Most modern reviews about bacterial cell wall properties use the traditional classification of prokaryotic envelopes as either gram positive or gram negative (27, 30). Mainly based on the variance of their peptidoglycan architecture, both envelope types show a characteristic difference when stained with crystal violet (Gram staining) (see reference 12). The multilayered peptidoglycan of gram-positive bacteria, with a thickness ranging from 20 to 40 nm, usually forms a physical barrier for the dye. In gram-negative bacteria, however, with their relatively thin sacculus of 2 to 6 nm, the stain can easily be washed out. Together with other characteristics, such as the occurrence of accessory cell wall polymers or the presence of an outer membrane, the peptidoglycan architecture largely determines the different properties of the two cell wall types in terms of mechanical stability, permeability, and resistance toward chemical substances (106). Although this classification is generally useful, it oversimplifies the concept of bacterial cell walls. It also ignores, for example, that the largest and perhaps most diverse group of bacteria, the cyanobacteria, possess cell envelopes with a combination of these features.
Despite their overall gram-negative structure, the peptidoglycan layer found in cyanobacteria is considerably thicker than that of most gram-negative bacteria (Fig. 1). In unicellular strains such as Synechococcus, its thickness is about 10 nm (44), reaching 15 to 35 nm in filamentous species like Phormidium uncinatum and more than 700 nm in large cyanobacteria like Oscillatoria princeps (56). Chemical analysis of cyanobacterial peptidoglycans has revealed further unusual characteristics. The degree of cross-linking between the peptidoglycan chains within the murein of Synechocystis sp. strain PCC 6714 is far higher than the usual 20 to 33% found in most gram-negative bacterial peptidoglycans (42), and in fact, the extent of cross-linking (56 to 63%) (66) is more similar to the values reported for gram-positive bacteria (76). On the other hand, most pentapeptides involved in cross-linkage contained only the typical gram-negative bacterial diamino acid meso-diaminopimelic acid, in contrast to l-diaminopimelic acid or l-lysin in gram-positive peptidoglycan tetrapeptides (107), although l-lysin has been found in the pentapeptides of the cyanobacterium Anabaena cylindrica (75). Clearly, more studies are needed to establish the general composition of cyanobacterial peptidoglycans. Another typical constituent of gram-positive peptidoglycans, teichoic acid, is also missing in cyanobacterial cell walls. Nevertheless, the cyanobacterial peptidoglycan is complexed with specific polysaccharides (62, 67) in a fashion very similar to that of gram-positive bacterial peptidoglycan; this feature is also found in the peptidoglycan of the closely related prochlorophytes (64).
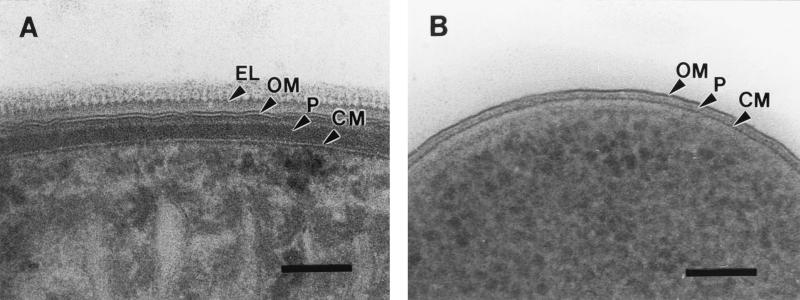
FIG. 1.
Electron microscopical comparison of the gram-negative cell envelopes of the cyanobacterium P. uncinatum (A) and E. coli (B). Both bacteria were identically processed using cryosubstitution procedures. Note the combination of gram-positive and gram-negative features present in the cyanobacterial cell wall, like the thick peptidoglycan layer and the outer membrane, respectively. The external layer of Phormidium is composed of an S-layer (see Fig. 3) and oscillin fibrils creating a serrated surface topography. CM, cytoplasmic membrane; EL, serrated external layer; OM, outer membrane; P, peptidoglycan layer. Bars, 100 nm.
The unique mosaic of different bacterial cell wall features is also found in the cyanobacterial outer membranes: the lipopolysaccharides (LPS) of cyanobacteria not only contain small amounts of bound phosphate (111) but also often lack ketodeoxyoctonate (86, 127), a common LPS component of gram-negative bacterial outer membranes. On the other hand, the cyanobacterial O antigen is reminiscent of the Escherichia coli O antigen (69, 130) and is responsible for cyanophage adsorption (108) or endotoxic activity in aquatic environments (71); S. Fukuoka, Q. Adil, H. Kakita, and H. Obika, J. Endotoxin Res. 3:35, 1996). Furthermore, the outer membranes of cyanobacteria contain constituents not usually present in gram-negative bacteria. Among these components are carotenoids (91, 102), unusual fatty acids, such as β-hydroxypalmitic acid which are found in the lipid A moiety (112), or porins, which are anchored to the underlying peptidoglycan layer via bridge-like coiled-coil domains (49). This list of cyanobacterium-specific cell envelope features is not complete but rather gives an idea how these cell walls might have changed as a consequence of specific adaptations during their (assumed) nearly 3.5 billion years of evolution (25). Our minireview will therefore focus on some of these features and discuss them with respect to general concepts of transenvelope transport, cell wall design, motility, or evolution of this important group of bacteria. By necessity, we have selected a few aspects of the cyanobacterial envelopes, and we apologize that due to the limited space, certain topics such as the complex differentiation processes of cyanobacterial cell walls during heterocyst formation have been omitted.
CYANOBACTERIAL CELL WALLS AND ENDOSYMBIOSIS
Probably the most exciting recent results of cyanobacterial studies are hints indicating that properties once thought to be exclusive to higher plants have their roots back to cyanobacterial ancestors of today's chloroplasts. Originally, the concept of a cyanobacterium-chloroplast transition during evolution was based on the discovery of cyanelles, chloroplast-like cyanobacterial endosymbionts found in a number of taxonomically unrelated eukaryotes (78). The cyanobacterial nature of these cyanelles was evident from their peptidoglycan-containing envelopes and their usage of phycobilisomes as light-harvesting complexes (79). More direct evidence for the cyanobacterial nature of chloroplasts came later with the discovery of the prokaryote-type chloroplast genetic system and protein translation machinery, especially their 70S ribosomes, and their double-layered envelopes. The identification of further links accelerated with the availability of the first completely sequenced cyanobacterial genome (87). Using the sequence of Synechocystis sp. strain PCC 6803, it could be shown that plant phytochromes might have evolved from phytochrome-like molecules in cyanobacteria (131; J. Hughes, T. Lamparter, F. Mittmann, E. Hartmann, W. Gartner, A. Wilde, and T. Borner, Letter, Nature 386:663, 1997; for a review, see reference 95) and that these proteins control similar light-dependent processes (24, 70), despite their prokaryotic organization in two-component regulatory systems (93).
Such links could also be established for the cyanobacterial cell walls. The analysis of the complete sequence of Synechocystis sp. strain PCC 6803 allowed the identification of three open reading frames (ORFs), named synToc75, synToc34, and synTic55, with significant homology to components of the peptide-translocating system of the pea chloroplast (16, 103). This peptide-translocating system is used by the chloroplast for the import of cytosolically synthesized proteins. This process became necessary as most of the genes of the prokaryotic chloroplast's ancestors were transferred after endosymbiosis to the cell nucleus. In pea chloroplasts, the protein translocation system is composed of two separate complexes, Toc and Tic, which are located in the outer (Toc) and inner (Tic) envelope of the chloroplast, respectively (81). The Toc complex consists of at least three proteins designated Toc86, Toc75, and Toc34, but only the function of Toc75 has been elucidated in some detail. This protein forms a voltage-gated channel in lipid membranes in vitro (52), a property which has also recently been demonstrated for the Synechocystis homologue synToc75 (51). The gating behavior of both channels changes dramatically in the presence of preproteins, suggesting that they form a major part of the protein translocation pore. No function is known for the second homologue, synToc34, which has the same distribution of GTP-binding sites and the same membrane anchor domain as its pea homologue.
Finally, the third ORF, synTic55, has a homologue in the chloroplast's inner envelope translocation complex, Tic, made up of the proteins Tic110, Tic55, Tic22, and Tic20. The structure and function of this translocon are less clear, as only a few components have been characterized so far. Nevertheless, synTic55 contains the same Rieske-type iron-sulfur cluster and mononuclear iron-binding site as the pea Tic55, indicating a similar function (23). This function is, however, not yet known, but chemical modification with diethylpyrocarbonate arrests the translocation of the preprotein at the level of the inner membrane. As no other genes encoding potential homologues of the Toc and Tic machinery have yet been identified in Synechocystis, we can only speculate about the origin of the other proteins. Altogether, these results suggest that the chloroplast protein import machinery was recruited from a preexisting transport system present in the cyanobacterial cell wall and thus support the hypothesis of a prokaryotic origin of the chloroplasts.
Further relations between cyanobacteria and plant chloroplasts were demonstrated to be present in the cyanobacterial outer membrane. This outer membrane usually contains a species-specific repertoire of carotenoids, in addition to different lipids and proteins (65, 91, 102). The fact that the chloroplast outer envelope membrane also contains such carotenoids (28, 61) supports the speculation that this plastid envelope is another relic of the endocytic event that led to the development of this organelle (29). It has been suggested that these carotenoids might protect the cyanobacterial cells from high light intensity, particularly in the UV range (92), a hypothesis supported by the facts that the carotenoid synthesis in cyanobacteria is enhanced under these light qualities (32) and that transcription of genes involved in the carotenoid synthesis such as crtB (phytoene synthetase) and crtP (phytoene desaturase) reaches its highest level under these light conditions (34). However, carotenoids are not the only potential photoprotective substances found in cyanobacterial envelopes. In many species like Nostoc commune, the carbohydrate sheaths (see “Cyanobacterial cell walls and external layers” below) contain aromatic pigments like scytonemin (99) or oligosaccharide mycosporine amino acids (OS-MAAs) (15, 110), which can effectively absorb UV light with their extended π electron systems (25). In this organism, different qualities of UV light even lead to synthesis of different sunscreen pigments: UV-B (<315-nm wavelength) induced the formation of OS-MAAs, whereas UV-A (315 to 425 nm) induced almost exclusively scytonemin synthesis (32). Most likely, these UV protection mechanisms have their origin in the conditions found on Earth 3.5 billion years ago, when the evolution of cyanobacteria began. The lack of an ozone layer at that time forced all organisms to adopt to extreme UV radiation. Even today, these mechanisms enable cyanobacteria to flourish in an extreme array of habitats, many of which, like tropical pools, rock surfaces, or terrestrial habitats of the Arctic and Antarctic, are characterized by intense solar radiation. However, some species like the antarctic cyanobacterium Oscillatoria priestleyi try to avoid this radiation rather than tolerate it and simply escape from UV light by gliding motility (100).
CYANOBACTERIAL CELL WALLS AND TRANSPORT
One of the major functions of every bacterial cell envelope is to allow sufficient transport of nutrients and metabolites into and out of the cell, a function especially delicate in cyanobacteria as their thick, multilayered envelopes form a considerable mechanical and permeability barrier for most larger molecules. Consequently, cyanobacteria have developed different transport systems, and as in other bacteria these transport systems can be discriminated according to the energy source used for transport, the complexity of the transport machinery, or the chemical nature of the translocated substrates (reviewed in reference 94). Compared to E. coli, much less experimental data are available for most of these transport systems, and we will therefore discuss some of their general properties using the following examples: (i) cyanobacterial porins as examples for diffusion-based import; (ii) ATP-binding cassette (ABC) transporters as examples of oligomeric transporters; (iii) the question of “Bayer bridges” acting as transport routes for the translocation of components within the cell wall (transmigration); and (iv) the junctional pore complex (JPC) as an example for a multicomponent export machinery.
Cyanobacterial porins, outer membrane proteins with tails.
The permeability characteristics of the outer membrane of gram-negative bacteria are largely determined by two types of molecules: porins, which are nonspecific diffusion channels for small solutes (11), and transport systems for substrates which are unable to diffuse passively through hydrophilic pores (94). In most gram-negative bacteria, porins are unusually stable trimers formed by polypeptides of 30 to 40 kDa (115, 128). The porins characterized so far in cyanobacterial cell walls are bigger and composed of monomers of about 50 to 70 kDa. In contrast, however, the measured single-channel conductance of these porins in lipid bilayer experiments is much smaller, e.g., 0.5 nS for Synechococcus SomA (48) versus 2 to 3.5 nS for typical gram-negative bacterial porins, such as E. coli OmpC, PhoE, or OmpF, or Rhodobacter capsulatus porin (88). This discrepancy between higher molecular mass and low conductance has also been found in other cyanobacterial species (10, 63, 113; A. Hansel and E. Hoiczyk, unpublished data). As there are no selectivity measurements for cyanobacterial porins, we can only speculate about reasons for the observed smaller conductances. They might reflect the photoautotrophic “lifestyle” of cyanobacteria, which require porins that are only large enough to facilitate the uptake of small solutes such as ions from their environment, whereas organic compounds are synthesized by the bacteria themselves. As cyanobacteria live mostly in low-nutrient environments, the strict molecular size cutoff of their porins would also protect the cells better from harmful agents such as toxins or antibiotics. Heterotrophic bacteria, however, such as E. coli or R. capsulatus depend on the uptake of larger molecules such as sugars or amino acids (26) for which they clearly need larger porins. Nevertheless, certain cyanobacterial strains are able to take up sugars from their environment (105), but the specific larger porins for this process have not yet been characterized.
Why then are cyanobacterial porins larger (50 to 70 kDa) than their eubacterial counterparts (30 to 40 kDa)? The deduced protein sequences of the two porins, SomA and SomB, of Synechococcus sp. strain PCC 6301 (48, 49, 123) gave the first clue to the answer. Both porins contained a 120-amino-acid (aa)-long domain not yet observed in this class of outer membrane proteins (59) N-terminally preceding the typical porin β-barrel region formed by 14 to 16 membrane-spanning segments. This domain accounted for the higher molecular weight and consists of two α-helices, flanked by a short β-sheet and followed by a coiled-coil motif. Similar domains have been described for S-layer proteins (surface layer homology [SLH] domains) (33, 82), where they connect these proteins to the peptidoglycan (89) or to secondary cell wall polymers (104) and thereby stabilize the cell wall. Apparently, this motif is a widespread feature of cyanobacterial porins, as the complete sequence of Synechocystis sp. strain PCC 6803 (87) encodes six homologues of SomA and SomB, all with highly conserved SLH domains (49). Sequence alignments of this and other SLH domains show similarities between the cyanobacterial porins and cell wall proteins of the evolutionary very ancient Thermus group, a result which fits well into the hypothesis that these two prokaryotic groups are rather closely related (22, 46). Compared with other SLH domains in bacterial S-layers or extracellular proteins, the cyanobacterial and Thermus SLH domains contain different highly conserved amino acids in their 55-aa-long binding motif (33). Also, the proteins contain only one copy of the cyanobacterial and Thermus SLH domains, whereas S-layers and extracellular proteins contain at least two to three complete repeats of the motif. This suggested close phylogenetic relationship between cyanobacteria and the Thermus group was recently also proposed using sequence comparisons of the heat shock proteins Hsp60 and Hsp70 (46).
Cyanobacterial ABC transporters.
Only a few cyanobacterial ABC transporters have so far been studied in some detail. One of these transporters might be encoded in Synechocystis by the ORFs designated slr0040, slr0041, slr0043, and slr0044, which show homology to nrtA to D (87) a nitrate transport system studied in Synechococcus sp. strain PCC 7942 (90), Anabaena sp. strain PCC 7120 (40), and Phormidium laminosum (84). In all these species, the system is required for nitrate permeation into the cells and is for that reason induced under nitrogen depletion and inhibited by ammonium. The function of the product of slr0042 in this nitrate permease system is not clear, but based on its sequence homology, it could constitute a nitrate-specific pore-forming outer membrane component of the transporter, a hypothesis which definitely requires experimental proof.
Studies of the formation of heterocysts which specifically differentiated cells for N2 fixation (reviewed in references 43 and 129) in certain filamentous cyanobacteria have identified another ABC transporter, encoded by devBCA. In Anabaena sp. strain PCC 7120, this ABC transporter is required for the export of glycolipids to the heterocyst cell walls, an impregnation process necessary to protect the nitrogenase from oxygen (50). DevA is a protein with high similarity to ATP-binding cassette proteins, while DevB and DevC show similarities to membrane fusion and membrane-spanning proteins of ABC transporters, respectively (35). Anabaena strains carrying mutations in any of the three proteins lack the typical heterocyst glycolipid layer and also fail to induce the hetM gene, suggesting that completion of the heterocyst envelope is a developmental checkpoint (43). Another protein revealing homology to an ABC transporter protein is encoded by hetC. Its expression is required for the formation of proheterocysts, the first microscopically visible stage of these highly differentiated cells (74).
Do cyanobacteria use adhesion sites (Bayer bridges) for the transport of components within the cell wall?
Besides the import and export of substances, gram-negative bacteria must also translocate components of the outer membrane through the intermembrane space. While in some model systems, such as the outer membrane protein OmpA (37, 38), specific export signals have been identified, nothing is known about the physical route used by these proteins to reach the outer membrane. Since there is no direct contact between the two membranes, the existence of discrete adhesion sites in E. coli has been proposed (5, 6, 7). Attempts to isolate these structures have revealed no unequivocal results (8, 9), and their existence has therefore been questioned repeatedly (53, 72). However, the existence of very thin connections between the two membranes has not yet been verified or ruled out. Cyanobacteria use the same machinery as E. coli (i.e., the Sec proteins) to transport substances across the cytoplasmic membrane (97), and one can imagine that they will also use the same transport routes to bridge the intermembrane space. However, structures which could be interpreted as putative adhesion sites have never been seen in electron micrographs of cyanobacterial cell walls (Fig. 1). This observation confirms those of other studies (77) and might be a consequence of the larger distance between the two membranes, which prevents the optical illusion of adhesion sites which is easily created by a superposition effect of two membranes in close proximity (described in reference 72). Moreover, the study of negatively stained isolated cell walls showed no pores besides the canals of the JPCs. The existence of lipid bridges traversing the peptidoglycan layer is therefore difficult to imagine. How, then, do the components of the outer membrane cross the intermembrane space? We think one plausible concept is a simple diffusion-based model, which would work without proteinaceous pores in the peptidoglycan layer for which no evidence exists. Similar to the transport of molecules through the thick peptidoglycan layer of gram-positive bacteria (2), proteins of the outer membrane could simply diffuse through the peptidoglycan layer, with only a delay from encountering the cell wall during their passage. More difficult to explain is the translocation of LPS or lipids, the outer membrane “building blocks.” However, these amphipathic molecules have the tendency to form micelles in aqueous environments like the periplasmic space. If their concentration in this compartment were above their critical micelle concentration, these molecules would be almost entirely micellar, allowing their diffusion through the hydrated meshwork of the peptidoglycan. This model could principally function like the translocation of lipoteichoic acids through the cell walls of gram-positive bacteria (36).
The JPC, a complex carbohydrate export machine.
In recent years, it has become more and more evident that gram-negative bacteria possess highly sophisticated translocation machines which enable them to transport large substrates in a single step across their complex envelopes. The flagellar basal body (83), the type III secretion apparatus (41), and DNA transfer machines (120) are examples of such translocation machines. So far, none of these systems have been reported for cyanobacteria. However, recently a novel type of transport complex, termed the JPC, has been found in some motile cyanobacteria (Fig. 2) (58). This complex forms either rows or girdles, which encircle the filaments on both sides of each cross wall and were first detected after acid treatment of isolated sacculi (85). After isolation, the JPC consists of two substructures: a straight tube-like part, 13 nm in diameter, and a terminal pore part with an overall length of 32 nm and a maximal width of about 20 nm. All in all, the whole complex in P. uncinatum is about 70 to 80 nm long, long enough to span the entire multilayered cell wall of this cyanobacterium. As indicated by staining experiments using India ink, these pores are the actual sites of mucilage secretion, a process which directly generates the necessary thrust for the locomotion of these bacteria (58). Furthermore, these experiments have shed some light on the transport capacity of these carbohydrate secretion organelles. Individual slime bands emerging from the pores are elongated at a speed of about 3 μms−1. Similar secretion rates have so far been reported only for the cellulose secretion process of the bacterium Acetobacter xylinum (19, 132). Interestingly, the pore complexes used by this bacterium not only match the dimensions and the electron microscopical appearance of the JPCs but also share their highly oriented spatial arrangement. Each Acetobacter cell possesses about 50 individual pore complexes forming a row on the “ventral” site of the cell's body (132). Obviously, the close proximity of these structures increases the efficiency with which the individually emerging carbohydrate fibrils can interact to form higher-ordered bundles that propel these cells along (discussed below).
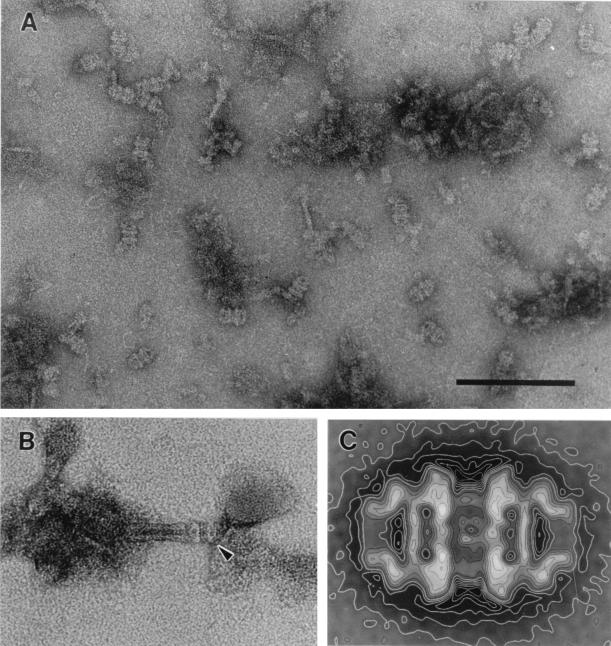
FIG. 2.
Electron micrographs of negatively stained JPC of P. uncinatum, the carbohydrate secretion organelles which generate the thrust for locomotion in cyanobacteria. (A) Highly enriched fraction of isolated pore complexes. Bar, 150 nm. (B) Appearance of the holo-organelle consisting of a tube-like part and the attached pore complex. (C) Average of side-view projections of the pore complex. The overall length of the complex is 32 nm, measuring 8 nm at the terminal opening. Reproduced from reference 58, with permission of Elsevier Science.
CYANOBACTERIAL CELL WALLS AND LOCOMOTION
Most bacteria that are able to move do so by means of flagella, small rotary motors (83), which have never been found in cyanobacteria (47). Nevertheless, many cyanobacteria are capable of a slow, surface-associated motility characterized as gliding (reviewed in reference 21). Although the precise mechanism of gliding is not understood, recent studies have revealed certain structural features invariably linked to this process. Among these features are distinct surface topographies, novel bacterial organelles (JCPs [discussed above]) and the continuous secretion of mucilage.
In most members of the family Oscillatoriaceae, the surface topographies consist of parallel, helically arranged protein fibrils (56), although another type of fibril has recently been described for some Oscillatoria strains (1). In all species studied so far, the arrangement of these surface fibrils correlates with the path of the filaments during locomotion. It has therefore been proposed that the surface fibrils serve as a screw thread guiding the rotation of the filaments, with the necessary thrust for locomotion being derived from the secretion of slime from the junctional pores (58). In this model, the frequent reversal of movements of the filaments can be explained by the alternation of the set of junctional pores used. The importance of the protein fibrils and the secretion of slime for motility are substantiated by the observation that spontaneous mutants lacking these features are nonmotile (57).
Only in Phormidium have the protein fibrils been characterized at the molecular level (57). They are formed by oscillin, a 646-aa glycoprotein (GenBank accession no. AF002131) which contains multiple repeats of a Ca2+-binding nonapeptide (3), a motif also found in SwmA (GenBank accession no. U48223), a surface protein of swimming Synechococcus (17, 18, 125), and HlyA, a partial ORF of Anabaena (GenBank accession no. U13767) with unknown function. However, only for SwmA has a role in motility been established (17). Compared with the role of oscillin in gliding, the role of SwmA in swimming is not so clear (31, 96). The fact that a mutant lacking SwmA is still able to rotate indicates that the protein plays a role in the generation of thrust but not torque, but how the cells produce the thrust for their locomotion is not known.
CYANOBACTERIAL CELL WALLS AND EXTERNAL LAYERS
Frequently, bacterial cell envelopes are covered by external surface layers such as S-layers and carbohydrate structures (13, 126). S-layers are two-dimensional crystalline arrays formed by a single species of (glyco)protein which covers the entire surface of a cell (117). They function as protective coats or molecular sieves or are involved in cell adhesion and recognition (14, 118). The first observation of S-layers in cyanobacteria was reported about 30 years ago (60), but there have been only a few detailed studies since then (68, 73, 80). As can be seen in Fig. 3, the structures of S-layers can be surprisingly well resolved using electron microscopy in conjunction with image analysis techniques (4). In addition to Phormidium (56), S-layers have been identified on more than 20 strains of Synechococcus (124), Synechocystis and Microcystis spp. (119), Gloecapsa alpicola (60), Cyanothece minerva (45), Aphanothece halophytica (116), and on a Chroococcidiopsis strain (20). It has also been shown that cyanobacterial S-layers can participate in the formation of fine-grain minerals (114), a process involved in the formation of stromatolites (122).
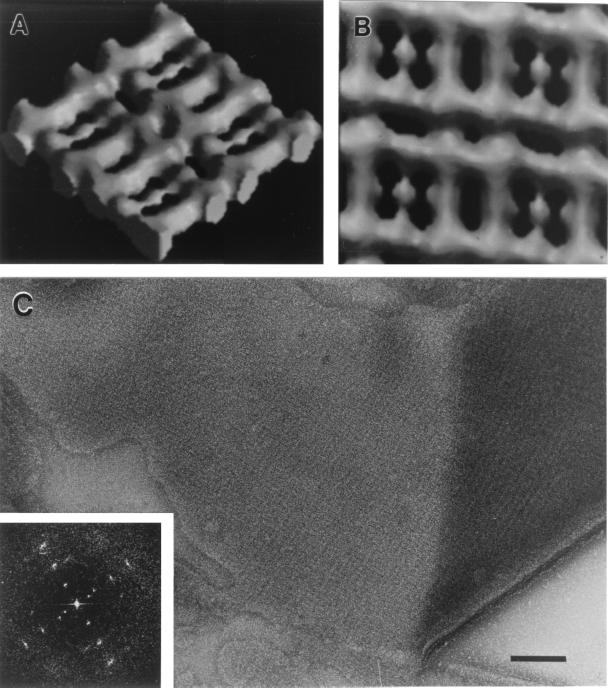
FIG. 3.
Structure of the oblique S-layer of P. uncinatum (54): Computer-generated side (A) and top (B) view of a surface-shaded, solid three-dimensional model of one side of the S-layer. According to the distribution of the measured data in Fourier space, the resolution of the reconstruction used for the model was about 2.0 nm. The lattice constants of the S-layer are a is 10 nm, b is 9.6 nm, and γ is 97.5°. (C) Appearance of the P. uncinatum cell wall after mechanical preparation of the S-layer (also see reference 56). The inset shows the corresponding optical diffraction pattern (power spectrum) which has been used for the reconstruction of the S-layer.
In response to various environmental factors, many cyanobacteria produce diverse external carbohydrate structures (Fig. 4) (126) which sometimes contain pigments such as scytonemin (32) and OS-MAAs (15). Some, like the slime used for gliding motility are readily secreted (discussed above), whereas others such as the sheath of the same species are more firmly attached to the cell (55). Only a few cyanobacterial exopolysaccharides have been defined structurally, although some details of their composition are known (126). The sheaths of P. uncinatum and Nostoc commune contain cellulose-like homoglucan fibrils which are cross-linked by minor monosaccharides (39, 55). Microcystis flos-aquae synthesizes an exopolysaccharide with a composition similar to that of pectin, containing up to 83% galacturonic acid (121). Information about whether its structure also resembles that of pectin is not yet available. Anabaena flos-aquae synthesizes two different polysaccharides: a xyloglucan containing glucose and xylose in a molar ratio of 8:1 and a more complex polysaccharide containing uronic acid, glucose, xylose, and ribose in the molar ratio of 10:6:1:1 (121). This list is far from complete but should highlight the similarity between some cyanobacterial and plant carbohydrates. This observation provokes an exciting speculation. According to the so-called endosymbiont hypothesis, early plant cells started out as amoeboid anaerobic cells without photosynthesis. Only after the establishment of a stable endosymbiosis with a photosynthetic (cyano)bacterium could the early plant cells subvert the endosymbiont's ability to produce carbohydrate for their own use. Today's plant carbohydrates might, therefore, be a consequence of this event and might have evolved from common ancient exopolysaccharides in cyanobacteria. This leads to the question what are the main functions of extracellular carbohydrates in cyanobacteria? Besides being another protective coat for the cells and the photoprotective function discussed earlier, these carbohydrates embed the cells in an extremely hydrated gel-like matrix, which can retain humidity effectively even under water stress. In doing so, the carbohydrates might even prevent dehydration of the cells during temporary dryness. If, however, the unfavorable conditions continue, the delayed desiccation of the carbohydrate matrix gives the cells enough time to activate more specific protection mechanisms. Among these mechanisms are the synthesis of membrane-protective sugars like trehalose (101) or the production of specific stress proteins (109) which help the cells to survive desiccation and rehydration processes. The resulting extraordinary drought resistance of cyanobacteria (reviewed in reference 98) explains their predominance in many desert and tropical soils, terrestrial and subaerial habitats, where an increased tolerance of desiccation is the only way to survive.
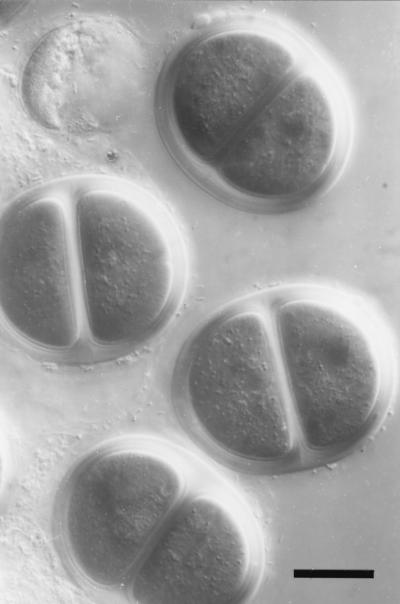
FIG. 4.
Differential interference contrast micrograph of the sheath of Chroococcus turgidus. This cyanobacterium forms regular aggregates of up to four individual cells which are held together by a multilayered, well-defined sheath. Note that the spherical cells become hemispherical as a consequence of compression applied to the cells by the surrounding sheath. Bar, 10 μm.
ACKNOWLEDGMENTS
We thank many of our colleagues for helpful discussions, and we thank our referees and Erica Johnson for critical reading of the manuscript.
This work was supported in part by a postdoctoral fellowship of the Deutsche Forschungsgemeinschaft (DFG) to E.H.
REFERENCES
- 1.Adams D G, Ashworth D, Nelmes B. Fibrillar array in the cell wall of a gliding filamentous cyanobacterium. J Bacteriol. 1999;181:884–892. doi: 10.1128/jb.181.3.884-892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archibald A R, Hancock I C, Harwood C R. Cell wall structure, synthesis and turnover. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria. Washington, D.C.: American Society for Microbiology; 1993. pp. 381–410. [Google Scholar]
- 3.Baumann U, Wu S, Flaherty K M, McKay D B. Three-dimensional structure of the alkaline protease of Pseudomonas aeruginosa: a two-domain protein with a calcium binding parallel beta roll motif. EMBO J. 1993;12:3357–3364. doi: 10.1002/j.1460-2075.1993.tb06009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumeister W, Engelhardt H. Three-dimensional structure of bacterial surface layers. In: Harris R, Horne R W, editors. Electron microscopy of proteins. Vol. 6. London, United Kingdom: Academic Press; 1987. pp. 109–154. [Google Scholar]
- 5.Bayer M E. Areas of adhesion between wall and membranes of Escherichia coli. J Gen Microbiol. 1968;53:395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- 6.Bayer M E. Ultrastructure and organization of the bacterial envelope. Ann N Y Acad Sci. 1974;235:6–28. doi: 10.1111/j.1749-6632.1974.tb43254.x. [DOI] [PubMed] [Google Scholar]
- 7.Bayer M E. Zones of membrane adhesion in the cryofixed envelope of Escherichia coli. J Struct Biol. 1991;107:268–280. doi: 10.1016/1047-8477(91)90052-x. [DOI] [PubMed] [Google Scholar]
- 8.Bayer M E, Bayer M H, Lunn C A, Pigiet V. Association of thioredoxin with the inner membrane and adhesion sites in Escherichia coli. J Bacteriol. 1987;169:2659–2666. doi: 10.1128/jb.169.6.2659-2666.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayer M H, Costello G P, Bayer M E. Isolation and partial characterization of membrane vesicles carrying markers of the membrane adhesion sites. J Bacteriol. 1982;149:758–767. doi: 10.1128/jb.149.2.758-767.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benz R, Böhme H. Pore formation by an outer membrane protein of the cyanobacterium Anabaena variabilis. Biochim Biophys Acta. 1985;812:286–292. [Google Scholar]
- 11.Benz R, Bauer K. Permeation of hydrophilic molecules through the outer membrane of gram-negative bacteria. Review on bacterial porins. Eur J Biochem. 1988;176:1–19. doi: 10.1111/j.1432-1033.1988.tb14245.x. [DOI] [PubMed] [Google Scholar]
- 12.Beveridge T J. Mechanism of gram variability in select bacteria. J Bacteriol. 1990;172:1609–1620. doi: 10.1128/jb.172.3.1609-1620.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beveridge T J, Graham L L. Surface layers of bacteria. Microbiol Rev. 1991;55:684–705. doi: 10.1128/mr.55.4.684-705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beveridge T J, Koval S F. Advances in paracrystalline surface layers. New York, N.Y: Plenum Press; 1993. [Google Scholar]
- 15.Böhm G A, Pfleiderer W, Böger P, Scherer S. Structure of a novel oligosaccharide-mycosporine-amino acid ultraviolet A/B sunscreen pigment from the terrestrial cyanobacterium Nostoc commune. J Biol Chem. 1995;270:8536–8539. doi: 10.1074/jbc.270.15.8536. [DOI] [PubMed] [Google Scholar]
- 16.Bölter B, Soll J, Schulz A, Hinnah S, Wagner R. Origin of a chloroplast protein importer. Proc Natl Acad Sci USA. 1998;95:15831–15836. doi: 10.1073/pnas.95.26.15831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahamsha B. An abundant cell-surface polypeptide is required for swimming by the non-flagellated marine cyanobacterium Synechococcus. Proc Natl Acad Sci USA. 1996;93:6504–6509. doi: 10.1073/pnas.93.13.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brahamsha B. Non-flagellar swimming in marine Synechococcus. J Mol Microbiol Biotechnol. 1999;1:59–62. [PubMed] [Google Scholar]
- 19.Brown R M, Jr, Willison J H M, Richardson C M. Cellulose biosynthesis in Acetobacter xylinum: visualization of the site of synthesis and direct measurement of the in vivo process. Proc Natl Acad Sci USA. 1976;73:4565–4569. doi: 10.1073/pnas.73.12.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Büdel B, Riehl E. A new cell wall structure in a symbiotic and a free-living strain of the blue-alga genus Chroococcidiopsis (Pleurocapsales) Arch Microbiol. 1985;143:117–121. [Google Scholar]
- 21.Burchard R P. Gliding motility. Annu Rev Microbiol. 1981;35:497–529. doi: 10.1146/annurev.mi.35.100181.002433. [DOI] [PubMed] [Google Scholar]
- 22.Bustard K, Gupta R S. The sequences of heat shock protein 40 (DnaJ) homologs provide evidence for a close evolutionary relationship between the Deinococcus-thermus group and cyanobacteria. J Mol Evol. 1997;45:193–205. doi: 10.1007/pl00006219. [DOI] [PubMed] [Google Scholar]
- 23.Caliebe A, Grimm R, Kaiser G, Lübeck J, Soll J, Heins L. The chloroplastic protein import machinery contains a Rieske-type iron-sulfur cluster and a mononuclear iron-binding protein. EMBO J. 1997;16:7342–7350. doi: 10.1093/emboj/16.24.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang G G, Schaefer M R, Grossman A R. Complementation of a red-light-indifferent cyanobacterial mutant. Proc Natl Acad Sci USA. 1992;89:9415–9419. doi: 10.1073/pnas.89.20.9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cockell C S. Ultraviolet radiation, evolution and the π-electron system. Biol J Linn Soc. 1998;63:449–457. [Google Scholar]
- 26.Cowan S W, Schirmer T, Rummel G, Steiert M, Ghosh R, Pauptit R A, Jansonius J N, Rosenbusch J P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992;358:727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- 27.Dijkstra A J, Keck W. Peptidoglycan as a barrier to transenvelope transport. J Bacteriol. 1996;178:5555–5562. doi: 10.1128/jb.178.19.5555-5562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douce R, Joyard J. Structure and function of the plastid envelope. Adv Bot Res. 1979;7:1–116. [Google Scholar]
- 29.Douce R, Joyard J. Does the plastid envelope derive from the endoplasmic reticulum? Trends Biochem Sci. 1981;6:237–239. [Google Scholar]
- 30.Doyle R J. Cell walls of bacteria. In: Lederberg J, editor. Encyclopedia of microbiology. Vol. 1. San Diego, Calif: Academic Press; 1992. pp. 479–493. [Google Scholar]
- 31.Ehlers K M, Samuel A D T, Berg H C, Montgomery R. Do cyanobacteria swim using traveling surface waves? Proc Natl Acad Sci USA. 1996;93:8340–8343. doi: 10.1073/pnas.93.16.8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehling-Schulz M, Bilger W, Scherer S. UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. J Bacteriol. 1997;179:1940–1945. doi: 10.1128/jb.179.6.1940-1945.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engelhardt H, Peters J. Structural research on surface layers: a focus on stability, surface layer homology domains, and surface layer cell wall interactions. J Struct Biol. 1998;124:276–302. doi: 10.1006/jsbi.1998.4070. [DOI] [PubMed] [Google Scholar]
- 34.Fernández-González B, Martínez-Férez I M, Vioque A. Characterization of two carotenoid gene promoters in the cyanobacterium Synechocystis sp. PCC 6803. Biochim Biophys Acta. 1998;1443:343–351. doi: 10.1016/s0167-4781(98)00239-5. [DOI] [PubMed] [Google Scholar]
- 35.Fiedler G, Arnold M, Hannus S, Maldener I. The DevBCA exporter is essential for envelope formation in heterocysts of the cyanobacterium Anabaena sp. strain PCC 7120. Mol Microbiol. 1998;27:1193–1202. doi: 10.1046/j.1365-2958.1998.00762.x. [DOI] [PubMed] [Google Scholar]
- 36.Fischer W. Physiology of lipoteichoic acids in bacteria. Adv Microb Physiol. 1988;29:233–303. doi: 10.1016/s0065-2911(08)60349-5. [DOI] [PubMed] [Google Scholar]
- 37.Freudl R, Schwarz H, Klose M, Movva N R, Henning U. The nature of information, required for export and sorting, present within the outer membrane protein OmpA of Escherichia coli K-12. EMBO J. 1985;4:3593–3598. doi: 10.1002/j.1460-2075.1985.tb04122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freudl R, Henning U. On the role of the mature part of an Escherichia coli outer membrane protein (OmpA) in translocation across the plasma membrane. J Mol Biol. 1988;203:517–519. doi: 10.1016/0022-2836(88)90018-6. [DOI] [PubMed] [Google Scholar]
- 39.Frey-Wyssling A, Stecher H. Über den Feinbau des Nostoc-Schleimes, Z. Zellforsch Mikrosk Anat Abt Histochem. 1954;39:515–519. [PubMed] [Google Scholar]
- 40.Frias J E, Flores E, Herrero A. Nitrate assimilation gene cluster from the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1997;179:477–486. doi: 10.1128/jb.179.2.477-486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galan J E, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284:1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 42.Glauner B, Höltje J V, Schwarz U. The composition of the murein of Escherichia coli. J Biol Chem. 1988;263:10088–10095. [PubMed] [Google Scholar]
- 43.Golden J W, Yoon H-S. Heterocyst formation in Anabaena. Curr Opin Microbiol. 1998;1:623–629. doi: 10.1016/s1369-5274(98)80106-9. [DOI] [PubMed] [Google Scholar]
- 44.Golecki J R. Studies on ultrastructure and composition of cell walls of the cyanobacterium Anacystis nidulans. Arch Microbiol. 1977;114:35–41. doi: 10.1007/BF00429627. [DOI] [PubMed] [Google Scholar]
- 45.Gromov B V, Gavrilova O V, Konovalov E S. Ultrastruktura kletok cianobakterij roda Cyanothece. Mikrobiologiya. 1986;55:821–824. [Google Scholar]
- 46.Gupta R S, Mukhtar T, Singh B. Evolutionary relationships among photosynthetic prokaryotes (Heliobacterium chlorum, Chloroflexus aurantiacus, cyanobacteria, Chlorobium tepidum and proteobacteria): implications regarding the origin of photosynthesis. Mol Microbiol. 1999;32:893–906. doi: 10.1046/j.1365-2958.1999.01417.x. [DOI] [PubMed] [Google Scholar]
- 47.Häder D-P. Photosensory behavior in procaryotes. Microbiol Rev. 1987;51:1–21. doi: 10.1128/mr.51.1.1-21.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansel A, Tadros M H. Characterization of two pore-forming proteins isolated from the outer membrane of Synechococcus PCC 6301. Curr Microbiol. 1998;36:321–326. doi: 10.1007/s002849900316. [DOI] [PubMed] [Google Scholar]
- 49.Hansel A, Pattus F, Jürgens U J, Tadros M H. Cloning and characterization of the genes coding for two porins in the unicellular cyanobacterium Synechococcus PCC 6301. Biochim Biophys Acta. 1998;1399:31–39. doi: 10.1016/s0167-4781(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 50.Hansel A, Lindblad P. Towards optimization of cyanobacteria as biotechnologically relevant producers of molecular hydrogen, a clean and renewable energy source. Appl Microbiol Biotechnol. 1998;50:153–160. [Google Scholar]
- 51.Heins L, Soll J. Chloroplast biogenesis: mixing the prokaryotic and the eukaryotic? Curr Biol. 1998;8:215–217. doi: 10.1016/s0960-9822(98)70129-0. [DOI] [PubMed] [Google Scholar]
- 52.Hinnah S C, Hill K, Wagner R, Schlicher T, Soll J. Reconstitution of a protein translocation channel of chloroplasts. EMBO J. 1997;16:7351–7360. doi: 10.1093/emboj/16.24.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hobot J A, Carlemalm E, Villiger W, Kellenberger E. Periplasmic gel: new concepts resulting from the reinvestigation of bacterial cell envelope ultrastructure by new methods. J Bacteriol. 1984;160:143–152. doi: 10.1128/jb.160.1.143-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoiczyk E. Untersuchungen zur gleitenden Bewegung von Cyanobakterien. Aachen, Germany: Shaker Verlag; 1996. [Google Scholar]
- 55.Hoiczyk E. Structural and biochemical analysis of the sheath of Phormidium uncinatum. J Bacteriol. 1998;180:3923–3932. doi: 10.1128/jb.180.15.3923-3932.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoiczyk E, Baumeister W. Envelope structure of four gliding filamentous cyanobacteria. J Bacteriol. 1995;177:2387–2395. doi: 10.1128/jb.177.9.2387-2395.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoiczyk E, Baumeister W. Oscillin, an extracellular, Ca2+-binding glycoprotein essential for the gliding motility of cyanobacteria. Mol Microbiol. 1997;26:699–708. doi: 10.1046/j.1365-2958.1997.5971972.x. [DOI] [PubMed] [Google Scholar]
- 58.Hoiczyk E, Baumeister W. The junctional pore complex, a prokaryotic secretion organelle, is the molecular motor underlying gliding motility in cyanobacteria. Curr Biol. 1998;8:1161–1168. doi: 10.1016/s0960-9822(07)00487-3. [DOI] [PubMed] [Google Scholar]
- 59.Jeanteur D, Lakey J H, Pattus F. The bacterial porin superfamily: sequence alignment and structure prediction. Mol Microbiol. 1991;5:2153–2164. doi: 10.1111/j.1365-2958.1991.tb02145.x. [DOI] [PubMed] [Google Scholar]
- 60.Jensen T E, Sicko L M. The fine structure of the cell wall of Gloeocapsa alpicola, a blue-green alga. Cytobiologie. 1972;6:439–446. [Google Scholar]
- 61.Jürgens U J, Weckesser J. Carotenoid-containing outer membrane of Synechocystis sp. strain PCC 6714. J Bacteriol. 1985;164:384–389. doi: 10.1128/jb.164.1.384-389.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jürgens U J, Weckesser J. Polysaccharide covalently linked to the peptidoglycan of the cyanobacterium Synechocystis sp. strain PCC6714. J Bacteriol. 1986;168:568–573. doi: 10.1128/jb.168.2.568-573.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jürgens U J, Benz R. Pore-forming activity of outer membrane extracts from the unicellular cyanobacterium Synechocystis PCC 6714. Z Naturforsch Sect C Biosci. 1989;44:165–169. [Google Scholar]
- 64.Jürgens U J, Burger-Wiersma T. Peptidoglycan-polysaccharide complex in the cell wall of the filamentous prochlorophyte Prochlorothrix hollandica. J Bacteriol. 1989;171:498–502. doi: 10.1128/jb.171.1.498-502.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jürgens U J, Mäntele W. Orientation of carotenoids in the outer membrane of Synechocystis PCC 6714. Biochim Biophys Acta. 1991;1067:208–212. doi: 10.1016/0005-2736(91)90045-a. [DOI] [PubMed] [Google Scholar]
- 66.Jürgens U J, Drews G, Weckesser J. Primary structure of the peptidoglycan of the cyanobacterium Synechocystis sp. strain PCC 6714. J Bacteriol. 1983;154:471–478. doi: 10.1128/jb.154.1.471-478.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jürgens U J, Golecki J R, Weckesser J. Characterization of the cell wall of the unicellular cyanobacterium Synechocystis PCC 6714. Arch Microbiol. 1985;142:168–174. [Google Scholar]
- 68.Karlsson B, Vaara T, Lounatmaa K, Gyllenberg H. Three-dimensional structure of the regularly constructed surface layer from Synechocystis sp. strain CLII. J Bacteriol. 1983;156:1338–1343. doi: 10.1128/jb.156.3.1338-1343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katz A, Weckesser J, Drews G, Mayer H. Chemical and biological studies on the lipopolysaccharide (O-antigen) of Anacystis nidulans. Arch Microbiol. 1977;113:247–256. doi: 10.1007/BF00492032. [DOI] [PubMed] [Google Scholar]
- 70.Kehoe D M, Grossman A R. Similarity of a chromatic adaptation sensor to phytochrome and ethylene receptors. Science. 1996;273:1409–1412. doi: 10.1126/science.273.5280.1409. [DOI] [PubMed] [Google Scholar]
- 71.Keleti G, Sykora J L. Production and properties of cyanobacterial endotoxins. Appl Environ Microbiol. 1982;43:104–109. doi: 10.1128/aem.43.1.104-109.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kellenberger E. The “Bayer-bridges” confronted with results from improved electron microscopy methods. Mol Microbiol. 1990;4:697–705. doi: 10.1111/j.1365-2958.1990.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 73.Kessel M. A unique crystalline wall layer in the cyanobacterium Microcystis marginata. J Ultrastruct Res. 1978;62:203–212. doi: 10.1016/s0022-5320(78)80017-3. [DOI] [PubMed] [Google Scholar]
- 74.Khudyakov I, Wolk C P. hetC, a gene coding for a protein similar to bacterial ABC protein exporters, is involved in early regulation of heterocyst differentiation in Anabaena sp. strain PCC 7120. J Bacteriol. 1997;179:6971–6978. doi: 10.1128/jb.179.22.6971-6978.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kodani S, Ishida K, Murakami M. Occurrence and identification of UDP-N-acetylmuramyl-pentapeptide from the cyanobacterium Anabaena cylindrica. FEMS Microbiol Lett. 1999;176:321–325. doi: 10.1111/j.1574-6968.1999.tb13678.x. [DOI] [PubMed] [Google Scholar]
- 76.Labischinski H, Maidhof H. Bacterial peptidoglycan: overview and evolving concepts. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier; 1994. pp. 23–38. [Google Scholar]
- 77.Leduc M, Frehel C, Siegel E, Van Heijenoort J. Multilayered distribution of peptidoglycan in the periplasmic space of Escherichia coli. J Gen Microbiol. 1989;135:1243–1254. doi: 10.1099/00221287-135-5-1243. [DOI] [PubMed] [Google Scholar]
- 78.Löffelhardt W, Bohnert H J. Molecular biology of cyanelles. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer; 1994. pp. 65–89. [Google Scholar]
- 79.Löffelhardt W, Bohnert H J, Bryant D A. The cyanelles of Cyanophora paradoxa. Crit Rev Plant Sci. 1997;16:393–413. [Google Scholar]
- 80.Lounatmaa K, Vaara T, Österlund K, Vaara M. Ultrastructure of the cell wall of a Synechocystis strain. Can J Microbiol. 1980;26:204–208. doi: 10.1139/m80-031. [DOI] [PubMed] [Google Scholar]
- 81.Lübeck J, Heins L, Soll J. Protein import into chloroplasts. Physiol Plant. 1997;100:53–64. [Google Scholar]
- 82.Lupas A, Engelhardt H, Peters J, Santarius U, Volker S, Baumeister W. Domain structure of the Acetogenium kivui surface layer revealed by electron crystallography and sequence analysis. J Bacteriol. 1994;176:1224–1233. doi: 10.1128/jb.176.5.1224-1233.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Macnab R M. The bacterial flagellum: reversible rotary propellor and type III export apparatus. J Bacteriol. 1999;181:7149–7153. doi: 10.1128/jb.181.23.7149-7153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Merchan F, Kindle K L, Llama M J, Serra J L, Fernandez E. Cloning and sequencing of the nitrate transport system from the thermophilic, filamentous cyanobacterium Phormidium laminosum: comparative analysis with the homologous system from Synechococcus sp. PCC 7942. Plant Mol Biol. 1995;28:759–766. doi: 10.1007/BF00021199. [DOI] [PubMed] [Google Scholar]
- 85.Metzner J. Zur Chemie und zum submikroskopischen Aufbau der Zellwände, Scheiden und Gallerten der Cyanophyceen. Arch Mikrobiol. 1955;22:45–77. [PubMed] [Google Scholar]
- 86.Mikheyskaya L V, Ovodova R G, Ovodova Y S. Isolation and characterization of lipopolysaccharides from cell walls of blue-green algae of the genus Phormidium. J Bacteriol. 1977;130:1–3. doi: 10.1128/jb.130.1.1-3.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakamura Y, Kaneko T, Hirosawa M, Myajima N, Tabata S. Cyanobase, a www database containing the complete nucleotide sequence of Synechocystis sp. strain PCC 6803. Nucleic Acids Res. 1998;26:63–67. doi: 10.1093/nar/26.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nikaido H. Porins and specific channels of bacterial outer membranes. Mol Microbiol. 1992;6:435–442. doi: 10.1111/j.1365-2958.1992.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 89.Olabarria G, Carrascosa J L, de Pedro M A, Berenguer J. A conserved motif in S-layer proteins is involved in peptidoglycan binding in Thermus thermophilus. J Bacteriol. 1996;178:4765–4772. doi: 10.1128/jb.178.16.4765-4772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Omata T. Structure, function and regulation of the nitrate transport system of the cyanobacterium Synechococcus sp. PCC7942. Plant Cell Physiol. 1995;36:207–213. doi: 10.1093/oxfordjournals.pcp.a078751. [DOI] [PubMed] [Google Scholar]
- 91.Omata T, Murata N. Isolation and characterization of three types of membranes from the cyanobacterium (blue green alga) Synechocystis sp. PCC 6714. Arch Microbiol. 1984;139:113–116. [Google Scholar]
- 92.Paerl H W. Cyanobacterial carotenoids: their roles in maintaining optimal photosynthetic production among aquatic bloom forming genera. Oecologia. 1984;61:143–149. doi: 10.1007/BF00396752. [DOI] [PubMed] [Google Scholar]
- 93.Parkinson J S, Kofoid E C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 94.Paulsen I T, Sliwinski M K, Saier M H., Jr Microbial genome analyses: global comparisons of transport capabilities based on phylogenies, bioenergetics and substrate specificities. J Mol Biol. 1998;277:573–592. doi: 10.1006/jmbi.1998.1609. [DOI] [PubMed] [Google Scholar]
- 95.Pepper A E. Molecular evolution: old branches on the phytochrome family tree. Curr Biol. 1998;8:117–120. doi: 10.1016/s0960-9822(98)70985-6. [DOI] [PubMed] [Google Scholar]
- 96.Pitta T P, Sherwood E E, Kobel A M, Berg H C. Calcium is required for swimming by the nonflagellated cyanobacterium Synechococcus strain WH8113. J Bacteriol. 1997;179:2524–2528. doi: 10.1128/jb.179.8.2524-2528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pohlschröder M, Prinz W A, Hartmann E, Beckwith J. Protein translocation in the three domains of life: variations on a theme. Cell. 1997;91:563–566. doi: 10.1016/s0092-8674(00)80443-2. [DOI] [PubMed] [Google Scholar]
- 98.Potts M. Desiccation tolerance of prokaryotes. Microbiol Rev. 1994;58:755–805. doi: 10.1128/mr.58.4.755-805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Proteau P J, Gerwick W H, Garcia-Pichel F, Castenholz R. The structure of sytonemin, an ultraviolet sunscreen pigment from the sheaths of cyanobacteria. Experientia. 1993;49:825–829. doi: 10.1007/BF01923559. [DOI] [PubMed] [Google Scholar]
- 100.Quesada A, Vincent W F. Strategies of adaptation by antarctic cyanobacteria to ultraviolet radiation. Eur J Phycol. 1997;32:335–342. [Google Scholar]
- 101.Reeds R H, Richardson D L, Warr S R C, Stewart W D P. Carbohydrate accumulation and osmotic stress in cyanobacteria. J Gen Microbiol. 1984;130:1–4. [Google Scholar]
- 102.Resch C M, Gibson J. Isolation of the carotenoid-containing cell wall of three unicellular cyanobacteria. J Bacteriol. 1983;155:345–350. doi: 10.1128/jb.155.1.345-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reumann S, Davila-Aponte J, Keegstra K. The evolutionary origin of the protein-translocating channel of chloroplastic envelope membranes: identification of a cyanobacterial homolog. Proc Natl Acad Sci USA. 1999;96:784–789. doi: 10.1073/pnas.96.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ries W, Hotzy C, Schocher I, Sleytr U B, Sára M. Evidence that the N-terminal part of the S-layer protein from Bacillus stearothermophilus PV72/P2 recognizes a secondary cell wall polymer. J Bacteriol. 1997;179:3892–3898. doi: 10.1128/jb.179.12.3892-3898.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rippka R, Deruelles J, Waterbury J B, Herdman M, Stanier R Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- 106.Rogers H J, Perkins H R, Ward J B. Microbial cell walls and membranes. London, United Kingdom: Chapman and Hall; 1980. [Google Scholar]
- 107.Salton M R J. The bacterial cell envelope—a historical perspective. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier; 1994. pp. 1–25. [Google Scholar]
- 108.Samimi B, Drews G. Adsorption of cyanophage AS-1 to unicellular cyanobacteria and isolation of receptor material from Anacystis nidulans. J Virol. 1978;25:164–174. doi: 10.1128/jvi.25.1.164-174.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scherer S, Potts M. Novel water stress protein from a desiccation-tolerant cyanobacterium. J Biol Chem. 1989;264:12546–12553. [PubMed] [Google Scholar]
- 110.Scherer S, Chen T W, Böger P. A new UV-A/B protecting pigment in the terrestrial cyanobacterium Nostoc commune. Plant Physiol. 1988;88:1055–1057. doi: 10.1104/pp.88.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schmidt W, Drews G, Weckesser J, Fromme J, Borowiak D. Characterization of the lipopolysaccharides from eight Synechococcus strains. Arch Microbiol. 1980;127:217–222. [Google Scholar]
- 112.Schrader M, Drews G, Weckesser J. Chemical analyses on cell wall constituents of the thermophilic cyanobacterium Synechococcus PCC 6716. FEMS Microbiol Lett. 1981;11:37–40. [Google Scholar]
- 113.Schulte S. Isolierung und Charakterisierung des Porins der äußeren Membran von Prochlorothrix hollandica. Diploma thesis. Freiburg, Germany: Universität Freiburg; 1993. [Google Scholar]
- 114.Schultze-Lam S, Harauz G, Beveridge T J. Participation of a cyanobacterial S layer in fine-grain mineral formation. J Bacteriol. 1992;174:7971–7981. doi: 10.1128/jb.174.24.7971-7981.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schulz G E. Bacterial porins: structure and function. Curr Opin Cell Biol. 1993;5:701–707. doi: 10.1016/0955-0674(93)90143-e. [DOI] [PubMed] [Google Scholar]
- 116.Simon R D. Gliding motility in Aphanothece halophytica: analysis of wall proteins in mot mutants. J Bacteriol. 1981;148:315–321. doi: 10.1128/jb.148.1.315-321.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sleytr U B, Messner P, Pum D, Sára M. Crystalline bacterial cell surface layers. Berlin, Germany: Springer-Verlag; 1988. [Google Scholar]
- 118.Sleytr U B, Messner P, Pum D, Sára M. Crystalline bacterial cell surface proteins. San Diego, Calif: Academic Press; 1996. [Google Scholar]
- 119.Šmarda J. S-layers in cyanobacteria. In: Sleytr U B, Messner P, Pum D, Sára M, editors. Crystalline bacterial cell surface layers. Berlin, Germany: Springer-Verlag; 1988. pp. 127–132. [Google Scholar]
- 120.Solomon J M, Grossman A D. Who's competent and when—regulation of natural genetic competence in bacteria. Trends Genet. 1996;12:150–155. doi: 10.1016/0168-9525(96)10014-7. [DOI] [PubMed] [Google Scholar]
- 121.Sutherland R J, Tait M I. Biopolymers. In: Lederberg J, editor. Encyclopedia of microbiology. Vol. 1. San Diego, Calif: Academic Press; 1992. pp. 339–349. [Google Scholar]
- 122.Thompson J B, Ferris F G, Smith D A. Geomicrobiology and sedimentology of the mixolimnion and chemocline in Fayetteville Green Lake, New York. Palaios. 1990;5:52–75. [Google Scholar]
- 123.Umeda H, Aiba H, Mizuno T. somA, a novel gene that encodes a major outer-membrane protein of Synechococcus sp. PCC 7942. Microbiology. 1996;142:2121–2128. doi: 10.1099/13500872-142-8-2121. [DOI] [PubMed] [Google Scholar]
- 124.Vaara T. The outermost surface structures in chroococcaceaen cyanobacteria. Can J Microbiol. 1982;28:929–941. [Google Scholar]
- 125.Waterbury J B, Willey J M, Franks D G, Valois F W, Watson S W. A cyanobacterium capable of swimming motility. Science. 1985;230:74–76. doi: 10.1126/science.230.4721.74. [DOI] [PubMed] [Google Scholar]
- 126.Weckesser J, Jürgens U J. Cell wall and external layers. Methods Enzymol. 1988;167:173–188. [Google Scholar]
- 127.Weckesser J, Katz A, Drews G, Mayer H, Fromme J. Lipopolysaccharide containing l-acofriose in the filamentous blue-green alga Anabaena variabilis. J Bacteriol. 1974;120:672–678. doi: 10.1128/jb.120.2.672-678.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weiss M S, Abele U, Weckesser J, Welte W, Schiltz E, Schulz G E. Molecular architecture and electrostatic properties of a bacterial porin. Science. 1991;254:1627–1630. doi: 10.1126/science.1721242. [DOI] [PubMed] [Google Scholar]
- 129.Wolk C P. Heterocyst formation. Annu Rev Genet. 1996;30:59–78. doi: 10.1146/annurev.genet.30.1.59. [DOI] [PubMed] [Google Scholar]
- 130.Xu X, Khudyakov I, Wolk C P. Lipopolysaccharide dependence of cyanophage sensitivity and aerobic nitrogen fixation in Anabaena sp. strain PCC 7120. J Bacteriol. 1997;179:2884–2891. doi: 10.1128/jb.179.9.2884-2891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yeh K-C, Wu S-H, Murphy J T, Lagarias J C. A cyanobacterial phytochrome two-component light sensory system. Science. 1997;277:1505–1508. doi: 10.1126/science.277.5331.1505. [DOI] [PubMed] [Google Scholar]
- 132.Zaar K. Visualization of pores (export sites) correlated with cellulose production in the envelope of the gram-negative bacterium Acetobacter xylinum. J Cell Biol. 1979;80:773–777. doi: 10.1083/jcb.80.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]