Abstract
The effect of growth in 2xYT medium on catabolite repression control in Pseudomonas putida has been investigated using the bkd operon, encoding branched-chain keto acid dehydrogenase. Crc (catabolite repression control protein) was shown to be responsible for repression of bkd operon transcription in 2xYT. BkdR levels were elevated in a P. putida crc mutant, but bkdR transcript levels were the same in both wild type and crc mutant. This suggests that the mechanism of catabolite repression control in rich media by Crc involves posttranscriptional regulation of the bkdR message.
The molecular mechanisms of catabolite repression have been well described in enteric bacteria, where enzymes of the phosphoenolpyruvate phosphotransferase system mediate catabolite repression control by regulation of cAMP concentration via adenylate cyclase activity (19). However, a similar mechanism does not appear to be present in Pseudomonas because adenylate cyclase activity and cAMP pools do not fluctuate with carbon source, nor does addition of cAMP relieve repression of catabolite responsive pathways (12, 18). The only protein thus far shown to be involved in catabolite repression in Pseudomonas is Crc of P. aeruginosa, but a function has not been identified (13). However, Crc does not appear to bind DNA (13), suggesting that it is not simply a DNA-binding negative regulator.
Crc is involved in catabolite repression of P. putida branched-chain keto acid dehydrogenase (BCKAD), glucose-5-phosphate dehydrogenase, and amidase by glucose and succinate in synthetic media (11). BCKAD is encoded by the four structural genes of the bkd operon, which is positively regulated by BkdR (15). BkdR is a homologue of Lrp (leucine-responsive protein), which is a global transcriptional regulator in Escherichia coli (4). However, pseudomonads and enteric bacteria live in complex media in nature and not in chemically defined media. Expression of lrp is downregulated in nutritionally rich media (6), which suggested that this might also be the case with bkdR. In this report, the effect of 2xYT medium on the expression of bkdR in wild type and in a crc mutant of P. putida was studied to determine if catabolite repression control of the bkd operon might be accomplished by controlling the level of BkdR in the cell.
Crc downregulates BCKAD activity in 2xYT.
The wild-type strains of P. putida and P. aeruginosa, their crc mutants, and the complemented mutants (11) were grown to an A660 of ∼0.6 in 100 ml of 2xYT plus 0.3% valine and 0.1% isoleucine (wt/vol) and then harvested; cell extracts were then prepared as described earlier (16). P. putida JS394 had five- to sixfold higher activity than either PpG2 or JS394 (pJRS196) (Table 1), and a similar result was obtained when BCKAD activity of PAO8020 was compared to the activities of PAO1 and PAO8020 (pPZ352). These results demonstrate that Crc is involved in catabolite repression control of BCKAD activity by 2xYT in both P. putida and P. aeruginosa. However, the BCKAD activities of the crc were much lower than that obtained in minimal media (11), indicating that something in addition to Crc is involved in catabolite repression control in synthetic medium.
TABLE 1.
Effect of mutations in crc on repression of BCKAD activity by 2xYT in P. putida and P. aeruginosa
| Straina | Host genotype | Relevant plasmid genesa | BCKAD activityb |
|---|---|---|---|
| P. putida | |||
| PpG2 | crc+ | 4 | |
| JS394 | crc mutant | 23 | |
| JS394(pJRS196) | crc mutant | crc+ (P. putida) | 2 |
| P. aeruginosa | |||
| PAO1 | crc+ | 3 | |
| 8020 | crc mutant | 18 | |
| 8020(pPZ352) | crc mutant | crc+ (P. aeruginosa) | 2 |
| 8020(pJRS196) | crc mutant | crc+ (P. putida) | 3 |
Strains and plasmids were prepared under the growth conditions described earlier (11).
BCKAD activity is given in nanomoles of NADH formed/min/mg of protein.
It was interesting to investigate whether the crc mutants could be complemented with the heterologous crc. P. aeruginosa PAO8020 was transformed by triparental mating (9) with pJRS196, which contains crc from P. putida cloned in pUCPM19 (11). BCKAD activity in P. aeruginosa 8020(pJR196) was similar to that seen in P. aeruginosa PAO1 and P. aeruginosa PAO8020(pPZ352) (Table 1). This demonstrates Crc has the same function in both species and that P. aeruginosa recognizes the P. putida crc promoter. Several attempts were made to complement the P. putida crc mutation with pPZ352, but for some reason, all these attempts were unsuccessful.
Crc reduces the level of BCKAD in 2xYT.
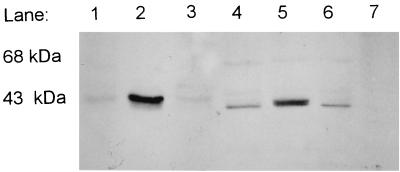
BCKAD is a multienzyme complex with three components. The E1 component of P. putida BCKAD is an αβ heterotetramer (10), the structure of which has just recently been determined (1). The E2 component is a transacylase (2), and the E3 component is a specific lipoamide dehydrogenase (3). In mammalian cells, BCKAD activity is regulated by a posttranslational modification: E1α contains two phosphorylation sites, and the phosphorylation state regulates activity of the complex (17). Although P. putida E1α is not phosphorylated (10), it is possible that catabolite repression of P. putida BCKAD activity could be the result of some other kind of posttranslational modification of BCKAD or could be the result of reduction in transcription of the bkd operon. To distinguish between these two possibilities first, Western blots with anti-E1α serum (10) were employed. P. putida PpG2, JS394, and JS394(pJRS196), and P. aeruginosa strains PAO1, PAO8020, and PAO8020(pJRS196) were grown to an A660 of ∼0.6 in 100 ml of 2xYT plus 0.3% valine and 0.1% isoleucine (wt/vol) and then harvested. Cell extracts were then prepared as described earlier (16). Five micrograms of protein was loaded onto a sodium dodecyl sulfate (SDS)–8.5% polyacrylamide gel electrophoresis (PAGE) gel, blotted to Hybond-enhanced chemiluminescence (ECL) membrane, and treated with anti-E1α serum. As seen in Fig. 1, E1α protein levels were greatly increased in the crc mutants P. putida JS394 and P. aeruginosa PAO8020 compared to the other four strains. The increase in E1α reflected the increased BCKAD activities found in these extracts (Table 1). Therefore, repression of BCKAD activity by Crc is due to a reduction in the amount of BCKAD. P. putida PpG2 grown in glucose minimal medium had no BCKAD activity, nor could E1α be detected by Western blots.
FIG. 1.
Western blot with anti-E1α serum (10) of crc mutants grown in 2xYT plus valine-isoleucine. The extracts in lanes 1 to 6 were from cultures grown in 2xYT plus 0.3% valine and 0.1% isoleucine (wt/vol); the extract in lane 7 was from a culture grown in glucose minimal medium. Each lane contained 5 μg of protein. Lane 1, P. putida PpG2; lane 2, P. putida JS394; lane 3, P. putida JS394(pJRS196); lane 4, P. aeruginosa PAO1; lane 5, P. aeruginosa PAO8020; lane 6, P. aeruginosa PA8020(pJRS196); lane 7, P. putida PpG2 grown in glucose. All cultures were grown to an A660 of between 0.6 and 0.8, and cell extracts were prepared as described before (16). Electrophoresis was done in an SDS–8.5% PAGE gel. Western blots were screened by using the ECL-Western blotting analysis system (Amersham Pharmacia Biotech) with Hybond-ECL nitrocellulose membranes according to the manufacturer's instructions. To determine whether the ECL detection method was quantitative, increasing amounts of PpG2 grown in valine-isoleucine-lactate medium were loaded on a gel and used for Western blotting with anti-E1α serum. The blot was scanned with a Molecular Dynamics densitometer, and pixel values versus micrograms of protein were graphed and shown to be a linear plot (data not shown).
Crc regulates the level of BkdR produced in 2xYT.
To characterize the role of BkdR in catabolite repression of BCKAD activity, Western blots with anti-BkdR serum were used to measure BkdR levels in wild type and the crc mutant of P. putida in grown in 2xYT plus valine-isoleucine and valine-isoleucine synthetic media. Then, 200 μg of cell extracts from P. putida PpG2, JS394, and JS394(pJRS196) grown in 2xYT plus 0.3% valine–0.1% isoleucine, along with P. putida PpG2 grown in 0.3% valine–0.1% isoleucine synthetic medium (16) alone or with 40 mM succinate, were loaded on an SDS–12% PAGE gel, blotted to Hybond-P membrane, and treated with anti-BkdR serum. As seen in Fig. 2, P. putida JS394 grown in 2xYT plus valine-isoleucine had higher levels of BkdR than either PpG2 or JS394(pJRS196) grown under the same conditions. This result demonstrates that Crc plays a major role controlling the level of BkdR in 2xYT and suggests that relief of catabolite repression control in crc mutants is due to a higher level of BkdR. BkdR levels of P. putida PpG2 were much higher in minimal media than in 2xYT (lanes 4 to 5 of Fig. 2), corresponding to the higher BCKAD activity in minimal medium (11). Also, the amount of BkdR was not repressed when succinate was added to the inducing medium, suggesting a different kind of control in minimal media. No BkdR was detected in P. putida JS386 which contains a bkdR-lacZ translational fusion (14).
FIG. 2.
Levels of BkdR in wild type and in the crc mutant of P. putida. These cultures were grown and harvested as in Fig. 1, and 200 μg of each cell extract was loaded onto an SDS–12% PAGE gel, blotted to Hybond-P membrane, and treated with anti-BkdR (14). More protein was used than in the experiment of Fig. 1 because of the low copy number of BkdR per cell. Hybond-polyvinylidene difluoride (PVDF) membranes (Amersham) were used in this experiment because PVDF has a better binding capacity for low-molecular-weight proteins. Proteins were separated by electrophoresis in an SDS–12% PAGE gel. Lane 1, P. putida grown in 2xYT plus 0.3% valine–0.1% isoleucine; lane 2, P. putida JS394 grown in 2xYT plus 0.3% valine–0.1% isoleucine; lane 3, P. putida JS394(pJRS196) grown in 2xYT plus 0.3% valine–0.1% isoleucine; lane 4, P. putida PpG2 grown in 0.3% valine–0.1% isoleucine synthetic medium; lane 5, P. putida PpG2 grown in 0.3% valine–0.1% isoleucine plus 40 mM succinate; lane 6, P. putida JS386 grown in 2xYT plus 0.3% valine–0.1% isoleucine; lane 7, 20 ng of purified BkdR.
Repression of BCKAD activity by 2xYT involves posttranscriptional regulation of bkdR expression.
Since both BCKAD and BkdR levels are elevated in the JS394 grown in 2xYT supplemented with valine and isoleucine (Fig. 1 and 2), mRNA levels of bkdR and bkdA1, which encodes E1α, were of interest in determining the mechanism of action of Crc in repression by 2xYT. P. putida PpG2, JS394, and JS394(pJRS196) were grown in 2xYT plus valine-isoleucine medium. P. putida JS382 (15), a mutant with a deletion in bkdR, which does not produce bkd operon mRNA, was grown under the same conditions for use as a negative control. At mid-log phase, total RNA was purified from each culture. Five micrograms total RNA was transferred to a GeneScreen Plus membrane by means of a vacuum suction HYBRI-SLOT Filtration Manifold (Life Technologies). The membrane was prehybridized for 1 h at 42°C and then hybridized overnight with 0.5 μCi of bkdA1 or bkdR mRNA probes at the same temperature. After a washing at 65°C, the membrane was exposed to a Phosphor Screen (Molecular Dynamics) overnight. The membrane was stripped by boiling for 30 min in 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) plus 1% SDS and then prehybridized and hybridized as described above with 0.3 μCi of 5′-end-labeled 16S RNA probe. The adjusted pixel value for each mRNA was obtained by normalizing each slot pixel value to its 16S RNA value and then averaging the duplicates and subtracting the average of the normalized P. putida JS382 pixel values. After hybridization and a washing, the membrane was exposed to a Phosphor Screen for 1 to 2 h.
Probes for bkdA1 and bkdR were generated from PCR fragments and were labeled with Ambion's Prime-A-Probe kit; a larger PCR fragment was primed internally with a third primer, so that the single-stranded DNA (ssDNA) probe could be gel purified away from the PCR product. A 286-bp bkdA1 PCR fragment was amplified with primers S73 (nucleotides [nt] 1615 to 1641) and S114 (nt 1901 to 1883). This fragment was primed internally with S87 (nt 1698 to 1681), yielding an 83-base ssDNA probe complementary to the bkd operon mRNA. A 511-bp bkdR PCR fragment was amplified with primers S88 (nt 916 to 935) and S58 (nt 1427 to 1411). This fragment was primed internally with two different primers: S40 (nt 1287 to 1300) produced a 140-base ssDNA probe, while S39 (nt 1022 to 1036) produced a 405-base probe, both complementary to bkdR mRNA. For the 16S RNA probe, the primer S179 (nt 33 to 10), which is complementary to the mRNA, was 5′-end-labeled with T4 kinase. The accession numbers for each sequence are as follows: bkd operon, M57613; and 16S RNA, D85995.
Two identical blots were prepared by loading each RNA sample in duplicate onto a slot blot. These blots were first probed with radiolabeled ssDNA probes to either bkdR or bkdA1 mRNA. After this, the blots were stripped and reprobed with a radiolabeled 16S RNA oligonucleotide to normalize blots for the amount of RNA loaded.
The levels of bkdA1 mRNA were typically four- to sixfold higher in P. putida JS394 than in PpG2 or JS394(pJRS196) (Table 2). This result, taken together with the BCKAD assays and the Western blots of E1α levels, indicates that regulation of bkd operon expression by 2xYT occurs by reducing transcription. However, bkdR mRNA levels in JS394 were always similar to or slightly lower than the levels seen in PpG2 and JS394(pJRS196) (Table 2). This suggests that the mechanism of regulation of bkdR expression by 2xYT occurs at a posttranscriptional level.
TABLE 2.
Analysis of mRNA levels and BCKAD activity in wild type and crc mutant of P. putida
| P. putida straina | bdkA1 messageb | bkdR messageb | BCKAD activityc |
|---|---|---|---|
| PpG2 | 38 | 10 | 4 |
| JS394 | 165 | 5 | 23 |
| JS394(pJRS196) | 38 | 7 | 2 |
See Table 2 for genotypes.
mRNA levels are expressed in pixels.
BCKAD activity is given in nanomoles of NADH formed/min/mg of protein.
The data in our related study demonstrated that Crc was involved in catabolite repression control of BCKAD, glucose-6-phosphate dehydrogenase, and amidase in synthetic media (11). In the present study, it has been shown that the amount of BkdR was elevated in the crc mutant, JS394 (Fig. 2) but that the amount of bkdR mRNA was unchanged (Table 2), which suggests that Crc acts posttranscriptionally in controlling BkdR levels. In contrast, bkdA1 mRNA, BCKAD activities (Table 2) and E1α protein levels (Fig. 1) were all elevated in the P. putida crc mutant, indicating that expression of the bkd operon was regulated at the transcriptional level. It was also shown in (11) that lacZ expression was increased two- to threefold in the mutant with transposon-inactivated crc (P. putida JS391) carrying a bkdR-lacZ translational fusion. However, lacZ is inserted after the 44th amino acid codon of BkdR (14), and this transcript would look very different to Crc than the normal bkdR message.
Crc shares sequence similarity with a group of DNA repair enzymes, although no endo- or exonuclease activity has been identified (13). It is possible that Crc's nuclease activity is very specific, such as acting only on secondary RNA structure, resulting in functional degradation of mRNA. Crc could effect posttranscriptional regulation of bkdR expression by affecting the efficiency of translation or the stability of functional mRNA. Two types of secondary structures are responsible for controlling mRNA stability: 5′ hairpins and 3′ hairpins. The 5′ untranslated region of the E. coli ompA transcript functions in vivo as a growth-rate-regulated mRNA stabilizer (7). The hairpin in this untranslated region is not only specific for the ompA gene but also confers stability when fused to other genes (5). The half-life of the mRNA was drastically reduced when the stem-loop structure was moved more than ten nucleotides away from the 5′ end (5). These results indicate that the stabilization provided by the hairpin is due to inhibition of endonuclease cleavage. Another secondary structure that can confer stability to a transcript is a 3′ hairpin. Stem-loop structures at the 3′ end of a transcript were originally thought to function only as ρ-independent transcriptional terminators, but more recently these structures have been shown to protect mRNA from degradation by the exonucleases RNase II and PNPase (8).
One possible explanation for the function of Crc in rich media is that there is a ligand in 2xYT which causes a conformational change in Crc, thereby activating it. Activated Crc would now have endonuclease activity which causes functional degradation of bkdR message. There is some support for this hypothesis, since Yuste et al. (20) showed that use of fresh Luria-Bertani medium resulted in catabolite repression of the alk operon of Pseudomonas oleovorans, whereas spent medium did not.
Acknowledgments
This research was supported by Public Health Service grant DK21737 and Presbyterian Health Foundation grant C5142801 (both to J.R.S.) and Environmental Protection Agency STAR fellowship grant U-915028-01-0 (to K.L.H.).
REFERENCES
- 1.Ævarsson A, Seger K, Turley S, Sokatch J R, Hol W G J. Crystal structure of 2-oxoisovalertate dehydrogenase and the architecture of 2-oxo acid dehydrogenase multienzyme complexes. Nat Struct Biol. 1999;6:785–792. doi: 10.1038/11563. [DOI] [PubMed] [Google Scholar]
- 2.Burns G, Brown T, Hatter K, Sokatch J R. Comparison of the amino acid sequences of the transacylase components of branched chain oxoacid dehydrogenase of Pseudomonas putida, and the pyruvate and 2-oxoglutarate dehydrogenases of Escherichia coli. Eur J Biochem. 1988;176:165–169. doi: 10.1111/j.1432-1033.1988.tb14264.x. [DOI] [PubMed] [Google Scholar]
- 3.Burns G, Brown T, Hatter K, Sokatch J R. Sequence analysis of the lpdV gene for lipoamide dehydrogenase of branched chain oxoacid dehydrogenase of Pseudomonas putida. Eur J Biochem. 1989;179:61–69. doi: 10.1111/j.1432-1033.1989.tb14521.x. [DOI] [PubMed] [Google Scholar]
- 4.Calvo J M, Matthews R G. The leucine-responsive regulatory protein, a global regulator of metabolism in Escherichia coli. Microbiol Rev. 1994;58:466–490. doi: 10.1128/mr.58.3.466-490.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrier T A, Keasling J D. Controlling mRNA stability in bacteria: strategies for engineering gene expression. Biotechnol Prog. 1997;13:699–708. doi: 10.1021/bp970095h. [DOI] [PubMed] [Google Scholar]
- 6.Chen C F, Lan J, Korovine M, Shao Z Q, Tao L, Zhang J, Newman E B. Metabolic regulation of lrp gene expression in Escherichia coli K-12. Microbiology. 1997;143:2079–2084. doi: 10.1099/00221287-143-6-2079. [DOI] [PubMed] [Google Scholar]
- 7.Chen L H, Emory S A, Bricker A L, Bouvet P, Belasco J G. Structure and function of a bacterial mRNA stabilizer: analysis of the 5′ untranslated region of ompA mRNA. J Bacteriol. 1991;173:4578–4586. doi: 10.1128/jb.173.15.4578-4586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deutscher M P. Ribonuclease multiplicity, diversity, and complexity. J Biol Chem. 1993;268:13011–13014. [PubMed] [Google Scholar]
- 9.Goldberg J B, Ohman D E. Cloning and expression in Pseudomonas aeruginosa of a gene involved in the production of alginate. J Bacteriol. 1984;158:1115–1121. doi: 10.1128/jb.158.3.1115-1121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hester K, Luo J, Burns G, Brasswell E H, Sokatch J R. Purification of E1α2β2 of Pseudomonas putida branched-chain-oxoacid dehydrogenase. Eur J Biochem. 1995;233:828–836. doi: 10.1111/j.1432-1033.1995.828_3.x. [DOI] [PubMed] [Google Scholar]
- 11.Hester K L, Lehman J, Najar F, Song L, Roe B A, MacGregor C H, Hager P W, Phibbs P V, Jr, Sokatch J R. Crc is involved in catabolite repression control of the bkd operons of Pseudomonas putida and Pseudomonas aeruginosa. J Bacteriol. 2000;182:1144–1149. doi: 10.1128/jb.182.4.1144-1149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hylemon P B, Phibbs P V., Jr Independent regulation of hexose catabolizing enzymes and glucose transport activity in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1972;48:1041–1048. doi: 10.1016/0006-291x(72)90813-3. [DOI] [PubMed] [Google Scholar]
- 13.MacGregor C H, Arora S K, Hager P W, Dail M B, Phibbs P V., Jr The nucleotide sequence of the Pseudomonas aeruginosa pyrE-crc-rph region and the purification of the crc gene product. J Bacteriol. 1996;178:5627–5635. doi: 10.1128/jb.178.19.5627-5635.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madhusudhan K T, Huang G, Sokatch J R. Characterization of BkdR-DNA binding in the expression of the bkd operon of Pseudomonas putida. J Bacteriol. 1995;177:636–641. doi: 10.1128/jb.177.3.636-641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madhusudhan K T, Lorenz D, Sokatch J R. The bkdR gene of Pseudomonas putida is required for expression of the bkd operon and encodes a protein related to Lrp of Escherichia coli. J Bacteriol. 1993;175:3934–3940. doi: 10.1128/jb.175.13.3934-3940.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall V P, Sokatch J R. Regulation of valine catabolism in Pseudomonas putida. J Bacteriol. 1972;110:1073–1081. doi: 10.1128/jb.110.3.1073-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paxton R, Harris R A. Isolation of rabbit liver branched chain α-ketoacid dehydrogenase and regulation by phosphorylation. J Biol Chem. 1982;257:14433–14439. [PubMed] [Google Scholar]
- 18.Phillips A T, Mulfinger L M. Cyclic adenosine 3′,5′-monophosphate levels in Pseudomonas putida and Pseudomonas aeruginosa during induction and carbon catabolite repression of histidase synthesis. J Bacteriol. 1981;145:1286–1292. doi: 10.1128/jb.145.3.1286-1292.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Postma P W, Lengeler J W, Jacobson G R. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuste L, Canosa I, Rojo F. Carbon-source-dependent expression of the PalkB promoter from the Pseudomonas oleovorans alkane degradation pathway. J Bacteriol. 1998;180:5218–5226. doi: 10.1128/jb.180.19.5218-5226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]