Abstract
Lignans have long been known for their abundant therapeutic properties due to their polyphenolic structure. Linseed is the richest plant source of lignans and has been studied widely for their properties. The most prevalent lignan, secoisolariciresinol diglucoside (SDG), is consumed with linseed and converted into mammalian lignans, enterodiol (END) and enterolactone (ENL), by the gut microbiota. SDG can easily be assessed using HPLC and its deglycosylated form viz secoisolariciresinol can be asses using GC–MS techniques. Variety of extraction and analysis methods has been reported for plant lignans. SDG is known to have therapeutic properties including anti-oxidant, anti-cancerous, anti-inflammatory, modulation of gene expression, anti-diabetic, estrogenic and anti-estrogenic. Despite a large number of bioactivities, strong evidences for the underlying mechanisms for most of the properties are still unknown. SDG is most studied for its anti-cancerous properties. But the use of lignans as anti-carcinogenic agent is limited and commercially not reported due to challenges of purification at commercial level, rapid metabolism, untargeted delivery and toxic compounds associated with lignans. Exploration of more prominent and active derivatives of SDG and their targeted drug delivery should be an important research toward the use of bioactive lignans of linseed.
Keywords: SDG, Polyphenols, Antioxidants, Phytochemical, Bioactive compounds, Phytolignan, Enterolignan, Anticancer
Introduction
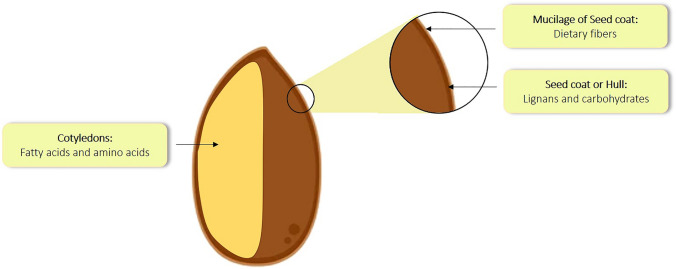
Plant lignans are polyphenols made up of coniferyl alcohols precursors. They are bi-phenolic compounds found in plants and have an oestrogen-like structure, thus called as phyto-oestrogens having anti-oxidant properties (Adlercreutz 2007). Fibre-rich foods (such as seeds, grains, vegetables, and fruits) are the most prevalent source of phytoestrogens in Western societies (Sonestedt and Wirfält 2010). In comparison to other sources, Linseed (Linum usitatissimum) has a high concentration of lignans (Smeds et al. 2007). Lignans are found in the secondary wall of sclerite cells of the linseed's outer integument (Attoumbré et al. 2011). In humans also, these phytoestrogens possess a week oestrogen activity. Plant lignans include a number of bi-phenolic compounds such as secoisolariciresinol diglucoside (SDG), secoisolariciresinol (SECO), pinoresinol (PINO), matairesinol (MATA), lariciresinol (LARI), medioresinol (MED), syringaresinol (SYR), sesamin (SES), 7′-hydroxymatairesinol and isoLARI (Smeds et al. 2007). Linseed is a particularly rich source of SDG, which is the glycosylated form of SECO (Qiu et al. 2020). Although other lignans such as MATA, PINO, LARI, PINO diglucoside, isoLARI, and a diastereomer of SDG are also present in linseed in small amounts. SDG is highly soluble in both water and alcohol (Bakke and Klosterman 1956). The UV absorption maxima of SDG and SECO are attained at 280 nm, which is typical of lignans (Fuentealba et al. 2015). SDG is found in the form of an oligomer made up of five interconnected SDG units. These SDG units are ester linked via 3-hydroxy-3-methylglutaric acid (HMGA). Five SDG residues are interconnected by 4 HMGA residues in a straight chain oligomeric structure (Ford et al. 2001; Kamal-eldin et al. 2001). Linseed contains 75–800 times the amount of SDG found in any other food (Hosseinian et al. 2006). Also, the concentration of SDG in linseed varies with different varieties, locations and growing conditions. The review delves in the details of the important aspects such as biosynthesis, biotransformation, therapeutic applications and challenges of the most prevalent lignans in linseed.
Cultivation and distribution of linseed
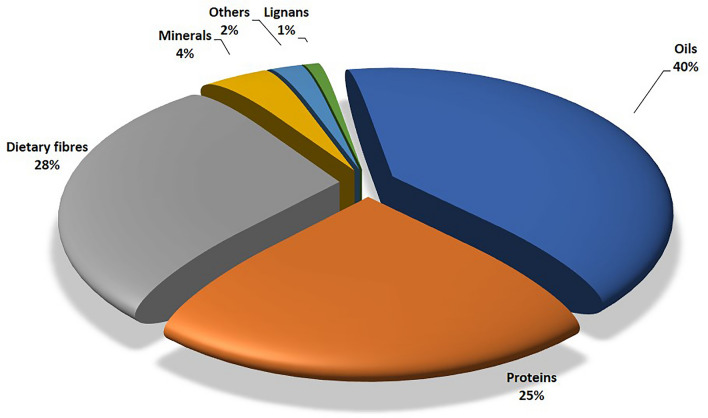
Linseed is one of the oldest crop grown for oil, fibre, and food since the dawn of civilization (Vaisey-Genser et al. 2003). It has long been valued for its oil, known as linseed oil, which is used for variety of applications in industries. Linseed and flaxseed are two words that are used interchangeably. Flaxseed refers to flax that is consumed by humans, while linseed is used when it is used for commercial purposes. Flaxseed is being consumed by the humans since ancient times. At present, it is cultivated in more than 50 countries around the world. The important flaxseed growing countries include Kazakhstan, Russia, Canada, China, United States of America, India, Ethiopia, France and United Kingdom (FAOSTAT 2020). India ranks sixth among the leading linseed producing countries in the world. According to the FAO 2020 statistics, India contributed about 121 million tonnes of linseed in 2020 of 3068 million tonnes world linseed production. Thus, India produced about 3.94% of the world’s linseed production (FAOSTAT 2020). It is interesting to know that linseed is native of India and was grown as a staple food crop. Though, it is no longer a staple crop, however, linseed is still used as a food and its use for therapeutic purposes has increased tremendously in recent times (Shakir and Madhusudhan 2007). The seed contains around 38–44% edible oil (Fig. 1) (Shim et al. 2014), which is primarily found in oleosomes known as oil bodies (Gui et al. 2012). They function as energy reserves during the germination and growth stages of plant, when metabolism is at its peak. A high content of omega-3 fatty acids, especially α-linolenic acid (ALA) (50–65%), provides significant dietary benefit linked to improvements in blood lipids (Shim et al. 2014). Linseed is also high in lignans, soluble and insoluble fibres, cyclic peptides, and a variety of minerals (Fig. 1). Linseed is gaining recognition in food supply of world as a functional food, characterized by its high levels of ALA, dietary fibres, and lignans. It contains about 28% of dietary fibres, 6% carbohydrates, 21–23% of proteins, up to 1% lignans and 4% of ash (Cloutier 2016). Linseed and its constituents provide physiological benefits and/or assistance in illness prevention and/or treatment. Due to the possible health benefits linked with some of its physiologically active components: oil containing high ALA and the presence of phyto-oestrogenic lignans Secoisolariciresinol Diglucoside (SDG), linseed has sparked greater interest in the field of food, nutraceuticals and medical research (Singh et al. 2011; Ivanov et al. 2011).
Fig. 1.
Nutrients composition of linseed
Lignans content is influenced by type of species, physiological and environmental conditions to which the species is exposed, and the genetic composition. The amount of lignan generated by plants is far less than the high demand for it in medicine. Increased lignan production may boost the crop's potential to meet pharmaceutical demand while also improving its nutritional and agronomic value (Rodríguez-García et al. 2019). Elicitation or metabolic engineering can be used to increase lignan content in potential sources.
Linseed lignans
Lignans are present all across the kingdom Plantae and are present in almost all the plants. Dietary lignans are fibre-associated lignans; thus, present in fibre rich foods including whole grains like rye (Secale cereale), oats (Avena sativa), barley (Hordeum vulgare), oilseeds like flax (Linum usitatissimum), sunflower (Helianthus annuus), and sesame (Sesamum indicum), cereal, fruits (particularly berries) and various processed foods (Thompson et al. 2006, 2009; Smeds et al. 2007). SECO and MATA were the first plant lignans discovered in foods, followed by PINO and LARI, which were more recently identified. About 75% of LARI and PINO and 25% of SECO and MATA was found to contribute in total lignan dietary intake (Gutte et al. 2015). As lignans are present in the secondary wall of sclerite cells, the bioavailability can be increased by crushing or milling the seeds (Venglat et al. 2011). Lignans aren’t present in the flaxseed oils unless the seeds are added to oil in ground form. Thus, they are not associated with oils of seeds. The concentration of lignans in different oil seeds, grains and cereals analyzed by various scientists using HPLC- mass spectrometry (Smeds et al. 2007; Milder et al. 2005).
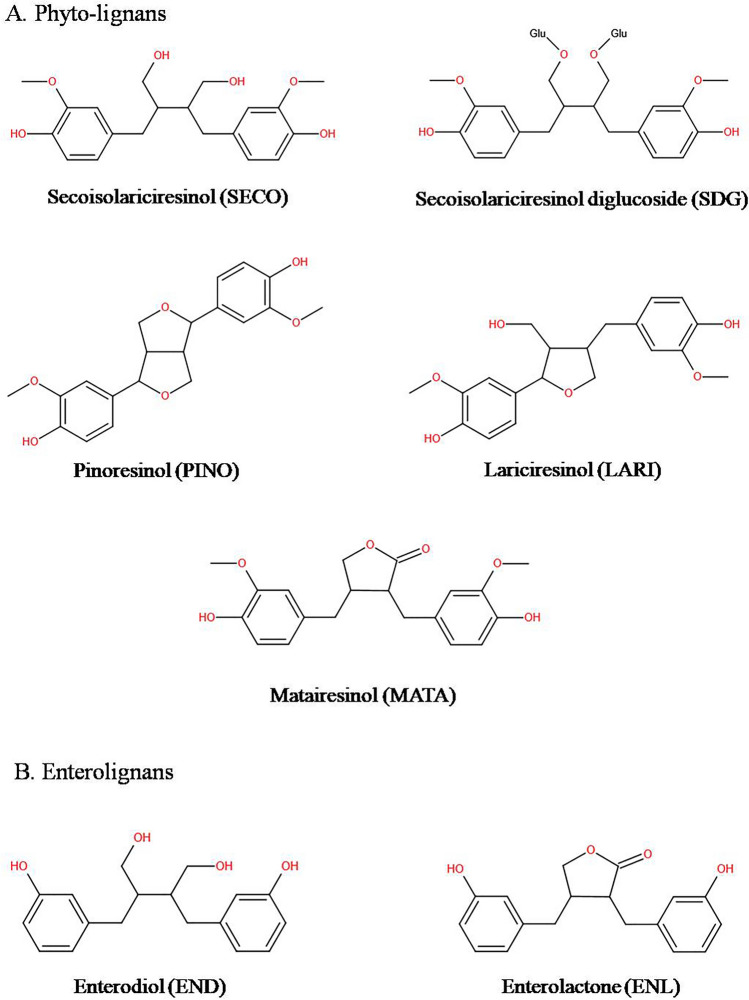
The most common lignan in flaxseed is SDG (MW = 686.7 Da), which depending on method of analysis often known as secoisolariciresinol or SECO (M.W = 362.3 Da), the aglycone of SDG (Toure and Xueming 2010). The molecular formula of SDG and SECO is C32H46O16 and C20H26O6. SDG exists in 2 isomeric forms in flaxseed as ( +)-SDG which is the major isomer (99%) and (−)-SDG which is a minor isomer (~ 1%) (Moree and Rajesha 2011). SECO is present in mono (SMG) or diglycosylated (SDG) forms. SDG or SMG are linked by 3-hydroxy-3-methyl-glutaryl units which are derived from 3-hydroxy-3-methyl-glutaric acid (HMGA). Thus, it forms a biopolymer where five SDG or SMG are interlinked with four HMG units by ester linkage in a straight chain. SDG isolated from a fat free linseed meal extract produces up to 3% yield. Once ingested, SDG is transformed to active mammalian lignans; ED and EL in colon by gut Microbiota and are identified as metabolic products of dietary lignans (Toure and Xueming 2010). Due to their colonic origin these metabolites (END and ENL) are generally called as enterolignans (Durazzo et al. 2013). SECO, SDG, MATA, PINO and LARI are some of the major lignans present in flaxseeds (Fig. 2).
Fig. 2.
Structures of linseed phytolignans and enterolignans
There are other related compounds which co-occur with lignans or neolignans known as nor-lignans and contain a C16–C17 core structure. Lignans can also exist in several different skeleton forms such as two bicyclic structures and three monocyclic structures and two tricyclic structures, one acyclic structure (Chhillar et al. 2020). These prominently help in reducing the growth of tumours, such as breast, prostate and endometrium cancerous tumour that is hormone sensitive (Toure and Xueming 2010). According to the oxygen incorporation and cyclization pattern, there are eight structural sub-groups of lignans such as, dibenzylbutyrolactol, dibenzocyclooctadiene, arylnaphthalene, aryltetralin, furan, furofuran, dibenzylbutane and dibenzylbutyrolactone. These groups are further divided according to non-propyl aromatic rings identities present on side chains and oxidation level of lignan molecule (Rodríguez-García et al. 2019). Dibenzylbutanes are considered as the simplest non-cyclic lignans and are found to be the dimers of phenylpropanes which have β-β’ linkages exhibiting antioxidant activity due to their multiple oxidation states accounting. Because of the multiple oxidation states throughout the butane chain, dibenzylbutane lignans have more variability (Cui et al. 2020). SECO is the main dibenzylbutane lignan present in linseed. On the other hand, furofuran lignans are classified as a large group of bisepoxy lignan consisting of 2,6-diaryl-3,7-dioxabicyclo octane skeleton and also have different substitutions at aryl group which further result in different structures. Furofurans exhibits several biological activities such as antioxidant, antimicrobial and anti-inflammatory activities. Furofurans can also be further classified as mono, di or non-oxidized based on the furofuran ring oxidation conditions. Some important furofuran lignans are Sesamin (SES), LARI, 4β-hydroxyasarinin-1-O-β-glucopyranoside, eudesmin, and PINO. Another subclass of lignans called dibenzocyclooctadiene constitutes the derivatives with C-6, C-6’biaryl bond. In dibenzocyclooctadienes, an eight membered ring structure is also present in addition to the biphenyl rings (Chhillar et al. 2020). Another subclass of lignans called dibenzylbutyrolactones are based on the dibenzylbutanes, with 9–9’ epoxy and C9 carbonyl. These are also known as lignan-β-β’–lactones or lignanolides. These are also important bioactive lignans with antimicrobial, cytotoxic and anti-inflammatory roles. MATA is the main dibenzylbutyrolactone present in linseed (Cui et al. 2020).
The highest lignans in linseed reported so far is 702,050 µg/100 g of linseed. Specifically in linseed, SECO was found to be 690,757 µg/100 g, which is highest than any other plant food, followed by 335 µg/100 g of LARI, 401 µg/100 g of PINO, 42.3 µg/100 g of MATA and small amounts of other minor lignans (Smeds et al. 2012). Currently, analysis of germplasm collection available in India is being analyzed for the lignan content under DBT supported Government of India project (Kaushik et al. 2022 unpublished data). The highest lignan content recorded so far in this project is 1395608 µg/100 g. Other than food products like sesame seeds and flaxseeds which are rich in lignans, wood knots in coniferous trees like Norway spruce (Picea abies) is found to be a concentrated source of lignan contain on average 5–10% lignans in the knots, with hydroxymatairesinol comprising 50–85% of the lignans (Holmbom et al. 2003).
Lignans in different food sources
Grain, cereals, beans, nuts, and seeds are high in fiber and lignan, making them excellent protective foods (Fig. 3). Flaxseed enriched bread was also found to be very good dietary form of consuming lignans. It was observed that even after baking, 89% of the original lignan content was present in such breads. A large variability was also found in the SDG concentration of whole flaxseed flour (WFF) and defatted flaxseed flour (DFF) as about 1.54% of SDG content was found in DFF on a dry matter basis. Thus, this result with previous findings was in agreement which showed the presence of SDG content between the range of 0.6 and 2.9% in DFF (Simbalista et al. 2012). LARI and PINO were found to have high concentration in most of the items as compared to SECO as different raw materials which were used have different refinement degree are mainly responsible for presence of variety of lignan concentration in cereals. The sum of LARI, SECO and PINO in breakfast cereals was found in a range between 209 and 764 μg/100 g wet basis (Durazzo et al. 2013). Other foods and food products like vegetables reported a total lignan content of the sum of SECO, MATA, LARI, and PINO 1325 µg/100 g for broccoli (Brassica oleracea), 185 µg/100 g for cauliflower (Brassica oleracea), 171 µg/100 g for carrot (Daucus carota), 58 µg/100 g for tomato (Solanum lycopersicum), and 48 µg/100 g for chicory (Cichorium intybus) of fresh edible weight. Another example of asparagus (Asparagus officinalis) showed different lignan concentration such as PINO upto 49 µg/100 g wet basis, LARI upto 47 µg/100 g wet basis, SECO upto183 µg/100 g wet basis, SYR upto 58 µg/100 g wet basis and MATA upto 2 µg/100 g wet basis while for food products like tomato (Solanum lycopersicum), and radish (Raphanus sativus) showed high LARI concentration. Sesame (Sesamum indicum) seeds was found to have PINO and LARI) as major lignans and with relatively high lignan concentration of about 29,331 mg/100 g while lignan concentration in grain products ranged from 7 to 764 mg/100 g. Vegetables from family Brassica contain high level of lignans mainly PINO and LARI with concentration of 185–2321 mg/100 g. in most cases the amount of LARI and PINO was high as compared to that of SECO and MATA (Milder et al. 2005).
Fig. 3.
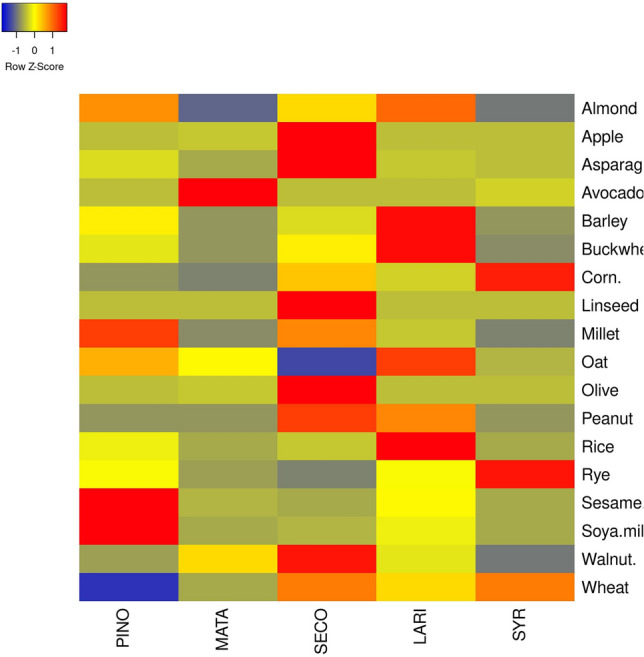
Heatmap of lignans composition in different food sources. Whereas, PINO pinoresinol, MATA matairesinol, SECO secoisolariciresinol, LARI lariciresinol, SYR syringaresinol
Biosynthesis of lignans
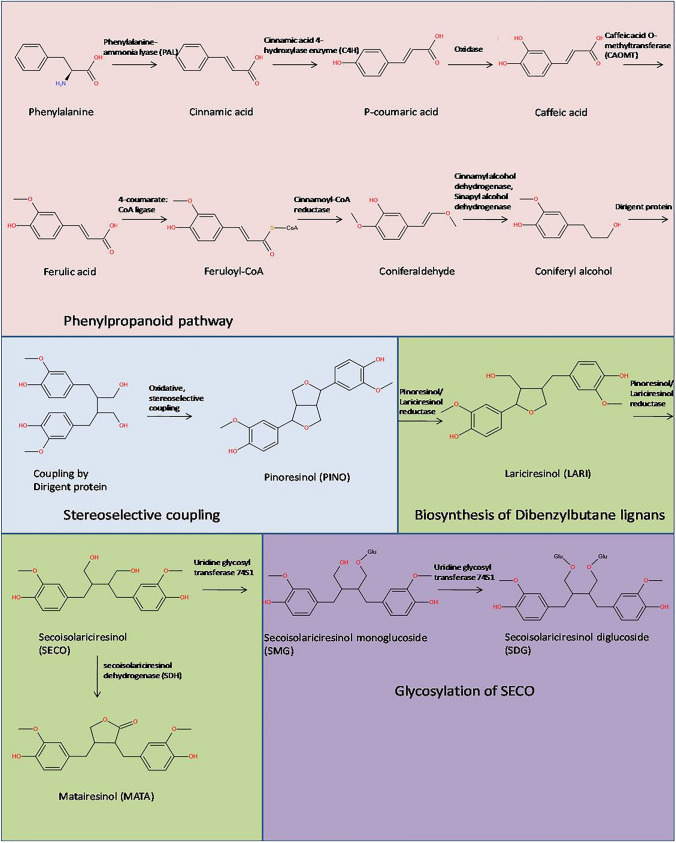
Lignans are synthesized by the regional- and stereospecific coupling of biomolecular phenoxy radicals, which is mediated by dirigent proteins (Pickel et al. 2010). They're synthesized from the phenylpropanoid pathway (Fig. 4), with coniferyl alcohol as a precursor. In the presence of dirigent proteins, two molecules of coniferyl alcohol undergo a coupling reaction with free radicals to form PINO. PINO further gets reduced stereo-selectively in sequential manner to form ( +)-LARI and later SECO. Two iso-functional forms of ( +)-PINO/( +)-LARI reductase catalyze this NADPH-dependent cascade of sequential reductive steps. SECO is then glycosylated to form SDG, this reaction is catalyzed by SECO diglucosyl transferase. Elucidation of the lignan biosynthetic pathway leads to the development of strategies for enhancing the levels of SDG in staple dietary foodstuffs and maximizing yields of lignans used to treat or protect against human disease. The biosynthesis of linseed involves four pathways which are (1) the phenylpropanoid pathway (2) stereospecific coupling by dirigent proteins (3) biosynthesis of dibenzylbutane lignans and (4) glycosylation of lignans into SDG.
Fig. 4.
Biosynthesis of Lignans
The phenyl propanoid pathway
Initiation of lignan biosynthesis is through phenylpropanoid pathway, which involves the formation of C6-C3 basic unit of lignans (Fig. 5). Cinnamic acid is synthesized in the first stage of this process by deamination of phenylalanine, which is mediated by the enzyme phenylalanine-ammonia lyase (PAL) (Struijs 2008). Cinnamic acid is converted to p-coumaric acid in an oxidation reaction, which can then be converted to caffeic acid by cinnamic acid 4- hydroxylase enzyme (C4H). Caffeic acid is further converted into ferulic acid by caffeic acid O-methyltransferases (CAOMT) activity (Boerjan et al. 2003). 4-coumarate: CoA ligase further converts ferulic acid to its coenzyme A-activated form, feruloyl-CoA. In the penultimate stage, cinnamoyl-CoA reductase reduces feruloyl-CoA to coniferaldehyde, which is then converted to coniferyl alcohol by the enzymatic activity of cinnamyl alcohol dehydrogenase and sinapyl alcohol dehydrogenase (Kezimana et al. 2018; Boerjan et al. 2003).
Fig. 5.
Distribution of bioactive and nutritive compounds in linseed
Stereo-selective coupling by dirigent proteins
The coniferyl alcohol synthesized in phenyl propanoid pathway then undergoes stereoselective radical dimerization by dirigent protein (Fig. 4). Elucidation of the pathway leading to the cancer chemopreventive agent SDG was first accomplished using Forsythia intermedia. In this pathway, two molecules of coniferyl alcohol undergoes oxidative (Pickel et al. 2010), stereoselective coupling mediated by the activity of dirigent protein which leads to the formation of PINO (Corbin et al. 2018). Following the production of PINO, lignan biosynthesis can take one of two routes. The furan structures are reduced in the first pathway, and dibenzylbutane lignans, such as SECO, are produced. The furan structures are preserved in the second pathway, and methylenedioxy bridged furanofuran lignans, such as SES, are generated (Suzuki and Umezawa 2007). The biosynthesis of flaxseed follows the first pathway.
Biosynthesis of Dibenzylbutane lignans
This pathway involves the formation of dibenzylbutane lignans such SECO and LARI (Fig. 4) (Kezimana et al. 2018; Schmidt et al. 2012). This process involves a bi-functional NADPH-dependent PINO/LARI reductase (PLR). This NADPH-dependent PLR catalyzes the conversion of PINO to LARI and then LARI to SECO in linseed (Hemmati et al. 2007; Hano et al. 2006). MATA is also synthesized in this pathway from SECO catalyzed by secoisolariciresinol dehydrogenase (SDH) (Ford et al. 2001; Xia et al. 2001). (See. Table 1)
Table 1.
Lignan composition in different food sources in (µg/100 g) dry weight
| Food sources | PINO | MATA | SECO | LARI | SYR | References |
|---|---|---|---|---|---|---|
| Almond | 208 | 24 | 159 | 233 | 40 | Smeds et al. (2007) |
| Apple | – | 0.027 | 1.79 | 0.01 | – | Rodríguez-García et al. (2019) |
| Asparagus | 122 | 14 | 743 | 92 | 58 | Landete (2012) |
| Avocado | – | 7.67 | 0.02 | 0.03 | 0.44 | Rodríguez-García et al. (2019) |
| Barley | 45 | – | 28 | 132 | – | Durazzo et al. (2013) |
| Black tea | 2.70–2.6 | 1.1–1.5 | 5.0–6.2 | 28.9–30.7 | – | Landete (2012) |
| Buckwheat | 92 | 1 | 131 | 362 | – | Durazzo et al. (2013) |
| Corn | 33 | 21 | 125 | 69 | 220 | Smeds et al. (2007) |
| Flaxseed | 3324 | 553 | 294,210 | 3041 | – | Milder et al. (2005) |
| Millet | 85 | 3 | 67 | 20 | – | Durazzo et al. (2013) |
| Oat | 567 | 440 | 90 | 766 | 297 | Landete (2012) |
| Olive | – | 2.7 | 55.9 | – | – | Landete (2012) |
| Peanut | 0 | 0 | 53 | 41 | – | Landete (2012) |
| Rice | 29 | – | 15 | 128 | – | Durazzo et al. (2013) |
| Rye | 1547 | 729 | 468 | 1505 | 3540 | Landete (2012) |
| Sesame seed | 47,136 | 1137 | 240 | 13,060 | 205 | Gutte et al. (2015) |
| Soya milk | 30.0 | 0.0 | 1.1 | 6.6 | – | Landete (2012) |
| Walnut | 34 | 60 | 99 | 47 | 24 | Smeds et al. (2007) |
| Wheat | 138 | 410 | 868 | 672 | 882 | Landete (2012) |
PINO pinoresinol, MATA matairesinol, SECO secoisolariciresinol, LARI lariciresinol, SYR syringaresinol
Glycosylation of SECO
Glycosylation is an important mechanism that defines the chemical complexity and diversity of plant natural products, as well as their chemical stability and water solubility, while lowering the reactivity and toxicity of compounds. Members of the glycosyl transferases (GTs) superfamily catalyze this process (Barvkar et al. 2012). The GT superfamily is divided into 94 families, the first of which is found in plants and pertains to the uridine glycosyl transferases (UGTs) (Wang and Hou 2009; Yonekura-sakakibara and Hanada 2011). UGTs transport UDP-activated sugar moieties to specified acceptor molecules, including UDP-glucose. 137 UGT genes have been discovered in flax. Of all, UGT74S1 gene expression is found to be high in the seed coat (Fig. 5), and it is discovered to glycosylate SECO, generating SECO monoglucoside (SMG) and subsequently SDG in that order (Fofana et al. 2017). Later in 2017, in another study of a mutagenized flax population with mutations in UGT74S1 and changed lignan glycosylation by (Fofana et al. 2017) demonstrated that the loss of function of SECO glycosylation into SDG is solely due to the UGT74S1 mutation. UGT74S4 and UGT74S3 may also play a role in delivering SMG as a substrate to UGT74S1 for the second glycosylation step to generate SDG to a modest level (Fofana et al. 2017).
Biotransformation of lignans
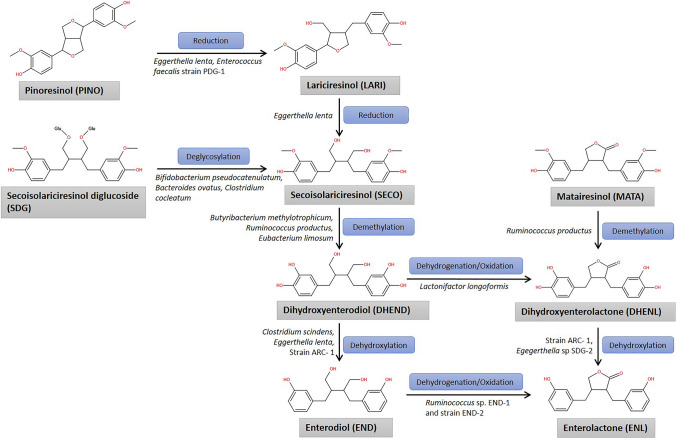
The flaxseed lignans SDG, SECO, PINO, LARI, and MATA are transformed to the mammalian lignans END and ENL by bacteria in the colon. Because END and ENL are synthesized in the guts of humans and other mammals, they are known as mammalian lignans or enterolignans (Wang et al. 2010). These are not found in plants. SDG is the major linseed lignan that Microbiota in the human (and other animal) colon converts to mammalian lignans known as enterodiol (END) and enterolactone (ENL) (Setchell et al. 2014). ED and EL are bioactive substances that are successively absorbed. Because of their plant origins and estrogenic and anti-estrogenic actions in humans, END and ENL were known as phyto-estrogens; they are also known as mammalian lignans or enterolignans. The bioavailability of lignans is dependent on the existence of the microorganisms needed to perform the necessary biochemical transformations, as these enterolignans are provided by the gastrointestinal Microbiota (Clavel et al. 2005). These bacteria include Eggerthella lenta (Clavel et al. 2006a), Bacteroides distasonis, Bacteroides fragilis, Bacteroides ovatus (Clavel et al. 2006c), Clostridium cocleatum (Clavel et al. 2005), Bifidobacterium pseudocatenulatum (Roncaglia et al. 2011), Butyribacterium methylotrophicum, Eubacterium callanderi, Eubacterium limosum, Ruminococcus productus, Peptostreptococcus productus (Clavel et al. 2005), Clostridium scindens, Clostridium saccharogumia, Blautia producta and Lactonifactor longoviformis (Woting et al. 2010). Due to lack of right type or sufficient gut bacteria in some humans, the plant lignans are not converted into mammalian lignans. Same is the case with antibiotics; taking antibiotics can virtually halt the production of ENL and END for several weeks.
Plant lignan transformation into enterolignans involves several biochemical processes, and bacteria consortia exchange metabolic intermediates. For example, bacteria perform different biochemical reactions to convert SDG to enterolactone END and ENL: O-Deglycosylation, O-demethylation, dehydroxylation, and dehydrogenation (Hameed et al. 2020). In first step, the intestinal microbiota hydrolyzes the sugar from the glycoside molecule (SDG) and the aglycone is released in O-deglycosylation process to give SECO (Clavel et al. 2006c) (Fig. 6). This step involves the bacteria of genera Bacteroides and Clostridium. Among these, Clostridium cocleatum, Clostridium ramosum, Clostridium saccharogumia, Bacteroides distasonis, Bacteroides fragilis, and Bacteroides ovatus were found to catalyze the reaction. The second step involves the O-demethylation reaction. SECO produced in the last reaction undergoes demethylation to give dihydroxyenterodiol (DHEND) intermediate, which further undergoes either dehydrogenation/oxidation reaction to give dihydroxyenterolactone (DHENL) or dehydroxylation reaction to give END (Eeckhaut et al. 2008). Demethylation reactions are carried out by intestinal bacteria such as Eggerthella lenta, Butyribacterium methylotrophicum, Eubacterium callanderi, Ruminococcus productus and Peptostreptococcus productus, Eubacterium limosum and Blautia product (Thumann et al. 2019). While dehydroxylation reactions are carried out by Eggerthella sp. SDG-2, strain ARC-1, Clostridium scinden and Eggerthella lenta (Clavel et al. 2005, 2006a; Jin et al. 2007). In third step, END and DHENL are further converted into ENL via dehydrogenation/oxidation and dehydroxylation reactions respectively, producing the final product of SDG metabolism. The dehydrogenation/oxidation reactions are catalyzed by Ruminococcus sp. END-1, strain END-2, Lactonifactor longoviformis (Jin et al. 2007; Clavel et al. 2006b). As a result of cooperation between diverse kinds of bacteria in a complex microbial community, ENL is produced. Thus, the bioactivity of lignans depends on their transformation by gut bacteria. The biotransformation of MATA follows two steps to form the final product i.e. ENL. MATA undergoes demethylation reaction to form DHENL which further undergoes dehydroxylation to give ENL (Landete et al. 2016). The bacteria population involved in these reactions are same to that required in SDG biotransformation for demethylation and dehydroxylation reactions. The biotransformation of PINO diglucoside and PINO to ENL is also similar to that of SECO and SDG except of two initial reduction reactions involving the reduction of PINO to LARI and further LARI into SECO (Heinonen et al. 2001; Landete et al. 2016). The SECO then follows the biotransformation reactions as discussed above. (See. Fig. 7)
Fig. 6.
Biotransformation of linseed lignans bu gut microbioata
Fig. 7.
Therapaeutic properties exhibited by linseed lignans
The ENL, so formed can have different fates; these can be excreted in faeces or are absorbed by the epithelial cells lining the human colon, where these are conjugated with sulphate or glucuronic acid and are either excreted in faeces or enter the circulation. They eventually proceed through enterohepatic circulation, where they are released into bile, reabsorbed from the intestine, and eliminated in conjugated form in the urine. The amount of enterodiol and enterolactone in faeces, blood, and urine is proportional to the quantity of plant lignans consumed in the food. High plant lignan intake results in high levels of these mammalian lignans in biological fluids. Crushing and grinding flax increases the bioavailability of mammalian lignans. The metabolism of lignans is significantly more complicated than previously imagined. Plant lignans are not completely converted into mammalian lignans, and some plant lignans, such as SECO, can be found in plasma.
Therapeutic application of linseed lignans
Flaxseed has been used by humankind for millennia for a variety of purposes. Flaxseed is one dietary source that contains a significant amount of phenolics, specifically lignans, which have health benefits (Barbary et al. 2010). In the food sector, plant extracts are extensively used and are generally recognized as safe. They normally contain more than one antimicrobial compound. As a result, using extracts requires taking advantage of all active compounds found in extracts (Barbary et al. 2010). SDG is a significant compound with antioxidant and anticancer properties (Akl et al. 2020). However, further studies, particularly clinical and toxicological trials in humans, is needed to back up this claim.
Antioxidant properties
Lignans are considered to be anti-carcinogenic. Flaxseed, along with a variety of other possible food ingredients, has been tested by the National Cancer Institute in the United States as an element of “designer foods” (Singh et al. 2011). The SDG present in flax and other foods is converted to the lignans enterodiol and enterolactone by bacteria in the gut, which have estrogenic and antiestrogenic properties as well as antioxidant properties (Adlercreutz 2007). Flax lignans have shown effectiveness in reducing the growth of cancerous tumours, particularly hormone-sensitive ones including breast, endometrium, and prostate cancers (Goyal et al. 2014). In a study, the results indicate that the non–oily component of flaxseed, not the α-linolenic acid, is responsible for the hypocholesterolemic effect. Flaxseed reduced hypercholesterolemic atherosclerosis by up to 73% due to its high SDG. SDG's anti-atherogenic role may be attributed to both its antioxidant and lipid-lowering properties (Singh et al. 2011). SDG and its metabolites are thought to mediate the total serum cholesterol, low density lipoprotein, cholesterol levels, and high-density lipoprotein ratio, resulting in fewer androgenic complications and antioxidative safety, according to animal and human studies (Zanwar et al. 2014).
The tendency of flaxseed to help maintain more early stages of cancer, according to the researchers, is due to the fact that flaxseed contains the highest amount of plant lignans (Goyal et al. 2014). Moreover, flax lignans may have a protective effect against breast cancer due to their low estrogenic activity and antioxidant properties (Truan et al. 2012). Plant lignans have antioxidant properties and have been shown to change oestrogen metabolism, potentially lowering the risk of ovarian cancer and improving overall health (McCann et al. 2007). When compared to a low-fat muffin without lignans, daily consumption of a low-fat muffin supplemented with a lignan complex for 6 weeks significantly dropped the C-reactive protein (CRP) levels(0.88 to 0.80 mg/L) in postmenopausal women (Hallund et al. 2008). The lignan-rich concentrate from linseed hulls has been studied as a potential treatment for chronic hormone disorders including benign prostatic hyperplasia (BPH) (Bisson et al. 2014). BPH was induced in rats using testosterone propionate, and they were fed a diet containing various amounts of extract. The lignan-rich extract was found to significantly reduce testosterone propionate-induced prostate size, with a dose-dependent effect (Bisson et al. 2014). Saleem et al. (2005) conducted research to study the effects of flaxseed and its constituent lignan SDG on plasma IGF-I levels in rats given N-methyl-N-nitrosourea or not (MNU). Flaxseed and SDG both decreased plasma IGF-I levels in MNU-free rats, which were inversely linked to urinary lignan excretion (Saleem et al. 2005). In mouse models of oxidative lung injury, flaxseed has been shown to be radio-protective. Pre-treatment with SDG protects lung cells from radiation-induced DNA damage and improves clonogenic survival. SDG also increases the gene expression and protein levels of antioxidant enzymes like HO-1, GSTM1, and NQO1 in lung cells, boosting their natural antioxidant capability (Velalopoulou et al. 2015). Flaxseed lignan's antioxidant properties have also been linked to a reduction in hypercholesterolemia, atherosclerosis, and diabetes (Touré and Xueming 2010). Various studies have indicated that the progression of N-methyl-N-nitrosourea-induced mammary neoplasia leads to carcinogenesis, and SDG has been found to slow this process down via modulating terminal end bud differentiation (Tan et al. 2004; Imran et al. 2015). There are numerous ways in which SDG can play a biological role in the delay or inhibition of carcinogenic phenomena. Plasma insulin-like growth factor I and endothelium growth factor are thought to be responsible for breast cancer development risks, and SDG can reduce those growth factors (Bergman et al. 2007).
Anti-cancerous properties
The phenolic extract of flaxseed meal had a significant cytotoxic effect with maximum inhibitory impact of IC50 = 22.3 and 22.6 μg/ml, attained on colon cancer and lung carcinoma cell lines, respectively. The liver cell line carcinoma is next to bare also inhibited with an IC50 of 39.4cμg/ml, subsequently, intestinal and breast cancer cell lines with IC50 values of 46.9 μg/ml and 49 μg/ml. Consumption of purified flaxseed lignan at 1.5 mg/kg/day for 20 weeks, beginning one day after administration with the carcinogen dimethylbenzanthracene, reduced the number of tumours per tumour-bearing rat by 37% (Thompson et al. 2009). In a study performed to find the mechanism of SDG in reducing MCF-7 tumour growth, the tumour was established in the mice and the mice were supplemented with Basal diet plus 1 g/kg diet of SDG against only basal diet in the control group for 8 weeks. It was observed that SDG reduced the tumour growth; also reduced the expression of PS2, BCL2, and IGF-1R mRNA (Saggar et al. 2010). In study to evaluate the effect of SDG lignan concentrate on Skin cancer induced by dimethyl Benz(a)anthracene and croton oil in female balb/c mice, 5% SDG lignan concentrate in diet showed decrease in tumour volumes. An increment in p53 mRNA and decrease in CDK4 mRNA was observed in SDG lignan concentrate administered mice (Patel and Patel 2021). Huang et al. (2018) suggested that SDG may target the JAK2 and may bind to the protein kinase domain of it. Molecular docking studies showed that SDG has a great affinity for JAK2 and it may act as a JAK2 agonist and halt cancer signalling. Research targeted to reveal the function of SDG in the modulation of levels of cyto-protective enzyme using irradiated lungs cells. By lowering mean comet tail length, SDG rescued cells from IR-induced mortality and mitigated DNA damage. SDG raised antioxidant HO-1, GSTM1, and NQO1 gene and protein levels considerably to exhibit the substantial radio protective capabilities, reducing DNA damage and increasing the antioxidant capacity of normal lung cells (Velalopoulou et al. 2015). Similarly, in research, when feeding of a standard diet was supplemented with 1 g/kg flaxseed lignan for 8 weeks breast tumour cell proliferation (or growth) was reduced without affecting tumour size in mice (Truan et al. 2012). The anticancer effect of Purified Flaxseed Hydrolysate (PFH), a lignan-rich fraction, was tested on a human breast cancer cell line (T47D) and in rats carrying tumours. The cytotoxicity of PFH-G9 against the ER-positive breast cell lines MCF7 and T47D was the most remarkable, with IC50 values of 13.8 and 15.8 μg/ml, respectively (Ezzat et al. 2018).Furthermore, mice with 5% flaxseed in their diet had less growth and development of transgenic prostate cancer (Lin et al. 2002). In a study, it was investigated that, combining flaxseed lignans with traditional chemotherapeutic treatments like Docetaxel and Carboplatin greatly increased the cytotoxic effect of these medications against the metastatic breast cancer cell lines, SKBR3 and MDA-MB-231 (Di et al. 2018). Scherbakov et al. (2021) showed Secoisolariciresinol and Secoisolariciresinol-4′, 4″-diacetate to inhibit the MCF-7 cell lines with IC50 values of 25 and 11 μM, respectively. Secoisolariciresinol-4′, 4″-diacetate also enhanced the effect of doxorubicin. More SDG derivatives can be promising anti-cancerous agents that can act alone or synergistically with other pro-apoptotic molecules or drugs.
Anti-inflammatory properties
SDG being the main lignan has been extensively studied for its many chemo preventive (Lowcock et al. 2013; Velalopoulou et al. 2015; Tannous et al. 2020) and biological effects. Anti-inflammatory effect is one of them (Bowers et al. 2019). In recent years, a surge has been seen in the demand of bioactive anti-inflammatory components due to less side effects and cost effectiveness. Cocktailing the different effective lignans can also be used to further enhance the effect of these lignans due to synergism as studied by (Bhaskar and Rajesha 2021). They used the lignans SDG (from flaxseed) and sesamin (from sesame seeds) to study the synergistic effect of anti-inflammatory properties in vitro of these lignans on Lipoxygenase (LOX) and human cyclogenase (COX-2) enzymes inhibition, which are known as pro-inflammatory enzymes. The cocktail showed the potential capacity in inhibiting 94.37% LOX activity with IC50 of 35 ± 0.53 μg comparing to SDG and SES with 68.26% and 67.07% inhibition. The similar results were seen with COX-2; SDG + SES showed to have inhibiting effect on COX-2 at relative lower concentrations then SDG and SES alone (Bhaskar and Rajesha 2021). In another study by (Bowers et al. 2019), SDG was found to have anti-inflammatory effect in mammary glands of SDG supplemented rats. SDG affects the inflammatory markers by decreasing the expression of Adgre1 (the gene for F4/80) and the prevalence of crown-like structures (CLS). But SDG showed to have no effect on systemic inflammation. (Pietrofesa et al. 2015) studied the anti-inflammatory effect of flaxseed lignans on asbestos treated mice. Mice supplemented with flaxseed lignans were found to show less inflammation compared to the control mice and found to have 58.3% less WBCs population in peritoneal lavage fluid of mice. The anti-inflammatory effect of flaxseed lignans when further characterized by determining the pro-inflammatory cytokines (IL-1ß, IL-6, HMGB1 and TNFα) in peritoneal lavage fluid resulted in decrease in these cytokines levels in peritoneal lavage fluid as well as decrease in the mRNA levels of these cytokines in WBCs present in the fluid. (Christofidou-solomidou et al. 2014) suggested the role of flaxseed lignans in reducing lung inflammation by down regulating miRNAs—miRs-142-3p and miRs-150. It is suggested by (During et al. 2012) that PINO found to have the highest anti-inflammatory activity in-vitro. It functions by blocking the NF-κβ signalling pathway.
Oestrogenic and anti-oestrogenic properties
ENL and END have structural similarities to oestradiol, the most prevalent and active form of oestrogen in the body, these lignans can bind to oestrogen receptors and have modest oestrogenic or anti-oestrogenic effects (Wang and Kurzer 1997). Evidence of dietary sources of lignans as modulators of oestrogen receptor signalling has been found in in-vivo studies (Penttinen‐Damdimopoulou et al. 2009). In addition, EL’s capacity to inhibit aromatase may be useful in oestrogen-responsive breast tumours (Adams and Chen. 2009). Lignans, like other phytoestrogens, bind to the same locations on cells where oestrogen binds to carry out their respective functions. When natural oestrogen is abundant in the body, lignans may diminish the effects of oestrogen by displacing it from cells. This hormone displacement could help prevent malignancies that rely on oestrogen to start and evolve, such as breast cancer (Dhirhi et al. 2016). The impact of flaxseed diet on postmenopausal women's urine oestrogen metabolites excretion was examined in a study by Haggans et al. (1999). The subjects were examined for their diet which included flaxseed (5 or 10 g/day) along with usual meal. In a dose-dependent manner, flaxseed intake raised urine 2-OHEstrogen excretion and the urine 2/16-OHE1 ratio (Haggans et al. 1999). A comparative study was conducted by Brooks et al. (2004), in which the effects of ingesting the same dose of flaxseed or soya on oestrogen metabolism were compared. The fraction of oestrogen metabolites (2-hydroxyestrone to 16-hydroxyestrone) was favourably linked with urinary lignan excretion in the flaxseed group, whereas there was no significant correlation in the soya group (Brooks et al. 2004).
Modulation of gene expression and /or enzyme properties
Enterolignans or their plant origins may impact hormonal state by modulating protein expression and breakdown. Enterolignans have the ability to alter gene expression and/or the activity of genes responsible in the health and metabolic processes in both normal and cancerous tissues. SDG plays an effective role in delaying or preventing the type-1 and type-2 diabetes (Prasad 2002). Increase in blood sugar levels (Diabetes mellitus), which is defined as a fasting plasma glucose level of 126 mg/dl or higher, is a major risk factor for cardiovascular illnesses. Diabetes mellitus is characterized by hyperglycemia and is linked to abnormalities in carbohydrate, protein, and lipid metabolism, which can lead to secondary problems. Diabetes, if left untreated, can lead to heart disease, renal failure, and blindness. A link has been discovered between high blood glucose levels and the risk of cardiovascular disease. Furthermore, diabetes is linked to additional risk factors such as obesity, hypertension, poor HDL cholesterol, and high triglyceride levels. The phosphoenolpyruvate carboxykinase (PEPCK) gene, which codes for a major enzyme called PEPCK, involved in glucose production (gluconeogenesis) in the liver, has been shown to be inhibited by flaxseed lignan SDG. Thus, hypoglycaemic effect of SDG is observed in type-2 diabetes via inhibition of PEPCK gene expression. Study performed by Prasad (2002) on cultured primary hepatocytes of rats reported that SDG of 100 µM concentration can suppress the gene expression of PEPCK completely in comparison to insulin, which can also completely suppress the expression of PEPCK gene in concentration of 10 nM. While another study conducted by Mani et al. (2011) on human subjects with type-2 diabetes, it reported that supplementation of 10 g of flaxseeds daily for a period of 1 month reduced the fasting blood glucose levels by 19.7% and glycated haemoglobin by 15.6%. The study also showed hypocholesterolemic effects. SDG of flaxseed being a phytoestrogen, also reported to have mild antihypertensive or hypotensive activity. SDG acts as the long-acting hypotensive agent which activates the guanylate cyclase (GC) pathways involved in the mechanism of controlling blood pressure (Prasad 2004). EL (mammalian counterpart of SDG) also shows to have anti-tumour activity in humans in a study conducted on the androgen-independent human prostate carcinoma PC-3 cells by (Chen et al. 2009). Enterolactone was found to block IGF-1/IGF-1R signalling at relevant concentrations of 20–60 mmol/L. It acts by inhibiting the IGF-1–induced tyrosine phosphorylation of IGF-1R and activation of AKT and ERK and result in the inhibition of proliferation and migration of prostate cancer cells.
Anti-diabetic properties
Secoisolariciresinol diglucoside is also shown to regulate the plasma-glucose homeostasis to exhibit the anti-diabetic activity (Toure and Xuemings 2010). The same has been tested in-vitro and against the animal models. Wang et al. (2015) studied the effect of SDG from linseed against the glucose homeostasis in the obese mice and found that SDG was able to reduce the fasting blood glucose, insulin and free fatty acid levels while improving oral glucose tolerance, insulin responsiveness. Another study on streptozotocin-induced diabetic rats that increased serum malondialdehyde (MDA), reduced levels of catalase (CAT), superoxide dismutase (SOD), and glutathione (GSH) are all linked to diabetes-related oxidative stress. In a 2-day single-dose (20 mg/kg) research, the synthetic SDG demonstrated a dose-dependent decrease in glucose levels, with a maximal effect of 64.62 percent at 48 h (p < 0.05), which is equivalent to the standard medicine tolbutamide (20 mg/kg) (Moree et al. 2013). In a similar study against streptozotocin (STZ)-induced diabetes in rats showed that SDG at the dose of 22 mg/kg body weight orally 3 days prior to STZ and 21 days thereafter prevented the development of diabetes by 75% (Prasad 2000). SDG also found to play renoprotective effects in STZ-induced diabetic rats.SDG administration boosted insulin levels while decreasing blood glucose, fructosamine, creatinine, and blood urea nitrogen levels. SDG also enhanced renal reduced glutathione, superoxide dismutase, and malondialdehyde and nitric oxide levels while decreasing malondialdehyde and nitric oxide levels. In addition, as compared to untreated diabetic control rats, SDG decreased kidney nuclear factor kappa-B (NF-B), tumour necrosis factor (TNF)-, and inducible nitric oxide synthase (iNOS) expression while increasing renal surviving and B-cell lymphoma-2 (Bcl-2) expression (Sherif 2014).In another study of the effect of SDG on the development of diabetes in diabetic prone BioBreeding rats, SDG found to prevent the development of diabetes. The diabetic incidences were only found to be 21.4% in the case of SDG treated group (Prasad et al. 2000).
Antibacterial properties
Escherichia, Klebsiella, Enterobacter, Serratia, and many other bacteria are responsible for many diseases in humankind (Guentzel. 1996). Moreover, Staphylococcus aureus can produce several types of enterotoxins that cause gastroenteritis, which is a major food-borne disease in most countries (Otto 2014). In a study conducted by Barbary et al. (2010), with a MIC value of 1.5 mg/ml, lignans extract from flaxseed was shown to have an effective antibacterial activity against Gram positive bacteria such as S. aureus and Vibrio sp. Similarly, due to the presence of polyphenols, including lignans, crude and hydrolyzed lignan extracts of flaxseeds displayed antibacterial activity against Gram-positive and Gram-negative pathogens (Pag et al. 2014). SDG extracts from two varieties of Indian linseed (LVF-01 and GVF-03) were tested. Among the fractions, LVF-01 showed maximum activity 31.5 mm at MIC 100 ppm and in the case of GVF-03, maximum activity with 31.9 at MIC 150 ppm were reported against E. coli (Rajesha et al. 2010). In agar well diffusion technique, 5 µl phenolic extracts of flaxseed meal demonstrated varying levels of inhibitory action against the various bacterial strains. Listeria monocytogenes, for example, was inhibited by phenolic extract of flaxseed meal, with a 26 mm inhibition zone diameter, followed by Salmonella typhimurium, Escherichia coli, and Pseudomonas aeruginosa with 25 mm, 22 mm, and 10 mm diameters of inhibition zone, respectively (Akl et al. 2020). The reduction levels of S. aureus and E. coli populations were observed with 1.23 and 1.83 log CFU/g, respectively, after 3 min of treatment with 0.7% flaxseed meal extract (FMC) in disc diffusion assay (Son and Song 2017). Furthermore, a combination treatment of 0.7% FME for 3 min at 50 °C, reduced the population of bacterial pathogens by 2.28 and 2.41 log CFU/g, respectively (Son and Song 2017). The aqueous extract of Flaxseeds showed the most antibacterial efficacy against both gram negative and positive bacteria. The lignans may bind the Ca+2 and Mg+2 ions, lowering the ion concentration from lipopolysaccharide of the outer membrane, triggering lipopolysaccharide release, and thereby weakening the membrane, thus increasing lignan activity (Narender et al. 2016).
Antifungal properties
Two fungi, Aspergillus flavus and Aspergillus niger produce aflatoxins, which are a strong hepatotoxin and carcinogen. They are pre-harvest infections of various major food crops. In farm animals, these chemicals can cause death or reduced productivity (Barbary et al. 2010). At 2.5–3.0 mg/ml, crude extracts of lignans demonstrated considerable (between 70 and 90%) antifungal efficacy against both Aspergillus flavus and Aspergillus niger (Barbary et al. 2010). Flaxseed constituents have been shown in a study to have a significant antifungal effect, specifically against Candida albicans growth. Furthermore, when compared to the commonly used Nystatin, lignan-enriched aqueous flaxseed extract showed significantly greater antifungal activity (Mustafa et al. 2018). Flax lignans stimulate the development of probiotics in the stomach and could also aid in the elimination of yeast and Candida, which cause fungal infections.
Challenges and limitation for use of lignan as therapeutic agents
Human and animal model observational and interventional research trials have shown that lignan consumption is linked to improved hormone levels and metabolism, improved blood lipid profiles, and lower risk of cardiovascular disease, osteoporosis, diabetes, and renal illness. Lignans have also been linked to a lower risk of cancer in the breast, prostate, skin, colon, thyroid, glioma, and stomach as they may be found in a variety of foods among which flax is found to be one of the richest sources of lignan in diet (Bisson et al. 2014). Such studies, however, cannot concentrate on which flaxseed components are responsible for the health benefits, because flaxseed contains at least three health-promoting components: soluble fibres or mucilage, high n-3 PUFA and the plant lignans majorly, SDG (Adolphe et al. 2010). Lignans are also found to be irregularly distributed in plant meals. Lignan analysis is complicated by the structural diversity of lignan aglycones, varied localization of lignans in plant diets and the variability of their conjugation pattern (Schwartz and Sontag 2011). Lignans have been shown to protect against a variety of clinical disorders. However, it has been studied that certain caution should be taken in premenopausal women and new-borns when it comes to timing and method of administration of dose, as inappropriate estrogenic activity might cause undesirable side effects (Mojzer et al. 2016). Although various animal studies have been conducted but the mechanisms of action of lignans and the actual bioactive lignan form (i.e. SDG, SECO, ED, and/or EL) delivering the effect are still unknown. Changes in species strain, age and sex affect the findings of these researches. As animal doses are virtually invariably higher than human doses for a variety of reasons, direct comparisons between species are impossible. The vast range of methodologies employed in human trials also makes it difficult to evaluate results, even if there is prominent evidence that SDG-enriched flaxseed products give health advantages (Adolphe et al. 2010). Thus, there is a much need of research to unveil the mechanisms of the therapeutic bioactivities of the lignans in animal studies. Also, there is need to take account of phytotoxic chemicals such as linatine, phytic acids, protease inhibitors, and cyanogenic glycosides present along the SDG in linseed. Consumption of these chemicals has been linked to reduced bioavailability of critical nutrients and/or health consequences in epidemiological studies. To make flaxseeds safe to eat, these components must be eliminated or inactivated to medically undetectable levels.
Extraction and quantification of lignans from plant sources can also be challenging sometime. The use of diverse extraction methods was blamed for the variations in the lignan values in various databases. Furthermore, the limitations of acidic and enzymatic hydrolysis, such as inadequate matrix extraction led to the results being underestimated. Although great progress has been made in the disciplines of lignan extraction and analysis over the last 10 years, efficient lignan extraction from plant matrices still remains a difficulty. The amount of lignan in flaxseed varies not only depend on variety but also on the growing region and year. These are natural obstacles that make lignan quantification in foods more difficult, as analyzing various farmed types of seeds and grains harvested in different sites and years would be impractical and time-consuming. The methods of lignan extraction and analysis are also some of the challenges that researchers can focus on (Nemes and Orsat 2012).
Conclusion and future prospects
Lignans seems to have crucial therapeutic activities involved anti-oxidant, anti-cancerous, anti-inflammatory, anti-microbial, anti-diabetic, estrogenic and anti-estrogenic activity. These properties of the lignans makes this class of molecules the trending topic of research. Although lignans are distributed all over the kingdom Plantae but linseed is reported to be the richest source of lignans, particularly Secoisolariciresinol diglucoside. SDG is further metabolized and bio-transformed into enterolignans that shows the characteristic properties of lignans in animals. Lignans being the ancient crop is being used from the ancient time for its valuable properties which are now being explored by the scientific research. To study and explore the lignans, it important to design the extraction, purification and identification approaches that are economically feasible, edible grade and higher yield. Green extraction and purification of lignans from linseed is needed to be studied and designed thoroughly. Although various animal studies have been conducted but the mechanisms of lignans used to mediate the potential health advantages and the actual bioactive lignan form (i.e. SDG, SECO, ED, and/or EL) are still unknown and needed to be studied. Linseed lignans and their therapeutic targets can be unveiled using in-silico studies, which will give an insight of target proteins and receptors of the cancer signalling that can be targeted using lignan as therapeutic agent. Also, various formulations can be prepared using the lignans of linseed to increase the bioavailability of lignans. Lignans of the linseed can also be explored for synthesizing its derivatives; using the lignans in combination to pharmaceutical drugs to enhance the therapeutic action against various cancers and inflammatory conditions.
Acknowledgements
Authors are grateful to Department of Biotechnology, Government of India for providing financial support under Project No. BT/Ag/Network/2019-2020/29.02.2020 awarded to Dr. Nutan Kaushik.
Author contribution
NK conceived the idea of manuscript. NSP, SA and AS wrote the manuscript. The figures were prepared by NSP. The review has been checked and finalized by NK.
Funding
The financial support was provided by Department of Biotechnology, Government of India under Project No. BT/Ag/Network/2019–2020/29.02.2020 awarded to Dr. Nutan Kaushik.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
We affirm that all the authors have seen, prepared, and agreed to the submission of paper and their inclusion of name(s) as co-author(s). We also declare that there are no conflicts of interests for the same.
References
- Adam LS, Chen S. Phytochemicals for breast cancer prevention by targeting aromatase. Front Biosci. 2009;14:3846–3863. doi: 10.2741/3493. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H. Lignans and human health. Crit Rev Clin Lab. 2007;44:483–525. doi: 10.1080/10408360701612942. [DOI] [PubMed] [Google Scholar]
- Adolphe JL, Whiting SJ, Juurlink BH, Thorpe LU, Alcorn J. Health effects with consumption of the flax lignan secoisolariciresinol diglucoside. Br J Nutr. 2010;103:929–938. doi: 10.1017/S0007114509992753. [DOI] [PubMed] [Google Scholar]
- Akl EM, Mohamed SS, Hashem AI, Taha FS. Biological activities of phenolic compounds extracted from flaxseed meal. Bull Natl Res Cent. 2020;44:1–8. doi: 10.1186/s42269-020-0280-x. [DOI] [Google Scholar]
- Attoumbré J, Laoualy AB, Bienaimé C, Dubois F, Baltora-Rosset S. Investigation of lignan accumulation in developing Linum usitatissimum seeds by immunolocalization and HPLC. Phytochem Lett. 2011;4:194–198. doi: 10.1016/j.phytol.2011.03.004. [DOI] [Google Scholar]
- Bakke JE, Klosterman HJ. A new di glucoside from flaxseed. Proc N D Acad Sci. 1956;10:18–22. [Google Scholar]
- Barbary OM, El-Sohaimy SA, El-Saadani MA, Zeitoun AM. Antioxidant, antimicrobial and anti-HCV activities of lignan extracted from flaxseed. J Agric Biol Sci. 2010;6:247–256. [Google Scholar]
- Barvkar VT, Pardeshi VC, Kale SM, Kadoo NY, Gupta VS. Phylogenomic analysis of UDP glycosyltransferase 1 multigene family in Linum usitatissimum identified genes with varied expression patterns. BMC Genom. 2012;13:1–3. doi: 10.1186/1471-2164-13-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman JM, Thompson LU, Dabrosin C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin Cancer Res. 2007;13:1061–1067. doi: 10.1158/1078-0432.CCR-06-1651. [DOI] [PubMed] [Google Scholar]
- Bhaskar A, Rajesha J. Synergistic anti-inflammatory and antioxidant activities of indian flax and sesame seed lignans. Aegaeum J. 2021;9:21. [Google Scholar]
- Bisson JF, Hidalgo S, Simons R, Verbruggen M. Preventive effects of lignan extract from flax hulls on experimentally induced benign prostate hyperplasia. J Med Food. 2014;17(6):650–656. doi: 10.1089/jmf.2013.0046. [DOI] [PubMed] [Google Scholar]
- Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annu Rev Plant Biol. 2003;54:519–546. doi: 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- Bowers LW, Lineberger CG, Ford NA, Rossi EL, Punjala A, Camp KK, Kimler BK, Fabian CJ, Hursting SD. The flaxseed lignan secoisolariciresinol diglucoside decreases local inflammation, suppresses NFκB signaling, and inhibits mammary tumor growth. Breast Cancer Res Treat. 2019;173:545–557. doi: 10.1007/s10549-018-5021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brglez Mojzer E, Knez Hrnčič M, Škerget M, Knez Ž, Bren U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules. 2016;21:901. doi: 10.3390/molecules21070901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JD, Ward WE, Lewis JE, Hilditch J, Nickell L, Wong E, Thompson LU. Supplementation with flaxseed alters estrogen metabolism in postmenopausal women to a greater extent than does supplementation with an equal amount of soy. Am J Clin Nutr. 2004;79:318–325. doi: 10.1093/ajcn/79.2.318. [DOI] [PubMed] [Google Scholar]
- Chen LH, Fang J, Sun Z, Li H, Wu Y, Demark-Wahnefried W, Lin X. Enterolactone inhibits insulin-like growth factor-1 receptor signaling in human prostatic carcinoma PC-3 cells. J Nutr. 2009;139:653–659. doi: 10.3945/jn.108.101832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhillar H, Chopra P, Ashfaq MA. Lignans from linseed (Linum usitatissimum L.) and its allied species: retrospect, introspect and prospect. Crit Rev Food Sci Nutr. 2020;61:2719–2741. doi: 10.1080/10408398.2020.1784840. [DOI] [PubMed] [Google Scholar]
- Christofidou-Solomidou M, Pietrofesa R, Arguiri E, McAlexander MA, Witwer KW. Dietary flaxseed modulates the miRNA profile in irradiated and non-irradiated murine lungs: a novel mechanism of tissue radioprotection by flaxseed. Cancer Biol Ther. 2014;15:930–937. doi: 10.4161/cbt.28905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel T, Henderson G, Alpert CA, Philippe C, Rigottier-Gois L, Doré J, Blaut M. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl Environ Microbiol. 2005;71:6077–6085. doi: 10.1128/AEM.71.10.6077-6085.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel T, Borrmann D, Braune A, Doré J, Blaut M. Occurrence and activity of human intestinal bacteria involved in the conversion of dietary lignans. Anaerobe. 2006;12:140–147. doi: 10.1016/j.anaerobe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Clavel T, Doré J, Blaut M. Bioavailability of lignans in human subjects. Nutr Res Rev. 2006;19:187–196. doi: 10.1017/NRR2006129. [DOI] [PubMed] [Google Scholar]
- Clavel T, Henderson G, Engst W, Doré J, Blaut M. Phylogeny of human intestinal bacteria that activate the dietary lignan secoisolariciresinol diglucoside. FEMS Microbiol. 2006;55:471–478. doi: 10.1111/j.1574-6941.2005.00057.x. [DOI] [PubMed] [Google Scholar]
- Cloutier S. Encyclopedia of food grains. 2. Oxford: Elsevier; 2016. Linseed; pp. 259–264. [Google Scholar]
- Corbin C, Drouet S, Markulin L, Auguin D, Lainé É, Davin LB, Cort JR, Lewis NG, Hano C. A genome-wide analysis of the flax (Linum usitatissimum L.) dirigent protein family: From gene identification and evolution to differential regulation. Plant Mol Biol. 2018;97:73–101. doi: 10.1007/s11103-018-0725-x. [DOI] [PubMed] [Google Scholar]
- Cui Q, Du R, Liu M, Rong L. Lignans and their derivatives from plants as antivirals. Molecules. 2020;25:183. doi: 10.3390/molecules25010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhirhi N, Shukla R, Patel NB, Sahu E, Gendley T, Mehta N. “Lignan”-antioxidant of linseed. Plant Arch. 2016;16:12–17. [Google Scholar]
- Di Y, De Silva F, Krol ES, Alcorn J. Flaxseed lignans enhance the cytotoxicity of chemotherapeutic agents against breast cancer cell lines MDA-MB-231 and SKBR3. Nutr Cancer. 2018;70:306–315. doi: 10.1080/01635581.2018.1421677. [DOI] [PubMed] [Google Scholar]
- Durazzo A, Zaccaria M, Polito A, Maiani G, Carcea M. Lignan content in cereals, buckwheat and derived foods. Foods. 2013;2:53–63. doi: 10.3390/foods2010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During A, Debouche C, Raas T, Larondelle Y. Among plant lignans, pinoresinol has the strongest antiinflammatory properties in human intestinal Caco-2 cells. J Nutr. 2012;142(10):1798–1805. doi: 10.3945/jn.112.162453. [DOI] [PubMed] [Google Scholar]
- Eeckhaut E, Struijs K, Possemiers S, Vincken JP, Keukeleire DD, Verstraete W. Metabolism of the lignan macromolecule into enterolignans in the gastrointestinal lumen as determined in the simulator of the human intestinal microbial ecosystem. J Agric Food Chem. 2008;56:4806–4812. doi: 10.1021/jf800101s. [DOI] [PubMed] [Google Scholar]
- Ezzat SM, Shouman SA, Elkhoely A, Attia YM, Elsesy MS, El Senousy AS, Choucry MA, El Gayed SH, El Sayed AA, Sattar EA, El Tanbouly N. Anticancer potentiality of lignan rich fraction of six flaxseed cultivars. Sci Rep. 2018;8:1–6. doi: 10.1038/s41598-017-18944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAOSTAT (2020) Food and Agriculture Organization of the United Nations. https://www.fao.org/faostat/en/
- Fofana B, Ghose K, McCallum J, You FM, Cloutier S. UGT74S1 is the key player in controlling secoisolariciresinol diglucoside (SDG) formation in flax. BMC Plant Biol. 2017;17:1–3. doi: 10.1186/s12870-017-0982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JD, Huang KS, Wang HB, Davin LB, Lewis NG. Biosynthetic pathway to the cancer chemopreventive secoisolariciresinol diglucoside—hydroxymethyl glutaryl ester-linked lignan oligomers in flax (Linum usitatissimum) seed. J Nat Prod. 2001;64:1388–1397. doi: 10.1021/np010367x. [DOI] [PubMed] [Google Scholar]
- Fuentealba C, Figuerola F, Estévez AM, González-Muñoz A, Muñoz O. Optimization of secoisolariciresinol diglucoside extraction from flaxseed (Linum usitatissimum L.) and isolation by a simple HPLC-UV method. CyTA-J Food. 2015;13:273–281. doi: 10.1080/19476337.2014.953209. [DOI] [Google Scholar]
- Goyal A, Sharma V, Upadhyay N, Gill S, Sihag M. Flax and flaxseed oil: an ancient medicine & modern functional food. J Food Sci Technol. 2014;51:1633–1653. doi: 10.1007/s13197-013-1247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel MN. Escherichia, Klebsiella, Enterobacter, Serratia, Citrobacter, and Proteus. In: Baron S, editor. Medical Microbiology. 4. Galveston: University of Texas, Medical Branch; 1996. [PubMed] [Google Scholar]
- Gui B, Shim YY, Reaney MJ. Distribution of cyclolinopeptides in flaxseed fractions and products. J Agric Food Chem. 2012;60:8580–8589. doi: 10.1021/jf3023832. [DOI] [PubMed] [Google Scholar]
- Gutte KB, Sahoo AK, Ranveer RC. Bioactive components of flaxseed and its health benefits. Int J Pharm Sci Rev Res. 2015;31:42–51. [Google Scholar]
- Haggans CJ, Hutchins AM, Olson BA, Thomas W, Martini MC, Slavin JL. Effect of flaxseed consumption on urinary estrogen metabolites in postmenopausal women. Nutr Cancer. 1999;33:188–195. doi: 10.1207/S15327914NC330211. [DOI] [PubMed] [Google Scholar]
- Hallund J, Tetens I, Bugel S, Tholstrup T, Bruun JM. The effect of a lignan complex isolated from flaxseed on inflammation markers in healthy postmenopausal women. NutrMetab Cardiovasc. 2008;18:497–502. doi: 10.1016/j.numecd.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Hameed AS, Rawat PS, Meng X, Liu W. Biotransformation of dietary phytoestrogens by gut microbes: a review on bidirectional interaction between phytoestrogen metabolism and gut microbiota. Biotechnol Adv. 2020;43:107576. doi: 10.1016/j.biotechadv.2020.107576. [DOI] [PubMed] [Google Scholar]
- Hano C, Martin I, Fliniaux O, Legrand B, Gutierrez L, Arroo RR, Mesnard F, Lamblin F, Lainé E. Pinoresinol–lariciresinol reductase gene expression and secoisolariciresinol diglucoside accumulation in developing flax (Linum usitatissimum) seeds. Planta. 2006;224:1291–1301. doi: 10.1007/s00425-006-0308-y. [DOI] [PubMed] [Google Scholar]
- Heinonen S, Nurmi T, Liukkonen K, Poutanen K, Wähälä K, Deyama T, Nishibe S, Adlercreutz H. In vitro metabolism of plant lignans: new precursors of mammalian lignans enterolactone and enterodiol. J Agric Food Chem. 2001;49:3178–3186. doi: 10.1021/jf010038a. [DOI] [PubMed] [Google Scholar]
- Hemmati S, Schmidt TJ, Fuss E. (+)-Pinoresinol/(−)-lariciresinol reductase from Linum perenne Himmelszelt involved in the biosynthesis of justicidin B. FEBS Let. 2007;581:603–610. doi: 10.1016/j.febslet.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Holmbom B, Eckerman C, Eklund P, Hemming J, Nisula L, Reunanen M, Sjöholm R, Sundberg A, Sundberg K, Willför S. Knots in trees–a new rich source of lignans. Phytochem Rev. 2003;2:331–340. doi: 10.1023/B:PHYT.0000045493.95074.a8. [DOI] [Google Scholar]
- Hosseinian FS, Muir AD, Westcott ND, Krol ES. Antioxidant capacity of flaxseed lignans in two model systems. J Am Oil Chem Soc. 2006;83:835. doi: 10.1007/s11746-006-5034-x. [DOI] [Google Scholar]
- Huang G, Huang X, Liu M, Hua Y, Deng B, Jin W, Yan W, Tan Z, Wu Y, Liu B, Zhou Y. Secoisolariciresinol diglucoside prevents the oxidative stress-induced apoptosis of myocardial cells through activation of the JAK2/STAT3 signaling pathway Int. J Mol Med. 2018;41:3570–3576. doi: 10.3892/ijmm.2018.3560. [DOI] [PubMed] [Google Scholar]
- Imran M, Ahmad N, Anjum FM, Khan MK, Mushtaq Z, Nadeem M, Hussain S. Potential protective properties of flax lignan secoisolariciresinol diglucoside. Nutr J. 2015;14:1–7. doi: 10.1186/s12937-015-0059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov S, Rashevskaya T, Makhonina M. Flaxseed additive application in dairy products production. Procedia Food Sci. 2011;1:275–280. doi: 10.1016/j.profoo.2011.09.043. [DOI] [Google Scholar]
- Jin JS, Zhao YF, Nakamura N, Akao T, Kakiuchi N, Min BS, Hattori M. Enantioselective dehydroxylation of enterodiol and enterolactone precursors by human intestinal bacteria. Biol Pharm Bull. 2007;30:2113–2119. doi: 10.1248/bpb.30.2113. [DOI] [PubMed] [Google Scholar]
- Kamal-Eldin A, Peerlkamp N, Johnsson P, Andersson R, Andersson RE, Lundgren LN, Åman P. An oligomer from flaxseed composed of secoisolariciresinol diglucoside and 3-hydroxy-3-methyl glutaric acid residues. Phytochemistry. 2001;58:587–590. doi: 10.1016/S0031-9422(01)00279-5. [DOI] [PubMed] [Google Scholar]
- Kaushik et al (2022) Biochemical characterization of Linseed, Quarterly Report
- Kezimana P, Dmitriev AA, Kudryavtseva AV, Romanova EV, Melnikova NV. Secoisolariciresinol diglucoside of flaxseed and its metabolites: biosynthesis and potential for nutraceuticals. Front Genet. 2018;9:641. doi: 10.3389/fgene.2018.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landete JM. Plant and mammalian lignans: a review of source, intake, metabolism, intestinal bacteria and health. Food Res Int. 2012;46:410–424. doi: 10.1016/j.foodres.2011.12.023. [DOI] [Google Scholar]
- Landete JM, Arqués J, Medina M, Gaya P, de Las RB, Muñoz R. Bioactivation of phytoestrogens: intestinal bacteria and health. Crit Rev Food Sci Nutr. 2016;56:1826–1843. doi: 10.1080/10408398.2013.789823. [DOI] [PubMed] [Google Scholar]
- Lin X, Gingrich JR, Bao W, Li J, Haroon ZA, Demark-Wahnefried W. Effect of flaxseed supplementation on prostatic carcinoma in transgenic mice. Urology. 2002;60:919–924. doi: 10.1016/S0090-4295(02)01863-0. [DOI] [PubMed] [Google Scholar]
- Lowcock EC, Cotterchio M, Boucher BA. Consumption of flaxseed, a rich source of lignans, is associated with reduced breast cancer risk. Cancer Causes Control. 2013;24:813–816. doi: 10.1007/s10552-013-0155-7. [DOI] [PubMed] [Google Scholar]
- Mani UV, Mani I, Biswas M, Kumar SN. An open-label study on the effect of flax seed powder (Linum usitatissimum) supplementation in the management of diabetes mellitus. J Diet Suppl. 2011;8:257–265. doi: 10.3109/19390211.2011.593615. [DOI] [PubMed] [Google Scholar]
- McCann SE, Wactawski-Wende J, Kufel K, Olson J, Ovando B, Kadlubar SN, Davis W, Carter L, Muti P, Shields PG, Freudenheim JL. Changes in 2-hydroxyestrone and 16α-hydroxyestrone metabolism with flaxseed consumption: modification by COMT and CYP1B1 genotype. Cancer Epidemiol Biomarkers Prev. 2007;16:256–262. doi: 10.1158/1055-9965.EPI-06-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milder IE, Arts IC, Putte B van de, Venema DP, Hollman PC. Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br J Nutr. 2005;3:393–402. doi: 10.1079/BJN20051371. [DOI] [PubMed] [Google Scholar]
- Moree SS, Kavishankar GB, Rajesha J. Antidiabetic effect of secoisolariciresinol diglucoside in streptozotocin-induced diabetic rats. Phytomedicine. 2013;20:237–245. doi: 10.1016/j.phymed.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Moree SS, Rajesha J. Secoisolariciresinoldiglucoside-a phytoestrogen nutraceutical of flaxseed: synthesis and evaluation of antioxidant potency. Free radicanti oxid. 2011;4:31–38. doi: 10.5530/ax.2011.4.6. [DOI] [Google Scholar]
- Mustafa BE, Subramaniam PK, Mustafa NS, Kashmoola MA, Mokhtar KI, Qaralleh H. The anti-fungal effect of flax seed on oral candidiasis: comparative in-vitro study. J Int Dent. 2018;11:580–586. [Google Scholar]
- Narender BR, Tejaswini S, Sarika M, Karuna N, Shirisha R, Priyanka S. Antibacterial and antifungal activities of Linum usitatissimum (Flax seeds) Int J Pharm Educ Res. 2016;3:4–8. [Google Scholar]
- Nemes SM, Orsat V. Evaluation of a microwave-assisted extraction method for lignan quantification in flaxseed cultivars and selected oil seeds. Food Anal Methods. 2012;5:551–563. doi: 10.1007/s12161-011-9281-6. [DOI] [Google Scholar]
- Otto M. staphylococcus aureus Toxins. Curr Opin. 2014;17:32–37. doi: 10.1016/j.mib.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pag AI, Radu DG, Draganescu D, Popa MI, Sirghie C. Flaxseed cake-a sustainable source of antioxidant and antibacterial extracts. Cellul Chem Technol. 2014;48:265–273. [Google Scholar]
- Patel YS, Patel BM. Secoisolariciresinol diglucoside lignan concentrate of flaxseeds exhibits chemoprotective role in non-melanoma skin cancer through inhibition of CDK4 and upregulation of p53. Indian J Exp Biol. 2021;59:688–696. [Google Scholar]
- Penttinen-Damdimopoulou PE, Power KA, Hurmerinta TT, Nurmi T, van der Saag PT, Mäkelä SI. Dietary sources of lignans and isoflavones modulate responses to estradiol in estrogen reporter mice. Mol Nutr Food Res. 2009;53:996–1006. doi: 10.1002/mnfr.200800487. [DOI] [PubMed] [Google Scholar]
- Pickel B, Constantin MA, Pfannstiel J, Conrad J, Beifuss U, Schaller A. An enantiocomplementary dirigent protein for the enantioselective laccase-catalyzed oxidative coupling of phenols. Angew Chem Int Ed. 2010;49(1):202–204. doi: 10.1002/anie.200904622. [DOI] [PubMed] [Google Scholar]
- Pietrofesa RA, Velalopoulou A, Arguiri E, Menges CW, Testa JR, Hwang WT, Albelda SM, Christofidou-Solomidou M. Flaxseed lignans enriched in secoisolariciresinol diglucoside prevent acute asbestos-induced peritoneal inflammation in mice. Carcinogenesis. 2015;37:177–187. doi: 10.1093/carcin/bgv174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K. Oxidative stress as a mechanism of diabetes in diabetic BB prone rats: effect of secoisolariciresinol diglucoside (SDG) Mol Cell Biochem. 2000;209:89–96. doi: 10.1023/A:1007079802459. [DOI] [PubMed] [Google Scholar]
- Prasad K. Suppression of phosphoenolpyruvate carboxykinase gene expression by secoisolariciresinol diglucoside (SDG), a new antidiabetic agent. Int J Angiol. 2002;11:107–109. doi: 10.1007/BF01616377. [DOI] [Google Scholar]
- Prasad K. Antihypertensive activity of secoisolariciresinol diglucoside (SDG) isolated from flaxseed: role of guanylate cyclase. Int J Angiol. 2004;13:7–14. doi: 10.1007/s00547-004-1060-4. [DOI] [Google Scholar]
- Prasad K, Mantha S, Muir A, Westcott N. Protective effect of secoisolariciresinol diglucoside against streptozotocin-induced diabetes and its mechanism. Mol Cell Biochem. 2000;206:141–150. doi: 10.1023/A:1007018030524. [DOI] [PubMed] [Google Scholar]
- Qiu C, Wang H, Guo Y, Long S, Wang Y, Abbasi AM, Guo X, Jarvis DI. Comparison of fatty acid composition, phytochemical profile and antioxidant activity in four flax (Linum usitatissimum L.) varieties. Oil Crop Sci. 2020;5:136–141. doi: 10.1016/j.ocsci.2020.08.001. [DOI] [Google Scholar]
- Rajesha J, Rao AR, Madhusudhan B, Karunakumar M. Antibacterial properties of secoisolariciresinol diglucoside isolated from Indian flaxseed cultivars. Curr Trends Biotechnol Pharm. 2010;4:551–560. [Google Scholar]
- Rodríguez-García C, Sánchez-Quesada C, Toledo E, Delgado-Rodríguez M, Gaforio JJ. Naturally lignan-rich foods: a dietary tool for health promotion. Molecules. 2019;24:917. doi: 10.3390/molecules24050917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncaglia L, Amaretti A, Raimondi S, Leonardi A, Rossi M. Role of bifidobacteria in the activation of the lignan secoisolariciresinol diglucoside. Appl Microbiol Biotechnol APPL. 2011;92:159–168. doi: 10.1007/s00253-011-3338-8. [DOI] [PubMed] [Google Scholar]
- Saggar JK, Chen J, Corey P, Thompson LU. The effect of secoisolariciresinol diglucoside and flaxseed oil, alone and in combination, on MCF-7 tumor growth and signaling pathways. Nutr Cancer. 2010;62:533–542. doi: 10.1080/01635580903532440. [DOI] [PubMed] [Google Scholar]
- Saleem M, Kim HJ, Ali MS, Lee YS. An update on bioactive plant lignans. Nat Prod Rep. 2005;22:696–716. doi: 10.1039/B514045P. [DOI] [PubMed] [Google Scholar]
- Scherbakov AM, Stasevich OV, Salnikova DI, Andreeva OE, Mikhaevich EI. Antiestrogenic and antiproliferative potency of secoisolariciresinol diglucoside derivatives on MCF-7 breast cancer cells. Nat Prod Res. 2021;35:6099–6105. doi: 10.1080/14786419.2020.1826479. [DOI] [PubMed] [Google Scholar]
- Schmidt TJ, Klaes M, Sendker J. Phytochemistry lignans in seeds of linum species. Phytochemistry. 2012;82:89–99. doi: 10.1016/j.phytochem.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Schwartz H, Sontag G. Analysis of lignans in food samples-impact of sample preparation. Curr Bioact Compd. 2011;7:156–171. doi: 10.2174/157340711796817904. [DOI] [Google Scholar]
- Setchell KD, Brown NM, Zimmer-Nechemias L, Wolfe B, Jha P, Heubi JE. Metabolism of secoisolariciresinol-diglycoside the dietary precursor to the intestinally derived lignan enterolactone in humans. Food Funct. 2014;5:491–501. doi: 10.1039/c3fo60402k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakir KA, Madhusudhan B. Hypocholesterolemic and hepatoprotective effects of flaxseed chutney: evidence from animal studies. Indian J Clin Biochem. 2007;22:117–121. doi: 10.1007/BF02912893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherif IO. Secoisolariciresinol diglucoside in high-fat diet and streptozotocin-induced diabetic nephropathy in rats: a possible renoprotective effect. J Physiol Biochem. 2014;70:961–969. doi: 10.1007/s13105-014-0364-x. [DOI] [PubMed] [Google Scholar]
- Shim YY, Gui B, Arnison PG, Wang Y, Reaney MJ. Flaxseed (Linum usitatissimum L.) bioactive compounds and peptide nomenclature: a review. Trends Food Sci Technol. 2014;38:5–20. doi: 10.1016/j.tifs.2014.03.011. [DOI] [Google Scholar]
- Simbalista RL, Frota KD, Soares RA, Arêas JA. Effect of storage and processing of Brazilian flaxseed on lipid and lignan contents. Food Sci Technol. 2012;32:374–380. doi: 10.1590/S0101-20612012005000037. [DOI] [Google Scholar]
- Singh KK, Mridula D, Rehal J, Barnwal P. Flaxseed: a potential source of food, feed and fiber. Crit Rev Food Sci Nutr. 2011;51:210–222. doi: 10.1080/10408390903537241. [DOI] [PubMed] [Google Scholar]
- Smeds AI, Eklund PC, Sjöholm RE, Willför SM, Nishibe S, Deyama T, Holmbom BR. Quantification of a broad spectrum of lignans in cereals, oilseeds, and nuts. J Agric Food Chem. 2007;55:1337–1346. doi: 10.1021/jf0629134. [DOI] [PubMed] [Google Scholar]
- Smeds AI, Eklund PC, Willför SM. Content, composition, and stereochemical characterisation of lignans in berries and seeds. Food Chem. 2012;134:1991–1998. doi: 10.1016/j.foodchem.2012.03.133. [DOI] [PubMed] [Google Scholar]
- Son HJ, Song KB. Antimicrobial activity of flaxseed meal extract against Escherichia coli O157: H7 and Staphylococcus aureus Inoculated on red mustard. J Microbiol Biotechnol. 2017;27:67–71. doi: 10.4014/jmb.1607.07036. [DOI] [PubMed] [Google Scholar]
- Sonestedt E, Wirfält E. Enterolactone and breast cancer: methodological issues may contribute to conflicting results in observational studies. Nutr Res. 2010;30:667–677. doi: 10.1016/j.nutres.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Struijs K. The lignan macromolecule from flaxseed: structure and bioconversion of lignans. Wageningen: Wageningen University and Research; 2008. [Google Scholar]
- Suzuki S, Umezawa T. Biosynthesis of lignans and norlignans. J Wood Sci. 2007;53:273–284. doi: 10.1007/s10086-007-0892-x. [DOI] [Google Scholar]
- Tan KP, Chen J, Ward WE, Thompson LU. Mammary gland morphogenesis is enhanced by exposure to flaxseed or its major lignan during suckling in rats. Exp Biol Med. 2004;229:147–157. doi: 10.1177/153537020422900203. [DOI] [PubMed] [Google Scholar]
- Thompson LU, Seidl MM, Rickard SE, Orcheson LJ, Fong HH. Antitumorigenic effect of a mammalian lignan precursor from flaxseed. Nutr Cancer. 1996 doi: 10.1080/01635589609514472. [DOI] [PubMed] [Google Scholar]
- Tannous S, Haykal T, Dhaini J, Hodroj MH, Rizk S. The anti-cancer effect of flaxseed lignan derivatives on different acute myeloid leukemia cancer cells. Biomed Pharmacother. 2020;132:110884. doi: 10.1016/j.biopha.2020.110884. [DOI] [PubMed] [Google Scholar]
- Thompson LU, Boucher BA, Liu Z, Cotterchio M, Kreiger N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr Cancer. 2006;54:184–201. doi: 10.1207/s15327914nc5402_5. [DOI] [PubMed] [Google Scholar]
- Thompson LU, Seidl MM, Rickard SE, Orcheson LJ, Fong HH. Antitumorigenic effect of a mammalian lignan precursor from flaxseed. Nutr Cancer. 2009 doi: 10.1080/01635589609514472. [DOI] [PubMed] [Google Scholar]
- Thumann TA, Pferschy-Wenzig EM, Moissl-Eichinger C, Bauer R. The role of gut microbiota for the activity of medicinal plants traditionally used in the European Union for gastrointestinal disorders. J Ethnopharmacol. 2019;245:112153. doi: 10.1016/j.jep.2019.112153. [DOI] [PubMed] [Google Scholar]
- Touré A, Xueming X. Flaxseed lignans: source, biosynthesis, metabolism, antioxidant activity, bio-active components, and health benefits. Compr Rev Food Sci Food Saf. 2010;9:261–269. doi: 10.1111/j.1541-4337.2009.00105.x. [DOI] [PubMed] [Google Scholar]
- Truan JS, Chen JM, Thompson LU. Comparative effects of sesame seed lignan and flaxseed lignan in reducing the growth of human breast tumors (MCF-7) at high levels of circulating estrogen in athymic mice. Nutr Cancer. 2012;64:65–71. doi: 10.1080/01635581.2012.630165. [DOI] [PubMed] [Google Scholar]
- Vaisey-Genser M, Morris DH. Introduction: history of the cultivation and uses of flaxseed. CRC Press; 2003. p. 33. [Google Scholar]
- Velalopoulou A, Tyagi S, Pietrofesa RA, Arguiri E, Christofidou-Solomidou M. The flaxseed-derived lignan phenolic secoisolariciresinol diglucoside (SDG) protects non-malignant lung cells from radiation damage. Int J Mol Sci. 2015;17:7. doi: 10.3390/ijms17010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venglat P, Xiang D, Qiu S, Stone SL, Tibiche C, Cram D, Alting-Mees M, Nowak J, Cloutier S, Deyholos M, Bekkaoui F. Gene expression analysis of flax seed development. BMC Plant Biol. 2011;11:1–5. doi: 10.1186/1471-2229-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hou B. Glycosyltransferases: key players involved in the modification of plant secondary metabolites. Front Biol (beijing) 2009;4:39–46. doi: 10.1007/s11515-008-0111-1. [DOI] [Google Scholar]
- Wang C, Kurzer MS. Phytoestrogen concentration determines effects on DNA synthesis in human breast cancer cells. Nutr Cancer. 1997 doi: 10.1080/01635589709514582. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Ma XQ, Yang DH, Guo ZR, Liu GR, Zhao GX, Tang J, Zhang YN, Ma M, Cai SQ, Ku BS. Production of enterodiol from defatted flaxseeds through biotransformation by human intestinal bacteria. BMC Microbiol. 2010;10:1–9. doi: 10.1186/1471-2180-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fofana B, Roy M, Ghose K, Yao X-H, Nixon M-S, Nair S, Nyomba GB. Flaxseed lignan secoisolariciresinol diglucoside improves insulin sensitivity through upregulation of GLUT4 expression in diet-induced obese mice. J Funct Foods. 2015;18:1–9. doi: 10.1016/j.jff.2015.06.053. [DOI] [Google Scholar]
- Woting A, Clavel T, Loh G, Blaut M. Bacterial transformation of dietary lignans in gnotobiotic rats. FEMS Microbio Ecol. 2010;72:507–514. doi: 10.1111/j.1574-6941.2010.00863.x. [DOI] [PubMed] [Google Scholar]
- Xia ZQ, Costa MA, Pélissier HC, Davin LB, Lewis NG. Secoisolariciresinol dehydrogenase purification, cloning, and functional expression: implications for human health protection*[S] J Biol Chem J BIOL. 2001;C276:12614–12623. doi: 10.1074/jbc.M008622200. [DOI] [PubMed] [Google Scholar]
- Yonekura-Sakakibara K, Hanada K. An evolutionary view of functional diversity in family 1 glycosyltransferases. Plant J. 2011;66:182–193. doi: 10.1111/j.1365-313X.2011.04493.x. [DOI] [PubMed] [Google Scholar]
- Zanwar AA, Hegde MV, Rojatkar SR, Sonawane KB, Rajamohanan PR, Bodhankar SL. Isolation, characterization and antihyperlipidemic activity of secoisolariciresinol diglucoside in poloxamer-407-induced experimental hyperlipidemia. Pharm Biol. 2014;52:1094–1103. doi: 10.3109/13880209.2013.877492. [DOI] [PubMed] [Google Scholar]