Abstract
Advanced malignant melanoma (MM) is treated with immune checkpoint inhibitor (ICI) therapy, which often results in several immune-related adverse events. Fulminant type 1 diabetes mellitus (T1DM) is a rare, rapidly progressive, life-threatening disease. Here, we summarize 8 cases of MM with ICI-induced T1DM and describe one case that developed fulminant T1DM due to nivolumab therapy. We retrospectively reviewed patients treated with ICI from 2014 to 2021 at our hospital. The clinical features and risk factors of ICI-induced T1DM were discussed. ICIs were administered to 426 MM patients at our hospital. Among these, nivolumab was administered in 5 cases, pembrolizumab in 1 case, and the combination of nivolumab and ipilimumab in 2 cases. The frequency of ICI-associated T1DM was 1.88%. The mean glycated hemoglobin level at T1DM onset was 8.0 ± 1.0%. Of the patients, 75% were diagnosed with fulminant T1DM, 62.5% developed diabetic ketoacidosis, and 25% had glutamic acid decarboxylase (GAD) antibodies (an early predictive marker for T1DM). The mean interval between the first ICI administration and T1DM development was 201 ± 187 days. The mean duration of resumption was 13 ± 7 days. We should monitor for T1DM development following treatment with ICIs. ICI can be continued to be used to treat MM if insulin therapy successfully controls T1DM.
A 67-year-old patient who received adjuvant nivolumab therapy developed fulminant T1DM and thyrotoxicosis 57 days later and tested positive for GAD antibodies. Subsequently, he developed hypophysitis and an isolated adrenocorticotropin deficiency. He continued receiving nivolumab along with self-injected insulin without developing recurrence.
Keywords: autoimmune polyendocrine syndrome, immune checkpoint inhibitor, immune-related adverse events, malignant melanoma, type 1 diabetes
1. Introduction
Following the approval of nivolumab for malignant melanoma (MM) in Japan in 2014, immune checkpoint inhibitors (ICIs) have been increasingly used to treat advanced MM. CD8 + T cells activated by ICIs clear tumors mainly by inducing cell death through perforin-granzyme and Fas-Fas ligand pathways.[1] Accordingly, multiple immune-related adverse events (irAEs) (such as type 1 diabetes mellitus [T1DM]) have been reported.[2] Type 1 diabetes is caused by the destruction of insulin-producing beta cells of the pancreas through an immune-mediated mechanism. Since program death 1 (PD-1) is a negative regulator of the immune reaction, abnormalities in the PD-1 pathway cause hyperactivity of immune function, leading to the development of autoimmune diseases, such as T1DM. Immune checkpoint inhibitor-induced T1DM is a severe side effect due to its rapid progression. It often presents as ketoacidosis and could be fatal without appropriate treatment.[3] In this report, we present a case of mucosal MM, complicated by fulminant T1DM, with nivolumab therapy, along with 7 other T1DM cases at our hospital. The clinical features and risk factors of ICI-induced T1DM were also discussed.
2. Case report
A 67-year-old Japanese man experienced repeated epistaxis. He had a history of chronic sinusitis and thyroiditis and did not take any medications. On consultation with an otolaryngologist, a black broad-based mass in the left inferior turbinate was detected and resected. The patient was diagnosed with MM without metastasis based on pathological examination and computed tomography. The left upper maxillary region was additionally excised and confirmed complete resection (stage III). Nivolumab was administered as adjuvant therapy.
Although the patient successfully completed the fourth cycle of nivolumab, he experienced extreme thirst, polyurea, and 6-kg weight loss 2 weeks after the fourth cycle of nivolumab. Blood tests revealed prominent hyperglycemia. He had a blood glucose level of 539 mg/dL, glycated hemoglobin (HbA1c) 7.1%, glycoalbumin 23.3%, insulin 8.2 µU/mL, C-peptide 2.6 ng/mL, anti–glutamic acid decarboxylase (GAD) antibodies > 2000 U/mL, adrenocorticotropic hormone 30.1 pg/mL, cortisol 15.28 mg/dL, thyroid-stimulating hormone 0.01 µIU/mL, triiodothyronine 4.49 pg/mL, and free thyroxine 1.7 ng/dL. The arterial blood pH was 7.40. He was diagnosed with fulminant type 1 diabetes (FT1DM) (Grade 4) and thyrotoxicosis (Grade 1). He had no previously reported abnormalities related to diabetes. He tested positive for DRB1*08:02-DQB*03:02.
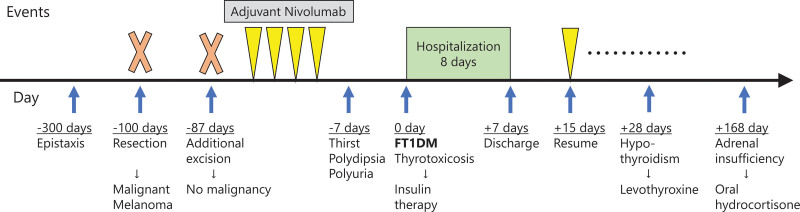
He was immediately hospitalized for hyperglycemia, and insulin treatment was initiated. His symptoms gradually improved with insulin treatment. He was discharged one week after the onset of irAE as his blood glucose level had stabilized. Two weeks after irAE onset, nivolumab was resumed because insulin could be supplied even if pancreatic function was lost. He successfully continued receiving nivolumab along with self-injected insulin without developing recurrence (Fig. 1).
Figure 1.
Clinical course of the present case report. FT1DM = fulminant type 1 diabetes.
He was in thyrotoxicosis when he developed hyperglycemia. Thyrotoxicosis spontaneously improved and hypothyroidism (Grade 2) progressed, and he started taking levothyroxine. This is a typical course for ICI-induced hypothyroidism. In particular, he may have had a high risk of developing hypothyroidism because of positive antithyroglobulin and anti-TPO antibodies. Six months later, it was suspected that he developed adrenal insufficiency due to malaise and loss of appetite. He was diagnosed with isolated adrenocorticotropin deficiency by ACTH 8.9 pg/mL, cortisol 1.51 µg/dL, and no pituitary swelling. He was prescribed oral hydrocortisone therapy and is currently alive with no recurrence 15 months after the surgery. He was diagnosed with autoimmune polyendocrine syndrome (APS) following functional impairment of pancreatic islets, thyroid glands, and adrenal glands.
3. Discussion
Fulminant type 1 diabetes is an independent subtype of type 1 diabetes that was discovered in Japan and clinically characterized by markedly rapid onset of hyperglycemia with ketoacidosis, near-normal levels of HbA1c despite remarkable hyperglycemia, and negative status of islet cell autoantibodies.[4] The Japan Diabetes Society reported the diagnostic criteria for FT1DM as pancreatic islets express PD-L1 and are susceptible to immune destruction; PD-1 inhibitors could induce diabetes.[5] ICI-related FT1DM sometimes includes acute-onset T1DM and progresses less rapidly than conventional FT1DM.[6] The frequency of ICI-induced T1DM was reportedly 0.2%.[7]
We have summarized 8 cases of MM with ICI-induced T1DM at our hospital between 2014 and 2021 (Table 1). ICIs were administered to 426 MM patients, and the frequency of ICI-induced T1DM in our hospital was 1.88%. This rate was higher than that recorded in previous clinical trials due to the inclusion of combination therapy. Also, the frequency of ICI-induced T1DM may be high in Japan due to the higher proportion of FT1DM there.[4] The average age at onset was 54 years. There were no notable differences between the sexes. Regarding the sites of the primary lesions, 4 cases were mucosal, 3 were cutaneous, and the site in one case was unknown. The rates and grades of irAE were comparable between the patients with mucosal MM and those with cutaneous MM.[8] Nivolumab was administered in 5 cases, whereas pembrolizumab was administered in 1 case. In 2 cases, the combination of nivolumab and ipilimumab was used. The mean HbA1c at T1DM onset was 8.0 ± 1.0% (mean ± standard deviation). There were 6 diagnosed cases of FT1DM (75%) and 2 diagnosed cases of acute-onset T1DM. Diabetic ketoacidosis, which could be fatal, occurred in 5 cases (62.5%). Three patients (37.5%) were transported by ambulance. There were no deaths in our cases but sudden death due to FT1DM has been previously reported.[9] It is essential to detect diabetic ketoacidosis before the onset or immediately thereafter. Signs of hyperglycemia and symptoms, such as malaise, thirst, and polyuria, should be recognized immediately. Two patients (25%) had anti-GAD antibodies; one was the Japanese patient described in the present case, and the other was a Malaysian case. A previous report showed that half of the ICI-induced T1DM patients were positive for T1DM-associated antibodies, the most common of which was the GAD antibody.[10] The GAD antibody positivity rate in ICI-induced T1DM was reportedly as low as 4.5% in Japan.[11] This racial difference in GAD antibody positivity rate might be related to the difference in human leukocyte antigen (HLA). The GAD antibody is an early predictive marker associated with a high risk of developing T1DM.[12] It might be useful for measuring GAD antibodies before or during ICI administration. The mean interval between ICI initiation and T1DM development was 201 ± 187 days, ranging from 22 to 484 days. In previous reports, the mean duration was 155 ± 123 days, and there was also a large variation.[11] Cases with a shorter duration had lower HbA1c levels, indicating a more rapidly progressive T1DM.[13] The statistical correlation is unknown because of the small number of cases. However, the development of ICI-induced T1DM should be considered during and after ICI administration. Four out of the 8 patients continued receiving the same ICI. The mean duration of resumption was 13 ± 7 days. The other patients reverted to the same ICI after using the other ICIs. The same ICI can be used to treat MM if insulin therapy successfully controls T1DM. Our case report had the HLA-DRB1*0802-HLA-DQB1*0302 haplotype. It was reported that DRB1*0802-DQB1*0302 was associated with acute-onset T1DM, but only DRB1*0405-DQB1*0401 was associated with fulminant T1DM.[14] Only one of our patients had DRB1*0405-DQB1*0401. Three patients experienced other irAEs aside from T1DM. This case report also had DRB1*08:02-DQB*03:02 and describes hypothyroidism and hypophysitis. The first patient had thyrotoxicosis and hypophysitis, while the second had colitis and hypothyroidism. These 3 cases were likely caused by APS.
Table 1.
Summary of the 8 Japanese patients developed immune checkpoint inhibitor-induced T1DM.
| No. | Age | Sex | Primary lesion | Drug | HbA1c (%) | Type | Diabetic ketoacidosis | Emergency transport | GAD antibody | Duration to diabetes (d) | Duration to resume (d) | Other irAEs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 74 | F | Nasal cavity | Nivolumab | NE | Fulminant | NE | + | NE | 122 | None | − |
| 2 | 46 | M | Foot sole | Pembrolizumab | 7.5 | Fulminant | + | + | − | 406 | None | − |
| 3 | 46 | M | Choroid | Nivolumab | 8.2 | Fulminant | + | − | >2000 | 39 | 21 | − |
| 4 | 48 | M | Head | Nivolumab | 9.2 | Acute-onset | + | + | − | 484 | None | − |
| 5 | 49 | F | Vagina | Nivolumab | 10 | Acute-onset | + | − | − | 373 | (291) | − |
| 6 | 63 | F | Unknown | Nivo/Ipi | 6.0 | Fulminant | − | − | − | 107 | 9 | Thyroiditis, hypophysitis |
| 7 | 36 | F | Head | Nivo/Ipi | 6.2 | Fulminant | + | − | − | 22 | 5 | Colitis, thyroiditis |
| 8 | 67 | M | Nasal cavity | Adj Nivolumab | 7.1 | Fulminant | − | − | >2000 | 57 | 15 | Thyroiditis |
Case 8 is our case report.
F = female, GAD = glutamic acid decarboxylase, HbA1c = glycated hemoglobin, irAEs = immune-related adverse events, M = male, NE = not evaluated, T1DM = type 1 diabetes mellitus.
First reported by Schmidt in 1926, APS is characterized by functional impairment of multiple endocrine glands.[15] APS type 2 is a polygenetic adult disease with 2 or 3 of the following endocrinopathies: autoimmune thyroid disease, type 1 diabetes, and adrenal insufficiency.[16] In a systematic review of 23 APS cases; endocrine organs, including the thyroid gland (78.3%), pancreatic islets (73.9%), pituitary gland (47.8%), and adrenal gland (8.7%), were reportedly affected by ICI administration.[17] Type 1 diabetes mellitus, a rare autoimmune disease, also occurred in most of the cases included in the report, indicating similarities between the pathogeneses of APS and autoimmune diabetes. Patients with diabetes should be aware that they may develop APS. APS was reportedly associated with HLA genotypes, and HLA testing can reveal the involvement of genetic factors.[17] In addition, a high therapeutic effect was observed in patients who developed APS.
The patient in our case report had a rare condition. He had mucosal melanoma, received ICI as postoperative adjuvant therapy, tested positive for GAD antibodies, and developed other endocrine irAEs along with T1DM. These factors were closely related to the risk of T1DM. Any irAEs were detected early, and immediate intervention helped the course run without any complications. He was probably GAD antibody-positive prior to nivolumab treatment, indicating his potential risk for developing T1DM.[14] The positive conversion of islet cell autoantibodies, including GAD antibodies, may have aided in the early detection of T1DM.[13] Although the incidence of T1DM as an irAE is low, measuring islet autoantibodies could be useful.
In conclusion, we reported a rare case of T1DM and summarized 8 cases. ICI-induced T1DM is a rare, but life-threatening irAE. Our study has some limitations, such as the small number of cases and the cases having significantly different patient backgrounds. We must pay attention to development of T1DM with ICIs, especially in the adjuvant setting. Immune-related adverse events can be detected at an early stage using regular blood tests and history taking, with ICIs administered to manage the irAE. Testing for HLA and pancreatic autoimmunity helps in the risk assessment and early detection of T1DM. Further studies are needed to reveal the pathogenic mechanism and risk factors of ICI-induced T1DM.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
Conceptualization: Akihiro Ishiguro, Dai Ogata
Data curation: Akihiro Ishiguro, Dai Ogata.
Investigation: Ken Ohashi, Kojiro Hiki, Kohei Yamakawa, Shunichi Jinnai, Keita Tsutsui, Akira Takahashi, Kenjiro Namikawa, and Naoya Yamazaki.
Writing—original draft: Akihiro Ishiguro.
Writing—review & editing: Dai Ogata, Ken Ohashi.
Abbreviations:
- FT1DM =
- fulminant type 1 diabetes
- GAD =
- glutamic acid decarboxylase
- HbA1c =
- glycated hemoglobin
- ICI =
- immune checkpoint inhibitor
- irAEs =
- immune-related adverse events
- MM =
- malignant melanoma
- T1DM =
- type 1 diabetes mellitus
How to cite this article: Ishiguro A, Ogata D, Ohashi K, Hiki K, Yamakawa K, Jinnai S, Tsutsui K, Takahashi A, Namikawa K, Yamazaki N. Type 1 diabetes associated with immune checkpoint inhibitors for malignant melanoma: A case report and review of 8 cases. Medicine 2022;101:35(e30398).
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Our institutional ethics committee for studies on human subjects approved the study protocol.
The authors have no conflicts of interest to disclose.
This work was supported in part by the National Cancer Center Research and Development Fund (2020-J-3).
Contributor Information
Akihiro Ishiguro, Email: shimaneizumoaki@gmail.com.
Ken Ohashi, Email: keohashi@ncc.go.jp.
Kojiro Hiki, Email: khiki@ncc.go.jp.
Kohei Yamakawa, Email: kyamakaw@ncc.go.jp.
Shunichi Jinnai, Email: sjinnai@ncc.go.jp.
Keita Tsutsui, Email: ktsutsui@ncc.go.jp.
Akira Takahashi, Email: atakahas@ncc.go.jp.
Kenjiro Namikawa, Email: knamikaw@ncc.go.jp.
Naoya Yamazaki, Email: nyamazak@ncc.go.jp.
References
- [1].Wang W, Green M, Choi JE, et al. CD8 + T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–48. [DOI] [PubMed] [Google Scholar]
- [3].Ikegami H, Kawabata Y, Noso S. Immune checkpoint therapy and type 1 diabetes. Diabetol Int. 2016;7:221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Imagawa A, Hanafusa T, Awata T, et al. Report of the Committee of the Japan Diabetes Society on the Research of Fulminant and Acute‐onset Type 1 Diabetes Mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus (2012). J Diabetes Investig. 2012;3:536–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kyriacou A, Melson E, Chen W, et al. Is immune checkpoint inhibitor-associated diabetes the same as fulminant type 1 diabetes mellitus?. Clin Med (Lond). 2020;20:417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Imagawa A. Two types of fulminant type 1 diabetes mellitus: Immune checkpoint inhibitor-related and conventional. J Diabetes Investig. 2021;12:917–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens. JAMA Oncol. 2018;4:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Otsuka M, Sugihara S, Mori S, et al. Immune-related adverse events correlate with improved survival in patients with advanced mucosal melanoma treated with nivolumab: a single-center retrospective study in Japan. J Dermatol. 2020;47:356–62. [DOI] [PubMed] [Google Scholar]
- [9].Baden MY, Imagawa A, Iwahashi H, et al. Risk factors for sudden death and cardiac arrest at the onset of fulminant type 1 diabetes mellitus. Diabetol Int. 2015;7:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Akturk HK, Kahramangil D, Sarwal A, et al. Immune checkpoint inhibitor-induced type 1 diabetes: a systematic review and meta-analysis. Diabet Med. 2019;36:1075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baden MY, Imagawa A, Abiru N, et al. Characteristics and clinical course of type 1 diabetes mellitus related to anti-programmed cell death-1 therapy. Diabetol Int. 2018;10:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tuomilehto J, Zimmet P, Mackay IR, et al. Antibodies to glutamic acid decarboxylase as predictors of insulin-dependent diabetes mellitus before clinical onset of disease. Lancet. 1994;343:1383–5. [DOI] [PubMed] [Google Scholar]
- [13].Lo Preiato V, Salvagni S, Ricci C, et al. Diabetes mellitus induced by immune checkpoint inhibitors: type 1 diabetes variant or new clinical entity? Review of the literature. Rev Endocr Metab Disord. 2021;22:337–49. [DOI] [PubMed] [Google Scholar]
- [14].Kawabata Y, Ikegami H, Awata T, et al. Differential association of HLA with three subtypes of type 1 diabetes: fulminant, slowly progressive and acute-onset. Diabetologia. 2009;52:2513–21. [DOI] [PubMed] [Google Scholar]
- [15].Schmidt MB. Eine biglandulare Erkrankung (Nebennieren und Schilddrüse) bei Morbus Adisonii. Verh Dtsch Ges Pathol. 1926;21:212–21. [Google Scholar]
- [16].Husebye ES, Anderson MS, Kampe O. Autoimmune polyendocrine syndromes. N Engl J Med. 2018;378:1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhao Z, Wang X, Bao XQ, et al. Autoimmune polyendocrine syndrome induced by immune checkpoint inhibitors: a systematic review. Cancer Immunol Immunother. 2021;70:1527–40. [DOI] [PMC free article] [PubMed] [Google Scholar]