Background:
Activation of venous flow has been shown with different types of electrical stimulation. The aim of this study is to compare the hemodynamic effects of transcutaneous electrical nerve stimulation (TENS), neuromuscular electrical stimulation (NMES), and sham stimulation on healthy young people.
Methods:
This randomized crossover study was conducted during June 2018 in the Faculty of Physical Therapy of A Coruña (Spain). Twenty-four university students (50% male) received in a randomized order 5 Hz-TENS, NMES, and sham stimulation on soleus muscle. Flow volume (FV) and peak velocity (PV) from popliteal vein were recorded via Doppler ultrasound, and relative changes from baseline were determined. Discomfort among the 3 stimulations was also compared.
Results:
The differences among the 3 stimulations were assessed using the ANOVA for repeated measured, the Friedman test and the Kendall tau test, according to the type of measurement to be compared. FV (mL/min) and PV (cm/s) increased significantly after NMES (percentual increase 37.2 ± 62.0%, P = .002; 264.4 ± 152.2%, P < .001, respectively) and TENS (226.2 ± 190.3%, P < .001; 202.7 ± 144.6%, P < .001, respectively). These percentual changes from basal level in hemodynamics were statistically different to those after placebo, which was ineffective enhancing hemodynamics. The improvements in FV were statistically higher with TENS than with NMES (P < .001), but there was no statistical difference in PV (P = .531). Despite NMES was applied at a significantly lower amplitude than TENS (P < .001), NMES protocol was the worst tolerated, though the differences in discomfort were not statistically significant.
Conclusion:
Both active electrical protocols but not sham stimulation increased hemodynamics in healthy people. TENS obtained higher flow volume increase from baseline than NMES, considered globally at not only in its on-time.
Keywords: hemodynamics, placebo, transcutaneous electric nerve stimulation, ultrasonography Doppler
1. Introduction
Pharmacological prophylaxis methods used in deep venous thrombosis can be contraindicated in some cases.[1] Therefore, more options should be evaluated. Electrical stimulation (ES) is a physical technique that has been shown to improve hemodynamics[2] and at the same time it could be safe and well tolerated.[3] However, it is underutilized and clarification for electrical parameters is needed for optimum effectiveness,[4] beginning with the type of ES. Neuromuscular electrical stimulation (NMES) and transcutaneous electrical nerve stimulation (TENS) are confusing terms as both modalities can be delivered transcutaneously and are able to stimulate muscles and nerves. For example, when looking for pain alleviation, TENS optimal intensities are described as strong sensations, allowing muscle twitches.[5] Therefore, the main difference between them is due to its original purpose, muscle strengthening for NMES, and pain relief for TENS. NMES consists of intermittent stimuli,[6] so duty cycle (on-off time) is a specific parameter for NMES[7] in order to elicit sustained muscle contractions and also to provide a resting time for minimizing fatigue.[8] In this sense, NMES provokes a rhythmic but more sustained contraction and resting time than brisk contraction using TENS. It should reflect better the active calf muscle pump.
Despite their differences, authors usually do not discriminate between TENS and NMES effects, even in recent reviews where the differences are categorized by placement only[4] TENS increases venous flow in healthy people[9] and may augment fibrinolysis[10] having a prophylactic role in the clinical context.[11] In a similar way, NMES enhanced venous return in healthy subjects[12] and in the patient population.[13] Comparisons among ES and other nonpharmacological methods as intermittent pneumatic compression (IPC) have been favorable to TENS[9] and NMES[12] in healthy people.
Despite the potential role for ES in hemodynamics, comparative studies between TENS and NMES are scarce. To our knowledge, there are only 2 studies that clearly compare venous return of TENS and NMES on healthy people, one testing both modalities on common peroneal nerve[14] and the other testing TENS on common peroneal nerve and NMES on soleus muscle.[15] Differences in hemodynamic responses to NMES have been found between upper and lower limbs[16] and they could also exist within different locations in the lower limb. A study testing soleus muscle and common peroneal nerve locations seemed to find better blood flow when TENS was applied at muscle site.[17] Therefore, the aim of this research is to compare TENS, NMES and sham stimulation on soleus muscle of young healthy individuals regarding hemodynamics improvements on venous return and tolerance parameters. The results may help selecting the type of ES more appropriated for stasis prevention.
2. Methods
2.1. Study design and subjects
This study was approved by the regional Clinical Research Ethics Committee of Galicia (Spain) in accordance with the Helsinki Declaration. The study adopted a randomized balanced within-subject’s crossover design stratified by sex to determine differences in hemodynamic effects and discomfort, and was registered in https://www.researchregistry.com (researchregistry3380). The study was funded by the College of Physical Therapists of Galicia.
Healthy young female and male students from the Faculty of Physical Therapy, University of A Coruña (Spain) were invited to participate in a testing session. Eligibility criteria were age between 18 and 39 years, nonsmoking, and body mass index (BMI) above 18 and below 30 kg/m2. A questionnaire, a physical examination, and a Doppler ultrasonography of the lower limbs were used to exclude any oral contraceptive use, recent surgery/trauma to lower limbs, diagnosed psychiatric, neurologic, rheumatologic, endocrine or metabolic diseases, history of cardiovascular disease or hematological disorders, and varicose, or ulceration of the lower limbs. Finally, from 25 volunteers who agreed to participate after providing signed consent, 12 men and 12 women were recruited for the study, and 1 was excluded.
2.2. Experimental procedure
The interventions were conducted during June 2018 in the Faculty of Physical Therapy, in a room in which temperature was controlled (22–24°C). Subjects rested from 15 to 20 minutes in prone position. All the subjects (24 volunteers) received the 3 stimulations (TENS, NMES, and sham stimulations). The order of the ES was randomly assigned from the 6 possible sequences. Each sequence was introduced by a researcher not involved in the study in opaque envelopes, and the participants selected 1 of the 6 available envelopes. All the ES were applied separated by a 5-minute period using 5 × 5 cm self-adhesive electrodes over motor points of soleus[18] and a TENSMED S82 (ENRAF NONIUS, Rotterdam, The Netherlands). Right emplacement was ensured by looking for the point where maximum contraction was found, using 2 Hz-TENS. A symmetrical biphasic square waveform with phase duration of 350 microseconds at a frequency of 5 Hz for TENS and 35 Hz for NMES was administered. The intensity was set to 10% below pain threshold.[17] The subjects were told that the session will be stopped in case of pain. Regarding NMES, ramp up and down times of 0.5 seconds with an active contraction time of 1 second were used.[12] The off-time of 10 seconds was selected to capture 1 muscle contraction at each Doppler measurement, while providing a comfortable calf stimulation.[19] Sham-TENS was delivered at 100 Hz, 350 microseconds phase duration, and amplitude was achieved in the same way as for both active types, but it was progressively decreased to 0.5 mA from 15 to 30 seconds after reaching the maximum but tolerable level previously determined. This protocol was adapted from transient TENS for pain relief studies.[20] The subjects were told that they could get used to some types of stimulation as TENS and perception may felt lowered or could be barely felt. Each ES modality was set for 1 minute before measurements. There were no adverse events during the study.
2.3. Study variables
2.3.1. Doppler ultrasound measurements.
The blood flow from popliteal vein of the nondominant leg was assessed with a Doppler ultrasound using a 6 to 13 MHz linear transducer (LOGIQ e BT12 General Electric Medical Systems) stabilized by an articulated arm at the popliteal fossa. The placement of the ultrasound probe was marked on the skin for consistency of the measures. The vein diameter was calculated from the Doppler image, at B-mode. The values were measured 3 times in 12 seconds’ periods at baseline and after ES, and the mean of them was considered for the analyses. The effect of the ES in the hemodynamic responses was evaluated as the difference of the ES minus baseline values, divided by the baseline and given as percentage. The difference between the ES was assessed by means of the difference of the corresponding ES outcomes, divided by the measure with second ES and given also as percentage. The same examiner made the measurements and recorded the peak venous velocity (PV) (cm/s) and the venous flow volume (FV) (mL/min). PV was obtained offline from the Doppler waveform, whereas FV was calculated by the Doppler´s unit software. Subjects were reminded to breath normally at a stable pattern and not to move during data collection.
2.3.2. Discomfort ratings.
A 100-mm modified visual analog score (VAS) determining ES discomfort from no sensation at all (0 mm) to pain onset (100 mm) was employed. Indeed, a 5-point verbal rating score (VRS) was used: 1, no sensation; 2, minimal discomfort; 3, mild discomfort; 4, moderate discomfort; and 5, severe discomfort. Several similar studies have used VAS and Likert scales to measure discomfort associated with ES.[17,21]
2.3.3. Change in sensation.
Participants were asked at the end of the experimental session if the sensation was decreased, maintained, or increased during the different types of ES.
2.4. Sample size calculation
The sample size was computed to detect, with α = 0.05 significance level and 95% power and using a 1 sample t test, a change in the ES effects of 10 percentage points, when the standard deviation is 14, which corresponds to the estimated standard deviation of the percentage of change of PV during the sham stimulation. The sample size to fit these requirements is 23.
2.5. Statistical analysis
Statistical analysis was carried out using IBM SPSS version 22. Mean with standard deviation and 95% confidence intervals, and median and interquartile range (IQR) are presented. The Kolmogorov–Smirnov–Lilliefors and Shapiro–Wilk tests were used to test for normal distribution of the data.
Physical baseline characteristics of participants were compared between men and women using the t test and Wilcoxon test for independent samples, and the difference in scores between baseline and stimulation, and between TENS and NMES, was tested by means of the relative differences (in percentage) with the t test and Wilcoxon tests for 1 sample. The differences among the 3 stimulations were assessed using the ANOVA for repeated measured, the Friedman test and the Kendall tau test, according to the type of measurement to be compared. Post hoc test with Bonferroni correction was used to identify differences. Correlation among variables was measured with the Spearman rank correlation coefficient. For all tests, the level for statistical significance was set to P < .05.
3. Results
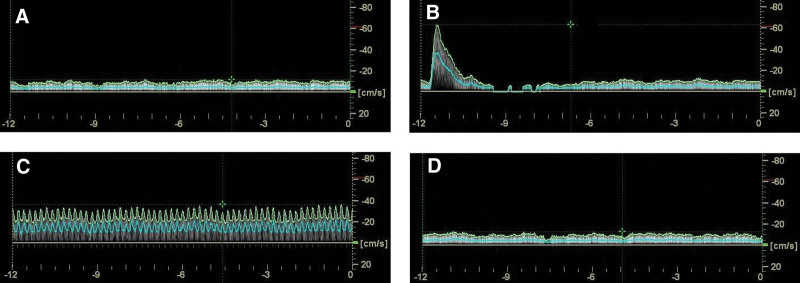
The study included 12 men and 12 women, median age 19.5 (IQR = 19–22) years. Physical characteristics of the participants as far as height, weight, BMI, and calf perimeter are summarized in Table 1. As expected, we found significant differences between men and women for all of them. Figure 1 shows a typical Duplex Doppler ultrasound capture window for blood flow measurements.
Table 1.
Physical baseline characteristics of participants, mean ± SD, and 95% confidence interval and median [Q1–Q3].
| Men (n = 12) | Women (n = 12) | P value | |
|---|---|---|---|
| Age (yr) | 22.17 ± 3.04 | 19.42 ± 0.90 | .014* |
| (20.24–24.10) | (18.84–19.99) | ||
| 22 [19.5–23.75] | 19 [19–19.7] | ||
| Height (m) | 1.76 ± 0.06 | 1.62 ± 0.06 | <.001† |
| (1.72–1.79) | (1.59–1.66) | ||
| 1.76 [1.71–1.79] | 1.61 [1.58–1.68] | ||
| Weight (kg) | 76.87 ± 9.52 | 59.87 ± 6.57 | <.001† |
| (70.82–82.91) | (55.70–64.05) | ||
| 76.5 [70.4–83.6] | 59.9 [54.2–66.7] | ||
| BMI | 24.78 ± 2.29 | 22.63 ± 2.01 | .023† |
| (23.33–26.24) | (21.36–23.91) | ||
| 24.7 [22.7–26.4] | 22.8 [20.9–24.3] | ||
| Calf perimeter (cm) | 36.51 ± 2.20 | 32.93 ± 2.64 | .002† |
| (35.11–37.90) | (31.25–34.61) | ||
| 36.3 [35.3–38.0] | 32.4 [31.1–35.1] |
BMI = body mass index.
Wilcoxon signed-rank test.
Independent samples t test.
Figure 1.
Representative Duplex ultrasound capture windows from 1 participant showing venous flow (A) in basal condition (B) in response to neuromuscular electrical stimulation (C) in response to transcutaneous electrical nerve stimulation (D) in response to placebo.
The Spearman correlation analysis between the change of the hemodynamic responses after stimulation and environmental variables (temperature, humidity) showed no significant effects. When analyzing relationship between the physical characteristics of the participants and the hemodynamic responses, the results showed significant relations between BMI (r = −0.54, P = .007) and calf perimeter (r = −0.53, P = 0.008) only with FV when using NMES.
When controlling by gender, neither the BMI nor the calf perimeter had a significant effect on the PV increments with any ES, nor on the FV increments with TENS. When using NMES, the change of FV with respect to baseline decreases significantly with high BMI (P = .036), and for larger calf parameter in women (P < .001), with no effects of the calf parameter for men (P = .952).
We confirmed that the placebo effect was not statistically significant neither in FV (−3.91% ± 10.45%, P = .080) nor in PV (−1.7 ± 13.6%, P = .212). FV increased significantly after NMES (37.2 ± 62.0%, P = .002) and TENS (226.2 ± 190.3%, P < .001), together with PV (NMES 264.4 ± 152.2%, P < .001; TENS 202.7 ± 144.6%, P < .001).
The increments of FV and PV after NMES and TENS stimulations were significantly higher when compared to those after sham stimulation (P < .001). When comparing both ES stimulations, the increment of FV was statistically higher with TENS than with NMES stimulation (P < .001), but there was no statistical difference in PV (P = .531). Results are summarized in Table 2.
Table 2.
Hemodynamic responses after placebo, NMES, and TENS, and relative differences (in percentage) between each ES and placebo, and between TENS and NMES given as mean ± SD and 95% confidence interval and median [Q1, Q3].
| Baseline | Stimulation | % change | P value† | P value‡ | ||
|---|---|---|---|---|---|---|
| FV (mL/min) | Placebo | 82.8 ± 34.7 | 78.9 ± 33.0 | −3.91 ± 10.45 | .080§ | |
| (68.15–97.47) | (64.94–92.83) | (−8.32 to 0.50) | ||||
| 76.3 [50.9–106.7] | 69.6 [47.6–107.1] | −4.36 [−13.13 to 3.48] | ||||
| NMES | 83.4 ± 35.3 | 101.5 ± 29.6 | 37.2 ± 62.0 | .002∥ | ||
| (68.45–98.31) | (88.97–113.99) | (10.98–63.36) | ||||
| 80.2 [49.3–115.9] | 102.3 [78.6–126.9] | 17.2 [−2.1 to 51.1] | ||||
| TENS | 78.6 ± 32.7 | 234.7 ± 109.0 | 226.2 ± 190.3 | <.001∥ | ||
| (64.83–92.42) | (188.67–280.72) | (145.79–306.52) | ||||
| 70.0 [48.7–107.4] | 214.8 [183.4,286.8] | 185.1 [113.7–266.6] | ||||
| NMES vs placebo vdPlaPlacebo | 42.57 ± 57.39 | .001∥ | ||||
| (18.34–66.81) | ||||||
| 26.6 [1.13–64.7] | ||||||
| TENS vs Placebo | 230.86 ± 203.34 | <.001∥ | ||||
| (144.99–316.73) | ||||||
| 170.4 [106.5–272.7] | ||||||
| TENS vs NMES | 139.28 ± 122.16 | <.001∥ | ||||
| (87.70–190.87) | ||||||
| 117.1 [85.2–151.6] | ||||||
| PV (cm/s) | Placebo | 15.57 ± 7.42 | 15.04 ± 6.64 | −1.77 ± 13.6 | .212∥ | |
| (12.44–18.71) | (12.24–17.85) | (−7.52 to 3.96) | ||||
| 12.66 [10.18–18.84] | 13.26 [9.95–17.49] | −3.47 [−10.74 to 2.86] | ||||
| NMES | 15.26 ± 6.88 | 49.54 ± 16.93 | 264.4 ± 152.2 | <.001§ | ||
| (12.35–18.17) | (42.39–56.69) | (200.14–328.71) | ||||
| 13.65 [10.45–17.86] | 46.6 [35.1–64.6] | 223.2 [122.8–404.5] | ||||
| TENS | 15.49 ± 6.39 | 44.2 ± 20.1 | 202.7 ± 144.6 | <.001∥ | ||
| (12.79–18.19) | (35.73–52.70) | (141.62–263.79) | ||||
| 14.55 [10.2–18.7] | 39.3 [27.6–58.4] | 148.9 [109.9–254.2] | ||||
| NMES vs placebo vdPlaPlacebo | 266.17 ± 150.42 | <.001∥ | ||||
| (202.66–329.69) | ||||||
| 235.8 [143.9–346.9] | ||||||
| TENS vs Placebo | 216.76 ± 160.27 | <.001∥ | ||||
| (149.09–284.44) | ||||||
| 166.8 [121.1–267.7] | ||||||
| TENS vs NMES | −5.55 ± 42.77 | .531§ | ||||
| (−23.61 to 12.50) | ||||||
| −10.1 [−36.2 to 24.6] |
NMES = neuromuscular electrical stimulation, TENS = transcutaneous electrical nerve stimulation.
*For each ES (ES − baseline) × 100/baseline. For each ES comparison, the differences of the corresponding change (%).
Based on the % of change of stimulation with respect to baseline.
Based on the % of change based on the differences in NMES vs Placebo, TENS vs Placebo and TENS vs NMES.
One sample t test for % change.
One sample Wilcoxon test for % change.
Values of the maximum tolerated intensity, given as amplitude (mA), and discomfort ratings VAS and VRS after finishing the 3 protocols are presented in Table 3. None of the subjects had to stop the intervention because of pain. As expected, the sham stimulation was the best tolerated one, with values of VAS and VRS significantly lower than those with NMES (P = .034 and .012 respectively), and TENS (P = .204 and .043, respectively). As for the NMES and TENS applications, despite ES was applied at a significantly lower amplitude in NMES than in TENS (P < .001) stimulations, NMES protocol was the worst tolerated, though those differences in VAS (P = 1.000) and VRS (P = .079) were not statistically significant. There were no significant differences in the maximum tolerated amplitude and discomfort ratings between men and women.
Table 3.
Maximum tolerated intensity and discomfort ratings (VAS and) after finishing the placebo, NMES, and TENS.
| Placebo | NMES | TENS | P value | |
|---|---|---|---|---|
| Intensity | 24.92 ± 5.48 | 14.98 ± 5.41 | 36.60 ± 11.93 | <.001* |
| (21.98–26.61) | (12.69–17.26) | (31.57–41.64) | ||
| 23.25 [19.12–29.87] | 13.75 [11.75–16.5] | 35.0 [30.25–40.87] | ||
| VAS | 40.92 ± 26.92 | 63.13 ± 27.81 | 56.25 ± 25.30 | .028† |
| (29.55–52.28) | (51.38–74.87) | (45.57–66.93) | ||
| 33.0 [19.75–53.0] | 70.0 [32.75–82.0] | 53.5 [34.75–77.75] | ||
| VRS | 2.79 ± 0.98 | 3.79 ± 1.10 | 3.46 ± 0.78 | .005‡ |
| (2.38–3.20) | (3.33–4.26) | (3.13–3.79) | ||
| 2.5 [2–3] | 4 [3–5] | 3 [3–4] |
The values are given as mean ± SD and 95% confidence interval and median [Q1–Q3].
ANOVA = analysis of variance, NMES = neuromuscular electrical stimulation, TENS = transcutaneous electrical nerve stimulation, VAS = visual analog score, VRS = verbal rating score.
Friedman test.
ANOVA for repeated measures.
Kendall tau test.
62.5% of the subjects experienced a decrease in the sensation of ES (40% of them only after placebo stimulation, 40% only after TENS or after NMES, and the remaining 20% after sham stimulation and either TENS or NMES). The mean reduction in perception was 50.3% (SD = 35.36, IQR = 20–90) after placebo stimulation, whereas it was only 16.0% after both TENS (SD = 6.5, IQR = 10–22.5) and NMES (SD = 10.84, IQR = 5–25). Regarding increments in sensation, only 16.7% of the subjects have felt an increase in any of the ES (from them, 25% after TENS, and 75% after NMES). The change in perception is not related to the type of ES applied in first place (P = .587 and .741 respectively), nor to sex (P = .500 and .705 respectively).
4. Discussion
To our knowledge, the results obtained in this study are the first to show a direct comparison in venous blood flow among TENS, NMES, and sham stimulation placed on soleus muscle. TENS and NMES increased PV and FV on healthy young people, whereas sham stimulation did not. Despite no differences in PV, TENS on muscle site has shown to achieve higher accumulated flow volume than NMES on the same location, considering for hemodynamics measurements not only the on-time of NMES, but the whole duty cycle. TENS was also better tolerated than NMES.
In a study, presumably testing TENS and NMES, similar flow enhancement results between both types of ES.[22] Some aspects make difficult comparing it to this research. First, main parameters used as amplitude and duty cycle for NMES were not specified. Second, the selected electrode location diverged from TENS and NMES, and was not accurately specified for the latter. Soleus stimulation with 1 channel is the most effective site to achieve hemodynamic improvement,[18] so this muscle placement has been chosen in the present study. Moreover, TENS was used at 10 pulses per second, a frequency which can produce a tetanic contraction, specially of soleus muscle, due to its predominance of slow-twitch fibers.[23] In the current study, a lower frequency of 5 Hz was used in order to allow the muscle to reach the resting position between contractions. This rate has shown a higher similitude to calf circumference of full active dorsiflexion than 1 Hz.[24]
The absence of difference between TENS and NMES on PV is compatible with the finding of Badger et al.[14] They compared TENS (GekoTM; FirstKind Ltd, UK) and NMES (Orthopaedic Microstim 2V2; Odstock Medical Ltd, UK)—and also an IPC device—over the common peroneal nerve of 12 healthy subjects and 5 stroke patients. Both ES were adjusted to produce a slight visible movement of the foot in the sample of healthy participants. Therefore, it means delivering TENS and NMES at a lower amplitude than in the current study. Geko works at 1 Hz, 27 mA, and pulse duration can be varied from 70 to 560 microseconds to achieve muscular contraction. NMES device was used at a pulse width of 300 microseconds and a pulse rate of 40 Hz, for half a second, every 2 seconds, thus meaning a duty cycle of 25%. NMES reached the highest values in FV compared to TENS, but without statistical significance. Significant differences with NMES were only found respect to baseline in PV and time-averaged mean velocity, whereas TENS did not show any significant difference from basal values in any variable. TENS at 1 Hz and at a low-level of amplitude may not improve blood flow adequately, whereas at a painful level may not be desirable as it can increase PV more than voluntary contraction[25] A limited increase might be a positive effect for using ES safely. In thrombosis patients compared to healthy subjects, a higher PV has been shown in the central jet of the stenotic region besides the formation of an eddy flow, thus favoring platelet aggregation.[26] Mild discomfort[21] to moderate discomfort[25] may be the required intensity to obtain an important enhancement of venous return. In the same direction, when NMES was applied at a higher amplitude (only in patient population), a greater increase in FV and PV was achieved.[14] A higher duty cycle than in the current study was delivered for NMES (25% vs 16.7%), although a fifth frequency was selected for TENS (1 vs 5 Hz). In the 14-second period of ultrasound recording, more NMES cycles, and only one fifth of the cycles concerning TENS, were measured than with our protocol. Therefore, the tendency to a higher FV with NMES than when using TENS may be because of the different parameters used. However, likely due to the small sample, and maybe due to the low amplitude and the nerve placement chosen in NMES, statistical differences were not found between both types of ES.
A recent report has compared TENS placed at common peroneal nerve and NMES on soleus muscle (motor points), besides voluntary contraction.[15] TENS (GekoTM; FirstKind Ltd) was applied at a mean pulse width of 200 microseconds for a foot twitch. NMES (Electrical & Electronic Engineering, NUI Galway, Galway, Ireland) protocol consisted of trains of biphasic pulses, 350 microseconds time duration, delivered at 36 Hz and 17V (maximum tolerable intensity). The cycle time was set at 23 seconds, with ramp up and down, and contraction times of 1 second each, and off-time of 20 seconds (13% of duty cycle). PV basal values (16.62 ± 9.1) and after TENS at 1 Hz (46.34 ± 18.3) were close to the values shown in the current study using TENS 5 Hz. However, these authors presented a higher PV using NMES (63.82 ± 18.2) than in the current research, which was also lower than the voluntary contraction.
Regarding the accumulated FV, contrarily to the current study revealing significant increments with both types of ES, Avvazadeh et al15] did not find significant improvements with any of them. This could be because of the different methodology used. Despite selecting a 23-second duration of duty cycle for NMES, Doppler recordings lasted only 12 seconds. It would make impossible to measure all the NMES period so it is not clear how they made comparisons among the different interventions. Moreover, TENS was selected at 1 Hz whereas in the current study used a frequency of 5 Hz, as in a previous research 5 Hz applied over the muscle was proved to obtain better blood flow increments.[17] Indeed, they compared pre–post values while subjects remained in sitting position. In the present investigation, the percentage of change respect to basal level was computed, and subjects adopted prone decubitus.
Other researches have obtained effective blood flow volume changes with NMES, but accounting for the on-time only.[12] It provides an indication of how much blood is mobilized when the muscle contraction takes place, ignoring the off-time and, thereby, overestimating NMES effects. The same reason has been argued in relation to IPC devices when measuring only during the inflation time.[26] Taking it into account, the measurement during the whole duty cycle provides a more realistic FV measurement for a determined NMES protocol.
Presumably, TENS has been favored in the present investigation because of the muscle electrode location and the increased cycling rate (5 brief contractions per second, 45 in each measurement sequence with Doppler ultrasound). TENS at common peroneal nerve activates calf muscles by passive stretch[27] but powerful muscles of the calf are poorly stimulated compared to active tip-toe and dorsiflexion maneuvers.[28] Moreover, when comparing TENS on common peroneal nerve versus soleus motor points, the latter was preferred in terms of tolerance of high amplitudes.[17] However, due to its nature, TENS provokes twitches to jerks (0.24 seconds of median duration), depending on the amplitude applied, low or high, respectively.[28] In the current research, NMES has a longer contraction time than TENS, to trigger a sustained contraction to ankle flexion. However, NMES was delivered employing an off-time of 10 seconds, 5 times higher than the on-time. It was done to include 1 contraction and the whole resting time cycle per each ultrasound measurement. Clear guidance on best parameters for improvement of the calf muscle pump has not been stablished. A variation in the NMES protocols may provide different results[29] as the relationship between on-time and off-time and the selected amplitude. A higher duty cycle, nearly 25%, and enough stimulus amplitude for obtaining the full range of movement might be helpful for optimizing NMES effects on hemodynamics.[14] However, the improvement in venous return could not be the only effect of ES. New evidence suggests that ES also could reduce the hypercoagulable state of the blood and the incidence of deep venous thrombosis.[3]
Similar to the current satisfaction results, TENS was rated the highest, without significant differences with NMES.[14] Other studies have found significant better tolerance with TENS than NMES.[22] Despite more subjects experienced an increase in sensation after NMES than after TENS in the present research, the proportion of subjects experiencing that increase in sensation can be assumed equal with both. In any case, when NMES protocols are extended by a period of days, tolerance may be improved.[19]
A third of the subjects perceived a reduction in the sensation after ES compared to initial tolerance. Similar percentages of subjects revealed a decreasing effect after sham stimulation than after active ES (NMES or TENS). However, a higher mean reduction was perceived after sham stimulation. Thus, a limited placebo effect may be resulted. This is the main strength of the study, the corroboration of an absence of a blood flow increases with a sham stimulation, whereas a significant enhancement in flow volume is acquired when applying active ES.
4.1. Limitations
The present study has several limitations. First, the small sample size may affect the power to detect differences between TENS and NMES regarding hemodynamics variables. Second, the ejected venous volume during on-time (single stimulus) was not calculated. For this reason, FV direct comparisons with other NMES protocols using different off-times are not possible. Third, the observations have been made in a short single session in young apparently healthy people, so direct translation to clinical area is not recommended.
In conclusion, an enhancement in hemodynamics can be achieved with NMES and TENS on soleus muscle at maximum tolerated intensity in apparently healthy people. Sham stimulation did not increase FV nor PV significantly. Also, a similar percentage of subjects with sham stimulation as with active ES felt a decreased sensation, yet at a higher mean reduction with the former. Considering the whole duty cycle of NMES (2 seconds of on-time and 10 seconds of off-time) for comparisons, TENS at 5 Hz significantly performed better improving flow volume, besides showing a better tolerance.
On-time and off-time must be explored in NMES specifically in this area, as hemodynamic results can very differently if they are modified, testing different amplitudes for improving acceptance. Future research should consider both the ejected volume of a single contraction during the on-time of NMES and flow volume during the whole period of on-time and off-time (duty cycle) for a more realistic FV mobilized in a determined period of time. These measurements will allow a better comparison among different types of ES and, also, in relation to voluntary contraction.
Author contributions
Conceptualization: Alicia Martínez-Rodríguez, Francisco Senin-Camargo, Marcelo Chouza-Insua.
Data curation: Alicia Martínez-Rodríguez, Isabel Raposo-Vidal.
Formal analysis: Alicia Martínez-Rodríguez, Isabel Raposo-Vidal, Marcelo Chouza-Insua.
Investigation: Francisco Senin-Camargo, Isabel Raposo-Vidal, Marcelo Chouza-Insua.
Methodology: Francisco Senin-Camargo, Marcelo Chouza-Insua.
Project administration: Alicia Martínez-Rodríguez, M. Amalia Jácome, Marcelo Chouza-Insua.
Resources: Alicia Martínez-Rodríguez.
Software: Alicia Martínez-Rodríguez.
Supervision: M. Amalia Jácome, Marcelo Chouza-Insua.
Validation: Francisco Senin-Camargo, M. Amalia Jácome.
Visualization: Francisco Senin-Camargo, Isabel Raposo-Vidal, Marcelo Chouza-Insua.
Writing – original draft: Alicia Martínez-Rodríguez, Francisco Senin-Camargo, Isabel Raposo-Vidal, M. Amalia Jácome.
Writing – review & editing: Alicia Martínez-Rodríguez, M. Amalia Jácome, Marcelo Chouza-Insua.
Abbreviations:
- BMI =
- body mass index
- ES =
- electrical stimulation
- FV =
- flow volume
- IPC =
- intermittent pneumatic compression
- IQR =
- interquartile range
- NMES =
- neuromuscular electrical stimulation
- PV =
- peak velocity
- TENS =
- transcutaneous electrical nerve stimulation
- VAS =
- visual analog score
- VRS =
- verbal rating score
FS-C and AM-R contributed equally to this work.
The study was funded by the College of Physical Therapists of Galicia. This research was supported by the Psychosocial and Functional Rehabilitation Research Group, based at the Faculty of Physical Therapy, Universidade da Coruña. In addition, this research has been supported by MINECO grant MTM2014-52876-R and by the Xunta de Galicia (Grupos de Referencia Competitiva ED431C-2016-015 and Centro Singular de Investigación de Galicia ED431G/01), all of them through the ERDF.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Senin-Camargo F, Martínez-Rodríguez A, Chouza-Insua M, Raposo-Vidal I, Jácome MA. Effects on venous flow of transcutaneous electrical stimulation, neuromuscular stimulation, and sham stimulation on soleus muscle: A randomized crossover study in healthy subjects. Medicine 2022;101:35(e30121).
Contributor Information
Francisco Senin-Camargo, Email: francisco.senin@udc.es.
Alicia Martínez-Rodríguez, Email: alicia.martinez@udc.es.
Isabel Raposo-Vidal, Email: isabel.raposo.vidal@udc.es.
M. Amalia Jácome, Email: maria.amalia.jacome@udc.es.
References
- [1].Chindamo MC, Marques MA. Bleeding risk assessment for venous thromboembolism prophylaxis. J Vasc Bras. 2021;20:e20200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hajibandeh S, Hajibandeh S, Antoniou GA, et al. Neuromuscular electrical stimulation for thromboprophylaxis: a systematic review. Phlebology. 2015;30:589–602. [DOI] [PubMed] [Google Scholar]
- [3].Jingwei LZ, Xuesong L, Hui J, et al. Clinical observation of neuromuscular electrical stimulation in prevention of deep venous thrombosis after total hip replacement. Chin J Bone Joint Injury. 2017;32:615–6. [Google Scholar]
- [4].Williams KJ, Ravikumar R, Gaweesh AS, et al. A review of the evidence to support neuromuscular electrical stimulation in the prevention and management of venous disease. Adv Exp Med Biol. 2017;906:377–86. [DOI] [PubMed] [Google Scholar]
- [5].Johnson MI, Jones G, Paley CA, et al. The clinical efficacy of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain: a protocol for a meta-analysis of randomised controlled trials (RCTs). BMJ Open. 2019;9:e029999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wegrzyk J, Ranjeva JP, Fouré A, et al. Specific brain activation patterns associated with two neuromuscular electrical stimulation protocols. Sci Rep. 2017;7:2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maffiuletti NA. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur J Appl Physiol. 2010;110:223–34. [DOI] [PubMed] [Google Scholar]
- [8].Glaviano NR, Saliba S. Can the use of neuromuscular electrical stimulation be improved to optimize quadriceps strengthening? Sports Health. 2016;8:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Williams KJ, Moore HM, Davies AH. Haemodynamic changes with the use of neuromuscular electrical stimulation compared to intermittent pneumatic compression. Phlebology. 2015;30:365–72. [DOI] [PubMed] [Google Scholar]
- [10].Barnes R, Madden LA, Chetter IC. Fibrinolytic effects of peroneal nerve stimulation in patients with lower limb vascular disease. Blood Coagul Fibrinolysis. 2016;27:275–80. [DOI] [PubMed] [Google Scholar]
- [11].Izumi M, Ikeuchi M, Aso K, et al. Less deep vein thrombosis due to transcutaneous fibular nerve stimulation in total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23:3317–23. [DOI] [PubMed] [Google Scholar]
- [12].Broderick BJ, O’Connell S, Moloney S, et al. Comparative lower limb hemodynamics using neuromuscular electrical stimulation (NMES) versus intermittent pneumatic compression (IPC). Physiol Meas. 2014;35:1849–59. [DOI] [PubMed] [Google Scholar]
- [13].Ojima M, Takegawa R, Hirose T, et al. Hemodynamic effects of electrical muscle stimulation in the prophylaxis of deep vein thrombosis for intensive care unit patients: a randomized trial. J Intensive Care. 2017;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Badger J, Taylor P, Papworth N, et al. Electrical stimulation devices for the prevention of venous thromboembolism: preliminary studies of physiological efficacy and user satisfaction. J Rehabil Assist Technol Eng. 2018;5:2055668318800218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Avazzadeh S, O’Farrell A, Flaherty K, et al. Comparison of the hemodynamic performance of two neuromuscular electrical stimulation devices applied to the lower limb. J Pers Med. 2020;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].De Macedo AC, Schein AS, Callegaro CC, et al. Hemodynamic responses to neuromuscular electrical stimulation and to metaboreflex activation. J Sports Med Phys Fitness. 2022;62:163–9. [DOI] [PubMed] [Google Scholar]
- [17].Martínez-Rodríguez A, Senin-Camargo F, Raposo-Vidal I, et al. Effects of transcutaneous electrical nerve stimulation via peroneal nerve or soleus muscle on venous flow: a randomized cross-over study in healthy subjects. Medicine (Baltimore). 2018;97:e12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Breen PP, Galvin O, Quondamatteo F, et al. Comparison of single- and two-channel neuromuscular electrical stimulation sites for enhancing venous return. IEEE Trans Neural Syst Rehabil Eng. 2012;20:389–94. [DOI] [PubMed] [Google Scholar]
- [19].Corley GJ, Breen PP, Bîrlea SI, et al. Hemodynamic effects of habituation to a week-long program of neuromuscular electrical stimulation. Med Eng Phys. 2012;34:459–65. [DOI] [PubMed] [Google Scholar]
- [20].Rakel B, Cooper N, Adams HJ, et al. A new transient sham TENS device allows for investigator blinding while delivering a true placebo treatment. J Pain. 2010;11:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jawad H, Bain DS, Dawson H, et al. The effectiveness of a novel neuromuscular electrostimulation method versus intermittent pneumatic compression in enhancing lower limb blood flow. J Vasc Surg Venous Lymphat Disord. 2014;2:160–5. [DOI] [PubMed] [Google Scholar]
- [22].Izumi M, Ikeuchi M, Mitani T, et al. Prevention of venous stasis in the lower limb by transcutaneous electrical nerve stimulation. Eur J Vasc Endovasc Surg. 2010;39:642–5. [DOI] [PubMed] [Google Scholar]
- [23].Bellemare F, Woods JJ, Johansson R, et al. Motor-unit discharge rates in maximal voluntary contractions of three human muscles. J Neurophysiol. 1983;50:1380–92. [DOI] [PubMed] [Google Scholar]
- [24].Tucker A, Maass A, Bain D, et al. Augmentation of venous, arterial and microvascular blood supply in the leg by isometric neuromuscular stimulation via the peroneal nerve. Int J Angiol. 2010;19:e31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yang S, Gong X, Wei S, et al. A hemodynamic study of the effect of neuromuscular electrical stimulation on enhancing popliteal venous flow. J Shanghai Jiaotong Univ Sci. 2014;19:706–11. [Google Scholar]
- [26].Bajd F, Vidmar J, Fabjan A, et al. Impact of altered venous hemodynamic conditions on the formation of platelet layers in thromboemboli. Thromb Res. 2012;129:158–63. [DOI] [PubMed] [Google Scholar]
- [27].Zhang Q, Styf J, Ekström L, Holm AK. Effects of electrical nerve stimulation on force generation, oxygenation and blood volume in muscles of the immobilized human leg. Scand J Clin Lab Invest. 2014;74:369–77. [DOI] [PubMed] [Google Scholar]
- [28].Lattimer CR, Zymvragoudakis V, Geroulakos G, et al. Venous thromboprophylaxis with neuromuscular stimulation: is it calf muscle pumping or just twitches and jerks? Clin Appl Thromb Hemost. 2018;24:446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wou J, Williams KJ, Davies AH. Compression stockings versus neuromuscular electrical stimulation devices in the management of occupational leg swelling. Int J Angiol. 2016;25:104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]