Abstract
The promoter selectivity of two extracytoplasmic function (ECF) subfamily ς subunits, ςE (ς24) and ςFecI (ς18), of Escherichia coli RNA polymerase was analyzed by using an in vitro transcription system and various promoters. The EςE holoenzyme recognized only the known cognate promoters, rpoEP2, rpoHP3, and degP, and the EςFecI recognized only one known cognate promoter, fecA. The strict promoter recognition properties of ςE and ςFecI are similar to those of other minor ς subunits. Transcription by EςE and EςFecI was enhanced by high concentrations of glutamate, as in the case of other minor ς subunits. The optimum temperature for transcription by EςFecI was low, around 25°C, apparently in agreement with the high rate of iron sequestration by E. coli at low temperatures. By quantitative Western blot analysis, the intracellular levels of ςE and ςFecI in the uninduced steady-state culture of E. coli W3110 (type A) were determined to be 0.7 to 2.0 and 0.1 to 0.2 fmol per μg of total proteins (or 3 to 9 and 0.4 to 0.9 molecules per cell), respectively, and less than 1% of the level of the major ς70 subunit.
The DNA-dependent RNA polymerase of Escherichia coli is composed of the core enzyme with the subunit structure α2ββ′ and one of seven molecular species of the ς subunit, ς70, ςN, ςS, ςH, ςF, ςE, or ςFecI (11, 13). Molecular properties and functional specificity have been studied in detail for all of these ς subunits except for two, ςE and ςFecI. The ςE subunit encoded by the rpoE gene controls transcription of the genes for extracytoplasmic stress response (3–5, 8, 25, 26, 31). The synthesis of ςE is induced upon exposure to heat shock or ethanol stress or following accumulation of unfolded proteins in the periplasm (8, 26, 29, 33). The holoenzyme EςE is responsible for transcription of at least 10 genes (32), of which 4 have been identified, including rpoH, which encodes ςH for transcription of the heat shock response genes (9, 31); degP, which encodes a periplasmic protease for degradation of misfolded proteins (14, 23, 31, 34); fkpA, which encodes a periplasmic peptidyl-prolyl isomerase (4); and rpoE itself (31). On the other hand, the fecI gene was originally identified as a regulatory gene for the ferric dicitrate transport system (30), but after sequencing, the FecI protein was recognized as a member of the extracytoplasmic function (ECF) subfamily of ς factor (hereafter referred to as ςFecI in this report) (1, 24). Transcription of the ferric dicitrate transport system of E. coli is repressed by Fe2+-Fur and activated by ferric dicitrate (2, 7, 12). Ferric dicitrate does not have to enter into the cytoplasm for transcription activation, but it initiates a signal transduction pathway by binding to the outer membrane receptor FecA (2, 12). The signal is then transmitted through the inner membrane-associated FecR, which ultimately activates the ςFecI subunit. The fecA promoter is the only one identified to date that is transcribed specifically by the EςFecI holoenzyme.
Here we performed the first systematic analysis of the promoter of ςE and ςFecI by using in vitro transcription assay systems. In addition, we determined the intracellular concentrations of these two ECF subfamily ς subunits in E. coli W3110 (A) growing at various phases.
Promoter selectivity of the EςE and EςFecI holoenzymes.
For analysis of the promoter selectivity of RNA polymerase holoenzymes containing ςE or ςFecI, two ECF family ς subunits were overexpressed in E. coli M15 by using plasmid pRPOE (14) or E. coli BL21(DE3) by using plasmid pETFecI. The plasmid pETFecI for the expression of ςFecI protein with a hexahistidine tag (His6) at the C terminus was constructed by insertion of PCR-amplified FecI-coding sequence into pET-21b (Novagen) between the NdeI and HindIII sites. Both ςE and ςFecI were extracted from the inclusion bodies with an extraction buffer containing 0.5% Triton X-100 and purified by Ni2+-nitrilotriacetic acid affinity chromatography. For reconstitution of the holoenzymes, we purified the ς-free core enzyme by chromatography of the purified RNA polymerase of E. coli W3350 (10) at least three times through phosphocellulose columns (6, 22). The repeated chromatography is essential for complete removal of traces of minor ς subunits. To detect the activity in vitro of purified ςE, we used truncated DNA templates, each containing one of the three known promoters, i.e., the 210-bp EcoRI-SphI rpoE promoter fragment, the 220-bp EcoRI-SphI rpoH fragment, or the 214-bp EcoRI-SphI degP fragment, each producing specific transcripts of 71 (rpoE), 81 (rpoH), and 74 (degP) nucleotides in length, respectively. For detection of the ςFecI activity, we used the only known FecI-dependent promoter, fecA, which produces RNA of 62 (fecA) nucleotides in length.
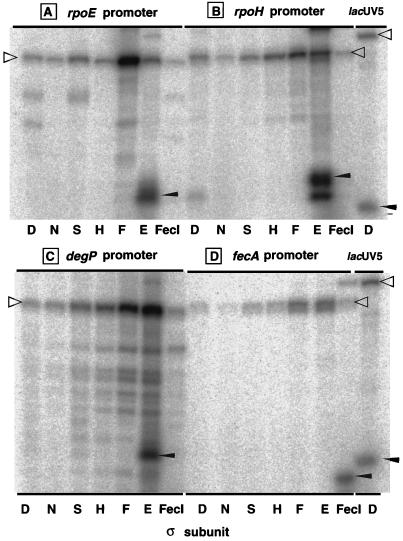
Under the standard transcription assay conditions for the Eς70 holoenzyme (E represents the core enzyme) (20), the reconstituted holoenzymes containing ςE and ςFecI produced specific transcripts directed by the respective cognate promoters (Fig. 1). None of the other holoenzymes containing ς70, ςN, ςS, ςH, or ςF, however, produced significant levels of transcripts from the ςE-dependent rpoE, rpoH, and degP promoters or from the ςFecI-dependent fecA promoter, even though all of these holoenzymes gave similar levels of the template-sized end-to-end transcripts, which migrated near the top of the gels (Fig. 1). On the other hand, both EςE and EςFecI holoenzymes were unable to transcribe the ς70-dependent lacUV5 (Fig. 1), trp, and rpsA promoters. Thus, we concluded that the ECF family ς subunits carry high selectivity for a specific set of the cognate promoters, as in the case of other minor ς subunits, ςN, ςH, and ςF. In contrast to the strict promoter selectivity characteristic of the minor ς subunits, the ς70 subunit recognizes in vitro most ςS-dependent promoters, and the ςS subunit recognizes some ς70-dependent promoters (21, 35).
FIG. 1.
Transcription in vitro of truncated DNA templates by RNA polymerase holoenzymes EςE and EςFecI. RNA polymerase holoenzymes were reconstituted by mixing the core enzyme with each ς subunit in a core-to-ς molar ratio of 1:4. Single-round transcription was carried out under the standard assay conditions (20) with 1 pmol each of seven different holoenzymes containing ς70, ςN, ςS, ςH, ςH, ςF, ςH, and ςFecI (the species of ς subunit used is shown at the bottom of each gel lane) and 0.1 pmol each of three ςE-dependent (rpoE [A], rpoH [B], and degP [C]) and one ςFecI-dependent (fecA [D]) promoter. RNA products were separated by 8% PAGE in the presence of 8 M urea, and gels were analyzed with a Bio-Imaging Analyzer BAS-2000 (Fuji). Arrowheads indicate the specific transcripts from the test promoters, while open triangles indicate the template-sized nonspecific transcripts.
Stimulation of ςE- and ςFecI-dependent transcription by potassium glutamate.
The reaction conditions such as DNA superhelicity, the species and concentrations of salts, trehalose, and polyphosphate, and the reaction temperature affect in vitro transcription in different ways for the different holoenzymes containing different ς subunits, presumably reflecting the difference in physiological conditions under which each ς subunit works (reviewed in references 15 and 16).
The standard reaction mixture to give maximum-level transcription of lacUV5 by the Eς70 holoenzyme contains 50 mM NaCl (Fig. 2C) (see also reference 20). The optimum concentrations of NaCl to give maximum transcription activity on trp, recA, and rpsA templates were between 50 and 100 nM (data not shown). The activity of Eς70 holoenzyme is, however, negligible at NaCl concentrations above 200 mM (Fig. 2C) (see also references 8 and 28). In contrast, transcription of rpoE by EςE (Fig. 2A) and of fecA by EςFecI (Fig. 2B) stays almost at the same level, between 50 and 300 mM NaCl, indicating that transcription by these two holoenzymes is relatively resistant to inhibition by high NaCl concentrations. Previously, we found that high concentrations of glutamate enhance transcription by the EςS and EςF holoenzymes (6, 22). Here we also examined the effect of increasing concentrations of potassium glutamate. As shown in Fig. 2A and B, transcription by both EςE and EςFecI holoenzymes was significantly enhanced upon increasing the potassium glutamate concentration up to at least 400 mM. The molecular mechanism underlying the activation of minor ς-dependent transcription by high concentrations of glutamate remains to be solved.
FIG. 2.
Effects of salt concentrations on in vitro transcription by RNA polymerase EςH and EςFecI holoenzymes. Single-round transcription was carried out by using 0.1 pmol of each of three test promoters, rpoE (A), fecA (B), and lacUV5 (C), and 1 pmol each of three different forms of the reconstituted holoenzyme, EςE (A), EςFecI (B), and Eς70 (C), under the standard reaction conditions except that 50 mM NaCl was replaced by the indicated concentrations of either NaCl or K glutamate. Transcripts were fractionated by 8% PAGE, and gels were examined with a Bio-Imaging Analyzer BAS2000 (Fuji). The maximum levels of transcription observed with each template are set at 100%.
Preference for low temperatures of ςFecI-dependent transcription.
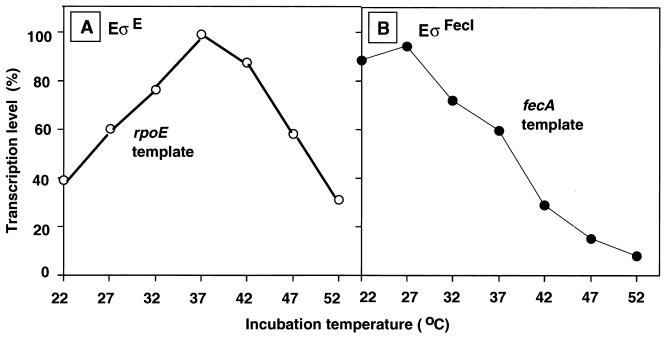
The optimum temperature for maximum-level transcription by the regular holoenzyme Eς70 is about 37°C, whereas the optimum temperature of transcription of certain promoters by EςH and EςF significantly deviates from this optimum temperature for the Eς70 holoenzyme (22, 36). We then examined the effect of reaction temperature on rpoE promoter-directed transcription by EςE and fecA promoter-directed transcription by EςFecI. As shown in Fig. 3A, the optimum temperature for maximum transcription by EςE was 37°C, but about half the maximum-level activity was retained above 50°C, indicating that transcription by EςE predominates at high temperatures, in good agreement with the expected role of ςE in response to extremely high temperatures (8).
FIG. 3.
Effect of temperature on in vitro transcription by RNA polymerase EςE and EςFecI holoenzymes. Single-round transcription was carried out with 0.1 pmol each of two test promoters, rpoE (A) and fecA (B), and 1 pmol each of either EςE (A) or EςFecI (B) holoenzyme under the standard reaction conditions in the presence of 400 mM K glutamate. Both preincubation for RNA polymerase-promoter complex formation and incubation for transcription were carried out at the various temperatures indicated. The maximum levels of transcription observed with each template are set at 100%.
In contrast, fecA promoter-directed transcription by EςFecI was at the maximum at around 25°C and linearly decreased thereafter up to 52°C, where the activity was almost negligible (Fig. 3B). Among seven species of the holoenzyme examined under the same conditions, the EςFecI species required the lowest temperature to give the maximum activity of transcription. The acquisition of iron by bacteria is known to be more efficient at low temperatures (37). The in vivo activation of ςFecI-dependent transcription at low temperatures awaits further analysis.
Intracellular levels of the ςE and ςFecI proteins.
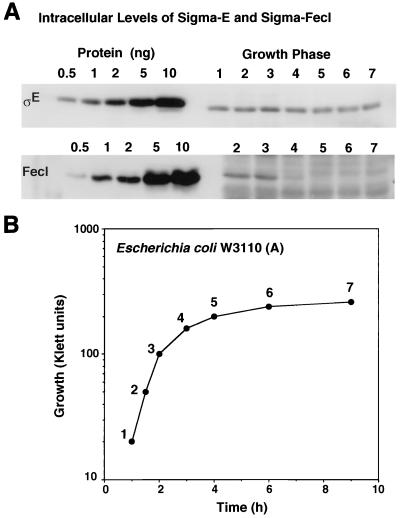
Previously, we determined the intracellular concentrations of ς70, ςN, ςS, ςH, and ςF in E. coli W3110 (type A lineage) by the quantitative Western blot method (17, 19). Here we determined the concentration of two ECF family subunits, ςE and ςFecI, in the same cell extracts of E. coli W3110 (A) used in our previous determination. In the exponential growth phase, the concentrations of ςE and ςFecI were 0.7 to 2.0 and 0.1 to 0.2 fmol per μg of total proteins, respectively (Fig. 4). In the same extract, the concentration of the major ς subunit, ς70, is 150 to 170 fmol/μg of total proteins (17). The number of ς70 molecules per cell is estimated to be around 700 (19). The numbers of ςE and ςFecI molecules per cell can then be calculated to be 3 to 9 and 0.4 to 0.9, respectively. The level of ςE stayed constant throughout the growth phase examined, but the ςFecI level further decreased in the stationary phase (Fig. 4). The critical factors leading to induction of the synthesis or activation of the ECF family ς subunits, however, remain unclear. Taken together with the previous determinations (17, 19), we conclude that under the steady state of cell growth, the uninduced levels of the minor subunits ςS, ςH, ςE, and ςFecI are all lower than 1% of the level of the major ς70 subunit.
FIG. 4.
Intracellular concentrations of ςE and ςFecI in E. coli W3110 (type A). E. coli W3110 (type A) was grown with shaking in Luria-Bertani medium at 37°C. Cell extract was prepared by the method of Jishage et al. (17, 19). The protein concentration of cell lysates was determined by using the Bio-Rad protein assay kit. Polyclonal antibodies against ςE and ςFecI were raised in rabbits by injecting the overexpressed and purified ς proteins. The quantitative Western blot analysis was employed for the measurement of ς subunits exactly as described in previous reports (17, 19). The immunostained blots were developed with 3,3′-diaminobenzidine tetrahydrochloride (Dojindo). Staining intensity was measured with a PDI image analyzer system equipped with a white light scanner. (A) Aliquots containing 10 μg of total proteins from cell lysates of E. coli W3110 (A) prepared at various times of the cell culture (see B for the growth curve) were subjected to quantitative Western blot analysis using anti-ςE and anti-ςFecI antibodies. (B) E. coli W3100 (type A) was grown in Luria-Bertani medium at 37°C under the same conditions employed in the determination of other ς subunits (17, 19), and growth was monitored by measuring the turbidity with a Klett-Summerson photometer. At the indicated time points labeled 1 to 7, aliquots were taken for preparation of the cell lysates.
In addition to the synthesis control, the activity is negatively regulated, at least in the case of ςE subunit, by a membrane-bound anti-ς factor, RseA (5, 27). Such an activity control of the ς subunit has been found for both ςF (28) and ς70 (18). However, the factor affecting the ςFecI activity has not yet been identified.
We thank S. Kusano and T. S. Kundu for preparation of ςS, ςH, and ςF and A. Iwata and S. Ueda for preparation of anti-ςE and anti-ςFecI antibodies.
This work was supported by Grants-in-Aid from the Ministry of Education, Science and Culture of Japan and from CREST (Core Research for Evolutional Science and Technology) of the Japan Science and Technology Corporation.
REFERENCES
- 1.Angerer A, Enz S, Ochs M, Braun V. Transcriptional regulation of ferric citrate transport in Escherichia coli. FecI belongs to subfamily of ς70-type factors that respond to extracytoplasmic stimuli. Mol Microbiol. 1995;18:163–174. doi: 10.1111/j.1365-2958.1995.mmi_18010163.x. [DOI] [PubMed] [Google Scholar]
- 2.Braun V. Surface signaling: novel transcription initiation mechanism starting from the cell surface. Arch Microbiol. 1997;167:325–331. doi: 10.1007/s002030050451. [DOI] [PubMed] [Google Scholar]
- 3.Connolly L, De Las Penas A, Alba B M, Gross C A. The response to extracytoplasmic stress in Escherichia coli is controlled by partially overlapping pathways. Genes Dev. 1997;11:2012–2021. doi: 10.1101/gad.11.15.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danese P N, Silhavy T J. The ςE and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 1997;11:1183–1193. doi: 10.1101/gad.11.9.1183. [DOI] [PubMed] [Google Scholar]
- 5.De Las Penas A, Connolly L, Gross C A. The ςE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of ςE. Mol Microbiol. 1997;24:373–385. doi: 10.1046/j.1365-2958.1997.3611718.x. [DOI] [PubMed] [Google Scholar]
- 6.Ding Q, Kusano S, Villarejo V, Ishihama A. Promoter selectivity control of Escherichia coli RNA polymerase by ionic strength: differential recognition of osmo-regulated promoters by EςD and EςS holoenzymes. Mol Microbiol. 1995;16:649–655. doi: 10.1111/j.1365-2958.1995.tb02427.x. [DOI] [PubMed] [Google Scholar]
- 7.Enz S, Braun V, Crosa J H. Transcription of the region encoding the ferric dicitrate-transport system in Escherichia coli: similarity between promoters for fecA and for extracytoplasmic function sigma factors. Gene. 1995;163:13–18. doi: 10.1016/0378-1119(95)00380-o. [DOI] [PubMed] [Google Scholar]
- 8.Erickson J W, Gross C A. Identification of the ςE subunit of Escherichia coli RNA polymerase: a second alternative ς factor involved in high-temperature gene expression. Genes Dev. 1989;3:1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- 9.Erickson J W, Vaughn V, Walter W A, Neidhardt F C, Gross C A. Regulation of the promoters and transcripts of rpoH, the Escherichia coli heat shock regulatory gene. Genes Dev. 1987;1:419–432. doi: 10.1101/gad.1.5.419. [DOI] [PubMed] [Google Scholar]
- 10.Fujita N, Nomura T, Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase: purification and properties of holoenzyme containing the heat-shock sigma subunit. J Biol Chem. 1987;262:1855–1859. [PubMed] [Google Scholar]
- 11.Gross C A, Lonetto M, Losick R. Bacterial sigma factors. In: Yamamoto K, McKnight S, editors. Transcriptional regulation. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 129–176. [Google Scholar]
- 12.Harle C, Kim I, Angerer A, Braun V. Signal transfer through three compartments: transcription initiation of the Escherichia coli ferric citrate transport system from the cell surface. EMBO J. 1995;14:1430–1438. doi: 10.1002/j.1460-2075.1995.tb07129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helmann J D, Chamberlin M J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- 14.Hiratsu K, Amemura M, Nashimoto H, Shinagawa H, Makino K. The rpoE gene of Escherichia coli, which encodes sigma E, is essential for bacterial growth at high temperature. J Bacteriol. 1995;177:2918–2922. doi: 10.1128/jb.177.10.2918-2922.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishihama A. Adaptation of gene expression in stationary phase bacteria. Curr Opin Genet Dev. 1997;7:582–588. doi: 10.1016/s0959-437x(97)80003-2. [DOI] [PubMed] [Google Scholar]
- 16.Ishihama A. Modulation of the nucleoid, the transcription apparatus, and the translation machinery in bacteria for stationary phase survival. Genes Cells. 1999;3:135–143. doi: 10.1046/j.1365-2443.1999.00247.x. [DOI] [PubMed] [Google Scholar]
- 17.Jishage M, Ishihama A. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of ς70 and ς38. J Bacteriol. 1995;177:6832–6835. doi: 10.1128/jb.177.23.6832-6835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jishage M, Ishihama A. A stationary-phase protein in Escherichia coli with binding activity to the major ς subunit of RNA polymerase. Proc Natl Acad Sci USA. 1998;95:4953–4958. doi: 10.1073/pnas.95.9.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jishage M, Iwata A, Ueda S, Ishihama A. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of four species of sigma subunit under various growth conditions. J Bacteriol. 1996;178:5447–5451. doi: 10.1128/jb.178.18.5447-5451.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kajitani M, Ishihama A. Determination of the promoter strength in the mixed transcription system: promoters of lactose, tryptophan and ribosomal protein L10 operons from Escherichia coli. Nucleic Acids Res. 1983;11:671–686. doi: 10.1093/nar/11.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolb A, Kotlarz D, Kusano S, Ishihama A. Selectivity of the Escherichia coli RNA polymerase Eς38 for overlapping promoters and ability to support CRP activation. Nucleic Acids Res. 1995;23:819–826. doi: 10.1093/nar/23.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kundu T K, Kusano S, Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase ςF holoenzyme involved in transcription of flagellar and chemotaxis genes. J Bacteriol. 1997;179:4264–4269. doi: 10.1128/jb.179.13.4264-4269.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipinska B, Fayet O, Baird L, Georgopoulos C. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J Bacteriol. 1989;171:1574–1584. doi: 10.1128/jb.171.3.1574-1584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lonetto M, Brown K L, Rudd K E, Buttner M J. Analysis of the Streptomyces coelicolor sigma-E gene reveals the existence of a subfamily of eubacterial RNA polymerase sigma factors involved in the regulation of extracytoplasmic functions. Proc Natl Acad Sci USA. 1994;91:7573–7577. doi: 10.1073/pnas.91.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mecsas J, Rouviere P E, Erickson J W, Donohue T J, Gross C A. The activity of ςE, an Escherichia coli heat-inducible ς-factor, is modulated by expression of outer membrane proteins. Genes Dev. 1993;7:2618–2628. doi: 10.1101/gad.7.12b.2618. [DOI] [PubMed] [Google Scholar]
- 26.Missiakas D, Betton J-M, Raina S. New components of protein folding in extracytoplasmic compartments of Escherichia coli SurA, FkpA and Skp/OmpH. Mol Microbiol. 1996;21:871–884. doi: 10.1046/j.1365-2958.1996.561412.x. [DOI] [PubMed] [Google Scholar]
- 27.Missiakas D, Mayer M P, Lemaire M, Georgopoulos C, Raina S. Modulation of the Escherichia coli ςE (RpoE) heat-shock transcription-factor activity by the RseA, RseB and RseC proteins. Mol Microbiol. 1997;24:355–371. doi: 10.1046/j.1365-2958.1997.3601713.x. [DOI] [PubMed] [Google Scholar]
- 28.Ohnishi K, Kutsukake K, Suzuki H, Iino T. A novel transcriptional regulation mechanism in the flagellar regulon of Salmonella typhimurium: an antisigma factor inhibits the activity of flagellum-specific factor, sigma F. Mol Microbiol. 1992;6:3149–3157. doi: 10.1111/j.1365-2958.1992.tb01771.x. [DOI] [PubMed] [Google Scholar]
- 29.Pogliano J, Lynch A S, Berlin D, Lin E C, Beckwith J. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 1997;11:1169–1182. doi: 10.1101/gad.11.9.1169. [DOI] [PubMed] [Google Scholar]
- 30.Pressler U, Staudenmaier H, Zimmermann L, Baun V. Genetics of the iron dicitrate transport system of Escherichia coli. J Bacteriol. 1988;170:2716–2724. doi: 10.1128/jb.170.6.2716-2724.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raina S, Missiakas D, Georgopoulos C. The rpoE gene encoding the ςE (ς24) heat shock sigma factor of Escherichia coli. EMBO J. 1995;14:1043–1055. doi: 10.1002/j.1460-2075.1995.tb07085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouviere P E, De Las Penas A, Meares J, Lu C Z, Rudd K E, Gross C A. rpoE, the gene encoding the second heat-shock sigma factor, ςE, in Escherichia coli. EMBO J. 1995;14:1032–1042. doi: 10.1002/j.1460-2075.1995.tb07084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rouviere P E, Gross C A. SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Genes Dev. 1996;10:3170–3182. doi: 10.1101/gad.10.24.3170. [DOI] [PubMed] [Google Scholar]
- 34.Strauch K L, Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci USA. 1988;85:1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka K, Kusano S, Fujita N, Ishihama A, Takahashi H. Promoter determinants for Escherichia coli RNA polymerase holoenzyme containing ς38 (the rpoS gene product) Nucleic Acids Res. 1995;23:827–834. doi: 10.1093/nar/23.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueshima R, Fujita N, Ishihama A. DNA supercoiling and temperature shift affect the promoter selectivity of Escherichia coli rpoH gene encoding the heat-shock sigma subunit of RNA polymerase. Mol Gen Genet. 1989;215:185–189. doi: 10.1007/BF00339716. [DOI] [PubMed] [Google Scholar]
- 37.Worsham P L, Konisky J. Effect of growth temperature on the acquisition of iron by Salmonella typhimurium and Escherichia coli. J Bacteriol. 1984;158:163–168. doi: 10.1128/jb.158.1.163-168.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]