Abstract
Background:
Although abstinence has traditionally been considered the only suitable outcome for alcohol treatment, reduced drinking is also associated with improved functioning and medical and psychiatric outcomes. The World Health Organization (WHO) risk drinking levels (RDLs) have been shown to be valid outcome measures in treatment trials for alcohol use disorder (AUD).
Methods:
We conducted a secondary analysis of two 12-week, randomized controlled trials (RCTs), in which a total of 308 individuals with problematic alcohol use received topiramate or placebo treatment. We compared the utility of the WHO RDLs with other treatment outcomes, including self-reported measures of alcohol consumption, alcohol-related problems, and quality of life, and the biomarker gamma-glutamyltransferase.
Results:
Topiramate treatment was associated with small effect sizes for both a 1-level (d=0.26) and a 2-level (d=0.19) reduction in WHO RDL, effects that were not significant after correction for multiple comparisons. No heavy drinking days, one of the outcome measures recommended by the US Food and Drug Administration for alcohol medication registration trials, also exhibited a small effect (0.21), while an effect size for abstinence could not be calculated. There were medium effects of topiramate on continuous measures of percent heavy drinking days (d=0.49) and alcohol-related problems (d=0.41).
Conclusions:
Topiramate is an efficacious pharmacotherapy for AUD. Although continuous measures of drinking and alcohol-related problems yielded larger effect sizes than the WHO RDLs, the latter capture changes in drinking behavior in response to treatment with topiramate and provide a categorical alternative for use in both clinical care and pharmacotherapy trials.
Keywords: alcohol use disorder, heavy drinking, topiramate, World Health Organization risk drinking levels
Introduction
Heavy drinking and alcohol use disorder (AUD) are prevalent worldwide and are associated with adverse physical, psychological, economic, and social consequences (Rehm et al. 2014) and increased mortality risk (World Health Organization, 2019). Despite AUD’s high prevalence (Grant et al. 2015), only a minority of affected individuals receives treatment, particularly evidence-based care, for the disorder (Cohen et al. 2007; Grant et al. 2017). A primary factor contributing to this disparity is the belief, common to both affected individuals and healthcare providers, that abstinence is the only acceptable goal of treatment (Keyes et al. 2010; Wallhed et al. 2014), which may discourage individuals from seeking care because they believe the goal to be unachievable or undesirable (Witkiewitz et al. 2016).
In response to the need for other metrics of successful treatment outcome, multiple studies have been undertaken with a goal of reduced drinking (Aubin and Daeppen 2013). These have shown that non-abstinent reductions, particularly in the frequency of heavy drinking, are associated with improved overall functioning and health (Kline-Simon et al. 2013; Laramée et al. 2015). Thus, the most recent guidance from the US Food and Drug Administration (FDA) considers both abstinence and no heavy drinking days (HDDs) as acceptable primary outcomes in pharmacotherapy registration trials (Food and Drug Administration, 2015). One non-abstinent treatment outcome measure that has recently been studied is a reduction in the World Health Organization (WHO) risk drinking levels (RDLs). The four WHO RDLs when, defined in terms of daily alcohol consumption, include: low (1-40 g for men; 1-20 g for women), medium (41-60 g for men; 21-40 g for women), high (61-100 g for men; 41-60 g for women), and very high (101+ g for men; 61+ g for women) (World Health Organization, 2000). Reductions in RDLs have been endorsed by the European Medicines Agency (2020) as an outcome for medication trials to treat AUD, as they are a quantifiable, clinically meaningful measure of changes in alcohol consumption (Falk et al. 2019).
Studies evaluating the validity of the WHO RDLs have used data from both the National Epidemiological Survey on Alcohol and Related Conditions (NESARC), a large population-based survey, and a variety of alcohol pharmacotherapy trials. These studies show that individuals who reduce their alcohol consumption by at least one risk level (e.g., from very high risk to high risk) have lower odds of AUD, drug use disorders, anxiety, depression, liver disease, cardiovascular disease, and a positive Alcohol Use Disorders Identification Test (AUDIT) score at follow-up (Hasin et al. 2017; Knox et al. 2018; Knox et al. 2019a; Knox et al. 2019b: Knox et al. 2020). In secondary analyses of the COMBINE study, a large clinical trial that compared different combinations of medication and psychosocial treatments, a reduction of at least one or two risk levels was associated with reduced blood pressure and liver enzyme levels, better quality of life, fewer alcohol-related consequences, and improved mental health (Witkiewitz et al. 2018; Witkiewitz et al. 2019).
Falk et al. (2019) conducted a secondary analysis of data from placebo-controlled, multi-center trials of naltrexone, varenicline, and topiramate to evaluate the utility of the WHO RDLs in gauging treatment response in AUD pharmacotherapy trials. Patients in the active treatment condition in all three trials were better able to achieve at least a 1- or 2-level reduction in RDLs than either abstinence or no HDD. Further, the effect sizes for a reduction in WHO RDLs for naltrexone and varenicline were larger than those for abstinence or no HDD. In the topiramate trial, however, the effect size was similar for a 2-level RDL reduction and no HDD, while abstinence showed the largest effect size.
Topiramate, first approved by the FDA in 1996 as an anticonvulsant, has been shown in a meta-analysis of seven RCTs to yield medium effects on abstinence and heavy drinking, and small effects on gamma-glutamyl transferase [GGT] concentration and craving (Blodgett et al. 2014). In large RCTs of topiramate for AUD, topiramate reduced alcohol consumption, craving, drinking-related consequences, and GGT concentration, and increased quality of life and physical well-being (Baltieri et al. 2008; Johnson et al. 2003; Johnson et al. 2008). Following the finding of an association with AUD of the rs2832407 single nucleotide polymorphism (SNP) located in GRIK1 (Kranzler et al. 2009), we conducted a post hoc examination of the moderating effect of this SNP on topiramate treatment response (Kranzler et al. 2014). In that study, European-American, C-allele homozygotes treated with topiramate reported significantly fewer heavy drinking days than A-allele carriers or those treated with placebo. A subsequent prospective trial (Kranzler et al. 2021A), failed to replicate that finding. In a recent combined analysis of the two RCTs, although both the number of heavy drinking days and alcohol-related problem severity were reduced in the topiramate-treated patients, the effects were not moderated by rs2832407 (Kranzler et al. 2021B).
We report here the results of a secondary analysis of two topiramate trials (Kranzler et al. 2014; Kranzler et al. 2021), in which we tested the utility of the WHO RDLs as an outcome measure. We hypothesized that patients treated with topiramate would be more likely to achieve 1- and 2-level reductions in WHO RDLs than placebo-treated patients, an effect that would be comparable in magnitude to the effects on other drinking outcome measures.
Methods
Patients and Procedures
Full descriptions of the procedures for both studies can be found in the primary publications (Study 1, n=138, Kranzler et al. 2014; Study 2, n=170, Kranzler et al. 2021A). In brief, 308 heavy drinking individuals were recruited to participate in one of two randomized, double-blind, 12-week trials in which they received up to 200 mg/day of topiramate or placebo. Patients were enrolled and treated at the University of Connecticut Health Center in Farmington, CT (Study 1 only, n=76), or two sites in Philadelphia–the University of Pennsylvania Perelman School of Medicine (Study 1 n=62, Study 2 n=164, total n=226) and the Corporal Michael J. Crescenz Veterans Affairs Medical Center (Study 2 only, n=6).
Patients were included if they were 18-65 years old (Study 1) or 18-70 (Study 2). In both studies, subjects were included if they reported heavy drinking (≥24 drinks/week for men, ≥18 drinks/week for women), read English at an 8th grade level, and, if a woman of childbearing potential, were using a reliable method of birth control. The majority of patients in Study 1 (n=113 or 92.6%) had a DSM-IV diagnosis of alcohol dependence (American Psychiatric Association, 2000) and each had a goal of reduced drinking. All patients in Study 2 had a diagnosis of DSM-5 AUD (American Psychiatric Association, 2013) and a goal of either reduced drinking or abstinence. In Study 2 we tested a pharmacogenetic hypothesis—namely, that rs2832407 moderates the response to topiramate—the randomization in that study was stratified on genotype. Exclusion criteria in both studies included significant physical or psychiatric comorbidities, a current DSM-IV diagnosis of drug dependence (excluding nicotine), or a clinical condition (e.g., a recent history of alcohol-related gastritis) that warranted abstinence from alcohol.
Patients in both studies were recruited through advertisements or clinical referrals and, if eligible after a telephone screening interview, were invited for an in-person visit, where they gave informed consent, provided a medical and psychiatric history, and underwent a physical examination and clinical laboratory testing. Because a combined analysis of the rs2832407 genotype in the two trials failed to support its effect as a moderator of the response to topiramate treatment (Kranzler et al, 2021B), we did not include genotype as a factor in the analyses reported here.
The same treatment protocol was used in the two studies. At the initial treatment visit, nurses dispensed the first dose of study medication and conducted the first psychosocial treatment session. Medication was initially dosed at 25 mg/day (or one placebo capsule) at bedtime and gradually increased to a maximum of 200 mg/day (or two placebo capsules) in two divided doses. For the first 6 weeks, patients attended weekly medication titration visits and by week 6 they reached the maximal dosage, after which there were three bi-weekly visits. At each treatment visit, patients received medical management (Pettinati et al. 2004), a brief intervention focused on medication adherence and counseling to reduce drinking and increase abstinent days.
Measures
We used the Timeline Follow-Back (TLFB) (Sobell and Sobell, 1992) to assess the quantity and frequency of alcohol consumption at each visit. The Short Index of Problems (SIP; Kiluk et al. 2013), a 15-item assessment, was used to measure alcohol-related problems over the preceding 3 months. Using the Short Form Health Survey (SF-12; Ware et al. 1996), a 12-item measure, patients rated their overall health and quality of life over the previous four weeks. Finally, gamma-glutamyl transferase (GGT), a liver enzyme and objective measure of alcohol intake, was assessed at baseline and at study completion.
Statistical Analysis
In the combined dataset comprising both trials, the WHO RDLs (World Health Organization, 2000) were determined for each patient as follows: low risk (1-40 g/day for males/1-20 g/day for females), medium risk (41-60 g/day for males/21-40 g/day for females), high risk (61-100 g/day for males/41-60 g/day for females), or very high risk (101+ g/day for males/61+ g/day for females). The amount of ethanol used to convert from standard drinks to risk drinking levels was 14 g. Following the method used by Falk et al. (2019), we added an “abstinence” category (no drinking during the study) to yield 5 WHO RDLs. We calculated the proportion of patients in the topiramate and placebo arms who, over the 12-week treatment period: 1) experienced a 1-level+ or a 2-level+ reduction in the WHO risk levels, 2) who experienced no HDDs, and 3) who completely abstained. Consistent with Falk et al. (2019), patients who discontinued treatment prematurely (8.2% of the present study sample) were coded as non-responders for all of the outcome analyses (i.e., no reduction in WHO risk level, HDD+, non abstinent). The difference in proportions of these categorical measures between treatment groups was converted to Cohen’s h. Additionally, three commonly used drinking measures were calculated from the TLFB across the 12 weeks of treatment: the percentage of HDDs, percentage of days abstained, and drinks per day. The difference in means between treatment groups on these measures was converted to Cohen’s d. We chose to analyze the drinking data over the entire 12-week period because in both RCTs on which the analysis is based, differences between the topiramate and placebo groups were evident as early as the first two weeks of the trials.
Regarding missing data, we used all data available to calculate the continuous outcomes. Using a Kaplan-Meier analysis, dropout rates did not differ by medication group (p=0.41) and the mean number of weeks in the study was similar between the treatment groups (topiramate=11.35, placebo=11.40). The consistency of our approach with that used in other studies of the WHO RDLs, as well as the lack of evidence of differential dropout by treatment group, lends confidence that the outcomes are not biased.
Using the combined dataset, both the logistic and linear regression models included treatment group as a factor and study as a covariate. We calculated the False Discovery Rate (FDR) using the Benjamini-Hochberg procedure for all outcomes to account for multiple comparisons, which we chose because of the high degree of intercorrelation among the outcome measures (Benjamini and Hochberg, 1995).
Results
Patients were mostly male (67.2%), pregominantly of European descent (94.8%), and middle-aged (mean=51.1 years, SD=10.3). The only significant difference between treatment groups on demographic variables was on age (Table 1).
Table 1:
Baseline Demographics and Drinking Measures for the Total Sample and Separately by Medication Group
| Total Sample (n=308) |
Placebo (n=156) | Topiramate (n=152) | p- value1 |
|||
|---|---|---|---|---|---|---|
| Study 1 (n=71) |
Study 2 (n=85) |
Study 1 (n=67) |
Study 2 (n=85) |
|||
| Sex (% male) | 207 (67.2%) | 41 (57.7%) | 60 (70.6%) | 45 (67.2%) | 61 (71.8%) | 0.54 |
| Race (% EA) | 292 (94.8%) | 66 (93.0%) | 85 (100%) | 56 (83.6%) | 85 (100%) | 1.0 |
| Age | 51.1 ± 10.3 | 52.8 ± 7.4 | 50.0 ± 12.8 | 49.3 ± 9.0 | 52.3 ± 10.5 | 0.02 |
| % Heavy Drinking Days | 69.3 ± 25.8 | 66.0 ± 27.0 | 69.1 ± 25.5 | 67.0 ± 27.0 | 74.2 ± 23.3 | 0.49 |
| % Days Abstinent | 14.3 ± 19.9 | 12.0 ± 15.0 | 15.6 ± 22.8 | 13.0 ± 16.0 | 16.1 ± 22.6 | 0.74 |
| Drinks per Day | 5.36 ± 3.03 | 4.61 ± 1.81 | 5.66 ± 3.19 | 4.53 ± 2.15 | 6.34 ± 3.88 | 0.26 |
| SIP | 14.7 ± 8.5 | 15.5 ± 6.7 | 14.3 ± 9.7 | 14.9 ± 8.6 | 14.2 ± 8.6 | 0.80 |
| SF-12 | 73.5 ± 17.2 | 74.6 ± 16.1 | 72.5 ± 16.6 | 74.7 ± 18.1 | 72.6 ± 18.0 | 0.98 |
| lnGGT | 3.71 ± 0.78 | 3.67 ± 0.76 | 3.73 ± 0.78 | 3.69 ± 0.90 | 3.73 ± 0.71 | 0.91 |
For the study by medication effect. Study 1, Kranzler et al. 2014; Study 2, Kranzler et al. 2021A; EA=European American; SIP=Short Index of Problems; SF-12=Short Form Health Survey; lnGGT=natural log of the gamma-glutamyl transferase concentration
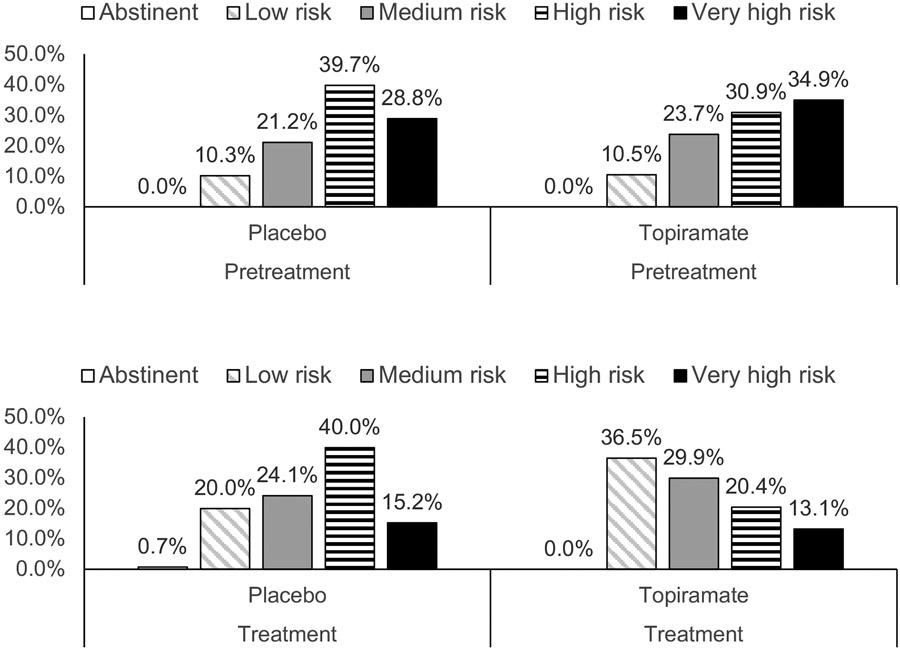
Figure 1 shows the percentage of patients in each WHO RDL during the pretreatment and treatment periods. At the outset of treatment, 67.2% of patients were in the two highest risk levels and there was no difference in the distribution of risk levels between medication groups (Cochran-Armitage Trend Test: Z= −0.266, two-sided p=0.79). Thirty-two (10.4%) of the patients began the study at the low-risk level, and thus could not show a 2-level reduction. However, none of these individuals were later classified as abstinent (they either remained low risk or withdrew from treatment) and thus were included in the analyses as showing no reduction in RDL. During treatment, there was a downward shift in risk classification, with the topiramate group showing a significantly greater reduction in RDLs than the placebo group (Cochran-Armitage Trend Test: Z=3.13, two-sided p=0.0017). This is evidenced by the reduction from the high- or very-high-risk groups from 65.8% to 34.5% (Δ=47.6%) for topiramate and from 68.5% to 55.2% (Δ=19.4%) for placebo, the topiramate group showing more than a doubling of the placebo difference on this measure.
Figure 1.
Percentage of Individuals at Different WHO Risk Drinking Levels by Treatment Group by Study Period
The percentage of patients that showed a 1-level reduction or a 2-level reduction, along with the effects on other drinking outcomes, are shown in Table 2. The effect sizes for topiramate on both the 1-level and 2-level WHO reductions were small (d=0.26 and d=0.19, respectively). For the FDA-recommended outcomes there was a small, nonsignificant effect of topiramate on the risk of no heavy drinking days (d=0.21), but no effect on the likelihood of abstinence. The effects of topiramate treatment on the continuous outcomes of percentage of heavy drinking days (d=0.49) and SIP score (d=0.41) approached the medium range of effects and both were significant following FDR correction (q=0.0003 and q=0.005, respectively). Small effects of topiramate on the percentage of days abstinent, SF-12 score, and GGT concentration were not significant.
Table 2:
Treatment Main Effects During the 12-week Treatment Period
| Measures | Placebo | Topiramate | Effect Size (95% CI) |
P-Value | FDR |
|---|---|---|---|---|---|
| WHO Risk Level Outcomes | N (%) | N (%) | |||
| 1-level reduction | 58 (37.2%) | 76 (50.0%) | 0.26 (0.03, 0.50) | 0.023 | 0.056 |
| 2-level reduction | 18 (11.5%) | 28 (18.4%) | 0.19 (−0.04, 0.42) | 0.090 | 0.113 |
| FDA Recommended Outcomes | N (%) | N (%) | |||
| No Heavy Drinking | 3 (1.9%) | 9 (5.9%) | 0.21 (−0.01, 0.44) | 0.083* | 0.113 |
| Abstinence | 1 (0.6%) | 0 (0%) | N/A | N/A | N/A |
| Continuous Drinking Outcomes | M (SD) | M (SD) | |||
| % Heavy Drinking Days | 46.7 ± 31.5 | 31.9 ± 28.2 | 0.49 (0.26, 0.72) | <0.001 | 0.0003 |
| % Days Abstinent | 20.5 ± 23.8 | 27.3 ± 28.6 | 0.26 (0.04, 0.49) | 0.028 | 0.056 |
| Drinks per Day | 4.09 ± 2.20 | 3.39 ± 2.41 | 0.30 (0.08, 0.53) | 0.011 | 0.037 |
| Other Outcomes | M (SD) | M (SD) | |||
| SIP | 10.4 ± 8.3 | 7.2 ± 7.5 | 0.41 (0.18, 0.65) | 0.001 | 0.005 |
| SF-12 | 78.0 ± 16.7 | 80.9 ± 14.7 | 0.18 (−0.05, 0.42) | 0.12 | 0.133 |
| lnGGT | 3.5 ± 0.77 | 3.4 ± 0.74 | 0.21 (−0.03, 0.45) | 0.089 | 0.113 |
Effect Size=Cohen’s d, Cohen’s h; 95% CI=95% confidence interval; FDR=false discovery rate; SIP=Short Index of Problems; SF-12=Short Form Health Survey; GGT=gamma-glutamyl transferase
Fisher exact test
To examine potential differences between the two studies, we reran models for the 10 outcomes in Table 2 in which a study-by-drug interaction term was included. Results showed that in no model was the interaction term significant (p-values ranged from 0.34 for the SIP to 1.00 for abstinence), with a similar effect of the drug between studies. To test for sex effects, we also reran the models by including a sex-by-drug interaction term. Results showed that in none of the models was the interaction significant (p-values ranged from 0.12 for no heavy drinking to 1.00 for abstinence) and the direction of effect was consistent between sexes.
Discussion
This secondary analysis of two RCTs of topiramate for treating heavy drinking or AUD examined the utility of WHO RDL reductions as a measure of treatment outcome. Topiramate produced a small effect on both the proportion of patients who achieved a WHO 1-level or a 2-level reduction, and although the the 1-level reduction was nominally significant, neither RDL reduction survived correction for multiple comparisons. There was also a small effect of the medication on the likelihood of a patient having no heavy drinking days (HDDs), one of the measures recommended by the FDA for use in clinical trials, as well as two outcomes that are often used in trials of medications for treating AUD: drinks per day and percent days abstinent. Of these measures, only the effect on drinks per day remained significant after FDR correction. The most significant outcome was a reduction in percent HDDs, which showed a moderate effect of medication. Small effects observed for the SF-12 and GGT levels, both favoring topiramate, may be limited by the relatively short follow-up period, as such effects may take longer than self-reported consumption to become evident.
The study does not provide information on the other FDA-recommended outcome, abstinence, as only one patient in the study achieved that goal, consistent with the focus of the trials being on the reduction of heavy drinking rather than the promotion of abstinence. In contrast, Falk et al. (2019) observed a small effect of topiramate on the 2-level WHO RDL (Cohen’s h =0.23) in a multi-center trial, with a similar effect for no HDDs, and a small-to-medium effect on abstinence (Cohen’s h=0.37). It should be noted that for the outcome of no HDDs there was an effect size similar to that of a 2+ level RDL reduction, only nine topiramate-treated patients achieved this outcome (compared to 28 who had the 2+ RDL reduction). This underscores the fact that the FDA-recommended outcomes are difficult to achieve and may limit the development of medications for AUD treatment. We also found that topiramate had medium-sized effects on the percentage of HDDs and the SIP score, measures that were not reported by Falk et al. A key difference between this study and that by Falk et al. (2019) is that we examined medication effects during the entire study period, while Falk et al. reported outcomes for only the final four weeks of treatment. Nonetheless, both studies support the efficacy of topiramate for treating AUD.
The results of this secondary analysis should be interpreted in the context of its limitations. Combining two studies, despite their similar design and inclusion and exclusion criteria, could introduce confounders. We sought to account for this by including study as a covariate in the analyses. Unlike many other clinical trials for substance use, neither study required patients to have a goal of complete abstinence from alcohol and only one patient achieved abstinence during the treatment period. The therapeutic benefit of topiramate in the context of an abstinence-oriented treatment trial cannot be ascertained from the current study. Participants included in this analysis were mostly male and of European ancestry, therefore the findings may not generalize to other groups. Primary measures of alcohol use and alcohol-related problems were self-reported and retrospective and thus subject to recall bias and underreporting. Because the objective measure that we used, GGT concentration, is not sensitive to changes in drinking behavior, subsequent trials should use a more sensitive biomarker such as phosphatidylethanol (Wurst et al. 2015). Investigation of whether gains made during the treatment period, in this case 12 weeks, are maintained over time are also warranted (Mejldal et al. 2021). We also did not examine the moderating effect of rs2832407, which one of these studies was prospectively designed to test. However, our recent analysis showed no moderating effect of this SNP on alcohol-related outcomes in EAs, suggesting that it is not a confounder in the present study (Kranzler et al. 2021B).
The present study also had a number of strengths. The inclusion of data from two placebo-controlled RCTs provided a larger sample in which to evaluate the utility of the WHO RDLs as a measure of treatment outcome than either trial alone. Further, both studies had good treatment retention and medication adherence, supporting the internal validity of the findings.
These results add to the growing literature on the potential utility of the WHO RDLs as an outcome measure in alcohol clinical trials. Unlike the current FDA-recommended outcomes of abstinence and no heavy drinking days, which are based on not exceeding a particular cutoff of consumption, the WHO RDLs capture reductions in drinking. This is useful from both a research and a clinical perspective insofar as reduced drinking, as captured by the WHO RDLs, may be a more desirable and feasible goal for individuals with problematic alcohol use and their treatment providers (Mann et al. 2017). Our findings indicate that more patients were able to achieve such reductions in drinking compared to the FDA endpoints. WHO RDL reductions have been associated with improved functioning, less psychiatric symptomatology, and better health (Knox et al. 2018; Knox et al. 2019a; Knox et al. 2019b: Knox et al. 2020; Witkiewitz et al. 2018; Witkiewitz et al. 2019). However, the effect of topiramate on the percentage of heavy drinking days and on the SIP score, a measure of alcohol-related problems, was substantially larger than the effect on either a 1-level or a 2-level reduction in RDLs, indicating that such continuous outcome measures may provide the greatest discriminatory power.
Supplementary Material
Acknowledgment
The study was funded by NIAAA grants P60 AA03510, R01 AA023192, and R01 AA025539 and the Mental Illness Research, Education and Clinical Center of the Veterans Integrated Service Network 4 at the Crescenz VAMC.
Footnotes
Disclosures: HRK is a member of a Dicerna Pharmaceuticals scientific advisory board and a consultant for Sophrosyne Pharmaceuticals. HRK and KW are members of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which during the past three years was supported by AbbVie, Alkermes, Dicerna, Ethypharm, Indivior, Lilly, Lundbeck, Otsuka, Pfizer, Arbor, and Amygdala Neurosciences. HRK and JG are named as inventors on PCT patent application #15/878,640 entitled: "Genotype-guided dosing of opioid agonists," filed January 24, 2018.
References
- Aubin HJ, Daeppen JB (2013) Emerging pharmacotherapies for alcohol dependence: a systematic review focusing on reduction in consumption. Drug Alcohol Depend 133:15–29. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013)Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA. [Google Scholar]
- American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders. 4th ed., text rev. Washington, DC. [Google Scholar]
- Baltieri DA, Daro FR, Ribeiro PL, de Andrade AG (2008) Comparing topiramate with naltrexone in the treatment of alcohol dependence. Addiction 103:2035–2044. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Stat Soc, Series B 57:289–300. [Google Scholar]
- Blodgett JC, Del Re AC, Maisel NC, Finney JW (2014) A meta-analysis of topiramate's effects for individuals with alcohol use disorders. Alcohol Clin Exp Res 38:1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Feinn R, Arias A, Kranzler HR (2007) Alcohol treatment utilization: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend 86:214–221. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. Guideline on the development of medicinal products for the treatment of alcohol dependence. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-development-medicinal-products-treatment-alcohol-dependence_en.pdf. Accessed December 23, 2020.
- Falk DE, O’Malley SS, Witkiewitz K, Anton RF, Litten RZ, Slater M, Kranzler HR, Mann KF, Hasin DS, Jonson B, Meulien D (2019) Evaluation of drinking risk levels as outcomes in alcohol pharmacotherapy trials: a secondary analysis of 3 randomized clinical trials. JAMA Psychiatry 76:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration (2015) Alcoholism: Developing drugs for treatment guidance for industry: draft guidance. https://www.fda.gov/downloads/Drugs/Guidancecomplianceregulatoryinformation/guidances/UCM433618.pdf Accessed March 29, 2019.
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS (2015) Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS (2017) Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 74:911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Wall M, Witkiewitz K, Kranzler HR, Falk D, Litten R, Mann K, O'Malley SS, Scodes J, Robinson RL, Anton R (2017) Change in non-abstinent WHO drinking risk levels and alcohol dependence: a 3 year follow-up study in the US general population. Lancet Psychiatry 4:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O'Malley SS, Swift RM; Topiramate for Alcoholism Advisory Board; Topiramate for Alcoholism Study Group (2007) Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA 298:1641–1651. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ (2003) Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet 361:1677–1685. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Hatzenbuehler ML, McLaughlin KA, Link B, Olfson M, Grant BF, Hasin D (2010) Stigma and treatment for alcohol disorders in the United States. Am J Epidemiol 172:1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiluk BD, Dreifuss JA, Weiss RD, Morgenstern J, Carroll KM (2013) The Short Inventory of Problems - revised (SIP-R): psychometric properties within a large, diverse sample of substance use disorder treatment seekers. Psychol Addict Behav 27:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline-Simon AH, Falk DE, Litten RZ, Mertens JR, Fertig J, Ryan M, Weisner CM (2013) Posttreatment low-risk drinking as a predictor of future drinking and problem outcomes among individuals with alcohol use disorders. Alcohol Clin Exp Res 37:E373–E380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox J, Wall M, Witkiewitz K, et al. (2018) Reduction in nonabstinent WHO drinking risk levels and change in risk for liver disease and positive AUDIT-C scores: Prospective 3-year follow-up results in the US general population. Alcohol Clin Exp Res 42:2256–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox J, Scodes J, Wall M, et al. (2019a) Reduction in non-abstinent WHO drinking risk levels and depression/anxiety disorders: 3-year follow-up results in the US general population. Drug Alcohol Depend 197:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox J, Wall M, Witkiewitz K, Kranzler HR, Falk DE, Litten R, Mann K, O’Malley SS, Scodes J, Anton R, Hasin DS (2019b) Reduction in non-abstinent World Health Organization (WHO) drinking risk levels and drug use disorders: 3-year follow-up results in the US general population. Drug Alcohol Depend 201:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox J, Scodes J, Witkiewitz K, Kranzler HR, Mann K, O’Malley SS, Wall M, Anton R, Hasin DS, Alcohol Clinical Trials (ACTIVE) Workgroup (2020) Reduction in World Health Organization risk drinking levels and cardiovascular disease. Alcohol Clin Exp Res 44:1625–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Gelernter J, Anton RF, Arias AJ, Herman A, Zhao H, Burian L, Covault J (2009) Association of markers in the 3' region of the GluR5 kainate receptor subunit gene to alcohol dependence. Alcohol Clin Exp Res 33:925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Feinn R, Armeli S, Tennen H, Arias AJ, Gelernter J, Pond T, Oncken C, Kampman KM (2014) Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry 171(4):445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Morris PE, Pond T, Crist RC, Kampman KM, Hartwell EE, Lynch KG (2021A) Prospective pharmacogenetic study of topiramate for treating alcohol use disorder. Neuropsychopharmacology doi: 10.1038/s41386-020-00945-9. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Hartwell EE, Feinn R, Pond T, Witkiewitz K, Gelernter J, Crist RC (2021B) Combined analysis of the moderating effect of a GRIK1 polymorphism on the effects of topiramate for treating alcohol use disorder. Drug Alcohol Depend 225:108762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laramée P, Leonard S, Buchanan-Hughes A, Warnakula S, Daeppen JB, Rehm J (2015) Risk of all-cause mortality in alcohol-dependent individuals: a systematic literature review and meta-analysis. EBioMedicine 2:1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Aubin HJ, Witkiewitz K (2017) Reduced drinking in alcohol dependence treatment, what is the evidence? Eur Addict Res 23:219–230. [DOI] [PubMed] [Google Scholar]
- Mejldal A, Andersen K, Behrendt S, Bilberg R, Bogenschutz M, Braun-Michl B, Buhringer G, Nielsen AS (2021) Stability of posttreatment reduction in World Health Organization (WHO) drinking risk levels and post-treatment functioning in older adults with DSM-5 alcohol use disorder: secondary data analysis of the elderly-study. Alcohol Clin Exp Res 45:638–649. [DOI] [PubMed] [Google Scholar]
- Pettinati H, Weiss R, Miller W, Donovan D, Ernst D, Rounsaville B (2004) COMBINE Monograph Series, Volume 2. Medical management treatment manual: a clinical research guide for medically trained clinicians providing pharmacotherapy as part of the treatment for alcohol dependence (DHHS Publication No. NIH 04-5289), Bethesda, MD. [Google Scholar]
- Rehm J, Dawson D, Frick U, Gmel G, Roerecke M, Shield KD, Grant B (2014) Burden of disease associated with alcohol use disorders in the United States. Alcohol Clin Exp Res 38:1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline follow-back. In Measuring alcohol consumption. Humana Press, Totowa, NJ, pp. 41–72. [Google Scholar]
- Wallhed Finn S, Bakshi AS, Andréasson S (2014) Alcohol consumption, dependence, and treatment barriers: perceptions among nontreatment seekers with alcohol dependence. Subst Use Misuse 49:762–769. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD (1996) A 12-item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 220–233. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Roos CR, Pearson MR, Hallgren KA, Maisto SA, Kirouac M, Forcehimes AA, Wilson AD, Robinson CS, McCallion E, Tonigan JS (2016) How much is too much? Patterns of drinking during alcohol treatment and associations with post-treatment outcomes across three alcohol clinical trials. J Stud Alcohol Drugs 78:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Falk DE, Litten RZ, Hasin DS, Kranzler HR, Mann KF, O'Malley SS, Anton RF(2019) Maintenance of World Health Organization risk drinking level reductions and posttreatment functioning following a large alcohol use disorder clinical trial. Alcohol Clin Exp Res 43:979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Kranzler HR, Hallgren KA, O'Malley SS, Falk DE, Litten RZ, Hasin DS, Mann KF, Anton RF (2018) Drinking Risk Level reductions associated with improvements in physical health and quality of life among individuals with alcohol use disorder. Alcohol Clin Exp Res 42:2453–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global Status Report on Alcohol and Health 2018 (2019) Geneva, Switzerland. [Google Scholar]
- World Health Organization. International guide for monitoring alcohol consumption and related harm. World Health Organization, Geneva, Switzerland; 2000. [Google Scholar]
- Wurst FM, Thon N, Yegles M, Schrück A, Preuss UW, Weinmann W (2015) Ethanol metabolites: their role in the assessment of alcohol intake. Alcohol Clin Exp Res 39:2060–2072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.