Abstract
Background and Objectives:
Laparoscopic sleeve gastrectomy has become one of the most popular bariatric surgeries in the United States with a low rate of morbidity and effective weight loss. However, staple line leak remains a feared complication requiring a lengthy and difficult treatment course until resolution. This study outlines the various treatment methods used within a high-volume bariatric practice for successful leak resolution without necessitating a conversion procedure.
Methods:
A retrospective review was conducted on all patients with staple line leak after laparoscopic sleeve gastrectomy in a three-surgeon bariatric practice from January 1, 2010 to December 31, 2019.
Results:
A total of 10 staple line leaks were identified with a leak rate of 0.9%. Patients presented on average 29.3 days postoperatively and were all diagnosed on computed tomography. Three patients were initially managed operatively with washout and drainage procedure. Six patients were managed endoscopically initially with either stent or over-the-scope clip placement. Most patients required multiple interventions with an average of 2.4 interventions per patient. Average time to leak resolution was 48.2 days (15–95 days).
Conclusion:
Management of staple line leaks after laparoscopic sleeve gastrectomy requires a multimodal approach usually requiring multiple interventions before leak resolution. We demonstrate effective utilization of varying interventions that lead to effective leak resolution and avoid conversion operations.
Keywords: Endoscopic treatment, Laparoscopic sleeve gastrectomy complication, Management staple line leak, Staple line leak
INTRODUCTION
The laparoscopic sleeve gastrectomy (LSG) now accounts for most bariatric surgery in the United States.4,5 The prevalence of the LSG has motivated surgeons to better understand the safety and efficacy of the procedure, in addition to the associated complications. Morbidities related to LSG include gastroesophageal reflux disease (GERD), insufficient weight loss, stricture or dilation of gastric tube, bleeding, and leak from the residual staple line.6 The most severe of these complications includes bleeding and staple line leaks.3,7 Bleeding is typically detected in the immediate or acute postoperative period, while staple line leaks tend to present later, often after the patient is discharged.1 The potential severity and increased mortality associated with staple line leaks warrants a better understanding of the etiology and management of this complication.
The pathophysiology of staple line leaks in LSG is thought to be due to two distinct phenomena: (1) mechanical failure of the staple line, and (2) ischemia in that area causing tissue breakdown.8,9 Mechanical failure is attributed to increased luminal pressures within the gastric tube postoperatively due to narrowing at the angularis incisura. Consequently, leaks tend to occur in the proximal portion of the stomach near the gastroesophageal junction.7–9 Ischemia at the staple line is presumably caused by both the dissection required to release the stomach, combined with the stapling itself. Damage to tissue during these maneuvers causes weakening and breakdown of the stomach wall, causing leakage. In all likelihood, the true etiology of leaks is a combination of both theories. Mild ischemia predisposes the staple line to injury and leak ensues from increased luminal pressure.
The management of staple line leaks is complicated by delayed presentation usually after the patient has been discharged.2,8 Initial management is typically determined by computed tomography (CT) imaging and then initial control of sepsis by either percutaneous or laparoscopic drainage.1,8,10,11 Subsequently, patients are managed either surgically or endoscopically based on clinical course. Many new endoscopic management techniques are being studied and it is becoming an increasingly popular option for minimally invasive management of leaks.12,13 Interventions include endoluminal stent placement, over-the-scope clips (OTSC), fibrin glue techniques, and endoluminal wound vacuum (E-Vac) placement.1,8,12 There is varying evidence on the efficacy of each technique for management of leaks. Surgical reconstruction is typically used as a last resort for management of chronic leaks from LSG. Reconstruction is done with Roux-en-Y gastric bypass or with Roux-en-Y esophagojejunostomy.14 These methods have been reportedly successful at treating leaks, but each come with their own set of associated morbidities. This study aims to examine management of staple line leaks at a single institution and compare our findings to those in the literature. Our study took place at an American College of Surgeon accredited, high-volume bariatric center, performed by surgeons who were past their learning curves.
METHODS
All patients from a three-surgeon bariatric group who were diagnosed with staple line leak after laparoscopic sleeve gastrectomy between January 1, 2010 and December 31, 2019 were included in the analysis. Leaks from other bariatric procedures such as Roux-en-Y gastric bypass procedures, laparoscopic gastric banding, or conversion of previous LSG to Roux-en-Y gastric bypass were excluded. Patients in whom leaks were suspected but not confirmed were also excluded from the study. Institutional review board approval was obtained for retrospective electronic chart review. For this type of study formal consent is not required. Variables retrospectively obtained from the electronic medical record included descriptive statistics of our patients, timing of leak presentation, modality of diagnosis, initial management, additional interventions, and time to leak resolution.
There are variations in surgical technique for performing an LSG. Within our practice, all surgeons used a 40-Fr bougie and Covidien Tristaple black reinforced stapler loads for the antrum starting 3–5 cm from pylorus. The rest of the staple line from the gastric body up to the angle of His was performed with Covidien Tristaple purple stapler loads. This portion of the staple line was handled with either reinforced stapler loads or oversewing, and with fibrin glue application dependent on surgeon preference. All surgeons performed air leak test with air insufflation and methylene blue dye. In all cases included, negative air leak test was confirmed.
RESULTS
Within our practice, 1,116 laparoscopic sleeve gastrectomies were performed between January 1, 2010 and December 31, 2019. Ten staple line leaks occurred within that time period, resulting in a leak rate of 00.9% within our practice compared to the reported national leak rate of 0–2.7%.5,15,16 Of these patients, three were male and seven were female. They had an average preoperative body mass index of 42.5. The most common comorbidities included hyperlipidemia (40%), hypertension (30%), and obstructive sleep apnea (30%) (Table 1).
Table 1.
Patient Characteristics
| Variable | Value |
|---|---|
| Age (years, range) | 39.4 (24–56) |
| Female (n, %) | 7 (70%) |
| Preoperative BMI (avg., range) | 43 (36–52) |
| Postoperative BMI (avg., range) | 33 (26–45) |
| Medical Comorbidities: (n, %) | |
|
3 (30%) |
|
4 (40%) |
|
3 (30%) |
|
2 (20%) |
|
2 (20%) |
|
2 (20%) |
|
7 (70%) |
Abbreviations: BMI, body mass index; PCOS, polycystic ovarian syndrome; TIA, transient ischemic attack; PTSD, post-traumatic stress disorder.
Initial management of patients who presented with leak was based on clinical presentation and initial CT imaging. Those who were septic were taken for diagnostic laparoscopy with washout and drainage. Those who were more stable on presentation were initially treated with esophagogastroduodenoscopy (EGD) and stenting with possible percutaneous drainage if a drainable collection was present. In our patient population, time to leak presentation varied from postoperative day five to day 111, with average presentation at 29.3 days. All patients were diagnosed with leak via CT imaging at presentation to the emergency department.
Of these 10 patients, three underwent initial surgical intervention for washout and drainage; two of the three subsequently underwent concurrent feeding jejunostomy tube placement. Two of these patients were managed laparoscopically while one required laparotomy due to small bowel serosal injury during washout. Three patients required operative intervention after initial management, one of whom was initially managed surgically and the other two were initially managed endoscopically. Re-operation included washout and drainage with one patient requiring feeding jejunostomy tube placement. One of these patients required additional reoperation with video-assisted thorascopic surgery due to development of persistent left pleural effusion requiring pneumolysis and partial decortication.
Six patients were initially managed endoscopically with EGD with either OTSC or endoscopic stent placement. One of these patients developed a chronic leak and eventually was managed with E-Vac placement. Three patients required stent replacement or removal due to stent migration or nausea. Four patients had OTSC placement in either initial or subsequent endoscopic interventions. Two patients eventually required operative intervention as described above. A list of each patient’s interventions before leak resolution is detailed in Table 2.
Table 2.
Interventions and Time to Leak Resolution
| Patient | Postoperative Day of presentation (days) | Time to resolution (days) | Number of interventions | Initial Management: Surgical or Endoscopic | Interventions |
|---|---|---|---|---|---|
| 1 | 12 | 25 | 1 | Endoscopic | 1) EGD with stent |
| 2 | 8 | 30 | 2 | Endoscopic | 1) EGD with stent |
| 2) EGD with stent replacement | |||||
| 3 | 5 | 95 | 4 | Surgical | 1) Laparoscopic drainage with Jejunostomy tube |
| 2) EGD with stent | |||||
| 3) Laparoscopic drainage | |||||
| 4) E-Vac placement | |||||
| 4 | 31 | 30 | 2 | Endoscopic | 1) EGD with OTSC |
| 2) EGD with stent | |||||
| 5 | 18 | 91 | 4 | Endoscopic | 1) EGD with OTSC |
| 2) Repeat EGD with OTSC | |||||
| 3) EGD with stent | |||||
| 4) Percutaneous drainage | |||||
| 6 | 25 | 55 | 5 | Surgical | 1) Laparoscopic washout and drainage |
| 2) Pigtail catheter placement | |||||
| 3) EGD with stent | |||||
| 4) Laparoscopic washout and drainage | |||||
| 5) Video-assisted thoracic surgery washout and decortication | |||||
| 7 | 111 | 38 | 2 | Surgical | 1) Laparotomy with washout, drainage, Jejunostomy tube placement, small bowel resection |
| 2) EGD with stent | |||||
| 8 | 35 | 32 | 2 | Endoscopic | 1) EGD with stent and OTSC |
| 2) Laparoscopic washout and jejunostomy tube placement | |||||
| 9 | 26 | 71 | 2 | Endoscopic | 1) EGD with stent |
| 2) EGD with stent removal and placement of OTSC | |||||
| 10 | 22 | 15 | 0 | Neither | No operative or endoscopic management |
| Average | 29.3 | 48.2 | 2.4 |
Abbreviations: EGD, esophagogastroduodenoscopy; OTSC, over-the-scope clip; E-Vac, endoluminal wound vacuum.
Recovery time ranged from 15 to 95 days, with an average recovery of 48.2 days, from the time of leak presentation. The longest recovery time was three months in a patient treated with E-Vac sponges whose course was complicated by acute respiratory failure requiring percutaneous tracheostomy placement for ventilatory weaning. The fastest recovery was 15 days in a patient who presented on postoperative day 22 and was able to be managed conservatively with antibiotics and total parenteral nutrition. Most patients required multiple interventions for resolution of leak with multiple different treatment modalities. Eight patients required at least two interventions, which highlights the difficult nature of managing sleeve leaks.
To evaluate leak resolution, three patients had repeat CT imaging, two patients underwent repeat EGD, one patient was evaluated with upper gastrointestinal series imaging (fluoroscopic radiologic imaging), and the remaining four demonstrated clinical improvement. Of the patients who improved clinically, two had jejunostomy tubes that were removed once clinically improved and tolerating an oral diet. The methods for leak management and time to resolution are compared in Table 2.
DISCUSSION
At our institution, we have used various methods reported in the literature to mitigate staple line leak. Such strategies range from initial operative management with drainage to exclusively endoscopic intervention with stent placement. The variety of treatment strategies, tailored to different patient-types, suggests that there is not a single best method to manage staple line leaks from LSG. As with many other studies, the decision for operative versus endoscopic intervention was based on patient’s presenting clinical picture, with more acute presentations requiring initial operative management.
Management of staple line leaks in LSG is complicated by the timing of leak presentation. With the advent of the enhanced recovery protocol, time to discharge has decreased to one or two days after surgery.17 However, most leaks become symptomatic after a patient is discharged.2,8,18 Our average presentation of leak was 29.3 days. This is consistent with the variation seen in literature with some reported averages of seven days, 30 days, and even leaks occurring seven months after surgery.1,10,21 A large review analysis including 4888 patients demonstrated 79% of patients presented after 10 days.8 One patient in our study presented very late at 111 days after surgery. EGD evaluation demonstrated distal stricture requiring dilation, which was a likely contributor to the development of late staple line leak secondary to increased intraluminal pressure.
When staple line leaks do occur, patients often present peritonitic and septic, and intervention is determined by the both the stability of the patient and the extent of leak. Leaks are first detected with CT imaging, which allows the surgeon to plan management strategies accordingly. Sepsis control is the initial priority; if the patient is unstable or septic, they will typically undergo diagnostic laparoscopy with washout, drainage, and occasionally feeding jejunostomy tube placement.1,8,10,11 If the patient is stable, CT or interventional radiology-guided drains are usually placed and the patient is monitored. In the long term, patients are either managed endoscopically or surgically for staple line leaks.
The experience at our institution demonstrates the success of various endoscopic management strategies, including OTSC, stenting, and E-Vac sponge techniques.1,12,13 First, the utilization of endoscopic stents has proven an effective treatment model, with complications including stent migration and nausea.13 While this method is often initially successful, in some cases patients may warrant further endoscopic modalities. Another method utilized at our institution was OTSC, which was utilized in four patients but only as the last intervention in one patient. Ongoing research demonstrates the efficacy of this treatment modality in appropriate patients without the complications associated with stent placement.12
Finally, there is limited research on the use of E-Vac placement for persistent staple line leaks. In a combined retrospective and prospective study of nine patients treated with E-Vac for staple line leak after LSG, the authors found that although the procedure is efficacious (rescue rate of 89%) it demands a longer length of stay (mean = 72.5 days).19 In the present study, we found E-Vacs to be most appropriate in a case of chronic leakage, where other treatment modalities, such as stenting or OTSC had previously failed. The use of E-Vac requires repeated EGDs for wound vac replacement, but nonetheless preserve the stomach without need for conversion to Roux-en-Y gastric bypass.
Despite leakage, all patients in this series experienced appropriate postoperative weight loss after LSG. Only one patient required conversion to Roux-en-Y gastric bypass for refractory nausea that persisted two years after leak resolution. Nausea, or more commonly persistent gastroesophageal reflux disease, are two indications for conversion of LSG to Roux-en-Y gastric bypass, regardless of the status of postoperative staple line leak from LSG.4 Of note, there was one mortality in this series of patients which occurred approximately two years after LSG and was unrelated to complications from staple line leak. Nonetheless, leaks after LSG have been associated with higher rates of mortality, ranging from 0–0.2% in the literature.15,20 In a study examining major complications after laparoscopic bariatric surgery, the adjusted odds ratio for one year mortality after leak was 35.8 (95% confidence interval [CI], 8.61–157.3) compared to 25.4 (95% CI, 17.2–37.5; P < .001) in laparoscopic Roux-en-Y gastric bypass.15
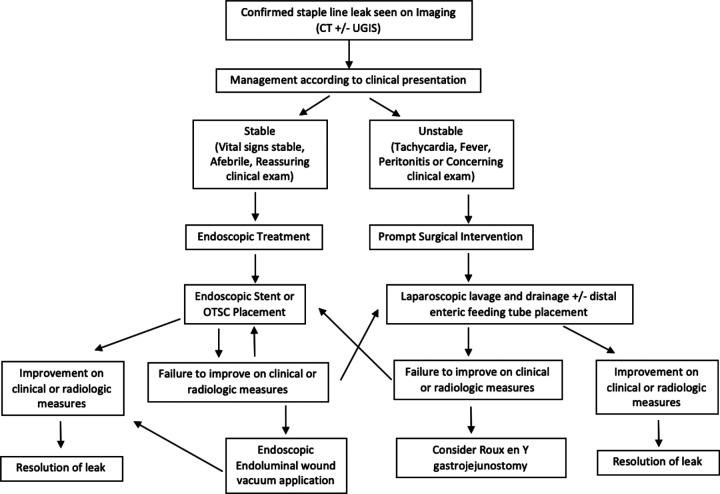
Overall, the experience at our institution, combined with findings in the literature, allow us to outline the various treatment options for leaks after LSG and suggest a strategy for approaching this rare patient population. Initially, all patients will be diagnosed on CT imaging; types of intervention then depend on the patient’s stability, regardless of timing of presentation, as illustrated in Figure 1. If the patient is unstable or septic, we recommend initial diagnostic laparoscopy for washout and drainage. If the patient is clinically stable, we recommend further evaluation with EGD with concurrent stent or OTSC placement. For those initially managed operatively, further endoscopic intervention is indicated if it appears that the leak has persisted. Endoscopic intervention again includes stent placement or OTSC placement. Repeat endoscopic intervention can be pursued if it appears initial intervention was unsuccessful. If the leak fails to resolve with stenting or clipping, E-Vac can be pursued as an alternative for closure. The progress of endoluminal wound vacuum will require multiple sponge changes based on the progression of tissue closure at the leak site. If the patient fails to improve with these interventions or develops worsening clinical picture or fluid collection, operative washout and drainage with jejunostomy tube placement should be pursued. Finally, there are studies demonstrating operative management can be pursued with conversion to Roux-en-Y gastric bypass or Roux-en-Y esophagojejunostomy as a last resort.14 However, conversion operations present a difficult challenge given the extent of disease and present the potential for their own known complications. Notably, in our patient population, we were able to avoid conversion by tailoring treatment strategies based on clinical presentation and progression. We present a comprehensive review of different treatment modalities that were successful in managing staple line leaks without necessitating conversion operation.
Figure 1.
Treatment algorithm. Abbreviations: UGIS, Upper Gastrointestinal Series; OTSC, Over the Scope Clip.
CONCLUSION
Management of staple line leaks after laparoscopic sleeve gastrectomy should first focus on medical resuscitation of the patient, followed by endoscopic evaluation and treatment (via stent placement, clipping, or endoluminal wound vacuum) or diagnostic laparoscopy with drainage and jejunostomy tube placement, if warranted. If these initial techniques fail, reintervention with either endoscopic evaluation or diagnostic laparoscopy should be considered as most patients will require multiple interventions for successful management.
Footnotes
Disclosure: none.
Conflict of interests: none.
Funding sources: none.
Informed consent: Dr. Yun Hwa Walter Wang declares that written informed consent was obtained from the patient/s for publication of this study/report and any accompanying images.
Contributor Information
Megan Parmer, Department of Surgery, Icahn School of Medicine at Mt Sinai, New York, NY..
Yun Hwa Walter Wang, Department of Surgery, Icahn School of Medicine at Mt Sinai, New York, NY..
Eliza H. Hersh, Department of Surgery, Brigham and Women’s Hospital, Boston, MA..
Linda Zhang, Department of Surgery, Icahn School of Medicine at Mt Sinai, New York, NY..
Edward Chin, Department of Surgery, Icahn School of Medicine at Mt Sinai, New York, NY..
Scott Q. Nguyen, Department of Surgery, Icahn School of Medicine at Mt Sinai, New York, NY..
References:
- 1.Moon RC, Shah N, Teixeira AF, Jawad MA. Management of staple line leaks following sleeve gastrectomy. Surg Obes Relat Dis. 2015;11(1):54–59. [DOI] [PubMed] [Google Scholar]
- 2.Aurora AR, Khaitan L, Saber AA. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc. 2012;26(6):1509–1515. [DOI] [PubMed] [Google Scholar]
- 3.ASMBS Clinical Issues Committee. Updated position statement on sleeve gastrectomy as a bariatric procedure. Surg Obes Relat Dis. 2012;8(3):e21–e26. [DOI] [PubMed] [Google Scholar]
- 4.Abraham A, Ikramuddin S, Jahansouz C, Arafat F, Hevelone N, Leslie D. Trends in bariatric surgery: procedure selection, revisional surgeries, and readmissions. Obes Surg. 2016;26(7):1371–1377. [DOI] [PubMed] [Google Scholar]
- 5.Gagner M, Kemmeter P. Comparison of laparoscopic sleeve gastrectomy leak rates in five staple-line reinforcement options: a systematic review. Surg Endosc. 2020;34(1):396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seki Y, Kasama K, Hashimoto K. Long-term outcome of laparoscopic sleeve gastrectomy in morbidly obese Japanese patients. Obes Surg. 2016;26(1):138–145. [DOI] [PubMed] [Google Scholar]
- 7.D’Ugo S, Gentileschi P, Benavoli D, et al. Comparative use of different techniques for leak and bleeding prevention during laparoscopic sleeve gastrectomy: a multicenter study. Surg Obes Relat Dis. 2014;10(3):450–454. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Azagury D, Eisenberg D, DeMaria E, Campos GM. ASMBS position statement on prevention, detection, and treatment of gastrointestinal leak after gastric bypass and sleeve gastrectomy, including the roles of imaging, surgical exploration, and nonoperative management. Surg Obes Relat Dis. 2015;11(4):739–748. [DOI] [PubMed] [Google Scholar]
- 9.Benedix F, Poranzke O, Adolf D, et al. Staple line leak after primary sleeve gastrectomy-risk factors and mid-term results: do patients still benefit from the weight loss procedure? Obes Surg. 2017;27(7):1780–1788. [DOI] [PubMed] [Google Scholar]
- 10.Sakran N, Goitein D, Raziel A, et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27(1):240–245. [DOI] [PubMed] [Google Scholar]
- 11.Safadi BY, Shamseddine G, Elias E, Alami RS. Definitive surgical management of staple line leak after sleeve gastrectomy. Surg Obes Relat Dis. 2015;11(5):1037–1043. [DOI] [PubMed] [Google Scholar]
- 12.Shoar S, Poliakin L, Khorgami Z, et al. Efficacy and safety of the over-the-scope clip (OTSC) system in the management of leak and fistula after laparoscopic sleeve gastrectomy: a systematic review. Obes Surg. 2017;27(9):2410–2418. [DOI] [PubMed] [Google Scholar]
- 13.Klimczak T, Klimczak J, Szewczyk T, Janczak P, Jurałowicz P. Endoscopic treatment of leaks after laparoscopic sleeve gastrectomy using MEGA esophageal covered stents. Surg Endosc. 2018;32(4):2038–2045. [DOI] [PubMed] [Google Scholar]
- 14.Mahmoud M, Maasher A, Al Hadad M, Salim E, Nimeri AA. Laparoscopic Roux-en-Y esophago-jejunostomy for chronic leak/fistula after laparoscopic sleeve gastrectomy. Obes Surg. 2016;26(3):679–682. [DOI] [PubMed] [Google Scholar]
- 15.Inaba CS, Koh CY, Sujatha-Bhaskar S, et al. One-year mortality after contemporary laparoscopic bariatric surgery: an analysis of the bariatric outcomes longitudinal database. J Am Coll Surg. 2018;226(6):1166–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoursheed M, Al-Bader I, Mouzannar A, et al. Postoperative bleeding and leakage after sleeve gastrectomy: a single-center experience. Obes Surg. 2016;26(12):2944–2951. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Tovar J, Muñoz JL, Gonzalez J, et al. C-reactive protein, fibrinogen, and procalcitonin levels as early markers of staple line leak after laparoscopic sleeve gastrectomy in morbidly obese patients within an Enhanced Recovery After Surgery (ERAS) program. Surg Endosc. 2017;31(12):5283–5288. [DOI] [PubMed] [Google Scholar]
- 18.Sethi M, Zagzag J, Patel K, et al. Intraoperative leak testing has no correlation with leak after laparoscopic sleeve gastrectomy. Surg Endosc. 2016;30:(3):883–891. [DOI] [PubMed] [Google Scholar]
- 19.Leeds SG, Burdick JS. Management of gastric leaks after sleeve gastrectomy with endoluminal vacuum (E-Vac) therapy. Surg Obes Relat Dis. 2016;12(7):1278–1285. [DOI] [PubMed] [Google Scholar]
- 20.Boza C, Daroch D, Barros D, León F, Funke R, Crovari F. Long-term outcomes of laparoscopic sleeve gastrectomy as a primary bariatric procedure. Surg Obes Relat Dis. 2014;10(6):1129–1133. [DOI] [PubMed] [Google Scholar]
- 21.Praveenraj P, Gomes RM, Kumar S, et al. Management of gastric leaks after laparoscopic sleeve gastrectomy for morbid obesity: A tertiary care experience and design of a management algorithm. J Minim Access Surg. 2016;12(4):342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]