BACKGROUND:
Microvascular decompression for trigeminal neuralgia (TN) may require sacrifice of the superior petrosal vein (SPV), with potential risks of ischemia and hemorrhagic complications due to impaired venous return.
OBJECTIVE:
To investigate methods for safely sacrificing the SPV.
METHODS:
We retrospectively reviewed 21 cases in 346 consecutive microvascular decompression surgeries for TN. They were intraoperatively identified as SPV and its tributaries being the offending vessels causing TN and were intentionally sacrificed.
RESULTS:
The transverse pontine vein (TPV) was sacrificed in 10 patients. The main trunk of the SPV was sacrificed using the TPV as a collateral flow pathway in 10 patients. No complications occurred related to impaired venous return.
CONCLUSION:
The venous flow conversion technique can be applied to safely sacrificing the SPV and its tributaries with the TPV acting as a collateral blood flow pathway to prevent postoperative impaired venous return.
KEY WORDS: Microvascular decompression, Trigeminal neuralgia, Superior petrosal vein, Transverse pontine vein, Venous complication, Sacrifice
ABBREVIATIONS:
- ABR
auditory brainstem response
- BNI
Barrow Neurological Institute Pain Intensity score
- MVD
microvascular decompression
- SCA
superior cerebellar artery
- SPV
superior petrosal vein
- TPV
transverse pontine vein.
Microvascular decompression (MVD) is a safe and effective treatment method for trigeminal neuralgia (TN).1 The MVD technique uses a Teflon prosthesis to relieve vessels' compression of the trigeminal nerve.2 However, the superior petrosal vein (SPV) may obscure the surgical field. The SPV and its tributaries may be part of the offending vessel complex.2-5 Sacrificing the SPV may be necessary to obtain adequate visualization of the trigeminal nerve and its related compressing vessels.2,4,5 However, sacrifice of the SPV may result in complications such as ischemia and hemorrhage due to impaired venous return.6-8 We have found that the transverse pontine vein (TPV) could be safely isolated from the SPV so that sacrifice of the SPV could be safely performed with the TPV acting as a collateral outflow.9 We report our experience which may suggest the optimal technique of MVD for TN caused by the SPV and its tributaries to safely and reliably improve patient pain.
METHODS
Our research protocol was approved by the ethics committees of our institution (No. 2741). Written informed consent was obtained from all individual participants from 2012 to 2021, and oral informed consent or medical record informed consent was obtained for all participants before 2012. The requirement for written informed consent was waived for participants before 2012 because of the retrospective design. All data identifying the patients were anonymized.
A total of 346 consecutive MVD surgeries were performed in 323 patients at our institution from April 2007 to July 2021. Twenty patients have undergone reoperations, including 1 patient who had 3 surgeries. Two patients also developed bilateral TN and underwent surgery for each. We retrospectively reviewed these surgeries by referring to medical records, surgical records, and surgical videos. We identified the surgeries in which the SPV or its tributaries were directly compressing or touching the trigeminal nerve and were treated by MVD by sacrifice of the offending vessels.
All patients underwent head computed tomography on the day after surgery and head magnetic resonance imaging within 1 month after surgery. Complications due to impaired venous return were evaluated based on clinical and imaging findings. Postoperative pain relief and facial numbness were assessed during hospitalization and at the last outpatient follow-up, and the Barrow Neurological Institute pain intensity score was used to assess pain relief.10
Surgeries were performed under general anesthesia in the park bench position by the retrosigmoidal approach. Auditory brainstem response (ABR) was used for intraoperative monitoring. If necessary, the great horizontal fissure of the cerebellum was dissected to obtain an intraoperative view of the entire trigeminal nerve and the SPV and its tributaries.
Surgery for the offending SPV or its tributaries used transposition techniques to preserve the main trunk of the SPV and its tributaries as far as possible, until certain criteria for sacrifice of the SPV were introduced in 2013. Subsequently, the TPV could be coagulated and cut. If the TPV could be used for collateral outflow, the SPV could also be coagulated and cut.
RESULTS
Patient characteristics are shown in Table. The SPV complex was the offending vessel in 21 cases (21 of 346, 6%): The TPV was sacrificed in 10 cases and the main trunk of the SPV in 11. Blood flow to the SPV was converted to the TPV as the SPV was sacrificed in 10 cases. In 1 case, both the SPV and the TPV were unintentionally sacrificed, and the flow to the SPV was converted to the vein of the middle cerebral peduncle. Nineteen patients showed improvement of symptoms (90%), and 2 patients had no change in symptoms immediately after surgery. Nine patients (43%) had facial numbness at discharge, and 5 patients (24%) had residual numbness at the last outpatient follow-up. Postoperative computed tomography and magnetic resonance imaging detected no cases of venous infarction or bleeding complications. The median follow-up period was 8 months (mean, 17 ± 22 months). Four second surgeries were included: initial surgery using transposition of the SPV with Teflon string but pain recurred after 2 years and 11 months in 2 patients (1 not treated in our practice); pain recurred after MVD for superior cerebellar artery in 1 patient after 3 months; and persistent pain continued just after transposition of the superior cerebellar artery in 1 patient (Figure 1). Two illustrative cases are shown in Figures 1 and 2.
FIGURE 1.
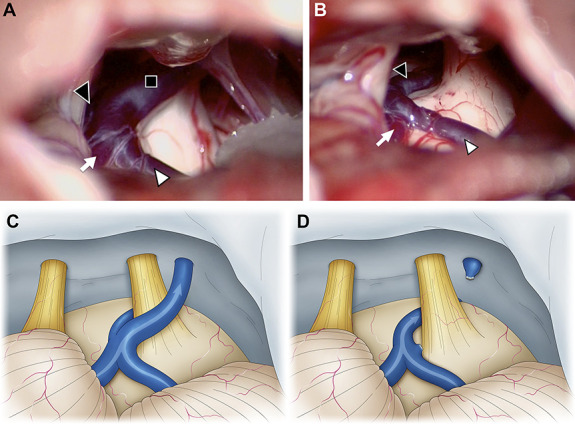
Intraoperative photographs of the second surgery of a 47-year-old woman who underwent microvascular decompression for left trigeminal neuralgia. At the initial surgery, the SCA seemed to be the offending vessel. We performed only transposition of the SCA, but the patient did not experience any postoperative pain relief. Therefore, we concluded that the responsible vessels were the SPV and performed the second surgery. A and C, The SPV (black square) contacts the trigeminal nerve, and its tributaries are the TPV (black arrowhead), the vein of the middle cerebral peduncle (white arrow), and the vein of the cerebellopontine fissure (white arrowhead). B and D, We determined that blood flow from the vein of the middle cerebral peduncle and vein of the cerebral pontine fissure could be drained into the TPV. The SPV was sacrificed, and the blood flow from the vein of cerebellopontine fissure and the vein of middle cerebral peduncle was diverted to the TPV. SCA, superior cerebellar artery; SPV, superior petrosal vein; TPV, transverse pontine vein.
FIGURE 2.
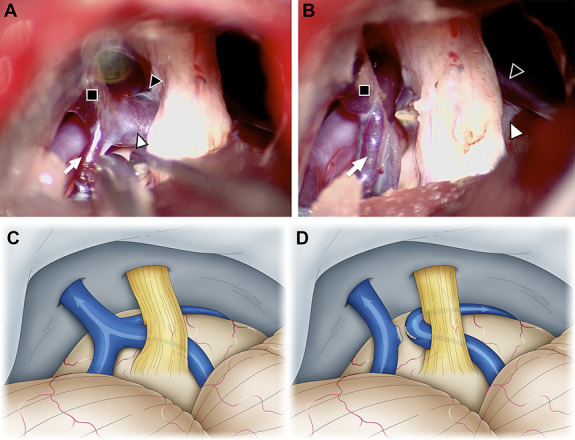
Intraoperative photographs of a 67-year-old man who underwent microvascular decompression for right trigeminal neuralgia. A and C, The vein of the cerebellopontine fissure (white arrowhead) penetrates the trigeminal nerve, the transverse pontine vein (black arrowhead) contacts the ventral side of the trigeminal nerve, and the trigeminal nerve is in traction. B and D, By cutting a part of the superior petrosal vein (SPV, black square), the blood flow from the pontotrigeminal vein (white arrow) to the SPV was maintained, and the blood flow from the vein of cerebellopontine fissure to the SPV was diverted to the transverse pontine vein. SPV, superior petrosal vein.
TABLE.
Clinical Characteristics of 21 Patients With Trigeminal Neuralgia Caused by Venous Compression
| Characteristic | Value |
|---|---|
| Age, mean (range), y | 55.2 ± 16 (26-79) |
| Sex, No. (%) | |
| Male | 4 (19) |
| Female | 17 (81) |
| Side of pain, No. (%) | |
| Right | 10 (48) |
| Left | 11 (52) |
| Pain distribution, No. | |
| V2 | 6 |
| V3 | 5 |
| V1/V2 | 5 |
| V2/V3 | 4 |
| V1/V2/V3 | 1 |
| Second surgery, No. | 4 |
| Sacrificed veins, No. | |
| Transverse pontine vein | 10 |
| Superior petrosal vein | 11 |
| Veins of blood flow conversion, No. | |
| Transverse pontine vein | 10 |
| Vein of middle cerebral peduncle | 1 |
| Pain relief, No. | |
| BNI I | 17 |
| BNI II | 2 |
| BNI IV | 2 |
| Numbness at discharge, No. (%) | 9 (43) |
| Numbness at final visit, No. (%) | 5 (24) |
| Complications, No. | 0 |
BNI, Barrow Neurological Institute Pain Intensity score
DISCUSSION
The feasibility of cutting the SPV during MVD surgery has been discussed since the time of Dandy.11 Major studies have shown that the incidence of postoperative complications caused by the sacrifice of the SPV is not high.2,4,5 However, some cases of serious complications,7,12,13 with a complication rate of up to 31% have been reported.14 Therefore, the impact of SPV sacrifice should not be ignored. Previous studies of low complication rates with SPV sacrifice did not support unnecessary sacrifice of the SPV and suggested that SPV sacrifice should depend on the intraoperative risk–benefit analysis.15,16
Venous compression of the trigeminal nerve is believed to cause 4% to 19% of cases of TN, with several cases involving the SPV.17,18 To preserve the vein as far as possible, the arachnoid membrane around the SPV is dissected to increase mobility,19 and the approach for MVD is adapted according to the position of the SPV complex.18 However, the most appropriate location (main trunk or branched part) for cutting the SPV has not been identified.
The SPV complex generally consists of the vein of the cerebellopontine fissure, the vein of the middle cerebellar peduncle, the TPV, the pontotrigeminal vein, and the anterior lateral marginal vein. The SPV shows considerable variation, with multiple tributaries branching from the SPV and even multiple SPVs (Figure 3).20 Various studies of preoperative imaging have found that the location of the SPVs and the composition and thickness of tributaries can be identified, but there is no consensus on determining which veins can be sacrificed.3,21
FIGURE 3.
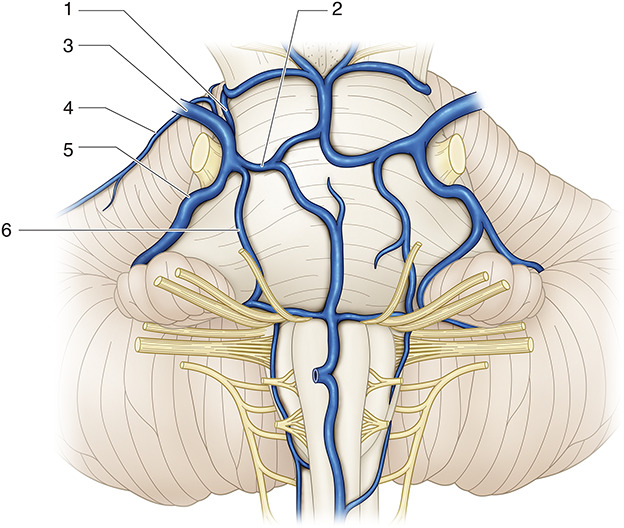
Comprehensive illustrations of the venous vessels comprising the superior petrosal vein complex from the ventral side of the brainstem. 1, pontotrigeminal vein; 2, transverse pontine vein; 3, superior petrosal vein; 4, anterior lateral marginal vein; 5, vein of cerebellopontine fissure; 6, vein of middle cerebellar peduncle.
We evaluated our cases requiring sacrifice of the SPV complex with 2 hypotheses in mind. The first is that the TPV can be sacrificed.9 The TPV often causes TN17,22-24 and sometimes has to be sacrificed. The TPV is connected to the terminal vein, the anterior pontomesencephalic venous system, and the contralateral TPV, and this wide network of anastomoses has sufficient collateral blood flow pathways.25 Therefore, we believe that these abundant collateral channels will compensate for the changes in blood flow caused by sacrifice of the TPV.
Our second hypothesis is that the SPV can be sacrificed by using the TPV as a compensatory mechanism for blood flow. As mentioned above, the network of venous return in the ventral part of the brainstem is extensive and the compensatory mechanisms are easily activated.21,25 However, loss of perfusion may not be compensated in vessels such as the pontotrigeminal vein and vein of the cerebellopontine fissure, which have the potential for extensive drainage from the dorsal side of the bridge and cerebellar hemispheres so increasing the risk for venous infarction and hemorrhage. Therefore, we believe that disruption of the continuity between these vessels and the main trunk of the SPV is feasible only if the continuity of these vessels can be maintained with the TPV.
The present study showed that no patients suffered cerebral infarction or hemorrhagic complications suspected to result from impaired venous return. Pure venous compression is an unfavorable prognostic factor for surgical outcomes after MVD for TN and is associated with the failure to attain complete pain relief. We presented that 90% of treated patients showed Barrow Neurological Institute Pain Intensity score I and II at the last follow-up. Our results were better than previous reports, despite the short follow-up period.17,26,27 Because the offending vessel is adequately transposed, the vessel does not contact the trigeminal nerve again and adhesions do not occur around the trigeminal nerve. In addition, we can treat without placing Teflon fibers between the nerve and the vessel. In fact, we have experienced 2 cases in which the SPV was transposed using a Teflon string at the initial surgery but recurred later. In those 2 cases, we successfully treated them by sacrificing the SPV.
The usefulness of cutting the TPV during MVD, including our report,9,28 has been investigated, but maintaining the venous return of the SPV is unclear. We here propose a hypothesis for the safe sacrifice of the SPV based on the TPV's ability to provide compensatory collateral flow pathways. We believe that cutting the SPV at a point that impairs venous return carries a risk of serious complications. Masuoka et al7 reported that the cerebellar infarction occurred after sacrificing the main trunk of the SPV and its tributaries consisting of the vein of the cerebellopontine fissure, the superior hemispheric vein, and the pontotrigeminal vein. The lack of a compensatory pathway such as the TPV may have caused the complication. Zhong et al29 confirmed the presence or absence of changes in the ABR by temporarily occluding the SPV; because perfusion of the lateral cerebellar hemisphere and the vein diameter were evaluated, tributaries were not identified. Changes in ABR were found in 8.6% of patients, who may not have had a compensatory collateral blood flow pathway such as the TPV. On the other hand, the complication rate caused by SPV sacrifice has been low with a large number of cases. Possibly even if the main trunk of the SPV is sacrificed, the blood flow to the SPV may have been converted to tributaries of the SPV that acts as a compensatory pathway.
Our series included 4 recurrent cases. Transposition of the SPV was used the string technique in 2 patients at the initial surgery. We believe that this can be avoided if steady decompression is achieved by coagulating and cutting the SPV at the appropriate location.
Limitations
The limitations of this study are the retrospective design, small number of cases, and short follow-up period. The number of cases in which the SPV is the offending vessel is inevitably small, so further accumulation of cases is necessary to confirm our results.
CONCLUSION
MVD for TN requiring cutting of the offending SPV and its tributaries is possible if the TPV could be sacrificed or used as a collateral blood flow pathway to prevent postoperative impaired venous return. This procedure can be applied for other cerebellopontine angle surgeries.
Acknowledgments
We thank Benjamin W Y Lo for assistance with manuscript preparation.
Contributor Information
Asami Kikuchi, Email: kikuchi.asami@twmu.ac.jp.
Hidenori Ohbuchi, Email: hide.obuchi@twmu.ac.jp.
Yuichi Kubota, Email: kubota.yuichi@twmu.ac.jp.
Hidetoshi Kasuya, Email: hkasuya@twmu.ac.jp.
Funding
This study did not receive any funding or financial support.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
COMMENT
In this detailed and comprehensive technical report, the authors characterize their technique modification for the sacrifice of the superior petrosal vein (SPV) for microvascular decompression of the trigeminal nerve in cases of venous contact etiology. They characterize this technique beautifully, with clear operative photos and illustrative figures. Pain relief was quite good, and there were no vascular complications in their series of 21 patients.
Sacrifice of the SPV has been debated significantly over the decades, with large series demonstrating vascular complications (ranging from 0.5% to 31%, but most around 5%). The current dogma is to protect this vein and its branches unless unavoidable or absolutely necessary for the operation at hand. For trigeminal neuralgia patients, who are effectively undergoing a quality-of-life surgery, even a 0.5% risk of hemorrhage or stroke is considered a significant incidence. This technique modification exemplified by the authors may very well be safer than traditional SPV sacrifice, though larger series will be necessary to prove safety with statistical significance. The authors should be commended for their work on this important technical nuance.
Garni Barkhoudarian
Santa Monica, California, USA
REFERENCES
- 1.Barker FG, II, Jannetta PJ, Bissonette DJ, et al. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med. 1996;334(17):1077-1083. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin MR, Jannetta PJ, Clyde BL, et al. Microvascular decompression of cranial nerves: lessons learned after 4400 operations. J Neurosurg. 1999;90(1):1-8. [DOI] [PubMed] [Google Scholar]
- 3.Basamh M, Sinning N, Kehler U. Individual variations of the superior petrosal vein complex and their microsurgical relevance in 50 cases of trigeminal microvascular decompression. Acta Neurochir (Wien). 2020;162(1):197-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pathmanaban ON, O’Brien F, Al-Tamimi YZ, et al. Safety of superior petrosal vein sacrifice during microvascular decompression of the trigeminal nerve. World Neurosurg. 2017;103:84-87. [DOI] [PubMed] [Google Scholar]
- 5.Xia Y, Kim TY, Mashouf LA, et al. Absence of ischemic injury after sacrificing the superior petrosal vein during microvascular decompression. Oper Neurosurg. 2020;18(3):316-320. [DOI] [PubMed] [Google Scholar]
- 6.Anichini G, Iqbal M, Rafiq NM, et al. Sacrificing the superior petrosal vein during microvascular decompression. Is it safe? Learning the hard way. Case report and review of literature. Surg Neurol Int. 2016;7(suppl 14):S415-S420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masuoka J, Matsushima T, Hikita T, Inoue E. Cerebellar swelling after sacrifice of the superior petrosal vein during microvascular decompression for trigeminal neuralgia. J Clin Neurosci. 2009;16(10):1342-1344. [DOI] [PubMed] [Google Scholar]
- 8.Tsukamoto H, Matsushima T, Fujiwara S, Fukui M. Peduncular hallucinosis following microvascular decompression for trigeminal neuralgia: case report. Surg Neurol. 1993;40(1):31-34. [DOI] [PubMed] [Google Scholar]
- 9.Kasuya H, Tani S, Kubota Y, et al. Characteristics and management of the offending veins in microvascular decompression surgery for trigeminal neuralgia. Neurosurg Rev. 2021;44(4):2337-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers CL, Shetter AG, Fiedler JA, et al. Gamma knife radiosurgery for trigeminal neuralgia: the initial experience of the Barrow Neurological Institute. Int J Radiat Oncol Biol Phys. 2000;47(4):1013-1019. [DOI] [PubMed] [Google Scholar]
- 11.Dandy WE. The treatment of trigeminal neuralgia by the cerebellar route. Ann Surg. 1932;96(4):787-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liebelt BD, Barber SM, Desai VR, et al. Superior petrosal vein sacrifice during microvascular decompression: perioperative complication rates and comparison with venous preservation. World Neurosurg. 2017;104:788-794. [DOI] [PubMed] [Google Scholar]
- 13.Singh D, Jagetia A, Sinha S. Brain stem infarction: a complication of microvascular decompression for trigeminal neuralgia. Neurol India. 2006;54(3):325-326. [DOI] [PubMed] [Google Scholar]
- 14.Narayan V, Savardekar AR, Patra DP, et al. Safety profile of superior petrosal vein (the vein of Dandy) sacrifice in neurosurgical procedures: a systematic review. Neurosurg Focus. 2018;45(1):E3. [DOI] [PubMed] [Google Scholar]
- 15.Fujimaki T, Kirino T. Coagulation of the petrosal vein for MVD. J Neurosurg. 1999;90(6):1148. [DOI] [PubMed] [Google Scholar]
- 16.Xia Y, Lim M. In reply: absence of ischemic injury after sacrificing the superior petrosal vein during microvascular decompression. Oper Neurosurg. 2021;20(3):E260. [DOI] [PubMed] [Google Scholar]
- 17.Inoue T, Hirai H, Shima A, et al. Diagnosis and management for trigeminal neuralgia caused solely by venous compression. Acta Neurochir (Wien). 2017;159(4):681-688. [DOI] [PubMed] [Google Scholar]
- 18.Wu M, Fu X, Ji Y, et al. Microvascular decompression for classical trigeminal neuralgia caused by venous compression: novel anatomic classifications and surgical strategy. World Neurosurg. 2018;113:e707-e713. [DOI] [PubMed] [Google Scholar]
- 19.Toda H, Iwasaki K, Yoshimoto N, et al. Bridging veins and veins of the brainstem in microvascular decompression surgery for trigeminal neuralgia and hemifacial spasm. Neurosurg Focus. 2018;45(1):E2. [DOI] [PubMed] [Google Scholar]
- 20.Huang YP, Wolf BS, Antin SP, Okudera T. The veins of the posterior fossa—anterior or petrosal draining group. AJR Am J Roentgenol. 1968;104(1):36-56. [DOI] [PubMed] [Google Scholar]
- 21.Bender B, Hauser TK, Korn A, et al. Depiction of the superior petrosal vein complex by 3D contrast-enhanced MR angiography. AJNR Am J Neuroradiol. 2018;39(12):2249-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumot C, Brinzeu A, Berthiller J, Sindou M. Trigeminal neuralgia due to venous neurovascular conflicts: outcome after microvascular decompression in a series of 55 consecutive patients. Acta Neurochir (Wien). 2017;159(2):237-249. [DOI] [PubMed] [Google Scholar]
- 23.Dumot C, Sindou M. Trigeminal neuralgia due to neurovascular conflicts from venous origin: an anatomical-surgical study (consecutive series of 124 operated cases). Acta Neurochir (Wien). 2015;157(3):455-466. [DOI] [PubMed] [Google Scholar]
- 24.Matsushima T, Huynh-Le P, Miyazono M. Trigeminal neuralgia caused by venous compression. Neurosurgery. 2004;55(2):334-337; discussion 338-339. [DOI] [PubMed] [Google Scholar]
- 25.Kiyosue H, Tanoue S, Sagara Y, et al. The anterior medullary-anterior pontomesencephalic venous system and its bridging veins communicating to the dural sinuses: normal anatomy and drainage routes from dural arteriovenous fistulas. Neuroradiology. 2008;50(12):1013-1023. [DOI] [PubMed] [Google Scholar]
- 26.Hong W, Zheng X, Wu Z, et al. Clinical features and surgical treatment of trigeminal neuralgia caused solely by venous compression. Acta Neurochir (Wien). 2011;153(5):1037-1042. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y, Zhang X, Yao J, et al. Microvascular decompression for trigeminal neuralgia due to venous compression alone. J Craniofac Surg. 2018;29(1):178-181. [DOI] [PubMed] [Google Scholar]
- 28.Inoue T, Shitara S, Goto Y, et al. Petrosal vein involvement in neurovascular conflict in trigeminal neuralgia: surgical technique and clinical outcomes. Oper Neurosurg. 2021;20(4):E264-E271. [DOI] [PubMed] [Google Scholar]
- 29.Zhong J, Li ST, Xu SQ, et al. Management of petrosal veins during microvascular decompression for trigeminal neuralgia. Neurol Res. 2008;30(7):697-700. [DOI] [PubMed] [Google Scholar]


