Abstract
Background
Body weight is defended by strong homeostatic forces. Several of the key biological mechanisms that counteract weight loss have been unraveled over the last decades. In contrast, the mechanisms that protect body weight and fat mass from becoming too high remain largely unknown. Understanding this aspect of energy balance regulation holds great promise for curbing the obesity epidemic. Decoding the physiological and molecular pathways that defend against weight gain can be achieved by an intervention referred to as ‘experimental overfeeding’.
Scope of the review
In this review, we define experimental overfeeding and summarize the studies that have been conducted on animals. This field of research shows that experimental overfeeding induces a potent and prolonged hypophagic response that seems to be conserved across species and mediated by unidentified endocrine factors. In addition, the literature shows that experimental overfeeding can be used to model the development of non-alcoholic steatohepatitis and that forced intragastric infusion of surplus calories lowers survival from infections. Finally, we highlight studies indicating that experimental overfeeding can be employed to study the transgenerational effects of a positive energy balance and how dietary composition and macronutrient content might impact energy homeostasis and obesity development in animals.
Major conclusions
Experimental overfeeding of animals is a powerful yet underappreciated method to investigate the defense mechanisms against weight gain. This intervention also represents an alternative approach for studying the pathophysiology of metabolic liver diseases and the links between energy balance and infection biology. Future research in this field could help uncover why humans respond differently to an obesogenic environment and reveal novel pathways with therapeutic potential against obesity and cardiometabolic disorders.
Keywords: Experimental overfeeding, Intragastric overfeeding, Obesity, Energy balance, Animal models, Leptin, Body weight, NASH
Highlights
-
•
Experimental overfeeding in animals involves intragastric delivery of an energy-dense (liquid) diet.
-
•
Experimental overfeeding rapidly increases body weight while suppressing voluntary food intake.
-
•
Overfeeding-induced hypophagia continues after overfeeding is stopped, driving body weight back to baseline.
-
•
Experimental overfeeding is a promising but underutilized resource for identifying weight gain defense mechanisms.
1. Prologue: when life depends upon programmed hyperphagia
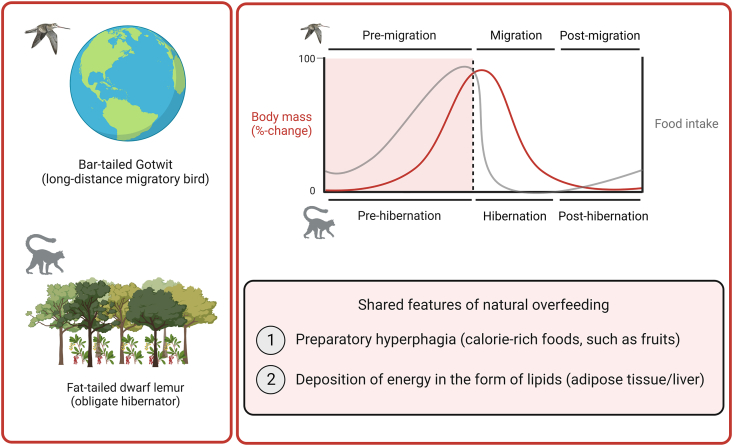
In order to survive on planet Earth, all animals must acquire and ingest sufficient amounts of food to fuel essential organ systems and reproductive physiology. However, these fundamental aspects of life are threatened by the seasonal changes that occur as summer turns into autumn and winter turns into spring. Certain species have developed exquisite survival strategies to cope with these environmental challenges, such as season-dependent hyperphagia that causes profound deposition of lipids in fat depots [1,2]. The bar-tailed Gotwit (Limosa Iapponica) and the fat-tailed dwarf lemur (Cheirogaleus medius) are illustrative examples of this fascinating evolutionary adaptation (Figure 1).
Figure 1.
Examples of seasonal overfeeding in nature. The bar-tailed Gotwit and the fat-tailed dwarf lemur are illustrative examples of seasonal overfeeding in nature. The bar-tailed Gotwit migrates between Alaska and New Zealand. The fat-tailed dwarf lemur is a primate hibernator that lives in Madagascar. The graph on the right shows a schematic of circannual cycles of changes in body mass (red line) and food intake (gray line) during the preparatory hyperphagic period and the subsequent migration/hibernation period in bar-tailed Gotwits and fat-tailed dwarf lemurs.
Bar-tailed Gotwits are shorebirds that live in New Zealand during the Southern hemisphere's winter season. However, they leave this feeding ground in spring and cross the Pacific Ocean to arrive in Alaska, an ecological niche in the Northern hemisphere that better supports their breeding [3]. For some subspecies of Gotwits, this journey involves a non-stop flapping flight covering a distance of up to 12,000 km [3]. Like many other migrating birds that cross cold mountain ranges and dry deserts, bar-tailed Gotwits cannot forage and feed during their flight across the open sea. Therefore, they are energetically dependent upon endogenous lipid substrates that are stored primarily in subcutaneous, intraperitoneal and hepatic fat depots. Hence, a critical part of their annual migration is the preparatory ‘fueling’ phase, which is initiated by the longer day length in spring and characterized by hyperphagia and higher energy assimilation efficiency, the sum of which leads to a large increase in body weight and fat mass [[4], [5], [6]]. Studies of bar-tailed Gotwits show not only that these birds can increase their body mass by around 90% within less than two months, but they also show that these birds consist of up to 55% body fat before embarking on their incredible journey across the Pacific Ocean [5,7,8].
Similar to migratory birds, hibernators also follow annual rhythms of body mass gain and loss. During summer, they ingest large quantities of fruits and other foods that are rich in fats and sugars. These accumulated calories are subsequently catabolized during winter torpor. The fat-tailed dwarf lemur is a primate hibernator that stores remarkable quantities of fat in its tail before shielding itself from the dry Madagascan winter by hibernating in tree holes for up to seven consecutive months [9]. This increase in adiposity is driven by changes in feeding behavior and energy intake and, to a lesser extent, by a reduction in locomotor activity [10]. The fattening period in fat-tailed dwarf lemurs that precedes hibernation can last up to eight months [11] and can cause an astonishing 200% increase in body weight before the onset of dormancy [12].
The hyperphagic period that allows migrating birds and hibernating lemurs to double their body weight before take-off and torpor, respectively, and their subsequent return to baseline body weight, are spectacular examples of seasonal adaptations in energetic physiology (Figure 1). These natural examples of ‘programmed’ hyperphagia are intriguing and ecological studies of these species might aid in unraveling the biological mechanisms that temporarily allow a rapid increase in body fatness [13]. In contrast to these examples of programmed hyperphagia, most mammals, including humans and laboratory rodents, are equipped with a permanent physiological feedback system that potently defends against rapid and substantial weight gain. This regulatory arm in body weight control has been documented and characterized since the early 20th century using an experimental approach termed ‘experimental overfeeding’. However, despite more than 100 years of research within this field, the molecular mechanisms that protect mammals from excessive weight gain are still largely unknown.
2. Experimental overfeeding in humans
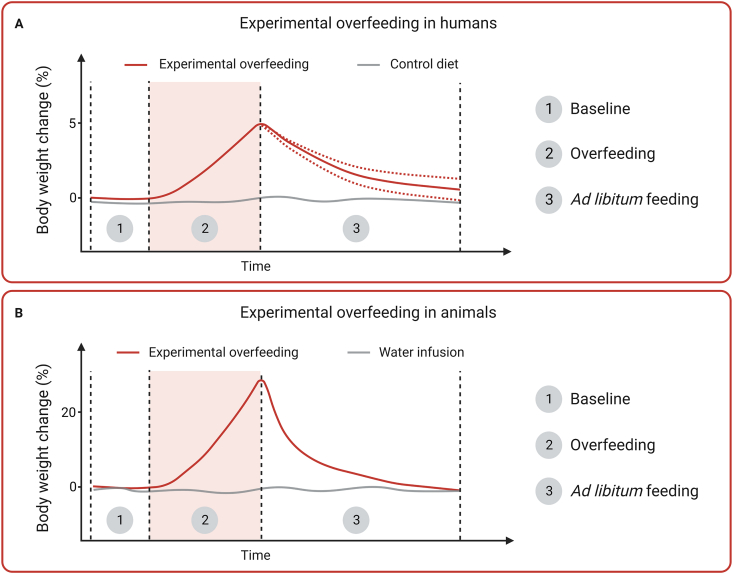
Experimental overfeeding is an intervention characterized by an energy intake that is higher than what is required for body weight stability. If calorie consumption is ‘clamped’ at a sufficiently high level, the intervention elicits a rapid increase in body weight and fatness and a potent suppression of appetite that counteracts the positive energy balance generated by the overfeeding intervention (Figure 2). Experimental overfeeding interventions in humans are sometimes referred to as ‘voluntary overfeeding’ or ‘conscious overfeeding’ [14,15] and the overfeeding interventions range from a single high-caloric meal [16] to a sustained excess caloric intake for several months [17]. The energetic surplus typically ranges from 30 to 50% over baseline caloric intake, corresponding to ingesting 130–150% of the energy needed for body weight stability [18]. The human overfeeding literature dates back to the middle of the 20th century, with over 110 studies disseminated in more than 300 scientific articles [18]. For more detailed insights on human experimental overfeeding, see e.g. Cuthbertson et al. [19] for a narrative review and Bray and Bouchard for a systematic review [18].
Figure 2.
Comparative changes in body weight during and after experimental overfeeding in humans and animals. Typical body weight trajectories reported from experimental overfeeding in humans (A) and experimental overfeeding in animals (B). Experimental overfeeding comprises three distinct phases: 1) Baseline, in which the caloric requirements for weight stability are estimated or measured; 2) experimental overfeeding that can vary in magnitude and duration depending on study objectives; and 3) ad libitum feeding, which typically is reflected by body weight recovery (loss of weight gained during overfeeding). The dotted red lines in phase 3 in (A) reflect the substantial inter-individual variation in post-overfeeding body weight recovery in humans.
3. Experimental overfeeding in animals
Similar to experimental overfeeding in humans, overfeeding in animals is an intervention in which a positive energy balance is introduced by controlling energy intake. In contrast to voluntary overeating in humans, animals are forced to gain body weight by intragastric overfeeding, either by gavage or via automated infusion of liquid food through a surgically implanted tubing system [20]. However, the term ‘overfeeding’ is loosely used in the animal literature and many studies incorrectly use ‘overfeeding’ to refer to voluntary overeating of palatable and calorie-dense diets that often have a high fat content. Another example of the loose use of the term ‘overfeeding’ can be seen in studies where manipulation of litter size by removing rodent pups from the litter after birth leads to an increased calorie intake in those pups that remain in the litter. Given these inconsistencies, we propose that the terms ‘overfeeding’ and ‘experimental overfeeding’ are used exclusively for animal studies with intragastric administration of calories beyond the energetic requirement for weight stability. The term ‘overeating’ would instead be useful for describing weight gain in animals that results from voluntary ingestion of high-caloric diets, such as the typical rodent high-fat diet (HFD).
Experimental overfeeding enables the study of controlled body weight gain and the subsequent weight recovery that occurs once overfeeding is stopped (Figure 2). A key feature of experimental overfeeding is the infusion of calories beyond what the animal voluntarily consumes. A calorie infusion of 100% corresponds to delivering an amount of energy that equals the basal caloric requirement for body weight stability, which is usually measured before overfeeding starts. Accordingly, a calorie infusion of 150% corresponds to an intragastric delivery of 1.5 times the baseline calorie intake. In mice, this leads to a rapid 25–35% increase in body weight within two weeks (unpublished observations). Others report similar effects, and this overfeeding-induced weight gain is associated with almost complete suppression of voluntary food intake [[21], [22], [23], [24], [25]]. Both the speed by which weight gain is induced and the potent counter-regulation in voluntary food intake induced by experimental overfeeding are in clear contrast to the much slower and less counteracted weight gain that results from providing rodents with ad libitum access to a palatable HFD. Hence, an important difference between experimental overfeeding and voluntary HFD overeating is that experimental overfeeding enables the study of the potent but currently unknown physiological and molecular mechanisms that govern overfeeding-induced hypophagia and thus protect animals (and humans) against obesity.
In addition to such strengths, experimental overfeeding also has some limitations. Intragastric infusion of food bypasses the oral cavity and the oropharyngeal part of the gastrointestinal tract and will therefore not elicit the same gustatory and cephalic effects seen in response to oral food intake [26]. Moreover, food is typically infused in liquid form into the stomach continuously throughout the day, as opposed to ingestion of solid food during distinct feeding episodes (meals) in ad libitum-fed animals. Such continuous infusion of nutrients, especially during the light phase when animals tend to sleep and rest, may alter and interact with circadian rhythms [27,28]. To our knowledge, the circadian aspect of experimental overfeeding has not yet been studied.
4. Literature search and review methodology
This review is focused on studies that fulfill our definition of experimental overfeeding in animals, i.e. studies that intragastrically administer calories beyond the energetic requirement of the animals. We performed a PubMed search to identify relevant literature on experimental overfeeding in animals, focusing on studies that have used mice and rats. We employed the following search query “overfeeding AND (rat OR mouse OR mice)”, which yielded 529 publications from 1970 to 2021. This list of publications was supplemented by additional studies, some in larger domestic animals, that were either identified by inspection of reference lists or had been found before performing the systematic search. A total of 105 references were included in this review, describing studies in mice (7) and rats (55) but also guinea pigs (1), swine (2), dogs (3), monkeys (4), and different species of birds (37) (Supplementary Table 1). Of note, the literature on experimental overfeeding in birds is quite extensive and because many of these studies were irrelevant for this review, not all bird studies that fit our definition of experimental overfeeding are being discussed.
5. Lessons learned from experimental overfeeding studies in animals
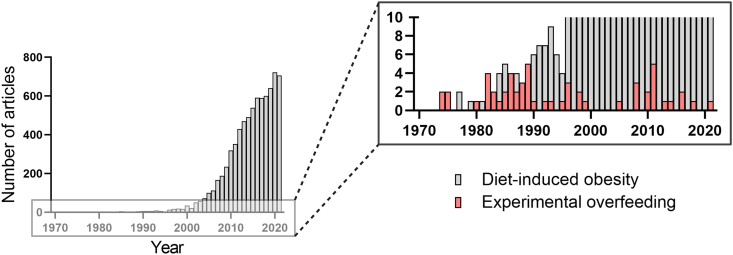
Experimental overfeeding in animals has a long but fragmented history. A few studies date back to the 1940s and 1950s, but most studies were conducted in the 1980s and 1990s. They have predominantly been performed in rats, which account for 55 out of our 105 identified studies. This amount of research in rats is in stark contrast to the merely seven experimental overfeeding studies that have been performed in mice (Supplementary Table 1). The overrepresentation of rats in experimental overfeeding research aligns with the historical preference for using rats to study biological aspects of obesity [29]. However, over the last two decades, mouse models have become increasingly popular, and the use of mice has increased to a point in which ∼50% of all preclinical animal research is conducted in experimental mice [30]. Moreover, research using (high-fat) diet-induced obese rodents has become highly popular within the last couple of decades, as illustrated by more than 500 annual scientific articles that now use these models of obesity (Figure 3). However, it is somewhat perplexing that the advancement of mouse models in metabolism and obesity research is not reflected by a parallel surge in experimental overfeeding studies performed in mice (Figure 3).
Figure 3.
Experimental overfeeding studies in small rodents have not increased over time. The yearly number of retrieved papers from Pubmed on experimental overfeeding in small rodents is shown in red bars. For comparison, the yearly number of retrieved papers from Pubmed on diet-induced obesity in small rodents is shown in gray bars.
The early experimental overfeeding studies from the 1940s used an intragastric infusion of high-carbohydrate diets in rats to demonstrate that body weight and fat mass gain are positively correlated with the infused amount of energy [31,32]. Since then, numerous studies have investigated the metabolic responses to experimental overfeeding and provided new insights into the regulation of body weight and energy metabolism. Other experimental overfeeding studies in animals have investigated how a forced positive energy balance affects adipose tissue morphology, development of metabolic liver disease, survival from infections, transgenerational health, and how different dietary components can modulate metabolic homeostasis. In the following sections, we will summarize and discuss key lessons learned from these experimental overfeeding studies.
5.1. Experimental overfeeding is followed by prolonged hypophagia
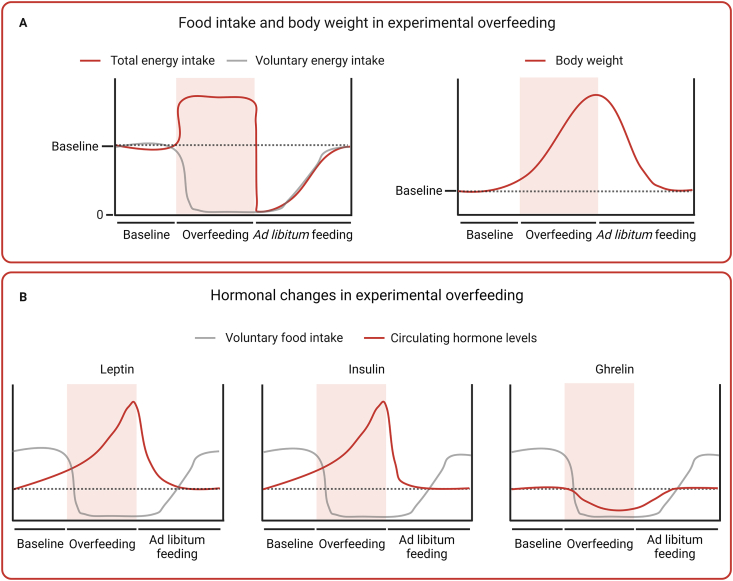
Experimental overfeeding in animals is characterized by a robust and rapid gain in body weight associated with a potent counter-regulatory decrease in voluntary food intake (Figure 4A). The first study that observed this phenomenon found that male dogs responded to one week of 133%-intragastric overfeeding by decreasing their voluntary food intake by 63%, on average. Importantly, this hypophagic response also involved a complete suppression of food intake on some days. In the two weeks after the overfeeding period, the dogs still showed a 13–26% reduction in food intake [33]. In a longer study that used a similar experimental approach, female dogs were overfed 175% for 14 weeks. Here, the authors observed that voluntary food intake was completely abolished most days during the overfeeding period. Voluntary eating was still much reduced one week after the cessation of overfeeding and only reached normal levels three weeks later [34].
Figure 4.
Food intake, body weight, and hormonal changes during and after experimental overfeeding in rodents. (A) Schematic graphs showing changes in energy intake and body weight during and after experimental overfeeding. Energy intake is divided into total energy intake (red) and voluntary energy intake (gray), highlighting the suppression of voluntary food intake during overfeeding and the prolonged period of voluntary hypophagia after the cessation of overfeeding coinciding with return of body weight to baseline. (B) Schematic graphs showing reported changes during and after experimental overfeeding (representation from different studies) in three hormones important for energy homeostasis: leptin, insulin, and ghrelin. The data highlight that the hormones change substantially during overfeeding, but also that they return to baseline plasma levels before food intake has normalized – indicating that they are dispensable for the prolonged hypophagia following overfeeding.
This potent and prolonged hypophagic response is a key physiological feature of experimental overfeeding that has also been observed in birds [35,36], rodents [[21], [22], [23], [24], [25], [37]], pigs [38], and monkeys [22,39]. Like in dogs, two studies in chickens observed that overfeeding-induced hypophagia continues after overfeeding has stopped [35,40]. In one of these experiments, the effect was rather remarkable, as voluntary food intake was almost abolished for at least five days after three weeks of overfeeding [35]. An even more prolonged hypophagic response was observed in a study on Rhesus monkeys in which successive increases in caloric infusion led to levels of overfeeding of 125%, 145%, and 165% [39]. Overfeeding caused a rapid increase in body weight and a complete suppression of voluntary food intake. Moreover, after overfeeding ended, the monkeys refused to ingest any food for periods that lasted between 14 and 35 days [39]. A similar observation has been made in male Holtzman rats that were overfed by 200% for three months. Here, some rats did not eat for 16 days after cessation of overfeeding [23]. Together, these observations highlight that post-overfeeding hypophagia can be a very potent and prolonged response that is conserved across the animal kingdom.
5.2. Searching for a circulating satiety factor of overfeeding
The mechanisms responsible for overfeeding-induced hypophagia and the rapid weight loss seen after cessation of overfeeding are unknown [[41], [43], [85]]. Parabiosis studies have provided key evidence that circulating molecules regulate mammalian energy balance [41,44,45], and experiments on genetically obese ob/ob and db/db mice paved the way for the discovery of leptin [46,47]. In this type of study, two animals (rodents) are surgically united in a manner that creates a shared circulatory system. This allows the investigator to explore physiological feedback systems by performing interventions in one rodent and subsequently observe how its parabiotic partner responds [48]. Using the parabiosis setup, Nishizawa and Bray overfed one rat by 160% via intragastric intubation and found that its non-overfed parabiotic partner decreased its food intake and lowered its fat pad mass and body weight [49]. Other parabiosis studies have also observed such decreases in body weight and fat mass in the parabiotic partners of overfed rats, but the hypophagic effect in these studies was less pronounced [[50], [52], [53], [54], [55]]. Thus, the changes in body weight and fat mass observed in the parabiotic partners of obese rats may involve additional mechanisms independent of food intake regulation. This includes other humoral agents [41], such as the suggested ‘antilipogenic factor’ by Ruth Harris [[50], [57]].
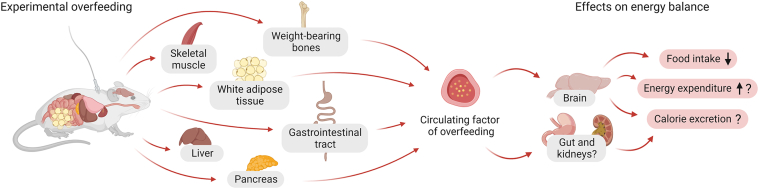
The search for the circulating factors that mediate the physiological defense against weight gain has been ongoing since at least the late 1950s, when Hervey published a landmark parabiosis study [44,56]. Yet, more than seven decades later, these factors remain unidentified. Some have observed that the serum from overfed rats and chickens inhibits lipogenesis in adipose tissue extracts from rats [57]. Others have homed in on a 5 kb mRNA that is highly expressed in adipose tissue and induced by overfeeding [22]. Moreover, in 1992, Hulsey and Martin identified an ‘adipose satiety factor’ in fat tissue extracts derived from overfed rats. This ‘factor’ has been reported to inhibit food intake in male rats when administered peripherally or centrally [58]. Based on the available evidence, we have previously discussed the molecular characteristics for these unidentified factors of overfeeding and highlighted several potential tissues and cell types from where they might be secreted [41] (Figure 5). In addition to promoting secretion of blood-borne mediators, nutrients also affect energy homeostasis through engaging vagal and spinal afferents [59,60].
Figure 5.
Potential tissue sources and effects on energy balance of circulating factors of overfeeding. Schematic diagram showing the hypothesized potential tissue sources of circulating factors of overfeeding. The potential sources represented in this figure are weight-bearing bones, skeletal muscle, white adipose tissue, gastrointestinal tract, liver and pancreas. Potent factors of overfeeding are predicted to exert regulation of energy balance via central nervous system mechanisms implicating inhibition of food intake and possibly also stimulation of energy expenditure and/or calorie excretion.
While many unanswered questions surround these unidentified blood-borne factors of overfeeding, there is also evidence to rule out several classical hormones as critical mediators of overfeeding-induced hypophagia (Figure 4B). Insulin, for example, could be considered a humoral factor of overfeeding [61]. It is a secreted hormone that circulates in the blood in proportion to adipose tissue mass, and it is known to act on hypothalamic neurons that regulate energy balance [62]. Moreover, several independent studies have reported that experimental overfeeding induces hyperinsulinemia [[63], [64], [65], [66]]. However, several studies have also shown that insulin levels return to baseline concentrations rapidly after experimental overfeeding and before food intake returns to normal [[21], [25], [50], [51], [67], [68]]. It is envisioned that overfeeding-induced satiety factors circulate at elevated levels during the entire hypophagic phase [41]. Thus, insulin is less likely to mediate the sustained hypophagic response to overfeeding. Another suggested candidate is the stomach-derived orexigenic peptide, ghrelin. It is well established that increased blood levels of this hunger-promoting hormone counteract weight loss [69]. Two weeks of 145%-overfeeding in male Sprague–Dawley rats resulted in lower plasma levels of ghrelin [70], which might contribute to the hypophagia seen after experimental overfeeding. However, two overfeeding studies in male Long Evans rats have shown that ghrelin levels quickly increase after overfeeding and return to baseline levels before food intake normalizes [[25], [68]], indicating that a decrease in ghrelin levels is not the primary mediator of post-overfeeding hypophagia. A blunted increase in circulating fibroblast growth factor 21 (FGF21) in response to overfeeding in humans [[71], [72], [73], [74]] has been associated with weight gain susceptibility [74,75]. Further work is needed to understand if FGF21 plays a direct role in the physiological protection against overfeeding-induced weight gain. Growth differentiation factor 15 (GDF15) might also be a candidate factor of overfeeding, given its role as a signal of somatic distress [76]. Yet, studies in humans show that circulating levels of GDF15 are not changed in response to acute (1-day) overfeeding [77], and that they are slightly reduced following chronic overfeeding (8-weeks) [78].
5.3. Evidence against leptin as a circulating factor of overfeeding
When leptin was discovered in the mid-1990s [46,79], it fulfilled the criteria for the molecular mediator of Kennedy's lipostatic hypothesis [80]. Because leptin was shown to be an adipose–secreted hormone that regulates food intake by targeting receptors in the brain, leptin was quickly portrayed as an afferent signal in a negative feedback loop. In other words, enlargement of adipose stores leads to higher levels of leptin in the blood, which, in turn, triggers hypophagic neuronal pathways and, thereby, a decrease in food intake [81]. This feedback model suggested that the hitherto unknown humoral satiety factor of overfeeding had been identified. However, several lines of evidence indicate that this is not the case. Leptin is a 16 kDa protein and thus does not fit the size of the putative ‘adipose satiety factor’, which was identified in a 30–100 kDa-fraction of fat tissue extract [58]. Moreover, it seems that leptin is not a potent physiological satiety signal but instead a ‘starvation signal’ that induces hunger when it circulates in low levels [41,43,[82], [83], [84]]. This might also explain why administration of exogenous leptin only has limited satiating and body weight-lowering effects, and, importantly, why the high blood levels of leptin often seen in individuals with obesity do not suppress appetite [83]. These observations and ideas point toward the existence of other adipostatic factors and suggest that non-leptin signals regulate the hypophagic and anorectic response to overfeeding [41,43,56,85,86].
Further evidence against the notion that leptin is the primary mediator of overfeeding-induced hypophagia can be found in the animal literature on experimental overfeeding. In 1998, a group of investigators studied whether conditioned media from ex vivo preparations of adipose tissue from overfed wild-type rats, leptin-deficient ob/ob mice and leptin receptor-deficient db/db mice and fa/fa rats would affect food intake [87]. As expected, media from the hyperleptinemic db/db mice and fa/fa rats suppressed food intake in the leptin-deficient ob/ob mice. However, injection of recombinant leptin at a dose equivalent to that present within the conditioned medium from db/db mice had no effect on food intake in ob/ob mice [87]. Therefore, the authors concluded that another factor secreted by the adipose tissue was responsible for suppressing food intake [87]. In 2010, White et al. showed that circulating levels of leptin were dramatically increased in male Long Evans rats at the end of a 17-day overfeeding period [25]. After overfeeding stopped, blood levels of leptin returned to baseline within just two days, yet the hypophagic response lasted for at least three days, highlighting that loss of appetite after overfeeding occurs in the presence of low circulating levels of leptin [25]. Similar observations have been made in another rat study [68], and a more recent experiment in mice provided even further evidence of a leptin-independent system that defends against weight gain [24]. In this study, leptin-deficient ob/ob mice were overfed while at the same time having blood leptin levels clamped at a low level by administration of exogenous leptin via subcutaneous osmotic pumps [24]. This experiment showed that keeping the blood concentration of leptin at a constant low level in ob/ob mice did not change the hypophagic response to overfeeding [24], thus reinforcing the idea that non-leptin factors mediate this effect.
In summary, experimental overfeeding studies in animals have investigated a series of well-characterized hormones and their potential role in controlling body weight, fat mass and food intake during and after an overfeeding intervention. However, as outlined both here and previously [41], the data obtained from these studies indicate that none of these hormones are among the hypothesized overfeeding factors that protect against obesity (Figure 4B).
5.4. Role of the hypothalamic melanocortin system in overfeeding-induced hypophagia
Two major neuronal populations within the arcuate nucleus of the hypothalamus play essential roles in the regulation of energy homeostasis. One population of neurons that express agouti-related protein (AgRP) and neuropeptide Y (NPY) are involved in orexigenic responses that increase appetite, whereas a second population of neurons that express cocaine- and amphetamine-related transcript (CART) and proopiomelanocortin (POMC) activate anorexigenic pathways that inhibit appetite [61]. POMC-positive neurons project to second-order neurons in the hypothalamus that express the receptors for melanocortin 3 and 4 (MC3R and MC4R). Melanocortin peptides generated after posttranslational processing of POMC act as agonists of MC3R and MC4R and initiate a coordinated response that inhibits food intake [82]. Besides the well-known role of MC4R in energy homeostasis [88,89], MC3R has also been implicated in the homeostatic response to both calorie restriction and HFD-induced energy surplus [90].
Surprisingly few studies have attempted to parse the neuronal pathways underlying homeostatic regulation of energy balance after experimental overfeeding. In 1996, Seeley et al. looked at selected transcripts in the arcuate nucleus of the hypothalamus of overfed rats but found no changes in the mRNA expression of the orexigenic-related transcript Npy (neuropeptide Y) in response to overfeeding [65]. At the same time, the authors reported increased corticotropin-releasing hormone (Crh) mRNA in response to overfeeding [65] but did not perform further assessments of putative changes in the Crh-related endocrine stress axis. Two years later, the same group of researchers reported increased Pomc expression in the arcuate nucleus of overfed rats and observed that hypophagia and weight loss following overfeeding could be blocked by MC3R/MC4R antagonism [91]. This finding suggests that the hypothalamic melanocortin pathway plays a major role in regulating food intake in response to overfeeding. Moreover, studies in rats made obese by lesions of the ventromedial hypothalamus have demonstrated that this hypothalamic region is not important for the hypophagic response seen during and after overfeeding [92,93]. Except for these few studies, the role of the central nervous system in overfeeding-induced hypophagia is largely unexplored, and future studies using modern neurobiological techniques are needed to explore and eventually map the neuronal circuits that underlie the profound loss of appetite and body weight seen after experimental overfeeding.
5.5. Experimental overfeeding and energy expenditure
As summarized above, the homeostatic correction of body weight that follows a period of experimental overfeeding seems to be primarily attributed to a robust hypophagic response. However, other biological aspects of energy balance regulation, such as energy expenditure [94], spontaneous physical activity [95,96], and calorie excretion via e.g. faeces, urine and skin [97,98], might also be involved in bringing back body weight to baseline levels after a period of overfeeding. Exploring these physiological processes might help to better understand why some humans are more weight gain susceptible than others, as demonstrated by different overfeeding studies [17,75,[99], [100], [101], [102], [103]]. Similar to what has been reported in these human overfeeding studies, some animal studies have reported an unexpectedly low body weight gain in response to overfeeding [34,37,65]. These observations might be explained by an ‘adaptive’ increase in energy expenditure. One study using female rats that were overfed by 130% for 30 days found an increase in resting O2 consumption and an elevated thermogenic response to norepinephrine during the hypophagic recovery period three and nine days after overfeeding, respectively [21]. Another study in male Long Evans rats overfed for 17 days also reported an increase in energy expenditure in the recovery period, but this effect was only seen on the first day post-overfeeding [25]. A couple of studies have reported changes in circulating factors that support the notion that overfeeding might increase energy expenditure. One study found increased circulating levels of norepinephrine in Wistar rats that were overfed for five weeks by 200% [63] and two other studies reported increased circulating levels of thyroid hormones during the hypophagic period in overfed rats [21,51].
In contrast to these findings, no evidence of increased energy expenditure was found in overfed C57BL/6J male mice immediately after 16 days of overfeeding [24]. This more recent finding agrees with three older studies in adult female rats that were overfed by >200%. Here the authors used respiration chambers to measure heat production and failed to detect increases in energy expenditure during overfeeding [[104], [105], [106]]. Thus, it is not entirely clear from the experimental overfeeding studies if adaptive thermogenesis is induced and thus contributes to lowering body weight after overfeeding. However, recent advances in indirect calorimetry systems for rodents enable concomitant high-resolution sampling of oxygen consumption, locomotion, body temperature, and food intake. In combination with measures of fecal and urinary energy content, experimental overfeeding studies in metabolic cages should enable the field to finally determine the extent to which energy intake, energy expenditure and energy excretion contribute to the homeostatic regulation of body weight during and after overfeeding.
5.6. Experimental overfeeding and its potential effects on long-term adiposity
Most overfeeding studies show a robust counter-regulatory hypophagic response during and after experimental overfeeding. This response usually brings body weight completely back to its pre-overfeeding level. However, there are also data to suggest that this recovery is sometimes incomplete and that overfeeding, in some cases, might have long-lasting effects on body weight and adiposity [23,39,51,67]. A previously discussed monkey study also found that post-overfeeding body weight stabilized at a level that was 14% higher than that of baseline body weight [39]. Cohn and Joseph noticed that previously overfed rats retained increased body fat levels compared to weight-matched controls (18% vs 11% body fat) two months after overfeeding [23]. An incomplete return of fat mass to baseline has been confirmed by other overfeeding studies in which rats were overfed by 160% for 21 days [51] or by 200% for 26 days [67]. One of the studies reported that although adipocyte size returned to baseline 41 days after overfeeding, total body weight and fat mass remained 10–20% higher [51]. In another study, body fat also remained higher in the overfed group (13% vs. 9% in controls) 36 days after the cessation of overfeeding, despite that body weight had returned to baseline [67]. These findings imply that the homeostatic mechanisms regulating body weight and adipose tissue expansion might be permanently altered by experimental overfeeding.
5.7. Effect of experimental overfeeding on adipose tissue morphology
Adipose tissue is a highly dynamic organ that undergoes expansion and remodeling in response to energy deprivation or excess. The adipose tissue of obese individuals is often characterized by immune cell infiltration, extracellular matrix expansion, and limited angiogenesis [107]. These changes in adipose tissue remodeling are considered critical pathogenic factors of obesity-related metabolic diseases [107]. Only a few studies have explored adipose tissue remodeling in response to experimental overfeeding. One study incubated preadipocytes with serum from male Sprague–Dawley rats that were overfed by 150% for four weeks and found that proliferation of preadipocytes isolated from the inguinal white fat depots was impaired while differentiation of preadipocytes into mature fat cells was enhanced [108]. A more recent study found that overfed and diet-induced obese C57BL/6J mice were similar in terms of adiposity, epididymal fat depot mass, and adipocyte size [24]. However, the transcriptional and immunological profiles in these two groups of obese mice were different [24]. Experimental overfeeding resulted in a lower percentage of crown-like structures and CD11c+ activated macrophages, together with a lower epression of inflammatory genes in adipose tissue compared to diet-induced obesity [24]. These results show that white adipose tissue is remodeled in response to experimental overfeeding and that this remodeling is different from diet-induced obesity. Further research on how longer-term overfeeding affects adipose tissue remodeling may enhance our knowledge regarding the development of metabolic disorders.
5.8. Experimental overfeeding as a model to study metabolic liver diseases
Not only adipose tissue but also the liver can store excess energy in the form of lipids. When exacerbated, this leads to hepatic steatosis. In the case of waterfowl birds, such as ducks and geese, the accumulation of liver fat is an advantageous trait that supports long migrations [109]. In order to produce foie gras, the food industry has exploited this biological phenomenon by force-feeding waterfowl and, in particular, certain species and breeds of geese that are more prone to develop hepatic steatosis. Studies in birds, rats, and mice have documented that experimental overfeeding increases liver weight [35,106,[110], [111], [112], [113], [114]], stimulates hepatic lipogenesis and fat deposition [35,67,[111], [112], [113], [114], [115], [116]], and inhibits hepatic fatty acid oxidation and ketogenesis [67,117,118]. Recent studies in overfed ducks and geese have aimed to reveal the underlying molecular changes in the liver following overfeeding using modern omics techniques [[119], [120], [121], [122], [123], [124]]. These studies have shown changes in circulating amino acids in overfed geese [123] and up-regulation of lipogenesis and down-regulation of fatty acid oxidation in the liver of overfed ducks [119]. However, significant translational progress has not yet been made, and the study of metabolic liver diseases, such as non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), has been limited by the lack of animal models that mimic the development and progression of these diseases [125].
Given that experimental overfeeding promotes hepatic lipid deposition in rodents, it might be a relevant model to study the pathophysiology of NASH. One study in male C57BL/6J mice reported that nine weeks of 185% overfeeding by intragastric infusion of a corn oil-based liquid diet led to severe obesity, impaired glucose metabolism, steatohepatitis and hepatic fibrosis [112]. These changes were accompanied by elevated circulating levels of alanine aminotransferase, a marker of liver damage [112]. Another overfeeding study in male C57BL/6J mice showed that intragastric overfeeding with a liquid high-fat diet accelerated the progression from hepatic steatosis to NASH [113]. Male Sprague–Dawley rats overfed with a corn oil-based high-fat liquid diet for 21 days also developed pathological and transcriptomic changes in the liver consistent with NASH [114]. Furthermore, another group of researchers has developed a model of NASH based on overfeeding rats with a liquid diet enriched in polyunsaturated fatty acids [126]. Using this model, they reported that the antioxidant N-acetyl cysteine slightly ameliorates NASH progression in rats without affecting overfeeding-induced hepatic steatosis [127]. Finally, the ability of alcohol to potentiate obesity-induced liver injury in humans can be mimicked by combining experimental overfeeding and ethanol administration in mice. As such, one study found that intragastric co-infusion of a high-fat diet (170% overfeeding) together with ethanol enhanced the liver-damaging effect of overfeeding, especially when a high dose of alcohol (32 g/kg/d) was used [128]. Together, these findings highlight that intragastric overfeeding of rodents can recapitulate critical pathophysiological features of human NASH [129] and suggest that experimental overfeeding might be a suitable model for exploring the molecular mechanisms that underlie the development of metabolic liver disease [125].
5.9. Overfeeding impairs infection tolerability
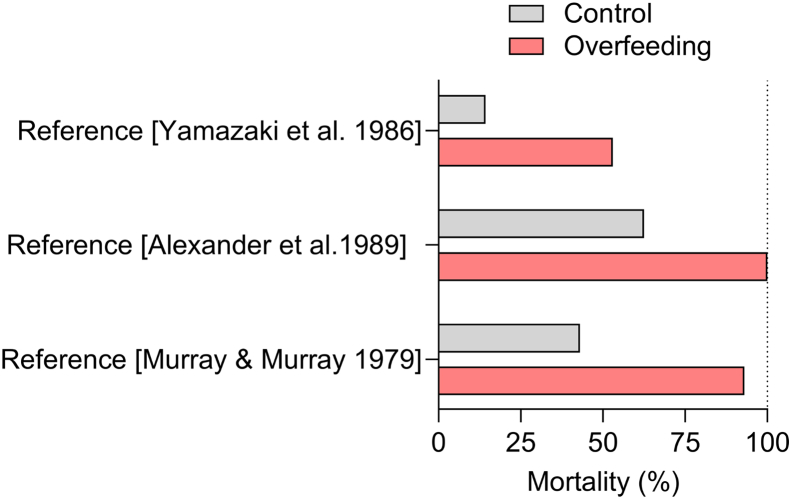
Loss of appetite is one of several features of sickness-induced behaviors [130]. Given that it is energetically costly to mount an immune response, this anorectic response is often regarded as paradoxical and maladaptive [131]. Yet, according to folklore, fevers can be ‘starved off’ [132], and it is clear that the acute hypophagic response to infectious diseases is a phenomenon that is conserved across the animal kingdom and capable of altering both disease resistance and tolerance, often in beneficial ways [131]. Therefore, overfeeding before and during infection may be harmful to the host, whereas underfeeding and fasting may help fight pathogens [131,133]. These notions are supported by evidence from studies on several different rodent species. A study in Swiss-Webster mice showed that intragastric eucaloric force-feeding by 100% during experimental infection with Listeria monocytogenes more than halved the mean survival time and caused death in 93% of the infected mice. In contrast, only 43% of the water-infused control mice succumbed to the infection [132]. Another study showed that intragastric overfeeding of guinea pigs increased their mortality rate in response to peritonitis caused by intraperitoneal infusion of live Escherichia coli and Staphylococcus aureus [134]. Guinea pigs infused with 150 or 175 kcal/kg/day showed a mortality rate of 100% after two weeks, whereas the mortality rate of guinea pigs infused with 125 kcal/kg/day was only 62.5% [134]. In contrast, underfeeding with intragastric infusions of a hypocaloric diet (100 kcal/kg/day) lowered the mortality rate of these infected guinea pigs to 42.8% [134]. Similarly, six days of 175% intragastric overfeeding in male Sprague–Dawley rats increased the mortality rate from experimentally-induced peritonitis (53% mortality in overfed rats vs. 14.3% mortality in control rats) [135]. This increase in mortality was associated with lower leucine incorporation into proteins in overfed rats compared to the 100%-fed rats [135]. These studies show that hypophagia is a beneficial response to some infections and illustrate that experimental overfeeding could be a valuable method to interrogate how energy balance interacts with the immune system and influences the tolerance to infectious diseases (Figure 6). Moreover, because specific nutrients like iron and branched-chain amino acids have been implicated in these processes [131], experimental overfeeding might also be relevant for investigating how various bioactive dietary components affect the defense against infectious diseases.
Figure 6.
Effect of experimental overfeeding on infection survival. Examples from literature in which the role of energy surplus in the form of experimental overfeeding has been evaluated for infection survival in animals.
5.10. Experimental overfeeding in early life: effects on growth trajectory and body composition in adulthood
There is a heightened interest in understanding how dietary components and energy balance during development affect metabolic health later in adulthood [136]. While some experimental overfeeding studies have explored the acute effects of overfeeding in young animals, other studies have evaluated the long-term obesogenic and metabolic effects of overfeeding pups during early postnatal life. One of these studies evaluated the effects of seven days of overfeeding by 150% via oral gavage in female Sprague–Dawley rats at 3, 5, 7, and 10 weeks of age [117]. Although overfeeding led to a more pronounced protein accretion in the two youngest age groups, there was no significant difference between groups in overall growth rate and fat mass accumulation [117]. In another study, Sprague–Dawley rat pups were overfed by oral gavage from delivery until weaning at 21 days of age, and body size and composition were subsequently studied in adulthood at 105 days of age [110]. This study showed that overfed rats, compared with sham-gavaged controls, displayed a 5.5% higher body weight gain (not significant) and higher carcass protein percentage (19.6 vs. 13.2%) at 105 days of age. In contrast, body fat levels were only increased during the overfeeding period and normalized in adulthood [110]. Interestingly, a significant obesogenic effect of overfeeding in early life has been reported in studies that overfed both male and female Long-Evans rat pups by intragastric infusion of a fat-supplemented milk formula from day 4 to day 18 after birth, followed by ad libitum feeding on a standard diet [137,138]. These studies showed that overfeeding accelerated growth rate in infancy and led to a more obesogenic growth trajectory in adulthood, as demonstrated in male rats by markedly larger fat pads and a 20% higher body weight than controls from day 150 to day 200 postpartum [137,138]. The mechanisms underlying these reported effects are unknown but related studies of postnatal overnutrition have shown that metabolic alterations caused by early-life overfeeding are associated with dysregulation of the melanocortin system [139] and hypothalamic inflammation [140].
5.11. Maternal overfeeding before conception might trigger obesity “programming” in offspring
In addition to investigating how a positive energy balance in early life affects adult metabolic health, studies have also employed experimental overfeeding to investigate if an excessive calorie intake in the parental generation affects energy metabolism in the offspring [141]. In one of these studies, female Sprague–Dawley rats were overfed by intragastric infusion of an obesogenic liquid diet for three weeks until weighing 21% more than control rats [142]. Afterwards, these overfed female rats were allowed to mate with lean males. In order to minimize exposure to obesogenic stimuli outside of the uterus, the offspring was reared by lean surrogate dams that were impregnated to give birth the same day as the infusion-fed dams [142]. Interestingly, male offspring from overfed dams displayed a higher susceptibility to diet-induced obesity, as they gained ∼20% more body weight and significantly more fat mass than the offspring of non-overfed dams when having ad libitum access to a high-fat diet for three months [142]. Follow-up studies using the same experimental model found transcriptional changes related to inflammatory and lipid metabolism pathways in the uterus of the overfed dams [143,144] and in the liver and adipose tissue of their offspring [145,146]. Yet, the causal mechanisms governing these intriguing transgenerational effects of overfeeding remain to be revealed.
5.12. The role of diet composition in experimental overfeeding: implications for obesity and type 2 diabetes
The role of diet composition in weight regulation and metabolic health is a topic of great interest and debate amongst obesity researchers [[147], [148], [149]]. A key methodological strength of experimental overfeeding is that diet composition and nutrient content can be controlled while keeping caloric intake clamped. Experimental overfeeding studies with variations in macronutrient composition have been performed in waterfowl [36,111,150], and they suggest that macronutrient composition has little impact on body weight gain in geese and chicks but also indicate that carbohydrate overfeeding can promote the development of hepatomegaly. Another area of research suggests a greater satiating effect of medium-chain triglycerides (MCT) compared to long-chain triglycerides (LCT) in both animals and humans [151,152] and overfeeding studies have also been performed in this field [52,[153], [154], [155]]. Rats overfed by 150% with an MCT-enriched diet showed around 15% lower body weight gain and around 35% less fat mass than rats overfed with an LCT diet [153]. Similar effects have been observed by the same group in another study [154]. Here, the MCT-diet induced changes in body composition associated with an elevation in resting- and norepinephrine-induced whole-body oxygen consumption of 40% and 22%, respectively [153]. This suggests that changes in energy expenditure may explain the lower body weight gain associated with an MCT diet, but the underlying mechanisms remain unknown. Studies suggest that neither changes in spontaneous physical activity nor heat production in interscapular brown fat mediate the weight gain-attenuating effect of MCTs [154,155]. However, given that energy intake is clamped in these studies and that MCTs are more efficiently absorbed in the intestine than LCTs [152], heat dissipation by other means could potentially mediate the anti-obesity effects of MCTs. Identifying these mechanisms might be done by future experimental overfeeding studies.
Experimental overfeeding studies have also investigated how diets with different fat content affect energy balance [55,114,156,157]. One study in male C57BL/6 mice showed that overfeeding with a high-fat diet (40.1% of total calories) or an isocaloric low-fat diet (8.6% of total calories) for six weeks caused a similar weight gain [156]. Yet, the group that was overfed with a high-fat diet displayed an exacerbated progression of glucose intolerance, hyperinsulinemia, and hyperleptinemia in addition to adipocyte hypertrophy, inflammation, and increased circulating levels of triglycerides and free fatty acids, as compared with mice overfed with a low-fat diet [156]. Similarly, another overfeeding study did not find any differences in weight gain and adiposity when comparing Sprague–Dawley rats overfed with either a low-fat diet (5% of total calories) or high-fat diets (70% of total calories) composed of different fat sources (olive oil, corn oil, or echium oil) [114]. However, the source of fat affected metabolic outcomes, as rats infused with the olive oil-enriched high-fat diet were protected from diet-induced liver injury even though they displayed hepatic steatosis to the same extent as the other high-fat diet-fed groups [114]. On one hand, these studies show that dietary lipids can affect metabolic health in the context of high-fat diet overfeeding. On the other hand, these data also indicate that dietary lipid composition does not modulate the body weight gain that is observed in response to overfeeding. However, this latter notion has recently been challenged by an overfeeding study in which intragastric infusion of a highly palatable high-fat liquid diet weakened the defense against weight gain and increased the voluntary intake of a less palatable low-fat diet in male C57BL/6 mice [157]. This novel finding is consistent with the idea that diets with high fat content promote the development of obesity [158] and suggests that the obesogenic effect of dietary fat might, at least partly, be attributed to its ability to impair the still unknown physiological mechanisms that protect against an excessive body weight gain.
6. Conclusions and perspectives
In 1922, Addison Gulick emphasized that “we do not yet know what mechanism there is to prevent the unlimited accumulation of potential energy in the form of an overload of adipose tissue” [159]. This early awareness of biological mechanisms that counteract body weight gain was in the 1960s corroborated by overfeeding interventions in more controlled settings, such as the Vermont State Prison experiments [160], and in the 1990s by ‘cultural overeating’ studies [103]. Today, hundreds of experimental overfeeding studies have been conducted [18,19], yet the biological mechanisms that defend against body weight gain remain unidentified, and the statement made by Gulick still stands more than 100 years after his publication in the American Journal of Physiology.
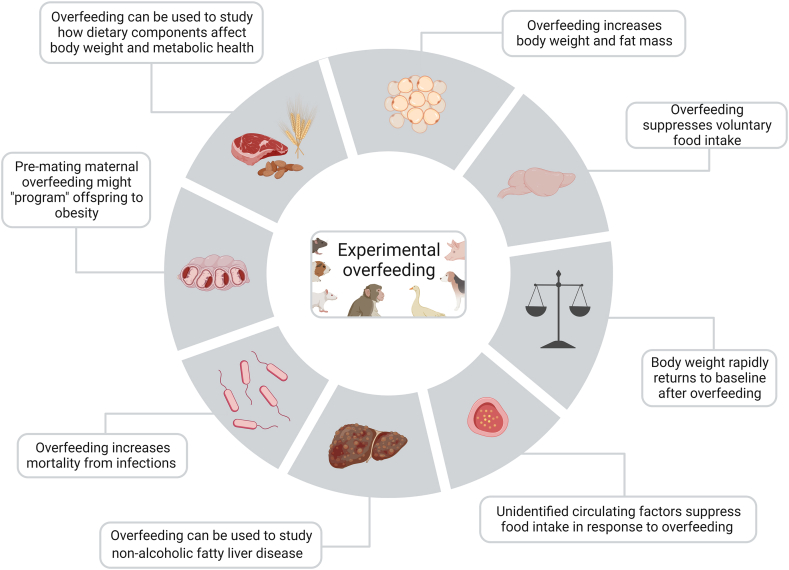
Ingenious utilization of animal models has been imperative for our current understanding of energy balance regulation and for the development of therapeutics to treat cardiometabolic disorders. As summarized in this review, data from experimental overfeeding studies in animals support that a yet-to-be-identified endocrine pathway is a critical component of the biological defense against weight gain. However, although experimental overfeeding studies in animals have an obvious potential to unravel new regulatory pathways underlying body weight homeostasis, the approach remains underutilized. Experimental overfeeding is also an underappreciated model for studying the etiology of metabolic liver diseases and an array of other pathologies in which perturbed energy balance affects health outcomes, such as during infections and in cancer cachexia. Finally, experimental overfeeding holds promise as a complementary model to experimentally interrogate how total energy intake versus dietary macronutrient composition versus meal timing affects energy balance, body weight and metabolic homeostasis [147,148,161] (Figure 7).
Figure 7.
Features and utility of experimental overfeeding in animals. Schematic overview of the major physiological underpinnings and suggested utility of experimental overfeeding in animals.
We believe that the time is ripe for experimental overfeeding in animals to emerge as a regularly used intervention for systematically mapping the physiology and molecular biology that counteracts weight gain [162]. Such work might ultimately inspire a new generation of therapeutics against obesity.
Acknowledgments
This work was supported by research grants from the Lundbeck Foundation (Fellowship R238-2016-2859) and the Novo Nordisk Foundation (grant numbers NNF17OC0026114). Novo Nordisk Foundation Center for Basic Metabolic Research is an independent Research Center, based at the University of Copenhagen, Denmark, and partially funded by an unconditional donation from the Novo Nordisk Foundation (www.cbmr.ku.dk) (Grant number NNF18CC0034900). Figures were created using BioRender.com.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2022.101573.
Contributor Information
Pablo Ranea-Robles, Email: pablo.ranea.robles@sund.ku.dk.
Jens Lund, Email: jens.lund@sund.ku.dk.
Christoffer Clemmensen, Email: chc@sund.ku.dk.
Conflict of interest
Christoffer Clemmensen is co-founder of Ousia Pharma ApS, a biotech company developing therapeutics for treatment of obesity.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Florant G.L., Healy J.E. The regulation of food intake in mammalian hibernators: a review. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2012;182(4):451–467. doi: 10.1007/s00360-011-0630-y. [DOI] [PubMed] [Google Scholar]
- 2.Bairlein F., Gwinner E. Nutritional mechanisms and temporal control of migratory energy accumulation in birds. Annual Review of Nutrition. 1994;14:187–215. doi: 10.1146/ANNUREV.NU.14.070194.001155. [DOI] [PubMed] [Google Scholar]
- 3.Alerstam T., Bäckman J. Ecology of animal migration. Current Biology. 2018;28(17):R968–R972. doi: 10.1016/J.CUB.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 4.Cornelius J.M., Boswell T., Jenni-Eiermann S., Breuner C.W., Ramenofsky M. Contributions of endocrinology to the migration life history of birds. General and Comparative Endocrinology. 2013;190:47–60. doi: 10.1016/J.YGCEN.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Bairlein F. How to get fat: nutritional mechanisms of seasonal fat accumulation in migratory songbirds. Die Naturwissenschaften. 2002;89(1):1–10. doi: 10.1007/S00114-001-0279-6. [DOI] [PubMed] [Google Scholar]
- 6.Scott I., Mitchell P.I., Evans P.R. Seasonal changes in body mass, body composition and food requirements in wild migratory birds. Proceedings of the Nutrition Society. 1994;53(3):521–531. doi: 10.1079/pns19940062. [DOI] [PubMed] [Google Scholar]
- 7.Piersma T., Gill R.E. Guts don't fly: small digestive organs in obese bar-tailed Godwits. The Auk: Ornithological Advances. 1998;115(1):196–203. doi: 10.2307/4089124. [DOI] [Google Scholar]
- 8.Piersma T., Jukema J. Budgeting the flight of a long-distance migrant: changes in nutrient reserve levels of bar-tailed godwits at successive spring staging sites. Ardea. 1990;78(1–2):315–337. doi: 10.5253/arde.v78.p315. [DOI] [Google Scholar]
- 9.Dausmann K.H., Glos J., Ganzhorn J.U., Heldmaier G. Physiology: hibernation in a tropical primate. Nature. 2004;429(6994):825–826. doi: 10.1038/429825a. [DOI] [PubMed] [Google Scholar]
- 10.Fietz J., Ganzhorn J.U. Feeding ecology of the hibernating primate Cheirogaleus medius: how does it get so fat? Oecologia. 1999;121(2):157–164. doi: 10.1007/S004420050917. [DOI] [PubMed] [Google Scholar]
- 11.Mohr S.M., Bagriantsev S.N., Gracheva E.O. Cellular, molecular, and physiological adaptations of hibernation: the solution to environmental challenges. Annual Review of Cell and Developmental Biology. 2020:315–338. doi: 10.1146/annurev-cellbio-012820-095945. [DOI] [PubMed] [Google Scholar]
- 12.Blanco M.B., Greene L.K., Schopler R., Williams C.V., Lynch D., Browning J., et al. On the modulation and maintenance of hibernation in captive dwarf lemurs. Scientific Reports. 2021;11(1) doi: 10.1038/s41598-021-84727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferris E., Gregg C. Parallel accelerated evolution in distant hibernators reveals candidate cis elements and genetic circuits regulating mammalian obesity. Cell Reports. 2019;29(9):2608–2620.e4. doi: 10.1016/J.CELREP.2019.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolaczynski J.W., Ohannesian J.P., Considine R.V., Marco C.C., Caro J.F. Response of leptin to short-term and prolonged overfeeding in humans. The Journal of Clinical Endocrinology and Metabolism. 1996;81(11):4162–4165. doi: 10.1210/JCEM.81.11.8923877. [DOI] [PubMed] [Google Scholar]
- 15.Unger R.H. Longevity, lipotoxicity and leptin: the adipocyte defense against feasting and famine. Biochimie. 2005;87(1):57–64. doi: 10.1016/J.BIOCHI.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Hengist A., Edinburgh R.M., Davies R.G., Walhin J.P., Buniam J., James L.J., et al. Physiological responses to maximal eating in men. British Journal of Nutrition. 2020;124(4):407–417. doi: 10.1017/S0007114520001270. [DOI] [PubMed] [Google Scholar]
- 17.Bouchard C., Tremblay A., Després J.-P., Nadeau A., Lupien P.J., Thériault G., et al. The response to long-term overfeeding in identical twins. New England Journal of Medicine. 1990;322(21):1477–1482. doi: 10.1056/nejm199005243222101. [DOI] [PubMed] [Google Scholar]
- 18.Bray G.A., Bouchard C. The biology of human overfeeding: a systematic review. Obesity Reviews. 2020;21(9) doi: 10.1111/obr.13040. [DOI] [PubMed] [Google Scholar]
- 19.Cuthbertson D.J., Steele T., Wilding J.P., Halford J.C., Harrold J.A., Hamer M., et al. What have human experimental overfeeding studies taught us about adipose tissue expansion and susceptibility to obesity and metabolic complications? International Journal of Obesity. 2017:853–865. doi: 10.1038/ijo.2017.4. [DOI] [PubMed] [Google Scholar]
- 20.Ueno A., Lazaro R., Wang P.Y., Higashiyama R., MacHida K., Tsukamoto H. Mouse intragastric infusion (iG) model. Nature Protocols. 2012;7(4):771–781. doi: 10.1038/nprot.2012.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almeida N.G., Levitsky D.A., Strupp B. Enhanced thermogenesis during recovery from diet-induced weight gain in the rat. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 1996;271(5):R1380–R1387. doi: 10.1152/ajpregu.1996.271.5.r1380. [DOI] [PubMed] [Google Scholar]
- 22.Wilson B.E., Meyer G.E., Cleveland J.C., Weigle D.S. Identification of candidate genes for a factor regulating body weight in primates. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 1990;259(6):R1148–R1155. doi: 10.1152/ajpregu.1990.259.6.r1148. [DOI] [PubMed] [Google Scholar]
- 23.Cohn C., Joseph D. Influence of body weight and body fat on appetite of “normal” lean and obese rats. The Yale Journal of Biology and Medicine. 1962;34(6):598–607. [PMC free article] [PubMed] [Google Scholar]
- 24.Ravussin Y., Edwin E., Gallop M., Xu L., Bartolomé A., Kraakman M.J., et al. Evidence for a non-leptin system that defends against weight gain in overfeeding. Cell Metabolism. 2018;28(2):289–299.e5. doi: 10.1016/j.cmet.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White C.L., Purpera M.N., Ballard K., Morrison C.D. Decreased food intake following overfeeding involves leptin-dependent and leptin-independent mechanisms. Physiology and Behavior. 2010;100(4):408–416. doi: 10.1016/J.PHYSBEH.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohn M. Satiation of hunger from food injected directly into the stomach versus food ingested by mouth. Journal of Comparative & Physiological Psychology. 1951;44(5):412–422. doi: 10.1037/h0061340. [DOI] [PubMed] [Google Scholar]
- 27.Panda S. Circadian physiology of metabolism. Science (New York, N.Y.) 2016;354(6315):1008–1015. doi: 10.1126/science.aah4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis P., Oster H., Korf H.W., Foster R.G., Erren T.C. Food as a circadian time cue – evidence from human studies. Nature Reviews. Endocrinology. 2020;16(4):213–223. doi: 10.1038/S41574-020-0318-Z. [DOI] [PubMed] [Google Scholar]
- 29.Lutz T.A., Woods S.C. Overview of animal models of obesity. Current Protocols in Pharmacology. 2012;(Suppl. 58) doi: 10.1002/0471141755.PH0561S58. chapter 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Commission E. 2018. Summary report on the statistics on the use of animals for scientific purposes in the member states of the European Union and Norway in 2018. [Google Scholar]
- 31.Ingle D.J. The production of alimentary glycosuria by forced feeding in the rat. Endocrinology. 1946;39:43–51. doi: 10.1210/endo-39-1-43. [DOI] [PubMed] [Google Scholar]
- 32.Ingle D.J., Nezamis J.E. The effect of insulin on the tolerance of normal male rats to the overfeeding of a high carbohydrate diet. Endocrinology. 1947;40(5):353–357. doi: 10.1210/ENDO-40-5-353. [DOI] [PubMed] [Google Scholar]
- 33.Share I., Martyniuk E., Grossman M.I. Effect of prolonged intragastric feeding on oral food intake in dogs. The American Journal of Physiology. 1952;169(1):229–235. doi: 10.1152/ajplegacy.1952.169.1.229. [DOI] [PubMed] [Google Scholar]
- 34.Janowitz H.D., Hollander F. The time factor in the adjustment of food intake to varied caloric requirement in the dog: a study of the precision of appetite regulation. Annals of the New York Academy of Sciences. 1955;63(1):56–67. doi: 10.1111/J.1749-6632.1955.TB36545.X. [DOI] [PubMed] [Google Scholar]
- 35.Lepkovsky S., Furuta F. The role of homeostasis in adipose tissues upon the regulation of food intake of White Leghorn cockerels. Poultry Science. 1971;50(2):573–577. doi: 10.3382/ps.0500573. [DOI] [PubMed] [Google Scholar]
- 36.Nir I., Nitsan Z., Vax A. The influence of force feeding and of protein supplementation to the diet on the metabolisable energy of diets, digestibility of nutrients, nitrogen retention and digestive enzymes output in geese. Annales de Biologie Animale, Biochimie, Biophysique. 1973;13(3):465–479. doi: 10.1051/rnd:19730312. [DOI] [PubMed] [Google Scholar]
- 37.Drewry M.M., Harris R.B.S., Martin R.J. The effect of increased adiposity on food intake of juvenile rats. Physiology and Behavior. 1989;45(2):381–386. doi: 10.1016/0031-9384(89)90144-3. [DOI] [PubMed] [Google Scholar]
- 38.Pekas J.C. Animal growth during liberation from appetite suppression. Growth. 1985;49(1):19–27. [PubMed] [Google Scholar]
- 39.Jen K.L.C., Hansen B.C. Feeding behavior during experimentally induced obesity in monkeys. Physiology and Behavior. 1984;33(6):863–869. doi: 10.1016/0031-9384(84)90220-8. [DOI] [PubMed] [Google Scholar]
- 40.Nir I., Nitsan Z., Dror Y., Shapira N. Influence of overfeeding on growth, obesity and intestinal tract in young chicks of light and heavy breeds. British Journal of Nutrition. 1978;39(1):27–35. doi: 10.1079/bjn19780008. [DOI] [PubMed] [Google Scholar]
- 41.Lund J., Lund C., Morville T., Clemmensen C. The unidentified hormonal defense against weight gain. PLoS Biology. 2020;18(2) doi: 10.1371/journal.pbio.3000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ravussin Y., Leibel R.L., Ferrante A.W. A missing link in body weight homeostasis: the catabolic signal of the overfed state. Cell Metabolism. 2014:565–572. doi: 10.1016/j.cmet.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hervey G.R. The effects of lesions in the hypothalamus in parabiotic rats. The Journal of Physiology. 1959;145(2):336–352. doi: 10.1113/jphysiol.1959.sp006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris R.B.S. Contribution made by parabiosis to the understanding of energy balance regulation. Biochimica et Biophysica Acta – Molecular Basis of Disease. 2013:1449–1455. doi: 10.1016/j.bbadis.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 47.Coleman D.L. A historical perspective on leptin. Nature Medicine. 2010:1097–1099. doi: 10.1038/nm1010-1097. [DOI] [PubMed] [Google Scholar]
- 48.Finerty J.C. Parabiosis in physiological studies. Physiological Reviews. 1952;32(3):277–302. doi: 10.1152/PHYSREV.1952.32.3.277. [DOI] [PubMed] [Google Scholar]
- 49.Nishizawa Y., Bray G.A. Evidence for a circulating ergostatic factor: studies on parabiotic rats. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 1980;8(2) doi: 10.1152/ajpregu.1980.239.3.r344. [DOI] [PubMed] [Google Scholar]
- 50.Harris R.B.S., Martin R.J. Specific depletion of body fat in parabiotic partners of tube-fed obese rats. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 1984;16(2) doi: 10.1152/ajpregu.1984.247.2.r380. [DOI] [PubMed] [Google Scholar]
- 51.Harris R.B.S., Kasser T.R., Martin R.J. Dynamics of recovery of body composition after overfeeding, food restriction or starvation of mature female rats. Journal of Nutrition. 1986;116(12):2536–2546. doi: 10.1093/jn/116.12.2536. [DOI] [PubMed] [Google Scholar]
- 52.Harris R.B.S., Martin R.J. Influence of diet on the production of a “lipid-depleting” factor in obese parabiotic rats. Journal of Nutrition. 1986;116(10):2013–2027. doi: 10.1093/jn/116.10.2013. [DOI] [PubMed] [Google Scholar]
- 53.Harris R.B.S., Martin R.J. Metabolic response to a specific lipid-depleting factor in parabiotic rats. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 1986;250(2):R276–R286. doi: 10.1152/ajpregu.1986.250.2.r276. [DOI] [PubMed] [Google Scholar]
- 54.Harris R.B.S., Martin R.J. Site of action of putative lipostatic factor: food intake and peripheral pentose shunt activity. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 1990;259(1):R45–R52. doi: 10.1152/ajpregu.1990.259.1.r45. [DOI] [PubMed] [Google Scholar]
- 55.Harris R.B.S., Martin R.J., Bruch R.C. Dissociation between food intake, diet composition, and metabolism in parabiotic partners of obese rats. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 1995;268(4):R874–R883. doi: 10.1152/ajpregu.1995.268.4.r874. [DOI] [PubMed] [Google Scholar]
- 56.Hervey G.R. Control of appetite. Personal and departmental recollections. Appetite. 2013;61(1):100–110. doi: 10.1016/J.APPET.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 57.Harris R.B.S., Bruch R.C., Martin R.J. In vitro evidence for an inhibitor of lipogenesis in serum from overfed obese rats. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 1989;257(2) doi: 10.1152/ajpregu.1989.257.2.r326. [DOI] [PubMed] [Google Scholar]
- 58.Hulsey M.G., Martin R.J. An anorectic agent from adipose tissue of overfed rats: effects on feeding behavior. Physiology and Behavior. 1992;52(6):1141–1149. doi: 10.1016/0031-9384(92)90473-F. [DOI] [PubMed] [Google Scholar]
- 59.Goldstein N., McKnight A.D., Carty J.R.E., Arnold M., Betley J.N., Alhadeff A.L. Hypothalamic detection of macronutrients via multiple gut-brain pathways. Cell Metabolism. 2021;33(3):676–687.e5. doi: 10.1016/j.cmet.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clemmensen C., Müller T.D., Woods S.C., Berthoud H.R., Seeley R.J., Tschöp M.H. Gut-brain cross-talk in metabolic control. Cell. 2017;168(5):758–774. doi: 10.1016/J.CELL.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwartz M.W., Woods S.C., Porte D., Seeley R.J., Baskin D.G. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 62.Woods S.C., Lutz T.A., Geary N., Langhans W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2006;361(1471):1219–1235. doi: 10.1098/rstb.2006.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balkan B., Strubbe J.H., Bruggink J.E., Steffens A.B. Overfeeding-induced obesity in rats: insulin sensitivity and autonomic regulation of metabolism. Metabolism. 1993;42(12):1509–1518. doi: 10.1016/0026-0495(93)90144-D. [DOI] [PubMed] [Google Scholar]
- 64.Harris R.B.S., Ramsay T.G., Smith S.R., Bruch R.C. Early and late stimulation of ob mRNA expression in meal-fed and overfed rats. Journal of Clinical Investigation. 1996;97(9):2020–2026. doi: 10.1172/JCI118637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seeley R.J., Matson C.A., Chavez M., Woods S.C., Dallman M.F., Schwartz M.W. Behavioral, endocrine, and hypothalamic responses to involuntary overfeeding. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 1996;271(3):R819–R823. doi: 10.1152/ajpregu.1996.271.3.r819. [DOI] [PubMed] [Google Scholar]
- 66.Bernstein I.L., Lotter E.C., Kulkosky P.J., Porte D., Woods S.C. Effect of force-feeding upon basal insulin levels of rats. Proceedings of the Society for Experimental Biology and Medicine. 1975;150(2):546–548. doi: 10.3181/00379727-150-39075. [DOI] [PubMed] [Google Scholar]
- 67.Harris R.B.S., Martin R.J. Changes in lipogenesis and lipolysis associated with recovery from reversible obesity in mature female rats. Proceedings of the Society for Experimental Biology and Medicine. 1989;191(1):82–89. doi: 10.3181/00379727-191-42893. [DOI] [PubMed] [Google Scholar]
- 68.Gloy V.L., Lutz T.A., Langhans W., Geary N., Hillebrand J.J. Basal plasma levels of insulin, leptin, ghrelin, and amylin do not signal adiposity in rats recovering from forced overweight. Endocrinology. 2010;151(9):4280–4288. doi: 10.1210/en.2010-0439. [DOI] [PubMed] [Google Scholar]
- 69.Cummings D.E. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiology and Behavior. 2006;89(1):71–84. doi: 10.1016/J.PHYSBEH.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 70.Williams D.L., Grill H.J., Cummings D.E., Kaplan J.M. Overfeeding-induced weight gain suppresses plasma ghrelin levels in rats. Journal of Endocrinological Investigation. 2006;29(10):863–868. doi: 10.1007/BF03349188. [DOI] [PubMed] [Google Scholar]
- 71.Willis S.A., Sargeant J.A., Yates T., Takamura T., Takayama H., Gupta V., et al. Acute hyperenergetic, high-fat feeding increases circulating FGF21, LECT2, and fetuin-A in healthy men. The Journal of Nutrition. 2020;150(5):1076–1085. doi: 10.1093/JN/NXZ333. [DOI] [PubMed] [Google Scholar]
- 72.Heilbronn L.K., Campbell L.V., Xu A., Samocha-Bonet D. Metabolically protective cytokines adiponectin and fibroblast growth factor-21 are increased by acute overfeeding in healthy humans. PLoS One. 2013;8(10) doi: 10.1371/JOURNAL.PONE.0078864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lundsgaard A.M., Fritzen A.M., Sjøberg K.A., Myrmel L.S., Madsen L., Wojtaszewski J.F.P., et al. Circulating FGF21 in humans is potently induced by short term overfeeding of carbohydrates. Molecular Metabolism. 2017;6(1):22–29. doi: 10.1016/j.molmet.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vinales K.L., Begaye B., Bogardus C., Walter M., Krakoff J., Piaggi P. FGF21 is a hormonal mediator of the human “thrifty” metabolic phenotype. Diabetes. 2019;68(2):318–323. doi: 10.2337/db18-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piaggi P. Metabolic determinants of weight gain in humans. Obesity. 2019;27(5):691–699. doi: 10.1002/oby.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lockhart S.M., Saudek V., O'Rahilly S. GDF15: a hormone conveying somatic distress to the brain. Endocrine Reviews. 2020;41(4):610–642. doi: 10.1210/ENDREV/BNAA007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klein A.B., Nicolaisen T.S., Ørtenblad N., Gejl K.D., Jensen R., Fritzen A.M., et al. Pharmacological but not physiological GDF15 suppresses feeding and the motivation to exercise. Nature Communications. 2021;12(1) doi: 10.1038/s41467-021-21309-x. 2020.10.23.352864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patel S., Alvarez-Guaita A., Melvin A., Rimmington D., Dattilo A., Miedzybrodzka E.L., et al. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metabolism. 2019;29(3):707–718.e8. doi: 10.1016/j.cmet.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Halaas J.L., Gajiwala K.S., Maffei M., Cohen S.L., Chait B.T., Rabinowitz D., et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 80.Kennedy G.C. The role of depot fat in the hypothalamic control of food intake in the rat. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1953;140(901):578–596. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- 81.Friedman J.M., Halaas J.L. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 82.Van Der Klaauw A.A., Farooqi I.S. The hunger genes: pathways to obesity. Cell. 2015;161(1):119–132. doi: 10.1016/J.CELL.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 83.Flier J.S., Maratos-Flier E. Leptin's physiologic role: does the emperor of energy balance have no clothes? Cell Metabolism. 2017;26(1):24–26. doi: 10.1016/j.cmet.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 84.Speakman J.R. The evolution of body fatness: trading off disease and predation risk. Journal of Experimental Biology. 2018 doi: 10.1242/jeb.167254. [DOI] [PubMed] [Google Scholar]
- 85.Harris R.B.S. Is leptin the parabiotic “satiety” factor? Past and present interpretations. Appetite. 2013;61(1):111–118. doi: 10.1016/J.APPET.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frühbeck G., Gómez-Ambrosi J. Rationale for the existence of additional adipostatic hormones. Federation of American Societies for Experimental Biology Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2001;15(11):1996–2006. doi: 10.1096/FJ.00-0829HYP. [DOI] [PubMed] [Google Scholar]
- 87.Weigle D.S., Hutson A.M., Kramer J.M., Fallon M.G.M., Lehner J.M., Lok S., et al. Leptin does not fully account for the satiety activity of adipose tissue-conditioned medium. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 1998;275(4):R976–R985. doi: 10.1152/ajpregu.1998.275.4.r976. [DOI] [PubMed] [Google Scholar]
- 88.Fan W., Boston B.A., Kesterson R.A., Hruby V.J., Cone R.D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385(6612):165–168. doi: 10.1038/385165A0. [DOI] [PubMed] [Google Scholar]
- 89.Huszar D., Lynch C.A., Fairchild-Huntress V., Dunmore J.H., Fang Q., Berkemeier L.R., et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. doi: 10.1016/S0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 90.Ghamari-Langroudi M., Cakir I., Lippert R.N., Sweeney P., Litt M.J., Ellacott K.L.J., et al. Regulation of energy rheostasis by the melanocortin-3 receptor. Science Advances. 2018;4(8) doi: 10.1126/sciadv.aat0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hagan M.M., Rushing P.A., Schwarte M.W., Yagaloff K.A., Burn P., Woods S.C., et al. Role of the CNS melanocortin system in the response to overfeeding. Journal of Neuroscience. 1999;19(6):2362–2367. doi: 10.1523/jneurosci.19-06-02362.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoebel B.G., Teitelbaum P. Weight regulation in normal and hypothalamic hyperphagic rats. Journal of Comparative & Physiological Psychology. 1966;61(2):189–193. doi: 10.1037/h0023126. [DOI] [PubMed] [Google Scholar]
- 93.Liu C.M., Yin T.H. Caloric compensation to gastric loads in rats with hypothalamic hyperphagia. Physiology and Behavior. 1974;13(2):231–238. doi: 10.1016/0031-9384(74)90039-0. [DOI] [PubMed] [Google Scholar]
- 94.Leibel R.L., Rosenbaum M., Hirsch J. Changes in energy expenditure resulting from altered body weight. New England Journal of Medicine. 1995;332(10):621–628. doi: 10.1056/nejm199503093321001. [DOI] [PubMed] [Google Scholar]
- 95.Schmidt S.L., Harmon K.A., Sharp T.A., Kealey E.H., Bessesen D.H. The effects of overfeeding on spontaneous physical activity in obesity prone and obesity resistant humans. Obesity. 2012;20(11):2186–2193. doi: 10.1038/oby.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Levine J.A., Eberhardt N.L., Jensen M.D. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science (New York, N.Y.) 1999;283(5399):212–214. doi: 10.1126/SCIENCE.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 97.Lund J., Gerhart-Hines Z., Clemmensen C. Role of energy excretion in human body weight regulation. Trends in Endocrinology and Metabolism. 2020;31(10):705–708. doi: 10.1016/j.tem.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 98.Choa R., Tohyama J., Wada S., Meng H., Hu J., Okumura M., et al. Thymic stromal lymphopoietin induces adipose loss through sebum hypersecretion. Science. 2021;373(6554) doi: 10.1126/science.abd2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sims E.A., Goldman F.R., Gluck C.M., Horton E.S., Kelleher P.C., Rowe D.W. Experimental obesity in man. Trans Assoc Am Physicians. 1968;81:153–170. [PubMed] [Google Scholar]
- 100.Norgan N.G., Durnin J.V.G.A. The effect of 6 weeks of overfeeding on the body weight, body composition, and energy metabolism of young men. American Journal of Clinical Nutrition. 1980;33(5):978–988. doi: 10.1093/ajcn/33.5.978. [DOI] [PubMed] [Google Scholar]
- 101.Forbes G.B., Brown M.R., Welle S.L., Lipinski B.A. Deliberate overfeeding in women and men: energy cost and composition of the weight gain. British Journal of Nutrition. 1986;56(1):1–9. doi: 10.1079/bjn19860080. [DOI] [PubMed] [Google Scholar]
- 102.Diaz E.O., Prentice A.M., Goldberg G.R., Murgatroyd P.R., Coward W.A. Metabolic response to experimental overfeeding in lean and overweight healthy volunteers. American Journal of Clinical Nutrition. 1992;56(4):641–655. doi: 10.1093/ajcn/56.4.641. [DOI] [PubMed] [Google Scholar]
- 103.Pasquet P., Brigant L., Froment A., Koppert G.A., Bard D., De Garine I., et al. Massive overfeeding and energy balance in men: the Guru Walla model. American Journal of Clinical Nutrition. 1992;56(3):483–490. doi: 10.1093/ajcn/56.3.483. [DOI] [PubMed] [Google Scholar]
- 104.McCracken K. vol. 34. Cambridge University Press; 1975. The effect of overfeeding on normal adult rats; pp. 15A–16A. (Proceedings of the nutrition society). [PubMed] [Google Scholar]
- 105.McCracken K., McNiven M. vol. 41. Cambridge University Press (CUP); 1982. Energy cost of deposition and maintenance of “obese tissue” in the adult rat; p. 31A. (Proceedings of the nutrition society). [Google Scholar]
- 106.McCracken K.J., McNiven M.A. Effects of overfeeding by gastric intubation on body composition of adult female rats and on heat production during feeding and fasting. British Journal of Nutrition. 1983;49(2):193–202. doi: 10.1079/bjn19830025. [DOI] [PubMed] [Google Scholar]
- 107.Sun K., Kusminski C.M., Scherer P.E. Adipose tissue remodeling and obesity. Journal of Clinical Investigation. 2011;121(6):2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jewell D.E., Drewry M.M., Martin R.J., Hausman G.J. Effect of sera from control and overfed rats on preadipocyte growth in culture. Journal of Nutrition. 1988;118(7):803–808. doi: 10.1093/jn/118.7.803. [DOI] [PubMed] [Google Scholar]
- 109.Mourot J., Guy G., Lagarrigue S., Peiniau P., Hermier D. Role of hepatic lipogenesis in the susceptibility to fatty liver in the goose (Anser anser) Comparative Biochemistry and Physiology – B Biochemistry and Molecular Biology. 2000;126(1):81–87. doi: 10.1016/S0305-0491(00)00171-1. [DOI] [PubMed] [Google Scholar]
- 110.Czajka-Narins D.M., Hirsch J. Supplementary feeding during the preweaning period. Effect on carcass composition and adipose tissue cellularity of the rat. Neonatology. 1974;25(3–4):176–185. doi: 10.1159/000240690. [DOI] [PubMed] [Google Scholar]
- 111.Nir I., Perek M. The effect of various protein levels in feed of goslings during the preparatory period on liver production and blood plasma components. Annales de Biologie Animale, Biochimie, Biophysique. 1971;11(4):645–656. [Google Scholar]
- 112.Deng Q.G., She H., Cheng J.H., French S.W., Koop D.R., Xiong S., et al. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology. 2005;42(4):905–914. doi: 10.1002/hep.20877. [DOI] [PubMed] [Google Scholar]
- 113.Gaemers I.C., Stallen J.M., Kunne C., Wallner C., van Werven J., Nederveen A., et al. Lipotoxicity and steatohepatitis in an overfed mouse model for non-alcoholic fatty liver disease. Biochimica et Biophysica Acta – Molecular Basis of Disease. 2011;1812(4):447–458. doi: 10.1016/j.bbadis.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 114.Ronis M.J.J., Baumgardner J.N., Marecki J.C., Hennings L., Wu X., Shankar K., et al. Dietary fat source alters hepatic gene expression profile and determines the type of liver pathology in rats overfed via total enteral nutrition. Physiological Genomics. 2012;44(22):1073–1089. doi: 10.1152/physiolgenomics.00069.2012. [DOI] [PubMed] [Google Scholar]
- 115.Harris R.B.S. Body composition and in vitro lipid metabolism of overfed hypophysectomized rats. International Journal of Obesity. 1989;13(5):647–660. [PubMed] [Google Scholar]
- 116.Shapira N., Nir I., Budowski P. Response of lipogenic enzymes to intensity and duration of overfeeding in the adult Japanese quail (Coturnix coturnix japonica) British Journal of Nutrition. 1978;39(2):289–295. doi: 10.1079/bjn19780038. [DOI] [PubMed] [Google Scholar]
- 117.Drewry M.M., Harris R.B.S., Martin R.J. Developmental changes in response to overfeeding: effect on composition of gain, liver metabolism and adipocyte cellularity in rats. Journal of Nutrition. 1988;118(2):194–198. doi: 10.1093/jn/118.2.194. [DOI] [PubMed] [Google Scholar]
- 118.Kasser T.R., Harris R.B.S., Martin R.J. Level of satiety: fatty acid and glucose metabolism in three brain sites associated with feeding. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 1985;17(4) doi: 10.1152/ajpregu.1985.248.4.r447. [DOI] [PubMed] [Google Scholar]
- 119.Hérault F., Houée-Bigot M., Baéza E., Bouchez O., Esquerré D., Klopp C., et al. RNA-seq analysis of hepatic gene expression of common Pekin, Muscovy, mule and hinny ducks fed ad libitum or overfed. BMC Genomics. 2019;20(1) doi: 10.1186/s12864-018-5415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang G., Jin L., Li Y., Tang Q., Hu S., Xu H., et al. Transcriptomic analysis between normal and high-intake feeding geese provides insight into adipose deposition and susceptibility to fatty liver in migratory birds. BMC Genomics. 2019;20(1) doi: 10.1186/s12864-019-5765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bax M.L., Chambon C., Marty-Gasset N., Remignon H., Fernandez X., Molette C. Proteomic profile evolution during steatosis development in ducks. Poultry Science. 2012;91(1):112–120. doi: 10.3382/ps.2011-01663. [DOI] [PubMed] [Google Scholar]
- 122.Lo B., Marty-Gasset N., Pichereaux C., Bravo C., Manse H., Domitile R., et al. Proteomic analysis of two weight classes of mule duck “foie gras” at the end of an overfeeding period. Frontiers in Physiology. 2020;11 doi: 10.3389/fphys.2020.569329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gong Y., Lyu W., Shi X., Zou X., Lu L., Yang H., et al. A serum metabolic profiling analysis during the formation of fatty liver in Landes geese via GC-TOF/MS. Frontiers in Physiology. 2020;11 doi: 10.3389/fphys.2020.581699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yu Y., Lyu W., Fu Z., Fan Q., Xiao Y., Ren Y., et al. Metabolic profiling analysis of liver in Landes geese during the formation of fatty liver via GC-TOF/MS. Frontiers in Physiology. 2022;12 doi: 10.3389/fphys.2021.783498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Farrell G., Schattenberg J.M., Leclercq I., Yeh M.M., Goldin R., Teoh N., et al. Mouse models of nonalcoholic steatohepatitis: toward optimization of their relevance to human nonalcoholic steatohepatitis. Hepatology. 2019:2241–2257. doi: 10.1002/hep.30333. [DOI] [PubMed] [Google Scholar]
- 126.Baumgardner J.N., Shankar K., Hennings L., Badger T.M., Ronis M.J.J. A new model for nonalcoholic steatohepatitis in the rat utilizing total enteral nutrition to overfeed a high-polyunsaturated fat diet. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2007;294(1) doi: 10.1152/ajpgi.00296.2007. [DOI] [PubMed] [Google Scholar]
- 127.Baumgardner J.N., Shankar K., Hennings L., Albano E., Badger T.M., Ronis M.J.J. N-acetylcysteine attenuates progression of liver pathology in a rat model of nonalcoholic steatohepatitis. Journal of Nutrition. 2008;138(10):1872–1879. doi: 10.1093/jn/138.10.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu J., Lai K.K.Y., Verlinsky A., Lugea A., French S.W., Cooper M.P., et al. Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK. Journal of Hepatology. 2011;55(3):673–682. doi: 10.1016/j.jhep.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Loomba R., Friedman S.L., Shulman G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184(10):2537–2564. doi: 10.1016/J.CELL.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hart B.L. Biological basis of the behavior of sick animals. Neuroscience & Biobehavioral Reviews. 1988;12(2):123–137. doi: 10.1016/S0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 131.Van Niekerk G., Isaacs A.W., Nell T., Engelbrecht A.M. Sickness-associated anorexia: mother nature's idea of immunonutrition? Mediators of Inflammation. 2016;2016 doi: 10.1155/2016/8071539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Murray M.J., Murray A.B. Anorexia of infection as a mechanism of host defense. American Journal of Clinical Nutrition. 1979;32(3):593–596. doi: 10.1093/ajcn/32.3.593. [DOI] [PubMed] [Google Scholar]
- 133.Hite J.L., Pfenning A.C., Cressler C.E. Starving the enemy? Feeding behavior shapes host-parasite interactions. Trends in Ecology & Evolution. 2020;35(1):68–80. doi: 10.1016/J.TREE.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 134.Alexander J.W., Gonce S.J., Miskell P.W., Peck M.D., Sax H. A new model for studying nutrition in peritonitis. The adverse effect of overfeeding. Annals of Surgery. 1989;209(3):334–340. doi: 10.1097/00000658-198903000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yamazaki K., Maiz A., Moldawer L.L., Bistrian B.R., Blackburn G.L. Complications associated with the overfeeding of infected animals. Journal of Surgical Research. 1986;40(2):152–158. doi: 10.1016/0022-4804(86)90117-4. [DOI] [PubMed] [Google Scholar]
- 136.Fall C.H.D., Kumaran K. Metabolic programming in early life in humans. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2019;374(1770) doi: 10.1098/RSTB.2018.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.West D.B., Diaz J., Woods S.C. Infant gastrostomy and chronic formula infusion as a technique to overfeed and accelerate weight gain of neonatal rats. Journal of Nutrition. 1982;112(7):1339–1343. doi: 10.1093/jn/112.7.1339. [DOI] [PubMed] [Google Scholar]
- 138.West D.B., Diaz J., Roddy S., Woods S.C. Long-term effects on adiposity after preweaning nutritional manipulations in the gastrostomy-reared rat. Journal of Nutrition. 1987;117(7):1259–1264. doi: 10.1093/jn/117.7.1259. [DOI] [PubMed] [Google Scholar]
- 139.Bouret S.G. Developmental programming of hypothalamic melanocortin circuits. Experimental & Molecular Medicine. 2022;54(4):403–413. doi: 10.1038/S12276-021-00625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Argente-Arizón P., Díaz F., Ros P., Barrios V., Tena-Sempere M., García-Segura L.M., et al. The hypothalamic inflammatory/gliosis response to neonatal overnutrition is sex and age dependent. Endocrinology. 2018;159(1):368–387. doi: 10.1210/EN.2017-00539. [DOI] [PubMed] [Google Scholar]
- 141.Fernandez-Twinn D.S., Constância M., Ozanne S.E. Intergenerational epigenetic inheritance in models of developmental programming of adult disease. Seminars in Cell & Developmental Biology. 2015:85–95. doi: 10.1016/j.semcdb.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shankar K., Harrell A., Liu X., Gilchrist J.M., Ronis M.J.J., Badger T.M. Maternal obesity at conception programs obesity in the offspring. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2008;294(2) doi: 10.1152/ajpregu.00316.2007. [DOI] [PubMed] [Google Scholar]
- 143.Ruebel M., Shankar K., Gaddy D., Lindsey F., Badger T., Andres A. Maternal obesity is associated with ovarian inflammation and upregulation of early growth response factor 1. American Journal of Physiology – Endocrinology and Metabolism. 2016;311(1):E269–E277. doi: 10.1152/ajpendo.00524.2015. [DOI] [PubMed] [Google Scholar]
- 144.Shankar K., Zhong Y., Kang P., Lau F., Blackburn M.L., Chen J.R., et al. Maternal obesity promotes a proinflammatory signature in rat uterus and blastocyst. Endocrinology. 2011;152(11):4158–4170. doi: 10.1210/en.2010-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Borengasser S.J., Zhong Y., Kang P., Lindsey F., Ronis M.J.J., Badger T.M., et al. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology. 2013;154(11):4113–4125. doi: 10.1210/en.2012-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Borengasser S.J., Kang P., Faske J., Gomez-Acevedo H., Blackburn M.L., Badger T.M., et al. High fat diet and in utero exposure to maternal obesity disrupts circadian rhythm and leads to metabolic programming of liver in rat offspring. PLoS One. 2014;9(1) doi: 10.1371/JOURNAL.PONE.0084209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ludwig D.S., Aronne L.J., Astrup A., De Cabo R., Cantley L.C., Friedman M.I., et al. The carbohydrate-insulin model: a physiological perspective on the obesity pandemic. The American Journal of Clinical Nutrition. 2021;114(6):1873–1885. doi: 10.1093/AJCN/NQAB270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hall K.D., Farooqi I.S., Friedman J.M., Klein S., Loos R.J.F., Mangelsdorf D.J., et al. The energy balance model of obesity: beyond calories in, calories out. The American Journal of Clinical Nutrition. 2022 doi: 10.1093/AJCN/NQAC031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Speakman J.R., Hall K.D. Carbohydrates, insulin, and obesity: insulin plays a role in body fat regulation independent of dietary carbohydrates. Science. 2021;372(6542):577–578. doi: 10.1126/science.aav0448. [DOI] [PubMed] [Google Scholar]
- 150.Shapira N., Nir I., Budowski P. Effect of glucose or oil supplementation on lipogenic enzymes in overfed chicks. Journal of Nutrition. 1978;108(3):490–496. doi: 10.1093/jn/108.3.490. [DOI] [PubMed] [Google Scholar]
- 151.Maher T., Clegg M.E. Dietary lipids with potential to affect satiety: mechanisms and evidence. Critical Reviews in Food Science and Nutrition. 2019;59(10):1619–1644. doi: 10.1080/10408398.2017.1423277. [DOI] [PubMed] [Google Scholar]
- 152.St-Onge M.P., Jones P.J.H. Physiological effects of medium-chain triglycerides: potential agents in the prevention of obesity. The Journal of Nutrition. 2002;132(3):329–332. doi: 10.1093/JN/132.3.329. [DOI] [PubMed] [Google Scholar]
- 153.Baba N., Bracco E.F., Hashim S.A. Enhanced thermogenesis and diminished deposition of fat in response to overfeeding with diet containing medium chain triglyceride. American Journal of Clinical Nutrition. 1982;35(4):678–682. doi: 10.1093/ajcn/35.4.678. [DOI] [PubMed] [Google Scholar]
- 154.Geliebter A., Torbay N., Bracco E.F., Hashim S.A., Van Itallie T.B. Overfeeding with medium chain triglyceride diet results in diminished deposition of fat. American Journal of Clinical Nutrition. 1983;37(1):1–4. doi: 10.1093/ajcn/37.1.1. [DOI] [PubMed] [Google Scholar]
- 155.Baba N., Bracco E.F., Hashim S.A. Role of brown adipose tissue in thermogenesis induced by overfeeding a diet containing medium chain triglyceride. Lipids. 1987;22(6):442–444. doi: 10.1007/BF02537276. [DOI] [PubMed] [Google Scholar]
- 156.Lackey D.E., Lazaro R.G., Li P., Johnson A., Hernandez-Carretero A., Weber N., et al. The role of dietary fat in obesity-induced insulin resistance. American Journal of Physiology – Endocrinology and Metabolism. 2016;311(6):E989–E997. doi: 10.1152/ajpendo.00323.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gallop M.R., Wilson V.C., Ferrante A.W. Post-oral sensing of fat increases food intake and attenuates body weight defense. Cell Reports. 2021;37(3) doi: 10.1016/j.celrep.2021.109845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hu S., Wang L., Yang D., Li L., Togo J., Wu Y., et al. Dietary fat, but not protein or carbohydrate, regulates energy intake and causes adiposity in mice. Cell Metabolism. 2018;28(3):415–431.e4. doi: 10.1016/j.cmet.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 159.Gulick A. A study of weight regulation in the adult human body during over-nutrition. American Journal of Physiology. 1922;60(2):371–395. doi: 10.1152/ajplegacy.1922.60.2.371. [DOI] [Google Scholar]
- 160.Sims E.A., Horton E.S. Endocrine and metabolic adaptation to obesity and starvation. The American Journal of Clinical Nutrition. 1968;21(12):1455–1470. doi: 10.1093/ajcn/21.12.1455. [DOI] [PubMed] [Google Scholar]
- 161.Johnston J.D., Ordovás J.M., Scheer F.A., Turek F.W. Circadian rhythms, metabolism, and chrononutrition in rodents and humans. Advances in Nutrition (Bethesda, Md.) 2016;7(2):399–406. doi: 10.3945/AN.115.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Speakman J.R., Elmquist J.K. Obesity: an evolutionary context. Life Metabolism. 2022 doi: 10.1093/lifemeta/loac002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.