Abstract
Background:
The increasing prevalence of obesity is a significant concern worldwide. Laparoscopic sleeve gastrectomy (LSG) is an effective and standard procedure for sustained weight loss. However, optimal pain control is essential for enhanced recovery after surgery. The aim of this randomized controlled study was to investigate the efficacy of a pre-incisional laparoscopic preperitoneal local anesthetic technique (PLPLAT) on recovery characteristics following LSG.
Methods:
A total of 120 obese patients scheduled to undergo LSG were randomized into the PLPLAT or placebo group (n = 60 patients in both groups). All patients received conventional intravenous or other analgesics postoperatively, as required. The primary outcome was the postoperative pain score. The secondary outcomes included morphine consumption, other analgesics, length of stay in the postanesthesia care unit (PACU), hemodynamic changes, postoperative nausea and vomiting (PONV), early mobilization, and length of hospital stay.
Results:
Pain scores in the PACU and at 12 hours after surgery in the ward were significantly lower in the PLPLAT group than in the placebo group (P < 0.05). The morphine consumption was significantly less in PLPLAT group with mean dosage of 2.95 mg (± 0.39) compared to 6.0 mg (± 0.4) in placebo group. PONV, mean arterial pressure, and PACU stay were significantly higher in the placebo group than in the PLPLAT group (P < 0.05).
Conclusion:
Intraoperative PLPLAT provide effective postoperative pain relief for patients undergoing LSG. The findings indicated the efficacy of PLPLAT in reducing postoperative pain, enhancing recovery, and facilitating early discharge.
Keywords: Enhanced recovery after surgery, Laparoscopic sleeve gastrectomy, Pain, Preperitoneal local anesthetic infiltration
INTRODUCTION
Obesity is a multifactorial disease with an alarming global incidence rate and worldwide prevalence of obesity has increased since 1980. Almost one-third of the world’s population is classified as overweight.1 Laparoscopic sleeve gastrectomy (LSG) is an effective treatment for sustained weight loss. Moreover, it ameliorates obesity-related comorbidities and is now the most commonly performed bariatric surgery worldwide.2
In patients undergoing LSG, the management of postoperative visceral pain as well as pain from the laparoscopic portal sites requires a multimodal approach. However, obese patients are sensitive to opioid overdose and are at risk of respiratory depression and obstructive sleep apnea.3 Periportal preperitoneal local anesthetic infiltration can decrease the postoperative opioid consumption after LSG.4 Although all medications have side effects, opioids have particularly concerning systemic, long-term, and short-term side effects, and may increase morbidity and prolong hospital admission.5 Perioperative multimodal analgesia uses a combination of analgesic medications that act on different sites and pathways in an additive or synergistic manner to achieve pain relief with minimal or no opioid consumption. Local infiltration analgesia (LIA) is widely used as a practical component of multimodal analgesia and represents a valuable option for controlling perioperative pain. LIA involves the injection and/or infusion of a local anesthetic near the surgical incision site to provide targeted analgesia.6,7 Preperitoneal local anesthetic infiltration is a novel technique first described by Dean et al.8 for pain relief in laparoscopic hernia repair and is now gaining popularity.9,10 Another study reported that continuous preperitoneal analgesia following radical cystectomy improved the postoperative inflammatory response and provided comparable overall analgesia to continuous epidural analgesia.11 Recently, an extensive systematic review and meta-analysis comparing preperitoneal or subcutaneous wound catheters with epidural analgesia in abdominal surgery showed that preperitoneal wound catheter pain control was comparable to epidural analgesia. However, recovery parameters and patient satisfaction seem to favor periportal wound catheters.12
In bariatric surgery, the clinical practice trends are now moving toward opioid-sparing anesthesia.13 Since the first description of preperitoneal local anesthetic infiltration almost two decades ago, only a few studies have tested its efficacy, especially in LSG patients. The bariatric surgical team in our center published many papers in the management of high-risk obesity patients and its surgical complications.14–16 The pre-incisional laparoscopic preperitoneal local anesthetic technique (PLPLAT) was also used successfully in one study,9 encouraging us to conduct a prospective study to validate the usage of this technique. Moreover, preperitoneal local anesthesia technique was used successfully in laparoscopic peritoneal dialysis catheter insertion for high-risk patients who are not candidates for general anesthesia.10 Therefore, in the present study, we hypothesize that the PLPLAT is an effective technique to reduce postoperative pain and promote early mobilization and patient satisfaction due to fewer side effects.
MATERIALS AND METHODS
Study Design
After obtaining institutional review board approval, this prospective, double-blind, controlled clinical study was conducted from December 1, 2020 to April 30, 2021. The randomization was done using sealed opaque envelopes. The inclusion criteria were American Society of Anesthesiologists physical status I–III, 18 – 60 years of age, and patients with body mass index ≥ 40 kg/m2 or 35 kg/m2 with comorbidities. Patients with severe cardiac diseases, severe renal failure, liver cirrhosis, and allergy to bupivacaine as well as chronic opioid users were excluded from the study.
All included patients were informed about the study, consented to LSG, and randomly divided into two groups: patients in group 1 (n = 60) received intraoperative PLPLAT using 00.5% bupivacaine further diluted in 100 mL of normal saline at a dose of 20.5 mg/kg body weight. In contrast, the control group (n = 60) received 100 mL of normal saline.
Upon arrival at the operating room, all eligible participants received an intravenous (IV) cannula in situ and underwent monitoring according to the Association of Anesthetists of Great Britain and Ireland, including evaluation with a peripheral nerve stimulator. Induction of anesthesia was achieved with IV 100 mcg fentanyl and propofol 2 mg/kg of body weight (BW) and tracheal intubation was facilitated using IV rocuronium 00.6 mg/kg BW. Anesthesia was maintained with one minimum alveolar concentration desflurane in a mixture of 50% oxygen in air. Controlled ventilation was adjusted to maintain an end-tidal CO2 between 35 and 40 mm Hg. Intraoperatively, all patients received IV dexamethasone (8 mg), paracetamol (1 g), lornoxicam (16 mg), and ondansetron (4 mg).
The patient was placed in the supine position on the bed with both arms secured to the footboard. Pneumoperitoneum was achieved via a Veress needle at the Palmer’s point. The incisions for trocar placement are illustrated in Figure 1. A camera incision was made 16 cm from the xiphoid process and 1 cm to the left midline. An 11 mm port trocar was inserted; a 5 mm camera size was used to explore the abdomen. With the guidance of the camera, the second skin incision was made for 15 mm port, 1 cm proximal, and 5 cm to the right of the first incision. The third incision (5 mm) was made 1 cm proximal and 5 cm to the left side of the patient. The fourth incision (5 mm) was made 1 – 3 cm to the left of the xiphoid process (Figure 1). After skin incisions were made, a Veress needle was inserted until it reached the preperitoneal space under laparoscopic guidelines with solution infiltration (Figure 2). The periperitoneum space was infiltrated adequately from all the quadrants around each trocar. Only the camera port was infiltrated after the trocar placement. The infiltration takes 20 – 30 seconds for each port. The fascial wounds of A and B were closed by sutures via a fascial closure device under laparoscopic guidance. This was followed by closure of the skin. After tracheal extubation, all the patients were transferred to the PACU. The primary and secondary outcome variables were recorded in the PACU and followed up in the ward at 12 h after surgery by health care providers blinded to group allocation. Patients were transferred to the ward when they achieved a modified Aldrete score of 9 on two sequential measurements at 10-min intervals. A detailed description of the PLPLAT has been reported by Aldohayan et al.9 Upon completion of surgery IV, Sugammadex 2 mg/kg BW was given and the trachea was extubated. The patient fully awake was transferred to the postanesthetic recovery room (PACU) for further follow up, then discharged to the normal surgical floor with stable vital signs.
Figure 1.

Port placement in sleeve gastrectomy.
Figure 2.
Injection of fluid in the retroperitoneal space through a veress needle.
Postoperative Pain Control
All patients were prescribed IV morphine 2 mg boluses if indicated with a maximum of 10 mg, and IV tramadol 50 – 100 mg for breakthrough pain in the PACU. Pain control in the ward was achieved with IV paracetamol 1 g every 6 – 8 hours, IV lornoxicam (nonsteroidal anti-inflammatory medication) 8 mg every 12 hours if indicated, and IV tramadol 50 – 100 mg every 12 hours.
Outcome Measures
The numerical rating scale (NRS) measures pain intensity on a scale from 0 (no pain) to 10 (worst pain possible). The primary outcome variable was the postoperative pain score measured using the NRS. The secondary outcome variables were opioids and any other analgesics, hemodynamics, length of stay in the PACU, referred shoulder pain, PONV, early mobilization, and length of hospital stay (LOS).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 8.40.3 for Windows (GraphPad Software, La Jolla, CA, USA). Where appropriate, group mean values with the standard error of the mean and sample size were reported. Assuming an annual rate of 400 patients for weight loss surgery for an identified surgical team, a power analysis indicated that a sample size of 117 patients was sufficiently large to detect a type-I error of 0.05, and a power of 80% [https://www.calculator.net/sample-size-calculator.html]. An additional three patients were included to compensate for attrition during the study period, resulting in a sample size of 120 patients. Differences between groups for all tests were reported as exact p-values. Differences were considered statistically significant at an α level of less than 0.05. Unpaired Student's t test and Mann–Whitney U test were used to calculate group differences between parametric data and nonparametric or categorical data, respectively.
RESULTS
No significant differences were observed in the demographic characteristics of the two groups (P > .05) (Table 1 and Figure 3). The two groups also showed no significant differences in referred shoulder pain, heart rate, use of other analgesics, CO2 insufflation pressure, early mobilization, and LOS (P > .05; Table 2). Mean arterial pressure (MAP) was significantly higher in the placebo group than in the PLPLAT group (P < .05). Morphine consumption was significantly higher in the placebo group than in the PLPLAT group (P < .0001). More patients experienced PONV in the placebo group than in the PLPLAT group (P < .05). The duration of stay in the PACU was significantly longer in the placebo group than in the PLPLAT group (P < .05) (Table 2). The mean pain score in the PACU was significantly lower in the PLPLAT group (30.4 ± 00.2) than in the placebo group (4.95 ± 0.25) (P < .0001). The mean pain score on mobilization after 12 hours in the ward was significantly lower in the PLPLAT group (1.95 ± 00.2) than in the placebo group (30.3 ± 00.2; P < .0001) (Figure 4). Twenty-two patients in the PLPLAT group were discharged on the same day.
Table 1.
Demographic Data
| PLPLAT (n = 60) | Placebo (n = 60) | P Value | |
|---|---|---|---|
| †Age (Years) | 35.48 ± 1.5 | 33.18 ± 1.46 | 0.2 |
| ‡Sex (Females/Males) | 38/22 | 36/24 | 0.7 |
| ‡ASA (II/III) | 52/8 | 45/15 | 0.1 |
| †BMI (kg/m2) | 46.97 ± 1.2 | 45.10 ± 1.25 | 0.2 |
Abbreviations: PLPLAT, pre-incisional laparoscopic preperitoneal local anesthetic technique; ASA, American Society of Anesthesiologists; BMI, body mass index.
Values are presented as mean ± standard error of the mean and frequency (n) where appropriate. *P < .05 significant; † by unpaired Student’s t test; ‡ by χ Square test.
Figure 3.

Consolidated standards of reporting trial flow diagram. Abbreviations: ASA, American Society of Anesthesiologists; SICU, surgical intensive care unit; BMI, body mass index; PLPLAT, combined periportal and preperitoneal local anesthetic infiltration.
Table 2.
Intraoperative and Postoperative Data
| PLPLAT (n = 60) | Placebo (n = 60) | P Value | |
|---|---|---|---|
| †Intraoperative CO2 insufflation (mm Hg) | 18.23 ± 0.20 | 18.35 ± 0.18 | 0.6 |
| †Heart rate | 83.18 ± 1.3 | 86.7 ± 1.3 | 0.06 |
| †Mean arterial pressure (mm Hg) | 98.92 ± 1.4 | 104.0 ± 1.9 | 0.03* |
| †Morphine consumption (mg) | 2.95 ± 0.39 | 6.0 ± 0.4 | < 0.0001* |
| ‡Other analgesics (no/yes) | 12/48 | 6/54 | 0.1 |
| ‡PONV (no/yes) | 39/21 | 28/32 | 0.04* |
| †PACU stay (Minutes) | 50.6 ± 1.1 | 58.2 ± 2.2 | 0.002* |
| ‡Referred shoulder pain (no/yes) | 56/4 | 57/3 | 0.6 |
| §Length of hospital stay (days) | .58 | 1.2 | 0.1 |
Abbreviations: PLPLAT, pre-incisional laparoscopic preperitoneal local anesthetic technique; PONV, postoperative nausea and vomiting; PACU, postanesthesia care unit.
Values are presented as mean ± standard error of the mean and frequency (n), where appropriate. *P < .05 significant; † Unpaired Student’s t test; ‡ by χ Square test; § by Mann–Whitney U test.
Figure 4.
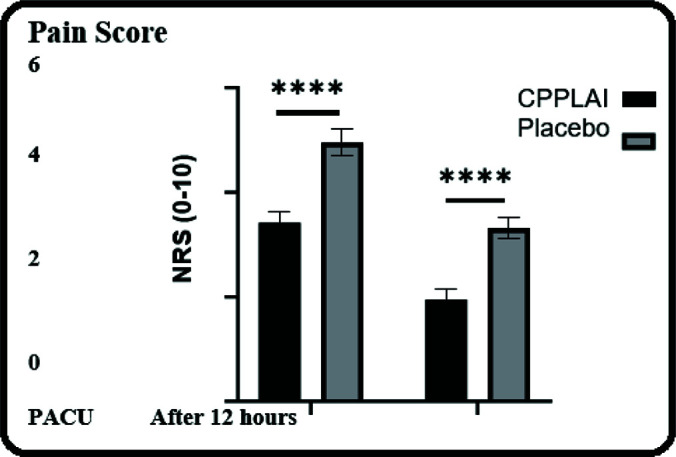
Postoperative pain score in the postanesthesia care unit and at 12 hours post-surgery in the ward using a zero to ten numeric rating scale. Not Significant, *P ≤ .05, **P ≤ .01, ***P ≤ .001, and ****P ≤ .0001. Abbreviations: PACU, Post-Anesthesia Care Unit; NRS, Numerical Rating Scale; PLPLAT, combined periportal and preperitoneal local anesthetic infiltration.
DISCUSSION
In the current study, the patients who received PLPLAT showed significantly lower pain scores in the PACU and at 12 hours after surgery in the ward compared to the placebo group. On the other hand, the patients in the placebo group had a higher MAP, received more postoperative morphine, had more PONV, and stayed longer in PACU.
LSG requires four incisions on the anterior abdominal wall for the placement of surgical ports and a laparoscopic camera.17 Local anesthetic infiltration at the surgical site is a well-known technique for reducing postoperative pain and opioid consumption.18 In this study, we performed local anesthetic infiltration from the skin to the abdominal wall muscles and up to the peritoneum. Under direct laparoscopic camera visualization, the Veress needle was inserted percutaneously until it reached the preperitoneal space, which was followed by normal saline injection through the needle to create the preperitoneal space. After confirmation, bupivacaine was injected (Figure 2). The surgeon performed this technique at the port sites before trocar insertion under direct visualization. None of the patients experienced any complications related to this technique. The implementation of enhanced recovery after surgery protocols is an evidence-based pathway that reduces pain, early mobilization, and shortened LOS.19,20 Due to better pain control and less opioid consumption, most of our patients were discharged on the same day of surgery or the first postoperative day.
In addition to PLPLAT, other regional anesthesia techniques that can reduce postoperative pain following laparoscopic surgery include ultrasound-guided transversus abdominis plane (TAP) block and erector spinae plane (ESP) block. The TAP block is a feasible and effective technique for multimodal analgesia following LSG,21,22 while the ESP block23,24 is an effective but relatively new technique to block the spinal nerves innervating the anterior abdominal wall to control postoperative pain after laparoscopic surgery.25,26 Currently, there is insufficient literature comparing ultrasound-guided TAP or ESP blocks with the PLPLAT in terms of efficacy and time required to perform the blocks.27,28 Further studies are needed to assess the efficacy and duration of procedure between PLPLAT and TAP block.
The major limitations of this study are the small number of patients and the noninclusion of the third arm of the TAP block group. Future three-arm studies, including PLPLAT, TAP, and control groups, will identify the merits of PLPLAT and will facilitate its use in future practice.
CONCLUSION
Intraoperative PLPLAT is an effective technique for postoperative pain relief in LSG patients and may facilitate considering LSG as a day surgery procedure. However, any laparoscopic procedure would potentially benefit from this technique.
Footnotes
Acknowledgment: I would like to express my special thanks of gratitude to SaudiLS for support from the beginning of the research.
Funding sources: none.
Disclosure: none.
Conflict of interests: none.
Informed consent: Dr. Sulaiman Alshammari declares that written informed consent was obtained from the patient/s for publication of this study/report and any accompanying images.
Contributor Information
Abdullah Aldohayan, Department of Surgery, University Medical City, King Saud University, Riyadh, Saudi Arabia..
Sulaiman Alshammari, Department of Surgery, University Medical City, King Saud University, Riyadh, Saudi Arabia..
Ahmed Binjaloud, Department of Surgery, University Medical City, King Saud University, Riyadh, Saudi Arabia..
Fahad Bamehriz, Department of Surgery, University Medical City, King Saud University, Riyadh, Saudi Arabia..
Abdul Sattar Narejo, Anesthesia Department, University Medical City, King Saud University, Riyadh, Saudi Arabia..
Mansoor Aqil, Anesthesia Department, University Medical City, King Saud University, Riyadh, Saudi Arabia..
Nahlah Aldahian, Anesthesia Department, University Medical City, King Saud University, Riyadh, Saudi Arabia.; Pharmacology Department, Alfaisal University, Riyadh, Saudi Arabia.
Abdulaziz Aldabaeab, Department of Surgery, University Medical City, King Saud University, Riyadh, Saudi Arabia..
Abdelazeem Eldawlatly, Anesthesia Department, University Medical City, King Saud University, Riyadh, Saudi Arabia..
References:
- 1.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. [DOI] [PubMed] [Google Scholar]
- 2.Chung AY, Thompson R, Overby DW, Duke MC, Farrell TM. Sleeve gastrectomy: surgical tips. J Laparoendosc Adv Surg Tech A. 2018;28(8):930–937. [DOI] [PubMed] [Google Scholar]
- 3.Belcaid I, Eipe N. Perioperative pain management in morbid obesity. Drugs. 2019;79(11):1163–1175. [DOI] [PubMed] [Google Scholar]
- 4.Boerboom SL, de Haes A, Vd Wetering L, et al. Preperitoneal bupivacaine infiltration reduces postoperative opioid consumption, acute pain, and chronic postsurgical pain after bariatric surgery: a randomized controlled trial. Obes Surg. 2018;28(10):3102–3110. [DOI] [PubMed] [Google Scholar]
- 5.Beverly A, Kaye AD, Ljungqvist O, Urman RD. Essential elements of multimodal analgesia in enhanced recovery after surgery (ERAS) guidelines. Anesthesiol Clin. 2017;35(2):e115–e143. [DOI] [PubMed] [Google Scholar]
- 6.Merritt CK, Mariano ER, Kaye AD, et al. Peripheral nerve catheters and local anesthetic infiltration in perioperative analgesia. Best Pract Res Clin Anaesthesiol. 2014;28(1):41–57. Mar [DOI] [PubMed] [Google Scholar]
- 7.Joshi GP, Machi A. Surgical site infiltration: a neuroanatomical approach. Best Pract Res Clin Anaesthesiol. 2019;33(3):317–324. [DOI] [PubMed] [Google Scholar]
- 8.Deans GT, Wilson MS, Brough WA. Controlled trial of preperitoneal local anaesthetic for reducing pain following laparoscopic hernia repair. Br J Surg. 1998;85(7):1013–1014. [DOI] [PubMed] [Google Scholar]
- 9.Aldohayan A, Eldawlatly A. Combined preincisional periportal and preperitoneal infiltration with bupivacaine in pain relief after laparoscopic surgery. Saudi J Anaesth. 2017;11(2):135–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aldohayan A, AlSehli R, Alosaimi MM, et al. Preperitoneal local anesthesia technique in laparoscopic peritoneal dialysis catheter placement. JSLS. 2022;26(1):e2021.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Othman AH, Ahmed DG, Abd El-Rahman AM, El Sherif FA, Mansour S, Aboeleuon E. Effect of preperitoneal versus epidural analgesia on postoperative inflammatory response and pain following radical cystectomy: a prospective, randomized trial. Clin J Pain. 2019;35(4):328–334. [DOI] [PubMed] [Google Scholar]
- 12.Mungroop TH, Bond MJ, Lirk P, et al. Preperitoneal or subcutaneous wound catheters as alternative for epidural analgesia in abdominal surgery: a systematic review and meta-analysis. Ann Surg. 2019;269(2):252–260. [DOI] [PubMed] [Google Scholar]
- 13.Lirk P, Rathmell JP. Opioid-free anaesthesia: con: it is too early to adopt opioid-free anaesthesia today. Eur J Anaesthesiol. 2019;36(4):250–254. [DOI] [PubMed] [Google Scholar]
- 14.BaMehriz F, Alali MN, Arishi H, et al. Characteristics of morbid obese patients with high-risk cardiac disease undergoing laparoscopic sleeve gastrectomy surgery. Saudi J Anaesth. 2020;14(2):182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Althuwaini S, Bamehriz F, Alobaid O, Barry M, Somily A, Aldohayan A. Identification of bacterial and fungal pathogens in patients with post-laparoscopic sleeve gastrectomy leakage. Obes Surg. 2018;28(12):3965–3968. [DOI] [PubMed] [Google Scholar]
- 16.Althuwaini S, Bamehriz F, Aldohayan A, Alaqel MA, Bassas R, AlJunidel RA. Comparison of laparoscopic sleeve gastrectomy outcomes between elderly and young patients. Saudi J Laparosc. 2020;5(1):18–21. [Google Scholar]
- 17.Hayes K, Eid G. Laparoscopic sleeve gastrectomy: surgical technique and perioperative care. Surg Clin North Am. 2016;96(4):763–771. [DOI] [PubMed] [Google Scholar]
- 18.Pavlidis TE, Atmatzidis KS, Papaziogas BT, Makris JG, Lazaridis CN, Papaziogas TB. The effect of preincisional periportal infiltration with ropivacaine in pain relief after laparoscopic procedures: a prospective, randomized controlled trial. JSLS. 2003;7(4):305–310. [PMC free article] [PubMed] [Google Scholar]
- 19.Prabhakaran S, Misra S, Magila M, et al. Randomized controlled trial comparing the outcomes of enhanced recovery after surgery and standard recovery pathways in laparoscopic sleeve gastrectomy. Obes Surg. 2020;30(9):3273–3279. [DOI] [PubMed] [Google Scholar]
- 20.Jones DB, Abu-Nuwar MRA, Ku CM, Berk LS, Trainor LS, Jones SB. Less pain and earlier discharge after implementation of a multidisciplinary enhanced recovery after surgery (ERAS) protocol for laparoscopic sleeve gastrectomy. Surg Endosc. 2020;34(12):5574–5582. [DOI] [PubMed] [Google Scholar]
- 21.Mittal T, Dey A, Siddhartha R, Nali A, Sharma B, Malik V. Efficacy of ultrasound-guided transversus abdominis plane (TAP) block for postoperative analgesia in laparoscopic gastric sleeve resection: a randomized single blinded case control study. Surg Endosc. 2018;32(12):4985–4989. [DOI] [PubMed] [Google Scholar]
- 22.Ari DE, Ar AY, Karip CS, et al. Ultrasound-guided subcostal-posterior transversus abdominis plane block for pain control following laparoscopic sleeve gastrectomy. Saudi Med J. 2017;38(12):1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kot P, Rodriguez P, Granell M, et al. The erector spinae plane block: a narrative review. Korean J Anesthesiol. 2019;72(3):209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Cassai A, Bonvicini D, Correale C, Sandei L, Tulgar S, Tonetti T. Erector spinae plane block: a systematic qualitative review. Minerva Anestesiol. 2019;85(3):308–319. [DOI] [PubMed] [Google Scholar]
- 25.Mostafa SF, Abdelghany MS, Abu Elyazed MM. Ultrasound-guided erector spinae plane block in patients undergoing laparoscopic bariatric surgery: a prospective randomized controlled trial. Pain Pract. 2021;21(4):445–453. [DOI] [PubMed] [Google Scholar]
- 26.Daghmouri MA, Akremi S, Chaouch MA, et al. Bilateral erector spinae plane block for postoperative analgesia in laparoscopic cholecystectomy: a systematic review and meta-analysis of randomized controlled trials. Pain Pract. 2021;21(3):357–365. [DOI] [PubMed] [Google Scholar]
- 27.Coşkun M, Yardimci S, Arslantaş MK, et al. Subcostal transversus abdominis plane block for laparoscopic sleeve gastrectomy, is it worth the time? Obes Surg. 2019;29(10):3188–3194. [DOI] [PubMed] [Google Scholar]
- 28.Albrecht E, Kirkham KR, Endersby RV, et al. Ultrasound-guided transversus abdominis plane (TAP) block for laparoscopic gastric-bypass surgery: a prospective randomized controlled double-blinded trial. Obes Surg. 2013;23(8):1309–1314. [DOI] [PubMed] [Google Scholar]


