Abstract
Autonomic neurons commonly respond to injury/axotomy with an increased expression of neuropeptides including galanin and pituitary adenylyl cyclase-activating polypeptide (PACAP). The increased peptide expression may enhance neuronal survival and axonal regeneration. Using quantitative (Q) PCR and immunocytochemistry, the present study tested whether galanin expression increased in male mouse major pelvic ganglia (MPG) neurons in response to injury. Galanin transcript expression increased significantly in MPG neurons following 72 h in explant culture and 72 h after unilateral transection of the cavernous nerve. Under both conditions, the increase in galanin transcript levels was greater than the increase in PACAP transcript levels. In control MPG, galanin-IR nerve fibers formed pericellular arrangements around MPG neurons although few galanin-IR cells were evident and many of the galanin-IR cells may be small intensely fluorescent (SIF) cells. In 3-day-cultured MPGs, many more galanin-IR cells and nerve fibers were noted. The increased galanin expression was most apparent in neurons that were also immunoreactive for neuronal nitric oxide synthase, rather than tyrosine hydroxylase. Some explant-cultured MPG neurons exhibited immunoreactivity to galanin and PACAP. As reported previously for PACAP, there is an injury-induced increase in MPG galanin expression, which occurs preferentially in the parasympathetic postganglionic neurons.
Keywords: Autonomic neurons, Injury response, Parasympathetic neurons, Immunocytochemistry, Quantitative PCR
Introduction
Many of the synaptic connections relaying centrally derived control of urogenital organs and components of the lower bowel are made in the major pelvic ganglia (MPG) (Keast 2006). The MPG is a mixed ganglion containing both catecholaminergic sympathetic postganglionic and cholinergic parasympathetic postganglionic neurons with each type of postganglionic neuron receiving a unique preganglionic innervation. In humans, it is common during pelvic surgeries such as hysterectomies, prostatectomies, and tumor removal from the lower bowel for injury to MPG neurons or their axons to occur (Keast 2006).
Many peripheral autonomic and sensory neurons assume a regenerative phenotype following injury resulting in the upregulation of a number of neuropeptides, transcription factors, and growth-associated molecules thought to support cell survival and regeneration (Makwana and Raivich 2005; Keast 2006; Navarro et al. 2007). However, the response to injury is not as well defined for MPG neurons as it is for other autonomic neurons such as the superior cervical ganglia (SCG). Consequently, we have begun studies to assess changes in the chemical phenotype of mouse MPG neurons that occur with injury. Our initial study (Girard et al. 2010) assessed the increase in expression of activating transcription factor 3 (ATF-3), a well-established injury marker (Tsuijino et al. 2000), and pituitary adenylate cyclase activating polypeptide (PACAP). These studies were completed using explant culture of mouse MPG as an in vitro injury model in which all of the neurons are axotomized. PACAP is suggested to support cell survival and regeneration in both sympathetic and parasympathetic postganglionic neurons (Moller et al. 1997; Girard et al. 2007).
Galanin is another neuropeptide that increases in many axotomized neurons, including sympathetic neurons (Mohney et al. 1994; Schreiber et al. 1994). Consequently, the present study tested whether galanin expression increases in the explant-cultured MPG. Furthermore, we compared any changes to those observed with PACAP. Finally, we determined whether MPG galanin and PACAP transcript levels increase following transection of the cavernous nerve.
Our results demonstrate that galanin transcript levels increase significantly in explant-cultured MPG and following transection of the cavernous nerve. In both conditions, the change in transcript level is greater for galanin than PACAP. We also found that the majority of galanin immunoreactive neurons in the cultured MPG are parasympathetic postganglionic neurons, an observation similar to that reported previously for PACAP (Girard et al. 2010).
Materials and Methods
Preparation
Experiments were performed in vitro on whole-mount preparations of MPG isolated from male C57BL6 mice (4–6 months of age). Protocols for use of mice were approved by the University of Vermont IACUC and followed NIH guidelines.
Ipsilateral Cavernous Nerve Transection
A unilateral transection of the cavernous nerve was performed 72 h prior to euthanasia and removal of the MPG. The procedures followed those described previously (Nangle and Keast 2009). In brief, the mice were anesthetized with isofluorane (3–4 %), and using sterile surgical techniques, one of the cavernous nerves was detached from the urethra below the prostate, transected and a small section (1–2 mm) removed to prevent regeneration. The animal was allowed to recover from surgery for 3 days.
Explant Culture
For explant culture, the MPG were quickly removed under sterile conditions and maintained at 37°C in culture media consisting of DMEM-F12 (1:1) containing 10 % horse serum, gentamicin (10 g/ml), amphotericin B (3.75 g/ml), penicillin (100 U/ml), and streptomycin (100 g/ml; Sigma, St. Louis, MO). The preparations were placed on a wave platform shaker in a 5 % CO2–95 % air incubator (37°C) and kept for 72 h, with the culture media replaced every 24 h.
Immunohistochemistry
Immunocytochemcial staining was completed on whole mount preparations of the MPG as previously described (Girard et al. 2010; Tompkins et al. 2010). Control and cultured MPG whole-mount preparations were fixed for 2 hours at 4°C in 2 % paraformaldehyde containing 0.2 % picric acid. Once fixed, the MPG whole mounts were washed in phosphate-buffered saline, permeabilized with 0.5 % Triton X-100, and incubated at 4°C overnight with combinations of primary antisera. The preparations then were washed, incubated for 2 h at room temperature with fluorescein isothiocyanate (FITC)-conjugated and/or indocarbocyanine (Cy3)-conjugated secondary antiserum (Jackson Immunoresearch Laboratories, West Grove, PA), washed again, and mounted with Citifluor (UKA Chemical Laboratory, Canterbury, England). The MPG whole mounts were viewed with an Olympus AX70 fluorescence microscope equipped with HBO 100-W UV light source and filters for FITC and Cy3. Digital images were obtained with a CCD camera (MagnaFire SP; Optronics; Optical Analysis Corp., Nashua, NH) and imported into Adobe Photoshop CS3 (Adobe Corporation, Mountain View, CA) to assemble figures, which were minimally adjusted for contrast and brightness.
Antibodies
Primary antisera used in this study included: a rabbit antigalanin, 1:1000, from Phoenix Pharmaceuticals (Burlingame, CA; lot No. R#509–3); a mouse monoclonal anti-PACAP 1:10, from Dr. Jan Fahrenkrug, Copenhagen, Denmark; a mouse anti-brain nitric oxide synthase (nNOS), 1:500, from Sigma (St Louis, MO; lot No. 037K4765); and a mouse anti-tyrosine hydroxylase (TH), 1:400 from RBI (Natick, MA; lot # TOW-1298UU).
All antisera used are well-characterized and have been used in our prior studies (Mawe et al. 1996; Calupca et al. 2000a, b; Zvarova et al. 2004; Zvarova and Vizzard 2006; Young et al. 2008; Girard et al. 2010). Neuronal staining was not observed when whole mounts were treated only with primary or secondary antisera.
Real-time Quantitative Reverse Transcription-Polymerase Chain Reaction
MPG preparations were dissected under RNase-free conditions, and total RNA was extracted from individual preparations using Tri reagent (Sigma). The total RNA quantity for each whole-mount preparation was determined with a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE). One μg of RNA per sample was used to synthesize complementary DNA using a mix of random hexamer and oligo-dT primers with M-MLV reverse transcriptase (Promega Corp.) in a 25-μl final reaction volume. Amplified MPG DNA product from specific primers was ligated into pCR2.1 TOPO using TOPO TA cloning kit (Invitrogen, Carlsbad, CA) to generate plasmid standards. The nucleotide sequences of the inserts were verified by automated fluorescent dideoxy dye terminator sequencing (Vermont Cancer Center DNA Analysis Facility), and 10-fold serial dilutions of stock plasmid were prepared to generate assay standard curves. Amplification of the mouse cDNA templates and plasmid standards was performed using HotStart IT® SYBR® Green qPCR Master Mix (USB). Real-time quantitative PCR was performed on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Norwalk, CT). For explant-cultured MPG, the data are presented as a fold change compared with the average 0-day time-point level. To determine changes in transcript levels following unilateral cavernous nerve section, the transcript levels from the ipsilateral MPG are compared with those obtained for the contralateral MPG. All quantitative data were normalized to L32 expression.
Amplification of the mouse cDNA templates and plasmid standards was performed using the following primers: galanin forward (CACAGATCATTTAGCGACAAGCAT), reverse (GACGATATTGCTCTCAGGCAG); PACAP forward (CATGTGTAGCGGAGCAAGGTT) , reverse (GTCTTGCAGCGGGTTTCC) ; L3 2 forward (CCTGGCGTTGGGATTGGTGA), reverse (GAAAAGCCATCGTAGAAAGA); GalR1forward (TAATCATCTGCATAAAAAGCT), reverse (ACATGATGGGGCAGCCAGGA; GalR2 forward (TATCCTCTGCGTGTGGT), reverse (TTGGCATAAGATACTAGATGTGA); and GalR3 forward (ATCTTCCTGTTGGGCATGGTG) ; reverse (GGTGAAGCTGCTGGCATACA).
Statistical Analyses
Student’s t test was used to evaluate differences among groups. Differences were considered statistically significant at P≤0.05.
Results
Galanin-IR Fibers and a Few Galanin-IR Neurons Are Present in Control MPG
Previously, it has been reported that many MPG neurons in male mice exhibit immunoreactivity to vasoactive intestinal polypeptide (VIP), nNOS, and TH (Wanigasekara et al. 2003; Girard et al. 2010; Tompkins et al. 2010). However, no PACAP-IR fibers or neurons were noted in control whole mounts (Girard et al. 2010). In contrast to that noted for PACAP, galanin immunoreactivity was evident in nerve fibers and a few cells in control MPG (3–25 cells/MPG, 12 preparations). Immunoreactivity was more prevalent within fibers than cells and was evident both in nerve connectives attached to the MPG and in nerve bundles within the MPG (data not shown). Within control MPG, galanin-IR fibers quite often formed pericellular arrangements around individual neurons (Fig. 1(A)).
Fig. 1.
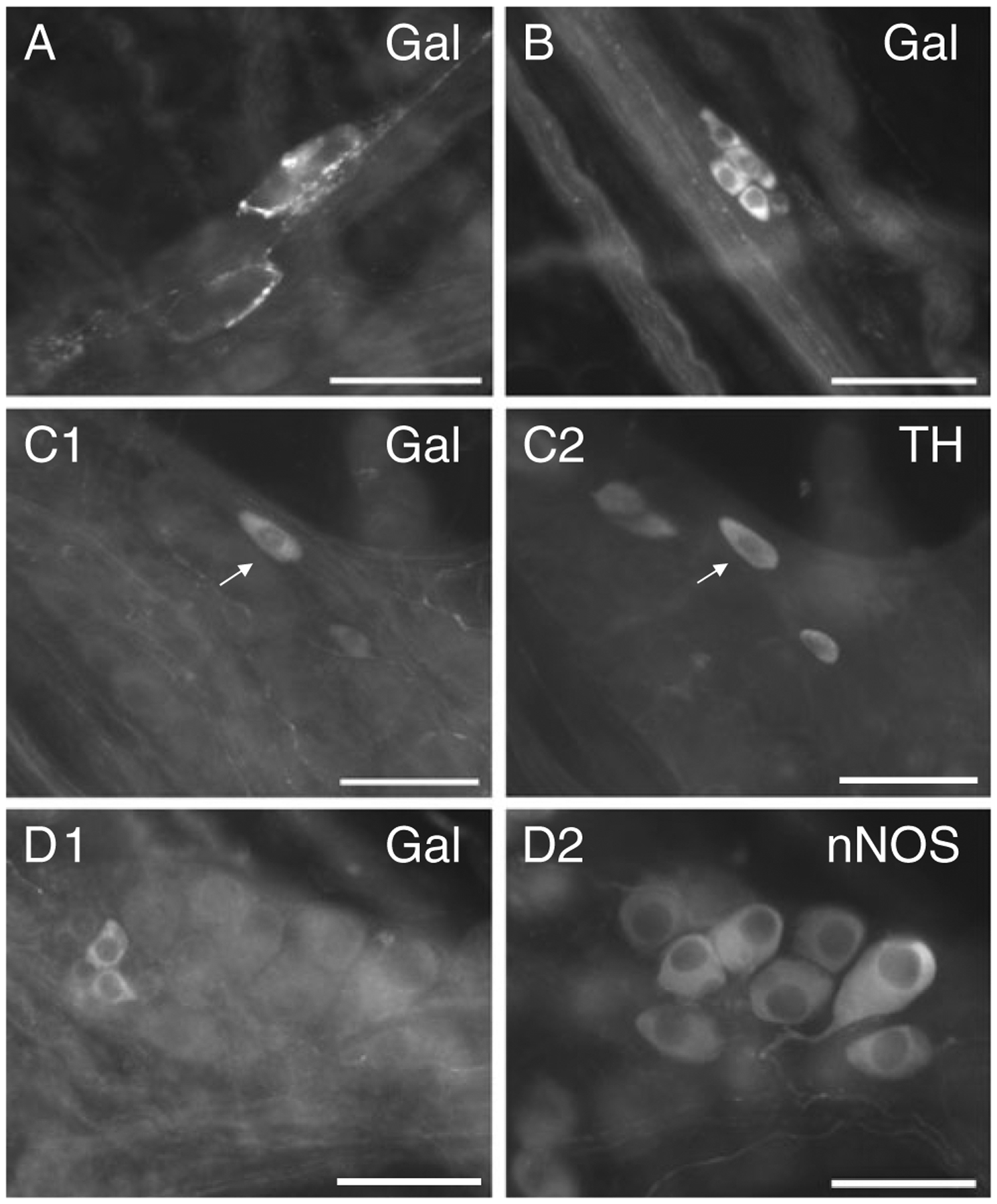
Galanin-IR cells and fibers in control MPG whole mount preparations. A Galanin-IR fibers form pericellular arrangements around two neurons. B A cluster of small galanin-IR cells. These cells were also TH-IR (data not shown) and are suggested to be SIF cells. C1 A galanin-IR neuron which was also TH-IR (C2). D A cluster of small galanin immunoreactive cells (D1) that did not exhibit nNOS imunoreactivity (D2) although larger nNOS-IR cells were evident. Calibration equals 50 μm
Many of the galanin-IR cells in control MPG were small, oval shaped and often clustered together, characteristics reminiscent of small intensely fluorescent cells (SIF) cells (Fig. 1(B)). Furthermore, in whole mounts double labeled with antiserum directed against galanin and antiserum against TH, essentially all of the very small galanin-IR cells were also TH immunoreactive. In the example shown in Fig. 1(B), there were nine small cells clustered together; six of the nine were galanin immunoreactive, and all nine were TH immunoreactive (TH staining not shown). Less frequently, larger neurons exhibited galanin immunoreactivity (Fig. 1(C1)). However, when galanin immunoreactive, the larger cells also were TH immunoreactive (Fig. 1(C2)). Three additional control MPGs were double labeled with antiserum directed against galanin and antiserum directed against nNOS. None of the galanin-IR cells identified were also nNOS immunoreactive (Fig. 1(D)).
We conclude that there are limited numbers of galanin-IR cells in control MPG. While most of the smaller galanin-IR cells are very likely SIF cells, the majority of the larger cells are sympathetic postganglionic neurons.
Galanin Transcript Expression and Numbers of Galanin-IR Cells Increase in Explant-Cultured MPG
Next, using QPCR we tested whether transcript levels for galanin increased during explant culture of the MPG. As shown in Fig. 2, transcript levels for galanin increased by 19-fold (P≤0.0001) after 3 days in culture. As shown previously, PACAP transcript levels also increased significantly after 3 days in culture, but the increase was less than that noted for galanin (4.2-fold; P≤0.03).
Fig. 2.
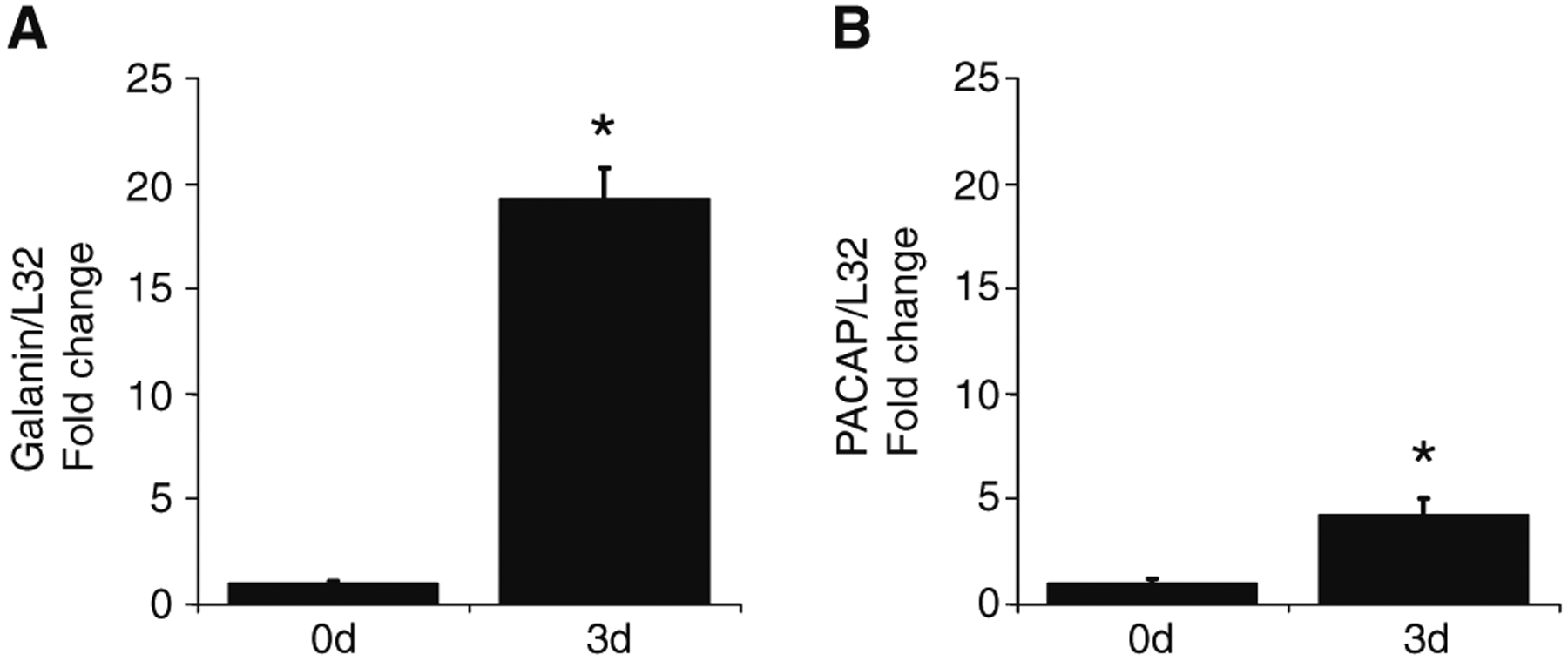
MPG Galanin and PACAP mRNA levels increase following explant culture. The transcript levels were normalized to the transcript level for L32. Transcript levels were significantly increased in 3-day-cultured MPGs for galanin (19-fold, P=0.0001) and PACAP (4.2-fold, P=0.03). Data represent mean±SEM from six acutely isolated ganglia (0d) and six 3-day-cultured ganglia (3d)
We also immunostained MPG that were cultured for 3 days. In the cultured MPG, there were many more galanin-IR cells than noted in control preparations and numerous galanin-IR fibers (Fig. 3a, b). Galanin-IR fibers were observed within the MPG and in nerve connectives, but no galanin-IR fibers made pericellular arrangements around the postganglionic neurons as commonly occurred in control MPG. In addition, the intensity of fluorescent staining for galanin was most intense at the ends of the fibers, where regeneration processes would be initiating (Fig. 3c). In the explant-cultured MPG, both small and large cells exhibited galanin immunoreactivity. The increase in number of large cells exhibiting immunoreactivity for galanin was reminiscent of that reported previously for PACAP (Girard et al. 2010).
Fig. 3.
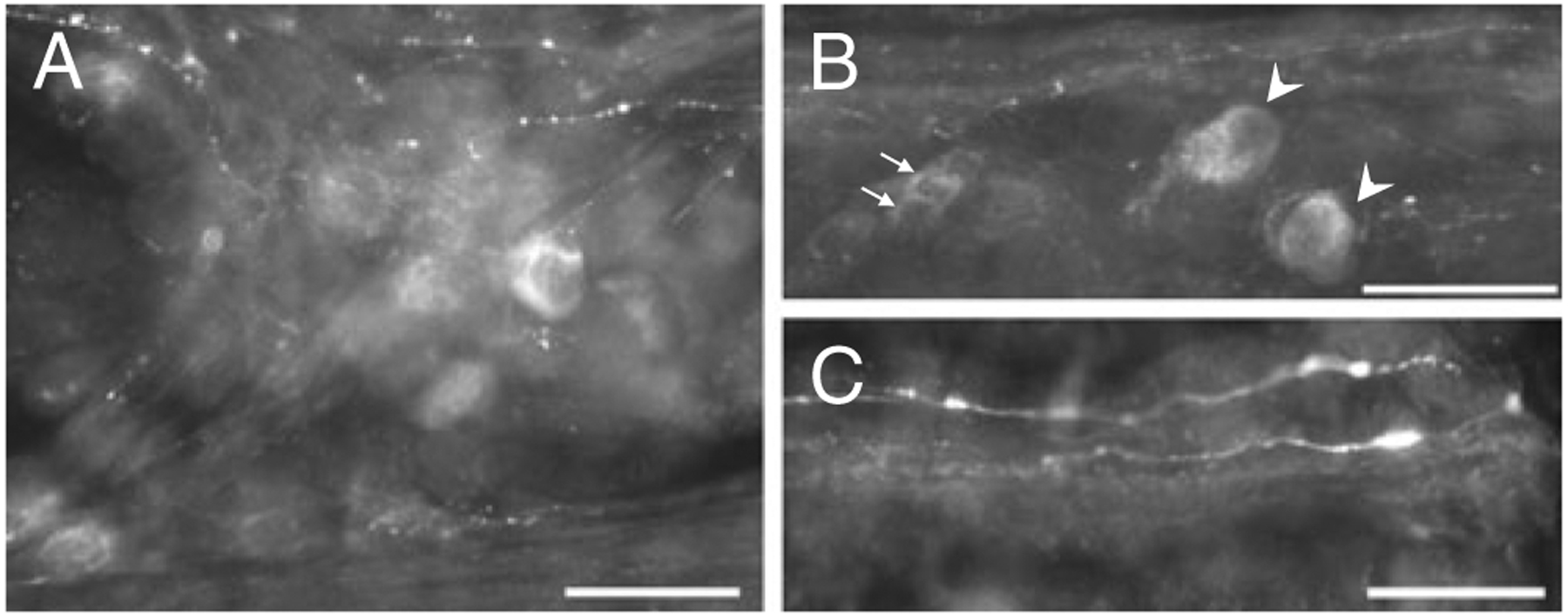
Galanin (a–c) immunoreactive cells and fibers in 3-day-cultured MPGs. a A cluster of galanin-IR cells and fibers in a 3-day-cultured MPG. b Both small cells (indicated by arrows) and larger cells (indicated by arrowheads) exhibit galanin immunoreactivity in 3-day-cultured MPGs. c Galanin immunoreactivity in fibers at the cut end of a nerve bundle. Calibration equals 50 μm
Increased Galanin Expression in the 3-Day-Cultured MPG Occurs Primarily in Parasympathetic Postganglionic Neurons
Previously, we demonstrated that the increase in PACAP expression in MPG neurons during explant culture primarily occurred in parasympathetic postganglionic neurons (Girard et al. 2010). Consequently, we determined whether the increased expression of galanin occurred in both sympathetic and parasympathetic postganglionic neurons or preferentially in one. Three-day-cultured MPG whole mounts were double labeled with antiserum directed against galanin and antiserum directed against either TH to mark sympathetic postganglionic neurons or nNOS to label parasympathetic postganglionic neurons. The majority of the larger galanin-IR cells were not TH immunoreactive (Fig. 4(A1, 2)). However, in whole mounts double labeled with antiserum directed against galanin and antiserum directed against nNOS, many of the larger galanin-IR neurons were immunoreactive for nNOS (Fig. 4(B1, 2)). Also, there were some larger galanin-IR neurons that were not nNOS immunoreactive (Fig. 4(C1, 2)).
Fig. 4.
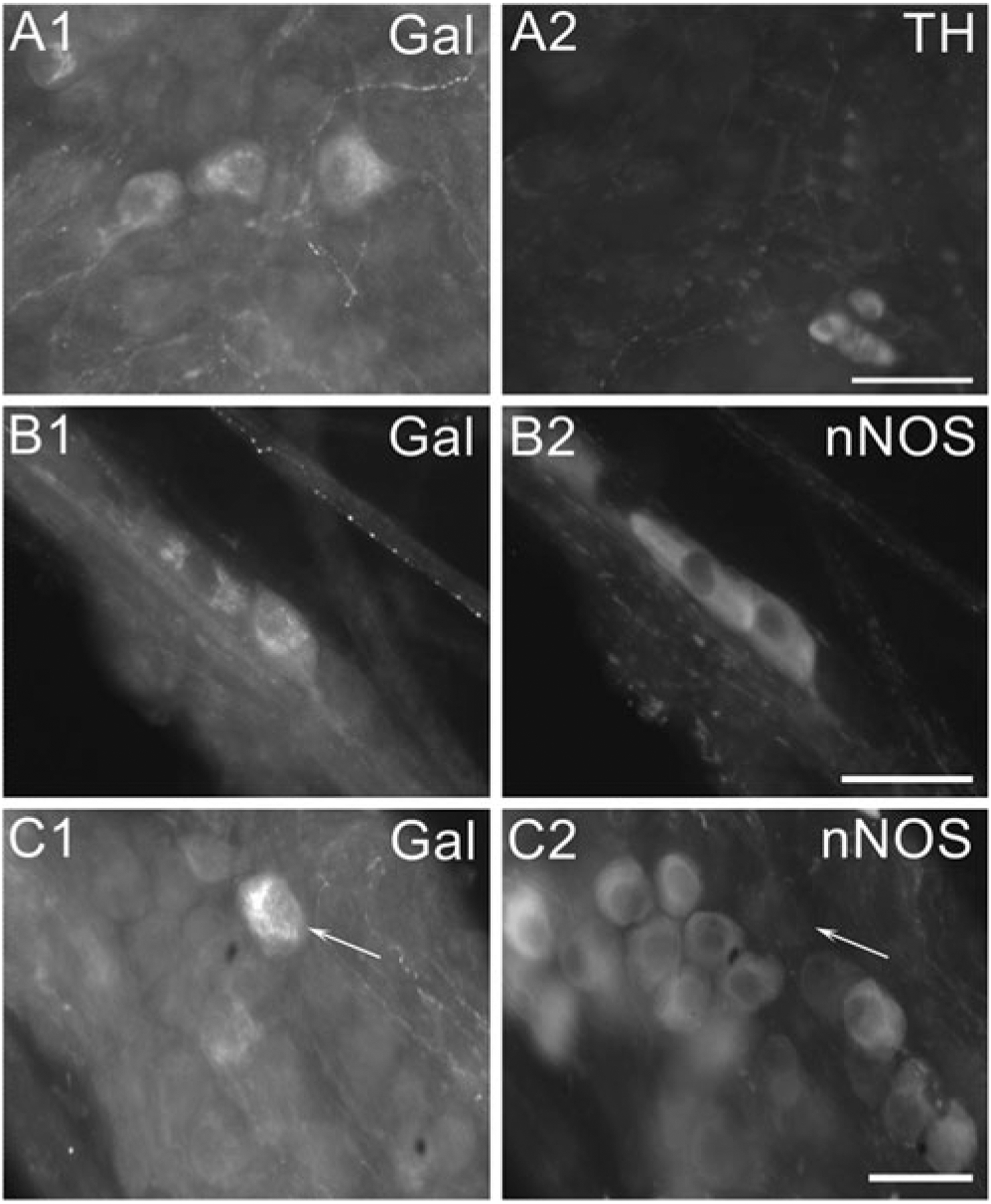
In 3-day-cultured MPG neurons, galanin is often co-localized with nNOS but rarely with TH. A In MPG double labeled with antiserum directed against galanin and antiserum directed against TH, the large galanin-IR cells (A1) are not immunoreactive for TH (A2). Note the small SIF cells in (A2) are TH-IR but not galanin-IR. B In a MPG double labeled with antiserum directed against galanin and antiserum directed against nNOS, two galanin-IR neurons (B1) also are immunoreactive for nNOS (B2). C In a MPG double labeled with antiserum directed against galanin and antiserum directed against nNOS, a galanin-IR neuron (indicated by arrow in (C1)) does not exhibit immunoreactivity for nNOS (indicated by arrow in (C2)). Calibration equals 50 μm
Occasionally, a cluster of very small galanin-IR cells also were TH immunoreactive (data not shown). These likely are galanin-IR SIF cells as was noted in control MPG whole mounts.
Galanin and PACAP Can Be Expressed in the Same 3-Day-Cultured MPG Neurons
The prior series of experiments suggested that the increased expression of galanin during explant culture occurred primarily in MPG parasympathetic postganglionic neurons. Previously, we concluded that the increased expression of PACAP during explant culture also occurred in parasympathetic rather than sympathetic postganglionic MPG neurons. Consequently, we determined whether galanin and PACAP immunoreactivity was evident in the same neurons. Three-day-cultured MPG whole mounts were double labeled with antiserum directed against galanin and antiserum directed against PACAP. Galanin and PACAP were co-localized in some cells, but many were only galanin immunoreactive or PACAP immunoreactive (Fig. 5(A1, 2)). Typically, the small galanin-IR cells, which were often clustered together, did not exhibit PACAP immunoreactivity (Fig. 5(B1, 2)).
Fig. 5.
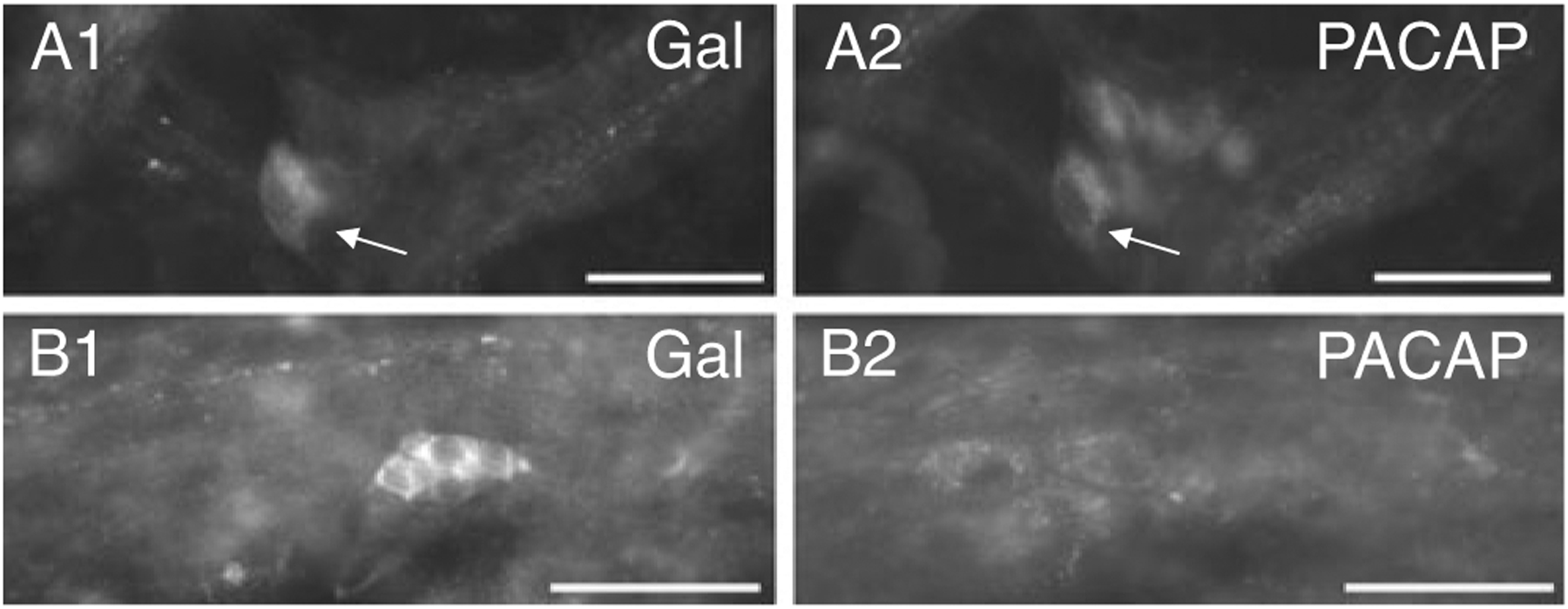
Galanin and PACAP can be co-localized in neurons in 3-day MPG explants. A A galanin-IR neuron indicated by arrow in (A1) is also immunoreactive for PACAP indicated by arrow in (A2). Note other PACAP-IR neurons in (A2) that were not galanin immunoreactive. B A cluster of small galanin-IR cells (B1) that did not exhibit PACAP immunoreactivity (B2) although other larger PACAP-IR neurons are evident. Calibration equals 50 μm
Transcript Expression for Galanin, But Not PACAP, Increases Significantly in the MPG Following Transection of the Cavernous Nerve
Essentially all of the parasympathetic nerve fibers projecting to the penis are axons derived from neurons in the MPG (Keast 2006). Consequently, we tested whether transcript levels for galanin and PACAP were increased in the ipsilateral MPG 3 days following unilateral transection of the cavernous nerve. Transcript expression from the contralateral MPG was used as a control for comparison with transcript levels from the ipsilateral MPG. As shown in Fig. 6, galanin transcript levels were increased 2-fold in the ipsilateral MPG from the lesioned side when compared with the levels in the contralateral MPG (P=0.028). PACAP transcript levels increased as well, but the increase did not reach statistical significance (P=0.15).
Fig. 6.
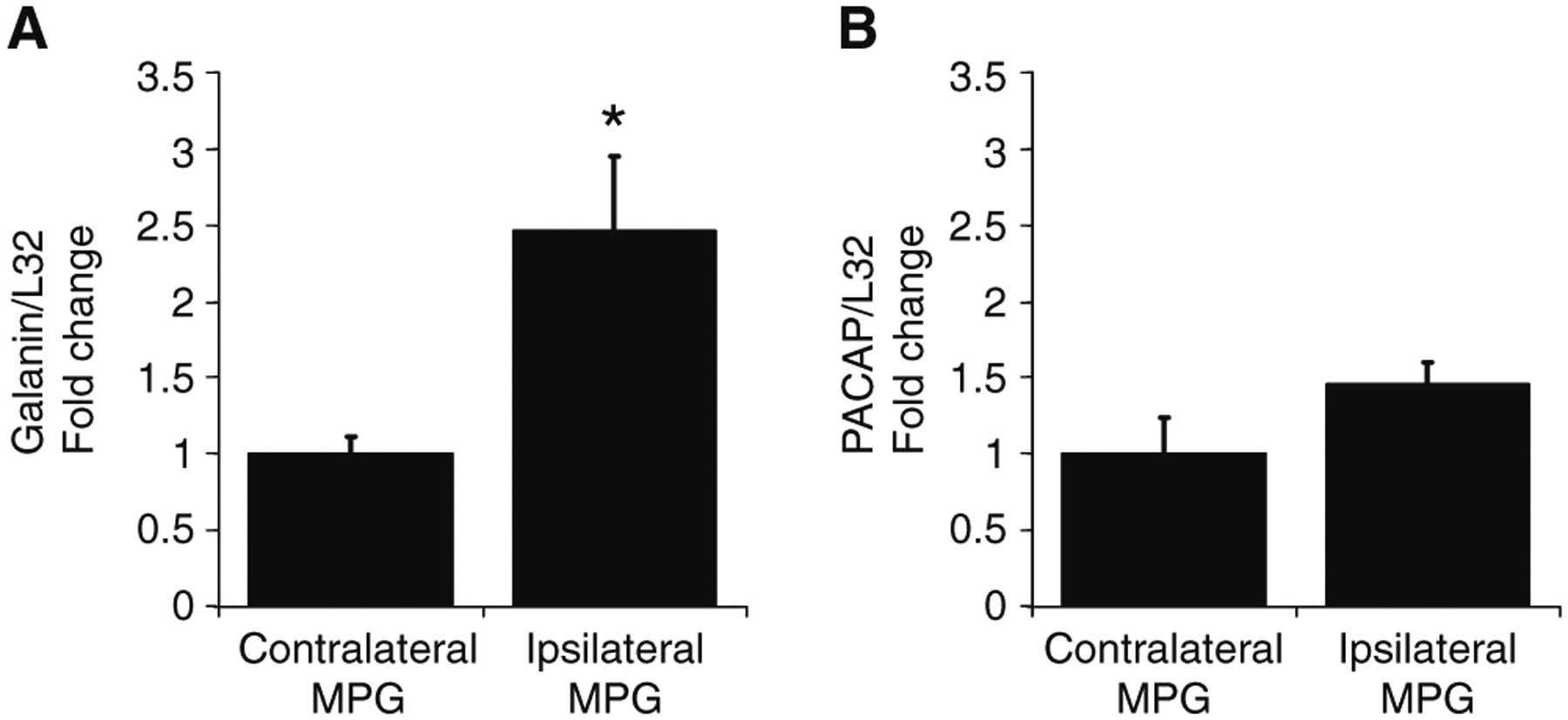
Galanin transcript levels, but not PACAP transcript levels, increase significantly in the ipsilateral MPG when compared with the contralateral MPG, 72 h following unilateral section of the cavernous nerve. Galanin and PACAP transcript levels were normalized to the transcript level for L32. Only galanin transcript levels were significantly greater in the ipsilateral MPG compared with the contralateral MPG (galanin, P=0.028; PACAP, P=0.15). Data represent mean±SEM for extracts from six MPG in each group
Transcript Levels for GalR3 Are Significantly Decreased in MPG Following Cavernous Nerve Transection
Our results demonstrate that galanin is present mostly in fibers innervating neurons in freshly isolated MPG. However, galanin transcript levels increased markedly after explant culture or axotomy. In the final series of experiments, we tested whether there was a change in transcript levels for galanin receptors following axotomy.
Using QPCR, we determined expression levels of the three galanin receptors, GalR1, GalR2, and GalR3, in extracts from freshly isolated MPG. As indicated in Fig. 7a, transcripts for all three galanin receptors were present, albeit at quite different levels. Transcript levels for GalR3 and GalR2 were more prominent than the transcript level for GalR1. In subsequent experiments we tested whether the transcripts levels were affected following transection of the cavernous nerve. The transcript levels for the galanin receptors, normalized to L32 transcript levels, were compared in MPG from the ipsilateral operated and contralateral unoperated side. GalR1 transcript expression was too low to quantify the comparison. However, transcript levels for both GalR2 and GalR3 decreased following cavernous nerve transection, but only the change in GalR3 was significantly reduced (Fig. 7b).
Fig. 7.
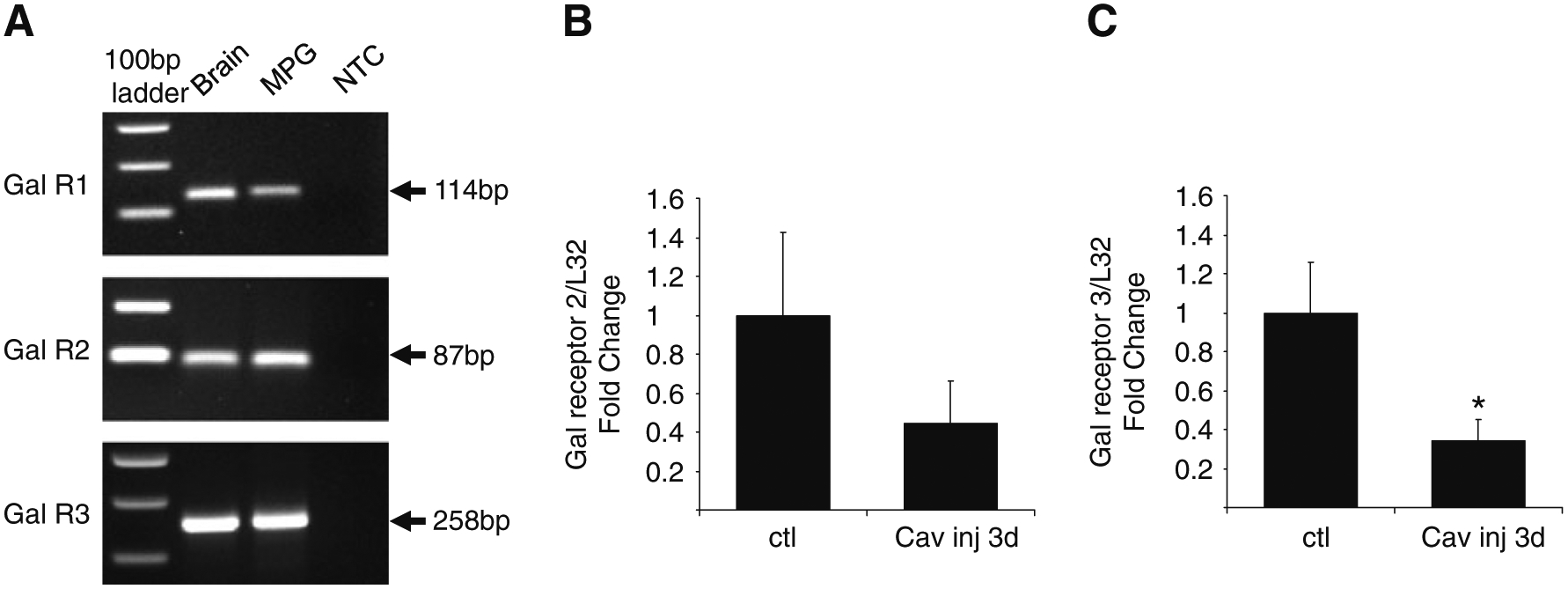
Multiple GalR transcripts are expressed by male mouse MPG neurons. a PCR products viewed on an ethidium bromide gel after amplification of freshly dissected MPG whole mounts and brain cDNA. All reactions were processed on a 7500 ABI fast cycler using a USB Sybr Green mix. All reactions were run at 55–58°C for 45 cycles. Extracts obtained from MPG whole mounts expressed transcripts for GalR1, GalR2, and GalR3. NTC no template control is a negative control assessing the absence of primer dimers or other contaminants. b, c Change in GalR2 (b) and GalR3 (c) mRNA levels 3 days following transection of the cavernous nerve. All transcript levels were normalized to the transcript level for L32 and the level of transcript in the ipsilateral MPG following unilateral cavernous nerve section was compared with that of the contralateral un-injured MPG. GalR3 transcript levels were significantly decreased increased (P<0.05). Although GalR2 transcript levels were reduced following cavernous nerve section, the decrease was not significant (P=0.29). Data represent mean±SEM from four MPG in each group
We also tested whether transcript levels of GalR2 and GalR3 decreased following explant culture for 3 days. The results indicated that after 3 days in explant culture transcript levels for both GalR2 and GalR3 were decreased relative to extracts from freshly isolated MPG. However, the decrease following explant culture was only significant for GalR2 (data not shown).
Discussion
The MPG is unique in that it contains both adrenergic sympathetic and cholinergic parasympathetic postganglionic neurons (Keast 2006). In the mouse MPG, the cholinergic parasympathetic postganglionic neurons also can express VIP and nNOS, the synthetic enzyme for nitric oxide generation (Wanigasekara et al. 2003; Girard et al. 2010; Tompkins et al. 2010). Results obtained in this study show that in the male mouse MPG some SIF cells and a few neurons exhibit galanin immunoreactivity. Previously, no PACAP-IR cells or fibers were noted in the control male mouse MPG (Girard et al. 2010).
Transcript levels for both galanin and PACAP increased during explant culture of the MPG (this study; Girard et al. 2010). In contrast, in 3-day-cultured MPGs, VIP transcript levels did not change significantly (Girard et al. 2010). Similarly, no significant change in substance P transcript levels occurred in 3-day-cultured MPGs (Girard, unpublished observations). These latter observations indicate that the increase in galanin and PACAP transcript levels is not simply a nonspecific effect common to many neuropeptides that results from the culture conditions.
Consistent with the increased transcript levels, the number of galanin-IR cells increased in cultured MPG. The presence of numerous PACAP-IR neurons in cultured MPG was demonstrated previously (Girard et al. 2010). Furthermore, the increased expression of these two neuropeptides appears to occur preferentially in the parasympathetic postganglionic neurons (this study; Girard et al. 2010). This conclusion was supported by the finding that in 3-day-cultured neurons galanin immunoreactivity (this study) and PACAP immunoreactivity (Girard et al. 2010) was noted primarily in nNOS-IR neurons rather than TH-IR neurons.
Based on prior studies using the rat and mouse SCG (Mohney et al. 1994; Schreiber et al. 1994; Moller et al. 1997), we had expected an increased expression of galanin and PACAP to occur in sympathetic postganglionic MPG neurons as well as in parasympathetic neurons within the MPG. The apparent increase of both neuropeptides in explant-cultured mouse parasympathetic neurons poses an intriguing question that will need to be explored in future studies. However, we know from our prior study that both neuron types responded to axotomy under the conditions of the culture experiments as ATF-3 expression occurred in both TH-IR and nNOS-IR neurons (Girard et al. 2010).
In control MPG, galanin-IR fibers often made pericellular arrangements around individual postganglionic neurons. These pericellular connections were not noted in explant-cultured MPG. We suggest that the galanin-IR fibers making connections with neurons in the control MPG are axons derived from neurons located outside the MPG (e.g., dorsal root ganglia) and further that they degenerate during culture. As the physical arrangement of the fibers around the neurons is not similar to the preganglionic innervation pattern we suggest that these extrinsic galanin-IR fibers may be sensory axons passing through the MPG.
Galanin and PACAP were co-localized in some neurons. However, there were many instances in which cells were either galanin or PACAP immunoreactive, but not immunolabeled by both antisera. This observation suggests that different subpopulations of the MPG parasympathetic postganglionic neurons may express one or both of these neuropeptides in response to injury/axotomy. The distinction of which neuropeptide is expressed may be determined by the target tissue innervated. This hypothesis needs to be evaluated in future in vivo experiments in which the effect of axotomy to specific targets is analyzed in more detail.
Galanin transcript levels increased significantly in the ipsilateral MPG following unilateral transection of the cavernous nerve. Because the parasympathetic nerve fibers projecting through the cavernous nerve to penile tissues are exclusively axons of MPG parasympathetic postganglionic neurons (Keast 2006), it was expected that galanin transcript levels would increase following axotomy. Although PACAP transcript levels tended to increase following cavernous nerve transection, the values did not reach significance.
Interestingly, although galanin transcript levels increased following axotomy, there was a decrease rather than increase in transcript levels for some of the galanin receptors following nerve injury. Although we were unable to quantify changes in GalR1 transcript levels, we did see a significant decrease in transcript levels for GalR3. GalR2 transcript levels were also less in MPG from the operated side compared with MPG from the contralateral unoperated side, but the difference was not significant. Similar to the results with cavernous axotomy, transcript levels for GalR2 and GalR3 were decreased following explant culture. Although transcripts for both decreased, the difference was only significant for GalR2 following explant culture. The extent of the decrease in GalR3 transcript level was comparable to that seen for GalR2, but the variability for the freshly dissected preparations was larger and this likely contributed to the decrease not reaching significance.
In sum, one goal of the present study was to test whether the explant-cultured MPG recapitulates changes in neuronal phenotype that are known to occur following axotomy/injury. Combined with the results reported previously (Girard et al. 2010), the present findings demonstrate that the explant-cultured MPG neurons increase expression of two neuropeptides, galanin, and PACAP, that are thought to promote neuronal survival and regeneration (Mohney et al. 1994; Schreiber et al. 1994; Moller et al. 1997; Girard et al. 2007). Thus, our results reinforce the view that the explant-cultured MPG whole mount preparation is a convenient in vitro model system in which to analyze injury-induced changes in the molecular and chemical properties of MPG neurons. Although it is known that the peripheral nervous system has the ability to regenerate after injury, the innate regenerative capacity of MPG neurons is limited and often does not result in the full recovery of target organ function (Palma and Keast 2006). Results obtained with this model system should provide essential information elucidating mechanisms that support and enhance the regenerative capacities of the pelvic ganglion neurons.
Acknowledgments
The study was supported in part by NIH grants R01 DK060481 and R01 DK051369 to MAV and NIH grants P20 RR16435, P30 RR032135, and P30 GM103498 to RLP. We thank Dr. Jan Fahrenkrug for kindly providing the PACAP antiserum.
Contributor Information
Beatrice M. Girard, Department of Anatomy and Neurobiology, College of Medicine, University of Vermont, Burlington, VT 05405, USA
Jonathan R. Galli, Department of Anatomy and Neurobiology, College of Medicine, University of Vermont, Burlington, VT 05405, USA
Margaret A. Vizzard, Department of Anatomy and Neurobiology, College of Medicine, University of Vermont, Burlington, VT 05405, USA Department Neurology, College of Medicine, University of Vermont, Burlington, VT 05405, USA.
Rodney L. Parsons, Department of Anatomy and Neurobiology, College of Medicine, University of Vermont, Burlington, VT 05405, USA
References
- Calupca MA, Vizzard MA, Parsons RL (2000a) Origin of pituitary adenylate cyclase-activating polypeptide (PACAP)-immunoreactive fibers innervating guinea pig parasympathetic cardiac ganglia. J Comp Neurol 423:26–39 [PubMed] [Google Scholar]
- Calupca MA, Vizzard MA, Parsons RL (2000b) Origin of neuronal nitric oxide synthase (nNOS)-immunoreactive fibers in guinea pig parasympathetic cardiac ganglia. J Comp Neurol 426:493–504 [PubMed] [Google Scholar]
- Girard BM, Young B, Buttolph T, White S, Parsons R (2007) Regulation of pituitary adenylate cyclase-activting polypeptide (PACAP) expression during culture of guinea-pig cardiac ganglia. Neuroscience 146:584–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard BM, Galli JR, Young BA, Vizzard MA, Parsons RL (2010) PACAP expression in explant cultured mouse major pelvic ganglia. J Mol Neurosci 42:70–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keast JR (2006) Plasticity of pelvic autonomic ganglia and urogenital innervation. Int Rev Cytol 248:141–208 [DOI] [PubMed] [Google Scholar]
- Makwana M, Raivich G (2005) Molecular mechanisms in successful peripheral regeneration. FEBS J 272:2628–2638 [DOI] [PubMed] [Google Scholar]
- Mawe GM, Talmage EK, Lee KP, Parsons RL (1996) Expression of choline acetyltransferase immunoreactivity in guinea pig cardiac ganglia. Cell Tissue Res 285:281–286 [DOI] [PubMed] [Google Scholar]
- Mohney RP, Siegel RE, Zigmond RE (1994) Galanin and vasoactive intestinal peptide messenger RNAs increase following axotomy of adult sympathetic neurons. J Neurobiol 25:108–118 [DOI] [PubMed] [Google Scholar]
- Moller K, Reimer M, Ekblad E et al. (1997) The effects of axotomy and preganglionic denervation on the expression of pituitary adenylate cyclase activating peptide (PACAP), galanin and PACAP type 1 receptors in the rat superior cervical ganglion. Brain Res 775:166–182 [DOI] [PubMed] [Google Scholar]
- Nangle MR, Keast JR (2009) Deafferentation and axotomy each cause neurturin-independent upregulation of c-Jun in rodent pelvic ganglia. Exp Neurol 215:271–280 [DOI] [PubMed] [Google Scholar]
- Navarro X, Vivo M, Valero-Cabre A (2007) Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol 82:163–201 [DOI] [PubMed] [Google Scholar]
- Palma CA, Keast JR (2006) Structural effects and potential changes in growth factor signalling in penis-projecting autonomic neurons after axotomy. BMC Neurosci 7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RC, Hyatt-Sachs H, Bennett TA, Zigmond RE (1994) Galanin expression increases in adult rat sympathetic neurons after axotomy. Neuroscience 60:17–27 [DOI] [PubMed] [Google Scholar]
- Tompkins JD, Girard BA, Vizzard MA, Parsons RL (2010) VIP and PACAP effects on mouse major pelvic ganglia neurons. J Mol Neurosci 42:390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujino H, Kondo E, Fukuoka T et al. (2000) Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: a novel neuronal marker of nerve injury. Mol Cell Neurosci 15:170–182 [DOI] [PubMed] [Google Scholar]
- Wanigasekara Y, Kepper ME, Keast JR (2003) Immunohistochemical characterisation of pelvic autonomic ganglia in male mice. Cell Tissue Res 311:175–185 [DOI] [PubMed] [Google Scholar]
- Young BA, Girard BM, Parsons RL (2008) Neurturin suppresses injury-induced neuronal activating transcription factor 3 (ATF-3) expression in cultured guinea pig cardiac ganglia. J Comp Neurol 508:795–805 [DOI] [PubMed] [Google Scholar]
- Zvarova K, Vizzard MA (2006) Changes in galanin immunoreactivity in rat micturition reflex pathways after cyclophosphamide-induced cystitis. Cell Tissue Res 324:213–224 [DOI] [PubMed] [Google Scholar]
- Zvarova K, Murray E, Vizzard MA (2004) Changes in galanin immunoreactivity in rat lumbosacral spinal cord and dorsal root ganglia after spinal cord injury. J Comp Neurol 475:590–603 [DOI] [PubMed] [Google Scholar]


