Abstract
The development of C(sp3)−H functionalization reactions that use common protecting groups and practical oxidants remains a significant challenge. Herein we report a monoprotected aminoethyl thioether (MPAThio) ligand‐enabled β‐C(sp3)−H lactamization of tosyl‐protected aliphatic amides using tert‐butyl hydrogen peroxide (TBHP) as the sole oxidant. This protocol features exceedingly mild reaction conditions, reliable scalability, and the use of practical oxidants and protecting groups. Further derivatization of the β‐lactam products enables the synthesis of a range of biologically important motifs including β‐amino acids, γ‐amino alcohols, and azetidines.
Keywords: C−H Activation, Carboxylic Acids, Lactamization, Ligand Design, Palladium
PdII‐catalyzed β‐C(sp3)−H lactamization of aliphatic amides is reported. This protocol features the use of practical and inexpensive oxidants and protecting groups, mild conditions, and reliable scalability. Further derivatization of β‐lactam products enables synthesis of a range of biologically important motifs such as β‐amino acid, γ‐amino alcohol, and azetidine.
Since the discovery of penicillin by Fleming in 1928, β‐lactams have featured widely among different classes of antibiotics. Although classic β‐lactam antibiotics consist of bicyclic rings, monocyclic β‐lactam variants, known as monobactams, have shown promising biological activity (Scheme 1A). [1] Among methods to synthesize β‐lactams, [2] β‐C(sp3)−H lactamization of amides provides a new route to access β‐lactams from ubiquitous and inexpensive aliphatic carboxylic acids (Scheme 1B).[ 3 , 4 , 5 , 6 ] The first example of β‐lactamization of aliphatic amides was reported by the Shi group in 2013, using the bidentate 2‐(pyridine‐yl)isopropyl (PIP) amine as a directing group (DG). [4a] Further studies on β‐lactamization were focused on the use of various DGs[ 4b , 4d , 4e , 4f ] and first‐row transition metals. [5] These protocols typically employed quinoline(pyridine)‐amide based bidentate DGs to promote cyclometalation and subsequent C−N reductive elimination. To accommodate ligand acceleration, especially enantiocontrol by bidentate ligands, we have focused on the use of simple monodentate functional groups or common protecting groups [7] to direct C−H lactamization. Despite recent success in ligand‐enabled β‐lactonization, [8a] analogous transformations using simple monodentate amides have not been developed thus far. Additionally, previously reported β‐lactamization methodologies using bidentate DGs pose practical limitations such as the use of silver salts[ 4c , 4d , 4e , 4f , 5c , 5d , 5e ] and harsh conditions (microwave irradiation[ 4d , 4e ] or temperatures as high as 160 °C[ 4c , 4d , 4e , 4f , 5 ]).
Scheme 1.

β‐C(sp3)−H lactamization of aliphatic amides.
The past two decades have witnessed dramatic developments in new carbon‐carbon and carbon‐heteroatom bond‐forming strategies using PdII/PdIV catalysis. [9] The inherent ability of PdIV to incorporate up to six ligands for participation in the reductive elimination has led to diverse transformations that are impossible using conventional PdII chemistry. Our group is particularly interested in using the bystanding oxidant strategy in PdII/PdIV chemistry to promote the otherwise difficult reductive elimination step required for the synthesis of small heterocycles. [9c] Recently, we reported an unprecedented β‐C(sp3)−H lactonization of free carboxylic acids. [8a] The use of tert‐butyl hydrogen peroxide (TBHP) as a bystanding oxidant and a β‐amino acid ligand is crucial to promote the selective reductive elimination to yield highly strained β‐lactones. However, despite their potential utility as targeted covalent inhibitors, β‐lactones are less stable both in solution at pH 7 and in serum, compared with their homologous β‐lactams. [10] Although we have recently developed a ligand enabled γ‐C(sp3)−H lactamization of amino acid derived native amides using TBHP as the terminal oxidant, [8b] this ligand was not reactive for β‐C−H lactamization. Thus, we embarked on the development of a ligand to β‐lactamization from abundant aliphatic carboxylic acid derived amides using a practical oxidant. [11]
Herein, we report a β‐C(sp3)−H lactamization of aliphatic amides using common sulfonyl‐based protecting groups (Ts, Mbs, Ns, Cs, SES, and Ms) (Scheme 1C). Using a monoprotected aminoethyl thioether (MPAThio) ligand proved crucial to the success of this reaction. Moreover, the use of the inexpensive oxidant TBHP (70 % in water, $5/mol) renders this reaction both practical and scalable. Compared to other C−H activation protocols, this reaction protocol features exceedingly mild conditions, reliable scalability, the use of practical oxidants and protecting groups, and exclusive monoselectivity. Further derivatization of β‐lactam products enables the synthesis of a range of biologically important motifs including β‐amino acids, γ‐amino alcohols, and azetidines.
Following our recent disclosure of the β‐C(sp3)−H lactonization of free carboxylic acids using TBHP, [8a] we initiated our investigation of β‐lactamization by selecting tosyl (Ts)‐protected aliphatic amide 1 a as a model substrate, because acyl sulfonamides are commonly employed as carboxylic acid bioisosteres based on their similar pK a values (5–6). [12] Under the optimal conditions of the aforementioned β‐lactonization reaction using Ligand L10, we were delighted to observe a 20 % 1H NMR (nuclear magnetic resonance) yield of the desired β‐lactam product 2 a with a considerable amount of the ring opened product mediated by reaction with the solvent hexafluoroisopropanol (HFIP) and oxidative side products (see Table S1 and Scheme S1A). Further investigation of the reaction conditions revealed that the byproduct could be significantly reduced using 0.1 equiv Cs2CO3 and the less acidic solvent 2,2,2‐trifluoroethanol (TFE). [13] In light of recent advances in ligand‐accelerated PdII‐catalyzed C−H activation, we next searched for ligands that could substantially improve the reactivity of the catalyst (Table 1). [14] Guided by pyridine ligand‐enabled C(sp3)−H activation reactions using monodentate amide directing groups, we tested the range of pyridine ligands L1–L5 developed in our laboratory. [15] While β‐lactam product 2 a was observed with 2‐pyridone ligands L4 and L5 that previously promoted γ‐lactamization of native amides, [9] yields were unsatisfactory (20 % and 21 %, respectively). In our previous publication, we employed a bidentate amino acid directing group with 2‐pyridone ligand to achieve γ‐C−H lactamization. [8b] Under the current reaction conditions, the γ‐C(sp3)−H bonds are not activated as six‐membered cyclopalladation is much less favored. However, β‐C−H lactamization can be challenging due to the difficulty in the reductive elimination step forming strained four‐membered ring. Bearing in mind the difficulties inherent to C(sp3)−H activation reactions directed by weakly coordinating monodentate amides and the sluggish reductive elimination of strained four‐membered rings from PdIV, we envisioned that bidentate ligands which were successful for previously reported weakly coordinating directing groups might merit further investigation, because their bidentate character might favor reductive elimination.[ 14b , 16 ] As such, a series of MPAA ligands that had enabled the β‐lactonization of free carboxylic acids (L6–L11, with L10 being the optimal ligand from the reported β‐lactonization) were evaluated, with the Ac‐Val‐OH (L7) ligand improving the yield to 44 %. [14b] The bidentate ligands L12–L16 that had facilitated other C(sp3)−H activations directed by weakly coordinating groups were also investigated. [16] To our delight, the MPAThio ligand developed for the C(sp3)−H olefination [17a] and carbonylation [17b] of free carboxylic acids and C(sp3)−H functionalization of free cyclopropylmethylamines [17c] significantly improved the yield to 74 % (75 % isolated yield). The scalability of our method has been demonstrated by a gram‐scale β‐lactamization of 1 a (4.0 mmol, 1.08 g) with 73 % isolated yield. Further modifications to the backbone of the MPAThio ligand (L17 and L18) led to no improvement. Control experiments showed that no reaction occurred under ligandless conditions, indicating the importance of the MPAThio ligand for the observed reactivity. Although the MPAThio ligand L16 is completely oxidized to the corresponding sulfoxide L19 after the reaction, we believe that the actual catalytic species in the β‐lactamization reaction is still a Pd/MPAThio species based on the following observations: 1) a 1H NMR study showed that the MPAThio ligand L16 could be detected in the first 6 hours; 2) thioether ligands have shown unique reactivity in our previous C−H activation reactions, [17a] mostly likely due to electronic effect required for reductive elimination step. While this hypothesis remains to be elucidated, our control experiment showed clearly the importance of PdII coordination with the sulfur as the oxidized sulfoxide ligand L19 did not show any reactivity (Table 1).
Table 1.
Ligand investigation for the β‐C(sp3)−H lactamization.[a,b]

[a] Conditions: 1 a (0.1 mmol), Pd(CH3CN)2Cl2 (10 mol%), ligand (L) (10 mol%), Cs2CO3 (0.1 equiv), TBHP (70 % in water) (2.0 equiv), TFE, 60 °C, 12 h. [b] The yields were determined by 1H NMR analysis of the crude product using CH2Br2 as the internal standard. [c] Isolated yield. [d] The reaction was run on a 4.0 mmol scale (1.08 g).
With the optimal ligand and reaction conditions in hand, we next evaluated the scope of aliphatic amides (Table 2). Different sulfonyl‐based protecting groups, including tosyl (Ts) (2 a), 4‐methoxybenzenesulfonyl (Mbs) (2 b), 4‐Nitrobenzenesulfonyl (4‐Ns) (2 c), 4‐cyanobenzenesulfonyl (4‐Cs) (2 d), 2‐trimethylsilylethanesulfonyl (SES) (2 e), and mesyl (Ms) (2 f) groups were compatible with the optimized protocol, affording the corresponding β‐lactams in moderate to good yields (52–75 %). [18] Aliphatic amides containing α‐gem‐dimethyl groups with various aliphatic chains (1 g–1 r) were all well tolerated, providing β‐lactams 2 g–2 p in yields ranging from modest to good (40–81 %) with exclusive mono‐selectivity. Alkyl amides containing a single α‐methyl group (1 s–1 z) consistently afforded good yields (up to 92 %), without formation of γ‐ or δ‐lactams in the presence of potentially reactive primary γ‐ or δ‐C−H bonds. Phenyl groups (2 j–2 o, 2 u, and 2 v) were compatible with the TBHP system, and remained intact despite the potentially reactive aryl or benzylic C−H bonds. A range of functionalities such as fluoro (2 w), chloro (2 m and 2 p), phenolic ether (2 q and 2 r), ester (2 l) and methoxy (2 x) was well tolerated, with the ester and chloro moieties serving as useful synthetic handles for subsequent derivatization. The aliphatic amide 1 q derived from gemfibrozil, an oral drug used to lower lipid levels, was converted to the corresponding β‐lactam 2 q in good yield (77 %). [19] The spiro β‐lactam 2 z could also be accessed in a synthetically useful 52 % yield. For substrates that have low reactivity, majority of the starting materials were recovered. Side products included the ring‐opening amino esters or oxidative products, similar to what we observed using ligand L10 (see Scheme S1A). Substrates that bear an α‐hydrogen afforded β‐amino ester products (e.g. 2 ae′) using slightly different conditions (see Scheme S1B). It is well‐known that less hindered β‐lactam can be readily opened by alcohols. [20] Methylene C−H bonds in substrate 1 al were not reactive under these conditions.
Table 2.
Substrate scope of the β‐C(sp3)−H lactamization.[a,b]

[a] Conditions: 1 (0.1 mmol), Pd(CH3CN)2Cl2 (10 mol%), L16 (10 mol%), Cs2CO3 (0.1 equiv), TBHP (70 % in water) (2.0 equiv), TFE, 60 °C, 12 h. [b] Isolated yields.
From a practical standpoint, this reaction has several key advantages over other C−H activation protocols: 1) use of the inexpensive oxidant TBHP as the sole oxidant; 2) tolerance of both air and moisture; 3) ability to be reliably scaled up. Considering the sluggish reductive elimination of primary C−H bonds, our protocol offers a complementary approach to the synthesis of β‐unsubstituted β‐lactams which are generally inaccessible in β‐lactamizations using DGs.[ 4 , 5 ]
To demonstrate the synthetic applications, the β‐lactam products 2 a and 2 e were successfully transformed into three structurally distinct synthons (Scheme 2): 1) opening of β‐lactam ring of 2 a using NaOMe afforded the biologically important β‐amino ester 3 a in 95 % yield, providing a formal synthesis of the β‐amidated product in two steps from the parent acid; [21] 2) reduction of β‐lactam 2 a in the presence of LAH gave the γ‐amino alcohol 3 b in 97 % yield; 3) a subsequent Mitsunobu reaction of 3 b resulted in the azetidine product 3 c in 79 % yield over two steps from 2 a. Finally, deprotection of β‐lactam 2 e using TBAF in THF gave 3 d in a synthetically useful yield (40 %).
Scheme 2.
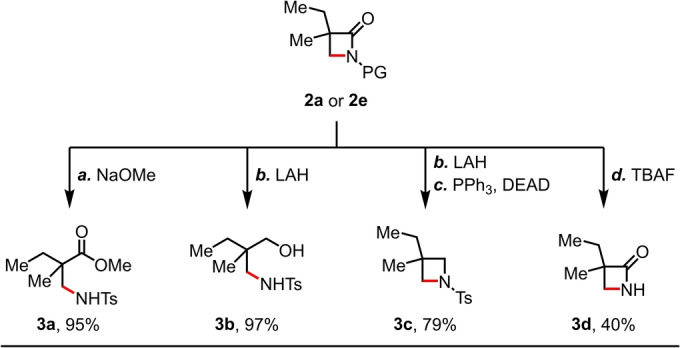
Synthetic applications. Conditions: a) 2 a (0.1 mmol), NaOMe (0.5 M in MeOH, 2.0 equiv), MeOH (0.5 mL), rt, overnight. b) 2 a (0.1 mmol), LAH (1.0 M in THF, 2.0 equiv), THF (1.0 mL), 0 °C, 1 h. c) 3 b (0.1 mmol), PPh3 (1.5 equiv), DEAD (1.5 equiv), THF (1.0 mL), rt, overnight. d) 2 e (0.1 mmol), TBAF (1.0 M in THF, 1.0 equiv), THF (1.0 mL), 0 °C, 1 h.
In summary, we have realized a β‐lactamization of aliphatic amides enabled by a PdII catalyst bearing a MPAThio ligand. The use of inexpensive TBHP as the sole oxidant and sulfonyl‐based common protecting groups renders this reaction highly practical and potentially amenable to large‐scale manufacturing. Derivatizations of β‐lactam products enable syntheses of a range of biologically important scaffolds such as β‐amino acid, γ‐amino alcohol, and azetidine.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
We gratefully acknowledge the NIH (NIGMS, R01GM084019), the Scripps Research Institute, and Bristol Myers Squibb for financial support. We thank Alastair N. Herron for proofreading.
Z. Zhuang, S. Liu, J.-T. Cheng, K.-S. Yeung, J. X. Qiao, N. A. Meanwell, J.-Q. Yu, Angew. Chem. Int. Ed. 2022, 61, e202207354; Angew. Chem. 2022, 134, e202207354.
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
References
- 1.
- 1a. Mehta P. D., Sengar N. P. S., Pathak A. K., Eur. J. Med. Chem. 2010, 45, 5541–5560; [DOI] [PubMed] [Google Scholar]
- 1b. Galletti P., Giacomini D., Curr. Med. Chem. 2011, 18, 4265–4283; [DOI] [PubMed] [Google Scholar]
- 1c. Decuyper L., Jukič M., Sosič I., Žula A., D′hooghe M., Gobec S., Med. Res. Rev. 2018, 38, 426–503; [DOI] [PubMed] [Google Scholar]
- 1d. Singh G. S., Mini-Rev. Med. Chem. 2004, 4, 69–92; [DOI] [PubMed] [Google Scholar]
- 1e. Pierrat O. A., Strisovsky K., Christova Y., Large J., Ansell K., Bouloc N., Smiljanic E., Freeman M., ACS Chem. Biol. 2011, 6, 325–335; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1f. Inokuma T., Fuller R. P., C. F. Barbas 3rd , Bioorg. Med. Chem. Lett. 2015, 25, 1684–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.For reviews on the syntheses of β-lactams, see:
- 2a. Brandi A., Cicchi S., Cordero F. M., Chem. Rev. 2008, 108, 3988–4035; [DOI] [PubMed] [Google Scholar]
- 2b. Pitts C. R., Lectka T., Chem. Rev. 2014, 114, 7930–7953; [DOI] [PubMed] [Google Scholar]
- 2c. Hosseyni S., Jarrahpour A., Org. Biomol. Chem. 2018, 16, 6840–6852; [DOI] [PubMed] [Google Scholar]
- 2d. Font M., Gulias M., Mascarenas J. L., Angew. Chem. Int. Ed. 2022, 61, 202112848; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2022, 134, 202112848; [Google Scholar]
- 2e. He C., Whitehurst W. G., Gaunt M. J., Chem 2019, 5, 1031–1058. [Google Scholar]
- 3. Zhang M., Wang Q., Peng Y., Chen Z., Wan C., Chen J., Zhao Y., Zhang R., Zhang A. Q., Chem. Commun. 2019, 55, 13048–13065. [DOI] [PubMed] [Google Scholar]
- 4.For Pd-catalyzed β-C(sp3)−H lactamizations using various directing groups, see:
- 4a. Zhang Q., Chen K., Rao W., Zhang Y., Chen F.-J., Shi B.-F., Angew. Chem. Int. Ed. 2013, 52, 13588–13592; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 13833–13837; [Google Scholar]
- 4b. Ling P.-X., Fang S.-L., Yin X.-S., Zhang Q., Chen K., Shi B.-F., Chem. Commun. 2017, 53, 6351–6354; [DOI] [PubMed] [Google Scholar]
- 4c. Zhou T., Jiang M.-X., Yang X., Yue Q., Han Y.-Q., Ding Y., Shi B.-F., Chin. J. Chem. 2020, 38, 242–246; [Google Scholar]
- 4d. Sun W.-W., Cao P., Mei R.-Q., Li Y., Ma Y.-L., Wu B., Org. Lett. 2014, 16, 480–483; [DOI] [PubMed] [Google Scholar]
- 4e. Zhang S.-J., Sun W.-W., Cao P., Dong X.-P., Liu J.-K., Wu B., J. Org. Chem. 2016, 81, 956–968; [DOI] [PubMed] [Google Scholar]
- 4f. Tong H.-R., Zheng W., Lv X., He G., Liu P., Chen G., ACS Catal. 2020, 10, 114–120. [Google Scholar]
- 5.For first-row transition metal-catalyzed β-C(sp3)−H lactamizations using different directing groups, see:
- 5a. Wu X., Zhao Y., Zhang G., Ge H., Angew. Chem. Int. Ed. 2014, 53, 3706–3710; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 3780–3784; [Google Scholar]
- 5b. Wu X., Zhao Y., Ge H., Chem. Eur. J. 2014, 20, 9530–9533; [DOI] [PubMed] [Google Scholar]
- 5c. Wu X., Yang K., Zhao Y., Sun H., Li G., Ge H., Nat. Commun. 2015, 6, 6462; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5d. Wang Z., Ni J., Kuninobu Y., Kanai M., Angew. Chem. Int. Ed. 2014, 53, 3496–3499; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 3564–3567; [Google Scholar]
- 5e. Aihara Y., Chatani N., ACS Catal. 2016, 6, 4323–4329. [Google Scholar]
- 6.For other examples of the syntheses of β-lactams using C(sp3)−H activation strategy, see:
- 6a. Pedroni J., Boghi M., Saget T., Cramer N., Angew. Chem. Int. Ed. 2014, 53, 9064–9067; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 9210–9213; [Google Scholar]
- 6b. Willcox D., Chappell B. G. N., Hogg K. F., Calleja J., Smalley A. P., Gaunt M. J., Science 2016, 354, 851–857; [DOI] [PubMed] [Google Scholar]
- 6c. Dailler D., Rocaboy R., Baudoin O., Angew. Chem. Int. Ed. 2017, 56, 7218–7222; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 7324–7328; [Google Scholar]
- 6d. Cabrera-Pardo J. R., Trowbridge A., Nappi M., Ozaki K., Gaunt M. J., Angew. Chem. Int. Ed. 2017, 56, 11958–11962; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 12120–12124. [Google Scholar]
- 7.For examples of C(sp3)−H functionalization reactions of amides using common protecting groups, see:
- 7a. Yoo E. J., Wasa M., Yu J.-Q., J. Am. Chem. Soc. 2010, 132, 17378–17380; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7b. Cendón B., Font M., Mascareñas J. L., Gulías M., ACS Catal. 2020, 10, 3425–3430. For examples of amine substrates, see: [Google Scholar]
- 7c. Shao Q., He J., Wu Q.-F., Yu J.-Q., ACS Catal. 2017, 7, 7777–7782; [Google Scholar]
- 7d. Zheng Y., Song W., Zhu Y., Wei B., Xuan L., J. Org. Chem. 2018, 83, 2448–2454. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. Zhuang Z., Yu J.-Q., Nature 2020, 577, 656–659; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8b. Liu S., Zhuang Z., Qiao J. X., Yeung K.-S., Su S., Cherney E. C., Ruan Z., Ewing W. R., Poss M. A., Yu J.-Q., J. Am. Chem. Soc. 2021, 143, 21657–21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.For reviews on PdIV chemistry, see:
- 9a. Xu L.-M., Li B.-J., Yang Z., Shi Z.-J., Chem. Soc. Rev. 2010, 39, 712–733; [DOI] [PubMed] [Google Scholar]
- 9b. Sehnal P., Taylor R. J. K., Fairlamb I. J. S., Chem. Rev. 2010, 110, 824–889; [DOI] [PubMed] [Google Scholar]
- 9c. Engle K. M., Mei T.-S., Wang X., Yu J.-Q., Angew. Chem. Int. Ed. 2011, 50, 1478–1491; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 1514–1528; [Google Scholar]
- 9d. Topczewski J. J., Sanford M. S., Chem. Sci. 2015, 6, 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.
- 10a. Robinson S. L., Christenson J. K., Wackett L. P., Nat. Prod. Rep. 2019, 36, 458–475; [DOI] [PubMed] [Google Scholar]
- 10b. Reddy L. R., Saravanan P., Corey E. J., J. Am. Chem. Soc. 2004, 126, 6230–6231; [DOI] [PubMed] [Google Scholar]
- 10c. Hogan P. C., Corey E. J., J. Am. Chem. Soc. 2005, 127, 15386–15387. [DOI] [PubMed] [Google Scholar]
- 11.For other examples of C−H functionalization reactions using peroxides, see:
- 11a. Giri R., Liang J., Lei J.-G., Li J.-J., Wang D.-H., Chen X., Naggar I. C., Guo C., Foxman B. M., Yu J.-Q., Angew. Chem. Int. Ed. 2005, 44, 7420–7424; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2005, 117, 7586–7590; [Google Scholar]
- 11b. Vickers C. J., Mei T. S., Yu J.-Q., Org. Lett. 2010, 12, 2511–2513; [DOI] [PubMed] [Google Scholar]
- 11c. Wei Y., Yoshikai N., Org. Lett. 2011, 13, 5504–5507; [DOI] [PubMed] [Google Scholar]
- 11d. Duan S., Xu Y., Zhang X., Fan X., Chem. Commun. 2016, 52, 10529–10532; [DOI] [PubMed] [Google Scholar]
- 11e. Zhuang Z., Herron A. N., Fan Z., Yu J.-Q., J. Am. Chem. Soc. 2020, 142, 6769–6776; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11f. Zhuang Z., Herron A. N., Liu S., Yu J.-Q., J. Am. Chem. Soc. 2021, 143, 687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.
- 12a. Stansfield I., Pompei M., Conte I., Ercolandi C., Migliaccio G., Jairaj M., Giuliano C., Rowley M., Narjes F., Bioorg. Med. Chem. Lett. 2007, 17, 5143–5149; [DOI] [PubMed] [Google Scholar]
- 12b.Meanwell, J. Med. Chem. 2011, 54, 2529–2591; [DOI] [PubMed]
- 12c. Ballatore C., Huryn D. M., Smith A. B., ChemMedChem 2013, 8, 385–395; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12d. Ammazzalorso A., De Filippis B., Giampietro L., Amoroso R., Chem. Biol. Drug Des. 2017, 90, 1094–1105. [DOI] [PubMed] [Google Scholar]
- 13. Dandia A., Singh R., Joshi J., Kumari S., Mini-Rev. Org. Chem. 2014, 11, 462–476. [Google Scholar]
- 14.For reviews, see:
- 14a. He J., Wasa M., Chan K. S. L., Shao Q., Yu J.-Q., Chem. Rev. 2017, 117, 8754–8786; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14b. Shao Q., Wu K., Zhuang Z., Qian S., Yu J.-Q., Acc. Chem. Res. 2020, 53, 833–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.
- 15a. Wasa M., Chan K. S. L., Zhang X.-G., He J., Miura M., Yu J.-Q., J. Am. Chem. Soc. 2012, 134, 18570–18572; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15b. He J., Li S., Deng Y., Fu H., Laforteza B. N., Spangler J. E., Homs A., Yu J.-Q., Science 2014, 343, 1216–1220; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15c. Chen Y.-Q., Wang Z., Wu Y., Wisniewski S. R., Qiao J. X., Ewing W. R., Eastgate M. D., Yu J.-Q., J. Am. Chem. Soc. 2018, 140, 17884–17894; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15d. Xia G., Zhuang Z., Liu L.-Y., Schreiber S. L., Melillo B., Yu J.-Q., Angew. Chem. Int. Ed. 2020, 59, 7783–7787; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 7857–7861. [Google Scholar]
- 16.
- 16a. Chen G., Gong W., Zhuang Z., Andrä M. S., Chen Y.-Q., Hong X., Yang Y.-F., Liu T., Houk K. N., Yu J.-Q., Science 2016, 353, 1023–1027; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16b. Wu Q.-F., Shen P.-X., He J., Wang X.-B., Zhang F., Shao Q., Zhu R.-Y., Mapelli C., Qiao J. X., Poss M. A., Yu J.-Q., Science 2017, 355, 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.
- 17a. Zhuang Z., Yu C.-B., Chen G., Wu Q.-F., Hsiao Y., Joe C. L., Qiao J. X., Poss M. A., Yu J.-Q., J. Am. Chem. Soc. 2018, 140, 10363–10367; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17b. Zhuang Z., Herron A. N., Yu J.-Q., Angew. Chem. Int. Ed. 2021, 60, 16382–16387; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 16518–16523; [Google Scholar]
- 17c. Zhuang Z., Yu J.-Q., J. Am. Chem. Soc. 2020, 142, 12015–12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wuts P. G. M., Greene's Protective Groups in Organic Synthesis , 5th ed, Wiley, Hoboken, 2014. [Google Scholar]
- 19. Todd P. A., Ward A., Drugs 1988, 36, 314–339. [DOI] [PubMed] [Google Scholar]
- 20.
- 20a. Thaisrivongs S., Schostarez H. J., Pals D. T., Turner S. R., J. Med. Chem. 1987, 30, 1837–1842; [DOI] [PubMed] [Google Scholar]
- 20b. Meiries S., Marquez R., J. Org. Chem. 2008, 73, 5015–5021. [DOI] [PubMed] [Google Scholar]
- 21.For Pd-catalyzed β-C(sp3)−H amination reactions, see:
- 21a. He J., Shigenari T., Yu J.-Q., Angew. Chem. Int. Ed. 2015, 54, 6545–6549; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 6645–6649; [Google Scholar]
- 21b. Gou Q., Liu G., Liu Z.-N., Qin J., Chem. Eur. J. 2015, 21, 15491–15495; [DOI] [PubMed] [Google Scholar]
- 21c. Bai H.-Y., Ma Z.-G., Yi M., Lin J.-B., Zhang S.-Y., ACS Catal. 2017, 7, 2042–2046. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.



