Abstract
Disorders of the brain are the most debilitating situation affected globally with increased mortality rates every year, while brain physiology and cumbersome drug development processes exacerbate this. Although the blood-brain barrier (BBB) and its components are important for brain protection, their complexity creates major obstacles for brain drug delivery and the BBB is the primary cause of treatment failure leading to disease progression. Therefore, developing an ideal platform that can predict the behavior of a drug delivery system in the brain at the early development phase is extremely crucial. In this direction, in the last two decades, numerous in vitro BBB models have been developed and investigated by researchers to understand the barrier properties and how closely the in vitro models mimic in vivo BBB. In vitro BBB models are mainly the culture of endothelial cells or their co-culture with other perivascular cells either in two or three-dimensional platforms. In this article, we have briefly summarized the fundamentals of BBB and outlined different types of in vitro BBB models with their pros and cons. Based on the available reports, no model seems to be robust that can truly mimic the entire properties of the in vivo BBB microvasculature. However, human stem cells, co-culture, and three-dimensional models were found to mimic the complexity of the barrier integrity not completely but more precisely than other in vitro models. More studies aiming towards combining them would be needed to develop an ideal in vitro model that can overcome the existing limitations and unravel the mysterious BBB.
Keywords: Blood-brain barrier, in vitro models, tight junctions, endothelial cells, stem cells, perivascular cells
Graphical abstract
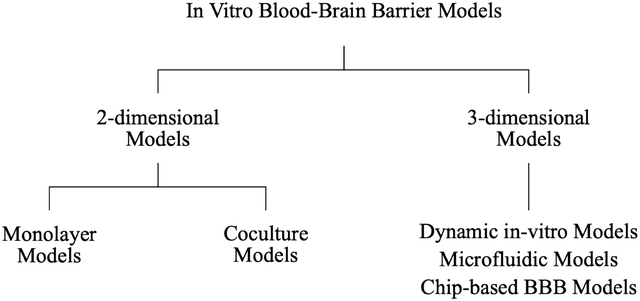
INTRODUCTION
Treatments of the central nervous system (CNS) disorders are facing numerous challenges such as limited therapeutics, complex physiology of the brain, and inadequate drug delivery to the brain, altogether leading to the increased mortality rate. The blood-brain barrier (BBB), a safeguard to the brain, is a complex interface between the blood circulation and the CNS, which strictly restricts the entry of potentially toxic substances and pathogens. However, it does allow the exchange of vital nutrients like glucose, iron, and blood gases, which are essential for brain functions. Overall, BBB possesses dual functions viz., barrier, and carrier [1]. On the one side, the barrier function of the BBB is of utmost importance for brain protection, but, on the other side, it is disadvantageous for CNS therapeutics as systemic delivery of more than 98% small molecules and nearly 100% large molecules cannot cross the BBB, leading to ineffective CNS treatments [2]. Therefore, there is an ample need to investigate and develop an ideal model which can characterize the permeation and/or penetration behavior of neurotherapeutics in the early developmental phase to develop a clinically effective CNS therapy.
Novel CNS entities and delivery techniques require detailed evaluation of in silico models, in vitro models, animal testing, and at last human trials. It has been stated that only 50% of the results obtained by testing in animal models are translated into human responses due to lack of consistent response, inter-species diversities, and differences in the expression of tight junction (TJ) proteins and transporters [3]. For example, Jamieson et al. stated that when compared to mice, humans have 1.85-fold higher and 2.33-fold lower expression of the breast cancer resistance protein (BCRP) and P-glycoprotein (P-gp), respectively, in the BBB [3]. Further details on the species difference concerning the BBB structure, protein expression levels, and impact on the permeability are covered in other review articles [4, 5]. Such differences across humans and rodents can significantly affect drug development, thus leading to unpredictable clinical translation. Taking aforesaid aspects into consideration, over the past several years, various in vitro BBB models have been developed and evaluated by numerous groups to predict the permeation mechanism and penetration measurement of CNS therapeutics across the BBB in vivo [6]. In addition to serving as very strong tools in drug development, in vitro BBB models are important to elucidate further physiological and pathophysiological molecular mechanisms [7]. To mimic the BBB, in vitro BBB models including mono- and multiple culture models, stem cell-based models, dynamic models, and microfluidic models have been developed.
In this review, initially, we outlined briefs on the fundamentals and structure of the BBB. The rest of the review covers a summary of various in vitro BBB models with the pros and cons of each model, the challenges associated and future directions towards optimal in vitro BBB models that would imitate the BBB in vivo.
STRUCTURE AND FUNDAMENTALS OF THE BBB
The BBB, a part of the neurovascular unit, is composed of endothelial cells (ECs), astrocytes (ACs), pericytes (PCs), neurons, and microglia. These ECs or brain microvascular endothelial cells (BMECs) are interconnected strongly through TJs and are responsible for the restriction and regulation of the transport of various molecules. As shown in Fig. 1a, neighboring ACs, PCs, and basement membranes surround the BMECs, providing structural support, membrane stability, and they all together form the impermeable BBB [3, 6]. As a result, only small molecules (< 400 Da) with high lipophilicity can cross the BBB, while the penetration of water-soluble substances is strictly restricted through TJs. In short, TJs are the obstructive structures of the BBB located at the apical side of the ECs, which primarily regulate paracellular permeability. TJs and the thick basal membrane result in the transendothelial electrical resistance (TEER, an indicator of the tightness of the junctions) of brain capillaries being ~2000 Ω cm2, in comparison to 2–20 Ω cm2 in peripheral capillaries [8, 9]. The molecular composition of TJs includes interactions between transmembrane and cytoplasmic proteins. Transmembrane proteins such as occludin, claudin, and junctional adhesion molecules bind to the cytoplasmic protein zonula occludens (ZO-I) linked to the actin cytoskeleton [1, 10]. In addition to TJs, adherens junctions composed of cadherins (transmembrane protein), catenins (cytoplasmic protein), and gap junctions also furnish barrier function and adhesion of ECs and restrict paracellular permeability (Fig. 1b). Molecules facing challenges in crossing the BBB through the paracellular pathways (between adjacent cells) utilize the transcellular pathways (through the cells) [11]. Besides these anatomical obstructions, many efflux transporters such as P-gp, BCRP, and multidrug resistance-associated protein-1 (MRP-1) are highly expressed in the BBB and concomitantly restrict the entry and accumulation of many drugs [12]. Expression profiles of these transporters largely determine the permeability properties and their functionality is an important requirement for the quality of the in vitro BBB models [1]. However, aforesaid physiological limitations are not the only rate-limiting step in determining drug transport and/or permeability across the BBB. The physicochemical properties of the drugs/formulations also influence drug transport.
Fig. 1.
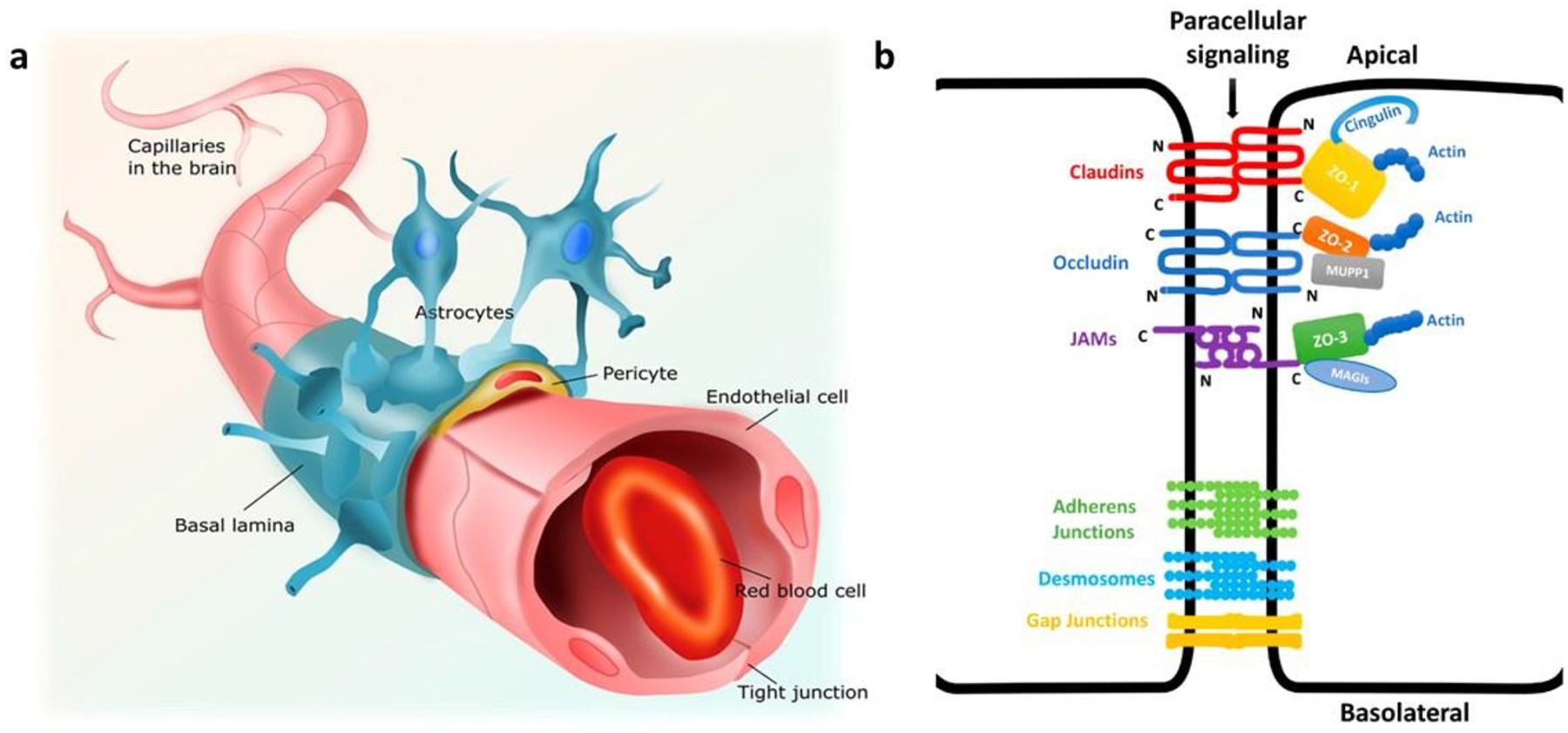
(a) Structure of the capillaries of neurovascular unit. The brain capillaries are lined with endothelial cells, which are connected by tight junctional complexes. The endothelium is partly covered with pericytes on the abluminal (brain facing) side. Endothelial cells and pericytes are covered almost completely by astrocytic end feet and together regulate brain endothelial cell function and phenotype. Adapted from [62]. (b) Schematic representation of interendothelial junctions composed of tight junctions, adherens junctions, and gap junctions. Tight junctions are mediated by adhesion proteins such as claudins, occludin, and junctional adhesion molecules (JAMs), whereas the zona occludin proteins (ZO-1, ZO-2, and ZO-3) connect adhesion molecules to the actin cytoskeleton. Adapted from [63].
IN VITRO BBB MODELS
The complexity of the BBB presents a plethora of options for constructing a BBB model to various degrees of physiological accuracy. In vitro BBB models, aimed at reliable and cost-effective models that would bypass and/or minimize the in vivo BBB testing conditions, have been established to investigate the physiological challenges of the BBB while designing the novel CNS therapy. These models range in complexity from a simple monoculture of cells to three-dimensional co-cultures using ECs, ACs, and PCs [13, 14]. In general, an ideal BBB model should meet the following conditions: (i) ease of culture, (ii) biologically realistic composition to maximize paracellular restriction, (iii) functional expression of TJ and transporters, and (iv) reproducibility of solute permeability [4].
The simplest in vitro BBB model used to test new molecules for brain diseases is the Caco-2 model that uses transwell chambers cultured with Caco-2 cells to mimic the BBB in terms of TEER and permeability. The correlation between the Caco-2 model and in vivo models was promising for small molecules that are transported by a passive diffusion mechanism [15]. Complex models or multicellular models more closely mimic the in vivo BBB to achieve better performance in terms of reduced monolayer permeability and physiological disease conditions. In addition to that, multicellular models maintain both a microvascular and cerebral environment, which is one of the crucial aspects of matching in vivo barrier functions, because cellular constituents like ACs, PCs, and to some extent, glial cells often connect with primary ECs to maintain the specific brain endothelial phenotype [4]. However, a consistent and reproducible source of these cells is a major limitation in the development of these models. To overcome this, human-induced pluripotent stem cell (HiPSCs)-based models have been developed to mimic the human BBB in vivo [3]. The aforementioned monolayer and co-culture models are part of static models or two-dimensional (2-D) models whereby in vivo shear stress caused due to regular blood flow is not imitated. Recently developed complex three-dimensional (3-D) models or so-called dynamic BBB models closely mimic the complexity of the in vivo BBB through imitating the shear stress and regulating the expression of TJ and transporters [6, 16]. Dynamic in vitro models, microfluidic models, and chip-based BBB models fall under this category. However, the complexity of such systems makes them more inconvenient, especially for screening applications. Thus, the underlying challenge of in vitro BBB model design is the striking balance between simplicity and performance [13]. Various in vitro BBB models are illustrated in Table (1) and their descriptions are summarized in the following sections.
Table 1.
Types, comparison, and compilation of in vitro BBB models.
| Models | Advantages | Disadvantages | Cellular type | Model properties (TEERa, Peb) | Remarks | Refs. |
|---|---|---|---|---|---|---|
| Monolayer | Simple and cheap, Drug transport, Molecular analysis, Scalable | No cellular contact, No shear stress, Limited barrier properties, Limited transporter expression, Very poor replicate to in-vivo BBB | Mouse primary BMECs | TEER: ~130 Pe: 6.5±0.8 |
ECs on top of transwell inserts. Limited expression of claudin and P-gp. | [49] |
| Immortalized mouse cerebral ECs | TEER: 405±20.47 | Higher expression of occludin and claudin. | [50] | |||
| Transwell double and triple co-culture | Cellular crosstalk, Enhanced barrier functions, Replicates confluent monolayer, Low-cost fabrication, Simple and scalable structure | No shear stress, Limited cellular differentiation, Lacks geometry and microvasculature, Large media volume | Rat primary BMECs with rat ACs | TEER: >60 | Noncontact model. <2-week time to achieve resistance. | [51] |
| Bovine BMECs with ACs | TEER: 352–857 Pe: 3.4±1.8 |
Contact type model. Very high TEER. High P-gp expression and actin localization. | [22] | |||
| Rat BMECs with PCs | TEER: 6.5±3.7 (noncontact), 87.0±7.4 (contact) | This TEER values are under hypoxic conditions. Contact model showed minimum dysfunction proving impact of the cross cell talk. | [52] | |||
| Porcine primary BMEC with PCs and ACs | TEER: >1000 Pe: ~1.9 (mannitol) |
PCs in contact with ECs and ACs at the bottom of the well. Low P-gp and high BCRP expression. | [53] | |||
| Immortalized human brain ECs (hCMEC/D3) with cerebral ACs and PCs | TEER: 40–45 | ACs in contact with ECs and PCs at the bottom of the well. Human serum supplements were used for culturing. Low TEER. Double co-culture models had improved TEER than triculture model. | [54] | |||
| Dynamic in-vitro models | High TEER, Allows effect of shear stress, Improved BMEC phenotype, Possibility of co-culture, Replicate 3-D environment | Invisible cellular interaction, Higher initial cell load, Lacks a thin dual cell layer interface, Expensive, Complex set-up, Long co-culture time, Need precise skills, Poor scalability | Bovine aortic ECs with glial cells | TEER: >500 | ACM was used as a culture medium. Flow-based model. TEER stability up to 10 days. | [55] |
| Primary hBMECs with ACs and smooth muscle cells | TEER: >700 Pe: <1.0 (sucrose) |
Modified DIV flow-based model with post hollow capillary to mimic capillary and venous regions. ACs on abluminal side of the fibers. Sheer stress: 1 dyne/cm2. | [56] | |||
| hBMESCs with ACs | TEER: 600–700 (flow) 100–150 (no flow) Pe: sucrose 0.005 (flow) 0.33 (no flow) |
Impact of shear stress/flow on BBB integrity. Nearly a two-fold higher expression for TJ proteins and transporters with flow. Shear stress: 1–6.2 dyne/cm2. | [57] | |||
| Microfluidic models | Mimics in vivo condition, Adequate initial cell load, Possibility of co-culture, Allow shear stress, Continuous TEER measurement, Improved BBB functions | Expensive, Complex fabrication, Limited studies and standardized protocols, Lacks cylindrical geometry, Reactivity of polydimethylsiloxane with other molecules | Mouse bEND.3 ECs with ACs | TEER: 250–300 | PDS sandwich model. 10 μm culture membrane. Shear stress: 10 dyne/cm2. TEER achieved in 3–4 days. | [58] |
| HiPSC-derived BMECs with rat ACs | TEER: 4000 | Pumpless microfluidic model. Very low shear stress (<1 dyne/cm2). High expression of claudin and ZO-I. TEER achieved in 3 days and remained ~3000 for 10 days. | [59] | |||
| BMECs and HiPSC with ACs and PCs | TEER: 35,000 | BBB on chip sandwich model. Three channels and thin (0.2 μm) polycarbonate membrane. Shear stress: 20 dyne/cm2. PC conditioned medium improved BBB integrity with high ZO-I expression. | [60] | |||
| hBMECs with ACs or PCs | Pe: 2.0 (3 kDa dextran) | BBB on chip. Shear stress: 1 dyne/cm2. Cylindrical geometry did not allow TEER measurement, but the model showed very high expression for ZO-I protein in AC co-culture. | [61] |
All TEER values are in Ω cm2 unit.
Pe (permeability coefficient): All values express multiplication with 10−6 cm/s factor.
2-Dimentional Models
Despite the limitations, 2-D models hold their potential for evaluating the chief barrier integrity functions viz., TEER, and permeability. Reliability, reproducibility, simplicity, and affordability are important aspects of 2-D models, while the incomplete imitation of the in vivo BBB is the major setback. However, during the initial screening and developmental phase whereby shear stress and blood flow mimicking are not truly essential, 2-D models are very swift and popular among researchers. Considering different cell types, 2-D models can be developed as a monolayer or co-culture-based systems as discussed below.
Monolayer Models
In early dates, studies on the BBB barrier functions were performed on monocultures in perti dishes. This method was cost-effective and allowed for large quantities of monocultures. However, limitations such as the absence of two compartments (blood and brain side), poor paracellular restrictions, and poor imitation of in vivo BBB made them unsuitable for studying drug transport across the BBB [17], which led to the introduction of a growing monolayer of ECs in a transwell chamber. This chamber comprises a microporous semipermeable membrane that separates the luminal side and the abluminal side, thereby allowing the exchange of solutes while restricting the movement of cells across the compartments [4, 18]. Transwell models offer several attributes such as scalability, low cost, selectivity of different membranes, and pore sizes [17]. Transwell models allow the use of ECs from different sources, but to mimic the BBB features truly, primary BMECs from humans are recommendable to increase the barrier functions. However, access to primary BMECs from the human brain is very challenging, time-consuming, costly, and restricted to biopsy or autopsy materials from patients with diseases such as epilepsy or brain tumors. Thus, primary ECs from pig, beef, mouse, and rat are considered as they are characterized by functionality, tight barrier integrity (TEER > 400 Ω cm2), and low permeability. However, in addition to the variation of isolation and yield, there are other issues associated with the models using primary ECs from animals. For example, our ongoing studies have faced difficulties in an in vitro model using primary ECs from fresh porcine brains. The source for the fresh porcine brain is very limited. For the fresh porcine brain, as we ordered, we cannot require storing the fresh brain in ethanol during transportation because of the regulation. Although several protocols for isolating porcine brain ECs are available in the literature, they all are based on different levels of centrifugation that are nonspecific for the isolation of primary ECs. Without further characterization, we are difficult to ensure that the collected fraction is the ECs. The isolated ECs grow very slowly, and we have taken 4 weeks to harvest enough cells for transwell culture. Moreover, potential contamination in cell culture is a big concern because the isolation process cannot be conducted in a sterile condition. To avoid the isolation of the primary cells, Myers et al. adapted commercially available bovine BMECs and normal human ACs to establish a co-cultured BBB model [13]. Besides the simplicity and moderate cost of the monoculture models, the absence of other cellular components, and inadequate properties such as relatively low TEER, high paracellular permeability, and poor expression of transporters makes this model a poor replicate of barrier functions. It has been reported that monolayer models are not ideal for studying BBB integrity parameters, as only a single type of cells are present and hence in vivo multicellular environment are paid more attention [18]. Hartmann et al. studied the impact of endogenously isolated ECs with ACs and PCs and reported improved barrier functions with an increase in the tightness of the co-cultured model compared to the monoculture model [19]. The authors also stated that in vitro co-culture of primary ECs with other cellular constituents of the extracellular matrix is very essential to ensure cellular interactions and to maintain the functional properties of the in vivo BBB [19]. Taking these aspects into account, complex co-culture models have been developed to mimic the in vivo anatomy of the BBB.
Co-Culture Models
Even though cerebral ECs are the primary constituents of the BBB, the regulatory and functional roles of the neighboring cells (ACs, PCs, and microglia) in the maintenance of the barrier properties of the BBB must not be ignored. The co-culture of ECs with these cells increases cellular interactions, expression of efflux transporters, and the potential applications of in vitro models beyond drug permeability screening [17]. These models include double (ECs+ACs and ECs+PCs) and triple (ECs+ACs+PCs) cultures in the transwell system [6, 16, 20]. In general, ECs are grown on the luminal side of the transwell membrane, while perivascular cells are grown at the abluminal side of the membrane (contact) or at the bottom of the chamber (noncontact) [10]. Both contact and non-contact methods are important and provide different aspects of cellular interactions or communications. Unlike monolayer models, co-culture models offer higher TEER values and lower permeability but still do not have complete in vitro phenotypes because of the lack of shear stress.
Co-culture of ECs with ACs:
Even though not in direct contact with ECs, ACs as part of the neurovascular unit, largely determine the TEER transporter expression and permeability functions. Therefore, the co-culture of ACs with ECs could partially establish the features of the BBB [4, 21]. ACs play a vital role in regulating the complexity of TJ. A comparison of noncontact and contact co-cultured models (bovine brain capillary ECs with rat ACs) with a monoculture model showed an improvement in the TEER values ranging from about 92.0 Ω cm2 (monoculture model) to 134.0 Ω cm2 (non-contact model) and then to more than four-fold i.e. 386.0 Ω cm2 (contact model) [22]. Interestingly, the authors also evaluated the paracellular permeability of a low molecular weight transport marker (376 Da) and a high molecular weight transport marker (4 kDa). The paracellular permeability for the monoculture model and the contact co-cultured model for low molecular weight was 11.0×10−6 cm/s and 6.0×10−6 cm/s, respectively, and for high molecular weight was 2.7×10−6 cm/s and 1.5×10−6 cm/s, respectively. Paracellular permeability of the co-culture model was almost two-fold lower than that of the monoculture model. In another study, Jeliazkova-Mecheva and Bobilya used porcine brain capillary ECs co-cultured with porcine ACs. They reported an increase in TEER values up to nine-fold in the contact co-culture model (108.0 – 139.0 Ω cm2) compared to the monoculture model (12.0 – 39.0 Ω cm2), while it was stated that ACs alone did not have barrier functions [23]. They also confirmed that the rate of inulin transport (0.5% per hour) in the co-culture model was identical with the reported value for tight in vitro BBB (TEER> 600.0 Ω cm2) under optimal conditions. The aforementioned studies demonstrated the influence of ACs on maintaining barrier functions. Moreover, pronounced results were observed when ACs were in contact with ECs, i.e., at the bottom of the membrane filter rather than being at the bottom of the transwell insert.
Co-culture of ECs with PCs:
PCs are localized in the basal membrane and cover between 22–32% of the endothelium. They are very crucial components of the neurovascular unit and are responsible for the regulation of endothelial proliferation and BBB permeability [1, 4]. PCs are the closest cells to the ECs. Few reports have even stated that PCs are more important and influential than ACs concerning the dynamic regulation of the BBB properties [10, 24]. Nakagawa et al. developed co-culture models (contact and non-contact) containing rat brain capillary ECs and rat cerebral PCs and reported a 3.6-fold increase in the tightness of the endothelial monolayers for the contact co-culture model (TEER ≈ 220.0 Ω cm2) over the monoculture model (TEER ≈ 60.0 Ω cm2), while the tightness of the noncontact model (TEER ≈ 205 Ω cm2) was close to that of the contact model [24]. Paracellular permeability of sodium fluorescein was in the order of monoculture model > noncontact co-culture model > contact co-culture model, i.e., (9.0 > 5.2 > 3.0 × 10−6 cm/s), indicting the leaky nature of the monoculture model. The low permeability of the co-culture models was attributed to the cellular interaction between ECs and PCs, resulting in PC-derived angiopoietin-1-enhanced occludin gene expression in the cerebral ECs [24]. Dohgu et al. established an in vitro co-culture model using immortalized mouse brain capillary ECs with rat brain PCs and reported a significant decrease in the permeability coefficient of the transport marker with the contact model (34.8% decrease) and the noncontact model (16.0% decrease) when compared to the monoculture model [25]. The accumulation of rhodamine 123 was reduced by 17.8% in the contact model and 7.8% in the noncontact model, compared to the monoculture of ECs, suggesting a positive role and active participation of PCs in the restriction of endothelial TJ. Transforming growth factor type I (TGF-β1) inhibition in the contact co-culture model showed PC-induced improvement of BBB functions when treated with TGF-β1 antibody, while the same treatment had no significant effect on the monolayer model. Based on these results, the authors concluded that PCs had a vast influence on the upregulation of BBB functions through interaction with ECs and continuous production of TGF-β1 [25].
Triple co-culture model:
The understanding of the co-culture models, whereby the presence of additional cell types (ACs or PCs) with the primary ECs can help in achieving more resemblance between in vitro and in vivo BBB, led to the development of in vitro triple co-culture models which hypothesize that crosstalk of ACs and PCs together with ECs would, even more, improve the quality of the co-culture and thereby truly mimic the in vivo BBB functions. However, the contact patterns of ECs with ACs and PCs, i.e. EC-AC-PC or EC-PC-AC, in triple culture models have shown an impact on the maintenance and regulation of in vitro BBB functions. In this direction, the impact of different cellular arrangements on detailed barrier characteristics in double and triple co-culture models has been compared by Nakagawa and the group [26]. Rat brain capillary ECs, cerebral ACs, and PCs were utilized to construct seven different models. Among all tested models, the monoculture model of ECs (E00) had the TEER value of <100 Ω cm2. In contrast, the TEER value of the triple co-culture model of EC-PC-AC (EPA) was the highest (350 Ω cm2) among all tested models. Surprisingly, there was no significant difference in the TEER (262 Ω cm2 vs 274 Ω cm2) and permeability (4.1 × 10−6 cm/s vs 4.2 × 10−6 cm/s) for the double contact co-culture model of ECs and PCs (EP0) and the triple co-culture model of EC-AC-PC (EAP), respectively. These results suggested that PCs had a dynamic role in regulating the tightness of the ECs; hence, the direct contact of PCs with ECs in double (EP0) and triple co-culture (EPA) models enhanced resistance over non-contact models E0P and EAP, respectively. Besides TEER, there was no significant difference in the permeability of the transport marker (sodium fluorescein) among models EPA, EAP, EP0, and EA0, suggesting that the presence of ACs or PCs in either double or triple co-culture systems significantly decreased the flux of the marker. The authors also confirmed the highest expressions of occludin, claudin, and ZO-I in the triple co-culture model of EPA against all other models through Western blot, suggesting the influence of ACs and PCs on the endothelial TJ proteins. Additionally, the expression of influx transporter (i.e., glucose transporter-1 (GLUT-1)) and efflux transporters (i.e., P-gp and MRP-1) in the EPA model were also confirmed by Western blot and immunohistochemistry. Permeability study with rhodamine 123 in the EPA model showed a strong efflux effect as the transport of rhodamine 123 from blood to the brain was almost 2.5 times lower than that from the brain to blood. Based on these results, the authors summarized that a triple co-culture model of EPA provided superior barrier integrity and resembled the maximum anatomical conditions of the BBB [26].
Stem Cell Models
Although the aforementioned models, especially co-culture models, showed satisfactory barrier functions, the cell type used in developing these models is still a question due to the limitations such as source reliability and reproducibility. HiPSCs as an alternative to primary and immortalized BMECs could overcome these limitations and enable the development of in vitro BBB model [3, 10, 16]. Because BMECs generated from HiPSCs express TJ proteins, adherens junction, and transporters, the BBB model constructed by HiPSC-derived BMECs exhibits biological resistance, which will be further increased when ACs and PCs are also collected from the same source of HiPSCs, altogether resulting in an ideal in vitro BBB model that imitates the in vivo BBB [3, 27, 28]. In a recent publication, HiPSCs were differentiated into a polarized monolayer that expressed BBB-specific proteins and had TEER values greater than 2500 Ω cm2. HiPSCs differentiation generated BMECs to produce an excellent barrier phenotype without the need for co-culture to improve barrier properties. The minimum TEER threshold to study the brain transport has been established as 500 Ω cm2 for sodium fluorescein and 900 Ω cm2 for IgG, though in vivo human permeability data are not publicly available [29]. The TEER threshold for one molecule is not statistically enough to represent other molecules considering the complication of molecule structure, the interaction of molecules with the BBB, and various disease conditions. The study raised the question again - how high TEER should be in the in vitro models to study drug transport through BBB? Although the answer is unknown, it is clear that disease conditions should be considered to study the TEER threshold.
BBB dysfunction is closely related to different pathological conditions of diseases such as cerebral ischemia, Alzheimer’s (AD), and Parkinson’s disease (PD). Ideally, an in vitro BBB model should be able to mimic the BBB under different disease conditions. HiPSCs have a great potential to promote physiological and medical studies. Using the HiPSC-derived BMECs, Kokubu et al. established an in vitro BBB disease model that mimicked the BBB in cerebral ischemia [30]. They examined the effect of oxygen-glucose deprivation (OGD) and OGD/reoxygenation on the BBB permeability. The results showed that OGD disrupted the barrier function, and the dysfunction was rapidly restored by resupply of oxygen and glucose. Interestingly, the authors proposed further studies to incorporate chronic disease conditions such as AD and PD into the model. Authors also stated that the present in vitro model was developed through monolayer culture only and therefore the impact of other perivascular cells (ACs, PCs, and glia) with HiPSC-derived BMECs must be determined to understand the neurological pathology precisely [30]. If this goal is achieved, the in vitro BBB model will be able to not only evaluate the permeability but also mimic real disease, leading to a more clinically relevant in vitro model to reflect the BBB in real disease than any preexisting in vitro models. HiPSC-derived cells can be resources instead of cells that are difficult to be constantly obtained from the body. Yamamizu et al. generated an in vitro BBB model by differentiating HiPSCs into human ECs, PCs, neurons, and ACs [28]. They demonstrated the existence of TJ and transporters in the BBB model. Moreover, the resulting model was validated with ten clinical drugs based on the permeability tests. However, the TEER of the model was rather low about 100 Ω cm2 and 120–180 days of co-culturing cells were needed to achieve this TEER. It is noteworthy that in vitro BBB models established with stem cells have the potential to mimic the brain conditions in diseases. If this model is established from stem cells that are specifically induced from patients with CNS disease, it should provide insights on how vascular dysfunction contributes to CNS diseases. The authors used multicellular cultures in a 2-D model that separated the luminal and abluminal sides. They anticipated that constructing in vitro 3-D multicellular models would have even more resemblance to in vivo BBB compared to the current 2-D models [28]. Recent applications and advances in the stem cell-based human BBB models with tabulated differences among transporters and TJ protein expression between human and non-human species were precisely summarized by Aday and group which will help in generating novel stem-cell based models [5].
3-Dimentional Models
While the existing 2-D models may recapitulate some of the important aspects of neurovascular function, they compromise physiological shear stress and fail to address the 3-D cellular organization, which is crucial to many cellular processes in vivo [31]. To overcome these challenges and as a step forward in mimicking BBB functions, complex 3-D models were introduced. In in vivo conditions, shear stress generated by the blood flow increases the expression of TJ proteins and reduces permeability, which leads to evaluate 3-D models with shear stress [18]. Additionally, using brain ECs and cerebral perivascular cells to establish a double or triple co-culture system is often time-consuming and technically challenging. Crisan et al. demonstrated strong similarities between mesenchymal stem cells (MSCs, a commercially available stem cell line) and PCs [32]. MSCs and PCs express many of the same cell surface markers, and CNS microvascular cells have been found to exhibit multipotential stem cell activities similar to that seen in MSCs. To simplify the model preparation and establish a 3-D model, Tian et al. substituted PCs by MSCs in an in vitro BBB model using brain capillary ECs (mouse bEND.3 cells) co-cultured with commercially available MSCs (Fig. 2). In this sandwich-like structure of bEND.3-MSC co-culture in a 3-D view (Fig. 2e), the authors also confirmed the higher expression of TJ protein ZO-I compared to monoculture bEND.3 (Fig. 2b). MSCs contributed similarly to PCs in a co-cultured 3-D model by increasing TEER and decreasing permeability against macromolecules [33]. However, the TEER in this BBB model established by MSCs was up to 200 Ω cm2.
Fig. 2.
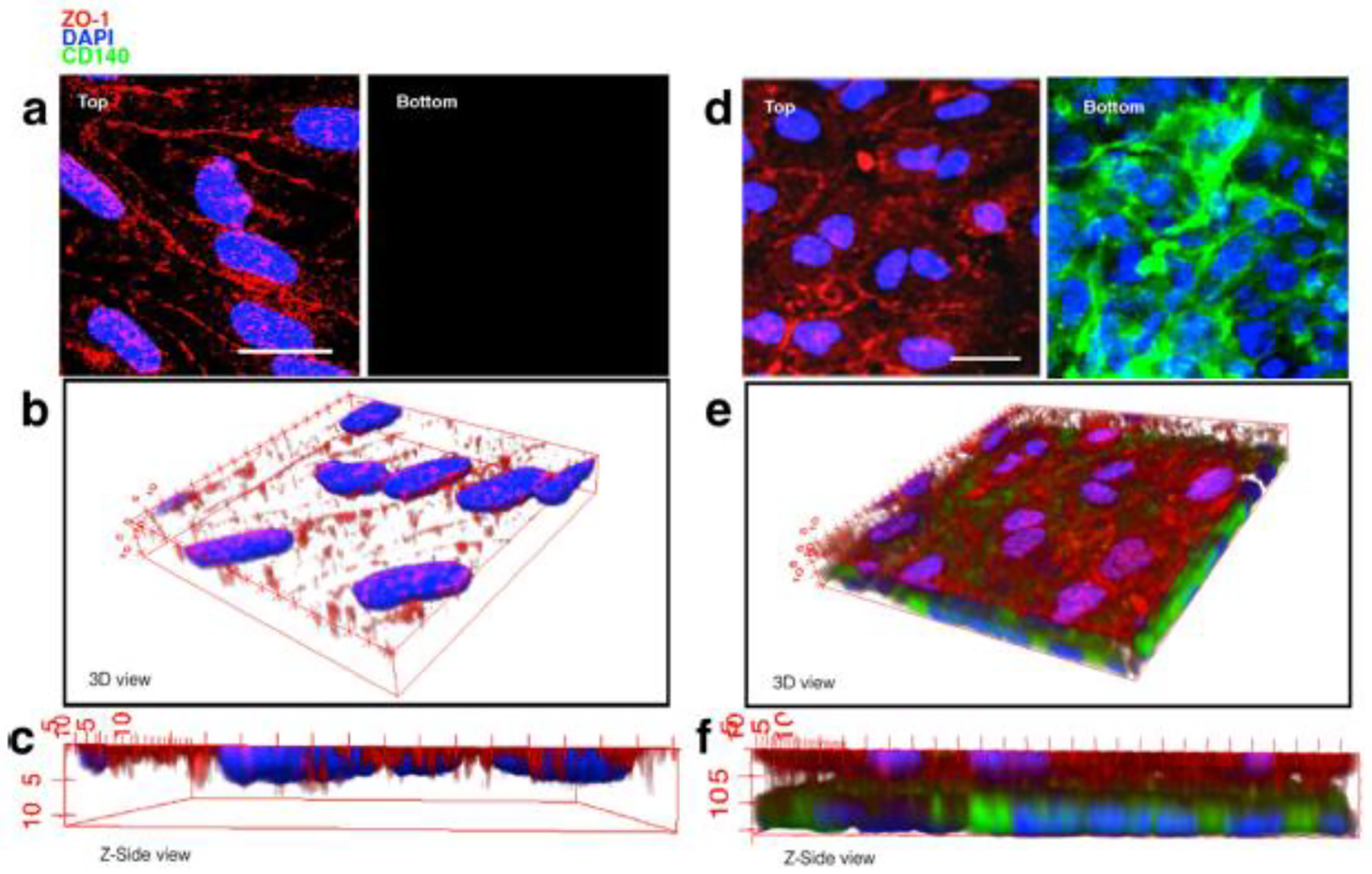
(a) bEND.3 monoculture 3-deminsional (3D) blood-brain barrier model with top and bottom view, (b) 3D reconstruction view, (c) Z-side view, (d) bEND.3/MSCs co-culture 3D model with top and bottom view, (e) 3D reconstruction view and (f) Z-side view. scale bars 20 μm. Adapted from Tian et al. [33].
Dynamic In Vitro Models
Dynamic in vitro (DIV) models are considered the first 3-D in vitro models containing artificial capillaries such as the structure of microporous polypropylene hollow fibers. Primary cells are cultured inside (luminal) of the capillaries, while perivascular cells are seeded on the outer side (abluminal) of the capillaries. Culture media is circulated in the capillaries through a pulsatile pump to create shear stress mimicking in vivo blood flow [1, 4, 16, 17]. In addition, the exchange of gases (oxygen and carbon dioxide) through a gas permeable tubing system helps in maintaining the stable microenvironment [18]. A flow-based DIV model of human cerebral ECs (hECs) was developed by Cucullo and the group [34]. In the presence of pulsatile flow, they cultured hECs in the lumen of a hollow microporous capillary with or without abluminal ACs. For comparison, the authors also developed a transwell co-culture model of hECs-ACs (contact 2-D model) and reported that the co-culture model had15- to 18-fold lower TEER than the DIV model (1000–1200 Ω cm2). Interestingly, there was no significant difference in the TEER values for DIV models with or without ACs. Thus, the results confirmed the importance of artificial shear stress in maintaining the in vitro BBB functions. Additionally, the authors reported transient (20–30 min) and completely reversible BBB opening in the DIV model [34]. Furthermore, to allow transendothelial migration of immune cells, they modified the DIV model by generating transmural microholes (<4 μm) in the hollow fibers and reported that their presence does not affect the tightness of the BBB as the TEER and the permeability of the modified DIV model were similar to that of original DIV model [35]. This result demonstrated that the differentiation of the vascular endothelium grown under stable shear stress and microenvironment is the major factor for the formation of a functional BBB in vitro and that this is not significantly affected by the properties of the supporting structure. Siddharthan et al. studied the flow-based in vitro model of hBMECs with human fetal ACs to examine the role of supporting factors, and the effect of astrocyte-conditioned medium (ACM) in place of ACs was also evaluated on the BBB properties. In the presence of shear stress, the TEER value for hBMECs with ACs or ACM was found comparable, but the permeability for dextran in the presence of ACM was 50% lower than ACs. The hBMECs with ACM showed two-fold higher expression of TJ protein ZO-I against ACs. Overall, the authors reported pronounced effects on BBB function in the presence of ACM in regulating the barrier properties of hBMECs through secreting AC factors into the medium [36]. Although DIV models showed realistic in vivo properties, precise technical skills, high cell load, long time for initial setup, presence of thick artificial basal membrane, long time required to achieve steady-state TEER, and visual obstruction of the endothelium compartment are some of the limitations that have been reported which prevent their scale-up and limit their applications [4, 6, 16, 17].
Microfluidic Models
To overcome the shortcomings of the DIV model, a microfluidic method was introduced as it can be processed with low cell load and allows improved cellular communication due to the presence of a thin membrane. This model encompasses semi-porous polycarbonate membranes sandwiched and sealed between two vertical/horizontal polydimethylsiloxane (PDS) channel networks. One channel acts as a vascular side to grow ECs while the second channel behaves as a parenchymal part to allow culturing perivascular cells. When a culture medium flows through the membrane, shear stress is generated and built-in electrodes measure the TEER [4, 16]. While designing this model, the thickness of the PDS membrane must be kept below 50 μm to make it more compatible with the native brain microvasculature. Moreover, parallel design of PDS channel allows enhanced cellular cross-talk [37, 38]. Adriani et al. developed a novel 3-D neurovascular microfluidic model consisting of primary rat ACs and neurons together with ECs [31]. The TEER was not measured in this model. EC functionality was confirmed by permeability testing of fluorescent dextran that demonstrated the selectivity of the endothelial monolayer, while neural functionality was demonstrated by calcium imaging. Besides the low fabrication cost, the 3-D neurovascular microfluidic model provides a basic form for developing a sophisticated and complex 3-D in vitro neurovascular model. However, the 3-D microfluidic models also have weaknesses. Yeon et al. studied permeability measurements for multiple drugs using microhole-based modified microfluidic devices and highlighted model deficiencies such as poor cellular communication and low expression of efflux transporters. Permeability results with the present 3-D models were not so promising over 2-D transwell models [39]. To better mimic the brain microvasculature, premix containing extracellular matrix hydrogel with ACs and neurons was injected into respective channels aligned parallelly. Authors reported that the hydrogel-based model not only supported the cells but also served as a physical barrier and better resembled the brain microvasculature in terms of thickness, stiffness, and composition [40]. Recently, it was stated that PDS being hydrophobic showed a poor affinity for cell adhesion. Plasma treatment or protein coating can improve cell adhesion by reducing the hydrophobicity of the PDS [41]. Furthermore, treated membranes also reduced the risk of leaching native monomer into culture medium that was known to affect cellular behavior [41, 42]. In this direction, Kim et al. developed an engineered brain microvascular structure with mouse brain ECs cultured on the luminal surface of the collagen channel coated with fibronectin [43]. The authors stated that adhesion and growth were improved on coated channels and thus showed enhanced barrier integrity and regulation of overall cell behaviors over non-coated collagen channels [43].
Chip-Based BBB Models
Another modified version of the microfluidic model is a “BBB on-chip” model which combines the features of microfluidic and synthetic microvasculature (SyM) models. This model is being developed to mimic even the physiological dimensions of the microvasculature. The SyM model mimics a typical in vivo microcirculation milieu with fluid flow and shear stress, while the complicated channel structure and lengthy design of the model do not allow measuring the TEER [16]. These models have the potential to be used as human-relevant disease models and, additionally, provide detailed insight into drug effects [44]. Moreover, they have been characterized by the dynamic permeability of drugs/markers, which make them more like in vivo permeability studies compared to the transwell system. The BBB maintains a unique homeostatic environment within the CNS and plays a critical role in mass transfer between the circulatory system and brain tissue [45]. Therefore, permeability measurements in BBB on-chip models and data comparison with corresponding in vivo studies are usually performed as a means of understanding whether the model can be a surrogate for the in vivo study and whether an appropriate in vitro to in vivo correlation can be established [45, 46]. A microfluidic chip model contains two PDS channels representing brain and blood sides that were separated by a microporous polycarbonate membrane. The upper channel contains a hollow fiber capillary lined by ECs while the bottom channel contains neighboring cells and the grooved area; both channels contain platinum electrodes for TEER measurement. To generate shear stress, a medium flows through the channels, and thereafter the barrier functions are evaluated. Complete fabrication setup with physical dimensions is described by Griep et al.. [44], and readers are encouraged to refer to the protocol for detailed understanding. Additionally, Oddo et al. have summarized a very interesting review on the chip-based microfluidic models covering model applications, possible design strategies of the chambers (sandwich, parallel, cylindrical structure, and vasculogenesis design), and current trends [40]. To maintain high TEER and enhanced expression of efflux pumps for a long duration, Park et al. developed an in vitro BBB on-chip microfluidic model incorporating HiPSC-derived BMECs interfaced with cerebral ACs and PCs [47]. Confocal images confirmed the direct contact of ACs with brain endothelium in a tight barrier expressing ZO-I, claudin, influx, and efflux transporters on the apical side. Interestingly, the authors reported that co-cultured HiPSC-derived BMECs under a hypoxic condition showed BBB impedance of ~25,000 Ω, which was close to the model developed under normoxic conditions (~24,000 Ω) but nearly 50-fold higher than a chip model lined by the primary human BMECs. Besides this, the hypoxic model-maintained BBB impedance value close to ~20,000 Ω up to one week, while the impedance value for the normoxic model was declined to ~10,000 Ω on day 3 and was ~6000 Ω on day 5, indicating that hypoxia triggered the in vitro barrier formation. The authors stated that such models were able to recapitulate in vivo human BBB functions in terms of an anatomical and physiological microenvironment and could facilitate vast applications in CNS drug discovery and delivery [47]. In a recently published study of a 3D self-organized microvascular model of the human BBB on-chip with ECs, PCs, and ACs, the permeability of 40 kDa fluorescent-dextran under mono-, co-, and tri-culture conditions was 6.6, 2.5, and 0.89 × 10−7 cm/s, respectively. These results showed that the presence of co- and tri-culture reduced the permeability of analyte [48]. Moreover, the permeability study of different molecular weight fluorescent-labeled dextran tracers in a recent study of iPSC-BMECs in a 3D microfluidic chip with the presence of primary ACs and PCs showed the permeability values inversely correlated with the size of the tracer (average Papp = 8.9, 1.1, and 0.24 × 10−8 cm/s for 3, 10, and 70 kDa dextrans, respectively) [47].
CONCLUSION AND FUTURE ASPECTS
This review has presented brief information on the BBB structure and categories of in vitro BBB models and discussed research reports published in the past few years based on monoculture, double co-culture, and triple co-culture with transwell and 3-D approaches. In vitro BBB models are the utmost significant tools used for target identification, lead optimization, and high-throughput screening at the initial stages of CNS drug development while used for concept testing in later stages. Understanding the anatomical arrangement of the BBB helps to design an ideal in vitro model that closely mimics the in vivo BBB microvasculature. However, there is no ideal model. Each type of model discussed herein has its pros and cons (Table 1). During this review, taking TEER and permeability coefficients into consideration, we were in a dilemma about the model superiority due to high controversy among monoculture models and double and triple co-culture models, wherein we found that cell source or species play a wide role in these parameters. As moved forward and with the increased demand of mimicking the BBB functions, 3-D models, particularly microfluidic and BBB on-chip, overtook 2-D models due to their additional configuration of multiple compartments, shear stress, and realistic microenvironment over simple TEER and permeability measurements. However, cost, complex design time, and poor scalability are the serious setbacks of 3-D models which limit their translation from lab to industry. Depending on the expected study output and model application, some of the model parameters may not be required to be considered. For example, to screen P-gp substrates and permeability assessments, 2-D transwell models are very precise, simple, economic, and swift. In contrast, 3-D models provide improved barrier integrity and dynamic changes in cell visualization. Considering interspecies differences, the influence of pathological conditions and to best mimic the BBB phenotype, hiPSCs have shown ample potential in generating promising in vitro models. Taking the high demand of in vivo correlation into account, BBB on-chip and microfluidic models can be futuristic models that will replace the current 2-D models. Compared with the previous in vitro models, the amalgamation of stem cells and microfluidic technology will boost the development of the next generation of in vitro models and give new insights associated with BBB dysfunction.
FUNDING
This work was supported by NIH 1R35GM138225-01 to Dong X.
ABBREVIATIONS
- ACs
astrocytes
- ACM
AC-conditioned medium
- AD
Alzheimer’s disease
- BBB
blood-brain barrier
- BCRP
breast cancer resistance protein
- BMECs
brain microvascular endothelial cells
- CNS
the central nervous system
- DIV
dynamic in vitro
- ECs
endothelial cells
- E00
monoculture model of ECs
- EAP
triple co-culture model of EC-AC-PC
- EPA
triple co-culture models of EC-PC-AC
- EP0
double contact co-culture model of ECs and PCs
- hECs
human cerebral ECs
- hiPSCs
human induced pluripotent stem cells
- GLUT-1
glucose transporter 1
- MRP-1
multidrug resistance-associated protein-1
- MSCs
mesenchymal stem cells
- OGD
oxygen-glucose deprivation
- PCs
pericytes
- PD
Parkinson’s disease
- PDS
polydimethylsiloxane
- P-gp
P-glycoprotein
- SyM
synthetic microvasculature model
- TEER
transendothelial electrical resistance
- 3-D
three-dimensional
- 2-D
two-dimensional
- TGF-β1
transforming growth factor type 1
- TJ
tight junction
- ZO-I
zonula occludens
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- [1].Wilhelm I; Fazakas C; Krizbai IA, In vitro models of the blood-brain barrier. Acta Neurobiol Exp (Wars) 2011, 71 (1), 113–28. [DOI] [PubMed] [Google Scholar]
- [2].Pardridge WM, Blood-brain barrier delivery. Drug Discov Today 2007, 12 (1–2), 54–61. [DOI] [PubMed] [Google Scholar]
- [3].Jamieson JJ; Searson PC; Gerecht S, Engineering the human blood-brain barrier in vitro. J Biol Eng 2017, 11, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wilhelm I; Krizbai IA, In vitro models of the blood-brain barrier for the study of drug delivery to the brain. Mol Pharm 2014, 11 (7), 1949–63. [DOI] [PubMed] [Google Scholar]
- [5].Aday S; Cecchelli R; Hallier-Vanuxeem D; Dehouck MP; Ferreira L, Stem Cell-Based Human Blood-Brain Barrier Models for Drug Discovery and Delivery. Trends Biotechnol 2016, 34 (5), 382–393. [DOI] [PubMed] [Google Scholar]
- [6].Bagchi S; Chhibber T; Lahooti B; Verma A; Borse V; Jayant RD, In-vitro blood-brain barrier models for drug screening and permeation studies: an overview. Drug Des Devel Ther 2019, 13, 3591–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Appelt-Menzel A; Cubukova A; Gunther K; Edenhofer F; Piontek J; Krause G; Stuber T; Walles H; Neuhaus W; Metzger M, Establishment of a Human Blood-Brain Barrier Co-culture Model Mimicking the Neurovascular Unit Using Induced Pluri- and Multipotent Stem Cells. Stem Cell Reports 2017, 8 (4), 894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Crone C; Christensen O, Electrical resistance of a capillary endothelium. J Gen Physiol 1981, 77 (4), 349–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Olesen SP; Crone C, Electrical resistance of muscle capillary endothelium. Biophys J 1983, 42 (1), 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wolff A; Antfolk M; Brodin B; Tenje M, In Vitro Blood-Brain Barrier Models-An Overview of Established Models and New Microfluidic Approaches. J Pharm Sci 2015, 104 (9), 2727–46. [DOI] [PubMed] [Google Scholar]
- [11].Dong X, Current Strategies for Brain Drug Delivery. Theranostics 2018, 8 (6), 1481–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Weksler BB; Subileau EA; Perriere N; Charneau P; Holloway K; Leveque M; Tricoire-Leignel H; Nicotra A; Bourdoulous S; Turowski P; Male DK; Roux F; Greenwood J; Romero IA; Couraud PO, Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J 2005, 19 (13), 1872–4. [DOI] [PubMed] [Google Scholar]
- [13].Myers JS; Hare J; Sang QA, A Simple Adaptable Blood-Brain Barrier Cell Model for Screening Matrix Metalloproteinase Inhibitor Functionality. Methods Mol Biol 2017, 1579, 287–296. [DOI] [PubMed] [Google Scholar]
- [14].Czupalla CJ; Liebner S; Devraj K, In vitro models of the blood-brain barrier. Methods Mol Biol 2014, 1135, 415–37. [DOI] [PubMed] [Google Scholar]
- [15].Dehouck MP; Jolliet-Riant P; Bree F; Fruchart JC; Cecchelli R; Tillement JP, Drug transfer across the blood-brain barrier: correlation between in vitro and in vivo models. J Neurochem 1992, 58 (5), 1790–7. [DOI] [PubMed] [Google Scholar]
- [16].Andjelkovic AV; Stamatovic SM; Phillips CM; Martinez-Revollar G; Keep RF, Modeling blood-brain barrier pathology in cerebrovascular disease in vitro: current and future paradigms. Fluids Barriers CNS 2020, 17 (1), 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sivandzade F; Cucullo L, In-vitro blood-brain barrier modeling: A review of modern and fast-advancing technologies. J Cereb Blood Flow Metab 2018, 38 (10), 1667–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bagchi S; Lahooti B; Chhibber T; Varahachalam SP; Mittal R; Joshi A; Jayant RD, In Vitro Models of Central Nervous System Barriers for Blood-Brain Barrier Permeation Studies. In Nanomedicines for Brain Drug Delivery, Morales JO; Gaillard PJ, Eds. Humana: New York, 2020; Vol. 157, pp 235–253. [Google Scholar]
- [19].Hartmann C; Zozulya A; Wegener J; Galla HJ, The impact of glia-derived extracellular matrices on the barrier function of cerebral endothelial cells: an in vitro study. Exp Cell Res 2007, 313 (7), 1318–25. [DOI] [PubMed] [Google Scholar]
- [20].Helms HC; Abbott NJ; Burek M; Cecchelli R; Couraud PO; Deli MA; Forster C; Galla HJ; Romero IA; Shusta EV; Stebbins MJ; Vandenhaute E; Weksler B; Brodin B, In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J Cereb Blood Flow Metab 2016, 36 (5), 862–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].DeBault LE; Cancilla PA, gamma-Glutamyl transpeptidase in isolated brain endothelial cells: induction by glial cells in vitro. Science 1980, 207 (4431), 653–5. [DOI] [PubMed] [Google Scholar]
- [22].Gaillard PJ; Voorwinden LH; Nielsen JL; Ivanov A; Atsumi R; Engman H; Ringbom C; de Boer AG; Breimer DD, Establishment and functional characterization of an in vitro model of the blood-brain barrier, comprising a co-culture of brain capillary endothelial cells and astrocytes. Eur J Pharm Sci 2001, 12 (3), 215–22. [DOI] [PubMed] [Google Scholar]
- [23].Jeliazkova-Mecheva VV; Bobilya DJ, A porcine astrocyte/endothelial cell co-culture model of the blood-brain barrier. Brain Res Brain Res Protoc 2003, 12 (2), 91–8. [DOI] [PubMed] [Google Scholar]
- [24].Nakagawa S; Deli MA; Nakao S; Honda M; Hayashi K; Nakaoke R; Kataoka Y; Niwa M, Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol 2007, 27 (6), 687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dohgu S; Takata F; Yamauchi A; Nakagawa S; Egawa T; Naito M; Tsuruo T; Sawada Y; Niwa M; Kataoka Y, Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res 2005, 1038 (2), 208–15. [DOI] [PubMed] [Google Scholar]
- [26].Nakagawa S; Deli MA; Kawaguchi H; Shimizudani T; Shimono T; Kittel A; Tanaka K; Niwa M, A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int 2009, 54 (3–4), 253–63. [DOI] [PubMed] [Google Scholar]
- [27].Canfield SG; Stebbins MJ; Morales BS; Asai SW; Vatine GD; Svendsen CN; Palecek SP; Shusta EV, An isogenic blood-brain barrier model comprising brain endothelial cells, astrocytes, and neurons derived from human induced pluripotent stem cells. J Neurochem 2017, 140 (6), 874–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yamamizu K; Iwasaki M; Takakubo H; Sakamoto T; Ikuno T; Miyoshi M; Kondo T; Nakao Y; Nakagawa M; Inoue H; Yamashita JK, In Vitro Modeling of Blood-Brain Barrier with Human iPSC-Derived Endothelial Cells, Pericytes, Neurons, and Astrocytes via Notch Signaling. Stem Cell Reports 2017, 8 (3), 634–647. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [29].Mantle JL; Min L; Lee KH, Minimum Transendothelial Electrical Resistance Thresholds for the Study of Small and Large Molecule Drug Transport in a Human in Vitro Blood-Brain Barrier Model. Mol Pharm 2016, 13 (12), 4191–4198. [DOI] [PubMed] [Google Scholar]
- [30].Kokubu Y; Yamaguchi T; Kawabata K, In vitro model of cerebral ischemia by using brain microvascular endothelial cells derived from human induced pluripotent stem cells. Biochem Biophys Res Commun 2017, 486 (2), 577–583. [DOI] [PubMed] [Google Scholar]
- [31].Adriani G; Ma D; Pavesi A; Kamm RD; Goh EL, A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood-brain barrier. Lab Chip 2017, 17 (3), 448–459. [DOI] [PubMed] [Google Scholar]
- [32].Crisan M; Yap S; Casteilla L; Chen CW; Corselli M; Park TS; Andriolo G; Sun B; Zheng B; Zhang L; Norotte C; Teng PN; Traas J; Schugar R; Deasy BM; Badylak S; Buhring HJ; Giacobino JP; Lazzari L; Huard J; Peault B, A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3 (3), 301–13. [DOI] [PubMed] [Google Scholar]
- [33].Tian X; Brookes O; Battaglia G, Pericytes from Mesenchymal Stem Cells as a model for the blood-brain barrier. Sci Rep 2017, 7, 39676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cucullo L; Couraud PO; Weksler B; Romero IA; Hossain M; Rapp E; Janigro D, Immortalized human brain endothelial cells and flow-based vascular modeling: a marriage of convenience for rational neurovascular studies. J Cereb Blood Flow Metab 2008, 28 (2), 312–28. [DOI] [PubMed] [Google Scholar]
- [35].Cucullo L; Marchi N; Hossain M; Janigro D, A dynamic in vitro BBB model for the study of immune cell trafficking into the central nervous system. J Cereb Blood Flow Metab 2011, 31 (2), 767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Siddharthan V; Kim YV; Liu S; Kim KS, Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res 2007, 1147, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Prabhakarpandian B; Shen MC; Nichols JB; Mills IR; Sidoryk-Wegrzynowicz M; Aschner M; Pant K, SyM-BBB: a microfluidic Blood Brain Barrier model. Lab Chip 2013, 13 (6), 1093–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Deosarkar SP; Prabhakarpandian B; Wang B; Sheffield JB; Krynska B; Kiani MF, A Novel Dynamic Neonatal Blood-Brain Barrier on a Chip. PLoS One 2015, 10 (11), e0142725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yeon JH; Na D; Choi K; Ryu SW; Choi C; Park JK, Reliable permeability assay system in a microfluidic device mimicking cerebral vasculatures. Biomed Microdevices 2012, 14 (6), 1141–8. [DOI] [PubMed] [Google Scholar]
- [40].Oddo A; Peng B; Tong Z; Wei Y; Tong WY; Thissen H; Voelcker NH, Advances in Microfluidic Blood-Brain Barrier (BBB) Models. Trends Biotechnol 2019, 37 (12), 1295–1314. [DOI] [PubMed] [Google Scholar]
- [41].Jiang L; Li S; Zheng J; Li Y; Huang H, Recent Progress in Microfluidic Models of the Blood-Brain Barrier. Micromachines (Basel) 2019, 10 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Menon NV; Chuah YJ; Cao B; Lim M; Kang Y, A microfluidic co-culture system to monitor tumor-stromal interactions on a chip. Biomicrofluidics 2014, 8 (6), 064118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kim JA; Kim HN; Im SK; Chung S; Kang JY; Choi N, Collagen-based brain microvasculature model in vitro using three-dimensional printed template. Biomicrofluidics 2015, 9 (2), 024115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Griep LM; Wolbers F; de Wagenaar B; ter Braak PM; Weksler BB; Romero IA; Couraud PO; Vermes I; van der Meer AD; van den Berg A, BBB on chip: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed Microdevices 2013, 15 (1), 145–50. [DOI] [PubMed] [Google Scholar]
- [45].Modarres HP; Janmaleki M; Novin M; Saliba J; El-Hajj F; RezayatiCharan M; Seyfoori A; Sadabadi H; Vandal M; Nguyen MD; Hasan A; Sanati-Nezhad A, In vitro models and systems for evaluating the dynamics of drug delivery to the healthy and diseased brain. J Control Release 2018, 273, 108–130. [DOI] [PubMed] [Google Scholar]
- [46].Bhalerao A; Sivandzade F; Archie SR; Chowdhury EA; Noorani B; Cucullo L, In vitro modeling of the neurovascular unit: advances in the field. Fluids Barriers CNS 2020, 17 (1), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Park TE; Mustafaoglu N; Herland A; Hasselkus R; Mannix R; FitzGerald EA; Prantil-Baun R; Watters A; Henry O; Benz M; Sanchez H; McCrea HJ; Goumnerova LC; Song HW; Palecek SP; Shusta E; Ingber DE, Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat Commun 2019, 10 (1), 2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shi L; Zeng M; Sun Y; Fu BM, Quantification of blood-brain barrier solute permeability and brain transport by multiphoton microscopy. J Biomech Eng 2014, 136 (3), 031005. [DOI] [PubMed] [Google Scholar]
- [49].Shayan G; Choi YS; Shusta EV; Shuler ML; Lee KH, Murine in vitro model of the blood-brain barrier for evaluating drug transport. Eur J Pharm Sci 2011, 42 (1–2), 148–55. [DOI] [PubMed] [Google Scholar]
- [50].Silwedel C; Forster C, Differential susceptibility of cerebral and cerebellar murine brain microvascular endothelial cells to loss of barrier properties in response to inflammatory stimuli. J Neuroimmunol 2006, 179 (1–2), 37–45. [DOI] [PubMed] [Google Scholar]
- [51].Abbott NJ; Dolman DE; Drndarski S; Fredriksson SM, An improved in vitro blood-brain barrier model: rat brain endothelial cells co-cultured with astrocytes. In Methods in Molecular Biology (Methods and Protocols), 2011/12/07 ed.; Milner R, Ed. Humana Press: 2012; Vol. 814, pp 415–30. [DOI] [PubMed] [Google Scholar]
- [52].Hayashi K; Nakao S; Nakaoke R; Nakagawa S; Kitagawa N; Niwa M, Effects of hypoxia on endothelial/pericytic co-culture model of the blood-brain barrier. Regul Pept 2004, 123 (1–3), 77–83. [DOI] [PubMed] [Google Scholar]
- [53].Thomsen LB; Burkhart A; Moos T, A Triple Culture Model of the Blood-Brain Barrier Using Porcine Brain Endothelial cells, Astrocytes and Pericytes. PLoS One 2015, 10 (8), e0134765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hatherell K; Couraud PO; Romero IA; Weksler B; Pilkington GJ, Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci Methods 2011, 199 (2), 223–9. [DOI] [PubMed] [Google Scholar]
- [55].Cucullo L; McAllister MS; Kight K; Krizanac-Bengez L; Marroni M; Mayberg MR; Stanness KA; Janigro D, A new dynamic in vitro model for the multidimensional study of astrocyte-endothelial cell interactions at the blood-brain barrier. Brain Res 2002, 951 (2), 243–54. [DOI] [PubMed] [Google Scholar]
- [56].Cucullo L; Hossain M; Tierney W; Janigro D, A new dynamic in vitro modular capillaries-venules modular system: cerebrovascular physiology in a box. BMC Neurosci 2013, 14, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cucullo L; Hossain M; Puvenna V; Marchi N; Janigro D, The role of shear stress in Blood-Brain Barrier endothelial physiology. BMC Neurosci 2011, 12, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Booth R; Kim H, Characterization of a microfluidic in vitro model of the blood-brain barrier (muBBB). Lab Chip 2012, 12 (10), 1784–92. [DOI] [PubMed] [Google Scholar]
- [59].Wang YI; Abaci HE; Shuler ML, Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol Bioeng 2017, 114 (1), 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Brown JA; Pensabene V; Markov DA; Allwardt V; Neely MD; Shi M; Britt CM; Hoilett OS; Yang Q; Brewer BM; Samson PC; McCawley LJ; May JM; Webb DJ; Li D; Bowman AB; Reiserer RS; Wikswo JP, Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics 2015, 9 (5), 054124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Herland A; van der Meer AD; FitzGerald EA; Park TE; Sleeboom JJ; Ingber DE, Distinct Contributions of Astrocytes and Pericytes to Neuroinflammation Identified in a 3D Human Blood-Brain Barrier on a Chip. PLoS One 2016, 11 (3), e0150360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Alcendor DJ, Human Vascular Pericytes and Cytomegalovirus Pathobiology. Int J Mol Sci 2019, 20 (6), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shi J; Barakat M; Chen D; Chen L, Bicellular tight junction and wound healing. Int J Mol Sci 2018, 19 (12), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


