was pooled over 10 rad intervals because minor variations in doses measured by surface monitors were unavoidable. Pooling did not occur for the 1 Mev x-radiation. The LD50/30 values were expressed as MTLD in rads or as exposure in rads or roentgens (R) for neutrons or x-rays, respectively and measured at the midpoint of the exposure volume (MEVD) in the absence of the canine. The MEVD of canines could be compared to depth doses obtained from phantom studies and converted to MTD. *The experimentally determined conversion factor was MTD = 0.72 MEVD.
Hematology. Eight canines were exposed to a neutron dose of 136 rads (MTD, range 132–142) to assess the hematological response over 73d post exposure.
Results.
A 14.6 Mev exposure resulted in an LD50/30 of 379 rads [349, 461] (MEVD). The dosimetric data supported an estimated LD50/30 of 281 rads (MTD) and the average survival time was 17.5 ± 1.9 d. The LD50/30 with 1 Mvp x-ray was 288 rads (MTD) (Fig. 9, Table 7). The average survival time was 23.7 ± 2.1 d.
Fig. 9.
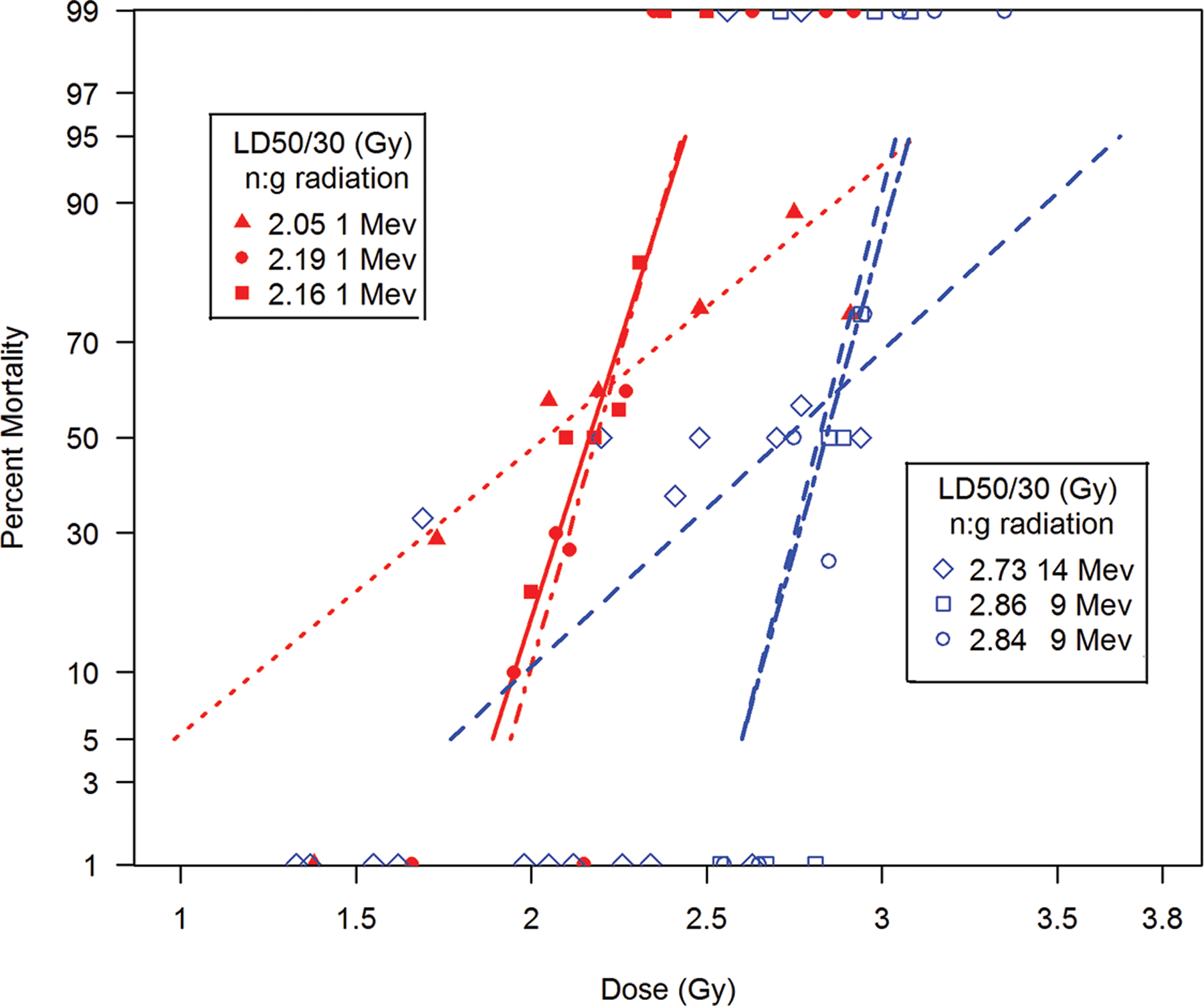
The comparative DRRs for exposure of canines to differential quality mixed neutron:gamma radiation of approximately 1 Mev versus 14.6 and 9 Mev delivered by bilateral, uniform exposure. Canines, beagles or mongrels, were exposed in separate studies to mixed neutron:gamma radiation of ~ 1 Mev neutrons relative to high energy neutrons 14.6, 9.0 Mev and 9.0 Mev (Bond et al. 1956; Alpen et al. 1960; Ainsworth et al. 1965; George et al. 1968; Earle et al. 1971; MacVittie and Jackson III 2020).
No canine succumbed earlier than 10 d post exposure. The MTD LD50/30 values provided an RBE for 14.6 Mev neutrons relative to 1 Mev x-radiation of 1.01. The database also included a depth dose curve for unilateral exposure of the 1 Mvp x- and 14.6 Mev neutron-radiation. The midplane depth doses for 1 Mvp x- and 14.6 Mev neutron-radiation were 71.7% and 76.6%, respectively.
Hematology. The WBC, in the cohort exposed to 136 rads (MTD) of the 14.6 Mev neutrons decreased over 6 d and recovered to within pre-irradiation levels by 49 d.
MacVittie and Jackson III (2020).
The authors performed a retrospective analysis of acute radiation-induced GI-ARS and H-ARS in canines (MacVittie and Jackson III 2020). Animals. Pure-bred male and female canines (Beagle), n = 60 total, 10–12 kg bw were treated to eliminate parasitic infections, immunized against distemper, hepatitis and rabies. Canines were quarantined for 2 wk prior to entering the experimental design. Canines were housed in temperature-controlled rooms, individual stainless-steel cages, water was ad libitum, fed kibbled dog food, supplemented once a wk with canned meat ration.
Radiation source and exposure.
AFRRI TRIGA reactor: Animals were exposed to mixed neutron:gamma (6:1), neutron energy approximately 1 Mev and average gamma energy of 0.9 Mev, operated in steady-state mode. The reference radiation was Co-60 gamma delivered from the AFRRI Cobalt source. Bilateral, uniform exposure was established with canines rotated 180 degrees at midline exposure at a dose rate of ~60 cGy min−1. Depth-dose measurements were made at the center of a cylindrical lucite, water-filled phantom of 15.2 cm diameter that was close to the average 16 cm diameter of 15 canines. The total neutron dose plus gamma dose measured at the phantom midline was 49% of that free-in-air. Animals, n = 60, were exposed to mixed neutron:gamma over the range of 150 cGy to 300 cGy at MTLD.
Co-60 gamma radiation.
The Co-60 gamma radiation was delivered bilateral at a dose rate of 0.1Gy min−1, MTD absorbed dose. Reference radiation exposure and established DRR for H-ARS were described previously (MacVittie et al. 1984; MacVittie et al. 1991).
Results.
The respective LD50/6 or LD50/30 values were: mixed neutron:gamma (6:1), LD50/6 ~ 283 cGy [276, 294], and LD50/30 ~ 216 cGy [206, 223] (Fig. 10). The reference Co-60 gamma radiation for the LD50/30 was ~ 260 cGy. Supportive care was not administered to the animals. These values projected an RBE ~ 1.20. The 1991 published study showed LD50/30 consequent to mixed neutron:gamma (6:1) of ~ 185 rads (MacVittie et al. 1991).
Fig. 10.
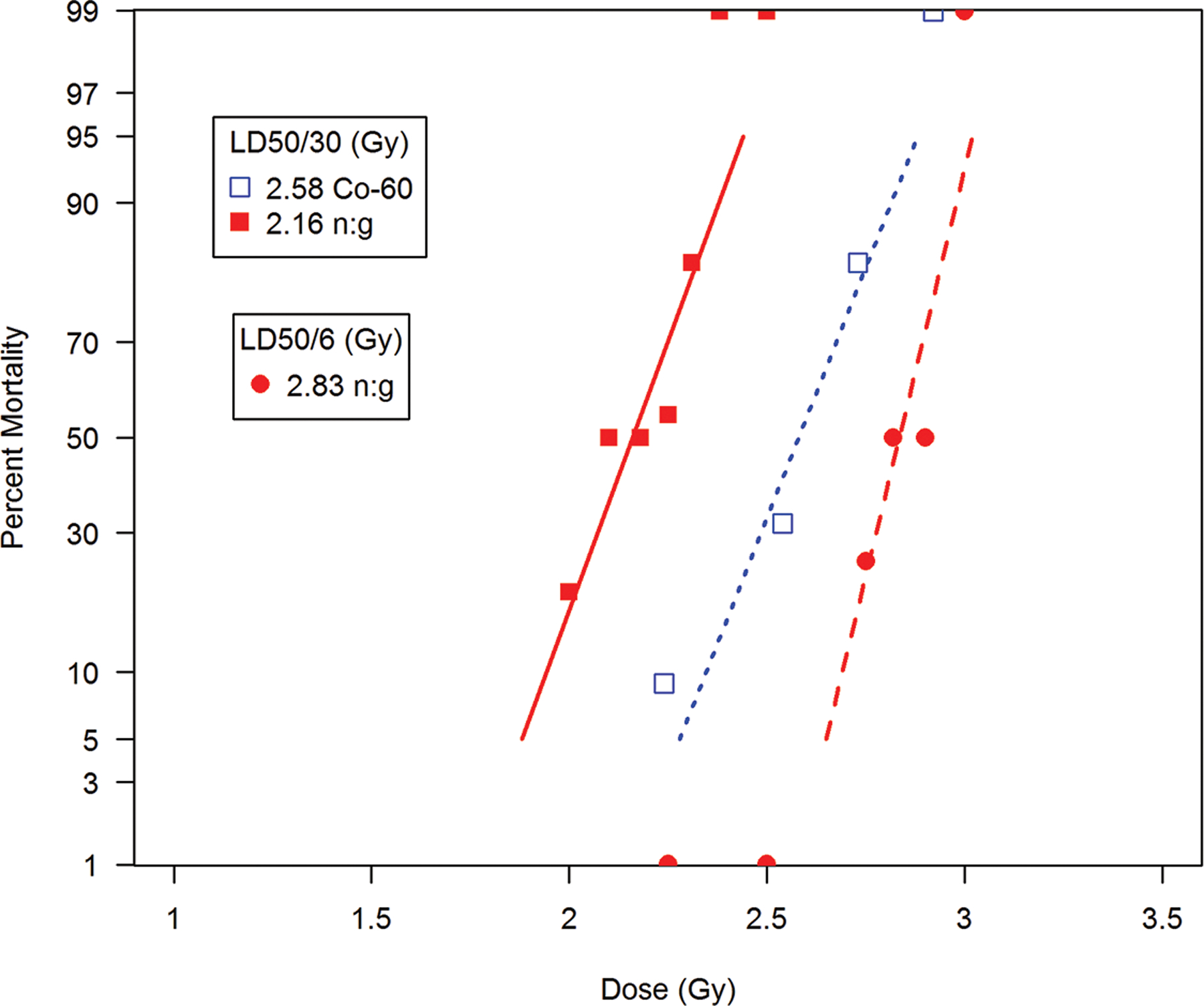
The comparative DRRs for acute H- and GI-ARS in canines induced by mixed neutron:gamma, bilateral, total-body irradiation relative to bilateral Co-60 gamma radiation. The dose response relationship (DRR) for H- and GI-ARS in the canine (beagle) derived from total-body, steady-state, bilateral, uniform, mixed neutron:gamma irradiation (5.4:1) with average neutron:gamma energy at 0.85/0.9 MeV (n = 78 total) (MacVittie and Jackson 2020) and a reference dataset of bilateral, uniform, total-body Co-60 gamma radiation (n = 61, male, female beagle) (Norris et al. 1968). The retrospective data sets are presented as probit percent mortality vs TBI as linear normal dose (Gy)
The DRRs for canines exposed to mixed neutron:gamma radiation relative to exposure aspect and uniformity, dose rate, neutron energy and reference radiation.
The systematic review herein provides a descriptive summary of primary studies designed to determine the DRR for canines under variable mixed neutron:gamma exposure conditions. The reference radiations were 250 kVp and 1 Mev x-ray and Co-60 gamma radiation. The DRRs are calculated from the data sets provided in the referenced studies. The dose is described as MTD in rad or Gy. Conversion factors were noted in the text for specific studies (Figs. 5–7, 9, 10).
The comparative DRRs for exposure of canines to mixed neutron:gamma relative to exposure aspect, dose rate, energy and reference radiation.
Canines, beagles or mongrels, were exposed to mixed neutron:gamma radiation in numerous studies designed to determine the DRRs for exposure to neutrons of variable energy from ~ 1 Mev to high energy, 9 Mev or 14.6 Mev using bilateral or unilateral exposure, variable neutron:gamma ratio, and dose rate, steady state vs pulse rate exposure. Reference radiation shown here was 1 Mev x-radiation and Co-60 gamma radiation.
MacVittie et al. (1984).
The authors presented an early, extended database for the 1991 manuscript described below (MacVittie et al. 1991). The study provided a dataset that described the hematopoietic and hematologic response to mixed neutron:gamma and Co-60 gamma radiation and mixed neutron:gamma radiation (MacVittie et al. 1984). The mortality dataset was preliminary relative to that provided in the 1991 manuscript (MacVittie et al. 1991).
Animals.
Pure-bred, male and female beagles (n = 51 total, 9 to 12 kg bw) were treated to eliminate parasitic infections, immunized against distemper, hepatitis and rabies 2 wk prior to entering experimental design. Canines were housed in temperature-controlled rooms in individual stainless-steel cages, fed kibbled dog food, water ad libitum and supplemented once a wk with canned meat ration.
Radiation exposure.
Animals were exposed using the AFRRI TRIGA, Mark F pool-type thermal research reactor. The exposure was steady-state, bilateral at a dose rate of 0.6 Gy min−1 with 180 degree rotation at mid exposure. The average neutron and gamma energies were 0.9 Mev and 1 Mev, respectively with a mixed neutron:gamma ratio of 6:1. The Co-60 gamma radiation MTD was delivered bilateral at a dose rate of 0.1 Gy min−1.
Dose and dosimetry.
Canines were exposed to MTD Co-60 gamma radiation at 1.50 and 2.50 Gy; mixed fission neutron:gamma radiation at 0.75 and 1.25 Gy. Depth-dose measurements made at center of a cylindrical phantom. The diameter of 15.2 cm was determined from diameter of 54 normal canines. The total neutron:gamma radiation dose at midline in phantom was ~ 49% of free-in-air dose
Medical management.
Antibiotic were administered [Ampicillin 500 mg (polycillin-N, Bristol labs and gentamicin sulfate, 30 mg (Garamycin, Schering Pharmaceutical Corp)] daily until the WBC level reached 1000 µL−1. Fluids (Ringer’s lactate) were administered intravenously as dictated by clinical symptoms. Platelets (from the product of platelet pheresis) obtained from donors were irradiated with 5,000 rad (Co-60 gamma radiation) and administered to canines at d 12, 15 and 18 post TBI.
Hematology, in vitro hematopoietic cultures.
BM aspirates were procured from ribs and iliac crest of anesthetized canines. Peripheral blood-derived CBC were assessed by hemacytometer. BM-derived mononuclear cells (MNC) were separated and assayed for hematopoietic progenitor cells identified as GM-CFC.
Results.
Mortality, LD50/30 values.
The respective dose response relationships for both neutron:gamma and Co-60 gamma radiation are described previously (MacVittie et al. 1991).
Peripheral WBC recovery. Comparative recovery kinetics relative to differential exposure to Co-60 gamma radiation (1.5 and 2.5 Gy) or mixed neutron:gamma radiation (0.75 and 1.25 Gy) were assessed through 30d post exposure (mean values n = 4 to 6). The mixed neutron:gamma exposures induced greater depth of nadirs, slower and more prolonged recovery kinetics through 30d post exposure relative to the Co-60 gamma radiation doses. The mixed neutron: gamma exposures demonstrated a dose effect whereas the leucocyte recovery kinetics for the cohorts exposed to Co-60 gamma radiation, were not different from each other. These data suggested a marked RBE for the mixed neutron:gamma exposures relative to the Co-60 gamma radiation. The recovery kinetics for platelets showed a similar, greater effect for the mixed neutron:gamma exposures than the Co-60 gamma radiation.
Hematopoietic progenitor cells, BM-derived GM-CFC.
The relative radiation sensitivity, D0 values. The percent survival of GM-CFC10−5 MNC relative to pre-irradiation values for each mixed neutron:gamma and Co-60 gamma radiations were assayed from BM aspirated at 24 h after exposure to doses over a range from 0.25 Gy to 3.50 Gy. The respective D0 values for BM-derived MNC obtained at 24 h post TBI were 0.73 and 0.30 Gy for Co-60 gamma and mixed neutron:gamma radiation, respectively.
BM-derived GM-CFC recovery kinetics post TBI.
The recovery kinetics of BM-derived GM-CFC reflected that of the circulating WBC. Recovery of GM-CFC after exposure to 0.75 Gy or 1.50 Gy mixed neutron:gamma and Co-60 gamma radiation respectively, were similar and reflected the noted RBE of the mixed neutron:gamma radiations. It required approximately 25–30 d for BM-derived GM-CFC to recover to within pre-irradiation values.
MacVittie et al. (1991).
This paper provided a relatively complete description of radiation dose delivery and dosimetry in two canine-specific phantoms (MacVittie et al. 1991). The dose distribution consequent to bilateral and unilateral exposures were well defined.
Animals.
Pure-bred male and female canines (Beagle), n = 144 total, 10 to 12 kg bw were treated to eliminate parasitic infections, immunized against distemper, hepatitis and rabies. Canines were quarantined for 2 wk prior to entering the experimental design. Canines were housed in temperature-controlled rooms, individual stainless-steel cages, water was ad libitum, fed kibbled dog food, supplemented once a wk with canned meat ration.
Radiation sources.
The AFRRI TRIGA, Mark F pool-type thermal research reactor was used for TBI at steady-state, bilateral exposure, at a dose rate of 0.6 Gy min−1 with 180 degree rotation at mid exposure. The average neutron and gamma radiation energies were 1 Mev and 0.9 Mev; the mixed neutron:gamma radiation ratio was 5.4:1 at entry dose. TBI with Co-60 gamma radiation was delivered as bilateral exposure with a dose rate of 0.1 Gy min−1, MTD absorbed dose.
Radiation exposure.
Canines were exposed to bilateral, MTD over a range of 0.25–4.0 Gy Co-60 gamma (n = 88) and 1.00 to 3.25 Gy mixed neutron:gamma radiation (n = 56). The Co-60 gamma cohort was further divided into those exposed without (n = 39) and with (n = 49) medical management. The mixed neutron:gamma exposed cohort was similarly divided into those with (n = 27) and without (n = 29) medical management. The animals were secured in Plexiglas holders for both types of exposures. Mixed neutron:gamma irradiation was achieved in a gadolinium-lined exposure room.
Radiation dosimetry.
Two canine phantoms, one each from AFRRI and The Radiobiological Institute TNO (Rijswijk, The Netherlands) contained canine skeletons, simulated lung tissue and contoured body mass. See article for a description of the canine phantoms. The paper provided a good presentation of gamma- and neutron-radiation dosimetry, neutron and gamma radiation depth-dose curves for bilateral and unilateral exposures and MTD dosimetry.
Hematology, hematopoietic cultures.
BM aspirates were procured from ribs and iliac crest of anesthetized canines. Peripheral blood-derived CBC were assessed by a hemacytometer. BM-derived MNC were separated and assayed for hematopoietic progenitor cells identified as GM-CFC.
Medical management.
Antibiotics [Ampicillin 500 mg (polycillin-N, Bristol labs; gentamicin sulfate, 30 mg (Garamycin, Schering Pharmaceutical Corp)] were administered daily until the WBC level was 1,000 mm−3. Fluids (Ringer’s lactate) iv dictated by clinical symptoms. Platelets (platelet pheresis product) from donors, irradiated to 5,000 rad (Co-60 gamma radiation) were administered to canines at d 12, 15, and 18 post TBI.
Results.
LD50/30 values.
The respective LD50/30 values for Co-60 gamma radiation and mixed neutron:gamma for bilateral, uniform exposure were 2.60 Gy [2.51, 2.89] and 1.53 Gy [1.31, 1.69] for clinically unsupported canines, respectively. The RBE for LD50/30 values was 1.69 [1.49, 1.92]. Administration of medical management significantly improved survival and increased the LD50/30 values independent of radiation quality. The resultant LD50/30 values for clinically supported animals exposed to Co-60 gamma- and mixed neutron:gamma radiation were 3.38 Gy [3.23, 3.55] and 1.85 Gy [1.52, 2.13], respectively. The RBE for LD50/30 was 1.82. The respective DRF’s for medical management were 1.3 (p < 0.001) and 1.21 (p < 0.04).
Radiation sensitivity of hematopoietic progenitors after in vivo exposure, D0 values.
The percent survival of GM-CFC 10−5 MNC relative to pre-irradiation values for each mixed neutron:gamma and Co-60 gamma radiations were assayed after exposure to doses over a range from 0.25 Gy to 4.00 Gy. The respective D0 values for BM-derived MNC obtained at 24 h post TBI were 0.67 ± 0.03 Gy and 0.3 ± 0.04 Gy (p < 0.001) for Co-60 gamma and mixed neutron:gamma radiation, respectively. The estimated RBE was 1.9 ± 0.2 (Carsten et al. 1976; Seed and Kaspar 1991).
The data suggested a significant RBE for the fission neutron spectrum radiation relative to Co-60 gamma radiation. The RBE is based on prescribed MTD to define a descriptive dose for the lethal H-ARS (Bond et al. 1957). Assessing accurate dose distribution to specific organ volume would relate the organ dose to the descriptive dose and consequent biology (Prado et al. 2015; Prado et al. 2017). The exposure of canines reported an entrance 5.4:1 mixed neutron:gamma ratio (0.8 – 1.0 Mev) free-in-air which decreased to 1.7:1 ratio at MTD with no apparent change in the effective neutron spectrum. The dose characteristics relative to specific organ volume are unknown. The physical characteristics and bw of canines would have marked effects on dose distribution. The accurate determination of the RBE for mixed neutron:gamma, Co-60 gamma, 0.25 – 1 Mev x-radiation or LINAC-derived 6 Mev photon exposures at MTD and organ-specific endpoints such as lethality due to acute GI-, H-ARS or delayed lung injury will be difficult when using a descriptive dose to the prescribed midline tissue target. Additionally, the paper presented a good discussion on dosimetry, use of phantoms, cadaveric canines and dose inhomogeneity due to tissue depth and organ volume.
Wang et al. (1991).
The authors focused on assessing the effect of mixed neutron:gamma radiation of different neutron:gamma ratios on induction of the GI- and H-ARS (Wang et al. 1991). The reference exposure was to Co-60 gamma radiation. The authors provided dosimetry information for unilateral depth dose as well as survival times relative to dose and estimated RBE’s for the GI- and H-ARS.
Animals.
Mongrel, male canines (n = 128), at 10–20 kg bw were used. A quarantine period was noted in the text and animals were examined regularly. The animals did not receive medical management.
Radiation sources.
Mixed fission neutron:gamma radiation was delivered by the Tsinghua University shielding experimental reactor (light water-cooled and moderated pool type). The effect of Co-60 gamma-irradiation was represented via an historical data base of canines that were total-body irradiated at 35 – 110 cGy min−1. A unilateral/bilateral coefficient was used to relate to unilateral mixed neutron:gamma radiation (Ainsworth et al. 1965).
Radiation exposure.
Canines were total-body, unilaterally irradiated in a rectangular, perforated aluminum canister at a dose rate of 9.7 – 59.4 cGy min−1. Lead plates with different thickness (10, 8, 5 cm) were used to moderate the incident beam and gamma radiation contribution. Mixed neutron beams were obtained with three different 1.33 Mev, neutron:gamma ratios: 90%, 50% and 15%. The average neutron energy was 1.33 Mev. The distribution of absorbed doses for neutrons and gamma radiation were determined in a canine-sized phantom (rectangular 6 cm Lucite container filled with tissue-equivalent liquid; 16 × 16 × 60 cm). The midline, entrance and exit doses Dm, Den, Dex, for neutron:gamma radiations and ratios Den/Dex, and Den - Dex/Dex were shown as an indication of dose inhomogeneity in dose distribution (Table 8). The ratio of entrance dose (Den) to exit dose (Dex) was 3.48.
Table 8.
Total-body, unilateral mixed neutron:gamma exposure of canines: Dose (D) rate: midline (m), entrance (en), and exit (ex) dose rates, and their ratios for different irradiation conditions.
| Conditions Parameter | Absorbed dose rate mGy min−1 kW) |
|---|---|
| Dm | 4.16 |
| Den | 8.60 |
| Dex | 2.47 |
| Den/Dex | 3.48 |
| (Den - Dex)/Dm | 1.47 |
Canines (male, mongrel), n=128, were exposed to mixed fission neutron/gamma radiation, unilaterally with their right sides facing the sources. Table shows distribution of absorbed doses for neutron:gamma radiation. The midline, entrance, and exit doses, Dm, Den, and Dex, for neutron plus gamma radiations, and the ratios Den/Dex and (Den - Dex)/Dm as an indication of non-homogeneity in dose distribution. The ratio of entrance dose (Den) to exit dose (Dex) was 3.48 (Wang et al.; 1991).
Hematologic exposures.
Three regimens of mixed irradiation were used with neutron:gamma ratios at the center of the empty animal holder of 10.6/1, 1.15/1, and 0.16/1, respectively (relative to the ~ 90, 50 and 15% neutron contribution). The respective dose rates were 9.7, 38.3 and 59.6 cGy min−1.
Results.
GI ARS:
The authors used mortality at 5 d post exposure as the time frame for lethal GI ARS after unilateral exposure. The threshold, 100% lethal doses for GI ARS were 3.7, 6.5 and 8.7 Gy for the mixed neutron:gamma exposures with 90%, 50% and 15% neutrons, respectively. The RBE values for mixed neutron:gamma exposures relative to 10 Gy Co-60 gamma radiation were ~ 2.7, 1.5 and 1.1, respectively. The LD50/5 values for the three cohorts of irradiated canines were 2.8, 5.0 and 7.0 Gy relative to the 90%, 50%, and 15% mixed neutron:gamma ratios. In this case, the respective RBE values for LD50/5 were 2.9, 1.6 and 1.1. The greater the % of neutrons, the greater was the amount of acute GI damage.
H ARS:
The increased neutron component reduced the absorbed, unilateral dose required for mortality due the hematopoietic syndrome. Mortality increased with absorbed dose. The LD50/30 values were 1.74, 2.32, and 2.80 Gy for the 90%, 50% and 15% neutron component exposures. The reference LD50/30 for Co-60 gamma radiation was 2.99 Gy. These respective values resulted in an RBE of 1.7, 1.3 and 1.1 for the H-ARS.
GI and H ARS:
The authors unilaterally irradiated each of four cohorts at 2.65 Gy MTD using the three different mixed neutron:gamma radiation ratios at 90%, 50% and 15% relative to Co-60 gamma radiation. The 30-day mortality and MST varied relative to the neutron:gamma ratio. The respective mortality (dead/total) at 2.65 Gy were: 90% neutron:gamma, (6/6), 100% mortality; 50% neutron:gamma, (8/10) 80% mortality; 10% neutron:gamma, (6/10) 60% mortality; Co60-gamma (2/6) 33.3% mortality. (MST 90%, 10.1d; 50% 14.9d; 10%, 19.5d; Co-60, 20.8d). The clinical signs of the GI ARS (loss of appetite, emesis, diarrhea, bloody diarrhea) increased with increasing percentage of neutrons. The onset of fever also increased with neutron percentage. The loss of leucocytes and platelets decreased more rapidly in the mixed neutron:gamma irradiated cohorts.
Wang and colleagues summarized critical points derived from their studies (1) The threshold dose for the GI-ARS decreased with increasing percentage of neutrons in the mixed neutron:gamma exposures, (2) The dose range for the H ARS and transition to GI ARS was reduced with higher neutron:gamma ratio, (3) The higher the neutron:gamma ratio, the more severe the H-ARS, (4) The RBE for GI ARS in canines was greater than three; the neutron component causes greater GI injury within the H ARS.
Pitchford and Thorp (1968).
Canine Beagles were exposed using the AFRRI TRIGA reactor with 1 Mev, mixed neutron:gamma, unilateral pulse exposure over the range of 155 – 236 rads, MLTD (Pitchford and Thorp 1968). The estimated LD50/30 was ~ 210 rads with the MST that ranged from 10 – 25 d. This is a valuable missing data set that is referenced in published studies.
A representative canine dataset shown below emphasized the dose-dependent LD50/30 to 250 kVp x-, 1 Mev x-radiation, and Co-60 gamma radiation relative to mixed neutron:gamma irradiation.
Secondary studies. Reports that provided a database of large animal studies using the canine to define the H-ARS consequent to mixed neutron:gamma radiations.
Alpen and Baum (1959).
The control cohort in this study provided evidence of lethality at the conditioning dose for BM transplant. The intent was to assess the GI ARS component of fast neutron exposure at the single dose of 470 rads for the autologous marrow transplant relative to the control cohort (Alpen and Baum 1959).
Animals.
Canine (mongrels), 11 to 15 kg bw, maintained at the laboratory stock facility. Animals underwent a period of quarantine, treated for intestinal parasite and immunized against distemper, hepatitis and rabies and housed in individual cages in preparation for irradiation and BM transplant.
Radiation source and exposure.
Bilateral, TBI was delivered by the neutron source [4Be9(p, n)5B9] at the 60 inch cyclotron of the Crocker Laboratory, University of California, Berkeley, CA (Tochilin and Kohler 1958). The mean neutron energy was 9 Mev. This was the same source used by Bond et al. and Alpen et al. (Bond et al. 1956; Alpen et al. 1960). The gamma ray dose at midline was ~ 26% of first collision dose. The total neutron dose at midline was 59% of the first collision dose. Depth dose measurements were provided in the text. The total tissue rads at any point in the animal was essentially equal at 85–88% of the first collision dose. The transplant (n = 11) cohort received 470 rads, untreated animals (n = 7) received 310 rads, all exposures were expressed as “first collision dose at the skin surface”.
Results: GI, H ARS.
The control untreated cohort exposed to 310 rads of fast neutrons of 9 Mev was 100% lethal (7/7) with a mean survival time of 14.3 d. The survival time indicated that the control, untreated cohort succumbed to the H ARS. The animals that received 470 rads and a marrow transplant had 36% lethality (4 of 11). Furthermore, the average survival time of the decedents after transplant was 3.7 d and indicative of the severe GI ARS. The clinical signs consisted of severe dehydration, diarrhea and emesis prior to death. The study suggested that the threshold for predominant GI injury after neutron (9 Mev) irradiation was greater than 310 rads and less than 470 rads. Seven of eleven (65%) animals were spared from the lethal GI or H ARS by the autologous marrow transplant. The authors note that the 470 rad “first collision dose at skin surface” translated to a MTLD of 410 rads, of which approximately 125 rads was gamma radiation.
Baum and Wyant (1970).
The authors described the hematopoietic recovery in irradiated dogs (Baum and Wyant 1970).
Animals.
Canines, pure-bred beagles, 1 – 2 y of age; immunized against distemper, hepatitis and rabies and eliminated of parasite infestation, Animals were transferred to temperature-controlled rooms at the AFRRI; maintained in individual stainless-steel cages, fed kibbled dog chow and fed high protein canned meat ration once a wk. A total of 252 canines were utilized; 24 non-irradiated controls, 114 exposed to 150 rads of x-rays and 114 exposed to mixed neutron:gamma radiation.
X-ray exposure.
A radial beam generator produced 250 kVp, 30 mA, 1.2 mm Be + 0.05 mm Cu filtration (HVL = 1.0 mm Cu). The absorbed dose rate at the center of the animals was 20 rads min−1. Animals were placed in Plexiglass containers and arranged in an equidistant circle from the source and rotated 180⁰ at half exposure.
Mixed neutron:gamma exposure.
The AFRRI TRIGA Reactor produced neutron:gamma tissue kerma rate free-in-air, bilateral exposure at 20 rads min−1. The absorbed dose is at the midline of the animal. The ratio of neutron to gamma tissue dose was not measured but estimated to be ~ 0.67 and the effective gamma energy was between 1 – 2 Mev.
The objective was to assess hematological recovery in dogs exposed once or repeatedly to either 150 rads x-radiation or 150 rads mixed neutron:gamma radiation. Demonstrated development of residual BM injury to the hematopoietic system.
Hematologic recovery.
Hematology baseline controls were assessed for Fe59 incorporation into recent erythrocytes, erythrocyte and plasma volume, hematocrits and peripheral blood leucocyte counts. Plasma iron concentration and clearance was measured for plasma iron turnover. Parameters were obtained from six different canines is each radiation cohort.
Residual injury parameter. Canines were irradiated for a second and third time at 90 d intervals after the initial exposure. All hematological tests were repeated for each cohort.
Results.
The authors showed that the hematological effects of 150 rads of x-radiation was greater than that of the neutron:gamma radiation. It was noted that the mixed neutron:gamma radiation was approximately 60% gamma radiation. The survival data after the repeated exposures indicated a greater degree of damage to the hematopoietic system after x-radiation than the mixed neutron:gamma radiation. Lethality after a single, second and third exposure of x-radiation increased from 2.6%, to 15% and 36% relative to respective mixed neutron:gamma-induced lethality of 1.8%, 2.6% and 8%.
Yu et al. (2011).
This study was designed to test the effects of recombinant human granulocyte colony-stimulating factor (rhG-CSF) in dogs which had received 2.3 Gy of ~ 1.4 Mev mixed fission neutron-gamme, unilateral irradiation, with a high ratio of neutrons (~ 90%) (Yu et al. 2011). A marked GI-ARS was noted in all irradiated cohorts. The rhG-CSF treatment induced 100% survival versus 60% in controls. The study design included medical management.
Experimental animals.
Healthy beagle dogs weighing 8.0 – 11.0 kg bw were purchased from the Experimental Animal Center of the Academy. Canines were housed individually in stainless steel cages in rooms with a reverse filtered air barrier, normal illumination rhythm, and stable temperature (18 – 22°C) and relative humidity (40 – 70%). They were fed commercially available primate chow and were provided with acidified drinking water. All canines were free of intestinal parasites and were seronegative for herpes B, simian T-lymphotrophic and simian immunodeficiency viruses. The housing, experiments and all other conditions were approved by an ethics committee in conformity with legal regulations in China.
Fission neutron irradiation.
The Tsinghua University shielding experimental reactor was used as the source of mixed fission-neutron:gamma radiation. This irradiation facility and the dosimetry have been described (Wang et al. 1991). The neutron energy spectrum in the irradiation compartment has an average neutron energy of 1.4 MeV at the empty irradiation position. The reactor was operated at 50 kW, resulting in a dose rate of 20.97 cGy min−1.
Radiation exposure.
Prostrate dogs placed in a rectangular perforated aluminum canister were irradiated unilaterally with their right sides facing the sources. They were exposed to mixed fission neutron and gamma radiation to a total MTLD of 2.3 Gy. The high neutron-gamma ratio (9:1) was achieved by imposing a 10 cm thick lead across the incident radiation beams. The midline, entrance, and exit doses, Dm, Den, and Dex, for neutron plus gamma radiations, and the ratios Den/Dex and (Den - Dex)/Dm as an indication of non-uniform dose distribution were established by Wang and colleagues (Wang et al. 1991).
Study design.
In the control groups, dogs were treated with the placebo solution and then received supportive care (n = 5) only or non-supportive care (n = 4). The treatment cohort received G-CSF.
Medical management.
Three days before irradiation, canines were placed in a laminar flow cabinet and the gastrointestinal tract was selectively decontaminated by administration of oral gentamicin and metronidazole (Hubei Huazhong Pharmaceutical Co., Hubei, China). An antibiotic regimen [penicillin 400,000 IU dog−1, intramuscularly, every day (qd) (North China Pharmaceutical Co., Hebei, China)], was initiated prophylactically at d 3 after irradiation until the WBC > 1 × 10−9 L for 3 consecutive days. Cefotaxime sodium [1.0 g, qd, (Yuekang Pharmaceutical Co., Beijing, China)] were administered intravenously when the WBC < 1 × 10−9 L and or the rectal temperature of animals was > 40°C. Reptilase was used when the platelet count was ≤ 100 × 10−9 L and or macroscopic hemorrhage appeared on the skin. Fresh, irradiated (20 Gy 60Co gamma irradiation) whole blood (approximately 60 mL per transfusion) from a random donor pool (canines of > 15 kg bw) was administered when platelet counts were below 50 × 10−9 L and the red blood cell counts were < 3 × 10−12 L. Dehydration and electrolyte disturbances were treated with appropriate fluid and electrolyte administered intravenously.
Results.
After 2.3 Gy neutron irradiation, all irradiated dogs showed early signs of GI damage including reduced food intake, diarrhea, and bloody diarrhea. Consistent with a previous study, the incidence of GI syndrome significantly increased in animals exposed to mixed neutron:gamma irradiation > 4.5 Gy (Wang et al. 1991; Farese et al. 1993; Farese et al. 1994). Due to severe GI syndrome, the animals experienced weight loss within 10 days after irradiation. The exposure of canines under current exposure conditions had neutron:gamma ratios at the center of the empty animal holder of 10.6:1 (about 90% neutrons). A previous study had found that the incidence of the early signs of GI syndrome increased after the mixed neutron:gamma irradiation with a high ratio of neutrons (Wang et al. 1991). Consistent with these data, our results indicated that the severity of injury in dogs after neutron-irradiation occurred with evident GI ARS in the early stage.
Tertiary Studies. Historical database; in vitro assay of granulopoietic and erythropoietic progenitor cells, dosimetry, absorbed dose, organ dose.
In vivo and in vitro analysis of granulopoietic and erythropoietic progenitor cells after in vitro and in vivo irradiation.
Seed and Kasper (1991).
The authors provided data on the RBE for hematopoietic progenitor cells assayed by in vitro methodology and exposed in vitro by 0.85 MeV mean energy JANUS Fission neutron to determine the Do for in vitro irradiated BM-derived hematopoietic progenitor cells (GM-CFC) (Seed and Kaspar 1991). The reference exposure was Co-60 gamma radiation at a dose rate of 25 cGy min−1. The respective values for Co-60 was D0 = 77 cGy and fission neutron = 28 cGy for an approximate RBE = 2.75.
Boyum and Carsten (1978).
The effect of in vitro irradiation with neutrons of increasing energy relative to x-radiation was assessed using human BM-derived granulopoietic progenitor cells (Boyum et al. 1978). Two culture techniques were used, an in vivo diffusion chamber (DC) culture method and an in vitro colony-forming unit culture (CFU-c) assay. X-irradiation resulted in D0 values of 80 rad and 117 rad for DC and CFU-c, respectively. Exposure to increasing neutron energy resulted in a diminishing RBE relative to the x-radiation D0 values: a) 0.44 Mev, DC = 3.7, CFU-c = 4.1; fission neutrons, DC = 2.6, CFU-c = 2.4; 6 Mev, DC = 1.8, CFU-c = 2.0; 15 Mev, DC = 1.6, CFU-c = 1.6.
Schwartz et al. (1986).
The authors assessed the effects of mixed neutron:gamma, in vitro exposure on two classes of BM-derived erythroid progenitor cells; burst-forming units, erythroid (BFU-e) and erythroid colony-forming cells (CFU-e) (Schwartz et al. 1986). The radiation sensitivity (Do value) was determined for CFU-e and for BFU-e in BM-cells exposed in vitro to 1 MeV fission neutron radiation or 250 kVp X rays. 1 MeV neutron exposure resulted in the D0 for CFU-e and BFU-e at 0.27 ± 0.01 Gy and 0.16 ± 0.03 Gy, respectively. The D0 values for CFU-e and BFU-e were, respectively, 0.61 ± 0.05 Gy and 0.26 ± 0.09 Gy for marrow cells subject to x-radiation. The RBE values for the marrow-derived hematopoietic, erythroid progenitor cells described therein were 2.3 for CFU-e and 1.6 for BFU-e.
MacVittie et al. (1991).
The authors assessed the radiation sensitivity via D0 values, of hematopoietic, granulocyte-macrophage (GM) progenitors after in vivo exposure (MacVittie et al. 1991). The percent survival of GM-CFC 10−5 MNC relative to pre-irradiation values for each mixed neutron:gamma and Co-60 gamma radiations were assayed after in vivo exposure to doses over a range from 0.25 Gy to 4.00 Gy. The respective D0 values for BM-derived GM-CFC assayed from separated mononuclear cells obtained at 24 h post TBI were 0.67 ± 0.03 Gy and 0.3 ± 0.04 Gy (p < 0.001) for Co-60 gamma- and mixed neutron:gamma radiation, respectively. The estimated RBE was 1.9 ± 0.2.
Depth dose assessment in the canine model.
Wingate et al. (1967).
The authors provided depth dose distribution data from canine cadaver and cylindrical phantoms for 1 Mvp x- and mixed neutron:gamma radiations (Wingate et al. 1967). It was noted that 1 Mvp x-radiation is considerably more penetrating than reactor-derived neutrons.
Cadaveric canine and cylindrical phantom.
A mongrel canine, 10 kg bw was euthanized and quickly frozen in appropriate shape for assessing depth-dose after exposure to radiation sources. The cadaver preparation was described in the text. The phantom was 6 inches of tissue-equivalent material used previously (Alpen et al. 1960; Simpson et al. 1963). All cadaver irradiations were unilateral (right to left side) depth-dose patterns from the TRIGA Mark F reactor for mixed neutron:gamma vs 1 Mev x-radiation. The respective mixed neutron and gamma energy was ~ 0.6 Mev and 1.25 Mev. The neutron:gamma ratio was 6:1.
Radiation sources.
Mixed neutron:gamma radiations were performed with General Atomics TRIGA (Model Mark F) at San Diego, CA. The facility and animal exposure geometry was described by Simpson and colleagues (Simpson et al. 1963). X-radiation (1 Mvp) was delivered by a General Electric machine operated at 15 ma, HVL of 9.5 mm Cu (photon energy ~ 540 keV) and dose rate of 10 R min−1.
Results.
Wingate and colleagues presented a thorough description of a depth-dose database consequent to mixed neutron:gamma vs 1 Mev x-radiation of a canine phantom relative to an actual canine cadaver. These studies confirmed the experimental accuracy of the MTD based on phantom dosimetry for exposed canines in earlier studies, as well as the quality factor of 1.4 based on MTD for LD50/30 values relative to neutron:gamma vs 1 Mev x-radiation (Alpen et al. 1960; Ainsworth et al. 1964; Ainsworth et al. 1965). The quality factor would be ~ 1.0 if based on midline-air-dose in the absence of the animal. The authors note the potential difference between using a descriptive dose such as MTD relative to a specific organ dose associated with the specific sub-syndrome.
Wingate et al., summarized key aspects of the isodose patterns and depth-dose curves from the canine cadaver analysis: (1) Depth-dose curves for 1 Mvp x-rays do not differ significantly in the head, chest and abdomen, (2) Depth-dose curves for total (neutron plus gamma) were not different in shape in different cross sections of the cadaver, (3) The lung tissue had a greater neutron dose component than other body sections. The gamma-ray dose was less, (4) The bony regions did not cause neutron isodose deviations, (5) The total (neutron plus gamma) dose was more rapidly attenuated than the 1 Mvp x-radiation.
Ainsworth et al. (1964).
Radiation lethality as a function of radiation dose rate has been extensively explored over the range of less than 1 rad to several hundred rad min−1, but comparatively little is known of the biological consequences at exposure intensities of the order of 105 to 106 rad min−1. Experiments with radiations produced by a TRIGA reactor were used to study the acute-mortality responses of dogs irradiated either at a moderate dose rate (23 rad min−1 for dogs) or by a single high dose-rate radiation pulse (~2.0 × 105 rad min−1 for dogs) (Ainsworth et al. 1964). In acute mortality studies conducted with unilaterally, mixed neutron:gamma-irradiated dogs, no significant differences in LD50/30 were found between groups irradiated at 23 rad min−1 or exposed to pulsed dose rates, in excess of 1.5 × 105 rad min−1.
Neutron and gamma radiations.
The doses are presented as MLTDs in rad. In the high dose-rate studies involving radiation pulses, dose-rate estimates were based on the rad doses at the mid-plane of the animal exposure volume, and the duration of the pulse intervals at half maximal height; the latter is estimated from reactor reactivity tracings.
The IAEA publication noted here provided data on unilateral vs bilateral depth doses consequent to pulse-rate and steady state mixed fission neutron and 1 Mev x-radiation.
Alpen (1991).
The author provided a description and discussion on radiation effects from published studies using canines and goats relative to the H-ARS; included discussion of RBE between H- and GI-ARS (Alpen 1991). Alpen also provided tabular data for MST to suggest GI component after neutron exposure.
Simpson et al. (1963).
The authors reported that the neutron:gamma ratio was 5:1 and used a tissue equivalent dog phantom (Simpson et al. 1963). They report that the neutron dose had dropped 2.3 times the gamma dose “in front” of the phantom. The neutron:gamma dose at midline was 44% of the dose “at the front” surface of the phantom.
Kaul and Scott (1984).
A thorough presentation was provided by two leaders in 1984 (Kaul and Scott 1984). The database provided a link between dose from gamma radiation and mixed neutron:gamma radiation relative to the marrow cell survival fraction over a range of energy from 14.0 – 0.1 MeV and 14.2 – 0.05 Mev for gamma radiation and mixed neutron:gamma radiation, respectively. The analysis included the dose range of expected LD50/60 values for the human.
DISCUSSION
The time-, dose-, exposure aspect- and radiation quality-dependent exposure in the immediate post-prompt irradiation environment following detonation of an improvised nuclear device (IND) varies significantly with size or yield of the device, air or ground detonation and physical environment, whether city (urban), rural or open. The neutron component of the prompt radiation has been considered marginal relative to inducing acute radiation effects since the concurrent blast and thermal components would likely cancel out the prompt radiation exposure within the area of the detonation. It has also been considered that the urban terrain, especially in context of a ground detonation would attenuate all three major components, blast, thermal and the radiation neutron and photon emissions. Recent reviews of the radiation exposure from an IND relative to the urban environment projected a significant component of neutrons in the neutron:gamma ratio in the near-exposure range while the associated blast and thermal are reduced. The neutron component then converged with the gamma component with increasing distance from the source (Kramer et al. 2016).
The understanding of acute radiation effects that define the H or GI ARS due to mixed neutron:gamma radiation remains incomplete relative to contemporary modeling of IND exposure parameters. The database in large animal models for mixed neutron:gamma exposure over a narrow neutron energy range has been focused on the H ARS with marginal effort on the GI ARS and complete lack of information on the delayed effects of acute exposure (DEARE), i.e., the lung, kidney, heart and prolonged GI injury. Knowledge of neutron- and or mixed neutron:gamma radiation-induced deterministic effects such as the ARS and MOI characteristic of the DEARE and its treatment will require more strategic and tactical approaches to achieve objectives focused on understanding mixed neutron:gamma exposure over a relevant energy range and respective dose distribution relative to prescribed dose, resultant organ-based descriptive dose and organ volume specific doses in valid animal models.
The systematic review herein focused on defining the DRR for the acute H ARS, induced by mixed neutron:gamma radiations in the canine and rhesus macaque exposed to TBI of mixed neutron:gamma radiation of differing quality, dose rate, exposure aspect or uniformity relative to comparable exposure parameters from reference radiation, e.g., x-radiation and Co-60 gamma radiation (MacVittie et al. 2015b; MacVittie et al. 2019b; MacVittie and Jackson III 2020). Secondary and tertiary data sets were identified that provided a comparative view of the dose threshold for H ARS and GI ARS consequent to mixed neutron:gamma and reference low LET radiation to include myelosuppression, recovery and the influence of the evolving, acute GI-ARS, on morbidity, mortality and MST of decedents.
The acute H ARS in response to TBI: mortality.
An effective, contemporary animal model research platform focused on low LET, LINAC-derived photons and Co-60 gamma radiation has been established that connected the seminal studies performed decades earlier that used mixed neutron:gamma and low LET radiations, relative to the definition of the acute radiation-induced H-ARS in the absence of medical management (MacVittie 2012; MacVittie 2014; MacVittie 2015). These early models established DRRs for the H ARS consequent to mixed neutron:gamma radiation, x-radiation of varied energy and Co-60 gamma radiation. The published database for established DRRs, spanned almost 6 decades. The database for the established DRRs for the H ARS in NHP was the subject of a recent review (MacVittie et al. 2015b). A subsequent review focused on the NHP provided estimated DRRs for the GI ARS, derived from the high-dose database and respective MSTs of decedents within the noted H-ARS studies (MacVittie et al. 2019b). The data sets for NHP exposed to mixed neutron:gamma radiation were summarized herein for comparative review of the DRRs noted for canines. Radiosensitivity relative to sex differential was not considered in the H ARS or GI ARS studies.
Rhesus macaque, mortality.
Four primary studies utilized reactor-derived mixed neutron:gamma radiation delivered by steady-state, bilateral exposure to include combined datasets that utilized unilateral, non-uniform exposure via reactor-derived “pulse” and two nuclear weapon detonation “prompt” dose rates (Zellmer and Pickering 1960; Stanley et al. 1966; Turbyfill et al. 1968; Wise and Turbyfill 1968). The respective LD50/30 values were 385 rad [357, 413], 395 rad [337, 432] and 412 rad [394, 441] for bilateral and unilateral steady-state exposure and combined “pulse-rate” and “prompt” nuclear weapon studies, respectively. There was no significant difference between the respective slopes (p = 0.57) or probit fits or intercepts (F, p = 0.57) therefore, there was no difference in LD or LD50/30 values (MacVittie et al. 2015b). The data are supported by the noted equivalent dose-rate effect for mixed neutron:gamma radiation in canine lethality studies relative to pulse-mode and a moderate dose rate of ~100 cGy min−1 (Ainsworth et al. 1965; Alpen 1991). The high-dose rate, unilateral, non-uniform exposures, noted in both NHP and canine models, induced equivalent, species-dependent DRRs for mortality relative to uniform bilateral or rotational exposures. Herein, we included the prompt exposure data from the nuclear weapon studies (Zellmer and Pickering 1960). These data underscored the importance of radiation dosimetry and assessing depth dose distribution relative to the radiation quality and the differential between prescribed dose and dose delivered to selected organ volume such as lung, kidney, GI and heart (Bond et al. 1957; Bond and Robinson 1967; Wingate et al. 1967; Vigneulle et al. 1990; Zeman et al. 1990; Kazi et al. 2014; Prado et al. 2015; Prado et al. 2017).
Relative biologic effect (RBE), rhesus macaque.
A probit analysis for the combined database of five studies for NHP, n = 338, exposed to 250 kVp x-radiation provided a composite LD50/30 of 520 rad (MacVittie et al. 2015b). The respective LD50/30 values noted above for both reactor-derived, steady-state and pulse-rate mixed neutron:gamma exposure and prompt nuclear detonation were 385 rad, 395 rad and 412 rad. The projected RBE relative to LD50/30 values was 1.35, 1.32 and 1.26. The respective LD50/30 values for Co-60 gamma- and 2 Mev x-radiation were 644 cGy and 671 cGy which projected an RBE of ~ 1.67 and 1.74 relative to the steady-state, mixed neutron:gamma LD50/30 value of 385 rad.
Two sites performed studies using comparative x-radiation and mixed neutron:gamma that emphasized the effect of an increased neutron component on mortality and thus RBE (Stanley et al. 1966; Broerse et al. 1978). Broerse et al., and Stanley et al., utilized respective 300 kVp or 250 kVp x-radiation, total-body, rotational exposure relative to variable mixed neutron:gamma exposure. The Broerse study estimated an RBE of 2.02 relative to an RBE of 1.29 within the Stanley et al., study. However, there are marked differences in the mixed neutron:gamma exposure parameters. The Broerse study used a mixed gamma:neutron ratio of approximately 25:75 with the mean neutron energy of 1.0 Mev. Stanley and colleagues used an approximate 60:40 ratio of gamma:neutron radiation.
Canine, mortality: Mixed neutron:gamma radiation.
LD50/30 values for canines exposed to bilateral or rotational geometry, uniform, moderate dose rates of mixed fission neutron:gamma radiation were conducted within six primary studies. The LD50/30 values were 218 rad, 216 rad, 153 cGy, 281 rad, 289 rad, and 239 rad (Bond et al. 1956; Alpen et al. 1960; Ainsworth et al. 1965; Earle et al. 1971; MacVittie et al. 1991; MacVittie and Jackson III 2020). The neutron energy ranged from 14.6 Mev to ~ 1 Mev. The LD50/30 values for the higher energy neutron exposures were 239 rad, 281 rad and 289 rad, with an average of ~ 270 rad. The lower energy neutron exposures had LD50/30 values of 153 rad, 203 rad, 218 rad, 216 rad. The mean was ~ 198 rad. The LD50/30 values for unilateral, non-uniform exposure were 230 rad for steady-state and 230 rad and 210 rad for pulse-rate exposures. The LD50/30 for unilateral 1 Mev x-radiation was ~ 338 rad relative to 280 rad (Ainsworth et al. 1965). As noted earlier, the database supported the conclusion that the dose-rate effect for mixed neutron:gamma radiation was approximately equivalent to moderate, steady state bilateral exposure. A marginal increase in LD50/30 values was noted between moderate, acute bilateral dose and a pulse-rate exposure. The higher energy neutron radiation administered via uniform TBI was less effective in inducing the H-ARS whereas the data suggested that TBI, unilateral, non-uniform exposure was somewhat less effective than TBI via uniform exposure as evidenced by the differential dose distribution to active marrow sites.
Reference radiation: 250 kVp x-, 1 Mev x-radiation, or Co-60 gamma radiation.
LD50/30 values for canines exposed to bilateral or rotational, uniform, moderate dose rates from reference quality radiation sources, i.e., 250 kVp x-, 1 Mev x-radiation, or Co-60 gamma radiation were 206 rad, 212 rad, 239 rad for 250 kVp, 280 rad, 288 rad for 1 Mev x-radiation and 260 cGy, 258 cGy, 263 cGy (combined five studies) for Co-60 gamma radiation for clinically unsupported canines (Gleiser 1953; Shively et al. 1958; Alpen et al. 1960; Ainsworth et al. 1965; George et al. 1968; Norris et al. 1968; Earle et al. 1971; Garner et al. 1974; MacVittie et al. 1991). The average LD50/30 values for 250 kVp, 1 Mvp and Co-60 exposures were ~ 219, 284 and 262 rad as reference radiation quality to assess an RBE for mixed neutron:gamma exposures. These values show the respective RBE for LD50/30 values of 1.29 and 1.2 for 250 kVp x-radiation relative 1 Mev x- and Co-60 gamma radiation.
Relative biologic effect (RBE), canine.
The established database indicated a negligible RBE between the Co-60 gamma radiation and 1 Mvp x-radiation but did estimate a small but significant RBE of 0.87 for these higher-energy radiations compared to the standard 200–250 kVp x-irradiation.
The estimation of an RBE for mixed neutron:gamma exposures must consider the source-dependent neutron energy and ratio of neutron to gamma radiations. Pitchford and Thorp reported on the neutron effectiveness of a 0.7:1 mixed neutron:gamma-ray exposure from the AFRRI TRIGA reactor operated at a steady-state dose rate of 14 rad min−1. The LD50/30 for mixed neutron:gamma-ray (0.7:1) exposure was 218 rad (midline tissue) compared to 206 rad for 250 kVp x-irradiation (Pitchford and Thorp 1968). The lower neutron component resulted in an equivalent bioeffect, relative to 250 kVp x-radiation. Compared to the Co-60 gamma radiation LD50/30 of 260 cGy, the RBE for neutron exposure would approach a value of 1.2. Whereas exposure to neutron energy of 9 – 14.6 Mev resulted in an estimated LD50/30 value of ~ 270 rad relative to the 260 cGy LD50/30 values for Co-60 gamma radiation.
The in vivo assessment of neutron RBE relative to LD50/30 values was also supported via the increased effect on GM-CFC derived from the BM of the rib of the irradiated canines, 24 h post exposure. The in vitro assay of marrow-derived GM-CFC after in vivo exposure to a range of doses from Co-60 gamma- or mixed neutron:gamma-radiations showed a significant RBE of ~ 1.9 for the respective D0 values of 67 cGy and 36 cGy (MacVittie et al. 1991). We note here that the role of radiation dosimetry and organ-specific depth-dose reconstruction is critical to place all study results in proper context relative to mean organ dose and prescribed dose such as MTD. The canines referenced above were bilaterally irradiated, left side/right side, at 10 cGy min−1 to a MTD. The depth-dose curves were symmetrical about the midline with the lowest doses noted at the midline. However, doses at the thorax were 20 – 35% higher compared to the abdomen. This relationship between exposure geometry, neutron energy and depth-dose must be considered relative to the noted D0 values derived from the rib BM. Seed and Kasper assayed the effect of in vitro exposure on marrow-derived GM-CFC. The respective D0 values were 77 cGy and 28 cGy for Co-60 and fission neutron exposure for an approximate RBE = 2.75 (Seed and Kaspar 1991). Boyum et al., performed similar studies with in vitro and in vivo BM culture techniques to assess the RBE of varied neutron energies on human BM-derived progenitor cells (Boyum et al. 1978). The RBE was inversely proportional to mono-energetic neutron energy levels. Fission neutrons were reported to have an RBE of ~ 2.4 relative to x-radiation. Schwartz et al., assessed the RBE of erythroid progenitors via assay of in vitro irradiated BM cells. Two classes of progenitor cells, CFU-e and BFU-e were assayed and showed RBEs for respective Do values of 2.3 and 1.6 (Schwartz et al. 1986).
Medical management and medical countermeasure (MCM) support.
The efficacy of medical management to mitigate the lethal H and GI ARS has been substantial and consistent in both canines and NHP as evidenced in historical and contemporary models. Selected MCM have been shown to significantly enhance survival from the lethal H-ARS in both canines and NHP.
Medical management support.
Administration of medical management with a regimen of subject-based fluids, antibiotics and whole blood transfusion mitigated mixed neutron:gamma induced mortality and myelosuppression (Broerse et al. 1978; MacVittie et al. 1991; Yu et al. 2011). The efficacy of timely administered medical management was demonstrated in studies performed over 50 y ago. Studies using canines showed the marked efficacy of supportive care centered on fluids, antibiotics and fresh platelet transfusions (Furth et al. 1953; Bagdasarov et al. 1959; Jackson et al. 1959; Sorensen et al. 1960; Perman et al. 1962). Sorenson et al., utilized fluids, antibiotics and whole blood transfusions to control dehydration, sepsis and bleeding in a canine model of the lethal H-ARS. The supportive care reduced lethality from an LD99/30 to an LD20/30 (Sorensen et al. 1960). Additional studies expanded the data base in canines, supporting the efficacy of timely medical management over the complete DRR for the canine H-ARS. MacVittie et al., demonstrated the significant effect on shifting the DRR for H-ARS with medical management (MacVittie et al. 1991; MacVittie et al. 2005). Similar effects on mitigating the lethal H-ARS were observed in NHP models (Farese et al. 2012; MacVittie et al. 2015a; Thrall et al. 2015; Yu et al. 2015).
Medical countermeasure (MCM).
Neupogen® (G-CSF), Neulasta® (pegylated G-CSF) and Leukine (GM-CSF) are Food and Drug Administration (FDA)-approved MCM under the FDA “animal rule”. These MCM used in combination with medical management are proven significantly efficacious in mitigating the lethal H-ARS in both the canine and NHP (Schuening et al. 1989; MacVittie and Monroy 1990; MacVittie et al. 2005; Yu et al. 2011; Farese et al. 2013; MacVittie et al. 2015a; Farese et al. 2019). The use of MCM and timely subject-based medical management to mitigate the H-ARS is supported through numerous clinical trials and reviews (Hughes et al. 1990; Hughes et al. 2002; Waselenko et al. 2004; Gafter-Gvili et al. 2005; Timmer-Bonte et al. 2005; Kuderer et al. 2007; Tomblyn et al. 2009).
Gaps in knowledge.
GI-ARS, canine.
The dose response relationship for mortality due to the acute GI ARS for low LET or high LET radiations in canines is marginal. There are no primary studies focused on determining the DRR relative to 250 kVp, 1–2 Mev x-radiation or Co-60 gamma radiation. A study reviewed herein used a modest, three dose range of 3.0 Gy, 3.44 Gy, and 3.68 Gy of mixed neutron:gamma radiations to suggest that the LD100 for the GI ARS was ~ 3.7 Gy. The associated dose-dependent mortality therefore suggested that the LD50/6 would be less than 3 Gy (Wang et al. 1991). Yu and colleages noted severe GI injury in canines exposed to 2.3 Gy, mixed neutron:gamma radiation. The 1.4 Mev neutron component was ~90% in the unilateral, nonuniform exposure (Yu et al. 2011). A retrospective study performed a DRR for canines exposed to ~ 1 Mev mixed neutron:gamma (6:1) radiations that estimated the LD50/6 at 2.83 Gy (MacVittie and Jackson III 2020). The lack of a DRR for the GI-ARS using low LET radiations prohibits a confident estimate of RBE for mixed neutron:gamma radiations relative to the LD50/6. The expected LD50/6–7 for Co-60 radiation would be greater than 7.0 Gy (unpublished dataset, MacVittie TJ et al).
Retrospective dose analysis, differential tissue depth dose, organ-specific dose.
There is significant value in assessing organ dose after unilateral, prompt or reactor-derived steady-state or pulse-rate, nonuniform mixed neutron:gamma and reference radiation exposures (Co-60 gamma and or LINAC-derived 6 Mev photon) relative to standard TBI, uniform exposure. Retrospective analysis of dose delivered to specific organ volume relative to the prescribed dose is lacking. Recent studies have described the use of computer tomography-based heterogeneity-corrected dose calculations to evaluate retrospective organ dose relative to the prescribed mid-thorax dose (Prado et al. 2015; Prado et al. 2017). The depth-dose pattern consequent to bilateral or unilateral exposure within the animal is critical and many authors have provided a knowledge base relative to the prescribed midline dose (Bond et al. 1957; Wingate et al. 1967; Kaul and Scott 1984; Vigneulle et al. 1990; Zeman et al. 1990; MacVittie et al. 1991; Wang et al. 1991; Yu et al. 2011). However, determination of the dose to the specific organ volume relative to the prescribed dose delivered to the target site would link organ-specific biology to that morbidity and mortality consequent to the prescribed target dose (Prado et al. 2015; Prado et al. 2017). A descriptive dose for morbidity and mortality belies definition of associated organ dose and contribution to the DRR. It is of interest that Zeman and colleagues used a canine model of partial-body, nonuniform irradiation with marrow shielding to focus on defining the dose differential to the small intestinal (Zeman et al. 1990). The model used an innovative in situ dosimetry protocol with thermoluminescent dosimeters to determine the radiation dose delivered as a function of position along a segment of the small intestine. This system made it possible to correlate the radiation dose delivered at a specific point along the small intestine with the macroscopic and microscopic appearance of the intestinal mucosa at that point. The histopathology was determined via biopsy using a fiberoptic endoscope.
Partial-body shielding (marrow-sparing).
A general assumption is that the radiation exposure consequent to a nuclear terrorist or accident event will be ill-defined as a result of body position (exposure aspect), random shielding, differential dose rate, the distance from the source and radiation intensity in the prompt exposure and short-term fallout field. These factors predict a non-uniform and heterogeneous exposure to victims. However, the circumstances described above, e.g., that exposure is non-uniform and heterogeneous predicts a differential dose distribution over the body, thus predicting a more favorable outcome of survival due of the consequent sparing of BM, GI-tissue and other critical organ volume such as lung, kidney and heart. Thus, given time, spontaneous recovery can occur especially in the context of medical management (provides the time component) and use of MCM (Monroy et al. 1988; MacVittie et al. 2012a; MacVittie et al. 2015a; Farese et al. 2019). Respective LD50/60 values in NHP exposed to 1 – 2 Mev gamma, x- or average 2 Mev LINAC-derived photons with or without medical management and 5% BM-sparing were: a) LD50/30, TBI, in the absence of medical management was 671/644 rads, b) LD50/60, TBI plus medical management was 7.54 Gy and c) LD50/60, with partial-body irradiation (5% BM spared) plus medical management was 10.88 Gy. A study performed in rhesus macaques by Accardi and colleages using Co-60 gamma radiation and sparing ~ 11% active BM in the oral cavity projected the LD50/15 to be ~ 12.0 Gy (Accardi et al. 2020).
Delayed effects of acute radiation exposure (DEARE).
The combination of non-uniform and heterogeneous exposure and use of medical management plus FDA-approved MCM will ensure survivors of the acute H and G ARS. Survivors will experience delayed MOI characterized by injury to the lung, kidney and heart to include the prolonged GI injury and immune suppression noted in contemporary studies.
Relative biologic effect (RBE).
The RBE for mixed neutron:gamma radiation database is lacking for any delayed effects MOI such as lung, heart, kidney or prolonged GI injury. The database for RBE of acute GI ARS is marginal. The relationship between neutron energy, its component fraction within the incident radiation, and the exposure aspect relative to clinical endpoints for morbidity and mortality is lacking.
Radiation/nuclear event models, what is the radiation exposure scenario relative to potential prompt vs fallout exposure?
Models relative to the nuclear radiation scenario should be further discussed especially in the context of prompt and or pulse rate exposure. Early models of the exposure scenario predicted a relatively minor prompt component from neutrons or low LET radiation relative to blast and thermal. Therefore, early fallout would be the predominant cause of radiation-induced sequelae. The relevant scenarios must be defined relative to expected neutron energy, mixed neutron:gamma ratio and exposure aspect. Definition of the radiation environment in the prompt exposure field is critical for large animal model development.
Biomarkers of organ and multi-organ radiation-induced pathology.
There are no FDA-approved biomarkers for MOI consequent to acute radiation-induced exposure given any of the multiple radiation-effects scenario. A multi-disciplinary approach is needed to identify biomarkers that are predictive of clinical outcome especially for the delayed effects of acute radiation exposure (MacVittie et al. 2019a; MacVittie et al. 2019b). Kane and colleagues have worked to advance the clinical and regulatory utility of candidate biomarkers by validating them for sensitivity and selectivity, robustness of response, and stability of response for radiation-induced injury (Jones et al. 2014; Jones et al. 2015a; Jones et al. 2015b). The potential for biomarkers to be diagnostic (absence/presence of radiation-induced disease state), prognostic (indicative of progression of natural history of radiation-induced disease state), or predictive (prediction of clinical endpoints at earlier time points) have been explored as well as the potential to serve as a pharmacodynamic biomarker (indicative of biological response to an intervention) (Carter et al. 2019; Huang et al. 2019a; Huang et al. 2019b; Jones et al. 2019a; Jones et al. 2019b).
Contemporary animal models, H-, GI-ARS and delayed MOI.
Well characterized and validated animal models are required for all aspects of research focused on mixed neutron:gamma radiation exposure to adhere to the criteria of the FDA “Animal Rule” and the respective FDA Guidance document (U. S. Food and Drug Administration 2002; U. S. Food and Drug Administration 2015). The model(s) should provide phenomenological, predictive and construct (mechanism of action) validity. They must accurately describe the organ-specific sub-syndrome(s) or injury, be predictive of the human response and treatment and understand the mechanism of action of radiation-induced injury and treatment. If prompt exposure is relevant to the nuclear radiation effect scenario, then, additional research (acute and delayed effects, MOI, biodosimetry, biomarkers, organ dose, MCM efficacy) may be required to provide a greater understanding of the following variables relative to non-uniform, unilateral exposure consequent to pulsed, mixed neutron:gamma dose rate, neutron energy and neutron:gamma ratio. Future studies should define the “best” radiation scenario and develop predictive models, likely within a validated NHP animal model research platform, that has defined the DRR for the H and GI ARS, to include models that define MOI characteristic of the DEARE (Farese et al. 2012; MacVittie et al. 2012a; MacVittie et al. 2012b; Garofalo et al. 2014; de Faria et al. 2015; MacVittie et al. 2015a; Thrall et al. 2015; Kramer et al. 2016; Cohen et al. 2017; Farese et al. 2019).
Space radiation exposure; mixed radiation species and relative radiation quality, dose rate and exposure scenarios.
The critical exposure scenarios involve variable, mixed radiation species, quality and prolonged exposure at low and variable dose rates. The neutron component and exposure aspects are not well defined let alone the exposure scenarios for incident radiations. A large animal model research platform relative to that established for the NHP ARS and DEARE is absent.
Summary.
This systematic review provided a substantial and consistent retrospective data set to establish the comparative DRRs for the acute H-ARS in canines and NHP exposed to mixed neutron:gamma radiations. The database herein included a comparative data set selected for exposure of the rhesus macaque to mixed neutron:gamma radiations from recent reviews of the H-ARS and GI-ARS in the NHP (MacVittie et al. 2015b; MacVittie et al. 2019b). The available studies established several comparable parameters for the canine and NHP, e.g., a.) the DRRs for the acute H ARS for canines exposed to uniform and non-uniform TBI with 250 kVp or 1 Mev x-radiation, Co-60 gamma radiation, and reactor-derived mixed gamma:neutron radiation at moderate to pulse-rate exposure, b.) the comparable DRRs for rhesus macaques exposed to reactor- and nuclear weapon-derived derived mixed gamma:neutron radiation, c) the time course for morbidity and mortality of the acute H ARS to include estimated parameters for the GI ARS, d.) an estimation of the RBE for respective H ARS endpoints to include the GI ARS radiation effects, e) the characteristic steep slopes for mortality vs dose of TBI, irrespective of species, radiation quality, dose rate and energy f) the longitudinal, dose-, time- and organ-dependent survival probability versus time post exposure demonstrated by the Kaplan-Meier plots.
Supplementary Material
Source of Funding
This project has been funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institute of Health under contracts HHSN272201000046C and SRI/NIAID Contract HHSN272201500013I.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest
References
- Accardi MV, Donini O, Rumage A, Ascah A, Haruna J, Pouliot M, Bujold K, Huang H, Wierzbicki W, Stamatopoulos J, Naraghi H, Measey T, Authier S. Characterization of a Partial-Body Irradiation Model With Oral Cavity Shielding in Nonhuman Primates. Int J Radiat Biol 96: 100–111; 2020. [DOI] [PubMed] [Google Scholar]
- Ainsworth EJ, Leong GF, Alpen EL. Early radiation mortality and recovery in large animals and primates. In: Broerse JJ, MacVitties TJ eds. Response of Different Species to Total Body Irradiation Dordrecht, the Netherlands: Martinus Nijhoff Publishers; 1984; 87–111. [Google Scholar]
- Ainsworth EJ, Leong GF, Kendall K, Alpen EL. The lethal effects of pulsed neutron or gamma irradiation in mice. Radiat Res 21: 75–85; 1964. [PubMed] [Google Scholar]
- Ainsworth EJ, Leong GF, Kendell K, Alpen EL. Comparative lethality responses of neutron and x irradiated dogs: Influence of dose rate and exposure aspect. Radiat Res 26: 32–43; 1965. [PubMed] [Google Scholar]
- Allen RG, Brown FA, Logie LC, Rovner DR, Wilson J, Zellmer RW. Acute effects of gamma radiation in the primate. Radiat Res 12: 532–559; 1960. [PubMed] [Google Scholar]
- Alpen EL. The Historical Background for Large-Animal Studies with Neurons of Various Energies. Radiat Res 128: S37–S41; 1991. [PubMed] [Google Scholar]
- Alpen EL, Baum SJ. Autologous bone-marrow implantation after fast neutron irradiation of dogs. Radiat Res 11: 383–9; 1959. [PubMed] [Google Scholar]
- Alpen EL, Jones DM, Hechter HH, Bond VP. The comparative biological responses of dogs to 250 kVp X rays and 100 kVp X rays. Radiology 70: 541–549; 1958. [DOI] [PubMed] [Google Scholar]
- Alpen EL, Shill OS, Tochilin E. The effects of total-body irradiation of dogs with simulated fission neutrons. Radiat Res 12: 237–50; 1960. [PubMed] [Google Scholar]
- Bagdasarov AA, Raushenbakk MO, Abdullaev GM, Beliaeva BF, Lagutina NY. The treatment of acute radiation sickness with packed platelets. Probl Hematol Blood Transfus 4: 1–5; 1959. [PubMed] [Google Scholar]
- Baum SJ, Wyant DE. Hematopoietic recovery in irradiated dogs Bethesda, MD: Armed Forces Radiobiology Research Institute; AFRRI SR70–2; 1970. [PubMed] [Google Scholar]
- Bertho JM, Prat M, Frick J, Demarquay C, Gaugler MH, Dudoignon N, Clairand I, Chapel A, Gorin NC, Thierry D. Application of autologous hematopoietic cell therapy to a nonhuman primate model of heterogeneous high-dose irradiation. Radiat Res 163: 557–570; 2005. [DOI] [PubMed] [Google Scholar]
- Bond VP, Carsten AL, Bullis J, Roth SP. Severity of organ injury as a predictor of acute mortality for disparate patterns of absorbed dose distribution. Radiat Res 128: S9–S11; 1991. [PubMed] [Google Scholar]
- Bond VP, Carter RE, Robertson JS, Seymour PH, Hetcher HH. The effects of total body fast neutron irradiation in dogs. Radiat Res 4: 139–153; 1956. [PubMed] [Google Scholar]
- Bond VP, Cronkite EP, Sondhaus CA, Imirie G, Obertson JS, Borg DC. The influence of exposure geometry on the pattern of radiation dose delivered to large animal phantoms. Radiat Res 6: 554–563; 1957. [PubMed] [Google Scholar]
- Bond VP, Robinson CV. A mortality determinant in nonuniform exposure of the mammal. Radiat Res 7: 265–275; 1967. [PubMed] [Google Scholar]
- Boyum A, Carsten AL, Chikkappa G, Cock L, Bullis J, Monikel L, Cronkite EP. The R.B.E. of different-energy neutrons as determined by human bone-marrow cell techniques. Int J Rad Biol 34: 201–212; 1978. [DOI] [PubMed] [Google Scholar]
- Broerse JJ, van Bekkum DW, Hollander CF, Davis JAG. Mortality of monkeys after exposure to fission neutrons and the effect of autologous bone marrow transplantation. Int J Rad Biol 34: 253–264; 1978. [DOI] [PubMed] [Google Scholar]
- Broerse JJ, Zoetelief J. The occurrence of radiation syndromes in rodents and monkeys in dependence on dose rate and radiation quality. In: Broerse JJ, MacVitties TJ eds. Response of different species to total body irradiation Boston: Martinus Nijhoff Publishers; 1984; 175–187. [Google Scholar]
- Byron JW, Haigh MV, Lajtha LG. Effect of an antibiotic regime on monkeys exposed to total-body irradiation. Nature 202: 977–979; 1964. [DOI] [PubMed] [Google Scholar]
- Carsten AL, Bond VP, Thompson K. The RBE of different energy neutrons as measured by the haematopoietic spleen-colony technique. Int J Rad Biol 29: 65–70; 1976. [DOI] [PubMed] [Google Scholar]
- Carter CL, Hankey KG, Booth C, Tudor GL, Parker GA, Jones JW, Farese AM, MacVittie TJ, Kane MA. Characterizing the natural history of acute radiation syndrome of the gastrointestinal tract: Combining high mass and spatial resolution using MALDI-FTICR-MSI. Health Phys 116: 454–472; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EP, Hankey KG, Bennett AW, Farese AM, Parker GA, MacVittie TJ. Acute and chronic kidney injury in a non-human primate model of partial-body irradiation with bone marrow sparing. Radiat Res 188: 661–671; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LJ, Haire HM, Alpen EL. Partial shielding of dogs: effectiveness of small external epicondylar lead cuffs against lethal x-radiation. Radiat Res 32: 54–63; 1967. [PubMed] [Google Scholar]
- Conard RA. Some effects of ionizing radiation on the physiology of the gastrointestinal tract:a review. Radiat Res 5: 167–188; 1956. [PubMed] [Google Scholar]
- Conard RA, Cronkite EP, Brecher G, Strome CPA. Experimental therapy of the gastrointestinal syndrome produced by lethal doses of ionizing radiation. J Appl Physiol 9: 227–233; 1956. [DOI] [PubMed] [Google Scholar]
- Dalrymple GV, Lindsay IR, Ghidoni JJ. The effect of 2-Mev whole-body x-radiation on primates. Radiat Res 25: 377–400; 1965. [PubMed] [Google Scholar]
- de Faria EB, Barrow K, Ruehle BT, Parker JT, Swartz E, Taylor-Howell C, Kieta KM, Lees C, Sleeper MM, Dobbin T, Baron AD, Mohindra P, MacVittie TJ. The evolving MCART multimodal imaging core: Establishing a protocol for computed tomography and echocardiography in the rhesus macaque to perform longitudinal analysis of radiation-induced organ injury. Health Phys 109: 479–492; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle JD, Ainsworth EJ, Leong GF. Lethal and hematologic effects of 14.6 MeV neutrons on beagles with estimation of RBE. Radiat Res 45: 487–498; 1971. [PubMed] [Google Scholar]
- Edmondson PW, Batchelor AL. Acute lethal responses of goats and sheep to bilateral or unilateral whole-body irradiation by gamma rays and fission neutrons. Int J Radiat Biol 20: 269–290; 1971. [DOI] [PubMed] [Google Scholar]
- Eldred E, Trowbridge WV. Radiation sickness in the monkey. Radiology 62: 65–73; 1954. [DOI] [PubMed] [Google Scholar]
- Eltringham JR. Recovery of the rhesus monkey from acute radiation exposure as evaluated by the split dose technique: preliminary results. Radiat Res 31: 533; 1967. [Google Scholar]
- Farese AM, Bennett AW, Gibbs AM, Hankey KG, Prado K, Jackson III W, MacVittie TJ. Efficacy of Neulasta or Neupogen on H- and GI-ARS mortality and hematopoietic recovery in nonhuman primates after 10 Gy irradiation with 2.5% bone marrow sparing. Health Physics 116: 339–353; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, Cohen MV, Katz BP, Smith CP, Gibbs A, Cohen DM, MacVittie TJ. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res 179: 89–100; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, Cohen MV, Katz BP, Smith CP, Jackson W III, Cohen DM, MacVittie TJ. A nonhuman primate model of the hematopoietic acute radiation syndrome plus medical management. Health Phys 103: 367–382; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, Myers LA, MacVittie TJ. Therapeutic efficacy of recombinant human leukemia inhibitory factor in a primate model of radiation-induced marrow aplasia. Blood 84: 3675–3678; 1994. [PubMed] [Google Scholar]
- Farese AM, Williams DE, Seiler FR, MacVittie TJ. Combination protocols of cytokine therapy with interleukin-3 and granulocyte-macrophage colony-stimulating factor in a primate model of radiation-induced marrow aplasia. Blood 82: 3012–3018; 1993. [PubMed] [Google Scholar]
- Finney DJ. Statistical Method in Biological Assay London: Charles Griffin & Co. Ltd.; 1952. [Google Scholar]
- Furth FW, Coulter MP, Miller RW, Howland JW, Swisher SN. The treatment of acute radiation syndrome in dogs with auromycin and whole blood. J Lab Clin Med 41: 918–928; 1953. [PubMed] [Google Scholar]
- Gafter-Gvili A, Fraser A, Mical P, Leibovici L. Meta-Analysis: Antibiotic Prophylaxis Reduces Mortality in Neutropenic Patients. Ann Int Med 142: 979–995; 2005. [DOI] [PubMed] [Google Scholar]
- Garner RJ, Phemister RD, Angleton GM, Lee AC, Thomassen RW. Effect of age on the acute lethal response of the beagle to cobalt-60 gamma radiation. Radiat Res 58: 190–195; 1974. [PubMed] [Google Scholar]
- Garofalo M, Bennett A, Farese AM, Ward A, Taylor-Howell C, Cui W, Gibbs A, Lasio G, Jackson WI, MacVittie TJ. The delayed pulmonary syndrome following acute high-dose irradiation: A Rhesus macaque model. Health Phys 106: 56–72; 2014. [DOI] [PubMed] [Google Scholar]
- George RE, Stanley RE, Wise D, Barron EL. The acute mortality response of beagles to mixed gamma-neutron radiations and 250 kVp x rays Bethesda, MD: Armed Forces Radiobiology Research Institute; AFRRI SR68–3; 1968. [Google Scholar]
- Gleiser CA. The determination of the lethal dose 50/30 of total body irradiation for dogs. Am J Vet Res 14: 284–286; 1953. [Google Scholar]
- Haigh MV, Paterson E. Effects of a single session of whole body irradiation in the rhesus monkey. Br J Radiol 29: 148–157; 1956. [DOI] [PubMed] [Google Scholar]
- Hansen CL, Michaelson SM, Howland SW. Lethality of upper body exposure in beagles. Health Phys 76: 242–246; 1961. [PMC free article] [PubMed] [Google Scholar]
- Henschke UK, Morton JL. Mortality of Rhesus monkeys after single total body irradiation. Am J Roentgenol Radium Ther Nucl Med 77: 889–909; 1957. [PubMed] [Google Scholar]
- Huang W, Yu J, Jones JW, Carter CL, Jackson IL, Vujaskovic Z, MacVittie TJ, Kane MA. Acute proteomic changes in lung after whole thorax lung irradiation in a mouse model: identification of potential initiating events for delayed effects of acute radiation exposure. Health Phys 116: 503–515; 2019a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Yu J, Jones JW, Carter CL, Pierzchalski K, Tudor GL, Booth C, MacVittie TJ, Kane MA. Proteomic evaluation of the acute radiation syndrome of the gastrointestinal tract in a murine total-body irradiation model. Health Phys 116: 516–528; 2019b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 34: 730–751; 2002. [DOI] [PubMed] [Google Scholar]
- Hughes WT, Armstrong D, Bodey GP, Feld R, et Mandell GL, Meyers JD, Pizzo PA, Schimpff SC, Shenep JL, Wade JC. Guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. J Infect Dis 161: 381–396; 1990. [DOI] [PubMed] [Google Scholar]
- Jackson DP, Sorensen DK, Cronkite EP, Bond VP, Fliedner TM. Effectiveness of transfusions of fresh and lyophiloized platelets in controlling bleeding due to thrombocytopenia. J Clin Inves 38: 1689–1697; 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Alloush J, Rajendran S, Chua HL, MacVittie TJ, Orschell CM, Kane MA. Effect of gender on biomarker response in a mouse model of the hematopoietic acute radiation syndrome. Health Phys 116: 484–502; 2019a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Bennett A, Carter CL, Tudor G, Hankey KG, Shea-Donohue T, Farese AM, Booth C, MacVittie TJ, Kane MA. Citrulline as a biomarker in the non-human primate total- and partial-body irradiation models: correlation of circulating citrulline to acute and prolonged gastrointestinal injury. Health Phys 109: 440–451; 2015a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Clifford Z, Li F, Tudor G, Farese AM, Booth C, MacVittie TJ, Kane M. Targeted metabolomics reveals metabolomic signatures correlating gastrointestinal tissue to plasma in a mouse total-body irradiation model. Health Phys 116: 473–483; 2019b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Scott AJ, Tudor G, Xu PT, Jackson IL, Vujaskovic Z, Booth C, MacVittie TJ, Ernst RK, Kane MA. Identification and quantitation of biomarkers for radiation-induced injury via mass spectrometry. Health Phys 106: 106–119; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Tudor GL, Li F, Tong Y, Katz B, Farese AM, MacVittie TJ, Booth C, Kane MA. Citrulline as a biomarker in the murine total-body irradiation model: correlation of circulating and tissue citrulline to small intestine epithelial histopathology. Health Phys 109: 452–465; 2015b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, George RE, West JE, Verrelli DM. The relative effectiveness of fission neutrons for gastrointestinal death in miniature pigs. Radiat Res 50: 504–18; 1972. [PubMed] [Google Scholar]
- Kaul DC, Scott WH. Dose and cell-survival calculations in anthropomorphic phantoms. In: Broerse JJ, MacVitties TJ eds. Response of Different Species to Total Body Irradiation Dordrecht, The Netherlands: Martinus Nijhoff; 1984; 29–42. [Google Scholar]
- Kazi AM, MacVittie TJ, Lasio G, Lu W, Prado KL. The MCART radiation physics core: The quest for radiation dosimetry standardization. Health Phys 106: 97–105; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer K, Li A, Madrigal J, Sanchez B, Millage K. Monte Carlo modeling of the initial radiation emitted by a nuclear device in the National Capital Region Fort Belvoir, VA: Defense Threat Reduction agency; DTRA-TR-13–045 (R1); 2016. [Google Scholar]
- Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol 25: 3158–3167; 2007. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ. The MCART Consortium animal models series. Health Phys 103: 340–342; 2012. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ. The MCART consortium animal models series: an evolving MCART. Health Phys 106: 1–6; 2014. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ. Commentary/Guest Editorial: MCART Animal Model Refinement and MCM Development: Defining organ dose, organ-specific tissue imaging, model validation and the natural history between the Acute Radiation Syndrome (ARS) and the Delayed Effects of Acute Radiation Exposure (DEARE). Health Phys 109: 335–341; 2015. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ, Bennett A, Booth C, Garofalo M, Tudor G, Ward A, Shea-Donohue T, Gelfond D, Mcfarland E, Jackson W III, Lu W, Farese AM. The prolonged gastrointestinal syndrome in rhesus macaques: the relationship between gastrointestinal, hematopoietic, and delayed multi-organ sequelae following acute, potentially lethal, partial-body irradiation. Health Phys 103: 427–453; 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVittie TJ, Bennett AW, Farese AM, Taylor-Howell C, Smith CP, Gibbs AM, Prado K, Tudor G, Booth C, Jackson WI. The effect of radiation dose and variation in Neupogen® initiation schedule on the mitigation of myelosuppression during the concomitant GI-ARS and H-ARS in a nonhuman primate model of high-dose exposure with marrow sparing. Health Phys 109: 427–439; 2015a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVittie TJ, Farese AM, Bennett A, Gelfond D, Shea-Donohue T, Tudor G, Booth C, Mcfarland E, Jackson W, III. The acute gastrointestinal subsyndrome of the acute radiation syndrome: a rhesus macaque model. Health Phys 103: 411–426; 2012b. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ, Farese AM, Jackson W, III. Defining the full therapeutic potential of recombinant growth factors in the post radiation-accident environment: the effect of supportive care plus administration of G-CSF. Health Phys 89: 546–555; 2005. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ, Farese AM, Jackson WI. The hematopoietic syndrome of the acute radiation syndrome in rhesus macaques: A systematic review of the lethal dose response relationship. Health Phys 109: 342–366; 2015b. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ, Farese AM, Kane MA. Commentary: The ARS, DEARE and multiple organ injury: A tactical and strategic approach to link radiation effects, model development, medical countermeasures and biomarker development to predict clinical outcome. Health Phys 116: 297–304; 2019a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVittie TJ, Farese AM, Parker GA, Jackson III W, Booth C, Tudor GL, Hankey KG, Potten C. The gastrointestinal syndrome of the acute radiation syndrome in rhesus macaques: A systematic review of the lethal dose response relationship with and without medical management. Health Phys 116: 305–338; 2019b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVittie TJ, Jackson III W. Acute Radiation-induced GI-ARS and H-ARS in a canine model of mixed neutron/gamma relative to reference Co-60 gamma radiation: A retrospective study. Health Phys In Press; 2020. [DOI] [PubMed]
- MacVittie TJ, Monroy R, Vigneulle RM, Zeman GH, Jackson WE. The relative biological effectiveness of mixed fission-neutron-gamma radiation on the hematopoietic syndrome in the canine: effect of therapy on survival. Radiat Res 128: S29–S36; 1991. [PubMed] [Google Scholar]
- MacVittie TJ, Monroy RL. Rescue of Lethally Irradiated Animals: Therapeutic use of rhG-CSF and rhGM-CSF in preclinical models of radiation-induced marrow aplasia. In: Browne D, Weiss JF, MacVittie TJ, Pillais MV eds. Treatment of Radiation Injuries New York: Plenum Press; 1990; 35–49. [Google Scholar]
- MacVittie TJ, Monroy RL, Patchen ML, Darden JH. Acute lethality and radiosensitivity of the canine hemopoietic system to cobalt-60 gamma and mixed neutron-gamma irradiation. In: Broerse JJ, MacVitties TJ eds. The Response of Different Species to High-Dose Total-Body Irradiation Dordrecht, The Netherlands: Martinus-Nijhoff; 1984; 113–130. [Google Scholar]
- Maillie HD, Krasavage W, Mermagen H. On the partial body irradiation of the dog. Health Phys 12: 883–887; 1966. [DOI] [PubMed] [Google Scholar]
- Monroy RL, Skelly RR, Taylor P, Dubois A, Donahue RE, MacVittie TJ. Recovery from severe hematopoietic suppression using recombinant human granulocyte-macrophage colony-stimulating factor. Exp Hematol 16: 344–348; 1988. [PubMed] [Google Scholar]
- Norris WP, Fritz TE, Rehfeld CE, Poole CM. The response of the beagle dog to cobalt-60 gamma radiation: Determination of the LD 50/30 and description of associated changes. Radiat Res 35: 681–708; 1968. [PubMed] [Google Scholar]
- Perman V, Sorensen DK, Usenik EA, Bond VP, Cronkite EP. Hemopoietic regeneration in control and recovered heavily irradiated dogs following severe hemorrhage. Blood 19: 738–742; 1962. [PubMed] [Google Scholar]
- Pitchford TL, Thorp JW. The acute mortality response of beagles to pulsed mixed gamma-neutron radiations Bethesda, MD: Armed Forces Radiobiology Research Institution; SR68–15; 1968. [Google Scholar]
- Prado C, Kazi A, Bennett AW, MacVittie TJ, Prado K. Mean organ doses resulting from non-human primate whole thorax lung irradiation prescribed to mid-line tissue. Health Phys 109: 367–373; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado C, MacVittie TJ, Bennet AW, Kazi A, Farese AM, Prado K. Organ doses associated with partial-body irradiation with 2.5% bone-marrow sparing of the non-human primate: A retrospective study. Radiat Res 188: 615–625; 2017. [DOI] [PubMed] [Google Scholar]
- Schlumberger HG, Vazquez JJ. Pathology of total body irradiation in the monkey. American J Path 30: 1013–1047; 1954. [PMC free article] [PubMed] [Google Scholar]
- Schuening FG, Storb R, Goehle S, Graham TC, Appelbaum FR, Hackman R, Souza LM. Effect of recombinant human granulocyte colony-stimulating factor on hematopoiesis of normal dogs and on hematopoietic recovery after otherwise lethal total body irradiation. Blood 74: 1308–1313; 1989. [PubMed] [Google Scholar]
- Schwartz GN, Vigneulle RM, MacVittie TJ. Survival of erythroid burst-forming units and erythroid colony-forming units in canine bone marrow cells exposed in vitro to 1 MeV neutron radiation or X rays. Radiat Res 108: 336–347; 1986. [PubMed] [Google Scholar]
- Seed TM, Kaspar LV. Probing altered hematopoietic progenitor cells of preleukemic dogs with JANUS fission neutrons. Radiat Res 128: S81–6; 1991. [PubMed] [Google Scholar]
- Shively JN, Michaelson SM, Howland JW. The responses of dogs to bilateral whole-body cobalt-60 irradiation. I. Lethal dose determinations. Radiat Res 9: 445–450; 1958. [PubMed] [Google Scholar]
- Simpson RE, Tochilin E, Goldstein N. Neutron and gamma-ray dosimetry in an animal exposure volume at a pulsed TRIGA reactor. Health Phys 9: 1021–30; 1963. [DOI] [PubMed] [Google Scholar]
- Sorensen DK, Bond VP, Cronkite EP, Perman V. An effective therapeutic regimen for the hemopoietic phase of the actue radiation syndrome in dogs. Radiat Res 13: 669–685; 1960. [Google Scholar]
- Stanley RE, Seigneur LJ, Strike TA. The acute mortality response of monkeys (Macaca mulatta) to mixed gamma-neutron radiations and 250 kVp X rays. Bethesda, MD: Armed Forces Radiobiology Research Institute, AFRRI SP66–23; 1966. [Google Scholar]
- Taketa ST. Water-electrolyte and antibiotic therapy against acute (3 to 5 day) intestinal radiation death in the rat. Radiat Res 16: 312–326; 1962. [PubMed] [Google Scholar]
- Terry NH, Travis EL. The influence of bone marrow depletion on intestinal radiation damage. Int J Radiat Oncol Biol Phys 17: 569–573; 1989. [DOI] [PubMed] [Google Scholar]
- Thrall KD, Love R, O’Donnell KC, Farese AM, Manning R, MacVittie TJ. An interlaboratory validation of the radiation dose response relationship (DRR) for the H-ARS in the rhesus macaque. Health Phys 109: 502–510; 2015. [DOI] [PubMed] [Google Scholar]
- Timmer-Bonte JN, de Boo TM, Smit HJ, Biesma B, Wilschut FA, Cheragwandi SA, Termeer A, Hensing CA, Akkermans J, Adang EM, Bootsma GP, Tjan-Heijen VC. Prevention of Chemotherapy-Induced Febrile Neutropenia by Prophylactic Antibiotics Plus or Minus Granulocyte Colony-Stimulating Factor in Small-Cell Lung Cancer: A Dutch Randomized Phase III Study. J Clin Oncol 23: 7974–7984; 2005. [DOI] [PubMed] [Google Scholar]
- Tochilin E, Kohler GD. Neutron beam characteristics from the University of California 60 in. Cyclotron. Health Phys 1: 332–9; 1958. [DOI] [PubMed] [Google Scholar]
- Tomblyn M, Chiller T, Einsele H. Guidelines for Preventing Infectious Complications among Hematopoietic Cell Transplantation Recipients: a global perspective. Biol Blood Marrow Transplant 15: 1142–1238; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullis JL, Lanson BG, Madden SG. Mortality in swine exposed to gamma radiation from an atomic bomb source. Radiology 62: 409–415; 1954. [DOI] [PubMed] [Google Scholar]
- Turbyfill CL, Pitchford TL, Cramer MB. Monkey (Macaca mulatta) survival time after pulsed gamma-neutron irradiation Bethesda, MD: Armed Forces Radiobiology Research Institute; SR 68–5; 1968. [Google Scholar]
- U. S. Food and Drug Administration. New Drug and Biological Drug Products; Evidence Needed to Demonstrate Effectiveness of New Drugs When Human Efficacy Studies Are Not Ethical or Feasible 67: 37988–37998; 2002. [PubMed] [Google Scholar]
- U. S. Food and Drug Administration. Guidance for Industry: Product Development Under the Animal Rule [online] Washington, DC: U.S. Department of Health and Human Services, Center for Drug Evaluation and Research (CDER), and Center for Biologics Evaluation and Research (CEBR); 2015. Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm399217.pdf. Accessed 05/19/2020. [Google Scholar]
- Vigneulle RM, Herrera J, Gage T, MacVittie TJ, Taylor P, Zeman G, Nold JB, Dubois A. Nonuniform irradiation of the canine intestine. I. Effects. Radiat Res 121: 46–53; 1990. [PubMed] [Google Scholar]
- Wang J, Wang B, Chen D, Luo Y. The response of dogs to mixed neutron-γ radiation with different n/γ ratios. Radiat Res 128: S42–S46; 1991. [PubMed] [Google Scholar]
- Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, Tsu H, Confer DL, Coleman CN, Seed T, Lowry P, Armitage JO, Dainiak N. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med 140: 1037–1051; 2004. [DOI] [PubMed] [Google Scholar]
- Wingate CL, Page NP, Ainsworth EJ. Comparison of dose patterns in a dog exposed to neutrons and x-rays. Radiat Res 32: 404–416; 1967. [PubMed] [Google Scholar]
- Wise D, Turbyfill CL. The acute mortality response of monkeys (Macaca mulatta) to pulsed mixed gamma-neutron radiation. Bethesda, MD; SR68–17; 1968. [PubMed] [Google Scholar]
- Wise D, Turbyfill CL. The acute mortality response of the miniature pig to pulsed mixed gamma-neutron radiations. Radiat Res 41: 507–515; 1970. [PubMed] [Google Scholar]
- Yu J-Z, Lindeblad M, Lyubimov A, Neri F, Smith B, Szilagyi E, Halliday L, MacVittie T, Nanda J, Bartholomew A. Subject-based versus population-based care after radiation exposure. Radiat Res 184: 46–55; 2015. [DOI] [PubMed] [Google Scholar]
- Yu ZY, Li M, Han AR, Xing S, Ou HL, Xiong GL, Xie L, Zhao YF, Xiao H, Shan YJ, Zhao ZH, Liu XL, Cong YW, Luo QL. RhG-CSF improves radiation-induced myelosuppression and survival in the canine exposed to fission neutron irradiation. Radiat Res 52: 472–480; 2011. [DOI] [PubMed] [Google Scholar]
- Zellmer RW, Pickering JE. Biologic effects of nuclear radiation in primates. Proj Rep USAF Sch Aviat Med 60: 1–11; 1960. [PubMed] [Google Scholar]
- Zeman GH, Mohaupt TH, Taylor PL, MacVittie TJ, Dubois A, Vigneulle RM. Nonuniform irradiation of the canine intestine. II. Dosimetry. Radiat Res 121: 54–62; 1990. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


