Abstract
Objective:
To examine the association between depressive symptoms, leukocyte telomere length, a marker of cellular aging, and survival among lung cancer patients.
Design:
Patients with non-small cell lung cancer were recruited from a university-affiliated cancer center clinic.
Main Outcome:
Patients (N = 67) reported on depressive symptoms and provided a blood sample for leukocyte telomere length assessment at baseline and at a 3-month follow-up. Survival status was tracked over three years.
Results:
Age at diagnosis and depressive symptoms, as measured by the CES-D, were associated with shorter leukocyte telomere length (p < .05), although only age at diagnosis contributed statistical significance to the model. Depressive symptoms predicted shorter survival from date of diagnosis (p < .01). Patients who reported experiencing clinically meaningful levels of depressive symptoms (CES-D scores ≥ 16) demonstrated shorter survival than those who reported subclinical levels of depressive symptoms (p < .05). Leukocyte telomere length did not emerge as a predictor of shorter survival.
Conclusion:
Clinically meaningful levels of depressive symptoms are associated with shorter survival among lung cancer patients. These findings support the ongoing efforts to screen all cancer patients for low mood and to investigate mechanisms linking depressive symptoms and shorter survival in cancer contexts.
Keywords: lung cancer, survival, depression, leukocyte telomere length
Lung cancer grows rapidly, spreads quickly, and often remains undiagnosed until the disease has progressed to a later stage (Yang et al., 2005). Lung cancer represents 11.4% of all new cancer cases globally (Sung et al., 2021). While the overall 5-year survival rate of lung cancer is estimated at 18%, those diagnosed at a later stage have a survival rate of less than 5% (Henningfield & Adjei, 2017; Siegel, Miller, & Jemal, 2016), and 65.1% of lung cancers are diagnosed at a late (III or IV) versus early (I or II) stage (Siegel, Ma, Zou, & Jemal, 2014). Despite efforts to improve diagnostic and treatment methods, the survival rate for lung cancer has remained relatively unchanged for the past three decades (Siegel, Miller, & Jemal, 2018). Although immunotherapy, a newer, molecularly targeted treatment, has improved the median survival in a limited group of non-small cell lung cancer patients, these therapies, unfortunately, do not work effectively for everyone (Massarelli, Papadimitrakopoulou, Welsh, Tang, & Tsao, 2014; Mielgo-Rubio, Uribelarrea, Cortés, & Moyano, 2021), emphasizing the need to identify additional strategies that improve survival rates.
Lung cancer patients experience many stressors and disease-specific challenges following a cancer diagnosis and throughout treatment. Lung cancer patients report being among the most distressed (Carlson et al., 2004) and depressed of all cancer patients. Estimated at approximately 12.5% (Linden, Vodermaier, Mackenzie, & Greig, 2012), depression rates across cancer types exceed those among non-medically ill populations by four times (Lutgendorf & Andersen, 2015; Raison & Miller, 2003). Among lung cancer specifically, the prevalence of depression has been estimated between 11– 44% (Caruso et al., 2017; Hopwood, Stephens, & Party, 2000; Massie, 2004), which is among the highest when compared to other cancers (Linden et al., 2012).
Mounting evidence points to depression as a predictor of shorter survival in cancer (Ko, Kim, Son, Park, & Park, 2019; Spiegel & Giese-Davis, 2003; Walker et al., 2021; Y.-H. Wang et al., 2020). Not only do meta-analyses indicate depression is predictive of earlier mortality across a number of cancers (Satin, Linden, & Phillips, 2009), including lung (Pinquart & Duberstein, 2010; Walker et al., 2021; Y.-H. Wang et al., 2020), depressive symptoms were the most consistent psychological predictor of shortened survival compared to cancer-related distress, anxiety, mood, sense of control, and perception of physical health, even after controlling for demographic (e.g., age, gender) and medical (e.g., cancer site and stage, treatment status) factors (Brown, Levy, Rosberger, & Edgar, 2003). Thus, depressive symptomatology appears to be a strong prognostic indicator of survival in cancer compared to other psychopathologies, including anxiety (Walker et al., 2021), and distress more broadly (Brown et al., 2003). Some have identified depressive symptoms to be as important as traditional prognostic indicators of survival including stage at diagnosis and disease recurrence (Zimmaro et al., 2018).
Despite the growing evidence for depressive symptoms as a predictor of earlier mortality across several cancers, the mechanisms by which depressive symptoms lead to shorter survival remains unclear. Behavioral pathways, such as treatment noncompliance, have been examined as potential explanations for this relationship given that depression-related motivation deficits can inhibit patients from attending appointments. Indeed, depressed patients are more likely to experience breaks in treatment (DiMatteo, Lepper, & Croghan, 2000), which can lead to increased risk for earlier mortality (Fesinmeyer, Mehta, Blough, Tock, & Ramsey, 2010). However, a recent study among head and neck cancer patients identified biological, opposed to behavioral, pathways as a significant mediator of the depression-survival relationship (Zimmaro et al., 2018). In this study, although depressive symptoms were associated with increased treatment disruption, treatment disruption did not mediate the depression-survival relationship, whereas treatment response (a dichotomous biological indicator of treatment success) did.
Most of the work to date examining the biological pathways linking depression and shorter survival have focused on the role of the immune and the neuroendocrine systems (Antoni et al., 2006; McFarland et al., 2020; Sephton & Spiegel, 2003) and significantly fewer studies have examined the role of telomeres. Telomere length is a biomarker of cellular aging, and has been linked to both depression and cancer independently, both of which have been posited as diseases of premature aging (Manoliu, Bosch, Brakowski, Brühl, & Seifritz, 2018); however the two have infrequently been examined simultaneously. Telomeres are DNA-based protein structures capping the ends of chromosomes that serve to maintain cellular integrity and stability (Blackburn, Epel, & Lin, 2015). Upon each cell division, telomeric DNA is lost, resulting in cumulative shortening of telomeres over time; a process that determines how fast cells age and when they die (Rivera-Tavarez, 2017). Thus, the slow, cumulative shortening of telomeres over the course of the lifespan is consistent with healthy aging; however, the loss of LTL can be accelerated by chronic stress (Epel et al., 2004) and depression (Vance et al., 2018) leading to an older biological than chronological age (Blackburn et al., 2015). Importantly, shorter peripheral LTL is a predictor of all-cause mortality in the general population (Needham et al., 2015; Rode, Nordestgaard, & Bojesen, 2015; Q. Wang, Zhan, Pedersen, Fang, & Hägg, 2018) and is associated with increased risk for tumorigenesis (Ma et al., 2011; Shao et al., 2007; Wentzensen, Mirabello, Pfeiffer, & Savage, 2011; Willeit et al., 2010; Wu et al., 2003), earlier mortality (Wentzensen et al., 2011; Willeit et al., 2010) and shorter survival (Callahan et al., 2017; Doherty et al., 2018; Renner, Krenn-Pilko, Gruber, Herrmann, & Langsenlehner, 2018; Valls, Piñol, Reñé, Buenestado, & Viñas, 2011; Weischer et al., 2013; Zhang et al., 2015) among patients with varying cancer types. Notably, lung cancer is significantly underrepresented comparatively in these samples. A growing number of meta-analyses and reviews have demonstrated a relationship between shorter telomeres and depression (Ridout, Ridout, Price, Sen, & Tyrka, 2016; Schutte & Malouff, 2015), pointing to telomeres as a potential pathway between depression and shorter survival in cancer.
To our knowledge, the relationship between depression, LTL, and survival has yet to be examined among lung cancer patients and has been examined in only one other study among bladder cancer patients (Lin et al., 2015). In this study, depressive symptoms and shorter LTL both independently and jointly predicted shorter survival, such that patients with both clinically meaningful levels of depression and shorter LTL had a four-times greater risk for mortality and shorter disease-free survival. This finding shows LTL accounted for some of the variance in the depression-survival relationship, although no association between depressive symptoms and LTL was observed (Lin et al., 2015). Only one other study, conducted by Sharpley and colleagues (2018), has examined the relationship between depressive symptoms and LTL among cancer patients, although this study did not examine survival. Taken together, despite evidence for depressive symptoms and telomere biology playing a role in tumorigenesis and contributing to poorer prognosis, and that they are associated with one another, there is paucity of investigations into these relationships. As such, it is plausible to hypothesize LTL is a pathway between depression and shorter survival among lung cancer patients.
To address this gap, the current investigation explored the association between depressive symptoms and LTL, and their prognostic value in the context of lung cancer. We hypothesized 1) greater (more severe) depressive symptoms would be associated with shorter LTL; 2) depressive symptoms would predict shorter survival from date of diagnosis and date of study entry; 3) shorter LTL would predict shorter survival from date of diagnosis and date of study entry; and 4) shorter LTL will mediate the association between greater (more severe) depressive symptoms and shorter survival calculated from date of diagnosis and study entry (see Figure 1).
Figure 1.
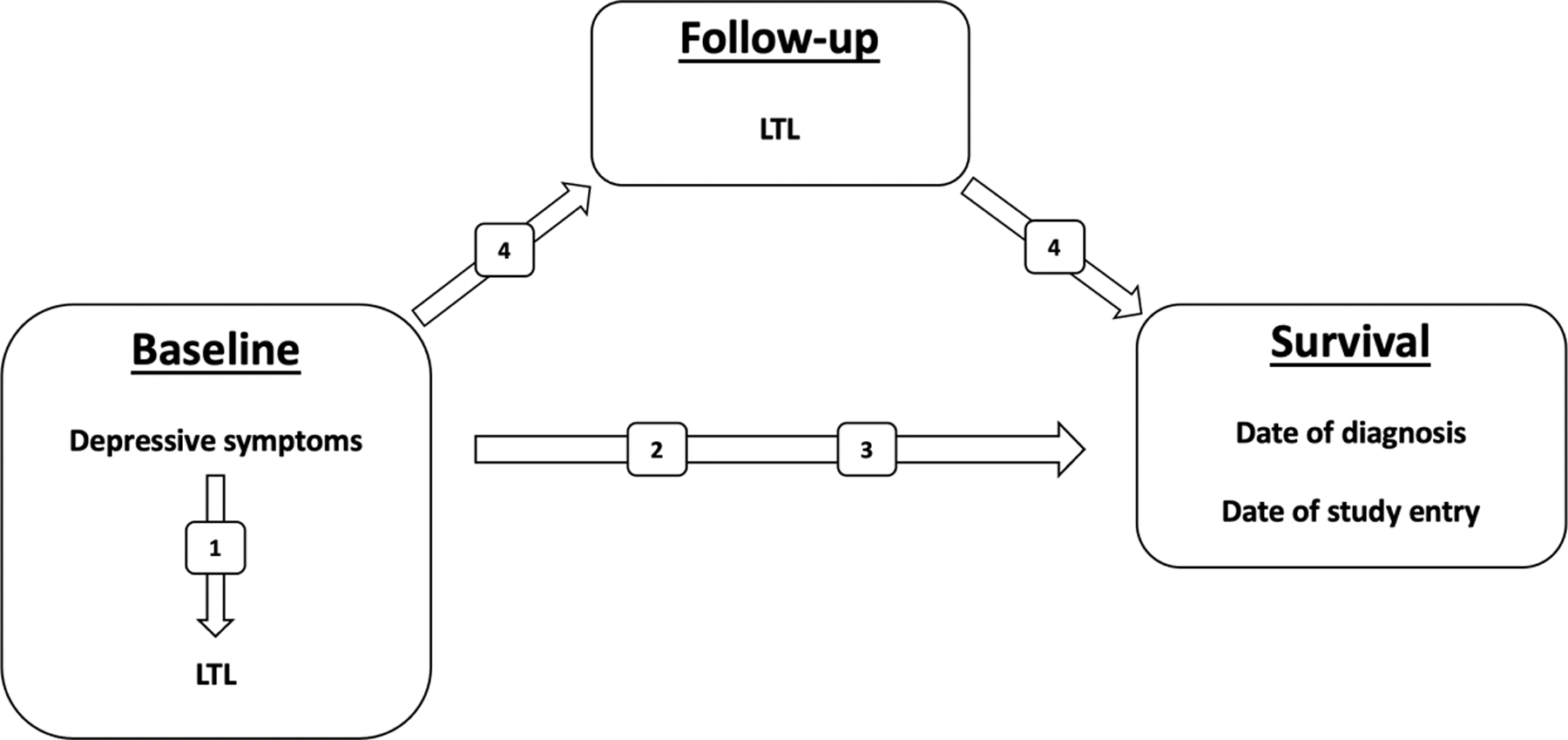
The theoretical study model and hypotheses, which are denoted by the numbers inside the boxes over the arrows. We hypothesized shorter leukocyte telomere length (LTL) would partially mediate the relationship between greater depressive symptoms and shorter survival.
Materials and Methods
Participants
Patients diagnosed with non-small cell lung cancer were recruited from a university-affiliated cancer center clinic as part of a larger study investigating the prognostic significance of circadian disruption and the preliminary feasibility of a digital mindfulness-based stress reduction intervention (IRB 13.0508). Eligible patients were diagnosed with non-small cell lung cancer within five years of study entry, were ages 18–85, resided within a 120-mile radius of the cancer center, had no medical diagnosis likely to influence six-month survival, no immune-compromising condition, and no recent history of psychiatric hospitalization or substance abuse.
The sample (N = 67) can be described as mostly female, white, aged 50 – 60 with a high school education, and an annual household income of less than $40,000. On average, patients were 60 years old at the time of diagnosis, which they received approximately 2 years (M = 22.6 months, SD = 17.82) prior to study entry. Consistent with the larger lung cancer population, 65% of which are diagnosed at a later stage (Siegel et al., 2014), most patients in the current sample also received an initial diagnosis of stage III or IV (65.7%). Patients diagnosed at a later stage have a poorer prognosis, and as such, several study participants in the current sample have since died (n = 31, 46.3%). Of those individuals, 83.9% were initially diagnosed with a later stage (III or IV). Medical and demographic information is summarized in Table 1.
Table 1.
Demographic and clinical characteristics of the study sample.
| Variable | Frequency (n) | Percent (%) |
|---|---|---|
| Total | 65 | 100 |
| Sex | ||
| Male | 21 | 31.3 |
| Female | 41 | 61.2 |
| * | 5 | 7.5 |
| Race/Ethnicity | ||
| Non-Hispanic White | 39 | 58.2 |
| Black/African American | 10 | 14.9 |
| Hispanic or Latino | 1 | 1.5 |
| Asian/Asian American | 1 | 1.5 |
| Other | 1 | 1.5 |
| * | 15 | 22.4 |
| Annual Household Income | ||
| ≥ $15,000 – 39,999 | 36 | 53.7 |
| $40,000 – 79,999 | 15 | 22.4 |
| $80,000 – 149,999 | 7 | 10.5 |
| $150,000 – 249,999 | 1 | 1.5 |
| * | 8 | 11.9 |
| Disease Stage at Diagnosis | ||
| I | 15 | 22.4 |
| II | 8 | 11.9 |
| III | 28 | 41.8 |
| IV | 16 | 23.9 |
| Early (I, II) | 23 | 34.3 |
| Late (III, IV) | 44 | 65.7 |
| Chemotherapy at study entry | ||
| Yes | 21 | 31.3 |
| No | 38 | 56.7 |
| * | 8 | 11.9 |
| Radiation at study entry | ||
| Yes | 3 | 4.5 |
| No | 57 | 85.1 |
| * | 7 | 10.4 |
| Chemotherapy ever | ||
| Yes | 48 | 71.6 |
| No | 12 | 17.9 |
| * | 7 | 10.4 |
| Radiation ever | ||
| Yes | 38 | 56.7 |
| No | 22 | 32.8 |
| * | 7 | 10.4 |
Note.
denotes missing data
Procedures
Study personnel confirmed eligibility criteria via patient medical records and alerted collaborating physicians to introduce patients to the study during their clinic appointment. Study personnel provided an overview of the study, answered questions, and obtained informed consent from patients. Participants received instructions and materials (e.g., packet of questionnaires) for at-home baseline data collection. Prior to leaving the clinic, blood was drawn by a phlebotomist for LTL. Participants attended a three-month follow-up during a scheduled appointment at the cancer center clinic. Following the same procedures as baseline data collection, patients were given materials for at-home data collection, which included the same packet of questionnaires. A second blood draw was conducted prior to leaving the clinic. Follow-up data collection materials were collected, and compensation was provided. All study procedures were conducted in accordance with University Human Subjects Protection Program guidelines.
Measures
Depressive symptoms.
Participants completed the Center for Epidemiological Studies – Depression (CES-D; Radloff (1977) at baseline and follow-up. The CES-D is composed of 20 items that assess depressive symptoms over the past week on a four-point scale ranging from “rarely or none of the time (less than one day)” and “most of all of the time (5–7 days).” The total score is the sum of the 16 depressive symptom items and the reverse score of the four positive affect items. Scores greater than 16 indicate risk for clinical depression. The CES-D is a commonly used measure of depressive symptoms among medically ill populations, including cancer. Among breast cancer patients, the CES-D has demonstrated high internal consistency (Cronbach α = 0.85), adequate test-retest reliability, and construct validity (Hann, Winter, & Jacobsen, 1999). In the present study, internal consistency was acceptable and good at baseline and follow-up (Cronbach α = .77; .83), respectively.
Leukocyte telomere length (LTL).
Blood samples were collected in K2 EDTA purple top tubes and were kept on ice until they were transferred to the laboratory where samples were centrifuged at 1300 RCF for 10 minutes at 4°C. Plasma and the remaining cell pellet (red blood cell + white blood cells) were aliquoted into separate microcentrifuge tubes that were kept on ice. All tubes were frozen at −80°C within two hours of draw. The average relative LTL was measured in PBMCs by quantitative PCR using a method adapted from the original published method (Cawthon, 2002; Lin et al., 2010). Detailed descriptions of the telomere length assay used in the current study are outlined by Lin and colleagues (2016). Telomere length was expressed as the ratio of telomere abundance versus a single copy gene (human β-globin) as T/S ratios. The average coefficient of variation (CV) was 2.4%.
Survival.
Survival status was tracked for approximately 3 years. A categorical variable (yes/no) indicating whether the patient had died (all-cause mortality) as of the date of tracking cessation (e.g., April 20, 2020) was created. The amount of time (in days) from date of study entry to date of death was calculated for patients who had died. For living patients, this tracking variable reflects the number of days from study entry to the date of cessation of tracking. Survival or tracking time (in days) was also calculated from each participant’s date of diagnosis to either date of death or the date of tracking cessation.
Statistical Analysis
Data preprocessing.
Data preparation and analyses were conducted using SPSS v27.0 (IBM, Armonk, NY). Data was entered by two research assistants separately in independent datasets and subsequently compared to ensure accuracy. Summary scores were calculated for the CES-D assessed at baseline and follow-up. For participants who responded to more than 50% of the CES-D, missing data points were replaced using the mean of their responses. If a participant missed more than half of the questionnaire, missing data was not replaced.
Preliminary analysis.
Data were explored and tested for assumptions of parametric data. Outliers, defined as scores > 4 standard deviations from the mean, were assessed for removal prior to analysis. Skewness and kurtosis statistics were calculated for continuous variables by dividing the statistic by the standard error. Those that failed to meet assumptions by visual inspection or by z-skewness values outside of the 95% confidence interval (values > 1.96) were assessed for transformation. Variables were transformed according to the parameters provided by Field (2013). All transformed variables were re-assessed for assumptions prior to analysis.
Analytic procedures followed a data driven approach and recommendations outlined by Kraemer and colleagues (2001). Prior to analysis, all continuous independent and control variables were median centered and binary variables were effects coded (i.e., −1/2, +1/2; Kraemer and Blasey (2004). Theoretically derived covariates were identified a priori (i.e., age at diagnosis, sex, disease stage, treatment, and smoking history), and Spearman’s rank-order correlations were conducted to identify the strength of the relationship between each covariate and dependent variable (LTL and survival). These traditional prognostic indicators variables were selected and evaluated for potential influence on LTL and survival. Covariates were included in the model if the relationship was r ≥ 0.3 to ensure contribution. Because the parent study included an optional, pilot intervention, it was also tested as a covariate in all models with a longitudinal dependent variable (i.e., survival). Due to the strong prognostic nature of disease stage, it was also included as a covariate in survival analyses.
Primary analysis.
To test the association between depressive symptoms and LTL assessed at baseline (hypothesis 1), a multiple regression was conducted. An ANCOVA was conducted to assess differences in LTL between those with scores above versus below the clinical cutoff on the CESD (scores ≥ 16) after adjusting for age at diagnosis. To test the prognostic significance of depressive symptoms assessed at baseline (hypothesis 2), two separate two-tailed Cox Proportional Hazards regressions were conducted with survival, one calculated from the date of study entry and one from the date of diagnosis. Two additional two-tailed Cox Proportional Hazards regressions were conducted to compare survival time from date of study entry and date of diagnosis between those with scores above and below the clinical cutoff on the CESD. To test the prognostic significance of LTL assessed at baseline (hypothesis 3), two separate two-tailed Cox Proportional Hazards regressions were conducted with survival calculated from the date of study entry and the date of diagnosis. Consistent with the definition of mediation (Kraemer, Kiernan, Essex, & Kupfer, 2008), and therefore, pending the result of tests for hypotheses 1 – 3, to test if LTL mediates depressive symptoms and survival (hypothesis 4), a multiple regression was planned, adjusting for history of chemotherapy, to test the association between depressive symptoms assessed at baseline and LTL assessed at follow-up. A two-tailed Cox Proportional Hazards regression was also planned to test the prognostic significance of LTL assessed at follow-up and survival from date of diagnosis and date of study entry.
Based on the effect sizes reported previously (Sephton et al., 2013), a power estimate of 66% was predicted for this sample. Further, a similar study involving fewer lung cancer patients (N = 43) with a greater survival rate (70%) reported depression predicted 6-month survival with a medium-to-large odds ratio of 5.30 (Pirl et al., 2008), suggesting the current larger sample with a 50% survival rate should attain adequate power.
Results
The results from the Spearman’s rank-order correlations testing the association between theoretically derived a priori covariates and outcome variables are summarized in Table 2. Older age at diagnosis and greater depressive symptoms were significantly associated with shorter log-transformed LTL, F(2, 54) = 3.197, p < .05, R2 = .106; however, only age at diagnosis, not depressive symptoms, contributed statistical significance to the model. Similarly, after splitting the sample between sub- and clinical levels of depression, depression was not significantly associated with LTL.
Table 2.
Spearman’s rank-order correlations between theoretically derived covariates and dependent variables.
| Correlations |
||||
|---|---|---|---|---|
| 7 | 8 | 9 | 10 | |
| 1. Age at diagnosis | −.312* | −.187 | −.056 | −.121 |
| 2. Sex | .136 | .057 | .229 | .192 |
| 3. Disease stage | .117 | .237 | −.252 | −.262 |
| 4. Chemotherapy | .246 | .330* | .149 | .109 |
| 5. Radiation | .062 | .141 | −.132 | .091 |
| 6. Smoking history | −.258 | −.233 | −.265 | −.069 |
| 7. TL at baseline | 1 | - | - | - |
| 8. TL at follow-up | - | 1 | - | - |
| 9. Survival from study entry | - | - | 1 | - |
| 10. Survival from date of diagnosis | - | - | - | 1 |
Note.
r ≥ 0.3
After adjusting for the non-randomized pilot intervention associated with the parent study and disease stage, both models predicting survival from date of diagnosis and date of study entry emerged as significant (p < .01); however, depressive symptoms only contributed statistical significance to the model predicting survival from date of diagnosis (Cox Proportional Hazards two-tailed p(Wald) = 5.201; hazard ratio = 1.046; 95% confidence interval (CI) = 1.006 – 1.087), not from study entry. After adjusting for the non-randomized pilot intervention associated with the parent study and disease stage, both models predicting survival from date of diagnosis and date of study entry emerged as significant (p < .01). Length of survival differed significantly between participants who reported clinically meaningful symptoms of depression (CES-D scores ≥ 16) and participants who reported non-clinical levels of depression both date of diagnosis (Cox Proportional Hazards two-tailed p(Wald) = 5.238; hazard ratio = .406; 95% confidence interval (CI) = .188 – .879) and date of study entry (Cox Proportional Hazards two-tailed p(Wald) = 5.718; hazard ratio = .377; 95% confidence interval (CI) = .169 – .839). As demonstrated in Figures 2 and 3, respectively, cancer patients who reported clinically meaningful levels of depressive symptoms demonstrated shorter survival from both date of diagnosis and date of study entry. Results from Spearman’s rank-order correlations revealed a weak and statistically insignificant association between disease stage and depressive symptoms measured continuously (r = −.006) and split by clinically meaningful scores (r = .002).
Figure 2.
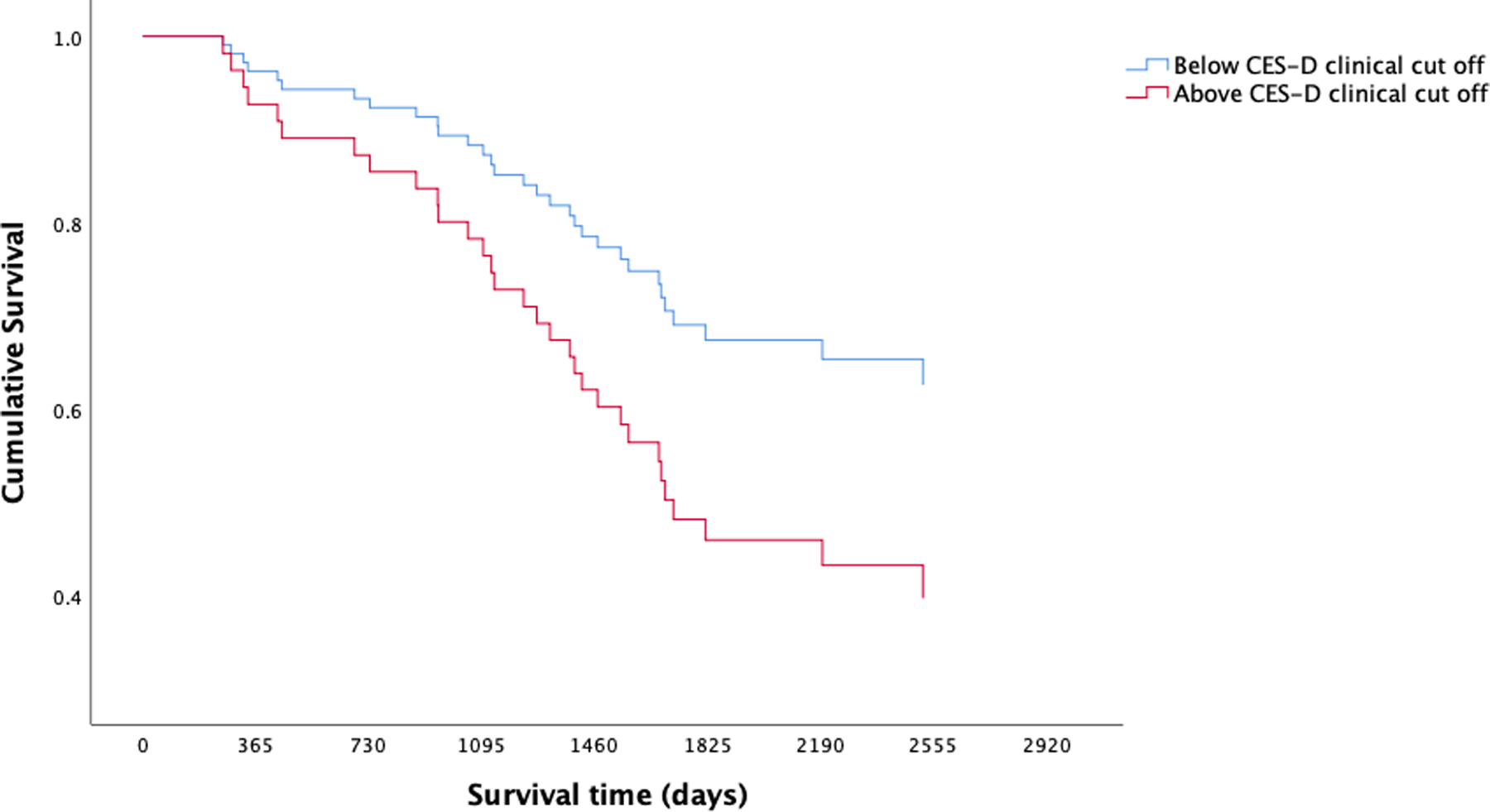
Kaplan-Meier survival curves from date of diagnosis for lung cancer patients split by clinically meaningful (CES-D scores ≥ 16) and sub-clinical levels of depressive symptoms. Cox Proportional Hazards models demonstrated clinically meaningful levels of depression were prognostic for lung cancer survival.
Figure 3.
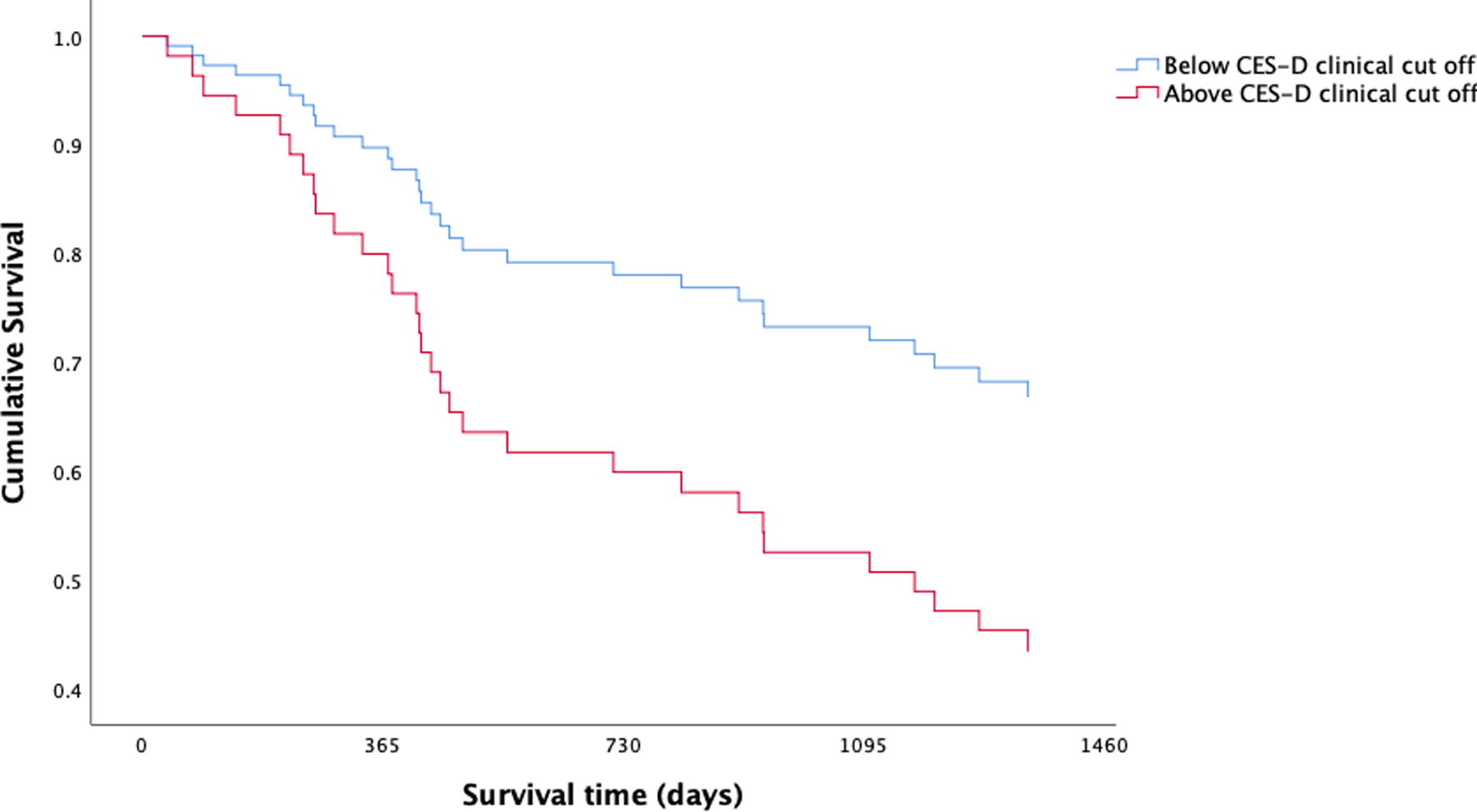
Kaplan-Meier survival curves from date of study entry for lung cancer patients split by clinically meaningful (CES-D scores ≥ 16) and sub-clinical levels of depressive symptoms. Cox Proportional Hazards models demonstrated clinically meaningful levels of depression were prognostic for lung cancer survival.
After adjusting for the non-randomized pilot intervention associated with the parent study and disease stage, although the models emerged as significant, LTL assessed at baseline and at 3-month follow-up did not predict shorter survival from date of diagnosis or date of study entry. Similarly, depressive symptoms assessed at baseline did not predict LTL at the 3-month follow-up. As such, LTL was not tested as a mediator between depressive symptoms and survival.
Discussion
Findings from the current investigation expand on those reported by Lin and colleagues (2015) among bladder cancer patients, such that lung cancer patients who reported clinical levels of depression (CES-D scores ≥ 16) exhibited shorter survival. Moreover, depressive symptoms predicted shorter survival from date of diagnosis, but not from date of study entry. This discrepancy is not surprising given that study entry was an arbitrary point in the current sample’s cancer trajectory, whereas time from date of diagnosis is more theoretically meaningful for length of survival. Importantly, given the weak association between disease stage and depressive symptoms, the predictive nature of depressive symptoms is likely not explained by extent of disease at diagnosis. Said otherwise, patients with greater disease growth were not significantly more depressed in the current sample.
These findings highlight the need to consider severity, specifically clinically meaningful levels of depressive symptomatology, when examining the association between depression and survival in cancer populations. These findings are, clearly, of great clinical importance and support the need for on-going efforts to routinely screen all cancer patients for indicators of low mood. Reports of sub- and clinical levels of depression should be taken seriously in cancer contexts. While the obvious focus of treatment among this population is oncologic, depression is a treatable and manageable condition that is not synonymous with the emotions that can and tend to accompany a cancer diagnosis and treatment (e.g., grief, sadness). Psychological intervention may not only be clinically warranted, but also key to achieving desired medical outcomes. Patients reporting subclinical levels of depression, despite exhibiting lower risk of earlier mortality, warrant as much clinical attention, as there may be an opportunity to prevent worsening of symptoms. These findings further motivate efforts to adapt efficacious interventions for cancer patient populations as well as elucidate mechanisms driving the association between depression and shorter survival.
Contrary to our hypotheses, depressive symptoms were not significantly associated with LTL. Null results may be explained by several factors, including the timing of the assessment of LTL relative to the assessment of depressive symptoms and the tumor itself. While the timeline between onset of depressive symptoms and observable telomere attrition is, to our knowledge, currently unknown, it is plausible the deleterious effects of depression on telomere biology exceeds three months. Although cell division, which leads to cumulative telomere attrition, occurs relatively quickly (within one day), observable changes in telomere length may require greater time. Additionally, it is plausible the tumor itself affected systemic LTL over the 3-month follow-up period. Future investigations should not only assess severity of depression, but also the timing of onset of symptoms to clarify the temporal nature of this relationship, and how solid tumors affect systemic LTL.
Contrary to our hypotheses, LTL did not predict shorter survival. While this was unexpected, evidence for the complexity of telomere biology within individuals with cancer is growing. Although telomere attrition has been linked with cancer incidence and earlier mortality (Willeit et al., 2010), recent studies suggest this relationship is likely tissue, disease, treatment, and timing specific (Gallicchio, Gadalla, Murphy, & Simonds, 2018). For example, Sanchez-Espiridin and colleagues (2014) found lung cancer patients with adenocarcinoma had longer peripheral LTL than controls, whereas patients with squamous cell carcinoma had shorter LTL, and Seow and colleagues (2014) reported longer LTL was associated with greater risk for lung cancer. Moreover, Doherty and colleagues (2018) found LTL predicted earlier mortality among small cell lung cancer patients, but in this sample, LTL was measured an average of 6 years prior to diagnosis. In the current sample, LTL was collected after a diagnosis of non-small cell lung cancer (on average, ~2 years post diagnosis), and 24 participants had active tumors at the time of study entry. Taken together, our current understanding of telomere biology in cancer is at an early stage and many questions remain unanswered. Future investigations into the prognostic nature and clinical utility of LTL as a marker in lung cancer should consider all these factors.
Limitations and Future Directions
Limitations to the current investigation should be noted. First, the medical characteristics of the sample are heterogeneous, potentially making associations with LTL, a biomarker, difficult to detect. Participants were diagnosed with cancer within 5 years of starting the study and some were actively receiving treatment (chemotherapy: n = 24; radiation: n = 3) at baseline, introducing considerable variability and increasing the difficulty in detecting associations. Second, the sample was limited in size and demographic variability, as it can be described as primarily white. Future investigations should either narrow inclusion criteria to reduce medical heterogeneity or increase power to examine nuanced associations between medical characteristics and LTL while increasing inclusion of patients that identify as black, indigenous, and as people of color (BIPOC). Given that LTL is a systemic, opposed to localized, marker of cellular aging, and experiences of discrimination have been linked to shorter LTL (Lee, Kim, & Neblett, 2017), future studies with larger samples and greater demographic heterogeneity should also account for the potential influence of discrimination on LTL, particularly when examining its relationship to depressive symptoms.
Additional gaps warrant greater attention, namely the role of telomerase, an enzyme that maintains and lengthens telomeres (Blackburn et al., 2015) as well as in depth assessments of depression, including severity, history, features, and subtypes, as relates to LTL and survival. The role of telomerase in cancer environments is complex due to its regulatory properties of genomic stability and cellular replication, yet it may be key to understanding telomere attrition systemically given that peripheral levels fluctuate following treatment in some cancers (Ganesh, Narayanan, & Kumar, 2020). Further investigation into the longitudinal change in peripheral LTL as relates to telomerase, treatment, and prognostic indicators would increase the utility of LTL as a clinical biomarker in cancer. Lastly, clarifying the timeline between onset of depressive symptoms and observable telomere attrition, as well as the features of depression (e.g., severity, subtypes) are important avenues for future investigation.
Conclusion
LTL has been linked to both depression and cancer outcomes independently; however, this study is the first to investigate the relationships between depression, LTL, and survival simultaneously in a lung cancer sample. Despite the attempt, the underlying biological mechanisms associated with the prognostic significance of depression in cancer remain unanswered and an important area of future inquiry. Nonetheless, the current investigation provided some important clues. First, the clinical significance, or severity, of depression emerged as an important factor in the depression-survival relationship. Second, the deleterious effects of depressive symptoms on LTL observed in prior studies may not emerge within 3 months. Little is known about the longitudinal changes in LTL across the cancer trajectory and how it is dynamically influenced by telomerase, tumorigenesis, and psychological factors, such as worsening depression, but results from the current investigation further support the importance of elucidating telomere biology in cancer contexts.
Acknowledgements
The authors wish to thank all institutions and patients who participated in this research.
Funding
This study was supported by the Kentucky Lung Cancer Research Program (PI, Sephton, S.E.). Dr. Chelsea Siwik was supported by a National Center for Complimentary and Integrative Health T32 Fellowship (5T32AT003997–13) at the University of California, San Francisco.
Footnotes
Disclosure of Interest
The authors report no conflict of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, S.E.S., upon reasonable request.
References
- Antoni Lutgendorf, S. K., Cole SW, Dhabhar FS, Sephton SE, McDonald PG, . . . Sood AK (2006). The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nature Reviews Cancer, 6(3), 240–248. doi: 10.1038/nrc1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn EH, Epel ES, & Lin J (2015). Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science, 350(6265), 1193–1198. [DOI] [PubMed] [Google Scholar]
- Brown KW, Levy AR, Rosberger Z, & Edgar L (2003). Psychological distress and cancer survival: A Follow‐Up 10 years after diagnosis. Psychosomatic Medicine, 65(4), 636–643. [DOI] [PubMed] [Google Scholar]
- Callahan CL, Schwartz K, Ruterbusch JJ, Shuch B, Graubard BI, Lan Q, . . . Rothman N (2017). Leukocyte telomere length and renal cell carcinoma survival in two studies. British Journal of Cancer, 117(5), 752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson Angen, M., Cullum J, Goodey E, Koopmans J, Lamont L, . . . Robinson J (2004). High levels of untreated distress and fatigue in cancer patients. British Journal of Cancer, 90(12), 2297–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso R, Nanni MG, Riba M, Sabato S, Mitchell A, Croce E, & Grassi L (2017). Depressive spectrum disorders in cancer: prevalence, risk factors and screening for depression: a critical review. Acta Oncologica, 56(2), 146–155. [DOI] [PubMed] [Google Scholar]
- Cawthon RM (2002). Telomere measurement by quantitative PCR. Nucleic acids research, 30(10), e47–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMatteo MR, Lepper HS, & Croghan TW (2000). Depression Is a Risk Factor for Noncompliance With Medical Treatment. Arch Intern Med, 160(14), 2101. doi: 10.1001/archinte.160.14.2101 [DOI] [PubMed] [Google Scholar]
- Doherty JA, Grieshober L, Houck JR, Barnett MJ, Tapsoba JDD, Thornquist M, . . . Chen C (2018). Telomere length and lung cancer mortality among heavy smokers. Cancer Epidemiology and Prevention Biomarkers, 27(7), 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel Blackburn, E. H., Lin J, Dhabhar FS, Adler NE, Morrow JD, & Cawthon RM (2004). Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the United States of America, 101(49), 17312–17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesinmeyer MD, Mehta V, Blough D, Tock L, & Ramsey SD (2010). Effect of radiotherapy interruptions on survival in medicare enrollees with local and regional head-and-neck cancer. Int J Radiat Oncol Biol Phys, 78(3), 675–681. doi: 10.1016/j.ijrobp.2009.08.004 [DOI] [PubMed] [Google Scholar]
- Field A (2013). Discovering statistics using IBM SPSS statistics: sage. [Google Scholar]
- Gallicchio L, Gadalla SM, Murphy JD, & Simonds NI (2018). The effect of cancer treatments on telomere length: a systematic review of the literature. JNCI: Journal of the National Cancer Institute, 110(10), 1048–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh M, Narayanan GS, & Kumar R (2020). Change of telomerase activity in peripheral blood of patients with head and neck squamous cell carcinoma pre and post curative treatment. Reports of Practical Oncology and Radiotherapy, 25(1), 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann D, Winter K, & Jacobsen P (1999). Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D). Journal of Psychosomatic Research, 46(5), 437–443. [DOI] [PubMed] [Google Scholar]
- Henningfield MF, & Adjei AA (2017). Lung Cancer Awareness Month—A Lot of Progress, But More Work Needs to Be Done In: Elsevier. [DOI] [PubMed] [Google Scholar]
- Hopwood P, Stephens RJ, & Party BMRCLCW (2000). Depression in patients with lung cancer: prevalence and risk factors derived from quality-of-life data. Journal of Clinical Oncology, 18(4), 893–893. [DOI] [PubMed] [Google Scholar]
- Ko A, Kim K, Son JS, Park HY, & Park SM (2019). Association of pre-existing depression with all-cause, cancer-related, and noncancer-related mortality among 5-year cancer survivors: a population-based cohort study. Scientific reports, 9(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer, & Blasey CM (2004). Centring in regression analyses: a strategy to prevent errors in statistical inference. International Journal of Methods in Psychiatric Research, 13(3), 141–151. doi:DOI 10.1002/mpr.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer Kiernan, M., Essex M, & Kupfer DJ (2008). How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychology, 27(2S), S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer Stice, E., Kazdin A, Offord D, & Kupfer D (2001). How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. American journal of psychiatry, 158(6), 848–856. [DOI] [PubMed] [Google Scholar]
- Lee DB, Kim ES, & Neblett EW (2017). The link between discrimination and telomere length in African American adults. Health Psychology, 36(5), 458. [DOI] [PubMed] [Google Scholar]
- Lin Blalock, J. A., Chen M, Ye Y, Gu J, Cohen L, . . . Wu X (2015). Depressive symptoms and short telomere length are associated with increased mortality in bladder cancer patients. Cancer Epidemiology and Prevention Biomarkers, 24(2), 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Epel, E., Cheon J, Kroenke C, Sinclair E, Bigos M, . . . Blackburn E (2010). Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. Journal of immunological methods, 352(1–2), 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Blalock JA, Chen M, Ye Y, Gu J, Cohen L, . . . Wu X (2015). Depressive symptoms and short telomere length are associated with increased mortality in bladder cancer patients. Cancer Epidemiology and Prevention Biomarkers, 24(2), 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Cheon J, Brown R, Coccia M, Puterman E, Aschbacher K, . . . Blackburn EH (2016). Systematic and cell type-specific telomere length changes in subsets of lymphocytes. Journal of immunology research, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden W, Vodermaier A, Mackenzie R, & Greig D (2012). Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord, 141(2–3), 343–351. doi: 10.1016/j.jad.2012.03.025 [DOI] [PubMed] [Google Scholar]
- Lutgendorf, & Andersen. (2015). Biobehavioral approaches to cancer progression and survival: Mechanisms and interventions. American psychologist, 70(2), 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Zhou Z, Wei S, Liu Z, Pooley KA, Dunning AM, . . . Shen M (2011). Shortened telomere length is associated with increased risk of cancer: a meta-analysis. PLoS ONE, 6(6), e20466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A, Bosch OG, Brakowski J, Brühl AB, & Seifritz E (2018). The potential impact of biochemical mediators on telomere attrition in major depressive disorder and implications for future study designs: A narrative review. Journal of Affective Disorders, 225, 630–646. [DOI] [PubMed] [Google Scholar]
- Massarelli E, Papadimitrakopoulou V, Welsh J, Tang C, & Tsao AS (2014). Immunotherapy in lung cancer. Translational lung cancer research, 3(1), 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie MJ (2004). Prevalence of Depression in Patients With Cancer. Journal of the National Cancer Institute Monographs, 2004(32), 57–71. doi: 10.1093/jncimonographs/lgh014 [DOI] [PubMed] [Google Scholar]
- McFarland DC, Saracino RM, Miller AH, Breitbart W, Rosenfeld B, & Nelson C (2020). Prognostic implications of depression and inflammation in patients with metastatic lung cancer. Future Oncology(0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielgo-Rubio X, Uribelarrea EA, Cortés LQ, & Moyano MS (2021). Immunotherapy in non-small cell lung cancer: Update and new insights. Journal of Clinical and Translational Research, 7(1), 1. [PMC free article] [PubMed] [Google Scholar]
- Needham BL, Rehkopf D, Adler N, Gregorich S, Lin J, Blackburn EH, & Epel ES (2015). Leukocyte telomere length and mortality in the National Health and Nutrition Examination Survey, 1999–2002. Epidemiology (Cambridge, Mass.), 26(4), 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart, & Duberstein. (2010). Depression and cancer mortality: a meta-analysis. Psychol Med, 40(11), 1797–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirl Temel, J. S., Billings A, Dahlin C, Jackson V, Prigerson HG, . . . Lynch TJ (2008). Depression after diagnosis of advanced non-small cell lung cancer and survival: a pilot study. Psychosomatics, 49(3), 218–224. [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement, 1(3), 385–401. [Google Scholar]
- Raison CL, & Miller AH (2003). Depression in cancer: new developments regarding diagnosis and treatment. Biological psychiatry, 54(3), 283–294. [DOI] [PubMed] [Google Scholar]
- Renner W, Krenn-Pilko S, Gruber H, Herrmann M, & Langsenlehner T (2018). Relative telomere length and prostate cancer mortality. Prostate cancer and prostatic diseases. [DOI] [PubMed] [Google Scholar]
- Ridout KK, Ridout SJ, Price LH, Sen S, & Tyrka AR (2016). Depression and telomere length: A meta-analysis. Journal of Affective Disorders, 191, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Tavarez CE (2017). Can We Increase Our Health Span? Physical Medicine and Rehabilitation Clinics, 28(4), 681–692. [DOI] [PubMed] [Google Scholar]
- Rode L, Nordestgaard BG, & Bojesen SE (2015). Peripheral blood leukocyte telomere length and mortality among 64 637 individuals from the general population. JNCI: Journal of the National Cancer Institute, 107(6). [DOI] [PubMed] [Google Scholar]
- Sanchez-Espiridion B, Chen M, Chang JY, Lu C, Chang DW, Roth JA, . . . Gu J (2014). Telomere length in peripheral blood leukocytes and lung cancer risk: a large case–control study in Caucasians. Cancer Research, 74(9), 2476–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satin JR, Linden W, & Phillips MJ (2009). Depression as a predictor of disease progression and mortality in cancer patients. Cancer, 115(22), 5349–5361. doi: 10.1002/cncr.24561 [DOI] [PubMed] [Google Scholar]
- Schutte NS, & Malouff JM (2015). The association between depression and leukocyte telomere length: a meta‐analysis. Depression and anxiety, 32(4), 229–238. [DOI] [PubMed] [Google Scholar]
- Seow WJ, Cawthon RM, Purdue MP, Hu W, Gao Y-T, Huang W-Y, . . . Hosgood HD (2014). Telomere length in white blood cell DNA and lung cancer: a pooled analysis of three prospective cohorts. Cancer Research, canres. 0459.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton Lush, E., Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, . . . Salmon P (2013). Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun, 30 Suppl, S163–170. doi: 10.1016/j.bbi.2012.07.019 [DOI] [PubMed] [Google Scholar]
- Sephton, & Spiegel. (2003). Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain, behavior, and immunity, 17(5), 321–328. [DOI] [PubMed] [Google Scholar]
- Shao L, Wood CG, Zhang D, Tannir NM, Matin S, Dinney CP, & Wu X (2007). Telomere dysfunction in peripheral lymphocytes as a potential predisposition factor for renal cancer. The Journal of urology, 178(4), 1492–1496. [DOI] [PubMed] [Google Scholar]
- Sharpley CF, Christie DR, Bitsika V, Agnew LL, Andronicos NM, & McMillan ME (2018). Associations between reduced telomere length, depressed mood, anhedonia, and irritability in prostate cancer patients: Further evidence for the presence of “male depression”? Psycho‐Oncology, 27(3), 1072–1074. [DOI] [PubMed] [Google Scholar]
- Siegel Ma, Zou, & Jemal. (2014). Cancer statistics, 2014. CA: A Cancer Journal for Clinicians, 64(1), 9–29. [DOI] [PubMed] [Google Scholar]
- Siegel Miller, & Jemal. (2016). Cancer statistics, 2016. CA: A Cancer Journal for Clinicians, 66(1), 7–30. [DOI] [PubMed] [Google Scholar]
- Siegel Miller, & Jemal. (2018). Cancer statistics, 2018. CA: A Cancer Journal for Clinicians, 68(1), 7–30. [DOI] [PubMed] [Google Scholar]
- Spiegel, & Giese-Davis. (2003). Depression and cancer: mechanisms and disease progression. Biological psychiatry, 54(3), 269–282. [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, & Bray F (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. [DOI] [PubMed] [Google Scholar]
- Valls C, Piñol C, Reñé J, Buenestado J, & Viñas J (2011). Telomere length is a prognostic factor for overall survival in colorectal cancer. Colorectal disease, 13(11), 1265–1272. [DOI] [PubMed] [Google Scholar]
- Vance MC, Bui E, Hoeppner SS, Kovachy B, Prescott J, Mischoulon D, . . . Worthington JJ (2018). Prospective Association between Major Depressive Disorder and Leukocyte Telomere Length over Two Years. Psychoneuroendocrinology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J, Mulick A, Magill N, Symeonides S, Gourley C, Burke K, . . . Toynbee M (2021). Major depression and survival in people with cancer. Psychosomatic Medicine, 83(5), 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhan Y, Pedersen NL, Fang F, & Hägg S (2018). Telomere length and all-cause mortality: a meta-analysis. Ageing research reviews, 48, 11–20. [DOI] [PubMed] [Google Scholar]
- Wang Y-H, Li J-Q, Shi J-F, Que J-Y, Liu J-J, Lappin JM, . . . Qiao Y-L (2020). Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Molecular psychiatry, 25(7), 1487–1499. [DOI] [PubMed] [Google Scholar]
- Weischer M, Nordestgaard BG, Cawthon RM, Freiberg JJ, Tybjærg-Hansen A, & Bojesen SE (2013). Short telomere length, cancer survival, and cancer risk in 47102 individuals. Journal of the National Cancer Institute, 105(7), 459–468. [DOI] [PubMed] [Google Scholar]
- Wentzensen IM, Mirabello L, Pfeiffer RM, & Savage SA (2011). The association of telomere length and cancer: a meta-analysis. Cancer Epidemiology and Prevention Biomarkers [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstätter A, . . . Kiechl S (2010). Telomere length and risk of incident cancer and cancer mortality. JAMA, 304(1), 69–75. [DOI] [PubMed] [Google Scholar]
- Wu X, Amos CI, Zhu Y, Zhao H, Grossman BH, Shay JW, . . . Spitz MR (2003). Telomere dysfunction: a potential cancer predisposition factor. Journal of the National Cancer Institute, 95(16), 1211–1218. [DOI] [PubMed] [Google Scholar]
- Yang Allen, M. S., Aubry MC, Wampfler JA, Marks RS, Edell ES, . . . Deschamps C (2005). Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest, 128(1), 452–462. [DOI] [PubMed] [Google Scholar]
- Zhang Chen, X., Li L, Zhou Y, Wang C, & Hou S (2015). The association between telomere length and cancer prognosis: evidence from a meta-analysis. PLoS ONE, 10(7), e0133174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmaro LA, Sephton SE, Siwik CJ, Phillips KM, Rebholz WN, Kraemer HC, . . . Cash ED (2018). Depressive symptoms predict head and neck cancer survival: Examining plausible behavioral and biological pathways. Cancer [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, S.E.S., upon reasonable request.


