Abstract
Platelet concentrates (PCs) are easily obtained from autogenous whole blood after centrifugation and have evolved through three generations of development to include platelet-rich plasma, platelet-rich fibrin, and concentrated growth factor. Currently, PCs are widely used for sinus floor elevation, alveolar ridge preservation, periodontal bone defects, guided bone regeneration, and treatment of gingival recession. More recently, PCs have been leveraged for tissue regeneration to promote oral soft and hard tissue regeneration in implant dentistry and regenerative periodontology. PCs are ideal for this purpose because they have a high concentration of platelets, growth factors, and cytokines. Platelets have been shown to release extracellular vesicles (P-EVs), which are thought to be essential for PC-induced tissue regeneration. This study reviewed the clinical application of PCs and P-EVs for implant surgery and periodontal tissue regeneration.
I. INTRODUCTION
An increasing number of people worldwide are becoming concerned about their periodontal health. Patients with severe problems are increasingly opting for treatment with oral implants to repair dentition defects and rehabilitate edentulous jaws. Possessing adequate alveolar bone in all three cardinal axes is the basic requirement for implant placement.1 Possessing sufficient quality and quantity of soft tissue is an effective guarantee of achieving long-term health of the implant.2,3 Artificial bone materials, soft tissue flap grafts, and autologous platelet concentrate (PC) products are now being used routinely to overcome the challenge of sufficiently augmenting soft and hard tissue for regenerative periodontology and implant dentistry.4
Platelets are an important component in the regulation of coagulation and hemostasis.5,6 A growing number of studies have demonstrated that platelets can also act as regulators in angiogenesis and tissue regeneration. Platelets are relatively easy to obtain for experimental or clinical purposes. Using differences in settling velocity of blood constituents, large numbers of platelets from autologous whole blood can be collected by differential centrifugation to obtain PCs.7 PC collection and separation techniques have been refined over the years, and researchers now can isolate three generations or main types of concentrates: platelet-rich plasma (PRP), platelet-rich fibrin (PRF), and concentrated growth factor (CGF). In addition to being rich in platelets, some types of PCs also contain fibrin and leukocytes. More importantly, many bioactive factors are released when PCs are applied topically for tissue repair. These releases include growth factors, cytokines, lysosomes, and adhesion proteins that initiate a signaling cascade, resulting in the binding to corresponding receptors, intracellular biochemical changes, and promotion of regeneration.8,9
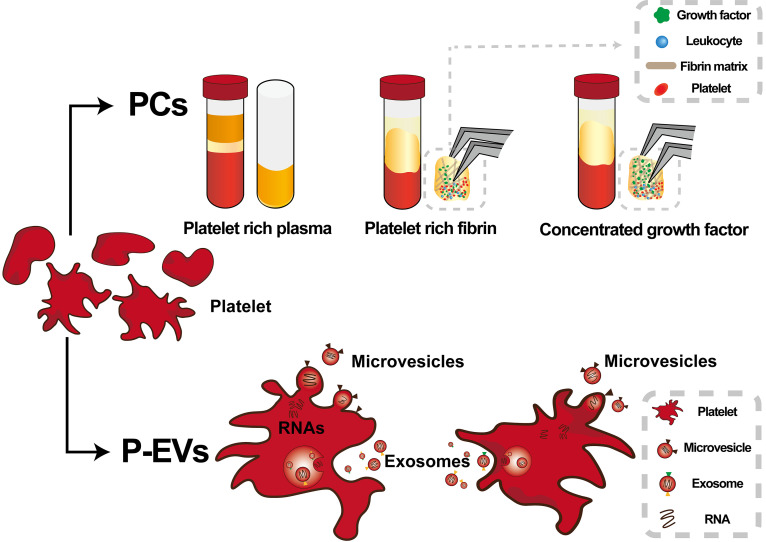
In recent years, the importance of extracellular vesicles (EVs) in intercellular communication has become more appreciated and understood. EVs can alter the phenotype and function of recipient cells by delivering various proteins, bioactive lipids, and even genetic information.10 Platelets can be released into the bloodstream during thrombosis. Upon activation, in addition to chemokines and cytokines, platelets release two different kinds of EVs—exosomes (EXOs) and microvesicles collectively referred to as platelet-derived extracellular vesicles (P-EVs). Currently, P-EVs are used for treating various pathophysiological conditions such as wound healing, inflammatory response, angiogenesis, and neuroregenerative response (Fig. 1).11–13
FIG. 1.
Schematic diagram of platelet concentrates (PCs) and platelet-derived extracellular vesicles (P-EVs).
II. PLATELET CONCENTRATES (PCS) AND PLATELET-DERIVED EXTRACELLULAR VESICLES (P-EVS)
A. Preparation of platelet concentrates (PCs)
PCs are obtained by gradient centrifugation of autologous venous blood and have shown positive results for tissue regeneration in periodontology and implant dentistry (Fig. 1). However, there is no international consensus on the centrifugal speed, centrifugal force, centrifugal time, and preparation temperature for the preparation of PC. Aside from that, some researchers have defined the preparation parameters in terms of rotational speed (rpm), while others have described them in terms of centrifugal force (g). Centrifugation parameters and several other procedural variables can affect efficacy and reproducibility. Each type of PC has its own sensitivities to preparation variables. PRP requires two tedious centrifugation steps, PRF requires only one step, and CGF preparation requires differential centrifugation.
1. Platelet-rich plasma (PRP)
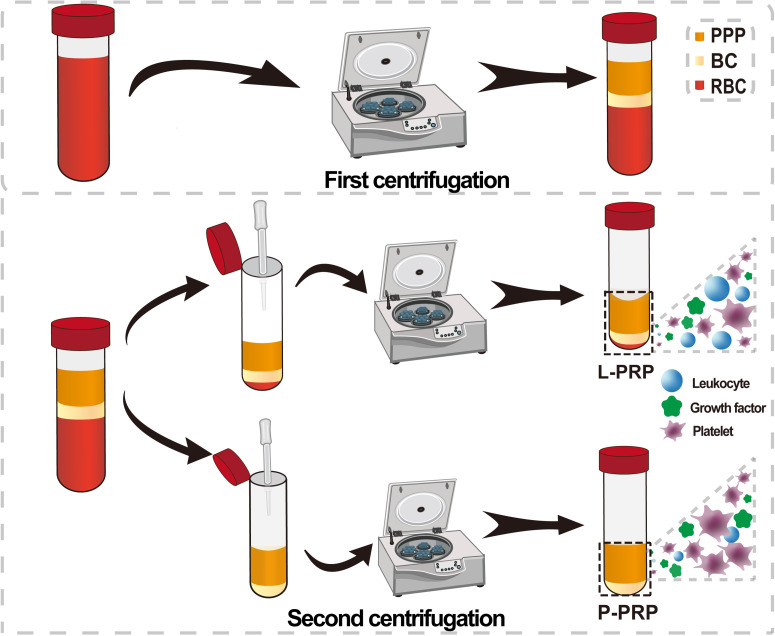
PRP is a first-generation PC, originally developed and refined in the 1980s. Normally PRP is obtained in liquid form through a tedious two-step centrifugation process. There is still no standard for the best protocol for PRP preparation. Regardless of the speed and time of centrifugation, three layers are obtained in this first gentle centrifugation step. This spin with low acceleration force and short spin time produces, from bottom to top, a red blood cell (RBC) layer, a buffy coat (BC) layer containing leukocytes and platelets, and a platelet-poor plasma (PPP) layer. At this step, PRP is processed differently to obtain either leucocyte- and platelet-rich plasma (L-PRP) or pure platelet-rich plasma (P-PRP) (Fig. 2). The main difference between L-PRP and P-PRP is that their leucocyte content differs.7 To obtain L-PRP, after first centrifugation at 160 g for 10 min, the PPP, BC layers, and some residual RBCs’ layer are carefully aspirated out of the centrifuge tube and centrifuged at 250 g for 15 min again.14 The resulting supernatant is mostly discarded, leaving a small amount of bottom sediment to obtain L-PRP. L-PRP contains platelets, growth factors, and leukocytes. P-PRP is obtained only by centrifugation of the original PPP layer and superficial BC layer and contains almost no leukocytes. Yin explored six centrifugation protocols and found that P-PRP obtained by first centrifugation at 160 g for 10 min and second centrifugation at 250 g for 15 min had the highest enrichment of platelets and growth factors.15
FIG. 2.
Schematic diagram of the preparation process of different types of PRP (L-PRP and P-PRP).
L-PRP and P-PRP can be activated to a gel state by adding calcium chloride solution, thrombin, collagen, or calcium gluconate. When procoagulants are added to activate the PRP solution into a gel, these procoagulants interact with pre-existing anticoagulants, and platelets are activated to release fibrinogen, which polymerizes into fibrin and connects to a mesh, forming a gel-like substance with some adhesion and strength. They can also be transformed into a gel via lysis by freezing.
2. Platelet-rich fibrin (PRF)
Obtaining PRF involves a simple one-step process: whole blood is collected in glass centrifuge tubes and centrifuged. As no anticoagulant is added, the whole blood coagulates naturally during centrifugation. Centrifugation produces an RBC layer at the bottom of the tube, followed by a PRF layer in the middle and a PPP layer at the top of the tube. Because PRF is rich in leukocytes, it is also known as leukocyte- and platelet-rich fibrin (L-PRF). The two most common L-PRF preparation protocols are centrifugation at 3000 rpm for 10 min proposed by Choukroun and 2700 rpm for 12 min proposed by Shahram.16,17 Advanced PRF (A-PRF) is obtained by reducing the centrifugation speed and centrifugation time at 1500 rpm for 10 min to achieve a more uniform distribution of leukocytes and platelets in the PRF,17 which can also be obtained by centrifugation at 1300 rpm for 14 min.18 Pure PRF (P-PRF) or leukocyte-poor PRF can only be obtained by processing whole blood using a commercial FibriNet® Platelet-Rich Fibrin Membrane (PRFM) kit (Cat. No. 510359; Royal Biologics; Hackensack, NJ, USA), which produces a leukocyte-free PRF.7 Another method to obtain PRF is to centrifuge whole blood in a titanium tube preferably at 3500 rpm for 15 min, called titanium-prepared PRF (T-PRF).19 One advantage to using titanium tubes is that T-PRF is free of silica contaminants, which can be found in PRF prepared in glass tubes and can affect patients receiving PRF.19 Horizontal PRF (H-PRF) is a new kind of PRF prepared by horizontal centrifugation at 700 g for 8 min.20 Since common PRF contains a strong fibrin matrix and is in the form of an active gel, it is usually compressed into a membrane for use. In 2015, whole blood was collected in hydrophobic plastic tubes with reduced centrifugal force and time at 60 g for 3 min to obtain injectable PRF in liquid form.21
3. Concentrated growth factor (CGF)
CGF is a modified form of PRF that contains higher levels of growth factors and platelets and a denser fibrin matrix.22 To obtain CGF, whole blood is subjected to repeated centrifugation at acceleration and deceleration rates to activate alpha particles in platelets, producing higher growth factor concentrations.22 According to Sacco’s protocol, whole blood was collected without anticoagulant and centrifuged using a variable speed centrifuge (MEDIFUGE™, Silfradent, Sofia, Italy) after 30 s of acceleration, 2700 rpm for 2 min, 2400 rpm for 4 min, 2700 rpm for 4 min, 3000 rpm for 3 min, and deceleration for 36 s to obtain CGF.23 Different forms of CGF can be prepared by centrifuging whole blood in different kinds of centrifuge tubes. A dense gel form of CGF is prepared in centrifuge tubes with rough inner walls; a loose gel form of CGF is prepared from tubes with smooth inner walls, and a liquid form of CGF is prepared by adding anticoagulant to whole blood prior to centrifugation.
B. Properties of PCs
PRP is low in fibrin content, making growth factors trapped in the fibrin reach their peak concentrations on the first day.24 PRP-derived growth factors are available and active in the early stages of regeneration and reconstruction.24 The rapid release of active factors means that PRP is unsuitable for complex and long-term regeneration processes. On the other hand, PRF releases growth factors for up to 7 to 10 days and has the potential for use in complex and long-term regeneration contexts. As PRF lacks anticoagulants, the natural cascade of coagulation reactions is not impeded, and cross-contamination associated with anticoagulants is avoided. More importantly, naturally agglutinated PRF forms a strong fibrin matrix network that traps many cytokines and growth factors initially secreted by activated platelets, which are gradually released as fibrin degrades.25 This makes PRF attractive for longer-term applications than PRP.26 Unlike PRP and PRF, A-PRF forms a more porous fibrous scaffold that contains more neutrophils.17 Compared with PRP and PRF, A-PRF releases more growth factors within 10 days.27 In addition, horizontal centrifugation not only avoids cell damage during angle centrifugation but also enables free migration of cells, resulting in a uniform distribution of cells in H-PRF with higher platelets and leukocytes and more excellent antibacterial effects.28 Among the PCs, CGF is best suited for long-term regeneration contexts, as its relatively higher adhesive and tensile strength (compared to PRP and PRF) provides better protection against hydrolysis of growth factors trapped within its fibrin matrix.29 This explains why the release of growth factors from CGF has been shown to last up to 28 days.29 CGF also promotes angiogenesis by recruiting CD34-positive stem cells from circulating blood.30,31
C. Mechanisms of PCs for tissue repair
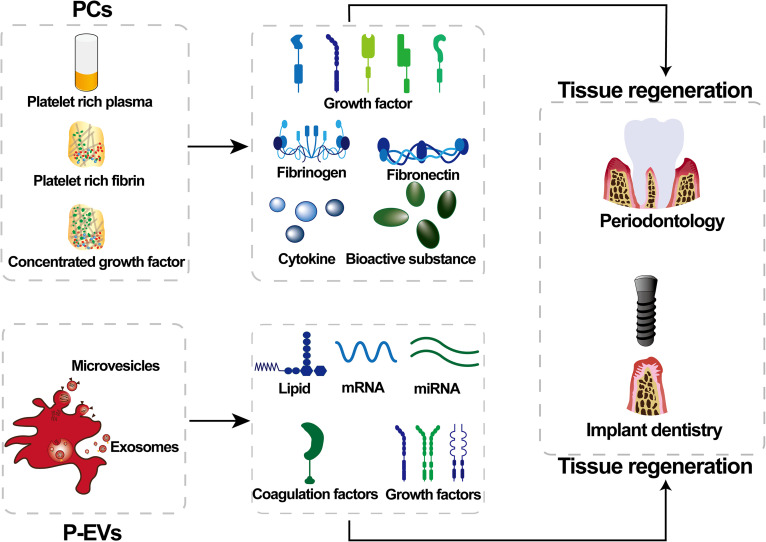
There are various mechanisms by which PCs can facilitate tissue repair. Platelets contain large amounts of alpha granules, major reservoirs of growth factors, and cytokines. Upon activation, platelets secrete transforming growth factor-beta, vascular endothelial growth factor (VEGF), epidermal growth factor, insulin-like growth factor-1, and bone morphogenetic protein, which act on target cells to promote cell proliferation and tissue repair.9,32 In addition, various bioactive substances, including serotonin, histamine, and adenosine, are released from dense granules.33 Fibrinogen and fibronectin within concentrated platelets form a flexible, progressively degradable protein scaffold that encases platelets, cytokines, and growth factors for gradual release, while the scaffold promotes cell adhesion, expansion, and differentiation properties through cell signaling, providing mechanical support for damaged tissue reconstruction.31,34 PCs also contain anti-inflammatory cytokines, including interleukin 1β, interleukin 4, interleukin 6, tumor necrosis factor α, and leukocytes, which play regulatory roles in inflammatory and antibacterial processes (Fig. 2).24
D. Platelet lysate (PL)
PLs, which can be obtained from PCs, are another source of growth factors useful for dental tissue repair and regeneration.35 The most common method of isolating PLs is to repeatedly freeze and thaw PCs after 3–5 freeze-thaw cycles of 80/37 °C.36 Ultrasonication of platelets at a frequency of 20 kHz for 30 min is an alternative method for the preparation of PL.37 Compared to PCs, PLs can be stored frozen until use, but its solution form barely forms a gel spontaneously. Processing liquid PL into gel allows for better performance at the surgical site. The primary method of gelation is the addition of thrombin to activate platelets, and there have also been studies of encapsulating PL into hydrogels or chemically cross-linking it with biomaterials.38
E. Platelet-derived extracellular vesicles (P-EVs)
In 1967, Peter Wolf isolated lipid-rich microparticles (MPs) from platelets by ultracentrifugation and discovered these MPs had procoagulant properties.39 Further studies revealed that these MPs are extracellular vesicles released by platelets upon activation (Fig. 1).40
PCs and PL are the sources of EVs, which can be isolated by differential ultracentrifugation. PRP is centrifuged at 2200 g for 20 min to collect the platelet pellet. The platelet pellet is resuspended and centrifuged at 4 °C at 4000 g for 10 min, 10 000 g for 30 min, and 100 000 g for 70 min to obtain P-EVs.41 Aside from that, centrifuging PL at 500 g for 10 min, 2000 g for 15 min, and 10 000 g for 30 min and then ultracentrifuge the supernatant at 30 000 rpm for 1 h is another method to obtain P-EVs.42
Based on the difference in diameter, there are two types of EVs, the first being MPs, also called microvesicles, and the second being platelet exosomes (P-EXOs). Microvesicles range from 100 nm to 1 µm in diameter, express surface markers such as Factor X and thrombospondin, and are formed by “budding” from the platelet membrane. P-EXOs are 40–100 nm in diameter and express the exosome-specific marker transmembrane protein CD63 on their surfaces. When the multivesicular bodies and alpha granules fuse with the platelet plasma membrane, the P-EXOs located therein are released by exocytosis.43 P-EVs are critical effectors for clotting with procoagulant capacity 50–100 times higher than that of activated platelets. Phosphatidylserine are adhesion receptors on the vesicle surface that provide binding sites for coagulation factor activation and thrombin production.44
Tissue repair studies have demonstrated that P-EVs specifically deliver to their targets bioactive molecules such as growth factors, lipids, coagulation factors, and mRNAs and microRNAs45 by explicitly recognizing membrane surface receptor-ligand proteins. These receptors mediate fusion with target cell membranes to regulate the translation of corresponding proteins, improve the physiological function of the target cells, and thereby promote tissue reconstruction.46 P-EVs stimulate endothelial cell proliferation and migration through VEGF, basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), and lipids to promote angiogenesis and significantly accelerated peri-implant angiogenesis was observed when P-EVs were filled around the implants.47 During hard tissue regeneration, especially osteogenesis, P-EVs enhance proliferation by increasing the incorporation of thymidine by osteoblasts, accelerating the mitogenic response of trabecular cells and promoting bone tissue mineralization and regeneration.48 P-EVs isolated from PL expressing bFGF, VEFG, and TGB-β, which are associated with bone healing and bone regeneration with a role in regulating osteoblast migration, proliferation, and differentiation.42 Concurrent findings suggest that miRNAs delivered by P-EVs can be involved in the transcription of intracellular signals and phosphorylation of tyrosine by regulating the cAMP, cGMP-PKG, and Rap1 signaling pathway, which, in turn, regulate osteoblast differentiation49 (Fig. 3).
FIG. 3.
Possible underlying mechanisms of platelet concentrates (PCs) and platelet-derived extracellular vesicles (P-EVs) for tissue regeneration in periodontology and implant dentistry.
In addition, to further improve the bioactivity of P-EVs, their presentation to recipient cells, and their ability to target binding at cell type-specific and tissue-specific levels, some studies have developed engineering P-EVs with the main engineering approaches being modifications of the membranes of extracellular vesicles. Platelet-mimetic EVs were fabricated by fusing the membranes of EVs with platelet membranes by extrusion. This engineering strategy offers an opportunity to design other targeted EVs fused with platelet membranes for therapeutic angiogenesis.50 The same team also introduced the platelet membrane-modified EVs based on the membrane fusion method to mimic the binding ability of platelets to monocytes. The new targeted delivery method of EVs to monocytes has the potential to improve immunoregulatory therapy.51
F. Potential for clinical applications
Currently, most clinical use PCs are prepared by simple centrifugation after fresh blood is drawn at the time of application. Although PL can be stored at 4 °C for 14 days and the clinical effect of PL is not worse than PC in clinical treatment, PL needs to be prepared by tedious freezing/thawing or ultrasound method.52 The complexity of the preparation process limits the application of PL in oral tissue regeneration to some extent. P-EVs are obtained by differentiated ultracentrifugation of PCs or PL. Although P-EVs’ clinical treatment is not as widespread as PC and PL, P-EVs as endogenous bio carriers can circulate to all human chambers and even the blood–brain barrier. Additionally, engineering P-EVs is a new approach to enhance the potential of P-EVs in biomedical applications such as targeted therapy toward angiogenesis.
III. CLINICAL APPLICATIONS OF PCs
A. Hard tissue regeneration
1. Sinus floor elevation
Maxillary sinus floor elevation is a dental surgical procedure that increases the amount of bone in the posterior maxilla. This procedure is done in preparation for, or in conjunction with, installation of dental implants because of bone atrophy or poor bone quality. Maxillary sinus floor augmentation is created by elevating the sinus membrane from the underlying sinus wall and by placing bone grafts to obtain sufficient bone volume to support the dental implants. As technology has evolved, various fillers have been used in order to ensure adequate new bone formation. For many years, autogenous bone grafts were considered the gold standard, but the grafting of autogenous bone inevitably caused damage to the donor site. This often-negative outcome has prompted the use of PCs to simplify the surgical procedure and avoid some complications.
Presently, there is still controversy about whether PRP alone can promote bone regeneration during sinus floor elevation. Some studies have concluded that no benefit was found with the addition of PRP to maxillary sinus floor elevation. However, it has also been shown that using PRP alone finds higher bone augmentation in sinus lifts.53,54 Aside from that, PRP can speed up healing after surgery and minimize complications associated with residual graft material.55 When PRP is used in combination with autologous bone or bone-graft material, it not only shortens the healing time but also accelerates bone regeneration through vascularization and enhances bone formation in the elevated maxillary sinus floor.56–59 Several studies have shown that the use of PRF or CGF alone, without mixing with bone material as a maxillary sinus lift-filling material, and immediate implant placement, can promote new bone formation and produce dense bone-like tissue.60–66 When implant surgery is performed immediately after maxillary sinus lift, combining PCs with bone material increases the bone volume at the implant margin and improves the survival and long-term stability of the implant.22,67,68 PRF and CGF can also provide protection for the sinus membrane during the use of bone chisels. Even in the case of sinus membrane perforation, the fibrin matrix can help with wound closure69–71 (Table I).
TABLE I.
Applications of PCs and P-EVs in oral hard tissue regeneration.
| Material | Comparisons | Effect of PCs | Reference |
|---|---|---|---|
| Maxillary sinus floor elevation | |||
| PRP | PRP | Leads to steady increase. | 53 |
| PRP vs alloplastic graft material | 54 | ||
| P-PRP + freeze-dried bone allograft (FDBA) vs FDBA | Shortens healing time. | 55 | |
| L-PRP + composite bone graft | When combined with PRP, new bone is formed. | 56 | |
| PRP + autologous bone grafts vs autologous bone grafts | 57 | ||
| PRP + iliac crest bone vs iliac crest bone | 58 | ||
| L-PRF + hydroxyapatite | 59 | ||
| PRF | PRF | Leads to endosinus bone gain. | 60–63 |
| PRF + deproteinized bovine bone mineral | Increases vertical bone height and stabilization. | 22, 68 | |
| L-PRF | Repair maxillary sinus membrane perforation. | 69, 70 | |
| A-PRF vs collagen membrane | 71 | ||
| CGF | CGF | Induces new bone formation under the elevated sinus membrane with vertical bone gain. | 64–66 |
| CGF + grafted with allograft vs grafted with allograft | Obtains vertical bone height stabilization. | 67 | |
| Alveolar ridge preservation | |||
| PRP | PRP | Reduces inflammation, promotes soft tissue healing. | 73, 74 |
| PRF | PRF vs natural healing | Promotes the healing of soft and bone tissues. reduces pain and discomfort. | 76–78 |
| PRF vs bone allografts | 79 | ||
| CGF | CGF vs natural healing | Reduces vertical and horizontal bone resorption and promotes new bone formation. | 80–82 |
| Guided bone regeneration (GBR) | |||
| PRP | PRP | Beneficial in the early healing phase of soft tissue wounds. No significant effect on bone height changes. | 85–89 |
| PRF | PRF | Improves implant stability, implant survival and marginal bone level. | 90, 91 |
| PRF + autogenous and xenogenous grafts + collagen membrane | 94 | ||
| PRF + autogenous bone + bovine inorganic bone graft | 95 | ||
| CGF | CGF | Promotes horizontal and vertical bone regeneration. | 31 |
| CGF vs collagen membrane | 96 | ||
| CGF + mineralized collagen vs mineralized collagen | 97 | ||
| CGF + bone graft matrix vs bone-shell technique | 98 | ||
| P-EVs | P-EVs | Promotes osteogenic differentiation. | 99, 100 |
| Periodontal intrabony defects repair | |||
| PRP | PRP + demineralized freeze-dried bone allograft vs demineralized freeze-dried bone allograft | Increases CAL and improves gingival recession, but no effect on the gain of hard tissue filling or new hard tissue formation. | 101 |
| PRP + bone mineral + GTR (guided tissue regeneration) vs bone mineral + GTR | 102 | ||
| PRP + β-TCP + GTR vs β-TCP + GTR | 103 | ||
| PRP + demineralized freeze-dried bone allograft vs demineralized freeze-dried bone allograft | 104 | ||
| PRF | PRF vs OFD | Reduces the bone defect depth and increases bone fill. | 108 |
| PRP + OFD vs OFD | 109 | ||
| PRF+ OFD vs autogenous bone graft + OFD | 110 | ||
| PRF+ freeze-dried bone allograft vs freeze-dried bone allograft | Combination of PRF is significantly beneficial to clinical defect depth reduction and defect filling. | 111 | |
| PRF vs PRF + Bovine porous bone mineral | 112 | ||
| PRF + bovine porous bone mineral + GTR vs bovine porous bone mineral + GTR | 113 | ||
| CGF | CGF | Reduces PD, increases CAL and bone level height, and fills bone defects. | 115 |
| CGF vs CGF +demineralized freeze-dried bone allograft | 116 | ||
| CGF + bovine bone mineral vs bovine bone mineral | Obtains better bone defect repair results. | 117 | |
| Flap surgery vs flap surgery + CGF vs flap surgery + Bio-Oss vs flap surgery + CGF + Bio-Oss | 118 | ||
2. Alveolar ridge preservation
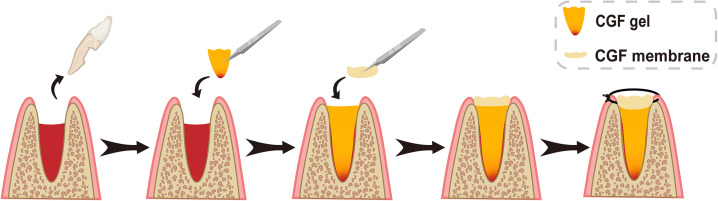
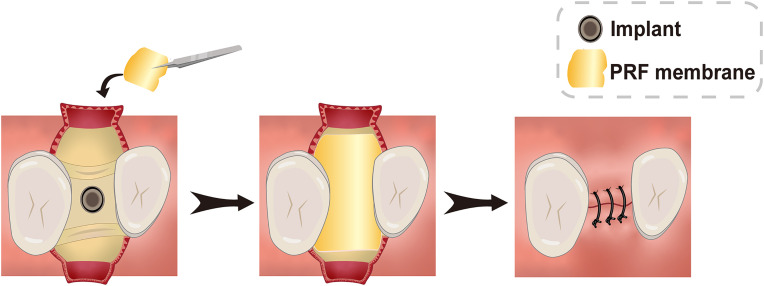
Alveolar ridge preservation is the process of filling the post-extraction socket with some bone material or scaffold to reduce bone resorption after extraction, preserve good alveolar ridge structure and soft tissue morphology, and ensure sufficient space for implant placement.72 After tooth extraction, filling with PRP can relieve pain and swelling, prevent food build-up, reduce oral odor, and to some extent promote the healing of hard and soft tissues.73,74 However, PRP has a low mechanical strength and is not easy to use it to fill sockets.75 PRF, on the other hand, possesses much better mechanical strength, and when used alone, it not only accelerates soft tissue wound healing but also reduces the resorption of alveolar ridge bone height and width. PRF also enhances bone density, as evidenced by both clinical results and radiographic measurements.76–79 CGF application following posterior tooth extraction may reduce vertical and horizontal bone resorption and promote new bone formation (Fig. 4).80 However, the use of CGF alone for alveolar ridge preservation is currently rare, and more data are needed to demonstrate that CGF used to fill extraction sockets significantly changes the quality of bone tissue81,82 (Table I).
FIG. 4.
Schematic diagram of the surgical process of CGF applied to alveolar ridge preservation after tooth extraction (sagittal view).
3. Guided bone regeneration (GBR)
Osseointegration after implant placement is the basis for successful implantation. Osseointegration after implant placement is the basis for successful implantation. One of the key factors for attaining osseointegration is the presence of an adequate osseous volume. The GBR technique is commonly used for bone augmentation in implant dentistry today. The critical issue with the GBR technique is the placement of an occlusive membrane that will prevent connective tissue cells from colonizing the defect and provide enough space to allow for bone regeneration of the entire defect volume.83,84
How do PC products contribute to this whole process? Only a transient and slight beneficial effect on early osseointegration was observed after the implant surface was treated with activated PRP impregnation prior to implant placement. During implantation, PRP liquid mixed with bone material and applied to the implant socket was less important for long-term survival of the implant.85–89 When GBR is used for dental implants, PRF and CGF can be compressed like a membrane for mineralizing blood clots while also preventing excess cells from entering the area of osseointegration and providing space for the migration of osteoblasts and angiogenic cells.90,91 The use of PRF and CGF improves the stability of the implant, provides for rapid osseointegration, and enhances the integration of the implant and the marginal bone volume around the implant.31,92–98
P-EVs are intrinsically osteoinductive and can induce osteogenic differentiation of stem cells in the absence of any external induction.99 Some have attempted to coat the surface of titanium implants with P-EVs, which improves the biocompatibility of titanium. It is gradually released over time, in the hope of improving the osteogenic properties of titanium implants100 (Table I).
4. Periodontal intrabony defects repair
Periodontal disease is a chronic inflammatory condition in which horizontal and vertical resorption of the alveolar bone results in varying degrees of intra-periodontal bone defects. These can ultimately result in loss of tooth support structures and loosening of teeth. Treatment of periodontal bone defects requires enhanced regeneration of the defective bone to maintain normal occlusal function. Currently, open flap debridement (OFD), GBR, and the use of grafts such as enamel matrix derivatives or bone material are currently used for periodontal intraosseous defects (IBDs) in order to promote regeneration.
For the repair of dental intraosseous defects, it is important to gain clinical attachment level (CAL) and gingival marginal level (GML) in addition to probing depth (PD) reduction. However, a reduction in the depth of the intraosseous defect (IBD) and an increase in bone fill (BF) are more important. For regeneration of intraosseous defects, at least four to six weeks are required to guide bone regeneration in order to block the epithelium. PRP is sometimes used to treat periodontal intraosseous defects. However, due to the rapid degradation of PRP, its beneficial effect on hard tissues is limited when it is used alone to treat periodontal intraosseous defects. Combining grafts with PRP or applying PRP in an OFD arrangement results in better soft tissue performance such as in the case of CAL gain and PD reduction in periodontal bone defects but is not better for hard tissue filling or new hard tissue formation.101–105
Studies show that in addition to the role of growth factors in periodontal bone defects, fibronectin in PRF also promotes the formation of new blood vessels and the conversion of undifferentiated mesenchymal cells in the blood into osteoblasts.106,107 Not only does the combined use of PRF achieve better CAL gain and PD reduction compared to OFD surgery alone, a superior reduction in the depth of the intraosseous defect is achieved, and an increase in bone filling is observed both clinically and radiologically.108–110 When PRF is used in combination with bone substitutes, better clinical outcomes result than with PRF alone.111–113 CGF still has a pro-osteogenic effect on human periodontal ligament cells in an inflammatory microenvironment that mimics periodontitis.114 When used alone, CGF reduces PD, enhances CAL, and good bone regeneration is achieved.115,116 This effect is even more pronounced when used in combination with bone graft material117,118 (Table I).
B. Soft tissue regeneration
1. Treatment of gingival recession
Gingival recession is one of the main symptoms of periodontitis, which can lead to extensive root exposure and tooth sensitivity. The main root-coverage procedures currently used to treat gingival recession are coronally advanced flap (CAF) and subepithelial connective tissue grafts (SCTGs).
In the case of gingival recession, attempts have been made to use PRP and PRF alone or combine with CAF or SCTG in Miller classes I and II gingival recessions. The application of PRP and PRF promotes early soft tissue healing and improves root coverage, depth of the gingival recession, and CAL.119–125 For gingival recession applications, PRF has advantages over PRP in liquid or gel form, as PRF can be compressed into a membrane and used alone as a barrier membrane to guide tissue regeneration to cover the surface of the exposed tooth root. Gingival fibroblasts are a significant component of gingival connective tissue. PRP and PRF promote the migration and proliferation of gingival fibroblasts and the expression of type I collagen by human gingival fibroblasts.126 However, some studies have concluded that PRP and PRF have very limited efficacy for improving the width of keratinized tissue, and only CGF films can significantly improve keratinized tissue.127–129
P-EVs are biocompatible in vitro systems, promote regeneration of gingival keratinocytes and granulocytes, and express genes related to gingival remodeling. P-EVs can also be combined with biomaterials such as hyaluronic acid to help maintain their regenerative effect while research seeks more applicable treatments130 (Table II).
TABLE II.
Applications of PCs and P-EVs in oral soft tissue regeneration.
| Material | Comparisons | Effect of PCs | References | |
|---|---|---|---|---|
| Treatment of gingival recession | ||||
| PRP | PRP + CTG vs CTG | Additional application of PRP reduces vertical recession depth and PD, improves CAL. | 119 | |
| PRP + CAF | 120 | |||
| PRP + CTG | 121 | |||
| PRF | PRF + CAF | Significantly improves PD and CAL, increases gingival thickness and improves root coverage. | 122 | |
| PRF + CAF vs CAF | 123, 124 | |||
| PRF + CAF vs CAF + CTG | 125 | |||
| CGF | CGF vs CTG + CAF | Increases the width of keratinized tissue | 127 | |
| CGF + CAF vs CAF | 128 | |||
| CGF + CAF vs PRF + CAF | 129 | |||
| P-EVs | P-EVs | Promotes regeneration of gingival keratinocytes and granulocytes, enhances gene expression during gingival healing. | 130 | |
| Periodontal soft tissue regeneration | ||||
| PRP | PRP + OFD | Combining with PRP increases CAL, reduces PD. | 131, 132 | |
| PRP + bone mineral + GTR vs bone mineral + GTR | 102 | |||
| PRP + β-TCP + GTR vs β-TCP + GTR | 103 | |||
| PRP vs PRP + collagen sponge | 134 | |||
| PRF | PRF + OFD vs OFD | Increases CAL, reduces PD. | 135 | |
| PRF + demineralized freeze-dried bone allograft vs demineralized freeze-dried bone allograft | 136 | |||
| PRF gel vs PRF gel + PRF membrane vs OFD | PRF alone promotes PD reduction and CAL gain. | 137 | ||
| PRF vs OFD | ||||
| 138 | ||||
| CGF | CGF | CGF alone promotes PD reduction, CAL gain. | 115 | |
| CGF vs CGF + demineralized freeze-dried bone allograft | ||||
| 116 | ||||
| P-EVs | P-EVs | Promotes angiogenesis, epithelial formation and wound healing. | 13, 142 | |
| Soft tissue augmentation around implants | ||||
| PRP | PRP | Promotes wound healing after implantation. | 89, 144 | |
| PRF | PRF | Increases peri-implant soft tissue thickness and keratinized tissue width. | 145 | |
| PRF vs CTG | 146 | |||
| PRF vs OFD | 147 | |||
| PRF vs free gingival graft | 148 | |||
| PRF | PRF | Almost negligible effect on thickening of the tissue around the neck implant. | 93, 149 | |
2. Periodontal soft tissue regeneration
Application of PCs provide various growth factors when used for regenerating periodontal soft tissues in the treatment of periodontal disease. PDGF can recruit progenitor cells from periodontal tissues, promote cell proliferation and migration and play an important role in periodontal healing. bFGF effectively promotes the proliferation and migration of periodontal membrane fibroblasts. VEGF recruits progenitor cells to periodontal wounds, promotes endothelial cell proliferation, and contributes to angiogenesis and hematopoietic reconstitution of periodontal tissue. Angiogenesis in this context plays a key role in mitigating the pathological process and in the regeneration of periodontal tissue. Several studies have found that beta-lysin and neutrophil activating protein 2 in PCs are anti-microbial.22
In restoring periodontal soft tissues, the use of PRP after open flap debridement (OFD) produced a higher CAL than OFD alone.131,132 The outcome is even better when combined with a guided tissue regeneration membrane.133 When applied in combination with bone material to treat periodontal defects, it has shown a positive clinical impact in terms of soft tissue defects in the periodontium.102,103,134 In treating periodontal soft tissue in combination with OFD, guided tissue regeneration (GTR), and bone graft material, PRF and CGF enhance attachment and reduce the depth of clinically treated periodontal pockets.116,135,136 PD reduction and CAL gain were also obtained with PRF or CGF alone.115,137,138 However, PRF and CGF are more likely to fill the defect in the form of a membrane, which not only stabilizes the blood clot for periodontal regeneration, but also acts as a competitive barrier, one that both blocks epithelial cell and facilitates the growth of the periodontal ligament into the defective area.139 More importantly, PRF and CGF have a significant regenerative effect on fixing defects within periodontal bone, providing attachment sites for new periodontal ligament fibers.140
Platelet lysate- (PL)-based materials can promote soft tissue healing in periodontal applications,38,141 and P-EVs are important effectors of PL activity.42 P-EVs facilitate wound healing by promoting the proliferation and migration of fibroblasts, which, in turn, stimulates re-epithelialization and angiogenesis. This suggests that P-EVs may be used in the clinic to promote periodontal tissue healing and regeneration13,142 (Table II).
3. Soft tissue augmentation around implants
Increasing the width of the peri-implant keratinized tissue and mucosal thickness can effectively improve the functional, esthetic, and biological outcome parameters after implant placement.143 Currently used surgical procedures for increasing the width of keratinized gingiva and mucosal thickness include apically positioned flap with vestibuloplasty or simultaneous use of autologous tissue or collagen matrix.
PRP facilitates early wound healing in soft tissues when used in implant surgery, but it is not effective for peri-implant soft tissue regeneration.89,144 Implant placement combined with PRF results in an increase in the thickness of the soft tissue around the implant (Fig. 5).145–148 However, the ability of PRF to increase the peri-implant tissue remains controversial.93,149 Although CGF has a strong effect on wound healing, most current studies have focused on the effectiveness of CGF in promoting bone regeneration around implants. More in-depth studies are needed to determine whether CGF has demonstrated capacity to augment soft tissues around implants (Table II).
FIG. 5.
Schematic illustration of the surgical procedure for thickening of peri-implant mucosa with PRF membrane (occlusal view).
IV. CONCLUSION AND PERSPECTIVES
PCs and P-EVs are safe, reliable products for bone augmentation and soft tissue augmentation. Originating from standard autologous blood collection procedures, they are easy to prepare and have rich and extensive applications. However, there are still no uniform standards in place for the preparation of PCs and P-EVs. Nor, is it completely understood yet how a patient’s systemic health affects their active ingredients. This latter gap in knowledge may partly explain the reported variability in their use for implant surgery and periodontal tissue regeneration. However, we still believe that they have potential and that there is an urgent need to develop uniform industry standards and clinical guidelines for their clinical use aimed to enhance tissue repair. Large randomized, controlled clinical trials in which patients’ systemic health status is documented are warranted to rigorously evaluate the potential of uniformly prepared PC products in regenerative periodontological and implant dentistry applications.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the support of the National Natural Science Foundation of China under Grant Nos. 81800935, 82071160, 81870806, and 81974152.
Note: This paper is part of the special issue on Drug/Gene Delivery and Theranostics.
Contributor Information
Derong Zou, Email: mailto:drzou@sjtu.edu.cn.
Jiayu Lu, Email: mailto:angelinelu@sjtu.edu.cn.
Chengqi Lyu, Email: mailto:loner_lcq@alumni.sjtu.edu.cn.
AUTHOR DECLARATIONS
Conflict of Interest
The authors have no conflicts to disclose.
Ethics Approval
Ethics approval is not required.
Author Contributions
Jiayue Sun and Yinghan Hu have contributed equally to this work and share first authorship.
Jiayue Sun: Data curation (equal); Formal analysis (equal); Writing – original draft (lead). Yinghan Hu: Data curation (equal); Formal analysis (equal); Methodology (equal); Visualization (lead). Yinxin Fu: Data curation (equal); Formal analysis (equal); Methodology (equal). Derong Zou: Conceptualization (equal); Funding acquisition (equal); Supervision (equal). Jiayu Lu: Conceptualization (equal); Data curation (equal); Funding acquisition (equal); Supervision (equal); Writing – review and editing (equal). Chengqi Lyu: Conceptualization (lead); Data curation (equal); Funding acquisition (equal); Project administration (lead); Supervision (equal); Writing – review and editing (lead).
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Fretwurst T., Nack C., Al-Ghrairi M., Raguse J. D., Stricker A., Schmelzeisen R. et al. , “ Long-term retrospective evaluation of the peri-implant bone level in onlay grafted patients with iliac bone from the anterior superior iliac crest,” J Cranio-Maxillofac. Surg. 43, 956–960 (2015). 10.1016/j.jcms.2015.03.037 [DOI] [PubMed] [Google Scholar]
- 2. Souza A. B., Tormena M., Matarazzo F., and Araujo M. G., “ The influence of peri-implant keratinized mucosa on brushing discomfort and peri-implant tissue health,” Clin. Oral Implants Res. 27, 650–655 (2016). 10.1111/clr.12703 [DOI] [PubMed] [Google Scholar]
- 3. Tavelli L., Barootchi S., Avila-Ortiz G., Urban I. A., Giannobile W. V., and Wang H. L., “ Peri-implant soft tissue phenotype modification and its impact on peri-implant health: A systematic review and network meta-analysis,” J. Periodontol. 92, 21–44 (2021). 10.1002/JPER.19-0716 [DOI] [PubMed] [Google Scholar]
- 4. Tolstunov L., Hamrick J. F. E., Broumand V., Shilo D., and Rachmiel A., “ Bone augmentation techniques for horizontal and vertical alveolar ridge deficiency in oral implantology,” Oral Maxillofac. Surg. Clin. North Am. 31, 163–191 (2019). 10.1016/j.coms.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 5. Nurden A. T., Nurden P., Sanchez M., Andia I., and Anitua E., “ Platelets and wound healing,” Front Biosci. 13, 3532–3548 (2008). 10.2741/2947 [DOI] [PubMed] [Google Scholar]
- 6. Leslie M., “ Cell biology. Beyond clotting: The powers of platelets,” Science 328, 562–564 (2010). 10.1126/science.328.5978.562 [DOI] [PubMed] [Google Scholar]
- 7. Dohan Ehrenfest D. M., Rasmusson L., and Albrektsson T., “ Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF),” Trends Biotechnol. 27, 158–167 (2009). 10.1016/j.tibtech.2008.11.009 [DOI] [PubMed] [Google Scholar]
- 8. Dohan D. M., Choukroun J., Diss A., Dohan S. L., Dohan A. J., Mouhyi J. et al. , “ Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates?,” Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 101, E51–E55 (2006). 10.1016/j.tripleo.2005.07.010 [DOI] [PubMed] [Google Scholar]
- 9. Everts P., Onishi K., Jayaram P., Lana J. F., and Mautner K., “ Platelet-rich plasma: New performance understandings and therapeutic considerations in 2020,” Int. J. Mol. Sci. 21, 7794 (2020). 10.3390/ijms21207794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Niel G., D'Angelo G., and Raposo G., “ Shedding light on the cell biology of extracellular vesicles,” Nat. Rev. Mol. Cell Biol. 19, 213–228 (2018). 10.1038/nrm.2017.125 [DOI] [PubMed] [Google Scholar]
- 11. Puhm F., Boilard E., and Machlus K. R., “ Platelet extracellular vesicles: Beyond the blood,” Arterioscler., Thromb., Vasc. Biol. 41, 87–96 (2021). 10.1161/ATVBAHA.120.314644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Antich-Rossello M., Forteza-Genestra M. A., Monjo M., and Ramis J. M., “ Platelet-derived extracellular vesicles for regenerative medicine,” Int. J. Mol. Sci. 22, 8580 (2021). 10.3390/ijms22168580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu N., Wang L., Guan J., Tang C., He N., Zhang W. et al. , “ Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model,” Int. J. Biol. Macromol. 117, 102–107 (2018). 10.1016/j.ijbiomac.2018.05.066 [DOI] [PubMed] [Google Scholar]
- 14. Hesseler M. J. and Shyam N., “ Platelet-rich plasma and its utility in medical dermatology: A systematic review,” J. Am. Acad. Dermatol. 81, 834–846 (2019). 10.1016/j.jaad.2019.04.037 [DOI] [PubMed] [Google Scholar]
- 15. Yin W. J., Xu H. T., Sheng J. G., Zhu Z. Z., Jin D. X., Hsu P. C. et al. , “ Optimization of pure platelet-rich plasma preparation: A comparative study of pure platelet-rich plasma obtained using different centrifugal conditions in a single-donor model,” Exp. Ther. Med. 14, 2060–2070 (2017). 10.3892/etm.2017.4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dohan D. M., Choukroun J., Diss A., Dohan S. L., Dohan A. J., Mouhyi J. et al. , “ Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution,” Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 101, e37–e44 (2006). 10.1016/j.tripleo.2005.07.008 [DOI] [PubMed] [Google Scholar]
- 17. Ghanaati S., Booms P., Orlowska A., Kubesch A., Lorenz J., Rutkowski J. et al. , “ Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells,” J. Oral Implantol. 40, 679–689 (2014). 10.1563/aaid-joi-D-14-00138 [DOI] [PubMed] [Google Scholar]
- 18. Fujioka-Kobayashi M., Miron R. J., Hernandez M., Kandalam U., Zhang Y. F., and Choukroun J., “ Optimized platelet-rich fibrin with the low-speed concept: Growth factor release, biocompatibility, and cellular response,” J. Periodontol. 88, 112–121 (2017). 10.1902/jop.2016.160443 [DOI] [PubMed] [Google Scholar]
- 19. Tunali M., Ozdemir H., Kucukodaci Z., Akman S., and Firatli E., “ In vivo evaluation of titanium-prepared platelet-rich fibrin (T-PRF): A new platelet concentrate,” Br. J. Oral Maxillofac. Surg. 51, 438–443 (2013). 10.1016/j.bjoms.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 20. Miron R. J., Chai J., Zheng S., Feng M., Sculean A., and Zhang Y., “ A novel method for evaluating and quantifying cell types in platelet rich fibrin and an introduction to horizontal centrifugation,” J. Biomed. Mater. Res., Part A 107, 2257–2271 (2019). 10.1002/jbm.a.36734 [DOI] [PubMed] [Google Scholar]
- 21. Miron R. J., Fujioka-Kobayashi M., Hernandez M., Kandalam U., Zhang Y., Ghanaati S. et al. , “ Injectable platelet rich fibrin (i-PRF): Opportunities in regenerative dentistry?,” Clin. Oral Investig. 21, 2619–2627 (2017). 10.1007/s00784-017-2063-9 [DOI] [PubMed] [Google Scholar]
- 22. Barbu H. M., Andreescu C. F., Comaneanu M. R., Referendaru D., and Mijiritsky E., “ Maxillary sinus floor augmentation to enable one-stage implant placement by using bovine bone substitute and platelet-rich fibrin,” BioMed Res. Int. 2018, 6562958. 10.1155/2018/6562958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yilmaz O., Ozmeric A., Alemdaroglu K. B., Celepli P., Hucumenoglu S., and Sahin O., “ Effects of concentrated growth factors (CGF) on the quality of the induced membrane in Masquelet’s technique: An experimental study in rabbits,” Injury 49, 1497–1503 (2018). 10.1016/j.injury.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 24. Oryan A., Alidadi S., and Moshiri A., “ Platelet-rich plasma for bone healing and regeneration,” Expert Opin. Biol. Ther. 16, 213–232 (2016). 10.1517/14712598.2016.1118458 [DOI] [PubMed] [Google Scholar]
- 25. Dohan D. M., Choukroun J., Diss A., Dohan S. L., Dohan A. J., Mouhyi J. et al. , “ Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features,” Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 101, e45–e50 (2006). 10.1016/j.tripleo.2005.07.009 [DOI] [PubMed] [Google Scholar]
- 26. Kobayashi E., Fluckiger L., Fujioka-Kobayashi M., Sawada K., Sculean A., Schaller B. et al. , “ Comparative release of growth factors from PRP, PRF, and advanced-PRF,” Clin. Oral Investig. 20, 2353–2360 (2016). 10.1007/s00784-016-1719-1 [DOI] [PubMed] [Google Scholar]
- 27. Masuki H., Okudera T., Watanebe T., Suzuki M., Nishiyama K., Okudera H. et al. , “ Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF),” Int. J. Implant Dent. 2, 19 (2016). 10.1186/s40729-016-0052-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feng M., Wang Y., Zhang P., Zhao Q., Yu S., Shen K. et al. , “ Antibacterial effects of platelet-rich fibrin produced by horizontal centrifugation,” Int. J. Oral Sci. 12, 32 (2020). 10.1038/s41368-020-00099-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu M., Wang X., Liu Y., and Qiao J., “ Cytokine release kinetics of concentrated growth factors in different scaffolds,” Clin. Oral Investig. 23, 1663–1671 (2019). 10.1007/s00784-018-2582-z [DOI] [PubMed] [Google Scholar]
- 30. Majka M., Janowska-Wieczorek A., Ratajczak J., Ehrenman K., Pietrzkowski Z., Kowalska M. A. et al. , “ Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner,” Blood 97, 3075–3085 (2001). 10.1182/blood.V97.10.3075 [DOI] [PubMed] [Google Scholar]
- 31. Pirpir C., Yilmaz O., Candirli C., and Balaban E., “ Evaluation of effectiveness of concentrated growth factor on osseointegration,” Int. J. Implant Dent. 3, 7 (2017). 10.1186/s40729-017-0069-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harrison P. and Cramer E. M., “ Platelet alpha-granules,” Blood Rev. 7, 52–62 (1993). 10.1016/0268-960X(93)90024-X [DOI] [PubMed] [Google Scholar]
- 33. Ambrosio A. L. and Di Pietro S. M., “ Storage pool diseases illuminate platelet dense granule biogenesis,” Platelets 28, 138–146 (2017). 10.1080/09537104.2016.1243789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wolberg A. S., “ Thrombin generation and fibrin clot structure,” Blood Rev. 21, 131–142 (2007). 10.1016/j.blre.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 35. Schallmoser K., Henschler R., Gabriel C., Koh M. B. C., and Burnouf T., “ Production and quality requirements of human platelet lysate: A position statement from the working party on cellular therapies of the International Society of Blood Transfusion,” Trends Biotechnol. 38, 13–23 (2020). 10.1016/j.tibtech.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 36. Burnouf T., Strunk D., Koh M. B. C., and Schallmoser K., “ Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation?,” Biomaterials 76, 371–387 (2016). 10.1016/j.biomaterials.2015.10.065 [DOI] [PubMed] [Google Scholar]
- 37. Bernardi M., Albiero E., Alghisi A., Chieregato K., Lievore C., Madeo D. et al. , “ Production of human platelet lysate by use of ultrasound for ex vivo expansion of human bone marrow-derived mesenchymal stromal cells,” Cytotherapy 15, 920–929 (2013). 10.1016/j.jcyt.2013.01.219 [DOI] [PubMed] [Google Scholar]
- 38. Babo P. S., Reis R. L., and Gomes M. E., “ Periodontal tissue engineering: Current strategies and the role of platelet rich hemoderivatives,” J. Mater. Chem. B 5, 3617–3628 (2017). 10.1039/C7TB00010C [DOI] [PubMed] [Google Scholar]
- 39. Wolf P., “ The nature and significance of platelet products in human plasma,” Br. J. Haematol. 13, 269–288 (1967). 10.1111/j.1365-2141.1967.tb08741.x [DOI] [PubMed] [Google Scholar]
- 40. Aatonen M. T., Ohman T., Nyman T. A., Laitinen S., Gronholm M., and Siljander P. R., “ Isolation and characterization of platelet-derived extracellular vesicles,” J. Extracell. Vesicles 3, 24692 (2014). 10.3402/jev.v3.24692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saumell-Esnaola M., Delgado D., Garcia Del Cano G., Beitia M., Salles J., Gonzalez-Burguera I. et al. , “ Isolation of platelet-derived exosomes from human platelet-rich plasma: Biochemical and morphological characterization,” Int. J. Mol. Sci. 23, 2861 (2022). 10.3390/ijms23052861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Torreggiani E., Perut F., Roncuzzi L., Zini N., Baglio S. R., and Baldini N., “ Exosomes: Novel effectors of human platelet lysate activity,” Eur. Cell Mater. 28, 137–151 (2014). 10.22203/eCM.v028a11 [DOI] [PubMed] [Google Scholar]
- 43. Heijnen H. F. G., Schiel A. E., Fijnheer R., Geuze H. J., and Sixma J. J., “ Activated platelets release two types of membrane vesicles: Microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules,” Blood 94, 3791–3799 (1999). 10.1182/blood.V94.11.3791 [DOI] [PubMed] [Google Scholar]
- 44. Caby M. P., Lankar D., Vincendeau-Scherrer C., Raposo G., and Bonnerot C., “ Exosomal-like vesicles are present in human blood plasma,” Int. Immunol. 17, 879–887 (2005). 10.1093/intimm/dxh267 [DOI] [PubMed] [Google Scholar]
- 45. Hunter M. P., Ismail N., Zhang X., Aguda B. D., Lee E. J., Yu L. et al. , “ Detection of microRNA expression in human peripheral blood microvesicles,” PLoS One 3, e3694 (2008). 10.1371/journal.pone.0003694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van der Meijden P. E. J. and Heemskerk J. W. M., “ Platelet biology and functions: New concepts and clinical perspectives,” Nat. Rev. Cardiol. 16, 166–179 (2019). 10.1038/s41569-018-0110-0 [DOI] [PubMed] [Google Scholar]
- 47. Moest T., Koehler F., Prechtl C., Schmitt C., Watzek G., and Schlegel K. A., “ Bone formation in peri-implant defects grafted with microparticles: A pilot animal experimental study,” J. Clin. Periodontol. 41, 990–998 (2014). 10.1111/jcpe.12295 [DOI] [PubMed] [Google Scholar]
- 48. Gruber R., Varga F., Fischer M. B., and Watzek G., “ Platelets stimulate proliferation of bone cells: Involvement of platelet-derived growth factor, microparticles and membranes,” Clin. Oral Implants Res. 13, 529–535 (2002). 10.1034/j.1600-0501.2002.130513.x [DOI] [PubMed] [Google Scholar]
- 49. Ferreira M. R. and Zambuzzi W. F., “ Platelet microparticles load a repertory of miRNAs programmed to drive osteogenic phenotype,” J. Biomed. Mater. Res., Part A 109, 1502–1511 (2021). 10.1002/jbm.a.37140 [DOI] [PubMed] [Google Scholar]
- 50. Li Q., Song Y., Wang Q., Chen J., Gao J., Tan H. et al. , “ Engineering extracellular vesicles with platelet membranes fusion enhanced targeted therapeutic angiogenesis in a mouse model of myocardial ischemia reperfusion,” Theranostics 11, 3916–3931 (2021). 10.7150/thno.52496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li Q., Huang Z., Wang Q., Gao J., Chen J., Tan H. et al. , “ Targeted immunomodulation therapy for cardiac repair by platelet membrane engineering extracellular vesicles via hitching peripheral monocytes,” Biomaterials 284, 121529 (2022). 10.1016/j.biomaterials.2022.121529 [DOI] [PubMed] [Google Scholar]
- 52. Astori G., Amati E., Bambi F., Bernardi M., Chieregato K., Schäfer R. et al. , “ Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: Present and future,” Stem Cell Res. Ther. 7, 93 (2016). 10.1186/s13287-016-0352-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Anitua E., Flores J., and Alkhraisat M. H., “ Transcrestal sinus lift using platelet concentrates in association to short implant placement: A retrospective study of augmented bone height remodeling,” Clin. Implant Dent. Relat. Res. 18, 993–1002 (2016). 10.1111/cid.12383 [DOI] [PubMed] [Google Scholar]
- 54. Steigmann M. and Garg A. K., “ A comparative study of bilateral sinus lifts performed with platelet-rich plasma alone versus alloplastic graft material reconstituted with blood,” Implant Dent. 14, 261–266 (2005). 10.1097/01.id.0000177412.84225.05 [DOI] [PubMed] [Google Scholar]
- 55. Kassolis J. D. and Reynolds M. A., “ Evaluation of the adjunctive benefits of platelet-rich plasma in subantral sinus augmentation,” J. Craniofac. Surg. 16, 280–287 (2005). 10.1097/00001665-200503000-00015 [DOI] [PubMed] [Google Scholar]
- 56. Mazor Z., Peleg M., Garg A. K., and Luboshitz J., “ Platelet-rich plasma for bone graft enhancement in sinus floor augmentation with simultaneous implant placement: Patient series study,” Implant Dent. 13, 65–72 (2004). 10.1097/01.ID.0000116454.97671.40 [DOI] [PubMed] [Google Scholar]
- 57. Torres J., Tamimi F., Martinez P. P., Alkhraisat M. H., Linares R., Hernandez G. et al. , “ Effect of platelet-rich plasma on sinus lifting: A randomized-controlled clinical trial,” J. Clin. Periodontol. 36, 677–687 (2009). 10.1111/j.1600-051X.2009.01437.x [DOI] [PubMed] [Google Scholar]
- 58. Bettega G., Brun J. P., Boutonnat J., Cracowski J. L., Quesada J. L., Hegelhofer H. et al. , “ Autologous platelet concentrates for bone graft enhancement in sinus lift procedure,” Transfusion 49, 779–785 (2009). 10.1111/j.1537-2995.2008.02036.x [DOI] [PubMed] [Google Scholar]
- 59. Poeschl P. W., Ziya-Ghazvini F., Schicho K., Buchta C., Moser D., Seemann R. et al. , “ Application of platelet-rich plasma for enhanced bone regeneration in grafted sinus,” J. Oral Maxillofac. Surg. 70, 657–664 (2012). 10.1016/j.joms.2011.04.027 [DOI] [PubMed] [Google Scholar]
- 60. Kanayama T., Horii K., Senga Y., and Shibuya Y., “ Crestal approach to sinus floor elevation for atrophic maxilla using platelet-rich fibrin as the only grafting material: A 1-year prospective study,” Implant Dent. 25, 32–38 (2016). 10.1097/ID.0000000000000327 [DOI] [PubMed] [Google Scholar]
- 61. Diss A., Dohan D. M., Mouhyi J., and Mahler P., “ Osteotome sinus floor elevation using Choukroun’s platelet-rich fibrin as grafting material: A 1-year prospective pilot study with microthreaded implants,” Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 105, 572–579 (2008). 10.1016/j.tripleo.2007.08.021 [DOI] [PubMed] [Google Scholar]
- 62. Toffler M., Toscano N., and Holtzclaw D., “ Osteotome-mediated sinus floor elevation using only platelet-rich fibrin: An early report on 110 patients,” Implant Dent. 19, 447–456 (2010). 10.1097/ID.0b013e3181f57288 [DOI] [PubMed] [Google Scholar]
- 63. Molemans B., Cortellini S., Jacobs R., Pinto N., Teughels W., and Quirynen M., “ Simultaneous sinus floor elevation and implant placement using leukocyte- and platelet-rich fibrin as a sole graft material,” Int. J. Oral Maxillofac. Implants 34, 1195–1201 (2019). 10.11607/jomi.7371 [DOI] [PubMed] [Google Scholar]
- 64. Kim J. M., Sohn D. S., Bae M. S., Moon J. W., Lee J. H., and Park I. S., “ Flapless transcrestal sinus augmentation using hydrodynamic piezoelectric internal sinus elevation with autologous concentrated growth factors alone,” Implant Dent. 23, 168–174 (2014). 10.1097/ID.0000000000000053 [DOI] [PubMed] [Google Scholar]
- 65. Sohn D. S., Heo J. U., Kwak D. H., Kim D. E., Kim J. M., Moon J. W. et al. , “ Bone regeneration in the maxillary sinus using an autologous fibrin-rich block with concentrated growth factors alone,” Implant Dent. 20, 389–395 (2011). 10.1097/ID.0b013e31822f7a70 [DOI] [PubMed] [Google Scholar]
- 66. Chen Y., Cai Z., Zheng D., Lin P., Cai Y., Hong S. et al. , “ Inlay osteotome sinus floor elevation with concentrated growth factor application and simultaneous short implant placement in severely atrophic maxilla,” Sci. Rep. 6, 27348 (2016). 10.1038/srep27348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Adali E., Yuce M. O., Gunbay T., and Gunbay S., “ Does concentrated growth factor used with allografts in maxillary sinus lifting have adjunctive benefits?,” J. Oral Maxilofac. Surg. 79, 98–108 (2021). 10.1016/j.joms.2020.07.217 [DOI] [PubMed] [Google Scholar]
- 68. Tanaka H., Toyoshima T., Atsuta I., Ayukawa Y., Sasaki M., Matsushita Y. et al. , “ Additional effects of platelet-rich fibrin on bone regeneration in sinus augmentation with deproteinized bovine bone mineral: Preliminary results,” Implant Dent. 24, 669–674 (2015). 10.1097/ID.0000000000000306 [DOI] [PubMed] [Google Scholar]
- 69. Barbu H. M., Iancu S. A., Hancu V., Referendaru D., Nissan J., and Naishlos S., “ PRF-solution in large sinus membrane perforation with simultaneous implant placement-micro CT and histological analysis,” Membranes 11, 438 (2021). 10.3390/membranes11060438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pinto G., Pigossi S. C., Pessoa T., Nícoli L. G., Araújo R., Marcantonio C. et al. , “ Successful use of leukocyte platelet-rich fibrin in the healing of sinus membrane perforation: A case report,” Implant Dent. 27, 375 (2018). 10.1097/ID.0000000000000731 [DOI] [PubMed] [Google Scholar]
- 71. Xin L., Yuan S., Mu Z., Li D., Song J., and Chen T., “ Histological and histomorphometric evaluation of applying a bioactive advanced platelet-rich fibrin to a perforated schneiderian membrane in a maxillary sinus elevation model,” Front. Bioeng. Biotechnol. 8, 600032 (2020). 10.3389/fbioe.2020.600032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Avila-Ortiz G., Chambrone L., and Vignoletti F., “ Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis,” J. Clin. Periodontol. 46(Suppl 21), 195–223 (2019). 10.1111/jcpe.13057 [DOI] [PubMed] [Google Scholar]
- 73. Alissa R., Esposito M., Horner K., and Oliver R., “ The influence of platelet-rich plasma on the healing of extraction sockets: An explorative randomised clinical trial,” Eur. J. Oral Implantol. 3, 121–134 (2010). [PubMed] [Google Scholar]
- 74. Rutkowski J. L., Johnson D. A., Radio N. M., and Fennell J. W., “ Platelet rich plasma to facilitate wound healing following tooth extraction,” J. Oral Implantol. 36, 11–23 (2010). 10.1563/AAID-JOI-09-00063 [DOI] [PubMed] [Google Scholar]
- 75. Al-Hamed F. S., Tawfik M. A., Abdelfadil E., and Al-Saleh M. A. Q., “ Efficacy of platelet-rich fibrin after mandibular third molar extraction: A systematic review and meta-analysis,” J. Oral Maxillofac. Surg. 75, 1124–1135 (2017). 10.1016/j.joms.2017.01.022 [DOI] [PubMed] [Google Scholar]
- 76. Mourao C. F. D. B., de Mello-Machado R. C., Javid K., and Moraschini V., “ The use of leukocyte- and platelet-rich fibrin in the management of soft tissue healing and pain in post-extraction sockets: A randomized clinical trial,” J. Cranio-Maxillofac. Surg. 48, 452–457 (2020). 10.1016/j.jcms.2020.02.020 [DOI] [PubMed] [Google Scholar]
- 77. Serafini G., Lollobrigida M., Fortunato L., Mazzucchi G., Lamazza L., Di Nardo D. et al. , “ Postextractive alveolar ridge preservation using L-PRF: Clinical and histological evaluation,” Case Rep. Dent. 2020, 5073519. 10.1155/2020/5073519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Suttapreyasri S. and Leepong N., “ Influence of platelet-rich fibrin on alveolar ridge preservation,” J. Craniofac. Surg. 24, 1088–1094 (2013). 10.1097/SCS.0b013e31828b6dc3 [DOI] [PubMed] [Google Scholar]
- 79. Azangookhiavi H., Ghodsi S., Jalil F., and Dadpour Y., “ Comparison of the efficacy of platelet-rich fibrin and bone allograft for alveolar ridge preservation after tooth extraction: A clinical trial,” Front Dent. 17, 1–6 (2020). 10.18502/fid.v17i1.3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ma F., Lin Y., Sun F., Jiang X., and Wei T., “ The impact of autologous concentrated growth factors on the alveolar ridge preservation after posterior tooth extraction: A prospective, randomized controlled clinical trial,” Clin. Implant Dent. Relat. Res. 23, 579–592 (2021). 10.1111/cid.13026 [DOI] [PubMed] [Google Scholar]
- 81. Buffoli B., Rosi S., Borsani E., Rodella L. F., and Mortellaro C., “ Effect of two different parts of CGF on post-extractive alveolar ridge preservation: A preliminary histomorphometric analysis in a Split-Mouth design,” J. Biol. Regul. Homeostatic Agents 35, 155–161 (2021). 10.23812/21-2supp1-15 [DOI] [PubMed] [Google Scholar]
- 82. Brignardello-Petersen R., “ Concentrated growth factor seems to have benefits in outcomes after extraction of partially impacted third molars,” J. Am. Dent. Assoc. 151, E29 (2020). 10.1016/j.adaj.2019.10.018 [DOI] [PubMed] [Google Scholar]
- 83. Allan B., Ruan R., Landao-Bassonga E., Gillman N., Wang T., Gao J. et al. , “ Collagen membrane for guided bone regeneration in dental and orthopedic applications,” Tissue Eng., Part A 27, 372–381 (2021). 10.1089/ten.tea.2020.0140 [DOI] [PubMed] [Google Scholar]
- 84. Retzepi M. and Donos N., “ Guided bone regeneration: Biological principle and therapeutic applications,” Clin. Oral Implants Res. 21, 567–576 (2010). 10.1111/j.1600-0501.2010.01922.x [DOI] [PubMed] [Google Scholar]
- 85. Simonpieri A., Del Corso M., Vervelle A., Jimbo R., Inchingolo F., Sammartino G. et al. , “ Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: Bone graft, implant and reconstructive surgery,” Curr. Pharm. Biotechnol. 13, 1231–1256 (2012). 10.2174/138920112800624472 [DOI] [PubMed] [Google Scholar]
- 86. Anand U. and Mehta D. S., “ Evaluation of immediately loaded dental implants bioactivated with platelet-rich plasma placed in the mandibular posterior region: A clinico-radiographic study,” J. Indian Soc. Periodontol. 16, 89–95 (2012). 10.4103/0972-124X.94612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ergun G., Egilmez F., Cekic-Nagas S., Karaca I. R., and Bozkaya S., “ Effect of platelet-rich plasma on the outcome of early loaded dental implants: A 3-year follow-up study,” J. Oral Implantol. 39, 256–263 (2013). 10.1563/AAID-JOI-D-11-00151 [DOI] [Google Scholar]
- 88. Tekin L., Akarsu S., and Ata E., “ Effect of platelet-rich-plasma (PRP) implant surface topography on implant stability and bone revisited,” J. Clin. Diagn. Res. 9, ZL01 (2015). 10.7860/JCDR/2015/.5547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Taschieri S., Lolato A., Ofer M., Testori T., Francetti L., and Del Fabbro M., “ Immediate post-extraction implants with or without pure platelet-rich plasma: A 5-year follow-up study,” Oral Maxillofac. Surg. 21, 147–157 (2017). 10.1007/s10006-017-0609-2 [DOI] [PubMed] [Google Scholar]
- 90. Cortese A., Pantaleo G., Borri A., Caggiano M., and Amato M., “ Platelet-rich fibrin (PRF) in implant dentistry in combination with new bone regenerative technique in elderly patients,” Int. J. Surg. Case Rep. 28, 52–56 (2016). 10.1016/j.ijscr.2016.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jo Y. Y. and Oh J. H., “ New resorbable membrane materials for guided bone regeneration,” Appl Sci. 8, 2157 (2018). 10.3390/app8112157 [DOI] [Google Scholar]
- 92. Tabrizi R., Arabion H., and Karagah T., “ Does platelet-rich fibrin increase the stability of implants in the posterior of the maxilla? A split-mouth randomized clinical trial,” Int. J. Oral Maxilofac. Surg. 47, 672–675 (2018). 10.1016/j.ijom.2017.07.025 [DOI] [PubMed] [Google Scholar]
- 93. Boora P., Rathee M., and Bhoria M., “ Effect of platelet rich fibrin (PRF) on peri-implant soft tissue and crestal bone in one-stage implant placement: A randomized controlled trial,” J. Clin. Diagn. Res. 9, ZC18–ZC21 (2015). 10.7860/JCDR/2015/.5788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. C. A. Amaral Valladão, Jr. , Freitas Monteiro M., and Joly J. C., “ Guided bone regeneration in staged vertical and horizontal bone augmentation using platelet-rich fibrin associated with bone grafts: A retrospective clinical study,” Int. J. Implant Dent. 6, 72 (2020). 10.1186/s40729-020-00266-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hartlev J., Schou S., Isidor F., and Nørholt S. E., “ A clinical and radiographic study of implants placed in autogenous bone grafts covered by either a platelet-rich fibrin membrane or deproteinised bovine bone mineral and a collagen membrane: A pilot randomised controlled clinical trial with a 2-year follow-up,” Int. J. Implant Dent. 7, 8 (2021). 10.1186/s40729-021-00289-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang X., Wang G., Zhao X., Feng Y., Liu H., and Li F., “ Short-term evaluation of guided bone reconstruction with titanium mesh membranes and CGF membranes in immediate implantation of anterior maxillary tooth,” Biomed. Res. Int. 2021, 4754078. 10.1155/2021/4754078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dai Y., Han X. H., Hu L. H., Wu H. W., Huang S. Y., and Lu Y. P., “ Efficacy of concentrated growth factors combined with mineralized collagen on quality of life and bone reconstruction of guided bone regeneration,” Regener. Biomater. 7, 313–320 (2020). 10.1093/rb/rbaa007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Barbu H. M., Iancu S. A., Rapani A., and Stacchi C., “ Guided bone regeneration with concentrated growth factor enriched bone graft matrix (sticky bone) vs. bone-shell technique in horizontal ridge augmentation: A retrospective study,” J. Clin. Med. 10, 3953 (2021). 10.3390/jcm10173953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Antich-Rossello M., Forteza-Genestra M. A., Calvo J., Gaya A., Monjo M., and Ramis J. M., “ Platelet-derived extracellular vesicles promote osteoinduction of mesenchymal stromal cells,” Bone Jt. Res. 9, 667–674 (2020). 10.1302/2046-3758.910.BJR-2020-0111.R2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Antich-Rossello M., Forteza-Genestra M. A., Calvo J., Gaya A., Monjo M., and Ramis J. M., “ Platelet-derived extracellular vesicle functionalization of Ti implants,” J. Visualized Exp. 174, e62781 (2021). 10.3791/62781 [DOI] [PubMed] [Google Scholar]
- 101. Agarwal A. and Gupta N. D., “ Platelet-rich plasma combined with decalcified freeze-dried bone allograft for the treatment of noncontained human intrabony periodontal defects: A randomized controlled split-mouth study,” Int. J. Periodontics Restor. Dent. 34, 705–711 (2014). 10.11607/prd.1766 [DOI] [PubMed] [Google Scholar]
- 102. Dori F., Huszar T., Nikolidakis D., Arweiler N. B., Gera I., and Sculean A., “ Effect of platelet-rich plasma on the healing of intra-bony defects treated with a natural bone mineral and a collagen membrane,” J. Clin. Periodontol. 34, 254–261 (2007). 10.1111/j.1600-051X.2006.01044.x [DOI] [PubMed] [Google Scholar]
- 103. Dori F., Huszar T., Nikolidakis D., Tihanyi D., Horvath A., Arweiler N. B. et al. , “ Effect of platelet-rich plasma on the healing of intrabony defects treated with Beta tricalcium phosphate and expanded polytetrafluoroethylene membranes,” J. Periodontol. 79, 660–669 (2008). 10.1902/jop.2008.070473 [DOI] [PubMed] [Google Scholar]
- 104. Piemontese M., Aspriello S. D., Rubini C., Ferrante L., and Procaccini M., “ Treatment of periodontal intrabony defects with demineralized freeze-dried bone allograft in combination with platelet-rich plasma: A comparative clinical trial,” J. Periodontol. 79, 802–810 (2008). 10.1902/jop.2008.070436 [DOI] [PubMed] [Google Scholar]
- 105. Saleem M., Pisani F., Zahid F. M., Georgakopoulos I., Pustina-Krasniqi T., Xhajanka E. et al. , “ Adjunctive platelet-rich plasma (PRP) in infrabony regenerative treatment: A systematic review and RCT’s meta-analysis,” Stem Cells Int. 2018, 9594235. 10.1155/2018/9594235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Duan X., Lin Z., Lin X., Wang Z., Wu Y., Ji M. et al. , “ Study of platelet-rich fibrin combined with rat periodontal ligament stem cells in periodontal tissue regeneration,” J. Cell Mol. Med. 22, 1047–1055 (2018). 10.1111/jcmm.13461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Li Q., Pan S., Dangaria S. J., Gopinathan G., Kolokythas A., Chu S. et al. , “ Platelet-rich fibrin promotes periodontal regeneration and enhances alveolar bone augmentation,” Biomed. Res. Int. 2013, 638043. 10.1155/2013/638043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Patel G. K., Gaekwad S. S., Gujjari S. K., and SC V. K., “ Platelet-rich fibrin in regeneration of intrabony defects: A randomized controlled trial,” J. Periodontol. 88, 1192–1199 (2017). 10.1902/jop.2017.130710 [DOI] [PubMed] [Google Scholar]
- 109. Bajaj P., Agarwal E., Rao N. S., Naik S. B., Pradeep A. R., Kalra N. et al. , “ Autologous platelet-rich fibrin in the treatment of 3-wall intrabony defects in aggressive periodontitis: A randomized controlled clinical trial,” J. Periodontol. 88, 1186–1191 (2017). 10.1902/jop.2017.120661 [DOI] [PubMed] [Google Scholar]
- 110. Mathur A., Bains V. K., Gupta V., Jhingran R., and Singh G. P., “ Evaluation of intrabony defects treated with platelet-rich fibrin or autogenous bone graft: A comparative analysis,” Eur. J. Dent. 9, 100–108 (2015). 10.4103/1305-7456.149653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bansal C. and Bharti V., “ Evaluation of efficacy of autologous platelet-rich fibrin with demineralized-freeze dried bone allograft in the treatment of periodontal intrabony defects,” J. Indian Soc. Periodontol. 17, 361–366 (2013). 10.4103/0972-124X.115663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lekovic V., Milinkovic I., Aleksic Z., Jankovic S., Stankovic P., Kenney E. B. et al. , “ Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects,” J. Periodontal Res. 47, 409–417 (2012). 10.1111/j.1600-0765.2011.01446.x [DOI] [PubMed] [Google Scholar]
- 113. Liu K. N., Huang Z., Chen Z. B., Han B., and Ouyang X. Y., “ Treatment of periodontal intrabony defects using bovine porous bone mineral and guided tissue regeneration with/without platelet-rich fibrin: A randomized controlled clinical trial,” J. Periodontol. 92, 1546 (2021). 10.1002/JPER.20-0860 [DOI] [PubMed] [Google Scholar]
- 114. Li X. J., Yang H. X., Zhang Z. J., Yan Z. H., Lv H. L., Zhang Y. et al. , “ Concentrated growth factor exudate enhances the proliferation of human periodontal ligament cells in the presence of TNF-α,” Mol. Med. Rep. 19, 943–950 (2019). 10.3892/mmr.2018.9714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lei L., Yu Y., Han J., Shi D., Sun W., Zhang D. et al. , “ Quantification of growth factors in advanced platelet-rich fibrin and concentrated growth factors and their clinical efficacy as adjunctive to the GTR procedure in periodontal intrabony defects,” J. Periodontol. 91, 462–472 (2020). 10.1002/JPER.19-0290 [DOI] [PubMed] [Google Scholar]
- 116. Vaid T., Kumar S., Mehta R., Shah S., Joshi S., Bhakkand S. et al. , “ Clinical and radiographic evaluation of demineralized freeze-dried bone allograft with concentrated growth factor versus concentrated growth factor alone in the treatment of intrabony defects,” Med. Pharm. Rep. 94, 220–228 (2021). 10.15386/mpr-1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Qiao J., Duan J., Zhang Y., Chu Y., and Sun C., “ The effect of concentrated growth factors in the treatment of periodontal intrabony defects,” Future Sci. OA 2, FS136 (2016). 10.4155/fsoa-2016-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Xu Y., Qiu J. L., Sun Q. F., Yan S. G., Wang W. X., Yang P. S. et al. , “ One year results evaluating the effects of concentrated growth factors on the healing of intrabony defects treated with or without bone substitute in chronic periodontitis,” Med. Sci. Monit. 25, 4384–4389 (2019). 10.12659/MSM.917025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jovicic B., Lazic Z., Nedic M., Matijevic S., and Gostovic-Spadijer A., “ Therapeutic efficacy of connective tissue autotransplants with periosteum and platelet rich plasma in the menagement of gingival recession,” Vojnosanit. Pregl. 70, 664–669 (2013). 10.2298/VSP1307664J [DOI] [PubMed] [Google Scholar]
- 120. Naik A. R., Ramesh A. V., Dwarkanath C. D., Naik M. S., and Chinnappa A. B., “ Use of autologous platelet rich plasma to treat gingival recession in esthetic periodontal surgery,” J. Indian Soc. Periodontol. 17, 345–353 (2013). 10.4103/0972-124X.115665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Srinivas B. V., Rupa N., Halini Kumari K. V., Prasad S. S., Varalakshmi U., and Sudhakar K., “ Root coverage using subepithelial connective tissue graft with platelet-rich plasma in the treatment of gingival recession: A clinical study,” J. Pharm. BioAllied Sci. 7, 530–538 (2015). 10.4103/0975-7406.163530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tunaliota M., Ozdemir H., Arabaciota T., Gurbuzer B., Pikdoken L., and Firatli E., “ Clinical evaluation of autologous platelet-rich fibrin in the treatment of multiple adjacent gingival recession defects: A 12-month study,” Int. J. Periodontics Restor. Dent. 35, 105–114 (2015). 10.11607/prd.1826 [DOI] [PubMed] [Google Scholar]
- 123. Padma R., Shilpa A., Kumar P. A., Nagasri M., Kumar C., and Sreedhar A., “ A split mouth randomized controlled study to evaluate the adjunctive effect of platelet-rich fibrin to coronally advanced flap in Miller’s class-I and II recession defects,” J. Indian Soc. Periodontol. 17, 631–636 (2013). 10.4103/0972-124X.119281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Dixit N., Lamba A. K., Faraz F., Tandon S., Aggarwal K., and Ahad A., “ Root coverage by modified coronally advanced flap with and without platelet-rich fibrin: A clinical study,” Indian J. Dent. Res. 29, 600–604 (2018). 10.4103/ijdr.IJDR_22_17 [DOI] [PubMed] [Google Scholar]
- 125. Oncu E., “ The use of platelet-rich fibrin versus subepithelial connective tissue graft in treatment of multiple gingival recessions: A randomized clinical trial,” Int. J. Periodontics Restor. Dent. 37, 265 (2017). 10.11607/prd.2741 [DOI] [PubMed] [Google Scholar]
- 126. Wang X., Zhang Y., Choukroun J., Ghanaati S., and Miron R. J., “ Behavior of gingival fibroblasts on titanium implant surfaces in combination with either injectable-PRF or PRP,” Int. J. Mol. Sci. 18, 331 (2017). 10.3390/ijms18020331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Akcan S. K. and Unsal B., “ Gingival recession treatment with concentrated growth factor membrane: A comparative clinical trial,” J. Appl. Oral Sci. 28, e20190236 (2020). 10.1590/1678-7757-2019-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bozkurt Dogan S., Ongoz Dede F., Balli U., Atalay E. N., and Durmuslar M. C., “ Concentrated growth factor in the treatment of adjacent multiple gingival recessions: A split-mouth randomized clinical trial,” J. Clin. Periodontol. 42, 868–875 (2015). 10.1111/jcpe.12444 [DOI] [PubMed] [Google Scholar]
- 129. Tazegul K., Dogan S. B., Balli U., Dede F. O., and Tayman M. A., “ Growth factor membranes in treatment of multiple gingival recessions: A randomized clinical trial,” Quintessence Int. 53, 288–297 (2022). 10.3290/j.qi.b2407765 [DOI] [PubMed] [Google Scholar]
- 130. Antich-Rosselló M., Munar-Bestard M., Forteza-Genestra M. A., Calvo J., Gayà A., Monjo M. et al. , Platelet Derived Extracellular Vesicles Promote In Vitro Gingival Wound Healing ( Research Square, 2021). [Google Scholar]
- 131. Pradeep A. R., Rao N. S., Agarwal E., Bajaj P., Kumari M., and Naik S. B., “ Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: A randomized controlled clinical trial,” J. Periodontol. 83, 1499–1507 (2012). 10.1902/jop.2012.110705 [DOI] [PubMed] [Google Scholar]
- 132. Bajaj P., Pradeep A. R., Agarwal E., Rao N. S., Naik S. B., Priyanka N. et al. , “ Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of mandibular degree II furcation defects: A randomized controlled clinical trial,” J. Periodontal. Res. 48, 573–581 (2013). 10.1111/jre.12040 [DOI] [PubMed] [Google Scholar]
- 133. Goyal B., Tewari S., Duhan J., and Sehgal P. K., “ Comparative evaluation of platelet-rich plasma and guided tissue regeneration membrane in the healing of apicomarginal defects: A clinical study,” J. Endod. 37, 773–780 (2011). 10.1016/j.joen.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 134. Christgau M., Moder D., Wagner J., Glassl M., Hiller K. A., Wenzel A. et al. , “ Influence of autologous platelet concentrate on healing in intra-bony defects following guided tissue regeneration therapy: A randomized prospective clinical split-mouth study,” J. Clin. Periodontol. 33, 908–921 (2006). 10.1111/j.1600-051X.2006.00999.x [DOI] [PubMed] [Google Scholar]
- 135. Sharma A. and Pradeep A. R., “ Treatment of 3-wall intrabony defects in patients with chronic periodontitis with autologous platelet-rich fibrin: a randomized controlled clinical trial,” J. Periodontol. 82, 1705–1712 (2011). 10.1902/jop.2011.110075 [DOI] [PubMed] [Google Scholar]
- 136. Agarwal A., Gupta N. D., and Jain A., “ Platelet rich fibrin combined with decalcified freeze-dried bone allograft for the treatment of human intrabony periodontal defects: A randomized split mouth clinical trail,” Acta Odontol. Scand. 74, 36–43 (2016). 10.3109/00016357.2015.1035672 [DOI] [PubMed] [Google Scholar]
- 137. Joseph V. R., Sam G., and Amol N. V., “ Clinical evaluation of autologous platelet rich fibrin in horizontal alveolar bony defects,” J. Clin. Diagn. Res. 8, ZC43 (2014). 10.7860/JCDR/2014/9948.5129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Thorat M., Pradeep A. R., and Pallavi B., “ Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: a controlled clinical trial,” J. Clin. Periodontol. 38, 925–932 (2011). 10.1111/j.1600-051X.2011.01760.x [DOI] [PubMed] [Google Scholar]
- 139. Feigin K. and Shope B., “ Use of platelet-rich plasma and platelet-rich fibrin in dentistry and oral surgery: Introduction and review of the literature,” J. Vet. Dent. 36, 109–123 (2019). 10.1177/0898756419876057 [DOI] [PubMed] [Google Scholar]
- 140. Bosshardt D. D., Stadlinger B., and Terheyden H., “ Cell-to-cell communication–periodontal regeneration,” Clin. Oral Implants Res. 26, 229–239 (2015). 10.1111/clr.12543 [DOI] [PubMed] [Google Scholar]
- 141. Babo P. S., Cai X., Plachokova A. S., Reis R. L., Jansen J., Gomes M. E. et al. , “ Evaluation of a platelet lysate bilayered system for periodontal regeneration in a rat intrabony three-wall periodontal defect,” J. Tissue Eng. Regener. Med. 12, e1277–e88 (2018). 10.1002/term.2535 [DOI] [PubMed] [Google Scholar]
- 142. Guo S. C., Tao S. C., Yin W. J., Qi X., Yuan T., and Zhang C. Q., “ Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model,” Theranostics 7, 81–96 (2017). 10.7150/thno.16803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Chackartchi T., Romanos G. E., and Sculean A., “ Soft tissue-related complications and management around dental implants,” Periodontology 2000 81, 124–138 (2019). 10.1111/prd.12287 [DOI] [PubMed] [Google Scholar]
- 144. Vishnu V. A., Sanyal P. K., Tewary S., Nilesh K., Suresh Prasad R. M., and Pawashe K., “ A split-mouth clinico-radiographic comparative study for evaluation of crestal bone and peri-implant soft tissues in immediately loaded implants with and without platelet-rich plasma bioactivation,” J. Dent. Res. Dent. Clin. Dent. 13, 117–122 (2019). 10.15171/joddd.2019.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lima V. C. D., Miguel M. M. V., Ferraz L. F. F., de Melo A. B., Jardini M. A. N., and Santamaria M. P., “ Use of platelet-rich fibrin membranes with single implant placement for peri-implant mucosal thickness augmentation: A case series study,” Clin. Adv. Periodontics 12, 17 (2021). 10.1002/cap.10143 [DOI] [PubMed] [Google Scholar]
- 146. Ustaoglu G., Paksoy T., and Gumus K. C., “ Titanium-prepared platelet-rich fibrin versus connective tissue graft on peri-implant soft tissue thickening and keratinized mucosa width: A randomized, controlled trial,” J. Oral Maxillofac. Surg. 78, 1112–1123 (2020). 10.1016/j.joms.2020.02.019 [DOI] [PubMed] [Google Scholar]
- 147. Cui A. M., Zhou J., Mudalal M., Wang Y., Wang J., Gong M. et al. , “ Soft tissue regeneration around immediate implant placement utilizing a platelet-rich fibrin membrane and without tightly flap closure: Two case reports,” Medicine 99, e22507 (2020). 10.1097/MD.0000000000022507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ibraheem W., “ Effect of platelet-rich fibrin and free gingival graft in the treatment of soft tissue defect preceding implant placement,” J. Contemp. Dent. Pract. 19, 895–899 (2018). 10.5005/jp-journals-10024-2353 [DOI] [PubMed] [Google Scholar]
- 149. Hehn J., Schwenk T., Striegel M., and Schlee M., “ The effect of PRF (platelet-rich fibrin) inserted with a split-flap technique on soft tissue thickening and initial marginal bone loss around implants: Results of a randomized, controlled clinical trial,” Int. J. Implant Dent. 2, 13 (2016). 10.1186/s40729-016-0044-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.