Abstract
Background:
We aimed to identify continuous electroencephalogram (cEEG) markers associated with survival and death in ECMO patients under standardized sedation cessation protocol.
Methods:
Prospectively collected records of adult patients (age ≥18 years) who were started on ECMO support in 7/2016–12/2020 at a single tertiary center were analyzed. cEEGs were performed on patients based on inclusion/exclusion criteria. Patients receiving sedation that affect cEEG reactivity at the start of cEEG recording, including propofol, ketamine, or benzodiazepines, were excluded. We allowed fentanyl and dexmedetomidine during cEEG monitoring. cEEGs were evaluated for frequency, amplitude, variability, reactivity, and state changes.
Results:
Of 290 patients, 40 underwent cEEG in absence of confounding sedation (median age: 60 years, 85% venoarterial-ECMO, 15% venovenous-ECMO). Median length of ECMO support and analyzable cEEG were 143 hours and 24 hours respectively. 27 underwent withdrawal of life sustaining therapies (WOLST) during ECMO support. Of the 13 weaned off ECMO, 9 underwent WOLST later in the hospitalization, and 4 survived at hospital discharge. Decisions of WOLST were not influenced by cEEG features’ results. Proportions of present EEG reactivity, present state changes, and fair/good variability were significantly higher in patients who survived compared to those deceased (odds ratios infinity, infinity, and 13.57, respectively; p-values <0.001, <0.001, and 0.0299, respectively). Sensitivity and specificity for survival at discharge were 100% and 91.67% for intact reactivity, 100% and 97.20% for present state changes, and 75% and 83.3% for fair/good variability.
Conclusions:
While future multi-center studies with larger patient cohorts are certainly warranted, we were able to validate the feasibility of protocolized sedation cessation and cEEG analyses in the absence of confounding effect from sedating medications. Moreover, we demonstrate some evidence that cEEG features of intact reactivity, present state changes, and fair/good variability in comatose ECMO patients may be associated with survival at hospital discharge.
Keywords: Extracorporeal Membrane Oxygenation, Coma, Electroencephalography, Neurophysiology, Prognostication, Survival
Introduction
Venoarterial (VA) and venovenous (VV) Extracorporeal Membrane Oxygenation (ECMO) are the two most common methods of cannulation for patients with refractory cardiac and respiratory failure. Although a life-saving intervention, ECMO carries significant risk of morbidity and mortality. The use of ECMO has been associated with various neurological complications, including ischemic stroke, intracranial hemorrhage, and hypoxic ischemic brain injury (1,2). In ECMO patients who develop these neurological complications or have refractory cardiopulmonary insufficiency, the mortality rates are significantly higher (1). In this setting, routine, protocolized neurologic evaluations of ECMO patients are crucial for prognostication. Applied correctly, they may affect the decision to continue life-supporting measures or undergo withdrawal of life-sustaining therapies (WOLST).
Electroencephalograms (EEG) have commonly been used for neurological prognostication of adult patients with postanoxic coma from cardiac arrest (3,4,5,6). Yet, only very few studies have evaluated the use of EEG in adult ECMO patients specifically. Our own pilot study of 13 adult ECMO patients, which included a protocol that eliminated sedating medications, revealed that the absence of reactivity, variability, and sleep features on continuous EEG (cEEG) monitoring may serve as predictors of poor neurological outcome at the time of hospital discharge (7,8). However, this study was limited by its small sample size. Other studies on EEG in the ECMO population also have significant limitations, including small sample sizes (9,10), solely using routine EEGs (rEEG) (11,12), solely using reduced-channel EEG monitoring (13), and perhaps most importantly, not accounting for confounding effects of sedating medications (12, 14, 15). We therefore aimed to expand upon our prior proof-of-concept study with a larger cohort of patients, and hypothesized that the presence of certain cEEG features, such as reactivity, variability, and/or state changes, may be associated with good neurological outcome in ECMO patients.
Methods
Study Design
Adult patients (age ≥18 years) who were started on VA- or VV-ECMO support between July 2016 and December 2020 were enrolled in a prospective observational study, consisted of multimodal monitoring including cEEG during ECMO to evaluate their neurological state and overall prognosis at a single tertiary care center. (Table 1) VA-ECMO was used primarily for post-cardiotomy shock, cardiogenic shock, and cardiac arrest, while VV-ECMO was exclusively used for patients with refractory hypoxic respiratory failure. This cohort included and expanded upon the patients that were analyzed in our prior studies (7,8). Per protocol, sedating medications were weaned off as tolerated for each patient, and cEEGs were performed. If both of these feats were achieved for a patient, relevant data were collected for analysis.
Table 1:
Demographics and outcome of comatose patients on extracorporeal membrane oxygenation with concurrent continuous electroencephalography without sedating medications
| Demographics | |
| Age at ECMO initiation, median, [Q1-Q3] | 60 years [46–66.5] |
| Gender, males, n | 18 (45%) |
| ECMO cannulation type, n | |
| VA-ECMO | 34 (85%) |
| Peripheral cannulation | 17 (42.5%) |
| Central cannulation | 17 (42.5%) |
| VV-ECMO | 6 (15%) |
| ECMO indication, n | |
| Post-cardiotomy shock | 19 (47.5%) |
| Cardiac arrest | 10 (25%) |
| Hypoxic respiratory failure | 7 (17.5%) |
| Cardiogenic shock | 4 (10%) |
| Hospitalization course | |
| Duration of ECMO, median [Q1-Q3] | 143 hours [81–254] |
| Reason for discontinuing ECMO | |
| WOLST during ECMO, n | 27 (67.5%) |
| Tolerated weaning of ECMO, n | 13 (32.5%) |
| Time from ECMO cannulation to cEEG initiation, median [Q1-Q3] | 57.5 hours [32.8–118.8] |
| Duration of cEEG with sedation cessation while on ECMO support, median [Q1-Q3] | 25 hours [21–45.3] |
| Patients given sedationa during cEEG recording, n | 9 |
| Duration of analyzable cEEG data, limited by sedationa administration during recording, median [Q1-Q3] | 24 hours [17–34.8] |
| Patients given dexmedetomidine or fentanyl during cEEG recording, n | 18 |
| Dexmedetomidine gtt, n (doses) | 7 (max 0.8, 1.4, 1.5 mcg/kg/hr) |
| Fentanyl gtt, n (doses) | 11 (max 25, 50, 100, 200, 550 mcg/hr) |
| Fentanyl bolus PRN, n (doses) | 11 (12.5, 25, 50mcg) |
| Seizures, n | 0 (0%) |
| Death at time of hospital discharge, n | 36 (90%) |
| Time from ECMO initiation until death, median [Q1-Q3] | 8 days [3.5–13.5] |
| WOLST during ECMO, n | 27 (67.5%) |
| WOLST after weaning off ECMO support, n | 9 (22.5%) |
| Alive at time of hospital discharge, n | 4 (10%) |
| Discharged home | 2 (5%) |
| Discharged to rehabilitation facility | 2 (5%) |
Abbreviations:
Infusions or bolus doses of propofol, ketamine, or benzodiazepines;
cEEG: continuous electroencephalogram; ECMO: extracorporeal membrane oxygenation; hr: hour; gtt: continuous infusion; n: number; PRN: as needed; Q1: first quartile; Q3: third quartile; VA: venoarterial; VV: venovenous; WOLST; withdrawal of life sustaining therapies
Patients who continued to receive sedating medications that significantly affect the EEG pattern, including propofol (16,17), ketamine (18,19), or benzodiazepines (20,21), at the start of cEEG recording were excluded from analysis. Administration of dexmedetomidine (21,22,23) and fentanyl (24,25) were allowed as they appear to have less of confounding effects on EEG recording, especially considering that they are not used for achieving burst suppression or seizure management. For the remaining patients, if sedating medications was required later during the cEEG recording, for example due to concerns for seizures or respiratory dysfunction while intubated, only the data up until the moment of sedation administration were analyzed.
This study was approved by the Johns Hopkins Medicine Institutional Review Board, and adhered to the ethics statement of the International Society for Heart and Lung Transplantation. Informed consent was obtained from the legally authorized representatives of all participants included in the study.
Study Protocol
All patients were followed by a neurocritical care consult team from day 1 of ECMO cannulation until hospital discharge or death. On day 1, patients were evaluated for their baseline neurological examination and their calculated APACHE II (Acute Physiology And Chronic Health Evaluation II) and SOFA (Sepsis-related Organ Failure Assessment) scores. Per institutional sedation protocol, patients were routinely given sedation holidays by day 3 to 5 of ECMO support, and attempts to wean off sedating medications were made on a daily basis. In order to address the high rates of WOLST in comatose patients, our study protocol included the continuation of ECMO for as long as the patients’ next of kin or other legal guardian agreed.
Inclusion criteria for performing cEEGs during ECMO consisted of patients with Glasgow Coma Scale (GCS) ≤8 when assessed off-sedation by day 3–5 yet remained clinically stable at 24 hours off-sedation; cardiac arrest patients as soon as possible, no later than 72 hours since ECMO support, and therefore typically on day 1; or as clinically indicated in patients with concern for seizures (8). Exclusion criteria for performing cEEGs included GCS >8 and extremely short ECMO support, such as only during the intraoperative setting. When cEEG was performed, it was monitored preferably for 24 hours or longer if clinically indicated. A full standard bipolar montage was preferentially used for cEEGs, while a reduced (double distance) montage was used if access to the head was limited. The entirety of all cEEGs were initially evaluated by one of three neurologists (JH, JB, or SMC) independently, the results of which were then reviewed and confirmed by one neurophysiology specialist (EKR) who analyzed every single cEEG. When disagreements on the EEG interpretation arose, we ultimately reached consensus upon group discussions. Persyst continuous monitoring software (Persyst Inc, Prescott, AZ, USA) was used to reduce artifact signals, such as those from the ECMO device and environment of the intensive care unit (ICU), as well as to analyze trends and patterns.
cEEG features used for analysis included frequency, amplitude, variability, reactivity, and state change. We also noted the presence of seizures or discharges on the ictal-interictal continuum (ICC). The predominant background frequency was categorized as suppression, burst suppression, discontinuous, delta, delta-theta, theta-delta, theta, or theta-alpha (26,27,28). Overall amplitude was categorized as very low, low, medium, or high, relative to a normal matched adult (28,29,30). Variability was categorized as absent, poor, fair, or good, based on spontaneous changes in the power spectrum composition during cEEG in the absence of any stimuli (26,31). For assessing reactivity, a standardized approach was used, which consisted of sequentially administered verbal stimulation, noxious stimulation of fingertips or toes, and nostril simulation, with a 30–60 second interval between each stimulus to allow the EEG signals to return to baseline prior to re-stimulation (26,28,32). Sternal stimulus and forced eye opening were avoided given that many patients had undergone open-chest procedures and/or presented with scleral edema respectively. Reactivity was categorized as absent or present (Figure 3). Any responsiveness in the cEEG tracing, except for the sole increase in muscle activity, was categorized as present reactivity. State change was categorized as absent or present, defined as at least two sustained types of background EEG pattern associated with different levels of alertness, such as rest or some form of alertness following stimulation (28). The presence or absence of stage II sleep transients was also examined, and if present, the patient was categorized as having a sleep state. Primary outcome of interest was the patients’ status at the time of discharge, categorized as alive or deceased.
Figure 3:
Electroencephalography Reactivity
Statistical Analysis
Demographic and clinical variables were reported as the number of counts with percentage or the median with interquartile range (IQR). Computing environment R (Foundation for Statistical Computing, Vienna, Austria) was used for statistical analysis. Fisher’s exact test was used for comparing proportions between two groups, given the relatively small sample size of our patient cohort. P-values less than 0.05 were considered to be statistically significant. Odds ratios and their associated 95% confidence intervals, sensitivities, specificities, positive predictive values, and negative predictive values of the variables were calculated.
Results
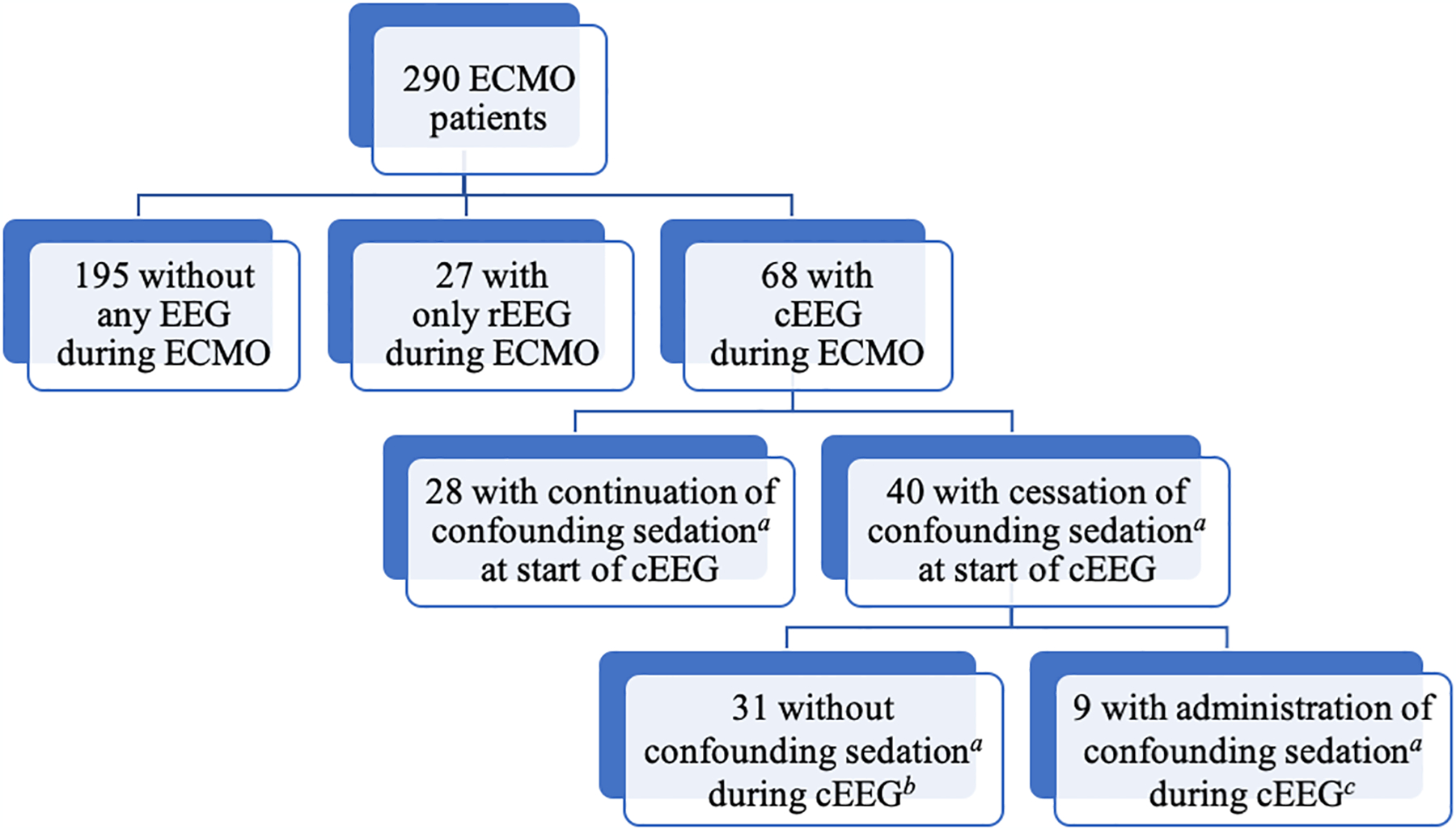
290 adult ECMO patients were initially collected. Of these, 222 patients were excluded based on inclusion and exclusion criteria. rEEGs (20 minutes) were performed on 27 patients, and cEEGs were performed on 68 patients. (Figure 1) However, of the 68 patients with cEEG recordings, 28 were further excluded as they received sedating medications at the start of cEEG recording. The cEEG data of the 40 remaining patients were then analyzed.
Figure 1:

Patient Selection
Abbreviations: cEEG: continuous electroencephalogram; ECMO: extracorporeal membrane oxygenation; EEG: electroencephalogram; rEEG: routine electroencephalogram
a: Infusions or bolus doses of propofol, ketamine, or benzodiazepines
b: Length of total cEEG recording = Length of analyzable cEEG data (median: 24 hours, IQR: 21–41 hours)
c: Length of total cEEG recording (median: 32 hours; IQR: 17–47 hours) ≠ Length of analyzable cEEG data (median: 22 hours; IQR 9.5–25.5 hours)
Forty patients (median age: 60 years, IQR: 46–66.5 years, 22 women, 18 men) who underwent cEEG after cessation of confounding sedating medications during ECMO were identified. (Table 1) The median time length of ECMO support was 143 hours (IQR: 81–254 hours). Seventeen patients (42.5%) underwent VA-ECMO central cannulation, 17 (42.5%) underwent VA-ECMO peripheral cannulation, and 6 (15%) underwent VV-ECMO cannulation. (Figure 2) The median time from ECMO cannulation to cEEG initiation was 57.5 hours (IQR: 32.8–118.8 hours). The median length of cEEG recording during ECMO was 25 hours (IQR: 21–45.3 hours). However, 9 patients required re-administration of confounding sedating medications during their cEEG recording as clinically indicated, which led to the early termination of their cEEG analysis for the purposes of this study. (Figure 1, Table 1) This led to a slight reduction in the overall median time length of analyzable cEEG data in the absence of any confounding sedating medications to 24 hours (IQR: 17–34.8 hours).
Figure 2:

Outcome of Comatose Patients on Extracorporeal Membrane Oxygenation
Abbreviations: cEEG: continuous electroencephalogram; ECMO: extracorporeal membrane oxygenation; rehab: rehabilitation center; s/p: status post; WOLST: withdrawal of life sustaining therapies; VA: venoarterial; VV: venovenous
a: Infusions or bolus doses of propofol, ketamine, or benzodiazepines
Twenty-seven patients (67.5%) could not be weaned off ECMO and underwent WOLST, with a median of 5.9 days (IQR: 2.9–9.0) of ECMO support. Among the 13 patients (32.5%) who were weaned off ECMO, 9 underwent WOLST later during the same hospitalization, while 4 survived and were discharged to a rehabilitation center or home. Importantly, decisions of WOLST were not influenced by the results of the analyzed cEEG variables.
The proportions of intact reactivity, present state changes, and fair/good variability were significantly higher in patients who survived at hospital discharge compared to those who had deceased (odds ratios infinity, infinity, and 13.57 respectively; p-values <0.001, <0.001, and 0.03 respectively). (Table 2) Sensitivity and specificity associated with survival for present reactivity were 100% and 91.67% respectively, for present state changes were 100% and 97.20% respectively, and for fair/good variability were 75% and 83.33% respectively. For the 4 patients who survived at hospital discharge, at least state changes and reactivity were present. In contrast, among the 36 patients who did not survive at hospital discharge, 28 (77.78%) had absent or poor findings in all three categories, while 34 (94.44%) had absent or poor findings in at least two categories. In particular, among the 9 patients who were weaned off ECMO but underwent WOLST later during the hospitalization, 8 (88.9%) had at absent or poor findings of at least two variables, 7 (77.8%) had absent reactivity, 9 (100%) had absent state changes, and 7 (77.8%) had absent/poor variability.
Table 2:
Association between intact continuous electroencephalography variables and survival at time of hospital discharge
| ORa | 95% CI | p-valueb | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| State Change | +∞ | [8.34, +∞] | <0.001 | 100% | 97.22% | 80% | 100% |
| Reactivity | +∞ | [4.44, +∞] | <0.001 | 100% | 91.67% | 57.14% | 100% |
| Variability | 13.57 | [0.92, 807.54] | 0.0299 | 75% | 83.33% | 33.33% | 96.77% |
| Amplitude | 1.55 | [0.10, 23.75] | 1 | 50% | 61.11% | 12.50% | 91.67% |
| Frequency | 2.02 | [0.03, 31.99] | 0.49 | 25% | 86.11% | 16.67% | 91.18% |
Abbreviations:
Interpreted as the odds of survival at time of hospital discharge in the group with present and at least fair quality of cEEG variables divided by the odds of survival at time of hospital discharge in the group with absent or poor cEEG variables;
Based on Fisher’s exact tests examining the association between the present and at least fair quality of the cEEG variable and survival at time of hospital discharge;
cEEG: continuous electroencephalogram; CI: confidence interval; +∞: positive infinity; OR: odds ratio; NPV: negative predictive value; PPV: positive predictive value.
None of the 40 patients had electrographic discharges or seizures. However, 3 patients had poorly formed discharges on the ICC in the form of triphasic waves (n=1) or generalized periodic discharges (n=2). Four patients had burst suppression, and 4 patients had suppressed backgrounds without periodic discharges, all of whom had no reactivity and ultimately died.
Discussion
The results of this study suggest that intact reactivity, present state changes, and fair/good variability on cEEG recordings during ECMO, when monitored due to coma, are associated with patient survival at hospital discharge. In fact, if all three features were present concomitantly, patients had a 100% chance of survival. On the other hand, absent reactivity and/or absent state changes (alone or in combination) were associated with ultimate death in all patients. Thirty (96.8%) out of 31 patients with absent/poor variability also died. However, poor variability with present reactivity and discernible state change was seen in one patient who survived. Taken together, this suggests that the collective presence of reactivity and state changes is 100% sensitive for survival, while the absence of reactivity and/or state changes is 100% sensitive for death. Importantly, the EEG analyses were performed in the absence of confounding sedating medications. These results confirm our previously reported findings on the poor prognosis of comatose ECMO patients in absence of reactivity, state changes, and variability, with higher statistical power due to the increased number of patients analyzed (7,8). In addition, our findings suggest that the presence of all three features is an important marker of a good outcome. Our study therefore highlights the importance of cEEG, as EEG markers may predict good outcome in the ECMO population.
It is encouraging that patients with preserved reactivity, state changes, and overall at least fair variability ultimately survived, even though all patients were comatose during cEEG monitoring. Notably, there were 9 patients who were successfully weaned off ECMO but later underwent WOLST during that hospitalization. These patients had poor reactivity, state changes, and/or variability on their cEEG while still on ECMO support. Their cEEG findings were therefore predictive of their ultimately poor outcome. This suggests that cEEG data obtained during ECMO may provide useful information for predicting the patients’ outcome, even beyond the period of ECMO support. This finding has not been reported in any other published studies to our knowledge.
In the cardiac arrest literature, prognostic EEG criteria for poor neurological outcome have been established. Studies have shown that “highly malignant” EEG patterns had 31–84% sensitivity and 91–100% specificity for predicting poor neurological outcome with a prevalence of 22–55% (3,33,34,35). These “highly malignant” patterns are defined as a suppressed background without discharges, suppressed background with continuous periodic discharges, or burst-suppression background, while “poor neurological outcome” is defined as severely disabled, comatose, or deceased, corresponding to cerebral performance category scores of 3–5. In our cohort, the prevalence of “highly malignant” patterns was only 20%, with 4 patients having burst suppression and 4 patients having suppressed backgrounds without periodic discharges. These 8 patients also had absent reactivity, absent state changes, and poor/absent variability, and were deceased at the time of hospital discharge. The presence of highly malignant EEG patterns as a predictor of poor outcome for our ECMO cohort therefore had a very low sensitivity of 19.44%, with a specificity of 100%. By contrast, the absence of reactivity and absence of state changes as predictors of poor outcome had a sensitivity of 91.67% and 97.22% respectively and specificity of 100%. For the ECMO population, evaluating the cEEG for reactivity, state changes, and variability therefore appears to be a better approach than relying on the presence of “highly malignant” patterns for neurological prognostication, which may be due to different patterns of brain injury in ECMO patients and cardiac arrest patients. Furthermore, studies of cardiac arrest patients report the presence of epileptiform discharges with a prevalence of 21–54.5%, although their prognostic value appears to be uncertain (3,4,6,36). Interestingly, none of our ECMO patients had any epileptiform discharges or seizures. This may point to differences in mechanism and patterns of brain injury between these patient populations.
One major strength of this study is that all EEG analyses were carried out after medications known to have major effects on EEG had been weaned off. Prior studies have also reported unreactive EEGs to be associated with poor neurological outcome in comatose ECMO patients (11,12,14), which has been challenging to interpret as sedating medications can often suppress the EEG signals, and more critically ill patients may receive heavier sedation, further confounding the association between EEG findings and patient outcome. Therefore, the findings from our study is important in that cEEG data were only evaluated when sedating medications were weaned off prior to cEEG monitoring.
Another strength of our paper is the carefully planned use of prolonged cEEG monitoring during ECMO support. While other studies using rEEGs in ECMO patients also revealed unreactive EEGs and suppressed backgrounds to be associated with poor neurological outcome, they did not account for the fact that EEG patterns often change significantly in the first few days of ECMO (15). This may be the reason why others were only able to predict poor but not favorable outcomes. Furthermore, state changes and variability are more difficult to capture on rEEGs, which are much briefer than cEEGs. Thus, we propose that a full day (>24 hours) of cEEG and absence of significantly confounding sedating medications in ECMO patients allow for more reliable prognostication based on EEG features, even after weaning off ECMO.
It is important to keep in mind that decisions about WOLST for comatose patients were made in setting of severe cardiopulmonary illness, often with concurrent multi-organ failure and inability to wean off ECMO. Given the controversial nature of WOLST and self-fulfilling prophecy (37,38,39), these decisions to implement WOLST were never guided by the results of the cEEG features analyzed. This was true for both patients who underwent WOLST at the time of ECMO support and those who had successfully weaned off ECMO but were still hospitalized. Based on the results of this study, the presence of reactivity, state changes, and/or variability on cEEG during ECMO support may potentially encourage providers and patients’ next of kin or other court-appointed guardian to continue with treatment and to delay WOLST that may have otherwise occurred too soon.
Lastly, cEEG recording during ECMO support while minimizing potentially confounding sedating medications appears to be a promising mode of prognostication of ECMO patients. No prior study has evaluated data matching these criteria before. The reproducibility of this protocol is further supported by our rigorous compliance in previously published studies (7,8,40). The cEEG’s predictive ability may be bolstered further when used in conjunction with other tools as part of a multimodal monitoring protocol for prognostication. For example, short-latency somatosensory evoked potentials (7,8,41), transcranial dopplers (7,42), pupillometry (42), and near-infrared spectroscopy (41) are all non-invasive modalities that are used collectively to improve the prognostic value in cardiac arrest patients, which can readily be adopted for use in ECMO patients. Future studies of cEEG in ECMO patients may consist of including those with GCS ≥8, those who continue to receive other sedating medications during cEEG recording for comparison, and/or those who were successfully weaned off ECMO and subsequently underwent a second cEEG monitoring during the same hospitalization. Doing so would not only increase the number of patient samples, but also help evaluate the neurologic evolution of ECMO patients. Additionally, further comparison studies involving the cardiac arrest and ECMO populations may allow a deeper understanding of their mechanistic differences related to neurological recovery and the comatose state associated with cardiac injury.
This study has limitations. First, the sample size of patients is still small, which prevents subgroup analyses for each specific indication for ECMO in setting of the heterogeneity of the ECMO patient population. Second, decisions for WOLST made by the patients’ next of kin or other court-appointed guardian curtailed further data collection related to the patients’ natural clinical course and cEEG monitoring. Third, we allowed dexmedetomidine and fentanyl during EEG monitoring as these medications have much less confounding effect on EEG, but nevertheless may still unintentionally affect EEG parameters, such as state changes. Fourth, the effects of sedating medications may linger beyond their typical half-lives in ECMO patients due to various metabolic derangements in setting of multi-system organ failure and/or targeted temperature management, which may have some confounding effects. Fifth, the timing of performing cEEG was not precisely consistent for all ECMO patients due to their individual abilities to tolerate cessation of sedating medications and differing medical indications for ECMO support. The optimal timing for performing cEEG on ECMO patients for neurological prognostication remains to be further studied.
Conclusion
We demonstrated that cEEG monitoring in comatose ECMO patients with standardized sedation cessation protocol may have the potential for neurological prognostication. While future multi-center studies with larger patient cohorts are certainly warranted, we were able to validate the feasibility of protocolized sedation cessation and cEEG analyses in the absence of confounding effect from sedating medications. Specifically, we further expanded upon our prior studies with higher statistical power, suggesting that intact reactivity, present state changes, and fair/good variability on cEEG recordings of ECMO patients are associated with survival at hospital discharge. Therefore, the cEEG data obtained during ECMO may remain useful for predicting the patients’ neurological outcome even after weaning off ECMO support.
Footnotes
This study was adherent to the use of ethical guidelines and informed consent, and was approved by the Johns Hopkins Medicine Institutional Review Board.
Conflict of Interest: Glenn Whitman is on the Data Safety Monitoring Board of Cytosorbent Corporation. Eva K Ritzl is a medical legal expert witness and council member of American Clinical Neurophysiology Society. The remaining authors have disclosed that they do not have any conflicts of interest.
References
- 1:Chapman JT, Breeding J, Kerr SJ, Bajic M, Nair P, Buscher H. CNS Complications in Adult Patients Treated With Extracorporeal Membrane Oxygenation. Crit Care Med. 2021; 49: 282. [DOI] [PubMed] [Google Scholar]
- 2:Cho SM, Farrokh S, Whitman G, Bleck TP, Geocadin RG. Neurocritical care for extracorporeal membrane oxygenation patients. Crit Care Med 2019;47:1773–1781. [DOI] [PubMed] [Google Scholar]
- 3:Westhall E, Rossetti AO, van Rootselaar AF, et al. Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology. 2016;86:1482–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4:Rossetti AO, Urbano LA, Delodder F, Kaplan PW, Oddo M. Prognostic value of continuous EEG monitoring during therapeutic hypothermia after cardiac arrest. Crit Care 2010; 14: R173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5:Admiraal MM, Horn J, Hofmeijer J, et al. EEG reactivity testing for prediction of good outcome in patients after cardiac arrest. Neurology. 2020; 95: e653–661. [DOI] [PubMed] [Google Scholar]
- 6:Cloostermans MC, van Meulen FB, Eertman CJ, Hom HW, van Putten MJ. Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: a prospective cohort study. Crit Care Med. 2012; 40: 2867–2875. [DOI] [PubMed] [Google Scholar]
- 7:Cho SM, Ziai W, Mayasi Y, et al. Noninvasive neurological monitoring in extracorporeal membrane oxygenation. ASAIO J. 2020; 66: 388–393. [DOI] [PubMed] [Google Scholar]
- 8:Cho SM, Choi C, Whitman G, et al. Neurophysiological Findings and Brain Injury Pattern in Patients on ECMO. Clin EEG Neurosci. 2021; 52: 462–469. [DOI] [PubMed] [Google Scholar]
- 9:Lee BK, Jeung KW, Lee DH, Cho YS, Jung YH. Use of amplitude-integrated electroencephalography in decision-making for extracorporeal membrane oxygenation in comatose cardiac arrest patients whose eventual neurologic recovery is uncertain. Clin Exp Emerg Med. 2019; 6: 362–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10:Kobata H, Tucker A, Sarapuddin G, Negoro T, Kawakami M. Continuous amplitude-integrated electroencephalography for prognostication of cardiac arrest patients undergoing extracorporeal cardiopulmonary resuscitation with targeted temperature management. Resuscitation. 2020; 156: 107–113. [DOI] [PubMed] [Google Scholar]
- 11:Kim YO, Ko RE, Chung CR, et al. Prognostic Value of Early Intermittent Electroencephalography in Patients after Extracorporeal Cardiopulmonary Resuscitation. J Clin Med. 2020; 9: 1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12:Magalhaes E, Reuter J, Wanono R, et al. Early EEG for Prognostication Under Venoarterial Extracorporeal Membrane Oxygenation. Neurocrit Care. 2020; 33: 688–694. [DOI] [PubMed] [Google Scholar]
- 13:Touchard C, Cartailler J, Vellieux G, et al. ; DINAMO Study Group. Simplified frontal EEG in adults under veno-arterial extracorporeal membrane oxygenation. Ann Intensive Care. 2021; 11: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14:Sinnah F, Dalloz MA, Magalhaes E, et al. Early Electroencephalography Findings in Cardiogenic Shock Patients Treated by Venoarterial Extracorporeal Membrane Oxygenation. Crit Care Med. 2018; 46: e389–e394 [DOI] [PubMed] [Google Scholar]
- 15:Cho SM, Ritzl E. Neurological Prognostication Using Electroencephalogram in Adult Veno-arterial Extracorporeal Membrane Oxygenation: Limitations and Recommendations. Neurocrit Care. 2020; 33: 652–654. [DOI] [PubMed] [Google Scholar]
- 16:San-Juan D, Chiappa KH, Cole AJ. Propofol and the electroencephalogram. Clin Neurophysiol. 2010; 121: 998–1006. [DOI] [PubMed] [Google Scholar]
- 17:Billard V, Gambus PL, Chamoun N, Stanski DR, Shafer SL. A comparison of spectral edge, delta power, and bispectral index as EEG measures of alfentanil, propofol, and midazolam drug effect. Clin Pharmacol Ther. 1997; 61: 45–58. [DOI] [PubMed] [Google Scholar]
- 18:Modica PA, Tempelhoff R, White PF. Pro- and Anticonvulsant Effects of Anesthetics (Part II). Anesth Analg. 1990; 70: 433–444. [DOI] [PubMed] [Google Scholar]
- 19:Akeju O, Song AH, Hamilos AE, et al. Electroencephalogram Signatures of Ketamine-induced Unconsciousness. Clin Neurophysiol. 2016; 127: 2414–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20:Brunner MD, Umo-Etuk J, Sharpe RM, Thornton C. Effect of a bolus dose of midazolam on the auditory evoked response in humans. Br J Anaesth. 1999; 82: 633–634. [DOI] [PubMed] [Google Scholar]
- 21:Aksu R, Kumandas S, Akin A, et al. The comparison of the effects of dexmedetomidine and midazolam sedation on electroencephalography in pediatric patients with febrile convulsion. Paediatr Anaesth. 2011; 21: 373–378. [DOI] [PubMed] [Google Scholar]
- 22:Purdon PL, Sampson A, Pavone KJ, Brown EN. Clinical Electroencephalography for Anesthesiologists Part I: Background and Basic Signatures. Anesthesiology. 2015; 123: 937–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23:Mason KP, O’Mahony E, Zurakowski D, Libenson MH. Effects of dexmedetomidine sedation on the EEG in children. Paediatr Anaesth. 2009; 19: 1175–1183. [DOI] [PubMed] [Google Scholar]
- 24:Montandon G, Horner RL. Electrocortical changes associating sedation and respiratory depression by the opioid analgesic fentanyl. Sci Rep. 2019; 9: 14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25:Sebel PS, Bovill JG, Wauquier A, Rog P. Effects of High-dose Fentanyl Anesthesia on the Electroencephalogram. Anesthesiology. 1981; 55: 203–211. [DOI] [PubMed] [Google Scholar]
- 26:Kane N, Acharya J, Beniczky S, et al. A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin Neurophysiol Pract. 2017; 2: 170–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27:Niedermeyer E, da Silva FHL. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 28:Hirsch LJ, Fong MWK, Markus L, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2021 version. J Clin Neurophysiol. 2021; 38: 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29:Kane N, Acharya J, Beniczky S, et al. A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin Neurophysiol Pract. 2017; 2: 170–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30:Niedermeyer E, da Silva FHL. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 31:Hasman A, Jansen BH, Landeweerd GH, van Blokland-Vogelesang AW. Demonstration of segmentation techniques for EEG records. Int J Biomed Comput. 1978;9:311–321. [DOI] [PubMed] [Google Scholar]
- 32:Tsetsou S, Novy J, Oddo M, Rossetti AO. EEG reactivity to pain in comatose patients: importance of the stimulus type. Resuscitation. 2015;97:34–37. [DOI] [PubMed] [Google Scholar]
- 33:Backman S, Cronberg T, Friberg H, et al. Highly malignant routine EEG predicts poor prognosis after cardiac arrest in the Target Temperature Management trial. Resuscitation. 2018; 131: 24–28. [DOI] [PubMed] [Google Scholar]
- 34:Beuchat I, Solari D, Novy J, Oddo M, Rosetti AO. Standardized EEG interpretation in patients after cardiac arrest: Correlation with other prognostic predictors. Resuscitation. 2018; 126: 143–146. [DOI] [PubMed] [Google Scholar]
- 35:Sivaraju A, Gilmore EJ, Wira CR, et al. Prognostication of post-cardiac arrest coma: early clinical and electroencephalographic predictors of outcome. Intensive Care Med. 2015; 41: 1264–72. [DOI] [PubMed] [Google Scholar]
- 36:Elmer J, Coppler PJ, Solanki P, et al. Sensitivity of Continuous Electroencephalography to Detect Ictal Activity After Cardiac Arrest. JAMA Netw Open. 2020; 3: e203751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37:Hemphill JC III, White DB. Clinical Nihilism in Neuro-Emergencies. Emerg Med Clin North Am. 2009; 27: 27–viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38:Izzy S, Compton R, Carandang R, Hall W, Muehlschlegel S. Self-Fulfilling Prophecies Through Withdrawal of Care: Do They Exist in Traumatic Brain Injury, Too? Neurocrit Care. 2013; 19: 347–363. [DOI] [PubMed] [Google Scholar]
- 39:Becker KJ, Baxter AB, Cohen WA, et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology. 2001; 56: 766–772. [DOI] [PubMed] [Google Scholar]
- 40:Ong CS, Etchill E, Dong J, et al. Neuromonitoring detects brain injury in patients receiving extracorporeal membrane oxygenation support. J Thorac Cardiovasc Surg. 2021; S0022–5223: 01508–7. [DOI] [PubMed] [Google Scholar]
- 41:Sandroni C, D’Arrigo S, Nolan JP. Prognostication after cardiac arrest. Crit Care. 2018; 22: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42:Heimburger D, Durand M, Gaide-Chevronnay L, et al. Quantitative pupillometry and transcranial Doppler measurements in patients treated with hypothermia after cardiac arrest. Resuscitation. 2016; 103: 88–93. [DOI] [PubMed] [Google Scholar]


