Background
The liver is of critical importance for the homeostasis of metabolic and immunomodulatory properties as well as the storage of vitamins, especially vitamin A. In this prospective analysis, the incidence of serological vitamin A deficiency and the association with disease severity as well as clinical complications in patients with liver cirrhosis were investigated.
Method
From May 2017 to May 2018, 159 patients with primarily alcohol-associated and non-alcoholic steatohepatitis (NASH)-associated preexisting liver cirrhosis were prospectively enrolled and vitamin A status was collected. Clinical complications and infections were followed and recorded over a period of 1-year follow-up. Selected findings were validated in an independent cohort of 44 patients.
Results
At study inclusion, 77% of patients showed decreased serological vitamin A. Suppressed vitamin A was more common in alcoholic (52 vs. 8%) and NASH-associated liver cirrhosis (16 vs. 9%) than in viral-associated liver cirrhosis. MELD score as well as Child-Pugh score were significantly associated with suppressed vitamin A (P < 0.001). The association between the degree of vitamin A suppression and liver function was confirmed in univariate and multivariate regression analysis. After 1 year of follow-up, 57 patients died and 21 patients received a liver transplant. In addition, low vitamin A levels were more commonly observed in patients with severe ascites (P = 0.001), hepatic encephalopathy (P = 0.002) and hepatorenal syndromes (P = 0.008). In addition, patients with reduced vitamin A showed an increased incidence of infections (P = 0.02), especially respiratory infections (P = 0.04).
Conclusion:
Suppressed serological Vitamin A is common in patients with liver cirrhosis and is associated with liver function. Clinical complications and infections are more frequent in patients with liver cirrhosis and vitamin A suppression.
Keywords: ACLF, decompensated liver cirrhosis, liver cirrhosis, vitamin A
Introduction
The liver plays a profound role in the body and organ homeostasis and is critical to regulate different cellular processes, including metabolisms, synthesis and immune defense, circulation as well as portal hemodynamics [1]. Among the different functions, the storage of essential components, including vitamins is one of the most important ones. In this context, the fat-soluble vitamin A is of vital importance not only for the visual process but also for several other processes, including immune cell function with especially the humoral and cellular immune response. Vitamin A can only be taken orally to the body, and as a fat-soluble vitamin, requires bile acids for absorption. Notably, vitamin A is stored up to 95% in the liver, and vitamin A storage is significantly impaired in chronic liver diseases [2]. Fibrogenesis results from increased production of extracellular matrix by activated hepatic stellate cells. In the context of HSC activation, these cells lose their vitamin A stores which explains the association between vitamin A and chronic liver disease [3]. Consistently, it is well known that chronic liver damage and impaired liver function are inversely correlated with the vitamin A status [4]. Furthermore, progressive malnutrition present in the majority of patients with impaired liver function, that is, liver cirrhosis, further aggravates the vitamin A deficiency [5]. In addition to malnutrition, alcohol consumption has also been identified as an independent risk factor for vitamin A deficiency [6]. As mentioned above Vitamin A is of critical relevance for the immune response due to a limited humoral and cell-mediated immunity and altered phagocytes as well as T cells function has been described in patients with vitamin A deficiency [6,7]. Given that patients with liver cirrhosis frequently suffer from infectious complications that commonly result in acute decompensation, acute - on - chronic liver failure and even death, vitamin A deficiency is of particular relevance for these patients [8]. This prospective study aims to evaluate the association between liver insufficiency and vitamin A status, investigate the frequency and severity of clinical complications and identify Vitamin A supplementation as a potential therapeutical target.
Methods
Study inclusion of the patients
In total after the screening of 280 patients, 159 inpatients and outpatients with primarily alcohol-associated and non-alcoholic steatohepatitis (NASH)-associated liver cirrhosis were prospectively recruited for this study between May 2017 and May 2018 at the Cirrhosis Center Mainz at the University Medical Center of the Johannes Gutenberg-University in Mainz, Germany. Patients with other chronic conditions with potential impact on vitamin A levels (heart disease NYHA III-IV, chronic obstructive pulmonary disease GOLD C and D, active malignancies, neurological comorbidities such as dementia or history of stroke) were excluded. In particular, patients with hepatocellular carcinoma or transjugular intrahepatic portosystemic shunt were excluded. Diagnosis of liver cirrhosis was established by typical laboratory findings and appearance on ultrasound, computed tomography/MRI or by liver biopsy. At presentation or inpatient admission, all patients received after informed consent a standardized medical history and a laboratory examination including vitamin A. The analysis of vitamin A in serum was performed by the central laboratory using HPLC. The reference interval was 300–700 ng/ml, so that a vitamin A decrease is defined as vitamin A content in serum less than 300 ng/ml. In addition to general epidemiological data such as age and sex, the etiology of liver cirrhosis was determined and liver function was assessed by the model of end-stage liver disease (MELD) and Child-Pugh score. For validation purpose, 44 patients with the same inclusion and exclusion criteria were enrolled at the University of Lübeck.
Assessment of the clinical course in the 1-year follow-up
After inclusion in the study, patients were monitored every 6 months as part of the regular hepatocellular carcinoma surveillance. During the presentation, clinical complications that led to inpatient therapy or changes in the existing therapy were assessed and documented. In addition, all infections requiring antibiotic therapy were documented. Patients who did not show up for the follow-up were contacted by telephone. In the absence of follow-up, patients were classified as lost to follow-up. Only decompensations and infections during the 1-year follow-up were included in our results. During the 1-year observation period, no supplementation with vitamin A took place in patients with diagnosed vitamin A deficiency.
Ethics
The study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008). The study protocol was approved by the ethics committee of the Landesärztekammer Rhineland-Palatine (Nr. 837.232.17 [11066]). Written informed consent was obtained from every participant.
Statistical analysis
The statistical analyses were performed with IBM SPSS Statistic Version 23.0 (IBM Corp., Armonk, New York, USA). Quantitative data are expressed as medians with interquartile ranges (IQR). Pairwise comparisons for quantitative variables were performed with an unpaired t-test or Mann–Whitney U-Test. Categorical variables are given as frequencies and percentages, respectively, and for the comparisons of two or more patients groups a chi-square test was applied. Regarding the endpoint of death/need for liver transplantation (mortality), survival curves were analyzed using Kaplan–Meier curves and a log-rank test. The correlation of clinical und epidemiological factors with vitamin A was assessed by means of univariate analyses. Variables with a P < 0.1 in the univariable analysis were subsequently considered in a multivariate linear regression model for each score. To reliably identify factors associated with vitamin A deficiency, final forward multivariate model was built based on a stepwise variable selection procedure for each score. Our complete data analysis is exploratory. Hence, no adjustments for multiple testing were performed. For all tests, we used a 0.05 level to define statistically relevant deviations from the respective null hypothesis.
Results
Baseline characteristics of the study cohort
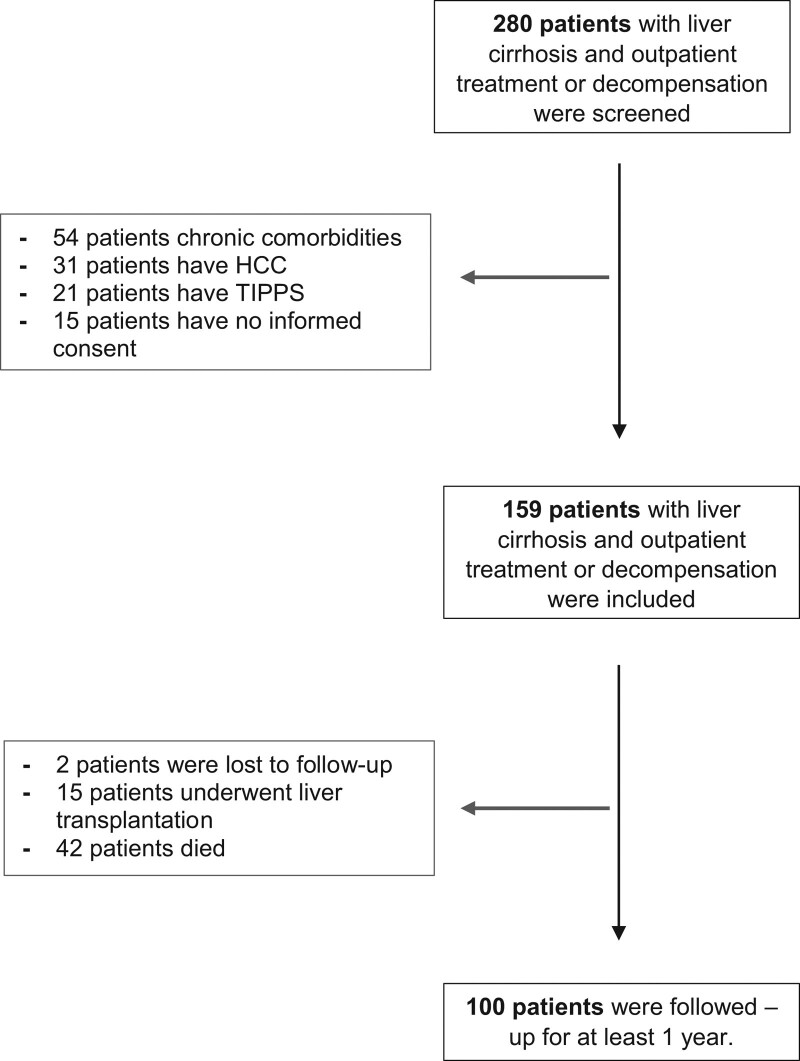
From May 2017 to May 2018, a total of 159 patients with liver cirrhosis were included and their vitamin A status was assessed. A total of 100 patients could be followed up after 1 year. During this period, 15 patients received a liver transplant, 42 patients died during the observation period and 2 were lost to follow-up. The remaining 41 patients survived the observation period without liver transplantation or death (Fig. 1). At the time of inclusion in the study, 123 patients showed a suppressed vitamin A status (77%). The median age was 58 years (53; 66) and the most common etiology of underlying liver disease was alcoholic cirrhosis (N = 60; 60%) followed by the NASH-associated cirrhosis (N = 41; 26%). The median MELD was 15 (10; 19) with an even distribution across Child-Pugh stages (Child-Pugh A, N = 52; Child-Pugh B, N = 54 and Child-Pugh C, N = 52) (Table 1).
Fig.1.
The flow chart of the study. In total 280 patients were screened and 159 patients with liver cirrhosis were prospectively included and followed up for at least 12 months. The median follow-up was 427 days. In follow-up, 21 patients underwent liver transplantation, 57 patients died and 23 patients were lost to follow-up.
Table 1.
The characteristics at the time of study inclusion divided between vitamin A deficiency and normal vitamin A
| Parameter | Total (N = 159) |
Suppressed vitamin A (N = 123) |
Normal vitamin A (N = 36) |
P value |
|---|---|---|---|---|
| Age (years) Median; IQR |
58 (53; 66) | 60 (53; 65) | 61 (51; 72) | 0.34 |
| Female gender (N; %) |
49 (31) |
35 (22) |
14 (9) |
0.23 |
| Alcoholic liver cirrhosis (N; %) |
95 (60) |
82 (52) |
13 (8) |
0.001 |
| NASH-associated liver cirrhosis (N; %) |
41 (26) |
26 (16) |
15 (9) |
0.013 |
| Viral liver cirrhosis (N, %) |
18 (11) |
15 (9) |
3 (2) |
0.52 |
| Metabolic/hereditary liver cirrhosis (N; %) |
1 (1) |
1 (1) |
0 (0) |
0.59 |
| Vascular liver cirrhosis (N; %) |
2 (1) |
1 (1) |
1 (1) |
0.35 |
| Autoimmune/cholestatic liver cirrhosis (N; %) |
18 (11) |
11 (7) |
7 (4) |
0.08 |
| Other cause of liver cirrhosis (N; %) |
1 (1) |
0 (0) |
1 (1) |
0.064 |
| MELD Median; IQR |
15 (10; 19) | 16 (11; 22) | 9 (7; 11) | < 0.001 |
| Sodium (mmol/l) Median; IQR |
136 (134; 140) | 137 (134; 140) | 140 (138; 141) | 0.005 |
| Creatinine (mg/dl) Median; IQR |
1.17 (0.75; 1.25) | 0.88 (0.76; 1.41) | 0.85 (0.74; 1) | 0.125 |
| Bilirubin (mg/dl) Median; IQR |
4.35 (1.14; 3.68) | 2.5 (1.5; 4.39) | 1.02 (0.74; 1.56) | < 0.001 |
| CRP (mg/l) Median; IQR |
16.7 (3.78; 22) | 9.65 (4.98; 25.5) | 4.2 (2.1; 12.3) | < 0.001 |
| Albumin (g/l) Median; IQR |
29 (24; 29) | 27 (23; 31) | 36 (33; 40) | <0.001 |
| INR Median; IQR |
1.52 (1.2; 1.7) | 1.5 (1.3; 1.8) | 1.1 (1.1; 1.2) | < 0.001 |
| Platelets (/nl) Median; IQR |
123 (66; 153) | 95 (60; 131) | 150 (105; 226) | < 0.001 |
| Child Pugh Stadium A (N; %) |
52 (33) |
22 (18) |
30 (83) |
< 0.001 |
| Child Pugh Stadium B (N; %) |
54 (34) |
48 (39) |
6 (17) |
|
| Child Pugh Stadium C (N; %) |
52 (33) |
52 (42) |
0 (0) |
Data are expressed as medians and IQRs or as frequencies and percentages.
CRP, C-reactive protein; INR, international normalized ratio; IQR, interquartile range; MELD, model of end-stage liver disease; NASH, non-alcoholic steatohepatitis; WBC, white blood cells.
Suppressed vitamin A is associated with liver function and the incidence of clinical decompensation
Patients with reduced vitamin A levels showed significantly worse liver function at the timepoint of study inclusion. Besides an increased MELD score (16 vs. 9; P < 0.001) patients with suppressed vitamin A levels consistently showed a higher Child-Pugh score (P value < 0.001) (Table 1). Consistently, all patients with a Child-Pugh C showed a vitamin A suppression. The univariate and multivariate regression analysis showed a significant association between vitamin A and MELD as well as Child-Pugh status.
For further analysis of the clinical course, 100 patients were followed up for at least 1 year. Although 82 patients in the follow-up showed vitamin A suppression at the time of study inclusion. Patients with suppressed vitamin A showed significantly higher incidence of ascites (63 vs. 22%; P = 0.001), more frequent episodes of hepatic encephalopathy (38 vs. 0%; P = 0.002) as well as hepatorenal syndrome (38 vs. 6%; P = 0.008). Importantly, infections were significantly more frequent in patients with suppressed vitamin A compared to patients within the normal status (39 vs. 11%; P = 0.02). In particular, respiratory infections were observed more frequently (20 vs. 0%; P = 0.04) (Table 4). In addition to the association of liver function and vitamin A status, patients with ultrasound proven steatosis display an association with vitamin A (univariate analysis: P ≤ 0.001; multivariate analysis: P = 0.01) and vitamin A suppression (univariate analysis: P = 0.022; multivariate analysis: P = 0.002) (Table 2).
Table 4.
Decompensation and infections in the 1-year follow-up divided between suppressed serological vitamin A and normative vitamin A
| Parameter | Total (N = 100) |
SuppressedVitamin A (N = 82) |
Normal Vitamin A (N = 18) |
P value |
|---|---|---|---|---|
| Ascites after inclusion (N; %) |
56 (56) |
52 (63) |
4 (22) |
0.001 |
| Hepatic encephalopathy after inclusion (N; %) |
31 (31) |
31 (38) |
0 (0) |
0.002 |
| Esophageal variceal bleeding after inclusion (N; %) |
11 (11) |
9 (11) |
2 (11) |
0.99 |
| SBP after inclusion (N; %) |
11 (11) |
10 (12) |
1 (6) |
0.41 |
| HRS after inclusion (N; %) |
32 (32) |
31 (38) |
1 (6) |
0.008 |
| Infection after inclusion (N; %) |
34 (34) |
32 (39) |
2 (11) |
0.02 |
| Respiratory infection after inclusion (N; %) |
16 (16) |
16 (20) |
0 (0) |
0.04 |
Data are expressed as medians and frequencies and percentages.
HRS, hepatorenal syndrome; SBP, spontaneous bacterial peritonitis.
Table 2.
Univariate and multivariate regression analysis of vitamin A and serological suppressed vitamin A
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| r | P value | β | P value | |
| Vitamin A | ||||
| MELD | −0.521 | <0.001 | −0.626 | 0.004 |
| Ultrasound Steatosis | 0.303 | <0.001 | 0.475 | 0.01 |
| Serological suppressed Vitamin A | ||||
| Etiology | 1.828 | 0.003 | 2.377 | 0.006 |
| Child Pugh | 0.110 | 0.003 | 0.110 | 0.002 |
| Ultrasound steatosis | 0.08 | 0.022 | 0.024 | 0.002 |
Factors not predictive of vitamin A in univariable analysis were etiology, age, sex, Sodium, INR, Creatinine, Bilirubin, CRP, Albumin, Platelets and Child Pugh. With the remaining factors, a multivariable linear regression model with inclusion variable selection was built.
Gender 1 for male, 2 for female; Ultrasound Steatosis 1 for ultrasound steatosis, 2 for no ultrasound steatosis.
Factors not predictive of vitamin A deficiency in univariable analysis were etiology, age, sex, INR, sodium, creatinine, bilirubin, CRP, albumin, platelets and MELD. With the remaining factors, a multivariable linear regression model with inclusion variable selection was built.
Gender 1 for male, 2 for female; Ultrasound Steatosis 1 for ultrasound steatosis, 2 for no ultrasound steatosis.
MELD, model of end-stage liver disease.
Validation of association between liver synthesis and vitamin a deficiency
In the validation cohort, 21 patients showed decreased vitamin A and 23 patients showed normal vitamin A. Albumin was significantly decreased in patients with suppressed vitamin A (29 vs. 38; P < 0.001). Similarly, the patients with decreased vitamin A showed a higher international normalized ratio (INR) (1.41 vs. 1.21; P = 0.05). A higher MELD value (14 vs. 12; P = 0.32) and a higher Child Pugh stage (P = 0.19) were detected in patients with decreased vitamin a, but without statistical significance. Hepatic encephalopathy (29 vs. 9%; P = 0.03) and esophageal variceal bleeding (4 vs. 0%; P = 0.03) were significantly more frequent in patients with vitamin A suppression (Supplementary Table 1, Supplemental digital content 1, http://links.lww.com/EJGH/A768).
Vitamin A deficiency is associated with reduced overall survival but is not an independent prognostic factor
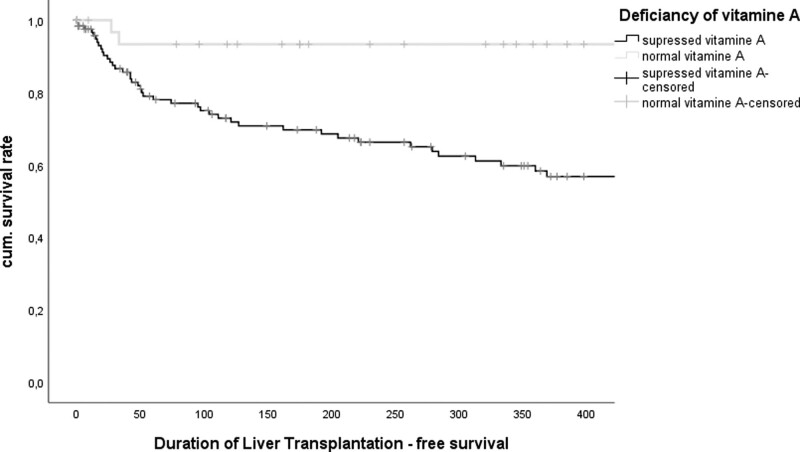
During the 1-year follow-up, 57 patients died and 21 received a liver transplant. The average follow-up time was 427 days. Patients with liver cirrhosis and suppressed vitamin A showed a significantly increased 1-year mortality compared to patients with normal vitamin A status (log-rank: 0.002) (Fig. 2). Similarly, in the Cox regression analysis, vitamin A was associated with the risk of death in the univariate analysis (P = 0.009). However, multivariate analyses did not confirm vitamin A as an independent prognostic factor. Besides vitamin A, INR could be determined as an independent variable in the univariate as well as in the multivariate Cox regression analysis (univariate analysis: P ≤ 0.001; multivariate analysis: P = 0.006). Interestingly, the inflammatory marker C-reactive protein could be confirmed as an independent marker both in the univariate as well as multivariate analysis (univariate analysis: P ≤ 0.001; multivariate analysis: P = 0.004) (table 3).
Fig. 2.
Kaplan–Meier-curve subdivides between patients with suppressed vitamin A and normal vitamin A (log-Rank test: P = 0.002).
Table 3.
Cox regression analysis of vitamin A
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| r | P value | β | P value | |
| CRP | 1.028 | <0.001 | 1.034 | 0.004 |
| INR | 4.825 | <0.001 | 4.853 | 0.006 |
| Vitamin A | 0.996 | 0.009 | 0.977 | 0.851 |
Factors not predictive of vitamin A in univariable analysis were etiology, age, sex, sodium, creatinine, bilirubin, albumin, platelets; ultrasound steatosis and Child Pugh. With the remaining factors, a multivariable linear regression model with inclusion variable selection was built.
Gender 1 for male, 2 for female; Ultrasound Steatosis 1 for ultrasound steatosis, 2 for no ultrasound steatosis.
CRP, C-reactive Protein; INR, international normalized ratio; MELD, model of end-stage liver disease.
Discussion
In this prospective clinical study, we investigated the association between reduced serum vitamin A and clinical course in patients with liver cirrhosis. We performed a detailed and comparative serological and clinical evaluation of patients with liver cirrhosis with normal and reduced serological vitamin A levels. We were able to show that reduced vitamin A is a common problem in patients with liver cirrhosis. In our study, almost 80% of the included patients showed reduced serum vitamin A levels. These data are largely consistent with other former analyses [9,10]. Interestingly, we were able to show that the frequency of vitamin A suppression is directly associated with the severity of liver insufficiency. In our analysis, only 18% of patients with liver cirrhosis in the Child-Pugh A stage showed reduced vitamin A levels, whereas all patients with advanced-stage C showed vitamin A suppression and no patient had normal vitamin A levels. Consistently, a recent study also showed that there is a close association between Child-Pugh score and serological levels of vitamin A [4]. In concordance with the reduced liver function, patients with reduced vitamin A also presented with a significantly higher MELD score than patients with normal vitamin A levels. Simbrunner et al. [4] showed that patients with portal hypertension, a hallmark of liver cirrhosis, were also more likely to have a vitamin A deficiency. Nevertheless, it is unclear whether vitamin A deficiency is a consequence of portal hypertension or whether fibrogenesis with loss of vitamin A storage leads to progression with the onset of portal hypertension. Further, reduced levels of albumin reflect impaired hepatic synthesis and are an indicator of increasing fibrogenesis with the development of liver cirrhosis. It is, therefore, likely that vitamin A is lost in the course of fibrogenesis, which leads to portal hypertension if disease progression continues. However, thrombocytopenia as a surrogate marker for portal hypertension was also significantly more frequent with suppressed vitamin A than with normal vitamin A levels.
Of note, it remains unclear whether malnutrition and limited absorption are the sole cause of the dependence on the Child-Pugh stage and vitamin A suppression. It is known that impaired liver function is closely associated with malnutrition, cachexia as well as sarcopenia [12]. In addition, alcohol consumption leads to further malnutrition and is a known risk factor for vitamin A deficiency [6]. Vitamin A metabolism requires transport proteins such as retinol-binding protein, as well as other trace elements such as zinc, which are often deficient in patients with liver cirrhosis [6,10]. In our cohort, alcoholic liver cirrhosis was the most frequent cause of the underlying liver disease. These patients with chronic alcohol consumption are particularly affected by malnutrition. This fact can certainly partly explain the association between liver cirrhosis and vitamin A deficiency. Not only malnutrition but also an unbalanced diet could be a cause of reduced vitamin A levels. Indeed, we could show a close association between ultrasound-proven steatosis and vitamin A and vitamin A deficiency. Similarly, significantly more patients with NASH-associated cirrhosis were in the vitamin A deficiency group. These data are consistent with a recent report delineating that vitamin A levels are inversely correlated with hepatic steatosis [13]. The combination between sarcopenia and visceral adipose tissue represents an important prognostic marker in patients with liver cirrhosis and especially with non-alcoholic steatohepatitis [14]. Similarly, it has been shown that after bariatric surgery and the normalization of body fat and hepatic steatosis, vitamin A levels frequently normalized [15]. This fact, in addition to the hypothesis of pure malnutrition, clearly favors vitamin A loss as the consequence of fibrogenesis through stimulation of the hepatic stellate cells.
For further analysis of the influence of vitamin A deficiency on the clinical course, patients were monitored over a 1-year follow-up after study inclusion. We could show that patients with reduced vitamin A more frequently develop liver-related complications. Notably, patients with vitamin A deficiency display more ascites, hepatic encephalopathy and hepatorenal syndrome during follow-up. Interestingly, the occurrence of gastrointestinal bleeding was comparable in vitamin A deficient patients and patients with normal levels. Hepatic encephalopathy, ascites and hepatorenal syndrome are known to have an inflammatory component with infections as a frequent trigger [16]. These observations potentially indicate the importance of vitamin A during inflammatory processes in addition to the progressive impairment of liver function. In addition, we were able to show that patients with vitamin A deficiency more frequently develop infectious complications, particularly respiratory infections. It is well known that vitamin A deficiency causes an altered cellular and humoral defense against infections [6]. In addition, animal experiments showed that the maturation of hematopoietic stem cells in the immune response is significantly influenced by vitamin A [7]. Importantly, hematopoietic stem cells are also considered to take part in the pathogenesis of acute-on-chronic liver failure [17]. Unfortunately, clinical studies on the stimulation of hematopoietic stem cells or direct transfusion recently failed to demonstrate a beneficial impact in patients with acute-on-chronic liver failure. Nevertheless, it is reasonable that vitamin A deficiency and the simultaneous occurrence of infectious complications could induce changes in the immune system as well as changes in the hematopoietic stem cell function. All these effects are certainly explained by the reduced liver function at the beginning of the study. At 1-year follow-up, patients with decreased serological vitamin A showed reduced overall survival. Nevertheless, vitamin A deficiency could not be identified as an independent prognostic factor in our study. These data suggest that vitamin A deficiency reflects impaired liver synthesis rather than a confounding factor.
Importantly, our study has several limitations. First, vitamin A deficiency was only assessed by serum levels, but not the hepatic vitamin A concentration. Vitamin A metabolism with protein-based transport as well as enzymatic activation steps is related to liver diseases. We were not able to investigate the possible influence of impaired metabolism due to impaired liver function on vitamin A measurement in more detail. In addition, hypoproteinemia with decreased retinol-binding protein may influence transport and thus hepatic availability [18]. In addition, we were unable to measure the level of vitamin A orally consumed. Regardless of this limitation, previous clinical studies have shown that oral vitamin A does not adequately refill hepatic vitamin A stores so the question arises whether monitoring the orally supplied amount of vitamin A would be useful. [19]. Second, we could not show that vitamin A suppression is an independent factor influencing patient survival. Established markers such as the Child-Pugh score and MELD score provide an accurate determination of prognosis. Although an association with shortened overall survival was shown, vitamin A reduction is not an independent prognostic factor. These results illustrate that vitamin A metabolism is multifaceted and that a single parameter such as vitamin A does not reflect the severity of the systemic disease liver cirrhosis. Other factors present in patients with liver cirrhosis play a crucial role. Decreased oral intake of vitamin A due to malnutrition, cachexia and malabsorption up to infections and dysregulated hepatic protein synthesis may play an important role. These points make it more difficult to use vitamin A as a possible therapeutic target. However, the main aim of this prospective study was to gain a better understanding of the Vitamin A metabolism and to improve the clinical course of patients with chronic liver disease. Prospective clinical trials of therapeutic vitamin A substitution are needed to further support these initial data. Besides the problem of insufficient hepatic vitamin A levels by oral supplementation, hepatic protein synthesis for vitamin A metabolism must be ensured. Not least, the question of hepatoxicity due to overdose must be answered.
Conclusion
In summary, we could show that suppressed vitamin A is commonly observed in patients with liver cirrhosis. Furthermore, we could demonstrate an association between vitamin A deficiency, liver insufficiency as well as clinical decompensation and, potentially, death. Thus, vitamin A could be a potential biomarker of imminent risk for complications and poor clinical outcomes.
Acknowledgements
The study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008). The study protocol was approved by the ethics committee of the Landesärztekammer Rhineland-Palatine (Nr. 837.232.17 [11066]). Written informed consent was obtained from every participant.
The data supporting the conclusion of this article is includes within the article. Any queries regarding these data may be directed to the corresponding author.
Authors’ Contribution
Performed research: M.N., M.A.W. and C.L.Contributed to acquisition of data: M.N. Designed the experiments and analyzed the data: M.N., C.L., J.M.S., J.U.M. and N.C.W. Contributed reagents/materials/analysis tools: M.N., P.R.G. and M.A.W. Wrote the article: M.N., J.U.M., M.A.W., C.L., C.C. and H.D. Statistical analysis: M.N. and C.L. All authors approved the final version of the article and the authorship list. Guarantor of the article: M.N.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.eurojgh.com.
References
- 1.Yoshiji H, Nagoshi S, Akahane T, Asaoka Y, Ueno Y, Ogawa K, et al. Evidence-based clinical practice guidelines for liver cirrhosis 2020. J Gastroenterol 2021; 56:593–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haaker MW, Vaandrager AB, Helms JB. Retinoids in health and disease: a role for hepatic stellate cells in affecting retinoid levels. Biochim Biophys Acta Mol Cell Biol Lipids 2020; 1865:158674. [DOI] [PubMed] [Google Scholar]
- 3.Venu M, Martin E, Saeian K, Gawrieh S. High prevalence of vitamin A deficiency and vitamin D deficiency in patients evaluated for liver transplantation. Liver Transpl 2013; 19:627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simbrunner B, Semmler G, Stadlmann A, Scheiner B, Schwabl P, Paternostro R, et al. Vitamin A levels reflect disease severity and portal hypertension in patients with cirrhosis. Hepatol Int 2020; 14:1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung K, Lee SS, Raman M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin Gastroenterol Hepatol 2012; 10:117–125. [DOI] [PubMed] [Google Scholar]
- 6.Koop AH, Mousa OY, Pham LE, Corral-Hurtado JE, Pungpapong S, Keaveny AP. An argument for vitamin D, A, and zinc monitoring in cirrhosis. Ann Hepatol 2018; 17:920–932. [DOI] [PubMed] [Google Scholar]
- 7.Cabezas-Wallscheid N, Buettner F, Sommerkamp P, Klimmeck D, Ladel L, Thalheimer FB, et al. Vitamin A-retinoic acid signaling regulates hematopoietic stem cell dormancy. Cell 2017; 169:807–823.e19. [DOI] [PubMed] [Google Scholar]
- 8.Van der Merwe S, Chokshi S, Bernsmeier C, Albillos A. The multifactorial mechanisms of bacterial infection in decompensated cirrhosis. J Hepatol 2021; 75 (Suppl 1):S82–S100. [DOI] [PubMed] [Google Scholar]
- 9.Peres WA, Chaves GV, Gonçalves JC, Ramalho A, Coelho HS. Vitamin A deficiency in patients with hepatitis C virus-related chronic liver disease. Br J Nutr 2011; 106:1724–1731. [DOI] [PubMed] [Google Scholar]
- 10.Chaves GV, Peres WA, Gonçalves JC, Ramalho A. Vitamin A and retinol-binding protein deficiency among chronic liver disease patients. Nutrition 2015; 31:664–668. [DOI] [PubMed] [Google Scholar]
- 11.Arantes Ferreira Peres W, Villaça Chaves G, Saraiva Gonçalves JC, Ramalho A, Moraes Coelho HS. Assessment of the relative dose-response test as indicators of hepatic vitamin A stores in various stages of chronic liver disease. Nutr Clin Pract 2013;28:95–100. [DOI] [PubMed] [Google Scholar]
- 12.Lai JC, Tandon P, Bernal W, Tapper EB, Ekong U, Dasarathy S, Carey EJ. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American association for the study of liver diseases. Hepatology 2021; 74:1611–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Chen H, Wang J, Zhou W, Sun R, Xia M. Association of serum retinoic acid with hepatic steatosis and liver injury in nonalcoholic fatty liver disease. Am J Clin Nutr 2015; 102:130–137. [DOI] [PubMed] [Google Scholar]
- 14.Habig G, Smaltz C, Halegoua-DeMarzio D. Presence and implications of sarcopenia in non-alcoholic steatohepatitis. Metabolites 2021; 11:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaves GV, Pereira SE, Saboya CJ, Spitz D, Rodrigues CS, Ramalho A. Association between liver vitamin A reserves and severity of nonalcoholic fatty liver disease in the class III obese following bariatric surgery. Obes Surg 2014; 24:219–224. [DOI] [PubMed] [Google Scholar]
- 16.Irvine KM, Ratnasekera I, Powell EE, Hume DA. Corrigendum: causes and consequences of innate immune dysfunction in cirrhosis. Front Immunol 2019; 10:818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newsome PN, Fox R, King AL, Barton D, Than NN, Moore J, et al. Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial. Lancet Gastroenterol Hepatol 2018; 3:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ukleja A, Scolapio JS, McConnell JP, Spivey JR, Dickson RC, Nguyen JH, O’Brien PC. Nutritional assessment of serum and hepatic vitamin A levels in patients with cirrhosis. JPEN J Parenter Enteral Nutr 2002; 26:184–188. [DOI] [PubMed] [Google Scholar]
- 19.Gropper SS, Smith JL, Groff JL. Advanced nutrition and human metabolism. 4th ed. Thomas Wadsworth; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.