Abstract
BACKGROUND:
Merkel cell carcinoma (MCC) is an aggressive cutaneous malignancy with a high risk of lymph node involvement. To the authors’ knowledge, few data have been published to date regarding the optimal regional therapy for lymph node-positive patients. This cohort study was performed to analyze the outcomes of patients with lymph node-positive MCC treated with lymph node irradiation as definitive therapy compared with completion lymphadenectomy (CLND).
METHODS:
Fifty patients with lymph node involvement of MCC at presentation and adequate follow-up data were included in this analysis. Forty-three of these patients were enrolled and followed prospectively. Twenty-six patients presented with microscopic lymph node disease, and 24 patients presented with palpable lymph node involvement.
RESULTS:
Regional control for patients with microscopically involved lymph nodes was 100% regardless of treatment modality—definitive lymph node irradiation (n = 19) or CLND ± radiotherapy (n = 7) with median follow-up of 18 months. Patients with clinically positive lymph nodes had 2-year regional recurrence-free survival rate of 78% and 73% in the definitive lymph node irradiation (n = 9) and CLND radiotherapy (n = 15) groups, respectively (P = .8) with a median follow-up of 16 months.
CONCLUSIONS:
To the best of the authors’ knowledge, the current study is the largest series published to date of radiation monotherapy as regional treatment for lymph node-positive MCC. Lymph node irradiation alone to positive regional lymph nodes was found to confer an excellent regional control rate that was comparable to CLND for both microscopic and palpable lymph node disease. There was no difference noted with regard to overall survival. Given their similar efficacy, the choice between these lymph node therapies may be based on the clinical scenario and anticipated side effect profiles.
Keywords: Merkel cell carcinoma, radiotherapy, lymphadenectomy, lymph node dissection
Merkel cell carcinoma (MCC) is an aggressive cutaneous malignancy with a predilection for lymph node involvement, distant metastases, and disease recurrence. It is a disease that primarily afflicts white elderly individuals and is associated with ultraviolet exposure, immunosuppression, and a newly described Merkel cell polyomavirus.1–3 The reported incidence of MCC has tripled in the past 20 years primarily due to increased detection through the development of immunohistochemical staining for cytokeratin-20, which is specific for MCC. In addition, increases in relevant risk factors (age >50 years, ultraviolet exposure, human immunodeficiency virus, solid organ transplantation) have likely resulted in true increases in the incidence of MCC.4
MCC is a rare disease, and therefore to the best of our knowledge no randomized controlled trials to define optimal treatment have been performed to date. Numerous retrospective studies have been performed assessing the role of radiation treatment5–13 with the growing consensus that radiotherapy confers local and regional control improvements. A recent population-based study suggested a survival benefit with adjuvant radiotherapy.14 Nonetheless, because of small sample sizes and heterogeneous patient populations of MCC series to date, optimal management of this malignancy continues to be controversial.
Since its first description in 1972,15 MCC has been known to have a high risk of lymphatic metastases. Patients present with lymph node disease in 19% to 33%9,13,16–18 of cases. Traditional recommended practice for the regional therapy for lymph node-positive disease is completion lymphadenectomy (CLND) with or without adjuvant radiotherapy. However, given the radiosensitivity of MCC19 as well as morbidity associated with full lymph node dissection, in more recent years, radiotherapy was used as definitive treatment to the primary lymph node basin in a cohort of patients with lymph node-positive disease. As such, the present study was performed to analyze the outcomes of lymph node-positive MCC patients treated with definitive lymph node irradiation compared with CLND.
MATERIALS AND METHODS
Patient Selection Criteria
After approval by the Institutional Review Board at the Fred Hutchinson Cancer Research Center, a Repository of Data and Specimens for MCC was created. Two hundred twenty-seven patients diagnosed with MCC from 1985 to 2007 were enrolled in this repository, mostly in a prospective manner since 2002. Eighty-six of these patients were found to have lymph node-positive disease at presentation after initial workup (Fig. 1). Eleven of these patients were found to have distant metastases, 5 patients were lost to follow-up, and specific information regarding lymph node status was missing in 20 patients. Thus, the 50 patients included in this analysis met the following criteria: 1) pathologic confirmation of lymph node involvement, 2) no evidence of distant metastases at presentation, and 3) available follow-up information regarding lymph node recurrence.
Figure 1.
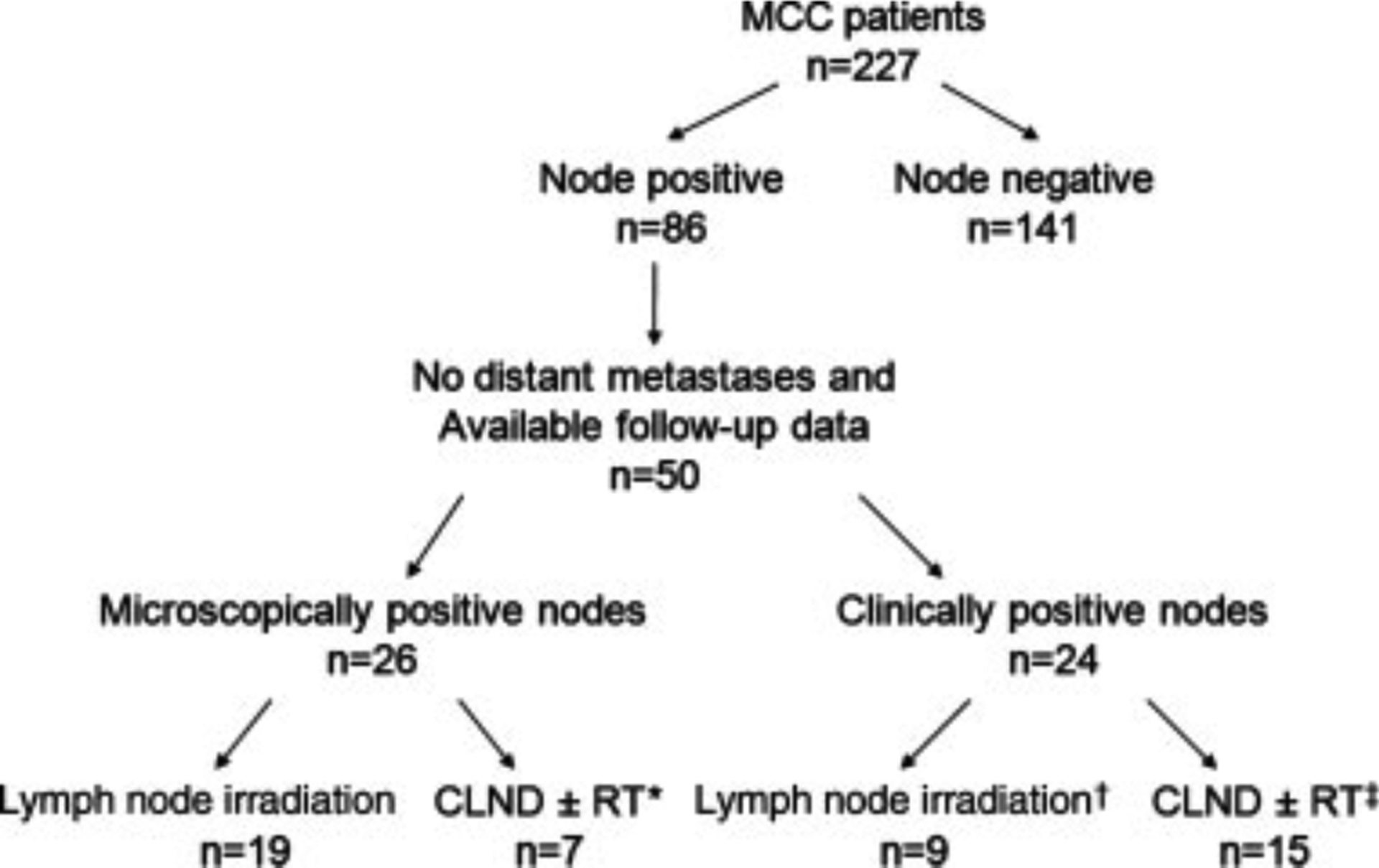
Patient selection is shown. MCC indicates Merkel cell carcinoma; CLND, completion lymphadenectomy; RT, radiotherapy. *3 patients received adjuvant lymph node irradiation; †3 patients underwent surgical debulking of palpable lymph node; ‡12 patients received adjuvant lymph node irradiation.
Data were collected prospectively in 43 patients and retrospective chart review was performed on the remaining 7 patients. All patients were seen at the authors’ institutions for consultation and treated at a mixture of academic and private institutions as a function of geography, insurance status, and patient preference. Because the vast majority of patients did not live geographically close to the authors’ institution, most or all of their treatment was provided by medical centers closer to their home. Therefore, there was no dominant institution or practitioner of surgical or radiation therapy for any particular group. Medical records were obtained from the patients’ treating physicians twice yearly and at the time of analysis of this cohort. The treatment modality for regional lymph nodes was at the discretion of the management team.
Statistical Analysis
The endpoints of this study were regional recurrence-free survival (RRFS), disease-specific survival (DSS), and overall survival (OS). RRFS was calculated as the time from diagnosis to regional disease recurrence, defined as recurrence in the primary lymph node basin or regional intransit lymphatics. In the case of an unknown primary tumor, regional disease recurrence was defined as recurrence within the lymph node region of initial presentation. DSS was defined as the time from diagnosis to death from MCC, and OS was defined as the time from diagnosis to death from any cause. The site of first failure was recorded as recurrence of local, regional, and/or distant disease that was the first to be detected in follow-up either by physical examination or imaging. The endpoints were calculated using Kaplan-Meier estimates. GraphPad Prism (GraphPad Software, La Jolla, Calif) was used to run statistical analyses.
RESULTS
Microscopic Cohort
Twenty-six patients meeting eligibility criteria had microscopic positive nodes as determined by sentinel lymph node biopsy (SLNB). Among these 26 patients, 16 were men and the median age was 68 years (range, 46–85 years). The median size of the primary tumor was 19 mm (range, 5–60 mm). The involved lymph node basin was in the head and neck in 3 patients, the axilla in 15 patients, and the inguinal lymph nodes in 8 patients.
Nineteen of 26 patients received definitive lymph node irradiation, and 7 underwent CLND. Four of these 7 patients also received adjuvant lymph node irradiation. No additional positive lymph nodes obtained from CLND were found on pathology in these 7 patients. The mean number of positive SLNs was 1.4 for both patients who received lymph node irradiation and patients who underwent CLND ± radiotherapy.
All 26 patients underwent local excision to the primary site, of whom 24 patients received adjuvant radiotherapy to the primary site. Six patients in this cohort received chemotherapy: 4 with cisplatin/carboplatin and etoposide; 1 with etoposide only; and 1 with cyclophosphamide, 5-fluorouracil (5-FU), and methotrexate. The median follow-up for this cohort was 18 months (range, 5–62 months).
Clinically Palpable Cohort
Twenty-four of the 50 patients included in this analysis presented with palpable lymph nodes. Fourteen patients were men, and the median age was 59 years (range, 35–89 years). The median size of the primary tumor was 30 mm (range, 6–120 mm). The involved lymph node basin was in the head and neck in 8 patients, the axilla in 6 patients, and the inguinal lymph nodes in 10 patients. Ten patients presented with lymph node disease and no detectable primary lesion.
As regional treatment, 9 of 24 patients received definitive lymph node irradiation, of whom 3 patients underwent excisional biopsy of a clinically apparent lymph node but did not proceed to CLND. Fifteen patients underwent CLND, 12 of whom received adjuvant lymph node irradiation. The mean number of dissected lymph nodes was 21, with an average of 5 positive lymph nodes.
Eleven patients underwent local excision with adjuvant radiotherapy to the primary site, 1 patient underwent local excision alone, and 2 patients received definitive radiotherapy to the primary site (the remaining 10 patients had unknown primary sites). Eight of 24 patients in this cohort received chemotherapy: 6 were treated with cisplatin/carboplatin and etoposide, 1 was treated with etoposide and doxorubicin, and the regimen was unknown in 1 patient. The median follow-up for this cohort was 16 months (range, 5–109 months).
Regional Control
For patients with microscopic lymph node involvement, the estimated 2-year RRFS was 100% regardless of treatment modality (Table 1) (Fig. 2). The 2-year RRFS for patients who presented with palpable lymph nodes was 78% and 73%, respectively, by Kaplan-Meier analysis in the definitive lymph node irradiation and CLND groups (P = .8).
Table 1.
Kaplan-Meier Estimated 2-Year Regional Recurrence-Free Survival, Disease-Specific Survival, and Overall Survival
| 2-Year RRFS | 2-Year DSS | 2-Year OS | |
|---|---|---|---|
| Microscopic | |||
| All (n=26) | 100% | 87% | 81% |
| Lymph node irradiation (n=19) | 100% | 83% | 75% |
| CLND ±RT (n=7) | 100% | 100% | 100% |
| Palpable | |||
| All (n=24) | 75% | 64% | 61% |
| Lymph node irradiation (n=9) | 78% | 73% | 63% |
| CLND ±RT (n=15) | 73% | 59% | 59% |
RRFS indicates regional recurrence-free survival, DSS, disease-specific survival; OS, overall survival; CLND, completion lymphadenectomy; RT, radiotherapy.
Figure 2.
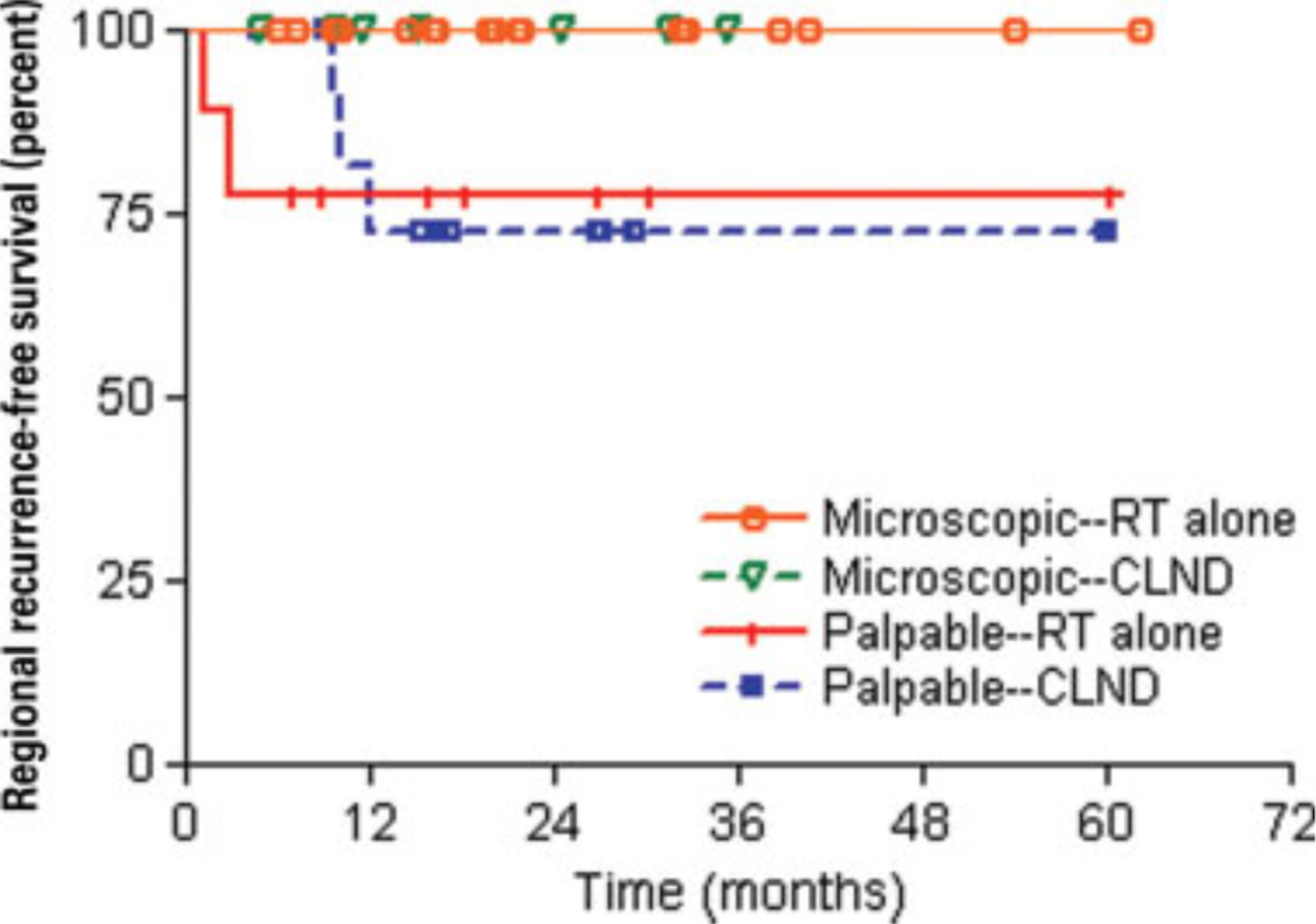
Kaplan-Meier curves for regional recurrence-free survival are shown by lymph node presentation and treatment. RT indicates radiotherapy; CLND, completion lymphadenectomy.
In total, 5 patients developed regional recurrence in the primary lymph node basin, all of whom had palpable lymph nodes at the time of initial presentation. There were no in-transit recurrences. Two of these 5 patients were treated with definitive lymph node irradiation. The first patient developed a local disease recurrence and distant skin metastasis at the time of lymph node recurrence, which occurred 1 month after completion of radiation treatment; this patient died 7 months later. The other patient developed local, regional, and distant failure 3 months after the completion of treatment and died 8 months later.
Three patients with palpable lymph node disease who underwent CLND developed lymph node recurrence. All 3 patients received adjuvant radiotherapy to the regional lymph nodes at the time of initial treatment. One patient had local, regional, and distant disease recurrences at 12 months and died 5 months later. One patient developed local and regional disease recurrences at 10 months, followed by distant metastasis at 14 months. The third patient developed a regional disease recurrence at 10 months and distant metastasis at 11 months.
Among patients treated with CLND, the mean number of pathologically involved lymph nodes identified at the time of dissection was 6 in those that developed a regional disease recurrence versus 2.6 in patients that did not develop a regional disease recurrence.
Survival
The 2-year DSS rate was 87% and 64% in patients with microscopic lymph node involvement versus clinically positive lymph nodes (P = .04) as shown in Figure 3. Among patients with microscopic lymph node involvement, the 2-year DSS rate was 83% and 100% in the lymph node irradiation group and CLND group, respectively (P = .7). The 2-year DSS rate for patients who presented with palpable lymph nodes was 73% and 59% in the lymph node irradiation group and CLND group, respectively (P = .9). Among patients who received CLND, the mean number of pathologically positive lymph nodes was 4.3 in patients who died of MCC versus 2.6 in patients who died of other causes.
Figure 3.
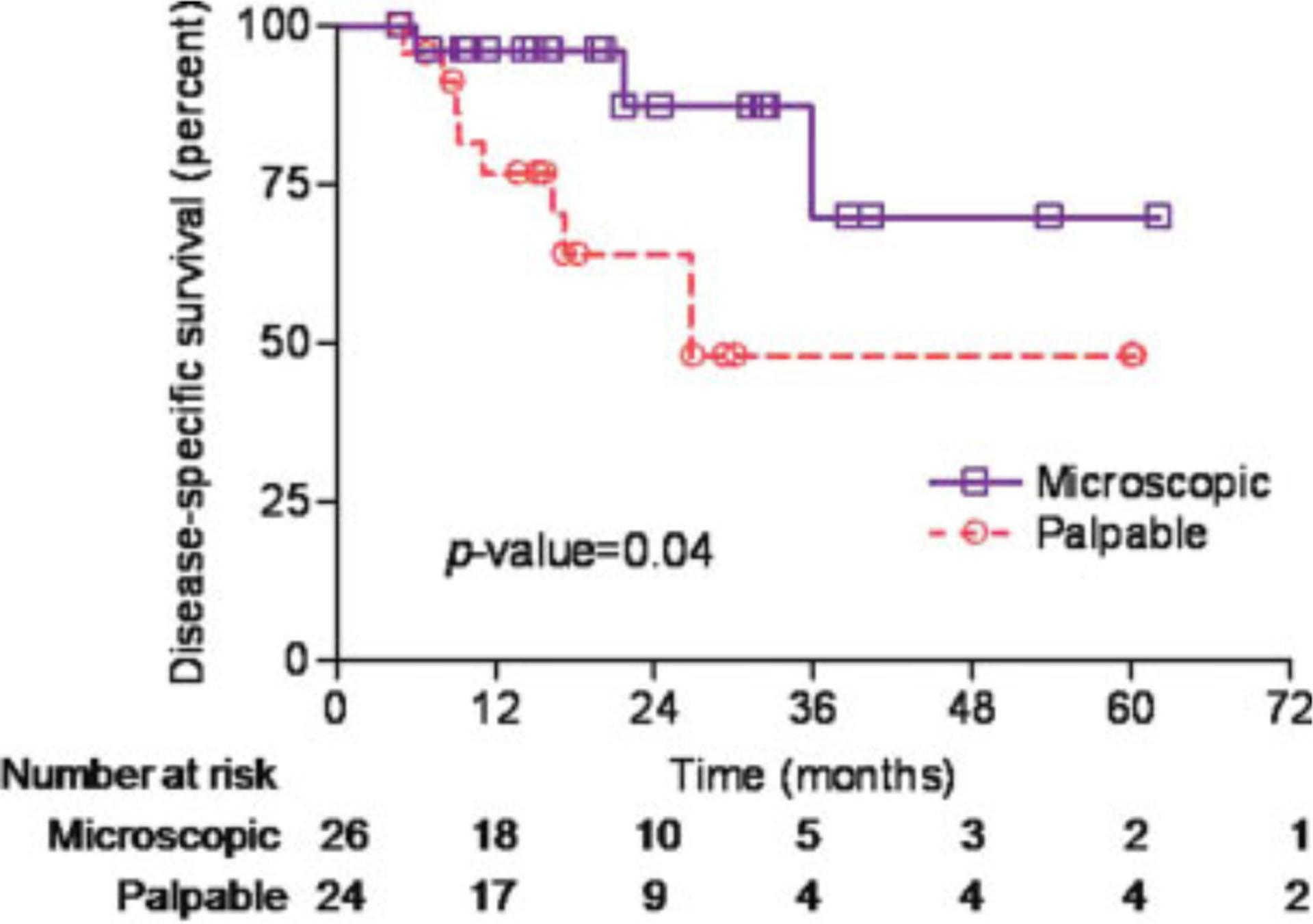
Kaplan-Meier curves for disease-specific survival are shown by lymph node presentation.
The estimated 2-year OS rate was 81% in patients with microscopic lymph node disease compared with 61% in patients who presented with palpable lymph nodes (P-value = .05) as shown in Table 1.
Patterns of First Failure
Distant metastasis was the most common site of first failure for all patients. Distant metastasis occurred in 8% (2 of 26) of patients with microscopic lymph node involvement and 50% (12 of 24) of patients with palpable lymph node involvement (Table 2). Regional failure occurred exclusively in patients with palpable lymph nodes at diagnosis (5 of 24).
Table 2.
Patterns of First Disease Recurrence
| Microscopic (n=26) | Palpable (n=24) | |
|---|---|---|
| Site of First Disease Recurrence | No. of Patients | No. of Patients |
| No recurrence | 22 (85%) | 9 (38%) |
| Any recurrence | 4 (15%) | 15 (62%) |
| Local alone | 2 (8%) | 0 |
| Regional alone | 0 | 1 (4%) |
| Local and regional | 0 | 2 (8%) |
| Local, regional, and distant | 0 | 2 (8%) |
| Distant alone | 2 (8%) | 10 (42%) |
| Total | 26 (100%) | 24 (100%) |
DISCUSSION
To date, the current study is the largest published series of lymph node-positive MCC patients treated definitively with lymph node irradiation without CLND (Table 3). There was a clear selection bias favoring CLND in the presence of palpable lymph node disease and for radiotherapy without CLND if lymph node involvement was identified solely by SLNB. Subgroup analysis revealed that there were no regional failures in any patient who presented with microscopic lymph node involvement. Among patients who presented with palpable lymph node disease, the 2-year RRFS rate was comparable at 78% in the lymph node irradiation group and 73% in the CLND group (P-value 0.8). It is interesting to note that the time to lymph node recurrence was shorter for the 2 patients in the radiation monotherapy group, neither of whom underwent surgical debulking. Although no conclusions can be drawn based on 2 cases, it is plausible that such a trend represents inferior short-term regional control of bulky lymph node disease by lymph node irradiation alone. Conversely, these data may be interpreted as CLND merely delaying lymph node recurrence.
Table 3.
Selected Series of Lymph Node-Positive Patients, Treatment, and Outcomes
| No. of Patients Studied | Regional Treatment | Lymph Node Involvement Detected by and No. of Patients | Crude Regional Recurrence Rate | |||
|---|---|---|---|---|---|---|
| XRT | CLND | CLND+XRT | ||||
| Current study | 50 | 28 | 7 | 15 | Clinical: 24 SLNB: 26 | 10% (5/50) |
| Pectasides 200720 | 5 | 0 | 1 | 4 | Clinical: 5 | 0% (0/5) |
| Jabbour 20078a | 29 | 2 | 8 | 17 | Clinical: 29 | Not reported |
| Senchenkov 200721 | 11 | 2 | 5 | 3 | Clinical: 5 SLNB: 6 |
18% (2/11) |
| Maza 200628 | 11 | 2 | 8 | 0 | SLNB: 11 | 0% (0/11) |
| Allen 20055b | 76 | 4 | 46 | 11 | Clinical: 60 SLNB: 12 ELND: 4 |
14% (10/73c) |
| Veness 200551 | 35 | 5 | 9 | 17 | Clinical: 35 | Not reported |
| Veness 200513 | 8 | 0 | 1 | 7 | Clinical: 8 | 25% (2/8) |
| Schmalbach 200530 | 2 | 2 | 0 | 0 | SLNB: 2 | 0% (0/2) |
| McAfee 200510d | 11 | 1 | 0 | 9 | Clinical: 8 ELND: 3 | 20% (2/10) |
| Gillenwater 20017e | 7 | 1 | 3 | 2 | Clinical: 7 | 57% (4/7) |
| Meeuwissen 199518f | 26 | 13 | 5 | 7 | Clinical: 26 | Not reported |
| Boyle 199532g | 10 | 4 | 2 | 2 | Clinical: 10 | Not reported |
| Morrison 199012 | 9 | 1 | 3 | 5 | Clinical: 4 ELND: 5 | Not reported |
| Shaw 1972–199052h | 23 | 5 | 11 | 5 | Clinical 23 | Not reported |
XRT indicates radiotherapy; CLND, completion lymphadenectomy; SLNB, sentinel lymph node biopsy; ELND, elective lymph node dissection.
Two patients did not receive treatment to the regional lymph nodes.
Ten patients underwent excisional biopsy alone, 1 patient did not receive lymph node treatment, and in 4 patients the lymph node treatment was not reported.
Excluded 3 patients who presented with clinically positive lymph nodes but these lymph nodes were not pathologically assessed.
In 1 patient, the treatment was unknown and patient was lost to follow-up.
One patient did not receive lymph node treatment.
One patient underwent excisional biopsy alone.
One patient received chemotherapy alone, and 1 patient underwent biopsy alone.
Two patients did not receive directed lymph node treatment.
There is a lack of randomized controlled trials to guide management of this aggressive disease, as is the case with other rare malignancies. Surgery, typically CLND with or without radiotherapy, has been accepted as standard therapy for regional lymphatics in patients with lymph node-positive MCC. Published regional outcomes are sparse, but available data report crude regional recurrence rates of 0% to 25% (Table 3).5,10,13,20,21 A series from The University of Texas M. D. Anderson Cancer Center reported a lymph node failure rate of 16% (2 of 12) in lymph node-positive patients treated with therapeutic dissections with or without radiotherapy.22 Allen et al published a single institution experience from Memorial Sloan-Kettering Cancer Center5 including 252 patients and reported a 14% (8 of 57) regional recurrence rate in pathologically lymph node-positive patients treated with surgery alone and a similar 13% rate (2 of 16) in patients treated with surgery and adjuvant radiotherapy. In the current series, the crude rate of regional recurrence in patients treated with CLND with or without radiotherapy was 14% (3 of 22) and thus similar to published outcomes.
In historic series of patients presenting with lymph node-positive MCC, the vast majority of reported patients were lymph node-positive by clinical examination. More recently, SLNB has become standard of care for MCC based on several reports and meta-analyses.23–25 It is particularly appropriate in this disease given its predilection for occult lymph node involvement. SLNB is now recommended in the National Comprehensive Cancer Network guidelines.26 Mehrany et al compiled all published cases of MCC patients who underwent SLNB and reported the grouped results of 60 patients.25 Approximately 33% (20 of 60) had a positive biopsy result and of these patients, only 1 had an isolated regional disease recurrence that occurred in the untreated contralateral neck.27 A series of previously unpublished cases from the Dana-Farber Cancer Institute of 30 patients who underwent SLNB reported a 30% (9 of 30) lymph node positivity rate,24 and there was 1 lymph node recurrence in this group. Maza et al reported on 11 patients with microscopic lymph node involvement by positive SLNB28; 3 patients developed recurrence, however, none of them were lymph node recurrences. Similarly, in this study, no patients with microscopic lymph node involvement found on SLNB developed a regional recurrence regardless of treatment modality. These data together suggest that patients with microscopic lymph node involvement have low regional failure rates when treated with radiation or surgery to the involved lymph node bed.
Although surgery with or without adjuvant radiotherapy is the mainstay of locoregional treatment, there is only a small body of literature regarding the management of MCC with radiotherapy alone. Mortier et al published a series of 9 patients with medically inoperable lymph node-negative MCC who were treated with radiotherapy alone to a median dose of 60 Gray (Gy).29 With a median follow-up of 3 years, there were no disease recurrences or deaths reported. The authors concluded that radiotherapy alone produced acceptable outcomes in these patients. Schmalbach et al reported on 10 patients with MCC of the head and neck evaluated with SLNB.30 Two patients were found to have micro-metastatic disease; they refused surgery and were treated with radiation alone to the lymph node basin. Both patients were free of disease at 38 months and 45 months, respectively. Other case reports of patients treated with radiation as monotherapy have been published with varying results,12,30–34 and small numbers have precluded the ability to draw any conclusions. In this series, 28 patients who received lymph node irradiation without CLND had control rates comparable to the surgical cohort for both microscopic and palpable lymph node presentations.
If lymph node irradiation without CLND can be effectively used as definitive regional treatment for lymph node-positive MCC, the potential morbidities of therapeutic lymph node dissection such as wound infection, lymphedema, pain, numbness, decreased range of motion, and nerve injury can be mitigated. The incidence of lymphedema after lymphadenectomy has been well-described in the literature, particularly for breast cancer and melanoma, and is in the range of 10% to 25%.35,36 The addition of radiotherapy to lymph node dissection can substantially increase the risk to as high as 38% to 77%37–40 and has been shown to negatively impact quality of life.41,42 In this series, 7 patients developed lymphedema (3 patients were treated with CLND and 4 patients with CLND followed by adjuvant radiation). No patients who received lymph node irradiation alone developed lymphedema, despite a similar distribution of affected lymph node basins. Because toxicity data were not collected in a systematic manner, robust conclusions cannot be drawn; however, the incidence is consistent with that reported in the lymphedema literature cited above. Lower rates of complications have been reported with SLNB or lymph node sampling plus radiotherapy compared with full dissections performed in breast cancer patients.43,44 Radiotherapy is not without its toxicities such as tissue fibrosis, brachial plexopathy, and lymphedema; however, long-term toxicity rates are expected to be low, particularly with doses of 50 to 55 Gy. Daily radiation treatment for 5 to 6 weeks is an inconvenience to the patient compared with surgery, although this may be an acceptable alternative if the risk of late toxicities are lowered.
The major limitations of the current study are its small sample size and nonrandomized nature leading to unidentified biases. Such is the difficulty with investigations of rare entities. Another shortfall is the absence of systematically collected information regarding toxicities; however, these data are consistent with what would be anticipated based on experience in melanoma and breast cancer. The median follow-up was 16 to 18 months, but the literature suggests that the majority of lymph node recurrences appear within 1 year of diagnosis.17,22,45–47 In the largest published MCC meta-analysis, it was found that the median time to lymph node recurrence was 7 months, with 75% of these events occurring within 12 months of initial treatment.9 Nevertheless, with longer follow-up, an increase in recurrence rates may be reported.
As we continue to refine regional management for lymph node-positive MCC, systemic failure remains a significant challenge, as is demonstrated in this series with distant failure as the most common site of first disease recurrence. Chemotherapy has been evaluated with disappointing results. A phase 2 study of concurrent and adjuvant cisplatin and etoposide in high-risk MCC patients initially reported favorable outcomes48; however, an update with further analyses demonstrated no significant improvement in OS or DSS when compared with historical controls.49 Given the known chemotherapy-related morbidity and mortality in conjunction with no clear evidence of improved outcomes, adjuvant chemo-therapy is currently not routinely recommended.50 Because metastatic disease will dictate the survival and ultimate outcomes of these patients, future investigations will have to be directed at improving systemic control.
Conclusions
It is imperative to define the optimal therapy for lymph node-positive MCC as the use of immunohistochemical staining and SLNB will increasingly identify patients with lymph node-positive disease. In addition, it is estimated that 30% to 50% of MCC patients will develop lymph node involvement over the disease course. This study found that lymph node irradiation to the primary lymph node basin in lymph node-positive disease confers an excellent regional control rate that is comparable to surgical outcomes with no detectable difference in OS. Definitive lymph node irradiation can thus be considered as a treatment option in patients with positive lymph nodes.
CONFLICT OF INTEREST DISCLOSURES
Supported by the American Cancer Society/Jerry Wachter Fund for Merkel Cell Carcinoma, Merkel Cell Carcinoma Patient Fund at the University of Washington and National Institutes of Health grant K24-CA139052 and American Cancer Society grant ACS-R56-08-115-01-CCE.
REFERENCES
- 1.Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet. 2002; 359:497–498. [DOI] [PubMed] [Google Scholar]
- 2.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717–1721. [DOI] [PubMed] [Google Scholar]
- 4.Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, Coit DG. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005; 23:2300–2309. [DOI] [PubMed] [Google Scholar]
- 6.Boyer JD, Zitelli JA, Brodland DG, D’Angelo G. Local control of primary Merkel cell carcinoma: review of 45 cases treated with Mohs micrographic surgery with and without adjuvant radiation. J Am Acad Dermatol. 2002;47:885–892. [DOI] [PubMed] [Google Scholar]
- 7.Gillenwater AM, Hessel AC, Morrison WH, et al. Merkel cell carcinoma of the head and neck: effect of surgical excision and radiation on recurrence and survival. Arch Otolaryngol Head Neck Surg. 2001;127:149–154. [DOI] [PubMed] [Google Scholar]
- 8.Jabbour J, Cumming R, Scolyer RA, Hruby G, Thompson JF, Lee S. Merkel cell carcinoma: assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in early-stage disease-results from a review of 82 consecutive cases diagnosed between 1992 and 2004. Ann Surg Oncol. 2007;14:1943–1952. [DOI] [PubMed] [Google Scholar]
- 9.Lewis KG, Weinstock MA, Weaver AL, Otley CC. Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol. 2006;142:693–700. [DOI] [PubMed] [Google Scholar]
- 10.McAfee WJ, Morris CG, Mendenhall CM, Werning JW, Mendenhall NP, Mendenhall WM. Merkel cell carcinoma: treatment and outcomes. Cancer. 2005;104:1761–1764. [DOI] [PubMed] [Google Scholar]
- 11.Medina-Franco H, Urist MM, Fiveash J, Heslin MJ, Bland KI, Beenken SW. Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1024 cases. Ann Surg Oncol. 2001;8:204–208. [DOI] [PubMed] [Google Scholar]
- 12.Morrison WH, Peters LJ, Silva EG, Wendt CD, Ang KK, Goepfert H. The essential role of radiation therapy in securing locoregional control of Merkel cell carcinoma. Int J Radiat Oncol Biol Phys. 1990;19:583–591. [DOI] [PubMed] [Google Scholar]
- 13.Veness MJ, Morgan GJ, Gebski V. Adjuvant locoregional radiotherapy as best practice in patients with Merkel cell carcinoma of the head and neck. Head Neck. 2005;27:208–216. [DOI] [PubMed] [Google Scholar]
- 14.Mojica P, Smith D, Ellenhorn JD. Adjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skin. J Clin Oncol. 2007;25:1043–1047. [DOI] [PubMed] [Google Scholar]
- 15.Toker C Trabecular carcinoma of the skin. Arch Dermatol. 1972;105:107–110. [PubMed] [Google Scholar]
- 16.Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003; 49:832–841. [DOI] [PubMed] [Google Scholar]
- 17.Brissett AE, Olsen KD, Kasperbauer JL, et al. Merkel cell carcinoma of the head and neck: a retrospective case series. Head Neck. 2002;24:982–988. [DOI] [PubMed] [Google Scholar]
- 18.Meeuwissen JA, Bourne RG, Kearsley JH. The importance of postoperative radiation therapy in the treatment of Merkel cell carcinoma. Int J Radiat Oncol Biol Phys. 1995;31: 325–331. [DOI] [PubMed] [Google Scholar]
- 19.Leonard JH, Ramsay JR, Kearsley JH, Birrell GW. Radiation sensitivity of Merkel cell carcinoma cell lines. Int J Radiat Oncol Biol Phys. 1995;32:1401–1407. [DOI] [PubMed] [Google Scholar]
- 20.Pectasides D, Papaxoinis G, Pectasides E, et al. Merkel cell carcinoma of the skin: a retrospective study of 24 cases by the Hellenic Cooperative Oncology Group. Oncology. 2007; 72:211–218. [DOI] [PubMed] [Google Scholar]
- 21.Senchenkov A, Barnes SA, Moran SL. Predictors of survival and recurrence in the surgical treatment of Merkel cell carcinoma of the extremities. J Surg Oncol. 2007;95:229–234. [DOI] [PubMed] [Google Scholar]
- 22.Goepfert H, Remmler D, Silva E, Wheeler B. Merkel cell carcinoma (endocrine carcinoma of the skin) of the head and neck. Arch Otolaryngol. 1984;110:707–712. [DOI] [PubMed] [Google Scholar]
- 23.Allen PJ, Busam K, Hill AD, Stojadinovic A, Coit DG. Immunohistochemical analysis of sentinel lymph nodes from patients with Merkel cell carcinoma. Cancer. 2001;92:1650–1655. [DOI] [PubMed] [Google Scholar]
- 24.Gupta SG, Wang LC, Penas PF, Gellenthin M, Lee SJ, Nghiem P. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: The Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142:685–690. [DOI] [PubMed] [Google Scholar]
- 25.Mehrany K, Otley CC, Weenig RH, Phillips PK, Roenigk RK, Nguyen TH. A meta-analysis of the prognostic significance of sentinel lymph node status in Merkel cell carcinoma. Dermatol Surg. 2002;28:113–117, discussion 17. [DOI] [PubMed] [Google Scholar]
- 26.Merkel Cell Carcinoma. NCCN Clinical Practice Guidelines in Oncology. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp 2009;1 Accessed on February 1, 2009. [Google Scholar]
- 27.Zeitouni NC, Cheney RT, Delacure MD. Lymphoscintigraphy, sentinel lymph node biopsy, and Mohs micrographic surgery in the treatment of Merkel cell carcinoma. Dermatol Surg. 2000;26:12–18. [DOI] [PubMed] [Google Scholar]
- 28.Maza S, Trefzer U, Hofmann M, et al. Impact of sentinel lymph node biopsy in patients with Merkel cell carcinoma: results of a prospective study and review of the literature. Eur J Nucl Med Mol Imaging. 2006;33:433–440. [DOI] [PubMed] [Google Scholar]
- 29.Mortier L, Mirabel X, Fournier C, Piette F, Lartigau E. Radiotherapy alone for primary Merkel cell carcinoma. Arch Dermatol. 2003;139:1587–1590. [DOI] [PubMed] [Google Scholar]
- 30.Schmalbach CE, Lowe L, Teknos TN, Johnson TM, Bradford CR. Reliability of sentinel lymph node biopsy for regional staging of head and neck Merkel cell carcinoma. Arch Otolaryngol Head Neck Surg. 2005;131:610–614. [DOI] [PubMed] [Google Scholar]
- 31.Ashby MA, Jones DH, Tasker AD, Blackshaw AJ. Primary cutaneous neuroendocrine (Merkel cell or trabecular carcinoma) tumour of the skin: a radioresponsive tumour. Clin Radiol. 1989;40:85–87. [DOI] [PubMed] [Google Scholar]
- 32.Boyle F, Pendlebury S, Bell D. Further insights into the natural history and management of primary cutaneous neuroendocrine (Merkel cell) carcinoma. Int J Radiat Oncol Biol Phys. 1995;31:315–323. [DOI] [PubMed] [Google Scholar]
- 33.Pacella J, Ashby M, Ainslie J, Minty C. The role of radiotherapy in the management of primary cutaneous neuroendocrine tumors (Merkel cell or trabecular carcinoma): experience at the Peter MacCallum Cancer Institute (Melbourne, Australia). Int J Radiat Oncol Biol Phys. 1988;14: 1077–1084. [DOI] [PubMed] [Google Scholar]
- 34.Pilotti S, Rilke F, Lombardi L. Neuroendocrine (Merkel cell) carcinoma of the skin. Am J Surg Pathol. 1982;6:243–254. [DOI] [PubMed] [Google Scholar]
- 35.Erickson VS, Pearson ML, Ganz PA, Adams J, Kahn KL. Arm edema in breast cancer patients. J Natl Cancer Inst. 2001;93:96–111. [DOI] [PubMed] [Google Scholar]
- 36.Petrek JA, Heelan MC. Incidence of breast carcinoma-related lymphedema. Cancer. 1998;83(suppl):2776–2781. [DOI] [PubMed] [Google Scholar]
- 37.Ballo MT, Strom EA, Zagars GK, et al. Adjuvant irradiation for axillary metastases from malignant melanoma. Int J Radiat Oncol Biol Phys. 2002;52:964–972. [DOI] [PubMed] [Google Scholar]
- 38.Starritt EC, Joseph D, McKinnon JG, Lo SK, de Wilt JH, Thompson JF. Lymphedema after complete axillary node dissection for melanoma: assessment using a new, objective definition. Ann Surg. 2004;240:866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens G, Thompson JF, Firth I, O’Brien CJ, McCarthy WH, Quinn MJ. Locally advanced melanoma: results of postoperative hypofractionated radiation therapy. Cancer. 2000;88:88–94. [DOI] [PubMed] [Google Scholar]
- 40.Kissin MW, Querci della Rovere G, Easton D, Westbury G. Risk of lymphoedema following the treatment of breast cancer. Br J Surg. 1986;73:580–584. [DOI] [PubMed] [Google Scholar]
- 41.Fleissig A, Fallowfield LJ, Langridge CI, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006; 95:279–293. [DOI] [PubMed] [Google Scholar]
- 42.Kwan W, Jackson J, Weir LM, Dingee C, McGregor G, Olivotto IA. Chronic arm morbidity after curative breast cancer treatment: prevalence and impact on quality of life. J Clin Oncol. 2002;20:4242–4248. [DOI] [PubMed] [Google Scholar]
- 43.Mathew J, Barthelmes L, Neminathan S, Crawford D. Comparative study of lymphoedema with axillary node dissection versus axillary node sampling with radiotherapy in patients undergoing breast conservation surgery. Eur J Surg Oncol. 2006;32:729–732. [DOI] [PubMed] [Google Scholar]
- 44.Chetty U, Jack W, Prescott RJ, Tyler C, Rodger A. Management of the axilla in operable breast cancer treated by breast conservation: a randomized clinical trial. Edinburgh Breast Unit. Br J Surg. 2000;87:163–169. [DOI] [PubMed] [Google Scholar]
- 45.Allen PJ, Zhang ZF, Coit DG. Surgical management of Merkel cell carcinoma. Ann Surg. 1999;229:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goessling W, McKee PH, Mayer RJ. Merkel cell carcinoma. J Clin Oncol. 2002;20:588–598. [DOI] [PubMed] [Google Scholar]
- 47.Hitchcock CL, Bland KI, Laney RG III, Franzini D, Harris B, Copeland EM III. Neuroendocrine (Merkel cell) carcinoma of the skin. Its natural history, diagnosis, and treatment. Ann Surg. 1988;207:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poulsen M, Rischin D, Walpole E, et al. High-risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a Trans-Tasman Radiation Oncology Group Study-TROG 96:07. J Clin Oncol. 2003; 21:4371–4376. [DOI] [PubMed] [Google Scholar]
- 49.Poulsen MG, Rischin D, Porter I, et al. Does chemotherapy improve survival in high-risk stage I and II Merkel cell carcinoma of the skin? Int J Radiat Oncol Biol Phys. 2006; 64:114–119. [DOI] [PubMed] [Google Scholar]
- 50.Garneski KM, Nghiem P. Merkel cell carcinoma adjuvant therapy: current data support radiation but not chemotherapy. J Am Acad Dermatol. 2007;57:166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veness MJ. Mekel cell carcinoma: improved outcome with the addition of adjuvant therapy. J Clin Oncol. 2005;23: 7235–7236; author reply 7237–7238. [DOI] [PubMed] [Google Scholar]
- 52.Shaw JH, Rumball E. Merkel cell tumour: clinical behaviour and treatment. Br J Surg. 1991;78:138–142. [DOI] [PubMed] [Google Scholar]


