Introduction:
Chronic traumatic encephalopathy (CTE) is a debilitating neurodegenerative disease, which is often the sequelae of repetitive head trauma. Although the definitive diagnosis of CTE is made postmortem, there are proposed clinical algorithms aimed at identifying characteristic features of CTE, based on a combination of clinical history, serum, cerebrospinal fluid and neuroimaging biomarkers. There are promising new advances in positron emission tomography neuroimaging, including tau specific ligands, which will potentially provide a robust assessment as well as an exploratory tool of the disease semiology and progression.
Case Report:
Here is a unique case of an ex-football player, who suffered multiple prior traumatic brain injuries throughout his career, and presented to our clinic with significant episodic memory, visuospatial and executive functioning deficits, as well as comorbid mood and behavioral changes in the absence of prior psychiatric history or substance use. His clinical presentation and biomarkers were consistent with a suspected diagnosis of CTE comorbid with Alzheimer disease, which comprises a significant portion of overall CTE cases.
Conclusion:
This case report presents a patient with a subtle case of dementia, which could be easily mistaken for behavioral variant frontotemporal dementia or primary progressive aphasia. This in turn highlights the importance of detailed longitudinal history taking, as well as rigorous biomarker studies.
Key Words: TBI, CTE, AD
BACKGROUND
There has been an increasing level of awareness regarding the association of popular sports including football, hockey and soccer, and development of chronic traumatic encephalopathy (CTE).1 First recognized in boxers who had suffered repeated head trauma by Dr Martland in 1928, the disease was initially termed “punch drunk syndrome”2,3 and was later named “dementia pugilistica.”4 CTE is currently characterized by accumulation of phosphorylated tau (p-tau) in sulci and perivascular region, with accompanying astrogliosis, microgliosis and TAR DNA-binding protein 43 (TDP-43).5
The pathologic progression of CTE is divided into 4 stages.6,7 In CTE stage I, the brain is grossly normal, with only a few p-tau deposits in the lateral and frontal cortices. As the disease progresses, there is wide-spread cortical deposition of p-tau and TDP-43. In the final CTE stage IV, there is marked atrophy of the frontal and medial temporal lobes, as well as the medial anterior thalami. The majority of patients in stage IV have septal abnormalities, including cavum septum pellucidum with or without fenestration, and pallor of locus coeruleus and substantia nigra.8
Although CTE is mainly a neuropathologic diagnosis, there are clinical manifestations corresponding to each progressive stage of CTE. In stage I, patients are either asymptomatic or might complain of mild memory and depressive symptoms, but as the diseases advances to stage III, there are prominent memory loss and executive functioning deficits. In the final CTE stage IV, profound cognitive, and motor deficits have been reported, which are often comorbid with psychotic symptoms.5 There are also reported CTE cases comorbid with Alzheimer disease (AD) pathology. In a cohort of CTE patients, reported by Stein and colleagues, amyloid β peptide (Aβ) deposition in diffuse or neuritic plaque form was reported in 52% of the cases. Despite the reported pathologic comorbidity of AD and CTE, there are relatively few case reports of patients with these comorbid diseases.9 Furthermore, the presence of neuritic plaques were found to be associated with increased CTE tauopathy, and comorbid Lewy body disease and dementia.10
CASE PRESENTATION
The patient is a 64-year-old right handed gentleman, Mr C.W., with history of multiple prior traumatic brain injuries (TBIs), who was seen for a neurocognitive assessment in the memory outpatient clinic at Yale New Haven Hospital. His community neurologist referred the patient to our clinic. The patient and his wife were hoping to receive the latest approved treatment for the patient’s progressive neurological decline. The clinical facility provides the additional pathway to the ongoing clinical trials for Alzheimer patients at our institution, one of few available research centers in the state.
The patient began to have significant short-term memory deficits 4 years before being seen in the clinic. His wife also started to notice behavioral changes, including unusual emotional reactivity and irritability in everyday situations. As his episodic memory symptoms continued to worsen, he had increasing difficulty with retaining details of conversations, and following multistep instructions. His wife emotionally recalled an episode, during which the patient was unable to remember the name of his nephew. A sociable person by nature, he started to become more reclusive and withdrawn, and actively avoided social gatherings within the first year of his neurocognitive changes. In addition, his demeanor morphed from more assertive to a more passive and deferential. In addition, he reported feeling depressed, despite no prior reported history of depression. Previously a social drinker, he started to consume alcohol excessively to cope with his mood symptoms. After repeated family interventions, the patient was able to stop drinking, and became sober a year before being seen in the clinic, He continued to avoid social settings, in turn becoming more withdrawn and isolated. He had a long-standing history of stuttering, but he reported worsening speech disturbances within the past 4 years. He had an extensive career as a business executive with an MBA degree, who had worked in multiple well-known companies managing purchasing, supply chains, and sustainability. He also managed his household financial affairs, but his wife had to take control of this because of his significant difficulties with processing numbers, calculations and multitasking.
Mr C.W. had his first concussion at the age of 5, and from the age of 5 until 1 year before being seen in the clinic, he suffered at least 20 concussions. The majority of them sustained playing in football teams, in his high school and college years playing in a quarterback position. In addition to his football involvement, Mr C.W. also played left field in baseball in high school, college, and a competitive league after college made up of former D1 college players, sustaining a few reported head injuries in collisions with other players and the outfield wall. His most recent concussion occurred at the age of 61 because of a fall, while walking with his dog. The concussions involved brief loss of consciousness, but the patient was unable to provide the exact durations. There was a reported maternal history of dementia, not formally diagnosed, and Mr C.W.’s sister is currently undergoing further assessment for mild cognitive impairment.
His Montreal Cognitive Assessment (MoCA) score was 13/30, with significant deficits involving multiple domains including episodic memory, visuospatial functioning, attention, executive functioning, and phonemic fluency. The more extensive neuropsychological assessment indicated significant dysnomia, significant deficits in auditory comprehension, verbal and phonemic verbal fluency, as well as deficits in working memory, clock drawing, cognitive estimation, and novel problem solving (Table 1).
TABLE 1.
Detailed Neuropsychological Assessment Reveals Global Cognitive Deficits
| Test | Raw | Standard Score | Percentile | ||
|---|---|---|---|---|---|
| Factor | |||||
| Verbal learning/memory | 40 | SS=54 | <1 | ||
| Visual learning/memory | 38 | SS=54 | <1 | ||
| Hopkins adult reading test | 23 | Std=111 | 77 | ||
| Trails A | 44 | t=41 | 18 | ||
| WAIS-IV digit span | |||||
| Digits forward | 6 | t=43 | 24 | ||
| Digits backward | 3 | t=27 | 1 | ||
| Cognitive estimation task | 14 | t=19 | <1 | ||
| Calibrated ideational fluency | |||||
| Phonemic fluency total | 11 | t=24 | <1 | ||
| Semantic fluency total | 10 | t=19 | <1 | ||
| Boston naming test-30 item | 22 | t=21 | <1 | ||
| NAB language module | |||||
| Language index | Std=51 | 0.05 | |||
| Oral production | 7 | t=19 | <1 | ||
| Auditory comprehension | 77 | t=19 | <1 | ||
| Naming | 23 | t=19 | <1 | ||
| Reading comprehension | 13 | 50 | |||
| Writing | 9 | t=42 | 21 | ||
| Bill payment | 10 | t=19 | <1 | ||
| Clock drawing | 2/5 | t=19 | <1 | ||
| Clock copy | 4/5 | t=20 | <1 | ||
| Rey complex figure-copy | 29 | t=38 | 12 | ||
| Hopkins verbal learning test | |||||
| Total recall | 2 | t=19 | <1 | ||
| Delayed recall | 0 | t=21 | <1 | ||
| % retention | 0 | t=25 | 1 | ||
| Recognition discrimination | 7 | t=30 | 2 | ||
| Brief visual | |||||
| Total recall | 1 | t=19 | <1 | ||
| Delayed recall | 2 | t=19 | <1 | ||
| % retention | 100 | t=53 | 62 | ||
| Recognition discrimination | 2 | t=19 | <1 | ||
| Geriatric depression scale | 2/15 | t | WNL | ||
| Wisconsin card sorting test-64 | |||||
| Categories | 0 | <1 | |||
| Perseverative responses | 13 | t=41 | 19 | ||
| Failure to maintain set | 0 | ||||
| Total errors | 32 | t=36 | 8 | ||
The neuropsychological battery demonstrated severe memory, executive functioning, language, and visuospatial deficits.
WAIS indicates Wechsler adult intelligence scale; WNL, within normal limits.
His serum levels for vitamin B12, folate, thyroid stimulating hormone, and free T4 were all within normal limits. The cerebrospinal fluid had significantly elevated total Tau (1045 pg/mL), phosphorylated Tau (125.05 pg/mL), and a decreased A-beta 42 to total Tau Index11 (0.25), which were consistent with diagnosis of AD.
The magnetic resonance imaging of brain without intravenous contrast with 3D volumetric analysis indicated total hippocampal volume measuring at the 11th percentile of the patient’s age-matched controls, with significant asymmetry (−12.1%) with smaller left hippocampal volume (Fig. 1). Moreover, the lateral ventricles were mildly enlarged with accompanying volume loss and a prominent cavum septum pellucidum (Fig. 2).
FIGURE 1.
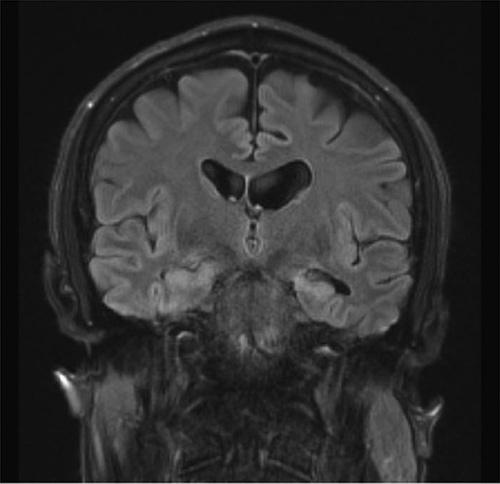
Magnetic resonance imaging reveals hippocampal and cortical atrophy. Coronal image shows significant hippocampal atrophy, with more asymmetrical loss of volume involving the left hippocampus.
FIGURE 2.
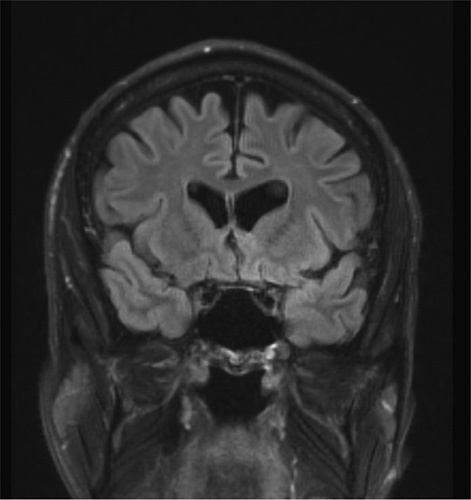
Magnetic resonance imaging reveals cavum septum pellucidum. Coronal image demonstrates generalized cortical atrophy and a demarcated cavum septum pellucidum.
DISCUSSION
This case report presents a unique but reported combination of memory, speech and executive functioning deficits comorbid with mood and subtle personality changes likely because of an underlying pathology of CTE and AD in a patient in his 60s.
The patient’s history of multiple mild TBI episodes with accompanying loss of consciousness in a subset of those episodes, is consistent with a history of CTE.5 Stern et al12 proposed that CTE presents clinically in 2 distinct subtypes. The first involves younger individuals who initially present with predominantly mood and behavioral symptoms; the second involving older patients with mostly memory and cognitive deficits. On the basis of this classification and consistent with the reported age onset, the presented case likely belongs to the second category.
Although CTE is a postmortem neuropathologic entity, the working diagnosis of the patient is “possible” CTE with comorbid AD, with features of “probable” CTE, based on the previously proposed CTE likelihood criteria by Montenigro et al.13,14 These suggestive features include a history of multiple impacts, leading to progressive memory and speech deficits over a time course lasting longer than 12 months, absence of prior psychiatric history including mood/anxiety disorders, post-traumatic stress disorder or substance use. There are additional supportive features including elevated cerebrospinal fluid phospho-tau/total-tau, cavum septum pellucidum, and cortical atrophy.14 There are multiple recent published cases of CTE comorbid with AD.9,15,16 However, the reported clinical presentation of dysarthria, dysnomia, personality alterations in a patient suffering from possible CTE with comorbid with AD is unique. This case highlights the importance of obtaining a detailed history of TBIs, capturing subtle presenting symptoms, as well as characteristic neuroimaging findings. CTE is increasingly being recognized to be a distinct tauopathy. The characteristic CTE neuropathologic lesions include perivascular accumulation of tau in neurons, astrocytes, and cell-processes in sulci depth. Although, the clinical presentation could resemble AD, or FTD, its tau pathology is distinct from AD, progressive supranuclear palsy, argyrophilic grain disease, corticobasal degeneration, Guamanian Parkinsonism Dementia Complex, and primary age-related tauopathy.10,17 Furthermore, according to a recent report by Falcon et al,18 using cryoelectron microscopy, tau filaments in 6 CTE patients was found to contains a distinct hydrophobic domain that is unique only to this pathology, and absent in AD patients. A recently published case report using 18F-MK6240 tau positron emission tomography ligand, has reported a frontally predominant pattern of tau/phosphor-tau accumulation, distinct from posterior temporoparietal predominant pattern of prodromal AD.16
Other diagnostic possibilities include behavioral variant frontotemporal dementia (bvFTD) and logopenic variant primary progressive aphasia. However, the patient presented here has overlapping symptoms of both bvFTD and logopenic variant primary progressive aphasia, making the individual diagnostic entities less likely. Furthermore, bvFTD patients typically do not develop speech-related deficits, or episodic memory deficits until late stages of the disease process.19
A number of promising tau specific neuroimaging positron emission tomography ligands, including 18F-MK6240,THK5317, THK5351, AV1451, PBB3, and flortaucipir could change the diagnostic and assessment landscape of CTE.16,20–22 Moreover, recent advances in serum markers of TBI will likely provide the opportunity to assess, and monitor injured patients over time, which, in turn, may provide a more comprehensive time window to the disease etiology, which is currently lacking. The wider utility of TBI biomarkers will also make future timely disease preventative interventions a viable possibility.23 As awareness and understanding of CTE evolves, preventative measures including safe practice techniques, destigmatizing the reporting of mild TBI symptoms in both sport and combat settings, as well as vigilant and timely care of patients post trauma will need to be incorporated in our everyday practice.
This case highlights the importance of early recognition of symptoms in light of prior history of brain injuries. The onset of new affective symptoms in the absence of any prior psychiatric history, as well as severe and progressive cognitive deficits including speech, memory and executive functioning decline, warrants extensive neuropsychiatric assessments. Given the comorbidity of AD in this case, it is entirely plausible that the patient could have benefitted from enrollment in current ongoing clinical trials using experimental regimens such as Aducanumab.24 This in turn highlights the importance of early recognition of symptoms, and exploring potential and appropriate clinical interventions in a timely manner.
ACKNOWLEDGMENTS
The author acknowledges the great support and mentorship of Dr Stephen Strittmatter.
Footnotes
The author declares no conflict of interest.
This study was approved by the office of Yale University IRB. The patient CW provided consent to participate in this study. The completed patient consent form can be provided upon request, as well as data used in this study.
REFERENCES
- 1.McKee AC, Daneshvar DH, Alvarez VE, et al. The neuropathology of sport. Acta Neuropathol. 2014;127:29–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Changa AR, Vietrogoski RA, Carmel PW. Dr Harrison Martland and the history of punch drunk syndrome. Brain. 2018;141:318–321. [DOI] [PubMed] [Google Scholar]
- 3.Martland HS. Punch drunk. J Am Med Assoc. 1928;91:1103–1107. [Google Scholar]
- 4.Millspaugh J. Dementia pugilistica. US Naval Med Bull. 1937;35:e303. [Google Scholar]
- 5.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mez J, Daneshvar DH, Kiernan PT, et al. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. Jama. 2017;318:360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fesharaki-Zadeh A. Chronic traumatic encephalopathy: a brief overview. Front Neurol. 2019;10:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKee AC, Stein TD, Keirnan PT, et al. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015;25:350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearce AJ, Sy J, Lee M, et al. Chronic traumatic encephalopathy in a former Australian rules football player diagnosed with Alzheimer’s disease. Acta Neuropathol Commun. 2020;8:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein TD, Montenigro PH, Alvarez VE, et al. Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol. 2015;130:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sunyer B, Patil S, Hoger H, et al. Barnes maze, a useful task to assess spatial reference memory in the mice. Nat Protoc. 2007;390:10–38. [Google Scholar]
- 12.Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81:1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montenigro PH, Baugh CM, Daneshvar DH, et al. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimer Res Ther. 2014;6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montenigro PH, Bernick C, Cantu RC. Clinical features of repetitive traumatic brain injury and chronic traumatic encephalopathy. Brain Pathol. 2015;25:304–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang C, Nag S, Xing G, et al. A clinicopathological report of a 93-year-old former street boxer with coexistence of chronic traumatic encephalopathy, Alzheimer’s disease, dementia with lewy bodies, and hippocampal sclerosis with TDP-43 pathology. Front Neurol. 2020;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnadas N, Dore V, Lamb F, et al. Case report: 18F-MK6240 Tau positron emission tomography pattern resembling chronic traumatic encephalopathy in a retired australian rules football player. Front Neurol. 2020;11:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falcon B, Zivanov J, Zhang W, et al. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature. 2019;568:420–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saint-Aubert L, Lemoine L, Chiotis K, et al. Tau PET imaging: present and future directions. Mol Neurodegen. 2017;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan SH, Wang SG. Emotional lability as a unique presenting sign of suspected chronic traumatic encephalopathy. Case Rep Neurol Med. 2018;2018:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stern RA, Adler CH, Chen K, et al. Tau positron-emission tomography in former national football League players. N Engl J Med. 2019;380:1716–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gan ZS, Stein SC, Swanson R, et al. Blood biomarkers for traumatic brain injury: a quantitative assessment of diagnostic and prognostic accuracy. Front Neurol. 2019;10:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sevigny J, Chiao P, Bussiere T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537:50–56. [DOI] [PubMed] [Google Scholar]


