Abstract
Traumatic brain injury (TBI) is a serious neurological disorder with increasing worldwide incidence. Emerging evidence has shown a significant therapeutic role of mesenchymal stem cells (MSCs) derived exosomes on traumatic brain injury with broad application prospects as a cell-free therapy. However, a comprehensive understanding of its underlying mechanism remained elusive. In this study, umbilical cord mesenchymal stem cells (UCMSCs)-derived exosomes (UC-MSCs-Exo) were isolated by ultracentrifugation and injected intraventricularly in a rat model of TBI. Our results showed that UC-MSCs-Exo promoted functional recovery and reduced neuronal apoptosis in TBI rats. Moreover, UC-MSCs-Exo inhibited the activation of microglia and astrocytes during brain injury, thereby promoting functional recovery. However, the effect of UC-MSCs-Exo on the content of plasma inflammatory factors in rats was not significant. Collectively our study suggested that UC-MSCs-Exo promotes the recovery of neurological function in TBI rats by inhibiting the activation of microglia and astrocytes, providing a theoretical basis for new therapeutic strategies for central nervous system diseases.
Keywords: Umbilical cord mesenchymal stem cells, Exosomes, Traumatic brain injury, Microglia, Astrocytes
1. Introduction
Traumatic Brain Injury (TBI) is the physiological damage to the brain structure or function caused by external force and attributed to high morbidity and mortality [1,2]. Patients with TBI often present severe motor and cognitive impairment [3]. It has been reported that during TBI external forces directly damage neurons, glial cells, blood vessels, axons, and dendrites, resulting in excitatory cytotoxicity, mitochondrial damage, inflammation, brain edema, and increased intracranial pressure, ultimately leading to the cascade of multiple pathophysiological mechanisms [4]. However, most drug treatments are limited by the blood–brain barrier. Whereas treatments such as mild hypothermia, hyperbaric oxygen therapy, and decompressive craniectomy can only relieve secondary reactions, but cannot effectively reduce neuronal apoptosis [5]. Nonetheless, in recent years regenerative medicine i.e., stem cell therapy has shown significant therapeutic efficiency in the treatment of traumatic brain injury [[6], [7], [8]]. Particularly, Mesenchymal stem cells (MSCs) hold the advantage over other stem cells due to their multi-directional differentiation potential, rapid proliferation, and differentiation ability, easy isolation and culture procedures, and autologous transplantation without immunogenicity [9,10]. Besides, Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) (derived from neonatal umbilical cord tissue) have been known to release various nutritional factors and cell repair factors, improve neurological function, reduce apoptosis, and promote angiogenesis, which suggest their significant repair effect on the neurological deficit caused by TBI [[11], [12], [13]], yet, the precise mechanism remained elusive.
Recently reported studies have shown that MSC- derived exosomes can effectively improve functional recovery and promote the brain remodelling [14]. Nonetheless, exosomes (that are extracellular vesicles with lipid bilayer membrane structure and an average diameter of 40–150 nm) possess biologically active substances such as proteins, lipids, and nucleic acids, and plays an important role in intercellular communication, maintenance of homeostasis and the occurrence and development of diseases [15]. Compared with MSCs, exosomes have the characteristics of good biocompatibility, long in vivo half-life, and easy extraction and storage, so they have gradually become a research hotspot [16,17]. Thus, in the present study, Umbilical Cord Mesenchymal Stem Cell-derived Exosomes (UC-MSC Exo) were isolated and injected into the ventricle of TBI rats to study the mechanism through which UC-MSCs-Exo repair the TBI.
2. Material and methods
2.1. Isolation and identification of UC-MSCs-Exo
UC-MSCs were provided by Guangdong VitaLife Biotechnology Co., Ltd (Foshan, China). Briefly, the UC-MSCs in the 3rd-5th generation were cultured in an exosome-free complete medium [MEM-a (Gibco, USA), 10% fetal bovine serum (ExCell, China) and 1% double antibody (Gibco, USA)], the cell culture supernatant was collected, and the UC-MSCs-Exo were isolated by ultracentrifugation and purified using an exosome purification reagent and concentration system (Exojuice, WeinaBio, China). The morphology of exosomes was observed by transmission electron microscopy, the particle size and concentration of exosomes were analyzed by flow nanometer, and the specific proteins (CD9, CD63, CD81, and TSG101) related to exosomes were detected by Western blot.
2.2. Animal experiments and behavioral assessment following TBI
All experimental procedures were approved by the Laboratory Animal Ethics Committee of Foshan University, and the experimental protocol were performed according to the ethical guidelines of animal protection and welfare. For an animal model of TBI, forty-five SPF-grade female SD rats weighing 200–220 g, were purchased from the Guangdong Provincial Medical Laboratory Animal Center. Before creating the TBI model, the rats fasted for 9 h without food or water. Furthermore, SD female rats were randomly divided into 3 groups i.e., UC-MSCs-Exo group, TBI group, and Sham (open skull without injury to the meninges) groups, with 15 rats in each group. The TBI model was established using an improved Feeney free-fall method, where a 20 g weight fell from a height of 30 cm to hit the dura mater. After 24 h, rats in the UC-MSCs-Exo group were injected with UC-MSCs-Exo (200ug) in a volume of 20 ul; rats in the TBI group were injected with an equal volume of PBS; rats in the Sham group were not treated. To evaluate the neurological deficits of the rats in each group, the behavioral function of the rats was evaluated by mNSS on 3, 7, 14, and 21 days after modeling by the field researcher (who did not know the experimental animal grouping).
2.3. qRT-PCR
Brain tissue was extracted by triazole method, using Takara's PrimeScript™ RT reagent kit with gDNA Eraser (Perfect Real Time) for reverse transcription into cDNA, and Takara's TB Green PCR Master reagent for real-time fluorescence quantitative PCR. The operation steps were carried out according to the manufacturer's instructions. The expression of Bcl-2, Bax, GFAP, and IBA1 genes in each group was detected and the PCR primers are shown in Table 1.
Table 1.
The forward (F-) and reverse (R-) primers used in this study.
| Gene | Gene names | primer (5′–3′) | Product length (bp) |
|---|---|---|---|
| GFAP | GFAP-F | AGAGGAAGGTTGAGTCGCTGGAG | 145 |
| GFAP-R | AGAGCCGCTGTGAGGTCTGG | ||
| Iba1 | Iba1-F | AGCGAATGCTGGAGAAACTTGGG | 84 |
| Iba1-R | CCTCGGAGCCACTGGACACC | ||
| Bcl-2 | Bcl-2-F | GAACTGGGGGAGGATTGTGG | 80 |
| Bcl-2-R | GGGGTGACATCTCCCTGTTG | ||
| Bax | Bax-F | GTCCTCACTGCCTCACTCAC | 189 |
| Bax-R | GTTTATTGGCACCTCCCCCA | ||
| GAPDH | GAPDH-F | TTCCTACCCCCAATGTATCCG | 270 |
| GAPDH-R | CCACCCTGTTGCTGTATCCATA |
2.4. ELISA
Three days after modeling, blood was collected by an orbital venous plexus puncture. After standing at room temperature for 30 min, samples were centrifuged at 3000 rpm for 5 min to separate plasma, followed by the detection of TNF-α, IL-6, and IL-10 content in plasma according to the instructions of the ELISA kit (Enzyme Immunobiology, China).
2.5. HE staining
For HE staining and tissue immunofluorescence, five rats in each group after modeling were taken, and the brain tissue (at the lesion site and the surrounding 3 mm area) was cut with a scalpel, fixed, dehydrated, embedded, and made into tissue sections with a thickness of 4 μm followed by HE staining and observed under the light microscope. The morphological and structural changes and the number of cells in the brain tissue were observed to determine the damage and recovery of the brain tissue of the rats in each group.
2.6. Immunohistochemical staining
Paraffin sections were deparaffinized, boiled in 10 mM citrate buffer for 10 min for antigen retrieval, 3% H2O2 solution was used to block the endogenous peroxidase. After serum blocking for 30 min, primary antibodies GFAP (1:200) and IBA1 (1:100) were added and incubated overnight at 4 °C. Thereafter, sections were rinsed with PBS 3 times followed by the addition of secondary antibody and incubated at room temperature for 2 h, observed and photographed after counterstaining with DAPI.
2.7. Western blot
10 μg of total exosome protein was resolved by 10% SDS-PAGE and then transferred to PVDF membrane (Millipore, USA). Then membranes were blocked with 5% skimmed milk for 2 h followed by incubation with primary antibody (diluted at 1:1 000) overnight at 4 °C. Thereafter, the membrane was washed 3 times with TBST, and incubated for 1 with respective horseradish peroxidase-conjugated secondary antibody (dilution ratio of 1:5 000). Then it was washed 3 times with TBST, and a chemiluminescent reagent (Tanon, China) was dropped into the membrane. Finally, protein expression was observed with a chemiluminescence gel imaging analyzer (Tanon, China) (Monoclonal antibodies to TSG101, CD9, CD63 and CD81 were from Affinity Biosciences Affinity, USA).
3. Results
3.1. Identification of UC-MSCs-Exo
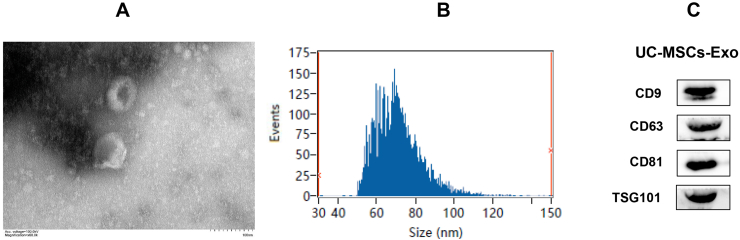
Our results from fluoroscopic electron microscope indicated that isolated and purified UC-MSCs-Exo was cup shape (Fig. 1A); NTA particle size analysis shows that its size is 30–150 nm, concentrated at 69.75 nm, and the average particle size was 71.20 nm (Fig. 1B). While western blotting results confirmed the expression of UC-MSCs-Exo specific exosome surface proteins i.e., TSG101, CD81, CD63, and CD9 (see Figure C), which proved that the isolated vesicles were exosomes.
Fig. 1.
Identification of UC-MSCs-Exo. A: The exosomes were cup-shaped as observed by fluoroscopy electron microscope; B: The size of NTA particle size was 30–150 nm, concentrated at 69.75 nm, and the average particle size was 71.20 nm; C: Western Blot detection showed that UC-MSCs-Exo expressed specific exosome surface proteins TSG101, CD81, CD63 and CD9.
3.2. Evaluation of neurological deficits by mNSS score in rats
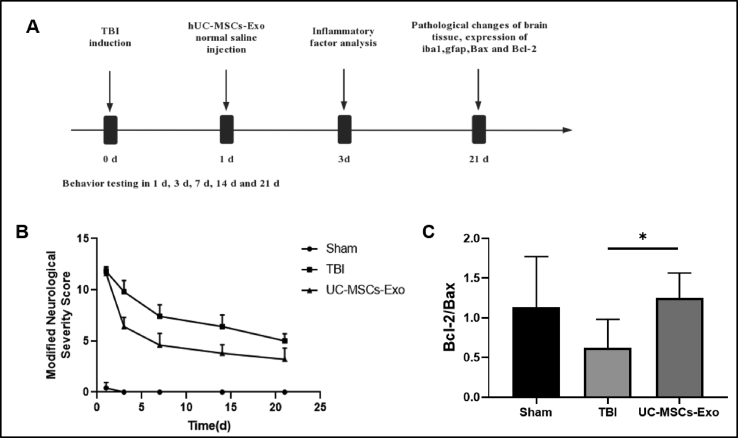
As shown in Fig. 2A, the mNSS scores of the rats in the UC-MSCs-Exo group were significantly lower than those in the TBI group at 3, 7, 14, and 21 d after modeling (P < 0.01), and were significantly higher than those in the Sham group (P < 0.01). 0.01).
Fig. 2.
UC-MSCs-Exo promotes neurological recovery in TBI rats. A: Schematic diagram of the experimental procedure. B: mNSS measures sensorimotor function. C: The expression of Bcl2/Bax apoptosis-related genes was detected by qPCR. ∗ indicates p < 0.05.
3.3. Expression of Bcl-2/Bax apoptosis-related genes
RT-qPCR results (Fig. 2C) showed that the expression of Bcl-2/Bax in the UC-MSCs-Exo group was significantly higher than that in the TBI group after 21 days of modeling (P < 0.01).
3.4. Expression of glial cell markers GFAP and IBA1
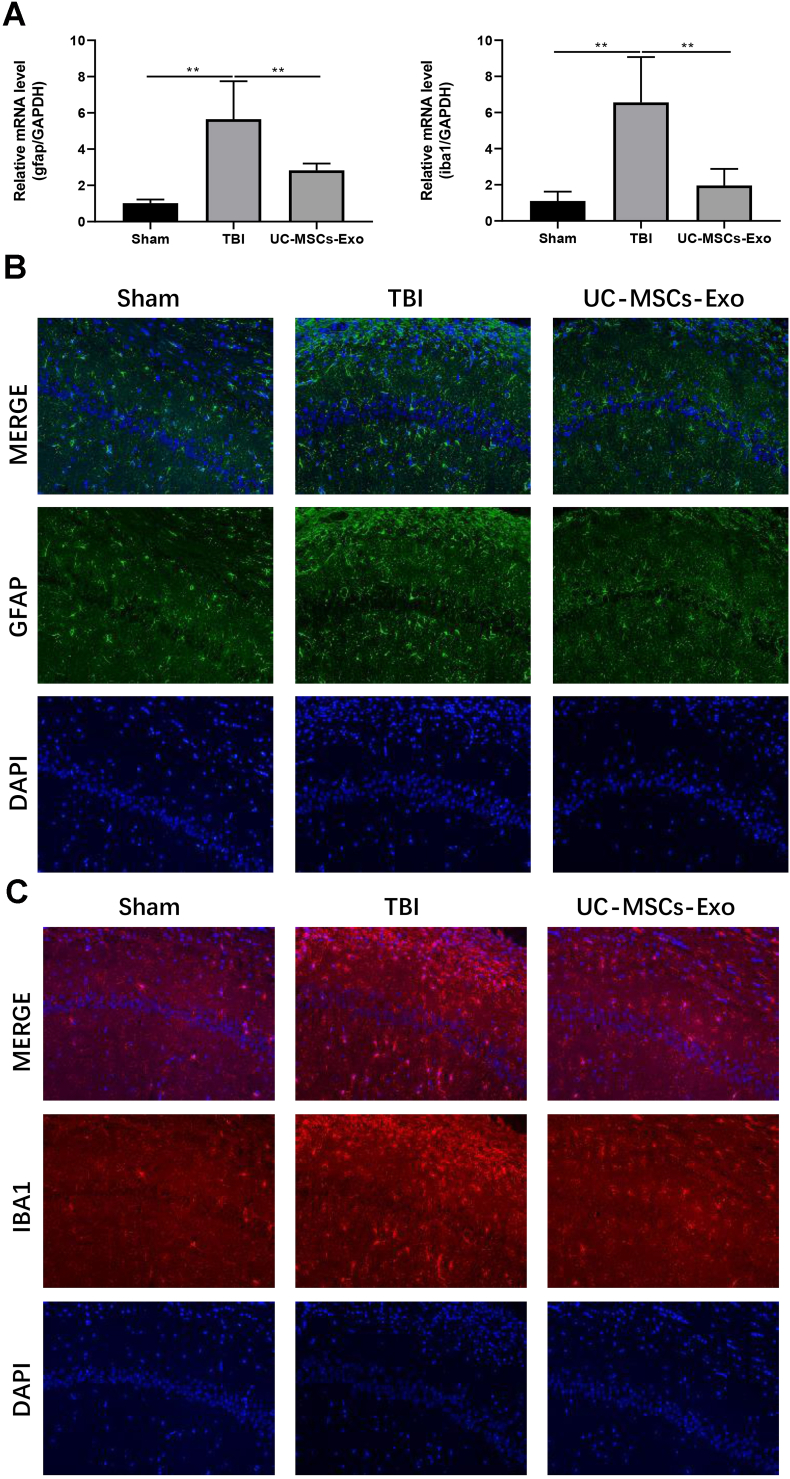
Further, we aimed to determine the expression of glial cell markers ie., GFAP and IBA. Our results from RT-qPCR showed (Fig. 3A) that 21 days after modeling, mRNA expressions of the GFAP and IBA1 in the UC-MSCs-Exo group were significantly lower than those in the TBI group (P < 0.01). Besides, immunofluorescence results showed that the fluorescence expression intensity of GFAP protein (Fig. 3B) and IBA1 protein (Fig. 3C) in the UC-MSCs-Exo group was lower than those in the TBI group after 21 days of modeling. Collectively, these results showed that the activation of glial cells in the rat brain was significantly reduced after the intervention of UC-MSCs-Exo, suggesting its beneficial role in neural repair.
Fig. 3.
UC-MSCs-Exo inhibits the expression of GFAP and IBA1. A: The mRNA expression of astrocyte marker GFAP and microglia marker IBA1 in brain tissue was detected by qPCR; B–C: The localized expression of astrocyte marker GFAP and microglia marker IBA1 protein was detected by immunofluorescence. ∗indicates p < 0.05; ∗∗indicates p < 0.01.
3.5. Expression of inflammatory factors TNF-α, IL-6, and IL-10
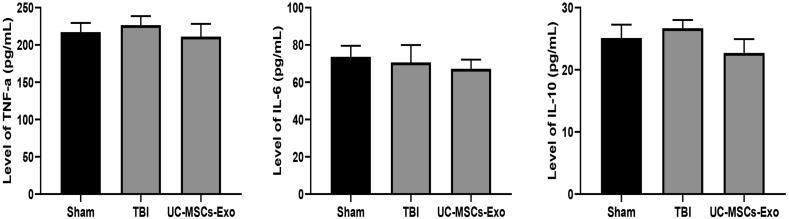
The expression of inflammatory factors TNF-α, IL-6, and IL-10 was determined by using the ELISA. Our results showed that there was no significant difference in the expression levels of inflammatory factors TNF-α, IL-6, and IL-10 in the plasma of the three groups of rats (P > 0.05) (Fig. 4).
Fig. 4.
UC-MSCs-Exo intervention did not affect the levels of inflammatory factors in rat plasma. There was no significant difference in the expression levels of inflammatory factors tumor necrosis factor α, interleukin 6 and interleukin 10 in the plasma of the three groups of rats (P > 0.05).
3.6. HE staining
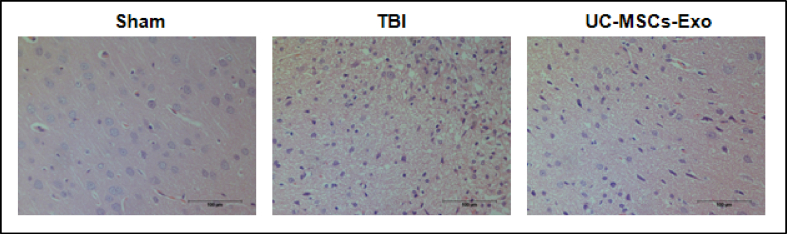
HE staining results indicated that the structure of the brain tissue of the rats in the Sham group was normal and significant pathological changes were found. Whereas rats in the TBI-model group exhibited large area defects and edema of brain tissue, a large number of glial cells were activated, the number of nerve cells was reduced, and the shape of the nucleus was irregular. A large number of activated glial cells were observed while the number of nerve cells was comparatively low, the shape of the nucleolus was not obvious, the cytoplasm showed dark red staining, a small part of neurons was necrotic, the nuclei were fragmented and condensed, and the nuclear staining was deepened. However, in the UC-MSCs-Exo group, we found that the damaged area of brain tissue, edema, and neuronal apoptosis was reduced to varying degrees as well as the number of the activated glial cell was less as compared to the TBI model without the treatment of UC-MSCs-Exo (Fig. 5).
Fig. 5.
HE staining of rat brain tissue. Rats in the TBI group had extensive brain tissue defects, edema, decreased number of nerve cells, irregular shape of nuclei, inconspicuous nucleoli, dark red staining of cytoplasm, necrosis of a small part of neurons, fragmented and condensed nuclei, nuclear staining deepened. The brain tissue defect area, edema and neuronal apoptosis of the UC-MSCs-Exo group were improved to varying degrees.
4. Discussion
Traumatic Brain Injury (TBI) is a common brain injury caused by mechanical force such as sudden acceleration or deceleration [18,19]. The occurrence and development of TBI are complex and due poor self-repairing ability of the central nervous system there is no standard treatment available to treat traumatic brain injury [20,21]. Nonetheless, recent studies have demonstrated that MSCs possess the multi-directional differentiation ability to promote tissue repair and regeneration, thus suggesting an effective treatment for the repair of TBI nerve injury [22,23]. Besides, recent studies have indicated the efficiency of MSCs-Exo, a cell-free treatment method, which holds advantages over MSCs therapy in terms of production, storage, transportation, and biological safety while ensuring the therapeutic effect [24,25]. Though preclinical studies using the TBI rat model have also shown therapeutic effects of MSCs-Exo derived from other sources i.e., bone marrow [26] and adipose tissues [27] on nerve repair by significantly promoting the recovery of nerve function, however, the underlying mechanism remained unclear. The present study shows that UC-MSCs-Exo is as efficient as MSCs-Exo derived from bone marrow and adipose, in the treatment of TBI.
It has been indicated that TBI disturbs the balance of Bcl-2/Bax which increases the apoptotic rate of neurons. However, in the present study, the level of Bcl-2/Bax was significantly increased after UC-MSCs-Exo intervention, reflecting that UC-MSCs reduced neuronal apoptosis. Moreover, was craniocerebral injury has also been attributed to the neuroinflammation-induced release of cytokines and excitatory cytotoxic substances; however, our data showed that UC-MSCs-Exo exhibited no significant effect on the release of inflammatory factors. Surprisingly, the results of real-time quantitative PCR and immunofluorescence analysis showed that the mRNA and protein levels of astrocyte and microglia markers GFAP and IBA1 were significantly decreased after UC-MSCs-Exo intervention, indicating that UC-MSCs- Exo has inhibitory effects on astrocyte and microglia hyperactivation.
Nonetheless, it has been indicated that glial cells in the central nervous system play key roles in neural development, tissue repair, and homeostasis [28,29]. Under normal physiological conditions, astrocytes and microglia function by clearing debris, recycling neurotransmitter molecules, and supporting transneuronal and cellular communication to maintain neuronal function [30,31]. However, the occurrence of traumatic brain injury can lead to excessive activation of astrocytes and microglia, which can lead to the formation of glial scarring, thereby aggravating the secondary brain injury [32]. This study found that UC-MSCs-Exo could exert a therapeutic effect on TBI by inhibiting the overactivation of astrocytes and microglia.
In conclusion, our study suggests that UC-MSCs-Exo can improve the neurological function of traumatic brain injury rats. Due to their key role in mediating intercellular signal transduction and information exchange, MSCs derived exosomes are considered as promising candidate for the diagnosis and treatment of traumatic brain injury. Recently, a growing number of studies have proved that miRNAs carried by UC-MSC-Exo play a critical role in tissue repair and remodeling as well as treatment of other diseases. Therefore, our next work will focus on sequencing and studying the miRNAs of UCMSC-Exo.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
L.X. C. designed the study, analyzed, and interpreted the data and prepared the manuscript. W. L., W.K. J., H.M. L., J.R. X., and X.C. L. performed the experiments and interpreted the data. B.Y. W., J.H. W., and G.Q. C. interpreted the findings.
Ethics statement
The animal study was reviewed and approved by the Animal Care and Use Committee of Foshan University.
Fundings
This work was funded by grants from the National Natural Science Foundation of China (81972335) and Foshan city climbing peak plan key project (2019(24)).
Declaration of competing interest
No.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine
Contributor Information
Jinhui Wang, Email: wangjinhui@vtlife.cn.
Guoqiang Chen, Email: 13929981788@139.com.
References
- 1.Galgano M., Toshkezi G., Qiu X., Russell T., Chin L., Zhao L.R. Traumatic brain injury: current treatment strategies and future endeavors. Cell Transplant. 2017;26(7):1118–1130. doi: 10.1177/0963689717714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khellaf A., Khan D.Z., Helmy A. Recent advances in traumatic brain injury. J Neurol. 2019;266(11):2878–2889. doi: 10.1007/s00415-019-09541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capizzi A., Woo J., Verduzco-Gutierrez M. Traumatic brain injury: an overview of epidemiology, pathophysiology, and medical management. Med Clin. 2020;104(2):213–238. doi: 10.1016/j.mcna.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Kaur P., Sharma S. Recent advances in pathophysiology of traumatic brain injury. Curr Neuropharmacol. 2018;16(8):1224–1238. doi: 10.2174/1570159X15666170613083606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasser M., Bejjani F., Raad M., Abou-El-Hassan H., Mantash S., Nokkari A., et al. Traumatic brain injury and blood-brain barrier cross-talk. CNS Neurol Disord: Drug Targets. 2016;15(9):1030–1044. doi: 10.2174/1871527315666160815093525. [DOI] [PubMed] [Google Scholar]
- 6.Schepici G., Silvestro S., Bramanti P., Mazzon E. Traumatic brain injury and stem cells: an overview of clinical trials, the current treatments and future therapeutic approaches. Medicina (Kaunas) 2020;56(3) doi: 10.3390/medicina56030137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dehghanian F., Soltani Z., Khaksari M. Can mesenchymal stem cells act multipotential in traumatic brain injury? J Mol Neurosci. 2020;70(5):677–688. doi: 10.1007/s12031-019-01475-w. [DOI] [PubMed] [Google Scholar]
- 8.Das M., Mayilsamy K., Mohapatra S.S., Mohapatra S. Mesenchymal stem cell therapy for the treatment of traumatic brain injury: progress and prospects. Rev Neurosci. 2019;30(8):839–855. doi: 10.1515/revneuro-2019-0002. [DOI] [PubMed] [Google Scholar]
- 9.Galipeau J., Sensebe L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10(6):709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Ding D.C., Chang Y.H., Shyu W.C., Lin S.Z. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24(3):339–347. doi: 10.3727/096368915X686841. [DOI] [PubMed] [Google Scholar]
- 12.Chen K.H., Shao P.L., Li Y.C., Chiang J.Y., Sung P.H., Chien H.W., et al. Human umbilical cord-derived mesenchymal stem cell therapy effectively protected the brain architecture and neurological function in rat after acute traumatic brain injury. Cell Transplant. 2020;29 doi: 10.1177/0963689720929313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang G., Wu H.L., Liu Y.P., Yan D.Q., Yuan Z.L., Chen L., et al. Pre-clinical study of human umbilical cord mesenchymal stem cell transplantation for the treatment of traumatic brain injury: safety evaluation from immunogenic and oncogenic perspectives. NEURAL REGEN RES. 2022;17(2):354–361. doi: 10.4103/1673-5374.317985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y., Chopp M., Meng Y., Katakowski M., Xin H., Mahmood A., et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122(4):856–867. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He C., Zheng S., Luo Y., Wang B. Exosome theranostics: biology and translational medicine. THERANOSTICS. 2018;8(1):237–255. doi: 10.7150/thno.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vizoso F.J., Eiro N., Cid S., Schneider J., Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18(9) doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendt M., Rezvani K., Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019;54(Suppl 2):789–792. doi: 10.1038/s41409-019-0616-z. [DOI] [PubMed] [Google Scholar]
- 18.Wang K., Cui D., Gao L. Traumatic brain injury: a review of characteristics, molecular basis and management. Front Biosci (Landmark Ed) 2016;21(5):890–899. doi: 10.2741/4426. [DOI] [PubMed] [Google Scholar]
- 19.Araki T., Yokota H., Morita A. Pediatric traumatic brain injury: characteristic features, diagnosis, and management. Neurol Med -Chir. 2017;57(2):82–93. doi: 10.2176/nmc.ra.2016-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandsmark D.K. Clinical outcomes after traumatic brain injury. Curr Neurol Neurosci Rep. 2016;16(6):52. doi: 10.1007/s11910-016-0654-5. [DOI] [PubMed] [Google Scholar]
- 21.Sandsmark D.K., Diaz-Arrastia R. Advances in traumatic brain injury research in 2020. Lancet Neurol. 2021;20(1):5–7. doi: 10.1016/S1474-4422(20)30455-5. [DOI] [PubMed] [Google Scholar]
- 22.Andrzejewska A., Dabrowska S., Lukomska B., Janowski M. Mesenchymal stem cells for neurological disorders. Adv Sci. 2021;8(7) doi: 10.1002/advs.202002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonilla C., Zurita M. Cell-based therapies for traumatic brain injury: therapeutic treatments and clinical trials. Biomedicines. 2021;9(6) doi: 10.3390/biomedicines9060669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui L., Saeed Y., Li H., Yang J. Regenerative medicine and traumatic brain injury: from stem cell to cell-free therapeutic strategies. Regen Med. 2022;17(1):37–53. doi: 10.2217/rme-2021-0069. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y., Ye Y., Su X., He J., Bai W., He X. MSCs-derived exosomes and neuroinflammation, neurogenesis and therapy of traumatic brain injury. Front Cell Neurosci. 2017;11:55. doi: 10.3389/fncel.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y., Zhang Y., Chopp M., Pang H., Zhang Z.G., Mahmood A., et al. MiR-17-92 cluster-enriched exosomes derived from human bone marrow mesenchymal stromal cells improve tissue and functional recovery in rats after traumatic brain injury. J Neurotrauma. 2021;38(11):1535–1550. doi: 10.1089/neu.2020.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y., Li J., Ma B., Li N., Wang S., Sun Z., et al. MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging (Albany NY) 2020;12(18):18274–18296. doi: 10.18632/aging.103692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Q.Q., Zhou J.W. Neuroinflammation in the central nervous system: symphony of glial cells. Glia. 2019;67(6):1017–1035. doi: 10.1002/glia.23571. [DOI] [PubMed] [Google Scholar]
- 29.Allen N.J., Lyons D.A. Glia as architects of central nervous system formation and function. SCIENCE. 2018;362(6411):181–185. doi: 10.1126/science.aat0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilton D.K., Dissing-Olesen L., Stevens B. Neuron-glia signaling in synapse elimination. Annu Rev Neurosci. 2019;42:107–127. doi: 10.1146/annurev-neuro-070918-050306. [DOI] [PubMed] [Google Scholar]
- 31.Kim S., Kim Y.E., Hong S., Kim K.T., Sung D.K., Lee Y., et al. Reactive microglia and astrocytes in neonatal intraventricular hemorrhage model are blocked by mesenchymal stem cells. Glia. 2020;68(1):178–192. doi: 10.1002/glia.23712. [DOI] [PubMed] [Google Scholar]
- 32.Wang J., Hou Y., Zhang L., Liu M., Zhao J., Zhang Z., et al. Estrogen attenuates traumatic brain injury by inhibiting the activation of microglia and astrocyte-mediated neuroinflammatory responses. Mol Neurobiol. 2021;58(3):1052–1061. doi: 10.1007/s12035-020-02171-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.