Abstract
Background
Worldwide, 1 % of the population receives anticoagulation therapy, with prevalence higher in older adults. Difficulties in the adequate management of these patients have led to the development of strategies focused on achieving therapeutic control and reducing adverse events with efficient use of resources.
Objective
To estimate the cost utility and budget impact on the Argentinean health system of implementation of anticoagulation clinics (ACs) (with and without use of point-of-care [POC] CoaguChek® devices [Roche Diagnostics International Ltd]) compared with the traditional laboratory method (non-AC settings) for the management of anticoagulated patients.
Methods
For the cost-utility analysis, a cohort-based state transition model was designed to compare costs and health outcomes of implementing ACs for outpatient management of anticoagulated patients. The budget impact analysis used an analytical model to estimate the differential costs of implementing an AC and the expected adverse events avoided, and the differential costs of an international normalized ratio (INR) determination using a POC device rather than a conventional laboratory.
Results
We calculated the study outcomes for a cohort of 1000 patients. Considering a 5 % discount rate, the use of ACs generated 13.9 additional quality-adjusted life-years (0.014 per patient) and 12.5 additional life-years (0.013 per patient). Incremental cost-effectiveness ratios of AC implementation with and without the use of POC devices compared with the scenario without ACs were dominant in both cases. In the probabilistic sensitivity analysis, nearly all simulated results were cost effective (i.e., below the 1 or 3 gross domestic product per capita thresholds). Budget impact analysis results showed AC implementation generated savings from the first year of implementation, with savings of AR $265,325 by year 5. The addition of POC devices in the ACs also generated savings as early as the first year of implementation, with savings of AR $488,072 by year 5 (AR $488 per patient).
Conclusions
Anticoagulation clinics are estimated to be cost effective and generate notable savings in the treatment of patients on long-term oral anticoagulant therapy when compared with non-AC settings. These savings are considerably higher when POC devices are added as part of the patient management, due to lower laboratory technician costs per INR determination.
Supplementary Information
The online version contains supplementary material available at 10.1007/s41669-022-00352-4.
Key Points for Decision Makers
| The results of this cost-utility analysis suggest that implementing anticoagulation clinics, with and without the use of point-of-care devices, can be cost effective in the Argentinean setting |
| The addition of point-of-care devices to anticoagulation clinics was shown to increase savings, mainly due to optimized extraction time and faster results. |
| This is the first economic study of its kind to analyze anticoagulation clinics and point-of-care devices implementation for anticoagulated patients from the perspective of payers in the Argentinean health system. |
Introduction
The use of oral anticoagulants is aimed at a diverse profile of patients. In Argentina, atrial fibrillation represents 60% of the indications for anticoagulation, followed by venous thromboembolic disease (30%) and mechanical valve replacement (4%) [1]. It is estimated that 1% of the population worldwide receives anticoagulation treatment, with prevalence higher in older adults [2, 3]. Indications for anticoagulation continue to increase [4, 5], and it is essential that patients monitor their coagulation status regularly to maintain safe and effective treatment [6, 7].
Available anticoagulation therapies are varied, ranging from vitamin K antagonists (VKAs) (warfarin, acenocoumarol) and heparin therapy in its different routes of administration to the new direct oral anticoagulants (dabigatran, apixaban, rivaroxaban). In Argentina, VKAs continue to be the most frequently used form of oral anticoagulant therapy, possibly due to their lower cost and greater acceptance by patients and the medical community [8]. However, VKAs have a pharmacodynamic profile that is sometimes difficult to predict, and they require frequent monitoring [9].
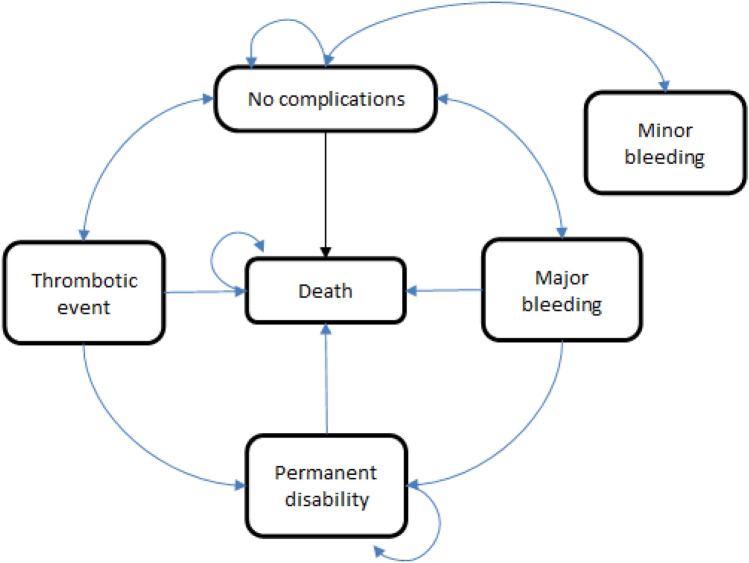
Difficulties in the adequate management of patients requiring anticoagulation have led to the development of strategies focused on achieving therapeutic control and reducing adverse events. One of these strategies was the development of anticoagulation clinics (ACs) [10, 11], which are specialized healthcare services focused on providing comprehensive and systematized care to patients on oral anticoagulation treatment [12–14]. Anticoagulation clinics involve coordinated and high-quality care monitored by a group of health professionals who treat patients receiving anticoagulation therapy. Their function is to achieve organized and systematic management of this labile population to avoid thrombotic and/or hemorrhagic complications.
The standard or traditional method of testing a patient's level of anticoagulation is by estimating the international normalized ratio (INR) via a blood sample, which is then processed at an on-site laboratory or sent off to another laboratory. Once the result is available, patients attend a consultation to (1) verify that the dose of anticoagulants they are receiving is correct, or (2) receive a new prescription or change of dose if the result is out of therapeutic range. The effectiveness of warfarin therapy has usually been measured via time in therapeutic range (TTR), the percentage of time a patient’s INR is within, above, or below the desired treatment range. TTR is typically assessed using the Rosendaal method [15], where the INR is assumed to change linearly from one INR check to the next. Time in therapeutic range is calculated by dividing the number of days with INRs of 2.0–3.0 by the total number of days.
An alternative method of monitoring INR is through the use of point-of-care (POC) devices. These are small, portable devices that process a small drop of capillary blood, with results available in approximately 1 min [16]. They have been shown to be safe and efficient in monitoring patients on dicoumarinics with no significant differences in adverse events when compared with traditional monitoring methods [17, 18]. These devices can be incorporated into the management of patients seen in ACs, where the patient must attend to record self-checks and to receive an evaluation of the equipment performance. Of the various POC devices, the CoaguChek® XS (Roche Diagnostics International Ltd, Rotkreuz, Switzerland) is the most widely used around the world, as demonstrated by various external quality assessment programs [19].
While the clinical effectiveness of POC devices is generally accepted [20–23], there is limited experience of their use in Argentina and a lack of literature regarding the economic or budget impact of shifting from a traditional to AC model of care where portable devices are used for anticoagulation monitoring. Health technology assessments conducted to inform resource allocation ideally require key data elements (e.g., health-related costs, socio-demographics) and information available at the local level [24]. Evaluating the economic impact of introducing POC devices in anticoagulated patients with local evidence may help inform health technology coverage decisions at both the public and private funding levels in Argentina.
The aim of this study was to estimate the cost-utility and budget impact on the Argentinean health system of implementation of ACs (with and without use of POC CoaguChek devices) compared with non-AC settings for the management of anticoagulated patients.
Methods
This study was a cost-utility analysis (CUA) and budget impact analysis (BIA) designed to evaluate the implications for both health outcomes and costs in four possible scenarios: (1) [ACs] versus [no ACs], (2) [no ACs + POC] versus [no ACs], (3) [ACs + POC] versus [No ACs], and (4) [ACs + POC] versus [ACs], from the perspective of payers in the Argentinean health system, following recommendations from regional guidelines for the economic evaluation of health technologies [25]. In non-AC settings, INR monitoring was assumed to be done in regular hospital settings; the interventions were INR monitoring in ACs, with and without POC devices. Informed consent and institutional review board approval were not required as no participants were recruited (modeling study only).
For the cost-utility analysis, taking into account that patients may have several health events in their lifetime, a state transition model was designed based on similar model structures used by the Canadian Agency for Drugs and Technologies in Health (CADTH) and the National Health System (NHS) in the UK for local health technology assessments [2, 16] (see Fig. 1). A key difference between our study and the aforementioned models was that patient self-management intervention (using POC device at home) was not analyzed in our study, as it is not common practice in Argentina. The model uses a cycle duration of 1 month, as it is assumed that patients undergo one INR measurement per month. Costs and quality-adjusted life years (QALYs) were calculated for a lifetime period, considering an annual discount rate of 5% according to local recommendations [26]. The target population comprised patients requiring long-term oral anticoagulation therapy [2, 27]. Patients entered the model in the state “no event/regular control” and based on inherent pathology risks they could have experienced one of three acute events: thrombosis, minor bleeding, or major bleeding. Patients experiencing an acute event resulted in either returning to the non-event state, permanent disability, or death. The risk of events changed depending on anticoagulation control. When patients were above range, the risk of bleeding increased; when below range, the risk of thrombosis increased.
Fig. 1.
State transition model. Argentina, 2021
The budget impact analysis model estimated the differential costs of implementing an AC and the expected adverse events avoided, and the differential costs of an INR determination using a POC device rather than processing in a conventional laboratory. Budget impact results were calculated on a 5-year time horizon, considering a gradual increase in the market share of 20% per year (100% at year 5); costs were not discounted according to recommendations [26]. Due to high inflation rates in Argentina, and to simplify the interpretation of results, we calculated results at a 0% inflation rate, so future costs should be interpreted according to 2021 values.
Both models were developed in Microsoft Excel. The complete list of model parameters can be found in Table 1, including base case estimations, minimum and maximum values considered for deterministic sensitivity analyses, probability distributions, and the respective data sources.
Table 1.
Parameters used in the economic evaluation
| Basea | Parameters for sensitivity analysis | Sourcesd | |||
|---|---|---|---|---|---|
| Minb | Maxb | Probability distributionc | |||
| Clinical and epidemiological | |||||
| Population | |||||
| (n) Patients followed per year | 1000 | – | – | – | Model assumption |
| Age of patients | 62.5 | 60 | 75 | Normal (62.50; 3.75) | Model assumption based on TERRA registry [1], Connock 2007 [2], CADTH 2014 [16] |
| Time in therapeutic range | |||||
| [AC] (%) TTR< (below range) | 15.20% | 7.9a% | 22.5% | Dirichlet | Tajer 2017 (TERRA registry) [1] |
| [AC] (%) <TTR< (in range) | 66.60% | 34.70% | 98.50% | Dirichlet | Tajer 2017 (TERRA registry) [1] |
| [AC] (%) <TTR (above range) | 18.20% | 9.5% | 26.9% | Dirichlet | Tajer 2017 (TERRA registry) [1] |
| (RR) Relative benefit of AC vs no AC for <TTR< | 1.11 | 1.09 | 1.2 | LogNormal (0.1; 0.0) | See Methods |
| Risks of events according to TTR | |||||
| (Pr) Thrombosis/TTR< | 2.72% | 2.00% | 3.40% | Beta (58.7; 2,100.3) | Connock 2007 [2] |
| (Pr) Thrombosis/<TTR< | 0.73% | 0.60% | 0.90% | Beta (65.3; 8,880.3) | Connock 2007 [2] |
| (Pr) Thrombosis/<TTR | 0.81% | 0.60% | 1.00% | Beta (65.1; 7,968.3) | Connock 2007 [2] |
| (Pr) Minor bleeding/TTR< | 6.10% | 4.60% | 7.60% | Beta (62.1; 955.2) | Connock 2007 [2] |
| (Pr) Minor bleeding/<TTR< | 4.80% | 3.60% | 5.90% | Beta (65.0; 1,302.5) | Connock 2007 [2] |
| (Pr) Minor bleeding/TTR< | 11.30% | 8.50% | 14.10% | Beta (57.6; 452.4) | Connock 2007 [2] |
| (Pr) Major bleeding/TTR< | 1.20% | 0.90% | 1.50% | Beta (60.1; 5,078.0) | Connock 2007 [2] |
| (Pr) Major bleeding/<TTR< | 0.90% | 0.70% | 1.20% | Beta (51.6; 5,554.7) | Connock 2007 [2] |
| (Pr) Major bleeding/TTR< | 3.40% | 2.50% | 4.20% | Beta (60.7; 1,741.1) | Connock 2007 [2] |
| (Pr) Permanent disability/major hemorrhagic event | 14.00% | 10.50% | 17.50% | Beta (54.9; 337.2) | Connock 2007 [2] |
| (Pr) Permanent disability/thrombosis | 63.80% | 47.80% | 79.70% | Beta (22.5; 12.8) | Connock 2007 [2] |
| (Pr) Death/major bleeding | 14.00% | 10.50% | 17.50% | Beta (54.9; 337.2) | Connock 2007 [2] |
| (Pr) Death/thrombosis | 21.00% | 15.80% | 26.30% | Beta (50.4; 189.4) | Connock 2007 [2] |
| (RR) Death/disability | 2.25 | 1.5 | 3 | LogNormal (0.8; 0.2) | Connock 2007 [2] & Sundberg 2003 [48] |
| Utilities | |||||
| (U) Argentinian population | – | Janssen 2019 [29] | |||
| Disutility due to anticoagulation therapy | 0.01 | 0.01 | 0.02 | Beta (98.7; 7,492.6) | Pink 2011 [30] |
| Ratio of U weights [Acute event minor bleeding]/[No event] | 0.98 | 0.78 | 1 | Gamma (100.00; 0.01) | Calculated from Connock 2007 [2] |
| Ratio of U weights [Acute event thrombosis]/[No event] | 0.77 | 0.62 | 0.93 | Gamma (100.00; 0.01) | Calculated from CADTH 2014 [16] |
| Ratio of U weights [Acute event major bleeding]/[No event] | 0.75 | 0.60 | 0.90 | Gamma (100.00; 0.01) | Calculated from CADTH 2014 [16] |
| Ratio of U weights [Permanent disability]/[No event] | 0.54 | 0.43 | 0.65 | Gamma (100.00; 0.01) | Calculated from CADTH 2014 [16] |
| Use of resources and costs (AR $) | |||||
| ($) CoaguChek INR determination | $164 | $139 | $245 | Gamma (38.60; 4.26) | Roche Diagnostics Argentina, 2021e |
| ($) LAB: Reagents (per sample) | $31 | $25 | $38 | Gamma (100.00; 0.31) | Roche Diagnostics Argentina, 2021e |
| ($) LAB: Syringe | $30 | $24 | $36 | Gamma (100.00; 0.30) | IECS BCU, 2021 [37] |
| ($) LAB: Needle | $20 | $16 | $24 | Gamma (100.00; 0.20) | IECS BCU, 2021 [37] |
| ($) LAB: Citrated tube | $42 | $27 | $50 | Gamma (51.11; 0.82) | IECS BCU, 2021 [37] |
| ($) LT total gross salary | $56,448 | $45,159 | $67,738 | Gamma (100.00; 564.48) | Collective labor agreement 2021 [49] |
| ($) Add monthly costs for AC per 1000 patients | $116,728 | $0 | $ 222,304 | Gamma (17.10; 6,824.39) | See Methods |
| ($) Permanent disability, monthly cost | $77,452 | $58,089 | $96,815 | Gamma (64.00; 1,210.19) | See Methods |
| ($) Thrombosis event | $406,018 | $242,219 | $560,616 | Gamma (26.02; 15,605.34) | Giorgi 2015 [31] (updated to 2021) |
| ($) Minor bleeding event | $293,871 | $107,350 | $183,652 | Gamma (237.33; 1,238.21) | Giorgi 2015 [31] (updated to 2021) |
| ($) Major bleeding event | $651,312 | $454,058 | $993,583 | Gamma (23.32; 27,932.76) | Giorgi 2015 [31] (updated to 2021) |
| LAB: LT time per extraction of each sample (min) | 10.4 | 8 | 12.8 | Gamma (75.11; 0.14) | Leung 2006 [50] |
| LAB: LT time per centrifugation of each sample (min) | 15 | 12 | 18 | Gamma (100.00; 0.15) | Funk 2008 CLSI [51] |
| LAB: Number of samples (per centrifugation) | 5 | 3 | 10 | Gamma (8.16; 0.61) | Assumption |
| LAB: LT processing time per sample (min) | 2 | 1 | 3 | Gamma (16.00; 0.13) | Model assumption |
| POC: LT time per extraction of each sample (min) | 2.2 | 1.8 | 2.7 | Gamma (100.00; 0.02) | Fitch 1999 [52] |
| POC: LT processing time per sample (min) | 1 | 0.5 | 2 | Gamma (7.11; 0.14) | CoaguChek XS user manual [19] |
| LT productivity (minutes worked per month) | 8400 | 6,300 | 8,400 | Gamma (256.00; 32.81) | Assumption |
| (n) INR determinations per year (per patient) | 12 | 9.6 | 14.4 | Normal (12.0; 1.20) | Model assumption |
| Others | |||||
| (%) Discount rate | 5% | 0% | 10% | – | Guías Mercosur [53] |
| (USD) GDPpc | 8442 | – | – | – | World Bank 2021 [54] |
| Exchange rate (AR per 1 USD) | $143 | $104 | $183 | Gamma (51.91; 2.75) | dolarhoy.com (Sep 8, 2021) [32] |
| (%) Market share AC year 1 | 20% | – | – | – | Assumption |
| (%) Market share AC year 2 | 40% | – | – | – | Assumption |
| (%) Market share AC year 3 | 60% | – | – | – | Assumption |
| (%) Market share AC year 4 | 80% | – | – | – | Assumption |
| (%) Market share AC year 5 | 100% | – | – | – | Assumption |
Base estimates, minimums, maximums, assumed probability distributions and sources. Costs expressed in local currency (Argentine pesos) Argentina, 2021
AC anticoagulation clinic, GDPpc gross domestic product per capita, INR international normalized ratio, LAB laboratory, LT laboratory technician, POC point of care, Pr probability, RR relative risk, TTR time in therapeutic range, TTR< below therapeutic range, <TTR< in therapeutic range, <TTR above therapeutic range, U utility
aValues used for the base (deterministic) estimates
bSee methods for more details on the calculation of minimum and maximum values
cProbability distributions were selected according to methodological recommendations. The parameters of the distributions were derived using the method of moments, assuming the mean values as the base values and the standard deviations as 1/4 of the width of the interval between the maximum and minimum values (see Methods)
dSee Methods and References sections for details of data sources.
eValues provided by Roche Diagnostics Argentina.
Clinical and Epidemiological Parameters
To simplify the interpretation of results, we calculated the study outcomes for a cohort of 1000 patients. It was assumed that patients entered the model at the age of 62.5 years, based on the estimates used in Connock [2] (65 years), CADTH 16 (50 years), and two local studies: TERRA [1], which reported anticoagulated patients to be on average 75 years old, and Labadet [28], which reported an average age of 68.8 years. It is worth mentioning that the latter two studies included only patients with atrial fibrillation. To evaluate the uncertainty around the estimation for the average age, we assumed a wide range in the sensitivity analysis of patients aged 60–75 years.
We used TTR estimates to characterize the percentage of time a patient’s INR was within, above, or below the desired treatment range. Local estimates of TTR for ACs were obtained from the TERRA registry [1], a multicentric study in Argentina designed to characterize anticoagulation levels of patients with non-valvular atrial fibrillation on oral anticoagulation (acenocoumarol or warfarin) in ACs. For the non-AC settings, the base case, minimum and maximum estimations of TTR were calculated by dividing the values from TERRA by values for the estimated relative benefit of ACs as reported in Connock 2007 and CADTH 2014 studies (base case: 68.9/62.32, min: 68.9/63.50 and max: 68.9/57.5) [2, 16]. For the POC intervention, we assumed no differences in terms of TTR.
Utilities
The model considered age-dependent utility weights to calculate QALYs (see Supplementary Fig. 1). The values for the general Argentine population were obtained from the 2019 Janssen et al study [29]. A disutility of − 0.013 due to anticoagulation therapy was applied [30]. Age-adjusted utility estimates for acute events of minor bleeding, major bleeding, thrombosis, and for disabilities, were calculated from the CADTH 2014 study [16] and the Connock 2007 study [2], taking into account the decrease in utility after experiencing an adverse event compared with the value for the general population.
Costs
The event costs of major hemorrhages, minor hemorrhages, and thrombosis were taken, in US dollars, from a 2012 study in Argentina [31], and updated for 2021 using an exchange rate of 143 Argentine pesos (AR) per US dollar, an average taken from the official and private market rates in September 2021 [32]. The cost of a minor hemorrhage was calculated as AR $293,871 and includes an emergency department consultation and subsequent medical consultation, without other added treatment. The cost of a major bleeding event was calculated as AR $651,312 and is a weighted average between cerebral hemorrhage, gastrointestinal bleeding, and epistaxis. Finally, the cost of a thrombotic event was calculated as AR $406,018 from a weighted average between pulmonary embolism and thrombotic stroke with prolonged hospitalization (see Supplementary Table 1).
To define monthly disability costs, the following parameters were estimated according to relevant literature: costs for rehabilitation, specialist consultation, and home care [33–35]. Rehabilitation costs were obtained from the nomenclature of medical benefits for people with disabilities in Argentina [36], with a value of AR $62,570 per month. For specialist consultation we considered six visits to the specialist per year, resulting in a cost of AR $625.57 per month [37]. Finally, home care costs were included. Considering that in Argentina, 50% of patients will have moderate–severe sequelae (i.e., they will require home care) after a thrombotic event [38], a cost of AR $13,965 per month for home care was estimated [39]. Thus, disability costs were estimated at AR $77,452 per month.
We considered additional monthly costs of ACs per 1000 patients, adapting the framework of activities defined by Izaguirre Ávila et al [40] to the Argentinean setting according to author opinion. However, only additional administrative costs and other running costs were evaluated (see Supplementary Table 2).
The budget impact analysis compared the costs of an INR determination by a POC device versus the use of a centralized laboratory. The cost of a POC device determination included the time needed for blood collection and sample processing, as well as the cost of a test strip and lancets. The cost of the POC devices was not included as it is common practice in Argentina for the devices to be provided on commodatum (no extra cost to payers) by the pharmaceutical company. For centralized laboratory use, the costs of blood collection, centrifugation, and processing of the sample as well as supplies (syringe, needle, and citrated tube) were included. All parameters used for this estimation are described in detail in the supplementary material (Appendix 1), and Supplementary Table 3 shows the details (in costs) of this comparison.
Sensitivity Analysis
The impact of parameter uncertainty on the results was analyzed with deterministic and probabilistic sensitivity analyses. The deterministic sensitivity analysis that allowed the identification of the drivers of results by means of univariate variations across the ranges of parameters was summarized in a tornado plot. The probabilistic sensitivity analysis consisted of simulating the result 1000 times using Monte Carlo simulations, assuming that the variation across the ranges of parameters had been distributed according to specific probability distributions. The probability distributions were selected according to technical recommendations [41], with the distribution parameters estimated from the mean values and standard deviations using the method of moments. The mean values were calculated as the base values of the parameters and the standard deviations were estimated as the difference between the maximum and minimum values divided by four. Probabilistic sensitivity analysis results were summarized in a cost-effectiveness plane and cost-effectiveness acceptability curves (see Supplementary Figs. 2 and 3).
Results
Cost-Utility Analysis
Results are shown in Table 2. In the discounted scenario, by implementing ACs (i.e., [ACs] vs [no ACs]), the health system would generate additional QALYs (0.0137 per patient), additional LYs (0.0125 per patient), and cost savings for the health system (AR $9255 per patient). The addition of POC devices to ACs (i.e., [ACs + POC] vs [no ACs]) would increase the savings (up to AR $12,209 per patient) and have no impact in terms of QALYs and LYs, as it was assumed that POC testing does not affect TTR. Finally, the addition of POC devices in AC settings (i.e., [ACs + POC] vs [ACs]) and non-AC settings (i.e., [no ACs + POC] vs [no ACs]) would also generate savings (AR $2954 and AR $2942 per patient, respectively) with no impact on QALYs and LYs. The incremental cost effectiveness ratios (ICERs) for all of the scenarios analyzed were dominant (i.e., the scenario without ACs is dominated in all cases).
Table 2.
Cost-utility analysis results (per patient)
| Undiscounted | Discounted | |||||
|---|---|---|---|---|---|---|
| LY | QALYs | Costs | LY | QALYs | Costs | |
| Monitoring strategies | ||||||
| [No ACs] | 8.09 | 6.59 | $508,936 | 6.25 | 5.12 | $377,625 |
| [No ACs + POC] | 8.092 | 6.585 | $505,133 | 6.25 | 5.12 | $374,683 |
| [ACs] | 8.113 | 6.606 | $496,208 | 6.26 | 5.13 | $368,370 |
| [ACs + POC] | 8.113 | 6.606 | $492,388 | 6.26 | 5.13 | $365,416 |
| Differences | ||||||
| [ACs] vs [No ACs] | 0.0203 | 0.0212 | − $12,728 | 0.0125 | 0.0137 | − $9255 |
| [No ACs + POC] vs [No ACs] | 0 | 0 | − $3,802 | 0 | 0 | − $2942 |
| [ACs + POC] vs [No ACs] | 0.0203 | 0.0212 | − $16,548 | 0.0125 | 0.0137 | − $12,209 |
| [ACs + POC] vs [ACs] | 0 | 0 | − $3,820 | 0 | 0 | − $2954 |
| ICERs | ||||||
| [ACs] vs [No ACs] | Dominant | Dominant | Dominant | Dominant | ||
| [No ACs + POC] vs [No ACs] | Dominant | Dominant | Dominant | Dominant | ||
| [ACs + POC] vs [No ACs] | Dominant | Dominant | Dominant | Dominant | ||
| [ACs + POC] vs [ACs] | Dominant | Dominant | Dominant | Dominant | ||
Anticoagulation clinic versus no anticoagulation clinic settings with and without POC devices. Costs expressed in local currency (Argentine pesos). Argentina, 2021
AC anticoagulation clinic, ICER incremental cost effectiveness ratio, LY life-year, POC point of care, QALY quality-adjusted life year
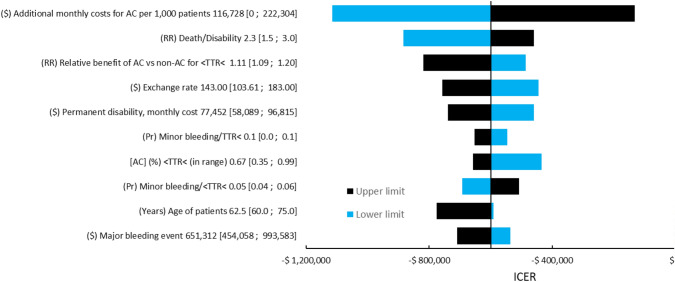
Figure 2 summarizes the deterministic sensitivity analysis using the discounted ICER of ACs compared with non-AC settings as a reference. All of the simulated ICERs were negative due to cost savings and QALY gains. The most influential parameters (i.e., drivers of results) were the monthly additional costs per 1000 patients for organizing an AC, the relative risk of death after an acute event, which results in disability for a patient, and the relative benefit of AC versus non-AC for patients in range. Supplementary Figure 4 shows the deterministic analysis of the comparison between ACs + POC and no ACs, while Supplementary Figure 5 shows the comparison between ACs + POC and ACs alone. The probabilistic sensitivity analysis is shown through a cost-effectiveness plane in Supplementary Fig. 2, where almost all of the simulated results analyses were cost effective (i.e., below the 1 or 3 gross domestic product per capita [GDPpc] thresholds). Finally, Supplementary Figure 3 shows the cost-effectiveness acceptability curve for the four scenarios analyzed. The probability of the implementation of ACs being cost effective is over 90% for a willingness to pay (WTP) of $0 per QALY and increases up to 100% for a WTP of 1 GDPpc. The other options analyzed also showed high probability of being cost effective at a 1 GDPpc threshold.
Fig. 2.
Deterministic sensitivity analysis on the incremental cost effectiveness ratio (ICER) for the implementation of anticoagulation clinics vs no anticoagulation clinics. Argentina, 2021. Values at the end of each variable line show base [min; max]. ($) Argentine pesos, AC anticoagulation clinic, LT, laboratory technician, POC point of care, (Pr) probability, (RR) relative risk, TTR time in therapeutic range, TTR< below therapeutic range, <TTR< in therapeutic range. All negative ICERs are cost-saving with QALY gains.
Budget Impact Analysis
Table 3 summarizes the results of the budget impact analysis for all scenarios analyzed. The number of adverse events avoided (and their associated costs) is greater with the intervention, both with and without POC use, than in the current scenario. In the case of implementation of ACs (i.e., [ACs] vs [no ACs]), savings were observed from the first year of implementation, with savings of AR $265,325 per 1000 patients by year 5. Adding POC in this context (i.e., [ACs + POC] vs [no ACs]) would increase savings up to AR $753,397 by year 5. Finally, the addition of POC devices in AC settings (i.e., [ACs + POC] vs [ACs]) and non-AC settings (i.e., [no ACs + POC] vs [No ACs]) would generate savings of AR $488,072 per 1000 patients by year 5 (AR $488 per patient).
Table 3.
Budget impact analysis results
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| Anticoagulation clinics [ACs] | |||||
| Current expenses from new structure (ACs) | $280,148 | $560,296 | $840,444 | $1,120,592 | $1,400,740 |
| Thrombosis attention | − $49,026 | − $98,051 | − $147,077 | − $196,103 | − $245,129 |
| Minor bleeding attention | − $156,187 | − $312,374 | − $468,560 | − $624,747 | − $780,934 |
| Major bleeding attention | − $120,035 | − $240,071 | − $360,106 | − $480,141 | − $600,176 |
| Disability | − $7965 | − $15,930 | − $23,895 | − $31,860 | − $39,825 |
| Total | − $53,065 | − $106,130 | − $159,194 | − $212,259 | − $265,324 |
| Use of point-of-care devices [POC] | |||||
| Use of POC devices (instead of central lab) | − $97,614 | − $195,229 | − $292,843 | − $390,458 | − $488,072 |
| Budget impact analyses | |||||
| [ACs] vs [No ACs] | − $53,065 | − $106,130 | − $159,195 | − $212,260 | − $265,325 |
| [No ACs + POC] vs [No ACs] | − $97,614 | − $195,229 | − $292,843 | − $390,458 | − $488,072 |
| [ACs + POC] vs [No ACs] | − $150,679 | − $301,359 | − $452,038 | − $602,717 | − $753,397 |
| [ACs + POC] vs [AC] | − $97,614 | − $195,229 | − $292,843 | − $390,458 | − $488,072 |
Anticoagulation clinics with and without POC devices and no anticoagulation clinics with and without POC devices. Costs expressed in local currency (Argentine pesos). Argentina, 2021
AC anticoagulation clinic, INR international normalized ratio, POC point of care
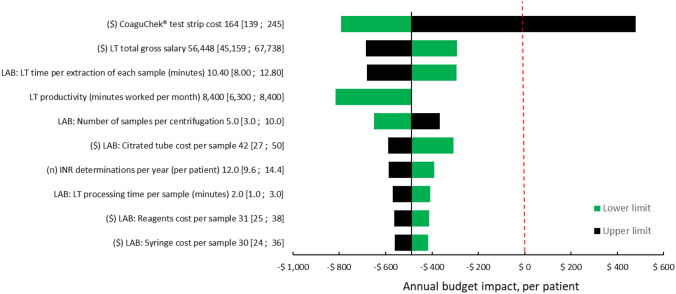
Figure 3 shows the deterministic sensitivity analysis for the budget impact analysis of the implementation of POC devices. The most influential parameters of the analysis were the costs of the CoaguChek INR determination (strips and lancets), and, to a lesser degree, the salary of the laboratory technician and the time taken for sample extraction. Supplementary Figure 6 shows the deterministic analysis for the implementation of ACs.
Fig. 3.
Deterministic sensitivity analysis on the annual budget impact for the use of POC devices vs central laboratory (in anticoagulation clinics or no anticoagulation clinics). Per patient, Argentina, 2021. Values at the end of each variable line show base [min; max]. ($) Argentine pesos, LAB laboratory, LT laboratory technician, POC point of care
Discussion
Many studies have shown that implementing ACs leads to fewer episodes of bleeding or thrombosis compared with individual monitoring by a primary care physician [42–45], but it was not clear whether these results could be applied in the Argentinean setting due to the characteristics specific to Argentina. Thus, our study objectives were to understand if implementing ACs with and without POC devices would be cost effective compared with non-AC settings, as well as to generate local evidence on the economic implications based on health-technology assessment recommendations and requirements [24]. To the best of our knowledge, this is the first economic study of its kind from the perspective of payers in the Argentinean health system.
The results of the CUA suggest that AC implementation, with and without the use of POC devices, can be cost effective for local payers, and the uncertainty analyses done suggest that these results are robust. Our findings align with the international evidence suggesting that implementing ACs is a cost-effective strategy [2, 16]; however, a key difference from our study when compared with CADTH 2014 [16], is that laboratory testing, in the base case analysis, was shown to be more costly than POC testing, mainly due to laboratory technician costs. On the clinical side, the benefits come from the increased average TTR and subsequent lower incidence of adverse events in the anticoagulated population. Although the implementation of an AC may require additional monthly expenses, the optimization of clinical management facilitates a reduction in thrombotic and hemorrhagic events.
Although we used models that were validated in previous studies [2, 16], it is important to interpret these results in the context of the specific parameters considered, both for the base case and for the expected variabilities. For example, the sensitivity analysis showed that savings from using POC devices depend heavily on the cost of test strips and the payer cost structure, so decision-makers should keep this in mind when analyzing results.
The addition of POC devices to ACs was shown to increase savings, mainly due to optimized extraction time and faster results, and no differences were seen in clinical outcomes in accordance with the conservative assumptions made. In addition to increased savings, we believe that the incorporation of POC devices also optimizes patient experience, as has been reported in the literature [46], although this parameter was not analyzed in the present study. In studies where a wider societal perspective has been adopted, self-management and self-testing strategies have proven to be more beneficial to society compared with standard clinic-based testing, as a result of lower time costs associated with fewer health service contacts [47].
This study has a number of limitations that make it necessary to interpret the results with caution. Although the parameters used were taken from specialized evidence, they may not necessarily reflect the reality of the entire spectrum of payers in Argentina. For this reason, several sensitivity analyses, both deterministic and probabilistic, were performed to analyze the impact of the uncertainty associated with the parameters. Although we used a local study as the basis for calculating the benefit of ACs versus non-AC settings [1], the relative benefit was estimated as a ratio of the average TTR of international studies, which is not free from bias, and we cannot ensure comparability with the health system in Argentina. It is worth mentioning the absence of local evidence for the utility weights of the model states. Our approach was to estimate them multiplying ratios to the health-related quality of life of the general Argentinian population, in order to have estimations adapted to the local setting instead of using international estimations, and to explore the uncertainty implication in the sensitivity analyses. Also, the main outcomes of the study, such as hemorrhagic and thrombotic events, were calculated using a model based on a surrogate outcome (i.e., TTR), while the optimal scenario would be to use evidence from controlled studies that analyze the outcomes of interest to the patient. Future investigations could include the use of POC devices in physician offices or by the patient at home. Additionally, education and training of anticoagulated patients could improve clinical outcomes; this was not assessed as part of the analysis. Other management strategies, such as self-monitoring, and adherence to the POC device, were also not considered as part of the model.
Another limitation is that the estimation of the additional monthly costs of implementing an anticoagulation clinic was based mainly on assumptions, as costs depend greatly on the baseline characteristics of payers and there are no hard-and-fast rules for estimating this (e.g., how many additional administrative staff would be required or if additional running costs would be needed). Therefore, and based on a pragmatic approach, we estimated these additional costs assuming a large variability in the parameters, which was reflected in the different sensitivity analyses performed.
Conclusions
The results of this study suggest that AC compared with non-AC settings may be a cost-effective strategy in Argentina and generate notable savings in the treatment of patients on long-term oral anticoagulant therapy. Using POC devices in ACs may result in additional savings to the health system due to lower laboratory technician costs per INR determination, depending on costs of test strips.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
This present study was supported by Roche Diagnostics International Ltd.
Prior presentations
N/A. COAGUCHEK, COBAS and COBAS T are trademarks of Roche.
Data availability
All data generated or analyzed during this study are included in this Published Article [And Its Supplementary Information Files].
Conflict of Interest
Osvaldo Ulises Garay and Gonzalo Guiñazú are employees of Roche Diagnostics. Yolanda Patricia Adamczuk and Cristina Duboscq do not report any conflict of interest.
Ethics approval
N/A
Consent to participate
N/A.
Consent for publication (from patients/participants)
N/A.
Code availability:
N/A
Authors’ contributions
Editorial assistance was provided by Erin Slobodian, BA, of Ashfield MedComms (Macclesfield, UK), an Ashfield Health company, funded by Roche Diagnostics International Ltd, Rotkreuz, Switzerland. Thanks to Daniel Vono, Analía Aimetta and Ludmila Frate for their valuable contributions. Study conception/Design: OUG, GG. Data acquisition: OUG, GG, YPA, CD. Data analysis and/or interpretation: OUG, GG. Manuscript writing and/or revision: OUG, GG, YPA, CD. Final version approval: OUG, GG, YPA, CD.
References
- 1.Tajer C, Ceresetto J, Bottaro FJ, Martí A, Casey M. Assessment of the quality of chronic anticoagulation control with time in therapeutic range in atrial fibrillation patients treated with vitamin K antagonists by hemostasis specialists: the TERRA registry: Tiempo en rango en la República Argentina. Clin Appl Thromb. 2017;23(5):445–453. doi: 10.1177/1076029615623378. [DOI] [PubMed] [Google Scholar]
- 2.Connock M, Stevens C, Fry-Smith A, et al. Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anticoagulation therapy: a systematic review and economic modelling. Health Technol Assess Winch Engl. 2007;11(38):iii–iv. doi: 10.3310/hta11380. [DOI] [PubMed] [Google Scholar]
- 3.Point-of-Care International Normalized Ratio (INR) Monitoring devices for patients on long-term oral anticoagulation therapy. Ont Health Technol Assess Ser. 2009;9(12):1–114. [PMC free article] [PubMed] [Google Scholar]
- 4.Levi M, Hobbs FDR, Jacobson AK, et al. Improving antithrombotic management in patients with atrial fibrillation: current status and perspectives. Semin Thromb Hemost. 2009;35(6):527–542. doi: 10.1055/s-0029-1240013. [DOI] [PubMed] [Google Scholar]
- 5.d’Arcy JL, Prendergast BD, Chambers JB, Ray SG, Bridgewater B. Valvular heart disease: the next cardiac epidemic. Heart Br Card Soc. 2011;97(2):91–93. doi: 10.1136/hrt.2010.205096. [DOI] [PubMed] [Google Scholar]
- 6.Eikelboom JW, Hart RG. Antithrombotic therapy for stroke prevention in atrial fibrillation and mechanical heart valves. Am J Hematol. 2012;87(Suppl 1):S100–107. doi: 10.1002/ajh.23136. [DOI] [PubMed] [Google Scholar]
- 7.Ryan F, Byrne S, O’Shea S. Managing oral anticoagulation therapy: improving clinical outcomes. A review. J Clin Pharm Ther. 2008;33(6):581–590. doi: 10.1111/j.1365-2710.2008.00959.x. [DOI] [PubMed] [Google Scholar]
- 8.Ceresetto J, Duboscq C, Korin J, Fondevila C, Casais P, Rossi A. Consenso argentino en gestión efectiva de clínicas de anticoagulación para uso de antagonistas de la vitamina K. Med B Aires. 2020;80(4):1–26. [PubMed] [Google Scholar]
- 9.Keeling D, Baglin T, Tait C, et al. Guidelines on oral anticoagulation with warfarin—fourth edition. Br J Haematol. 2011;154(3):311–324. doi: 10.1111/j.1365-2141.2011.08753.x. [DOI] [PubMed] [Google Scholar]
- 10.Ansell J, Hirsh J, Dalen J, et al. Managing oral anticoagulant therapy. Chest. 2001;119(1 Suppl):22S–38S. doi: 10.1378/chest.119.1_suppl.22s. [DOI] [PubMed] [Google Scholar]
- 11.Ansell JE, Hughes R. Evolving models of warfarin management: anticoagulation clinics, patient self-monitoring, and patient self-management. Am Heart J. 1996;132(5):1095–1100. doi: 10.1016/s0002-8703(96)90040-x. [DOI] [PubMed] [Google Scholar]
- 12.Berrettini M. Anticoagulation clinics: the Italian experience. Haematologica. 1997;82(6):713–717. [PubMed] [Google Scholar]
- 13.Pengo V, Denas G. Optimizing quality care for the oral vitamin K antagonists (VKAs) Hematol Am Soc Hematol Educ Program. 2018;2018(1):332–338. doi: 10.1182/asheducation-2018.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasan SS, Sunter W, Ahmed N, et al. A comparison of warfarin monitoring service models. Res Soc Adm Pharm RSAP. 2019;15(10):1236–1242. doi: 10.1016/j.sapharm.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 15.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–239. doi: 10.1055/s-0038-1651587. [DOI] [PubMed] [Google Scholar]
- 16.Point-of-Care Testing of International Normalized Ratio for Patients on Oral Anticoagulant Therapy: Systematic Review and Economic Analysis. Canadian Agency for Drugs and Technologies in Health; 2014. http://www.ncbi.nlm.nih.gov/books/NBK361419/. Accessed 14 Apr 2021. [PubMed]
- 17.Sunderji R, Gin K, Shalansky K, et al. Clinical impact of point-of-care vs laboratory measurement of anticoagulation. Am J Clin Pathol. 2005;123(2):184–188. doi: 10.1309/P2Y3VM4AXPVUDAW3. [DOI] [PubMed] [Google Scholar]
- 18.van den Besselaar AMHP, Péquériaux NCV, Ebben M, et al. Point-of-care monitoring of vitamin K-antagonists: validation of CoaguChek XS test strips with International Standard thromboplastin. J Clin Pathol. 2012;65(11):1031–1035. doi: 10.1136/jclinpath-2012-200934. [DOI] [PubMed] [Google Scholar]
- 19.CoaguChek XS System User Manual. https://diagnostics.roche.com/us/en/resource-center-pages/coaguchek-xs-system-user-manual-04837991001-07.html. Accessed 14 Apr 2021.
- 20.Bereznicki LR, Jackson SL, Peterson GM, Jeffrey EC, Marsden KA, Jupe DM. Accuracy and clinical utility of the CoaguChek XS portable international normalised ratio monitor in a pilot study of warfarin home-monitoring. J Clin Pathol. 2007;60(3):311–314. doi: 10.1136/jcp.2006.037820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobieraj-Teague M, Daniel D, Farrelly B, Coghlan D, Gallus A. Accuracy and clinical usefulness of the CoaguChek S and XS Point of Care devices when starting warfarin in a hospital outreach setting. Thromb Res. 2009;123(6):909–913. doi: 10.1016/j.thromres.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Wieloch M, Hillarp A, Strandberg K, Nilsson C, Svensson PJ. Comparison and evaluation of a Point-of-care device (CoaguChek XS) to Owren-type prothrombin time assay for monitoring of oral anticoagulant therapy with warfarin. Thromb Res. 2009;124(3):344–348. doi: 10.1016/j.thromres.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Nam MH, Roh KH, Pak HN, et al. Evaluation of the Roche CoaguChek XS handheld coagulation analyzer in a cardiac outpatient clinic. Ann Clin Lab Sci. 2008;38(1):37–40. [PubMed] [Google Scholar]
- 24.Pichon-Riviere A, Drummond M, García Martí S, Augustovski F. Aplicación de La Evidencia Económica En La Evaluación de Tecnologías Sanitarias y La Toma de Decisiones Sobre Asignación de Recursos Sanitarios En América Latina: Siete Temas Clave y Una Propuesta Preliminar de Implementación. Inter-American Development Bank; 2021. 10.18235/0003649.
- 25.MERCOSUR. Directrices para la evaluación económica de tecnologías en salud. Published online 2009. https://bancos.salud.gob.ar/sites/default/files/2018-10/0000000626cnt-4-guia-evaluacion-econ.pdf. Accessed 20 Apr 2021.
- 26.Augustovski F, Garay OU, Pichon-Riviere A, Rubinstein A, Caporale JE. Economic evaluation guidelines in Latin America: a current snapshot. Expert Rev Pharmacoecon Outcomes Res. 2010;10(5):525–537. doi: 10.1586/erp.10.56. [DOI] [PubMed] [Google Scholar]
- 27.WebINDEC-Sociedad/Salud/Indicadores de salud. https://sitioanterior.indec.gob.ar/nivel4_default.asp?id_tema_1=4&id_tema_2=32&id_tema_3=94. Accessed 28 Apr 2021.
- 28.Labadet C, Ferreirós ER, Toro DD, et al. Análisis de sobrevida a los 2 años de seguimiento del Primer Estudio Nacional, Multicéntrico y Prospectivo de Fibrilación Auricular Crónica en la República Argentina. Rev Argent Cardiol. 2005;73:9. [Google Scholar]
- 29.Janssen MF, Szende A, Cabases J, Ramos-Goñi JM, Vilagut G, König HH. Population norms for the EQ-5D-3L: a cross-country analysis of population surveys for 20 countries. Eur J Health Econ HEPAC Health Econ Prev Care. 2019;20(2):205–216. doi: 10.1007/s10198-018-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pink J, Lane S, Pirmohamed M, Hughes DA. Dabigatran etexilate versus warfarin in management of non-valvular atrial fibrillation in UK context: quantitative benefit-harm and economic analyses. BMJ. 2011;343:d6333. doi: 10.1136/bmj.d6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giorgi MA, Caroli C, Giglio ND, et al. Estimation of the cost-effectiveness of apixaban versus vitamin K antagonists in the management of atrial fibrillation in Argentina. Health Econ Rev. 2015;5:17. doi: 10.1186/s13561-015-0052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Registro de la cotización del precio histórico del Dólar en el mercado informal en Argentina del año 2021. Published 2021. https://www.cotizacion-dolar.com.ar/dolar-blue-historico-2021.php. Accessed 1 Nov 2021.
- 33.Fattore G, Torbica A, Susi A, et al. The social and economic burden of stroke survivors in Italy: a prospective, incidence-based, multi-centre cost of illness study. BMC Neurol. 2012;12:137. doi: 10.1186/1471-2377-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Bastida J, Oliva Moreno J, Worbes Cerezo M, Perestelo Perez L, Serrano-Aguilar P, Montón-Álvarez F. Social and economic costs and health-related quality of life in stroke survivors in the Canary Islands, Spain. BMC Health Serv Res. 2012;12:315. doi: 10.1186/1472-6963-12-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Araújo DV, Teich V, Passos RBF, Martins SCO. Análisis de costo-efectividad de la trombólisis con alteplase en el accidente vascular cerebral. Arq Bras Cardiol. 2010;95:12–20. doi: 10.1590/S0066-782X2010005000067. [DOI] [PubMed] [Google Scholar]
- 36.Nomenclador del Sistema Único de Prestaciones Básicas para Personas con Discapacidad. Actualización 2021. https://www.sssalud.gob.ar/padron/discapacidad/nomenclador_prestaciones.pdf. Accessed 5 July 2021.
- 37.Instituto de Efectividad Clínica y Sanitaria (IECS). Consorcio de costos en salud e impacto presupuestario. Published 2020. https://sheet2site.com/api/v3/index.php?key=1NwqZ8e_2Loe2SGhFTlZmEUBwN-UNkioailThHhWooVk. Accessed 18 May 2021.
- 38.Melcon CM, Melcon MO. Prevalence of stroke in an Argentine community. Neuroepidemiology. 2006;27(2):81–88. doi: 10.1159/000094978. [DOI] [PubMed] [Google Scholar]
- 39.Comisión Nacional de Trabajo en Casas Particulares 2021. https://www.boletinoficial.gob.ar/detalleAviso/primera/246035/20210625. Accessed 15 Aug 2021.
- 40.Raúl Izaguirre Ávila, Pablo Acevedo Gómez, Evelyn Cortina de la Rosa. Manejo de las clinicas de anticoagulantes: organización del tratamiento con antagonistas de la vitamina K. Permanyer; 2012.
- 41.Caldwell D, Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Int J Epidemiol. 2007;36(2):476–477. doi: 10.1093/ije/dym062. [DOI] [Google Scholar]
- 42.Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257–3291. doi: 10.1182/bloodadvances.2018024893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palareti G, Antonucci E, Migliaccio L, et al. Vitamin K antagonist therapy: changes in the treated populations and in management results in Italian anticoagulation clinics compared with those recorded 20 years ago. Intern Emerg Med. 2017;12(8):1109–1119. doi: 10.1007/s11739-017-1678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnes GD, Kline-Rogers E, Graves C, et al. Structure and function of anticoagulation clinics in the United States: an AC forum membership survey. J Thromb Thrombolysis. 2018;46(1):7–11. doi: 10.1007/s11239-018-1652-z. [DOI] [PubMed] [Google Scholar]
- 45.Fihn SD. Aiming for safe anticoagulation. N Engl J Med. 1995;333(1):54–55. doi: 10.1056/NEJM199507063330112. [DOI] [PubMed] [Google Scholar]
- 46.Duboscq C, Ceresetto JM, Stemmelin G, et al. Performance of a point of care device for determination of international normalised ratio in an anticoagulation clinic. Hematología. 2014;18(3):204–210. [Google Scholar]
- 47.Sharma P, Scotland G, Cruickshank M, et al. Assessment of cost-effectiveness. NIHR Journals Library; 2015. https://www.ncbi.nlm.nih.gov/books/NBK304015/. Accessed 18 Oct 2021.
- 48.Sundberg G, Bagust A, Terént A. A model for costs of stroke services. Health Policy. 2003;63(1):81–94. doi: 10.1016/S0168-8510(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 49.Sindicato Unico de Trabajadores de la Ciudad de Buenos Aires (SUTECBA). Grilla Salarial . 2021. http://sutecba.org.ar/grilla2021.php. Accessed 14 Apr 2021.
- 50.Leung ACP, Li SW, Tsang RHN, Tsao YC, Ma ESK. Audit of phlebotomy turnaround time in a private hospital setting. Clin Leadersh Manag Rev J CLMA. 2006;20(3):E3. [PubMed] [Google Scholar]
- 51.Funk DM, Clinical and Laboratory Standards Institute. Collection, Transport, and Processing of Blood Specimens for Testing Plasma-Based Coagulation Assays and Molecular Hemostasis Assays: Approved Guideline. Clinical and Laboratory Standards Institute; 2008.
- 52.Fitch JC, Mirto GP, Geary KL, Byrne DW, Hines RL. Point-of-care and standard laboratory coagulation testing during cardiovascular surgery: balancing reliability and timeliness. J Clin Monit Comput. 1999;15(3–4):197–204. doi: 10.1023/a:1009934804369. [DOI] [PubMed] [Google Scholar]
- 53.MERCOSUR. Guía Metodólogica para Estudios de Evaluación Económica de Tecnologías Sanitarias. Published online 2009. https://bancos.salud.gob.ar/sites/default/files/2018-10/0000000626cnt-4-guia-evaluacion-econ.pdf. Accessed 20 Apr 2021.
- 54.GDP per capita (current US$)-Argentina|Data. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=AR. Accessed 30 Nov 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this Published Article [And Its Supplementary Information Files].