Abstract
Ovarian atypical endometriosis (AE) is a premalignant lesion, and its potential to progress to endometriosis-associated ovarian cancer emphasizes its significance. However, the true risk of malignancy in AE remains unclear. Therefore, this study aimed to investigate the clinical outcomes of ovarian AE after ovarian cystectomy. We retrospectively reviewed the medical records and histopathological reports of 41 patients who had been diagnosed with ovarian AE between January 2011 and April 2020. We reviewed age, obstetric history, age at menarche, preoperative CA 125 level, C-reactive protein level, erythrocyte sedimentation rate, endometriosis stage, mean follow-up duration, postoperative hormonal therapy, and prognosis, including recurrence of endometriosis and malignant transformation. Among 41 patients with pathologically diagnosed ovarian AE, 26 were followed up after cystectomy only. The average follow-up period was 58.27 ± 33.22 months in cystectomy only patients. The mean age of the patients with cystectomy only versus that of patients with endometriosis-associated ovarian carcinoma was 32.73 ± 6.10 versus 48.29 ± 4.35 (P < .01) years. The preoperative CA 125 level was 115.63 ± 219.06 versus 225.75 ± 163.39 (P < .051) U/mL. Patients with endometriosis-associated ovarian carcinoma or other diseases and those who underwent oophorectomy were excluded. After surgery, hormone therapy was administered to 22 of 26 patients, and the remaining 4 patients were followed up without additional treatment. Endometriosis recurrence occurred in 5 patients, 1 of whom underwent second-line laparoscopic ovarian cystectomy. However, no malignant transformations were observed. Ovarian AE has a low possibility of malignant transformation. Conservative treatment is recommended after appropriate ovarian cystectomy, such as enucleation.
Keywords: atypical endometriosis, endometriosis-associated ovarian carcinoma, ovarian cystectomy, recurrence
1. Introduction
Endometriosis is a common gynecological disorder characterized by ectopic growth of endometrial glands and stroma.[1] Its prevalence in the general population ranges from 5% to 15% according to 1 study, and is increased among women with pelvic pain and/or infertility at a range of 30% to 50%.[2] Recently, in addition to surgical diagnosis, diagnosis using imaging modalities such as sonography and magnetic resonance imaging (MRI) has been introduced, and consequently the incidence rate has increased.
In 1925 and 1953, Sampson and Scott proposed the theory that endometriosis, a benign disease, could progress to malignancy.[3,4] They explained that when benign and malignant tissues coexist, gradual changes such as cytologic atypia, are seen in both malignant changes in endometriosis.
Accumulating evidence suggests that ovarian endometriosis can give rise to various ovarian malignancies, generally termed endometriosis-associated ovarian carcinomas (EAOCs). EAOC may refer to ovarian cancer with both cancer cells and endometriosis or the presence of both ovarian cancer and pelvic endometriosis.[5] Studies have shown that the risk of progression from endometriosis to malignancy is approximately 0.5% to 1%.[6]
Atypical endometriosis (AE) is diagnosed pathologically according to the endometriotic glands and whether cytologic atypia or architectural atypia, which is very rare, are present. AE is recognized as a premalignant lesion, and the existence of EAOC further emphasizes its importance.
However, the true risk of malignancy in AE versus typical ovarian endometriosis remains unclear. However, Fukunaga et al[7] recommended that close scrutiny of cellular atypia or hyperplasia in cases of endometriosis be applied to determine increased risk of concurrent or subsequent malignancy in the ovarian and extra-ovarian sites.
Many researchers have reported that AE possesses premalignant potential characterized by cytologic atypia and architectural atypia attributed to premalignant changes.[7–9] Even if no ovarian malignancy is present at the time of diagnosis, carcinoma may develop later from the remnant AE lesion. Therefore, close monitoring and long-term follow-up are recommended for patients diagnosed with AE.[10]
Although standards for management of AE have not yet been established, cystectomy or unilateral oophorectomy are most often performed when AE occurs in women of childbearing age. However, studies on the rate of recurrence or progression of AE to ovarian cancer are lacking.
Thus, this study aimed to investigate the clinical outcomes of ovarian AE after ovarian cystectomy.
2. Materials and Methods
2.1. Patients
We retrospectively reviewed the medical records and histopathology reports of 41 patients that had been diagnosed with AE at Kyungpook National University Chilgok Hospital between January 2011 and April 2020. This study was approved by the Institutional Review Board of our hospital (KNUCH 2022-01-002). The need for informed consent was waived owing to the retrospective design of the study. All procedures performed were in accordance with the ethical standards of the institution as well as the 1964 Declaration of Helsinki and its later amendments.
We searched for AEs using a clinical database and big data, BIG-CEN MED (version 2.0; SOFTCEN Ltd., Korea). We reviewed age, obstetric history, age at menarche, preoperative CA 125 level, C-reactive protein level, erythrocyte sedimentation rate, endometriosis stage, mean follow-up period, postoperative hormonal therapy, and prognosis such as recurrence of endometriosis or malignant transformation.
2.2. Atypical endometriosis and the stage of endometriosis
The diagnosis of AE was made based on the presence of epithelial cells with features previously suggested by Czernobilsky and Morris, and LeGrenade and Silverberg.[11,12] These features included large hyperchromatic or pale nuclei with moderate to marked pleomorphism, increased nuclear-to-cytoplasmic ratio, as well as cellular crowding, stratification, or tufting. All pathological sections were reviewed by qualified pathologists. The severity of endometriosis was assessed as stages I to IV according to the revised American Fertility Society classification, as amended in 1985.
2.3. Recurrence
Recurrence was diagnosed by pathological confirmation, and suspected recurrence was based on ultrasonography and/or MRI. Recurrence of ovarian endometrioma was considered when transvaginal or transrectal ultrasonography indicated the presence of a round cystic mass with a minimum diameter of 20 mm, thick walls, regular margins, homogeneous low echogenic fluid content with scattered internal echoes, and the absence of papillary proliferation.[13] If these lesions persisted for more than 2 months, they were considered recurrent lesions.
Patients with suspected uterine endometriosis and peritoneal endometriosis such as those with increased CA 125 or increased menstrual pain and those without ovarian lesions were considered to have no recurrence.
2.4. Statistical analysis
Continuous data were expressed as mean ± standard deviation, and categorical data were presented as frequencies and percentages. The follow-up date was calculated as the interval from the date of surgery to the date of the last outpatient visit. The Mann–Whitney U test was used to compare the 2 groups. All statistical analyses were performed using MedCalc® Statistical Software version 20.015 (MedCalc Software Ltd., Ostend, Belgium; https://www.medcalc.org; 2021).
3. Results
3.1. Patients’ distribution and postoperative treatment
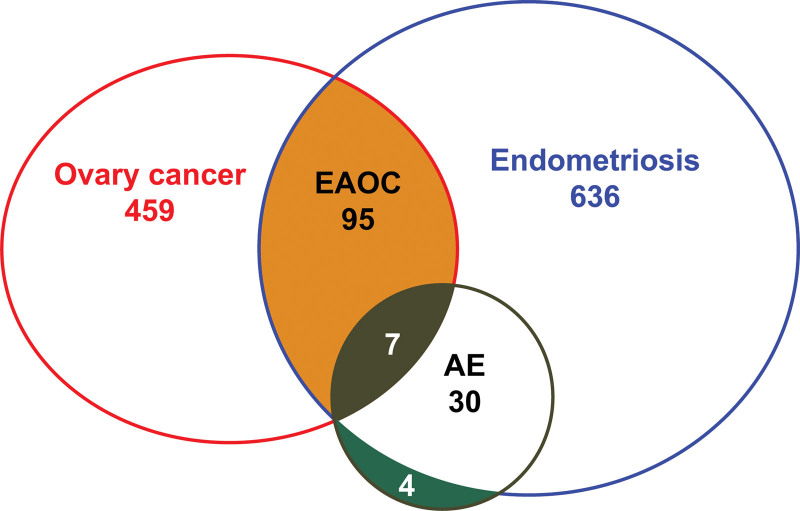
In total, data from 1231 patients with ovarian cancer or ovarian endometriosis were included in the analysis. Of these, 570 patients had epithelial ovarian cancer, 741 had ovarian endometriosis, and 103 had EAOC. Seven patients were diagnosed with EAOC and AE, 30 with AE only, and 4 with AE and breast or cervical cancer. A total of 41 patients had AEs (Fig. 1).
Figure 1.
Content overlap and differences between ovarian cancer and endometriosis. The green portion presents 4 patients with atypical endometriosis (AE) and other malignancies, such as breast or cervical cancer.
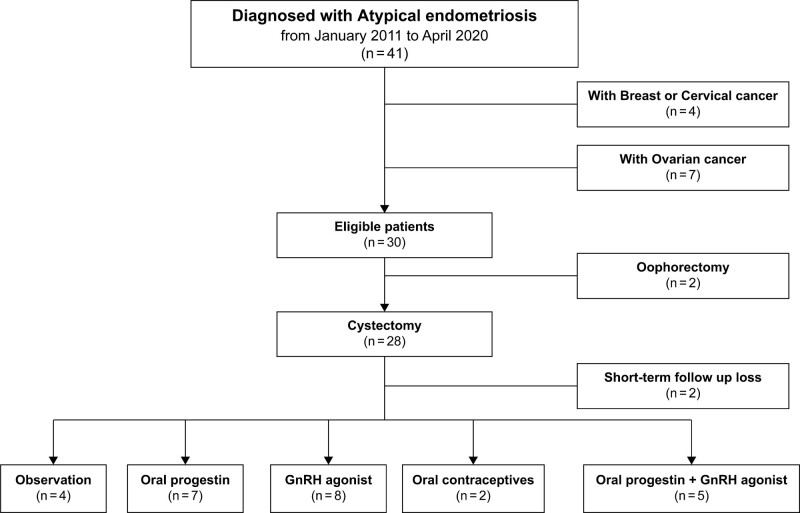
Among the 41 patients with pathologically diagnosed AE after surgery, 26 were followed up after undergoing cystectomy only; excluding 7 patients with EAOC, 4 with other malignancies, and 2 who had undergone oophorectomy. Among the patients who had undergone ovarian cystectomy, 2 were excluded because they were lost to follow-up immediately after surgery. Postoperative hormonal therapy was administered to 22 patients, and the remaining 4 patients were followed up without additional treatment. Hormonal therapy comprised oral progestin, gonadotropin-releasing hormone agonist, and oral contraceptives (Fig. 2).
Figure 2.
Selection of patients with atypical endometriosis.
3.2. Clinicopathologic characteristics and recurrence of cystectomy group
The average follow-up period was 58.27 ± 33.22 months in patients with cystectomy only. The mean age of the patients with cystectomy only versus that in patients with EAOC was 32.73 ± 6.10 versus 48.29 ± 4.35 (P < .01) years. The preoperative CA 125 level was 115.63 ± 219.06 versus 225.75 ± 163.39 (P < .051) U/mL (Table 1).
Table 1.
Characteristics of patients with atypical endometriosis.
| Variables | EAOC (n = 7) | Cystectomy (n = 26) | P value |
|---|---|---|---|
| Mean age (yr) | 48.29 ± 4.35 | 32.73 ± 6.10 | <.01 |
| Obstetric history | |||
| Gravity (n) | 2.28 ± 1.89 | 0.54 ± 0.86 | .01 |
| Parity (n) | 1.43 ± 0.98 | 0.42 ± 0.76 | .01 |
| Abortion (n) | 0.86 ± 1.22 | 0.12± 0.33 | .03 |
| Menarche (yr) | 13.71 ± 1.38 | 13.32 ± 1.46 | .48 |
| CRP (mg/dL) | 1.10 ± 0.90 | 0.74 ± 3.16 | <.01 |
| ESR (mm/hr) | 53.57 ± 43.17 | 19.88 ± 16.86 | .06 |
| HE4 (pmol/L) | 85.88 ± 46.59 | 42.00 ± 13.23 | .08 |
| Preoperative CA 125 (U/mL) | 225.75 ± 163.39 | 115.63 ± 219.06 | .05 |
| ASRM stage (n, %) | |||
| I | 0 (0) | ||
| II | 4 (16.0) | ||
| III | 3 (12.0) | ||
| IV | 18 (72.0) | ||
| Mean follow-up period (mo) | 58.27 ± 33.22 | ||
| Medication duration (mo) (total n =22) | |||
| Oral progestin (n = 7) | 19.71 ± 8.14 | ||
| GnRHa (n = 8) | 4.13 ± 1.55 | ||
| Oral contraceptives (n = 2) | 45.50 ± 50.20 | ||
| Oral progestin + GnRHa (n = 5) | 19.33 ± 7.42 | ||
ASRM = American Society for Reproductive Medicine, CA 125 = cancer antigen 125, CRP = C-reactive protein, EAOC = endometriosis-associated ovarian carcinoma, ESR = erythrocyte sedimentation rate, GnRHa = gonadotropin-releasing hormone agonist, HE4 = human epididymis protein 4.
Recurrence was suspected in 5 of the 26 patients who had undergone ovarian cystectomy based on ultrasonography and/or MRI findings. One patient underwent second-line laparoscopic ovarian cystectomy. The histological results were typical of ovarian endometriosis. No malignant transformation of AE to ovarian cancer was observed.
3.3 Histologic types of endometriosis-associated ovarian carcinoma
All histological types of the 7 patients with EAOC fell under the categories of clear cell or endometrioid carcinoma (Table 2).
Table 2.
Histologic type of ovarian carcinoma with atypical endometriosis.
| Histology | Number | Percentage (%) |
|---|---|---|
| Clear cell carcinoma | 4 | 57.1 |
| Endometrioid carcinoma | 1 | 14.3 |
| Mixed type | ||
| Clear cell carcinoma (50%) and endometrioid carcinoma (50%) | 1 | 14.3 |
| Endometrioid carcinoma (75%) and mucinous carcinoma (15%) | 1 | 14.3 |
| Total | 7 | 100 |
4. Discussion
This study aimed to investigate the practical risks of AEs in clinical practice. We reviewed the cases at our institution along with the existing literature to determine the safety of ovarian cystectomy using a minimally invasive surgical approach in AE, which is considered a precancerous lesion.
Among the 26 patients at our institution, 5 (19.2%) were suspected or surgically confirmed to have recurrence of typical ovarian endometriosis. No malignant transformation of AE to ovarian cancer was observed. Similarly, in 2 recently published papers, despite many patients with AE, malignant transformation from AE to ovarian cancer has not been reported.[14,15] These studies focused on comparing the characteristics and recurrence rate of AE with those of typical endometriosis. On the other hand, our study focused on whether it was safe to preserve the ovaries in young patients with AE.
In contrast, there have been previous reports on the transformation from AE to EAOC; 2 cases of AE that developed into ovarian cancer after surgery can be found in the literature,[9,16] wherein transformation occurred at 3 years to 68 months after the first surgery. The difference in age between patients with EAOC and AE suggests the need for long-term follow-up of 15 years or more in AE patients.
Through a review of cases from both our institution and the literature, it can be predicted that malignant transformation from AE to EAOC rarely occurs after surgery (Table 3).[8,14–16] However, several pieces of clinical, histological, and molecular biological evidence have suggested that AE is a disease with potential for malignant transformation. Among patients with endometriosis, a recent study reported that the prevalence of AE without ovarian cancer was 8.8% but increased to 34.6% in cases of ovarian cancer.[17] The literature reports an incidence of 1.7%[18] to 32.3% for AE in cases without ovarian cancer[19] and 4.4%[18] to 22.8%[20] for AE in cases with ovarian cancer. In this study, the incidence of AEs was 4.5% (30/666) in patients without ovarian cancer and 6.9% (7/102) in patients with ovarian cancer. These results suggest an association between endometriosis, AE, and ovarian cancer in clinical practice.
Table 3.
Clinical reports of atypical endometriosis
| Author | AE (N) | Follow-up (M) or (yr) | Malignant transformation† (%) | Endometriosis recur. N (%) | Surgery | |
|---|---|---|---|---|---|---|
| 2022 | Hong et al* | 41 | 58.27 | 0 (0 %) | 5 (19.2%) | Cystectomy: 26 |
| Oophorectomy:2 | ||||||
| 2021 | Won et al | 86 | 32.4 | 0 (0%) | 18 (20.9%) | n/a |
| 2021 | So et al | 98 | 44.5 | 0 (0%) | n/a | n/a |
| 2019 | Tanase et al | 9 | 68.0 | 1 (11.1%) | AE→EAOC | Cystectomy |
| 1990 | Moll et al | 1 | 3yrs | 1 | AE→EAOC | n/a |
AE = atypical endometriosis, EAOC = endometriosis associated ovarian carcinoma, n/a = none available.
*Current study.
Atypical endometriosis to ovarian cancer.
Differences have also been reported in the association between AE and ovarian cancer according to histology. In 1 study, the authors divided AE into AE with cytological (cellular) atypia and AE with architectural (hyperplasia) atypia. Cellular AE was observed in patients without ovarian cancer (71.4%), while architectural AE was seen in patients with ovarian cancer (88.9%) (P = .009). Ki-67 expression was significantly higher in patients with architectural AE than in those with cellular AE. This study reported that architectural AE has a stronger relationship with ovarian cancer than cytological AE, which accounts for most AE cases.[17]
Another study implicated ARID1A as a tumor-suppressor gene that is frequently altered in ovarian clear cell and endometrioid carcinomas. Since ARID1A mutations and BAF250a loss can be seen in preneoplastic lesions, the authors suggested that ARID1A mutations manifesting as BAF250a loss may be an important early event in the malignant transformation of endometriosis to ovarian cancer. In 2 patients, ARID1A mutations and loss of BAF250a expression were evident in the tumor and contiguous AE, but not in distant endometriotic lesions.[21]
Shin et al suggested that upregulated levels of tetraspanin 1 are an early event in the development of high-risk endometriosis that can progress to ovarian cancer. Tetraspanin 1 has also been found to increase endometrial cell growth and invasion by promoting AMP-activated protein kinase activity.[22]
Many reports have demonstrated the coexistence of AE and epithelial ovarian cancer, such as clear cell or endometrioid carcinoma; however, very few reports have been published on the occurrence of ovarian cancer after surgery for AE.
Clinically, the evaluation of additional surgery or treatment decisions and prognosis after AE diagnosis are very important. Determination of additional treatment takes into account the patient’s age, desire for further pregnancy, fear of cancer, previous surgical methods, such as complete enucleation or cauterization, and residual lesions after surgery.
Histologically, AE is most commonly of cellular type, and the expression rate of Ki-67, a cell proliferation index, is reported to be 5.93%, while loss of BAF250a is reported to be 9.1%.17 Therefore, the possibility of malignant transformation is very low, and most cases exhibit reactive or inflammatory cellular changes.[17] These lesions are limited to the cystic epithelium, and most are removed by enucleation without invasion, which means that residual lesions are unlikely to remain.
Considering these points, AE has a very low possibility of malignant transformation; however, if it does not affect future pregnancy and does not cause a decrease in quality of life due to early menopause, unilateral oophorectomy can also be recommended using an additional minimally invasive surgical approach.
Based on these research results, it is necessary to prepare an evaluation method for future risks when diagnosing AE, so that the risk of malignant transformation can be appropriately explained to patients.
Our study has several limitations. First, as a retrospective study, the number of patients included in the study was small. The following does not distinguish between cellular and architectural atypia in diagnosing AE pathologically. In addition, research on biomarkers has not been conducted in our institution’s research, which warrants future studies.
5. Conclusion
In conclusion, ovarian AE has a low possibility of malignant transformation. Conservative treatment with close and long-term follow-up is recommended after appropriate ovarian cystectomy.
Acknowledgments
We thank our colleagues Juhun Lee, Ji Young Park, Yoon Hee Lee, and Gun Oh Chong who provided insight and expertise that greatly assisted the research.
Author contributions
Conceptualization: JM Kim, J Lee, JY Park, YH Lee, GO Chong, DG Hong.
Data curation: JM Kim, J Lee, JY Park, YH Lee, GO Chong, DG Hong.
Formal analysis: JM Kim, J Lee, JY Park, YH Lee, GO Chong, DG Hong.
Funding acquisition: DG Hong.
Investigation: JM Kim, J Lee, JY Park, YH Lee, GO Chong, DG Hong.
Methodology: JM Kim, DG Hong.
Project administration: JM Kim, J Lee, JY Park, YH Lee, GO Chong, DG Hong.
Resources: JM Kim, J Lee, JY Park, YH Lee, GO Chong, DG Hong.
Software: JM Kim, DG Hong.
Supervision: JM Kim, J Lee, JY Park, YH Lee, GO Chong, DG Hong.
Validation: JM Kim, J Lee, JY Park, YH Lee, GO Chong, DG Hong.
Visualization: JI Kim, DG Hong.
Writing – original draft: JI Kim, DG Hong.
Writing – review and editing: JM Kim, J Lee, JY Park, YH Lee, GO Chong, DG Hong.
Conceptualization: Dae Gy Hong, Jong Mi Kim.
Data curation: Dae Gy Hong, Jong Mi Kim.
Formal analysis: Dae Gy Hong, Jong Mi Kim.
Funding acquisition: Dae Gy Hong.
Investigation: Dae Gy Hong, Jong Mi Kim.
Methodology: Dae Gy Hong, Jong Mi Kim.
Project administration: Dae Gy Hong, Jong Mi Kim.
Resources: Dae Gy Hong, Jong Mi Kim.
Software: Dae Gy Hong, Jong Mi Kim.
Supervision: Dae Gy Hong, Jong Mi Kim.
Validation: Dae Gy Hong, Jong Mi Kim.
Visualization: Dae Gy Hong, Jong Mi Kim.
Writing – original draft: Dae Gy Hong, Jong Mi Kim.
Writing – review & editing: Jong Mi Kim, Dae Gy Hong.
Abbreviations:
- AE =
- atypical endometriosis
- EAOCs =
- endometriosis-associated ovarian carcinomas
How to cite this article: Kim JM, Hong DG. Is ovarian cystectomy for atypical ovarian endometrioma safe?: A single center study. Medicine 2022;101:35(e30105).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Guo SW. Endometriosis and ovarian cancer: potential benefits and harms of screening and risk-reducing surgery. Fertil Steril. 2015;104:813–30. [DOI] [PubMed] [Google Scholar]
- [2].Zafrakas M, Grimbizis G, Timologou A, et al. Endometriosis and ovarian cancer risk: a systematic review of epidemiological studies. Front Surg. 2014;1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sampson JA. Endometrial carcinoma of the ovary arising in endometrial tissue in that organ. Am J Obstet Gynecol. 1925;9:111–4. [Google Scholar]
- [4].Scott RB. Malignant changes in endometriosis. Obstet Gynecol. 1953;2:283–9. [PubMed] [Google Scholar]
- [5].Grandi G, Toss A, Cortesi L, et al. The association between endometriomas and ovarian cancer: preventive effect of inhibiting ovulation and menstruation during reproductive life. Biomed Res Int. 2015;2015:751571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mikami Y. Endometriosis-related ovarian neoplasms: pathogenesis and histopathologic features. Diagn Histopathol. 2014;20:357–63. [Google Scholar]
- [7].Fukunaga M, Nomura K, Ishikawa E, et al. Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology. 1997;30:249–55. [DOI] [PubMed] [Google Scholar]
- [8].Moll UM, Chumas JC, Chalas E, et al. Ovarian carcinoma arising in atypical endometriosis. Obstet Gynecol. 1990;75(3 Pt 2):537–9. [PubMed] [Google Scholar]
- [9].Leng J, Lang J, Guo L, et al. Carcinosarcoma arising from atypical endometriosis in a cesarean section scar. Int J Gynecol Cancer. 2006;16:432–5. [DOI] [PubMed] [Google Scholar]
- [10].Fukunaga M. Atypical ovarian endometriosis. Pathol Case Rev. 2006;11:38–42. [Google Scholar]
- [11].Czernobilsky B, Morris WJ. A histologic study of ovarian endometriosis with emphasis on hyperplastic and atypical changes. Obstet Gynecol. 1979;53:318–23. [PubMed] [Google Scholar]
- [12].LaGrenade A, Silverberg SG. Ovarian tumors associated with atypical endometriosis. Hum Pathol. 1988;19:1080–4. [DOI] [PubMed] [Google Scholar]
- [13].Mais V, Guerriero S, Ajossa S, et al. The efficiency of transvaginal ultrasonography in the diagnosis of endometrioma. Fertil Steril. 1993;60:776–80. [DOI] [PubMed] [Google Scholar]
- [14].Won S, Cho YJ, Lee N, et al. Atypical endometriosis is related to a higher recurrence rate. Eur J Obstet Gynecol Reprod Biol. 2020;254:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].So KA, Hong SR, Kim NR, et al. Association between atypical endometriosis and ovarian malignancies in the real world. J Ovarian Res. 2021;14:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tanase Y, Kawaguchi R, Uchiyama T, et al. Long-term follow-up after surgical management for atypical endometriosis: a series of nine cases. Case Rep Oncol. 2019;12:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ñiguez Sevilla I, Machado Linde F, Marín Sánchez MDP, et al. Prognostic importance of atypical endometriosis with architectural hyperplasia versus cytologic atypia in endometriosis-associated ovarian cancer. J Gynecol Oncol. 2019;30:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Oral E, Ilvan S, Tustas E, et al. Prevalence of endometriosis in malignant epithelial ovary tumours. Eur J Obstet Gynecol Reprod Biol. 2003;109:97–101. [DOI] [PubMed] [Google Scholar]
- [19].Seidman JD. Prognostic importance of hyperplasia and atypia in endometriosis. Int J Gynecol Pathol. 1996;15:1–9. [DOI] [PubMed] [Google Scholar]
- [20].Ogawa S, Kaku T, Amada S, et al. Ovarian endometriosis associated with ovarian carcinoma: a clinicopathological and immunohistochemical study. Gynecol Oncol. 2000;77:298–304. [DOI] [PubMed] [Google Scholar]
- [21].Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shin HY, Yang W, Chay DB, et al. Tetraspanin 1 promotes endometriosis leading to ovarian clear cell carcinoma. Mol Oncol. 2021;15:987–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]