The efficacy of the Pfizer BioNTech BNT162b2 vaccine has been demonstrated in clinical trials and postvaccination observational studies. However, effectiveness had declined, and vaccine-induced immunity was waning.1 On November 17, 2021, the US Food and Drug Administration expanded the Emergency Use Authorization for the Pfizer and Moderna coronavirus disease 2019 (COVID-19) vaccines to include boosters. We recently described the results of studies of nationwide active surveillance for postvaccination myocarditis by the Israeli Ministry of Health. There were low but increased incidence rates, mainly in young male individuals, after the second vaccination, suggesting a causal relationship between second vaccine administration and myocarditis.2,3 These findings raised concerns about potential postbooster myocarditis.
After receipt of an Institutional Review Board waiver,2 all cases of myocarditis from July 31, 2021 (first day of booster), through November 5, 2021, regardless of vaccination status, were reported to the Ministry of Health by all hospitals in Israel. The analytical methods but not the data will be made available to other researchers by contacting the corresponding author. Clinical data were reviewed by a cardiologist and a rheumatologist and classified on the basis of the Brighton Collaboration Myocarditis Case Definition, as reported previously.2,3 Myocarditis risk for definite/probable cases after the booster dose was computed with the 95% CI, and risk differences (RDs) compared with previous vaccinations were calculated.
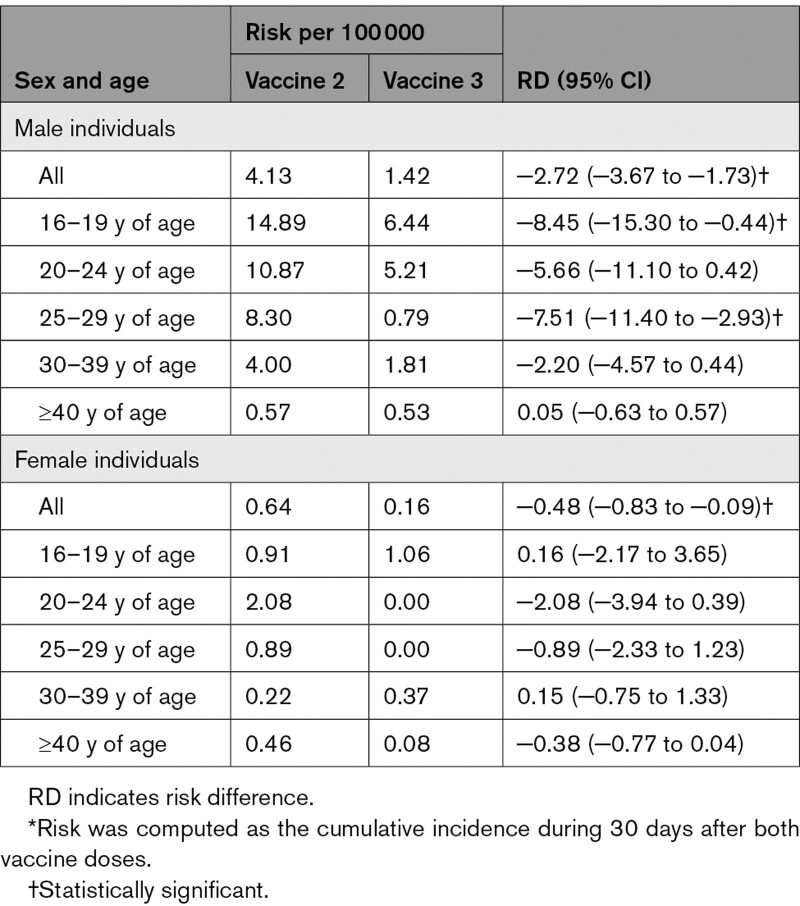
During the surveillance period, 3 944 797 individuals (1 919 453 male, 48.7%) received a booster dose. Ninety-one cases of myocarditis were reported, including 35 cases within 30 days of booster vaccination. Among them, 28 were probable or confirmed myocarditis; 18 of 28 occurred during the first week after the booster. All 28 cases were clinically mild, and patients recovered after an average 3.5-day (range, 1–6 days) hospitalization, with no readmissions after discharge during the 90-day follow-up. Risk estimates in the 30 days after the booster in male patients were 1.42 per 100 000 overall and 6.44 per 100 000 and 5.21 per 100 000 for male individuals 16 to 19 and 20 to 24 years of age, respectively. Compared with vaccine dose 2, for male individuals, the overall RD per 100 000 was −2.72 (95% CI, 3.67 to −1.73), driven mainly by male individuals 16 to 19 years of age (RD, −8.45 [95% CI, −15.30 to −0.44]); for female individuals, the overall RD was −0.48 (95% CI, −0.83 to −0.09; Table).
Table.
RDs Between the Third and Second Vaccine Doses by Age and Sex: 30-Day Follow-Up*
Compared with vaccine dose 1, for male individuals, the overall RD per 100 000 was 0.63 (95% CI, 0.03–1.27), largest in male individuals 16 to 19 years of age (RD, 5.25 [95% CI, 0.35–11.46]) and 20 to 24 years of age (RD, 2.26 [95% CI, −1.53 to 6.77]).
In our study, the risks in male individuals 16 to 19 and 20 to 24 years of age within 30 days after the booster dose were ≈1:15 625 and 1:19 409, respectively. The corresponding number in female individuals 16 to 19 years of age is 1:94 340; no cases were observed in women 20 to 24 years of age. Definitive/probable myocarditis was diagnosed without magnetic resonance imaging being systematically performed, and both diagnosis reviewers were not blinded to vaccination status.
As we previously reported in second-dose recipients, the clinical presentation was mild, with resolution of postbooster myocarditis in all cases, as judged by clinical symptoms, inflammatory markers, and troponin levels, ECG, echocardiogram normalization if abnormal (<55% ejection fraction), and a relatively short hospital stay. However, our follow-up period is relatively short. A longer follow-up is recommended.
In addition, and similar to our previous studies,2,3 postbooster myocarditis risk was associated with younger age and male sex, although current cases were distributed more evenly across 30 days of follow-up. Furthermore, the risk for individuals 16 to 29 years of age was lower compared with the second vaccine dose cases. Possible explanations for this observation may be related mainly to 2 factors. First, individual predisposition, either genetic or other, was severely reduced; individuals who developed myocarditis after the first or second vaccine dose did not receive a booster as a medical precaution. These patients were advised to avoid mRNA-based boosters and instead to have a non-mRNA booster vaccine. Indeed, none of the individuals who developed postbooster myocarditis in the current analysis had myocarditis after the first or second vaccine dose. Second, the interval between the second and third doses (20–24 weeks) was longer compared with the 3-week interval between the first and second vaccines. Tauzin et al4 have recently demonstrated longitudinal humoral responses against the D614G strain and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern in recipients who had 2 doses of the BNT162b2 mRNA vaccine with an interval of 16 weeks. Comparing these responses with those elicited in individuals having a shorter (4-week) dose interval showed that a 16-week interval induced more robust responses among naïve vaccinees. Thus, it may be suggested that a longer interval between vaccine doses does not compromise efficacy and may possibly reduce the occurrence of postvaccination myocarditis. The mechanism of vaccine-induced myocarditis is not understood but may be directly related to the active component of the vaccine, the mRNA sequence that codes for the spike (S) protein of SARS-CoV-2. mRNA vaccines tend to act as their own adjuvant and stimulate a further immune response that improves their efficacy.5
In conclusion, there was a mild decrease in the occurrence of vaccine-associated myocarditis after the third vaccine relative to the second, although the overall incidence is still low.
Article Information
Sources of Funding
None.
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- COVID-19
- coronavirus disease 2019
- RD
- risk difference
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
D. Mevorach and E. Anis contributed equally.
N. Cedar and T. Hasin contributed equally.
L. Goldberg and N. Levi contributed equally.
L. Keinan-Boker and S. Alroy-Preis contributed equally.
For Sources of Funding and Disclosures, see page 803.
Circulation is available at www.ahajournals.org/journal/circ
Contributor Information
Emilia Anis, Email: emilia.anis@moh.gov.il.
Noa Cedar, Email: noa.cedar@moh.gov.il.
Tal Hasin, Email: hasintal@gmail.com.
Michal Bromberg, Email: michal.bromberg@moh.gov.il.
Lital Goldberg, Email: lital.keinan2@moh.gov.il.
Nir Levi, Email: nir182@gmail.com.
Ofer Perzon, Email: ofer.perzon@gmail.com.
Nur Magadle, Email: NUR.MAGADLE@GMAIL.COM.
Barhoum Barhoum, Email: b.barhoum77@gmail.com.
Elchanan Parnassa, Email: elchanan.parnasa@mail.huji.ac.il.
Rita Dichtiar, Email: Rita.Dichtiar@moh.health.gov.il.
Yael Hershkovitz, Email: yael.hershkovitz@moh.gov.il.
Manfred S. Green, Email: manfred.s.green@gmail.com.
Nachman Ash, Email: nachmana@moh.gov.il.
Lital Keinan-Boker, Email: lital.keinan2@moh.gov.il.
Sharon Alroy-Preis, Email: sharon.alroy@moh.gov.il.
References
- 1.Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, Doolman R, Asraf K, Mendelson E, Ziv A, et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mevorach D, Anis E, Cedar N, Hasin T, Bromberg M, Goldberg L, Parnasa E, Dichtiar R, Hershkovitz Y, Ash N, et al. Myocarditis after BNT162b2 vaccination in Israeli adolescents. N Engl J Med. 2022;386:998–999. doi: 10.1056/NEJMc2116999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, Olsha-Castell S, Arad D, Hasin T, Levi N, et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N Engl J Med. 2021;385:2140–2149. doi: 10.1056/NEJMoa2109730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tauzin A, Gong SY, Beaudoin-Bussières G, Vézina D, Gasser R, Nault L, Marchitto L, Benlarbi M, Chatterjee D, Nayrac M, et al. Strong humoral immune responses against SARS-CoV-2 spike after BNT162b2 mRNA vaccination with a sixteen-week interval between doses. medRxiv. Preprint posted online September 21, 2021. doi: 10.1101/2021.09.17.21263532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C, Maruggi G, Shan H, Li J. Advances in mRNA vaccines for infectious diseases. Front Immunol. 2019;10:594. doi: 10.3389/fimmu.2019.00594 [DOI] [PMC free article] [PubMed] [Google Scholar]