Abstract
Cardiolipin (CL)-specific fluorescent dye 10-N-nonyl-acridine orange (NAO) was used to visualize CL distribution in Escherichia coli cells of different phospholipid compositions. In a filamentous mutant containing only anionic phospholipids, green fluorescent spots were observed along the filaments at approximately regular intervals. Three-dimensional image reconstruction obtained by optical sectioning and a deconvolution algorithm revealed NAO-binding domains in the plane of the cell membrane. Substantial red fluorescence emission of bound NAO supported labeling of CL-containing domains. These structures were not found in mutants deficient in CL biosynthesis. The domains were also observed mostly in the septal region and on the poles in cells of normal size with wild-type phospholipid composition.
The fluid mosaic model of Singer and Nicolson (17) assumed membrane lipid homogeneity. However, cells require a network of membrane domains that produce the specific environment for the action of membrane proteins (1). The existence of lipid domains in biological membranes has been demonstrated by several indirect biophysical techniques such as fluorescence depolarization (9), differential scanning calorimetry (2), and fluorescence redistribution after photobleaching (11). Gel and fluid lipid domains were directly visualized in mycobacteria by the use of fluorescent lipophilic probes (4). Uneven distribution of fluorescent steryl dye FM 4-64, which could be a reflection of lateral heterogeneity of phospholipid distribution in Escherichia coli membranes, was recently demonstrated by fluorescence microscopic imaging (6). In our work, we report the first attempt to visualize the distribution of cardiolipin (CL) in E. coli cells by staining living cells with the fluorescent dye 10-N-nonyl-3,6-bis(dimethylamino)acridine (10-N-nonyl acridine orange [NAO]). It has been shown that treatment of whole mammalian or yeast cells with NAO resulted in selective staining of the mitochondrial membrane due to specific binding of the dye to CL (3, 7, 15, 16). Replacing the NH group in position 10 of acridine orange by the N-nonyl group results in an increase in hydrophobicity of the reagent and the inability to form hydrogen bonds with DNA or RNA. Therefore, NAO only binds to anionic phospholipids of the cells owing to an interaction between its quaternary amine and the phosphate residue of phospholipids and an intercalation of the hydrophobic acridine moiety into the membrane bilayer (15). With CL, which contains two phosphate groups per molecule, the dye forms a dimer, but with monoacidic phospholipids, the stoichiometry is 1:1. Because of dimer formation, CL affinity for NAO (Ka = 2 × 106 M−1) is much higher than that of monoacidic phospholipids (Ka = 7 × 104 M−1) (15). We used NAO for treatment of E. coli cells of different phospholipid compositions to monitor CL distribution in bacterial membranes. A short version of this work was presented previously (E. Mileykovskaya and W. Dowhan, Abstr. 99th Gen. Meet. Am. Soc. Microbiol. 1999, abstr. K-134, p. 426, 1999).
Strains and growth conditions.
The E. coli strains used in this work are described below. AD90 (pss93::Km) is devoid of phosphatidylethanolamine (PE) and displays filamentous growth unless it carries plasmid pDD72 (pssA+ Cmr), which is temperature sensitive for replication (5). ADC90/pDD72 is a derivative of AD90/pDD72 containing the cls::Tn10 allele (18) that makes it deficient in CL biosynthesis. HDL11 [pgsA::kan Φ(lacOP-pgsA+) lpp2 zdg::Tn10] carries the pgsA gene, which is required for phosphatidylglycerol (PG) and CL synthesis, under lacOP regulation (8, 10). The strain also contains a mutation in the lpp gene encoding the major outer membrane lipoprotein. This mutation suppresses the normally lethal effect of a “leaky” pgsA mutation so that the mutant can grow in the absence of IPTG (isopropyl-β-d-thiogalactopyranoside). E614 (lpp2 zdg::Tn10) like HDL11 carries a mutant lpp allele that results in an outer membrane that is leaky to macromolecules (20). All strains were grown in Luria-Bertani (LB) medium at 30°C. In the case of AD90, the medium was supplemented with 50 mM MgCl2, which is absolutely required of all PE-deficient strains (5); addition of MgCl2 to wild-type cells had no effect on the results. In some experiments, 20 μg of cephalexin per ml was added to the growth medium to produce filamentous PE-containing cells. IPTG at 200 μM was used for induction of PG and CL biosynthesis in strain HDL11. Fresh overnight cultures were diluted between 1:50 and 1:1,000 and grown to an optical density at 600 nm (OD600) of 0.2 to 0.6.
Microscopic techniques.
For microscopic examination, cells from liquid cultures were stained directly in the growth medium with 200 nM NAO for 1 h at room temperature. Nucleoids of living cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) at a final concentration of 1 μg/ml. Cells were immobilized on a cover glass treated with poly-l-lysine. Cells were viewed in the presence of the dyes with an Olympus BX60 epifluorescence microscope equipped with a 100-W HBO lamp, a standard fluorescein isothiocyanate (FITC) filter set, and a 100× fluorite oil immersion objective. A standard DAPI filter was used for viewing DAPI stained cells. To increase the color contrast for overlay images, red-green-blue output cables were switched for red and blue so that DAPI staining appeared in red pseudocolor. Images were captured with an Optronics DEI-750 video camera and manipulated in Adobe PHOTOSHOP 3.0.
Three-dimensional image reconstruction of fluorescence was performed with a Delta Vision wide-field optical sectioning microscope (Applied Precision, Issaquah, Wash.) equipped with a 100× oil-immersion objective and visualized with a cooled charge-coupled device camera and a FITC filter set. Z-axis optical sections were taken at 0.1-μm intervals for a total of 20 sections. Deconvolution of raw data was performed with five rounds of integration.
Microscopy of NAO-stained E. coli cells.
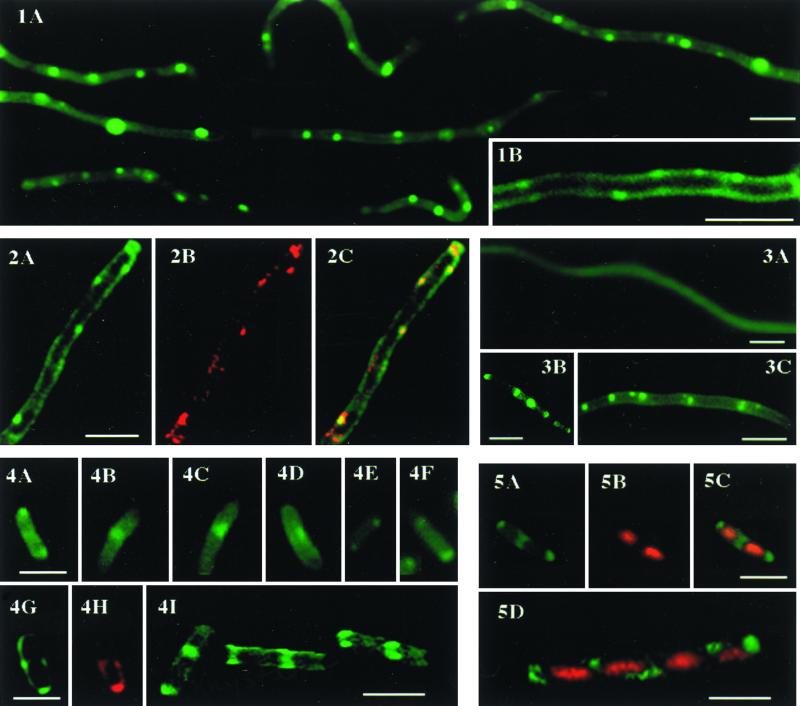
It was previously shown that NAO at a nanomolar concentration stained mitochondria in intact yeast and mammalian cells without inhibition of their functions or disruption of crista structures (7, 16). In our study, NAO at 100 to 200 nM in the growth medium resulted in staining of AD90/pDD72 (wild-type phospholipid composition, 83% PE, 12.7% PG, 3% CL, less than 0.3% PA) and AD90 (containing only anionic phospholipids, 63% PG, 13% CL, and 8% PA) (5), without any noticeable influence on their growth rates (data not shown). Figure 1A shows staining with NAO of living AD90 cells completely lacking amino-containing phospholipids and containing only the anionic phospholipids PG and CL. As found previously, pssA mutants are filamentous due to inhibition of the cell division process (5, 12). Figure 1A clearly demonstrates the uneven distribution of the dye in mutant cells. Green fluorescent spots are observed along the filaments at approximately regular intervals. The poles of the cells also produced stronger fluorescent signals. Figure 1B shows a single AD90 cell at a higher magnification, and Fig. 2A shows a deconvoluted image of an optical section of an AD90 cell (Fig. 2A). The images (Fig. 1B and 2A) are consistent with localization of fluorescent spots in the plane of the cell membranes. In addition, the red fluorescence of these domains (Fig. 2B) colocalizes with the green fluorescent domains (Fig. 2C). As mentioned above, NAO binding to CL results in dimerization of the dye (15) in a stacking organization in which dye molecules are in close proximity. The emitted fluorescence of the complex shifts to red (emission peak at 640 nm for dimer and 525 nm for monomer) due to the metachromatic properties of acridine molecules (14). Experimental titration curves of CL with NAO in isolated rat liver mitochondria using flow cytometry showed that at an NAO concentration of about 100 to 200 nM, the intensities of the red and green fluorescent signals were almost the same (14). In our experiments (Fig. 2A and C), the intensities of green (emission at 528 nm) and red (emission at 617 nm) fluorescence of NAO bound to the cells described above were also found to be about the same, consistent with NAO binding to CL rather than monoacidic phospholipids, which do not induce a red shift.
We demonstrated previously that disruption of the CLS (now named CRD) gene in yeast resulted in loss of CL and the inability of NAO to stain mitochondria in intact cells (3). In the present work, we applied the same control to E. coli cells. ADC90/pDD72 has about a 10- to 30-fold reduced level of CL (18), and growth in the presence of NAO revealed no fluorescent structures (Fig. 3A). Cells were grown in the presence of an inhibitor of cell division, cephalexin, to produce filaments. These data strongly suggest that CL is required for formation of the NAO-binding domains. We also used strain HDL11, in which the level of anionic phospholipids (PG and CL) is dependent on the level of IPTG in the growth medium (8, 10). In the cells grown in the absence of IPTG (90.5% PE, 1.8% PG, 1.3% CL, 6.3% phosphatidic acid) (10), the level of total fluorescence was similar to that seen in Fig. 3A (data not shown). Addition of IPTG (79.2% PE, 15.7% PG, 3.2% CL, 1.5% phosphatidic acid) (10) induced regularly distributed fluorescent structures along the filamentous cells (Fig. 3B, cells grown with cephalexin). Similar structures were seen in an NAO-treated lpp strain (E614) with wild-type phospholipid metabolism and composition (Fig. 3C). The results of these experiments show NAO-binding domains in cells with wild-type phospholipid composition as well as a correlation between the level of CL and the presence of these domains.
In strain AD90/pDD72 with a wild-type phospholipid composition, the NAO-binding structures were also observed in many cells of normal size (Fig. 4A to F), but they were better seen after additional manipulation of the contrast of the images. Fluorescent spots in these cells were localized at the poles and septal areas (Fig. 4). Figures 4G and H represent green and red fluorescence of a deconvoluted image of an optical section of such a cell. The localization of the fluorescent spots is consistent with the NAO-labeled domains being in the plane of the membrane. Figure 4I shows NAO fluorescence in a pole and septal area of another cell after three-dimensional reconstruction and rotation of the image at three different angles. In this cell, all of the septal area produces a high level of fluorescent signal. These results make it unlikely that NAO is labeling inclusion bodies but rather is labeling structures in the cell membrane.
Next, we attempted to localize the position of NAO-binding domains relative to the position of nucleoids by using double staining with NAO and DAPI. As can be seen in Fig. 5, NAO domains are apparently localized to the areas between nucleoids. This localization is more clear in the case of wild-type cells (Fig. 5A to C, septal localization) than in the case of the AD90 mutant cells, where some aberrations are seen (Fig. 5D). However, we previously showed that positioning of FtsZ in AD90 filamentous cells also exhibited some aberrations (12).
Conclusions.
Utilizing NAO as a tag for CL, we demonstrated for the first time the uneven distribution of this lipid along the E. coli cell with apparent localization in the plane of the cell membrane. Using NAO as a tool for localization of CL domains, we cannot completely exclude the possibility that treatment with the dye induces CL domain formation. However, we used extremely low concentrations of NAO, which for yeast mitochondria resulted in a ratio of CL to dye of about 1,000 (7) and in our experiments did not inhibit E. coli growth. In any case, the regular distribution of NAO-binding domains in cells is more consistent with the existence of specific zones in the membrane rather than random domain formation induced by the dye. The specificity for CL binding by NAO was established in eukaryotic cells where the complexity of membrane composition is at least on the level with the E. coli cell envelope. The red shift in the fluorescence spectrum and the lack of fluorescence in cells deficient in CL further validate the detection of CL-enriched domains. We can propose several possible explanations for the observed phenomenon. (i) There are CL-enriched domains in the E. coli membranes. (ii) There are phospholipid-enriched domains with a higher ratio of lipid to protein in these regions of membranes, and the NAO-CL complex is a marker of the discontinuous enrichment in phospholipid content over the surface of the membrane. (iii) There are sites in the E. coli envelope with higher permeability for NAO. In this case, we would have to propose a limited lateral diffusion of the lipids in these domains. This seems less likely since the outer membranes of lpp and pssA mutants are leaky to macromolecules and stained no differently than wild-type cells. Anionic phospholipid-enriched domains in E. coli have been implicated in protein translocation across the inner membrane (10) and initiation of DNA replication (19). A hypothetical model for participation of anionic phospholipids in formation of the septal domain in E. coli cells has been suggested (13). Our present experiments are directed toward the elucidation of the nature of NAO-binding domains and their possible functional role in E. coli.
FIG. 1-5.
Fig. 1. Staining of living AD90 filamentous cells with NAO. Cells were grown in LB medium supplemented with 50 mM MgCl2. (A) NAO was added to the cells in the exponential phase of growth, and pictures were taken after 1 h of incubation with the dye. (B) A part of filamentous AD90 cell at a higher magnification is shown. Bars, 2.5 μM. Exposure time, 0.25 s. Fig. 2. Deconvoluted images of an optical section of an AD90 cell. Cells were stained as described in the legend to Fig. 1. (A and B) Excitation was at 490 nm, and emission was at either 528 (A) or 617 (B) nm. Bar, 2.5 μM. Exposure time, 0.5 s. (C) Colocalization of green and red fluorescent domains. Fig. 3. Staining of living ADC/pDD72 (A), HDL11 (B), and E614 (C) cells with NAO. Cells were grown in LB medium supplemented with cephalexin and stained with NAO. Bars, 2.5 μM. Exposure times, 2 (A) and 1 (B and C) s. Fig. 4. Staining of living AD90/pDD72 cells with NAO. (A to F) Individual AD90/pDD72 cells. (G and H) Deconvoluted images of an optical section of an AD90/pDD72 cell. Excitation was at 490 nm and emission was at either 528 (G) or 617 (H) nm. (I) Three-dimensional picture of an AD90/pDD72 cell stained with NAO obtained by reconstruction of deconvolved optical sections. The same cell is shown at three different angles of rotation. Bars, 2.5 μM. Exposure time, 1 s. Fig. 5. AD90/pDD72 (A to C) and AD90 (D) cells stained with NAO and DAPI. Panels A and B show the same cell stained with NAO and DAPI, respectively. Panel C shows the overlay of the images in panels A and B. Panel D shows the overlay of NAO and DAPI staining. Stains, NAO (green) and DAPI (red). Bar, 2.5 μM.
Acknowledgments
This work was supported by NIH grant GM 20478 to W.D.
REFERENCES
- 1.Bergelson L. Special issue on domain organization in biological membranes. Introductory remarks. Mol Membr Biol. 1995;12:3. [Google Scholar]
- 2.Brasitus T A, Tall A R, Schachter D. Thermotropic transitions in rat intestinal plasma membranes studied by differential scanning calorimetry and fluorescence polarization. Biochemistry. 1980;19:1256–1261. doi: 10.1021/bi00547a033. [DOI] [PubMed] [Google Scholar]
- 3.Chang S-C, Heacock P N, Mileykovskaya E, Voelker D R, Dowhan W. Isolation and characterization of the gene (CLS1) encoding cardiolipin synthase in Saccharomyces cerevisiae. J Biol Chem. 1998;273:14933–14941. doi: 10.1074/jbc.273.24.14933. [DOI] [PubMed] [Google Scholar]
- 4.Christensen H, Garton N J, Horobin R W, Minnikin D E, Barer M R. Lipid domains of mycobacteria studied with fluorescent molecular probes. Mol Microbiol. 1999;31:1561–1572. doi: 10.1046/j.1365-2958.1999.01304.x. [DOI] [PubMed] [Google Scholar]
- 5.DeChavigny A, Heacock P N, Dowhan W. Sequence and inactivation of the pss gene of Escherichia coli. Phosphatidylethanolamine may not be essential for cell viability. J Biol Chem. 1991;266:5323–5332. [PubMed] [Google Scholar]
- 6.Fishov I, Woldringh C L. Visualization of membrane domains in Escherichia coli. Mol Microbiol. 1999;32:1166–1172. doi: 10.1046/j.1365-2958.1999.01425.x. [DOI] [PubMed] [Google Scholar]
- 7.Gallet P F, Maftah A, Petit J-M, Denis-Gay M, Julien R. Direct cardiolipin assay in yeast using the red fluorescence emission of 10-N-nonyl acridine orange. Eur J Biochem. 1995;228:113–119. doi: 10.1111/j.1432-1033.1995.tb20238.x. [DOI] [PubMed] [Google Scholar]
- 8.Heacock P N, Dowhan W. Alterations of the phospholipid composition of Escherichia coli through genetic manipulation. J Biol Chem. 1989;264:14972–14977. [PubMed] [Google Scholar]
- 9.Karnovsky M J, Kleinfeld A M, Hoover R L, Klausner R D. The concept of lipid domains in membranes. J Cell Biol. 1982;94:1–6. doi: 10.1083/jcb.94.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusters R, Dowhan W, de Kruijff B. Negatively charged phospholipids restore prePhoE translocation across phosphatidylglycerol-depleted Escherichia coli inner membranes. J Biol Chem. 1991;266:8659–8662. [PubMed] [Google Scholar]
- 11.Metcalf T N, Wang J L, Schindler M. Lateral diffusion of phospholipids in the plasma membrane of soybean protoplasts: evidence for membrane lipid domains. Proc Natl Acad Sci USA. 1986;83:95–99. doi: 10.1073/pnas.83.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mileykovskaya E, Sun Q, Margolin W, Dowhan W. Localization and function of early cell division proteins in filamentous Escherichia coli cells lacking phosphatidylethanolamine. J Bacteriol. 1998;180:4252–4257. doi: 10.1128/jb.180.16.4252-4257.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norris V. Phospholipid domains determine the spatial organization of the Escherichia coli cell cycle: the membrane tectonics model. J Theor Biol. 1992;154:91–107. doi: 10.1016/s0022-5193(05)80190-0. [DOI] [PubMed] [Google Scholar]
- 14.Petit J-M, Huet O, Gallet P F, Maftah A, Ratinaud M-H, Julien R. Direct analysis and significance of cardiolipin transverse distribution in mitochondrial inner membranes. Eur J Biochem. 1994;220:871–879. doi: 10.1111/j.1432-1033.1994.tb18690.x. [DOI] [PubMed] [Google Scholar]
- 15.Petit J-M, Maftah A, Ratinaud M-H, Julien R. 10-N-nonyl acridine orange interacts with cardiolipin and allows the quantification of this phospholipid in isolated mitochondria. Eur J Biochem. 1992;209:267–273. doi: 10.1111/j.1432-1033.1992.tb17285.x. [DOI] [PubMed] [Google Scholar]
- 16.Septinus M, Berthold T, Naujok A, Zimmermann H W. Uber hydrophobe Acridinfarbstoffe zur Fluorochromierung von Mitochondrien in lebenden Zellen. 3 Mitteilung: Spezifische Akkumulation des Farbstoffs NAO in die Mitochondrienmembranen von Hela-Zellen durch hydrophobe Wechselwirkung. Hemmung der Atmungsaktivitat, Veranderung der Ultrastruktur der Mitochondrien durch NAO. Intensivierung der Fluoreszenz vital gefarbter Mitochondrien in situ bei Bestrahlen. Histochemistry. 1985;82:51–66. doi: 10.1007/BF00502091. [DOI] [PubMed] [Google Scholar]
- 17.Singer S J, Nicolson G L. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 18.Xia W. Ph.D. thesis. Houston: University of Texas; 1994. [Google Scholar]
- 19.Xia W, Dowhan W. In vivo evidence for the involvement of anionic phospholipids in initiation of DNA replication in Escherichia coli. Proc Natl Acad Sci USA. 1995;92:783–787. doi: 10.1073/pnas.92.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yem D W, Wu H C. Physiological characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. J Bacteriol. 1978;133:1419–1426. doi: 10.1128/jb.133.3.1419-1426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]