Abstract
Introduction
Fibroblast growth factor-2 (FGF-2) has been reported to promote periodontal tissue regeneration. However, no study has investigated the long-term prognosis of periodontal regenerative therapy using FGF-2 to date. The aim of this study was to observe the long-term outcomes as well as to investigate the factors affecting the prognosis of periodontal regenerative therapy using FGF-2.
Methods
Sixty intrabony defects were prospectively investigated for three years after periodontal regenerative therapy with recombinant human FGF-2 (rhFGF-2) by evaluating probing pocket depth (PPD) and radiographic bone defect depth (RBD). The factors influencing RBD were assessed by conducting a multivariate linear regression analysis after adjusting for confounders.
Results
The mean age of the participants was 62.4 ± 13.4 years, and baseline PPD and RBD were 6.1 ± 1.9 mm and 4.5 ± 1.8 mm, respectively. At six months, one year, and three years after surgery, PPD and RBD had significantly improved to 4.2 ± 1.7, 3.7 ± 1.4, 4.0 ± 1.9 mm and to 3.08 ± 2.05, 2.73 ± 1.90, 2.51 ± 2.15 mm, respectively. At the three-year examination, a significant positive association was deteced between RBD reduction and RBD at baseline, while the association was not significant between RBD reduction and the radiographic bony angle, number of bony walls of the defect, or the furcation involvement at baseline.
Conclusions
rhFGF-2 was effective for alveolar bone regeneration in patients with periodontitis and maintained the improved parameters over the three-year observation period. The radiographic bone defect depth at baseline was found to be the factor affecting the periodontal regenerative therapy using rhFGF-2 in the intrabony defects.
Trial registration number
UMIN000027979.
Keywords: Periodontitis, Periodontal regenerative therapy, Fibroblast growth factor-2, rhFGF-2
Highlights
-
•
Mid-term observation following periodontal regenerative therapy using rhFGF-2.
-
•
Reductions in PPD and radiographic defect depth were maintained for 3 years.
-
•
Evaluation of prognostic factors of rhFGF-2 application in intrabony defects.
-
•
Preoperative radiographic defect depth predicts postoperative bone fill.
Abbreviations
- (BOP)
Bleeding on probing
- (CEJ)
Cemento-enamel junction
- (CI)
Confidence interval
- (EMD)
Enamel matrix derivatives
- (FGF)
Fibroblast growth factor
- (PPD)
Probing pocket depth
- (RBA)
Radiographic bone defect angle
- (RBD)
Radiographic bone defect depth
- (RCT)
Randomized controlled trial
- (rhFGF-2)
Recombinant human FGF-2
1. Introduction
The formation of intrabony defects surrounding the teeth is occasionally observed in periodontitis. The deep intrabony defect is recognized as a risk of tooth loss [1]. Multiple surgical approaches utilizing biomaterials, such as bone grafts, membranes, and biologics, have been explored to achieve periodontal tissue regeneration in the intrabony defects [2]. Fibroblast growth factor (FGF) is a large family of growth factors actively involved in angiogenesis, wound healing, and tissue regeneration. Of these, FGF-2 has been studied extensively with regard to periodontal regeneration [3]. FGF-2 has potent mitogenic and angiogenic effects due to its ability to bind heparin [4]. It has been reported that FGF-2 promotes bone formation by accelerating the differentiation of osteoprogenitor cells and accelerating the proliferation and migration of periodontal ligament cells, all of which may contribute to soft and hard tissue regeneration [[5], [6], [7], [8], [9], [10], [11]].
In Japan, recombinant human FGF-2 (rhFGF-2) was approved for use in periodontal tissue regenerative therapy in 2016 (Regroth®, Kaken Pharmaceutical Co., Ltd., Japan) and has been clinically applied ever since [12,13]. A meta-analysis has showed a significant effect of the regenerative therapy with rhFGF-2 on the defect bone fill for periodontal intrabony defects [14]. The phase 3 trial, which was a multicenter randomized controlled trial (RCT), revealed that rhFGF-2 possessed significantly higher bone regenerative effects in intrabony defects than enamel matrix derivatives (EMD) [15]. In this trial, the rhFGF-2 group showed radiographic bone fill of 34.4% (95% confidence interval [CI]: 29.8 to 39.0) while the EMD group showed radiographic bone fill of 23.3% (95% CI: 18.6 to 28.0) at 36 weeks postoperatively. In general, the verification of the validity of periodontal regenerative therapy is performed 6–12 months postoperatively. However, investigating subsequent changes and long-term prognosis of periodontal regenerative therapy is clinically important because periodontal disease is a chronic disease and is prone to recurrence. Regarding studies that used other regenerative materials, the ones that used EMD reported that the improvement of clinical parameters 1 year postoperatively is maintained until 5–10 years [16,17] and reported that the clinical parameters further improved at the long-term follow-up [18]. Our previous clinical study, in which we used EMD [19], also showed significant improvement in RBD between 1 and 3 years postoperatively. To investigate changes in clinical parameters after periodontal regenerative therapy using FGF-2 within a similar observation period, we conducted this 3-year follow-up study to allow maximum follow-up of cases with FGF-2 application since its launch at the end of 2016. Furthermore, limited studies have evaluated the factors that influence the clinical outcomes of periodontal regenerative therapy using rhFGF-2 [13,20].
This study aimed to examine the clinical outcomes of periodontal regenerative therapy using rhFGF-2 over a mid-term follow-up period and to investigate the factors that influence the bone regenerative potential of rhFGF-2 in the intrabony defects.
2. Methods
2.1. Study design and participants
This prospective cohort study was conducted using a registry of patients undergoing periodontal regenerative therapy with rhFGF-2 at the periodontal clinic of Tokyo Medical and Dental University Hospital. This study was approved by the Dental Research Ethics Committee of Tokyo Medical and Dental University (approval number: D2017-002) and was conducted in accordance with the Helsinki Declaration of 1975 as revised in 2013. This study was registered at the University Hospital Medical Information Network (UMIN: http//www.umin.ac.jp/) (clinical trial number: UMIN000027979). All patients provided informed consent. The selection criteria for this study were set according to the indications of rhFGF-2 for periodontal regenerative therapy. The inclusion criteria were as follows: (1) patients with periodontitis who completed cause-related periodontal therapy and demonstrated well-controlled oral hygiene, (2) teeth with residual deep periodontal pocket; probing pocket depth (PPD) ≥ 4 mm after non-surgical therapy, (3) presence of intrabony defects in the interproximal area on radiographs, irrespective of furcation involvement, and (4) patients under regular maintenance program for at least three years after surgery. The exclusion criteria were as follows: (1) smokers, (2) patients with diabetes mellitus, (3) teeth suspected with endo-perio lesions wherein the intrabony defect extended to the root apex, and (4) teeth with degree III furcation involvement [21]. The data of patients who underwent periodontal regenerative therapy using rhFGF-2 between December 2016 and December 2018 were included in the final analyses.
2.2. Clinical examinations
The medical and dental histories of all patients were obtained by interviewing them at the first visit. The operators routinely performed the following clinical examinations: (1) tooth mobility, (2) PPD, and (3) bleeding on probing (BOP). PPD was measured using a manual probe (15 UNC Color-Coded Probe, Hu-Friedy, USA) at six sites of each tooth and rounded off to the nearest millimeter. The site with the deepest PPD at baseline was registered as the subject site for each tooth. The number of bony walls and degree of furcation involvement were recorded during surgery.
Radiographic evaluations were performed according to the procedures employed in our previous studies [19,22]. Intraoral radiographs were acquired at baseline, six months, one year, and three years after surgery using the paralleling technique. The radiographic bone defect depth (RBD) was measured by subtracting the vertical linear distance of the long axis of the tooth from the cemento-enamel junction (CEJ) on the interproximal root surface and the alveolar crest to the point of bone-root contact on the radiographs [23]. As customized positioning stents were not used obtaining the radiographs, we calculated the ratio of the defect depth to root length (from the CEJ to the apex) using preoperative and postoperative radiographs to compensate for the reproducibility of the radiographic angulation. The radiographic bone defect angle (RBA) at baseline was measured according to the method described in a previous study [24]; the angle between the line connecting the CEJ to the bottom of the intrabony defect on the root and the line connecting the most coronal position of the alveolar bone crest of the intrabony defect to the bottom of the intrabony defect was measured. All radiographic measurements were performed independently and blindly by two experienced periodontists (SF and KT). The inter-examiner agreement values, calculated using the intraclass correlation coefficient, were 0.89 (95% CI: 0.68 to 0.97) for RBD and 0.95 (95% CI: 0.85 to 0.98) for RBA.
2.3. Surgical procedure
The surgical site was anesthetized with 2% lidocaine. An incision was created according to the modified [25] or simplified papilla preservation technique [26]. A full-thickness flap was elevated to facilitate appropriate instrumentation for accessing the intrabony defect. The Gracey curette (Rigid Gracey curette, Hu-Friedy, IL, USA) was used to debride the root surface and intrabony defect. The root surfaces were rinsed with normal saline, followed by application of 0.3% rhFRF-2 gel (Regroth®, Kaken Pharmaceutical Co., Ltd., Japan) to the exposed root surface. No bone grafts were used adjunctively. Modified vertical mattress sutures with adjunctive single sutures were used to obtain a tight wound closure. Post-surgically, antibiotics (Cefcapene pivoxil hydrochloride hydrate 300 mg per day) and analgesics were prescribed for 3 days. In addition, the patient was instructed to perform oral rinses with 0.2% benzethonium chloride (Neostelin Green 0.2% mouthwash solution, Nishika, Yamaguchi, Japan) for two weeks. The sutures were removed 2 weeks postoperatively. Each patient received professional tooth cleaning every month for the next six months. After the first six months, supportive periodontal therapy was provided every three months. The surgical procedures were performed by 16 periodontists in total. If the operator was clinically inexperienced, he or she was supervised by a well-experienced periodontist.
2.4. Statistical analysis
The data were presented as mean ± standard deviation and numbers (percentages) for continuous and categorical variables, respectively. The Wilcoxon Signed-Rank test with Bonferroni correction was used to compare the baseline and each time point clinical parameters. Multivariate linear regression analysis was performed with RBD reduction as the objective variable to investigate the factors affecting RBD reduction after adjusting for potential confounders, such as age, RBA at baseline, RBD at baseline, furcation involvement, and defect morphology. The amount of change in the RBD was calculated using the following formula: (RBD value at baseline) − (RBD value at the 3-year examination). Furcation involvement was classified into two categories: degrees I and II, and others. The defect morphology was classified on the basis of number of bony walls surrounding the bone defect into two categories: containing defects (3-wall) and non-containing defects (1- or 2-wall) according to the concepts by Cortellini and Tonetti [27]. P-values < 0.05 were considered statistically significant. Statistical analyses were performed using the STATA software (version 16.0; StataCorp, College Station, TX, USA).
3. Results
Of the 79 patients who met the selection criteria, 60 sites of 60 patients with complete clinical data, including radiographs and PPD for 3 years, were included in this study. The characteristics of the included patients and intraoral sites are shown in Table 1. The mean age of the included patients was 62.4 ± 13.4 years, and 43 (71.7%) were female. Eight (13.3%), 19 (31.7%), and 33 (55.0%) of the subjected sites were located on the incisors, premolars, and molars, respectively. The baseline PPD, the ratio of BOP-positive sites, RBD, and RBA, was 6.1 ± 1.9 mm, 60.0%, 4.46 ± 1.80 mm, and 34.7 ± 17.2°, respectively. Degree I or II furcation involvement was observed at 10 sites (16.7%). As assessed intraoperatively, the defect morphology classified by the number of surrounding bony walls, was of the containing type in 38 (63.3%) sites and non-containing type in 22 (36.7%) sites.
Table 1.
Characteristics of patients and preoperative periodontal parameters (N = 60).
| Age (year) | 62.4 ± 13.4 | |
| Female | 43 (71.7%) | |
| Subjected teeth | Incisor | 8 (13.3%) |
| Premolar | 19 (31.7%) | |
| Molar | 33 (55.0%) | |
| Tooth mobility | Degree 0 | 40 (66.7%) |
| Degree 1 | 16 (26.7%) | |
| Degree 2 | 4 (6.7%) | |
| PPD (mm) | 6.1 ± 1.9 | |
| BOP | Positive | 36 (60.0%) |
| RBD (mm) | 4.46 ± 1.80 | |
| RBA (°) | 34.65 ± 17.16 | |
| Furcation involvement | None or Degree 0 | 50 (83.3%) |
| Degree I or II | 10 (16.7%) | |
| Defect morphology | Containing | 38 (63.3%) |
| Non-containing | 22 (36.7%) |
PPD: probing pocket depth; BOP: bleeding on probing; RBD: radiographic bone defect depth; RBA radiographic bone defect angle.
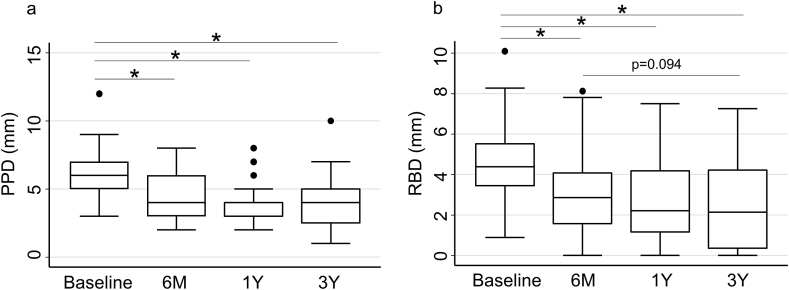
Fig. 1 shows the change in the clinical parameters after periodontal regenerative therapy with rhFGF-2. PPD decreased from 6.1 ± 1.9 mm preoperatively to 4.2 ± 1.7 mm, 3.7 ± 1.4 mm, and 4.0 ± 1.9 mm at six months, one, and three years postoperatively, respectively. The significant PPD reduction was observed at each time point compared to the preoperative value (p < 0.001). Similarly, RBD decreased from 4.46 ± 1.80 mm preoperatively to 3.08 ± 2.05 mm, 2.73 ± 1.90 mm, and 2.51 ± 2.15 mm at six months, one, and three years postoperatively, respectively. The significant RBD reduction was observed at each time point compared to the preoperative value (p < 0.001). Moreover, RBD subsequently decreased at three years compared with at six months (p = 0.094).
Fig. 1.
Postoperative change in PPD and RBD for three years. Black dots indicate the values that are more than 1.5 quarter deviations away from the 25th and 75th %tile. (a) PPDs were significantly reduced compared to baseline at each time point (p < 0.001). (b) RBDs were decreased significantly compared to baseline at each time point (p < 0.001). RBDs subsequently decreased at 3 years compared with at 6 months (p = 0.094).
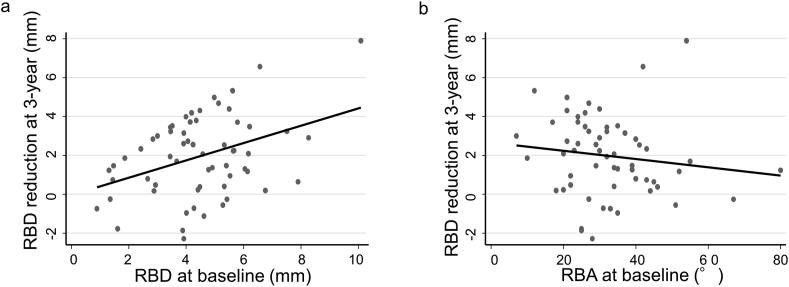
To explore the factors affecting RBD reduction after periodontal regenerative therapy with rhFGF-2, the correlation between clinical parameters at baseline and RBD reduction at three-year examination was analyzed. Fig. 2a shows the correlation between RBD at baseline and RBD reduction at the three-year examination, which showed a significant and positive correlation (Spearman's rho = 0.27, p = 0.038). A trend of negative correlation between RBA at baseline and RBD reduction at the three-year examination was observed but this was not significant (Spearman's rho = −0.24, p = 0.08). Multivariate linear regression analysis showed that RBD at baseline had a significant effect on RBD reduction at the three year examination (coefficient: 0.48, 95% CI: 0.17 to 0.78, p = 0.003), while age, RBA at baseline, furcation involvement, and morphology of bony defect had no significant effect (Table 2, multivariate model).
Fig. 2.
Correlation between RBD at baseline and RBD reduction at 3-year examination (a) they have significant and positive correlation (Spearman's rho = 0.27, p = 0.038). Correlation between RBA at baseline and RBD reduction at 3-year examination (b) a trend of negative correlation was observed but not significant (Spearman's rho = −0.24, p = 0.08).
Table 2.
Factors affecting RBD gain at 3-year examination.
| Univariate model |
Multivariate modela |
||||||
|---|---|---|---|---|---|---|---|
| Coef. | 95% CI | p-value | Coef. | 95% CI | p-value | ||
| Age (year) | −0.02 | −0.06 to 0.02 | 0.35 | −0.02 | −0.06 to 0.02 | 0.35 | |
| RBD at baseline (mm) | 0.48 | 0.19 to 0.77 | 0.002 | 0.48 | 0.17 to 0.78 | 0.003 | |
| RBA at baseline (mm) | −0.003 | −0.041 to 0.036 | 0.89 | −0.007 | −0.044 to 0.030 | 0.71 | |
| Furcation involvement | None or Degree 0 | Reference | Reference | ||||
| Degree I or | 0.13 | −1.34 to 1.61 | 0.86 | 0.19 | −1.39 to 1.77 | 0.81 | |
| Defect morphology | Containing | Reference | Reference | ||||
| Non-containing | −0.04 | −1.25 to 1.17 | 0.94 | −0.12 | −1.41 to 1.16 | 0.85 | |
Coef., coefficient; CI, confidence interval; RBA, radiographic bone defect angle; RBD, radiographic bone defect depth.
Adjusted for all covariates.
4. Discussion
This study showed that periodontal regenerative therapy using rhFGF-2 was clinically and radiographically effective for intrabony defects and that the positive effects were maintained for three years. The decrease in PPD reached a plateau in six months, but radiographical examination revealed that the gradual recovery of bone defects sustained for approximately one to three years, although the differences were not statistically significant. PPD had decreased by 2.1 mm, and RBD had reduced by 1.95 mm, three years after surgery. Furthermore, RBD at baseline was found to be a significant factor affecting RBD reduction; every 1 mm increase of RBD at baseline predicted a 0.5 mm reduction in RBD at three years postoperatively. The results of this study showed that the deeper the preoperative intrabony defects, the higher the expectation of increased bone regeneration. The multivariate analysis showed that the number of bony walls, the bony defect angle, or the presence of furcation involvement, which were previously assumed to be predictive factors of regenerative therapy, have no significant effects on the change in RBD. These findings reveal that, considering the indication for FGF-2, the clinical outcome of periodontal regenerative therapy using FGF-2 can be predicted based on the intrabony defect depth measured using simple dental radiographic examinations, regardless of the morphology of the bone defect.
Several studies have reported the efficacy of regenerative therapy with rhFGF-2 for intrabony defects. A meta-analysis focusing on the adjunctive use of rhFGF-2 with bone grafts for intrabony defects reported that 0.3% rhFGF-2 group showed significantly enhanced RBD reduction at six to nine months postoperatively (mean difference: 1.13 mm, 95% CI: 0.78 to 1.49, p < 0.00001) [28]. In a large multicenter RCT conducted by Kitamura et al. [15], RBD reduction was reported to be 1.32 mm at six months and 1.95 mm at nine months with the usage of 0.3% rhFGF-2 alone for intrabony defects, indicating that bone regeneration continued even after six months of operation. The present study demonstrated RBD reductions of 1.38 mm at six months, 1.73 mm at one year, and 1.95 mm at three years postoperatively, suggesting that bone regeneration occurs gradually after six months and over the course of one to three years following the procedure. While previous studies on regenerative therapy using FGF-2 have reported only short-term outcomes, this study, based on data from a medium-term observation period of 3 years, showed a trend of improvement in RBD even after 6 months postoperatively, which is consistent with the findings of our previous study in which EMD was used [19]. The RBD at baseline in this study was 4.46 ± 1.80 mm, which was shallower than that of 5.73 ± 1.61 mm in the previously mentioned RCT conducted by Kitamura et al. The present study revealed that, deeper the preoperative RBD, greater is the postoperative RBD reduction; therefore the results of our study might be comparable to those of the above mentioned RCT [15]. The selection criteria for this study were set according to general indications for periodontal regenerative therapy. In addition, all operators were not highly experienced periodontists because this study was performed in an educational institute. Nonetheless, the presented clinical outcomes were successfully comparable to those of previous RCTs, which might support the high efficacy of rhFGF-2 in periodontal regenerative therapy for intrabony defects.
Defect morphology has been shown to influence the clinical outcome of periodontal regenerative therapy [2,29]. The depth of bone defects and radiographic defect angles have been reported as essential factors for guided tissue regeneration based approaches [[30], [31], [32], [33], [34]]. Cosyn et al. [35] have demonstrated that a 1-wall defect is a risk factor for failure of periodontal regenerative therapy using a collagen-enriched bovine-derived xenograft. Tsitoura et al. [24] reported that the RBA at baseline affected clinical outcomes in the treatment of intrabony defects using EMD alone. On the other hand, Parashis et al. [36] reported that smoking and supracrestal soft tissue thickness were the only factors that significantly affected the amount of bone regeneration in intrabony defects with EMD alone, not the bone defect morphology, preoperative PPD, or bone defect angle. Owing to the great deal of variation among the findings of different studies, the factors that critically impact the outcome of periodontal regenerative therapy are still unclear. In addition, since each biomaterial used in periodontal regenerative therapy has different properties, the factors that influence the clinical outcomes might vary depending on the material utilized in a particular case. Aoki et al. [13] reported that RBD at baseline significantly affected the clinical attachment level gain two years after periodontal regenerative therapy using rhFGF-2 in combination with deproteinized bovine bone mineral. However, the above mentioned studies have not reported on the prognostic factors that might influence bone regeneration after periodontal regenerative therapy using rhFGF-2. The present study showed that RDB at baseline was a significant predictor of RBD reduction at the three-year examination, whereas the space-maintaining factors, such as RBA and the number of bony walls, had no significant effect. Nakayama et al. [20] also reported that the shape of bone defects did not have a significant influence on the rate of bone fill after regenerative therapy with rhFGF-2 in a 12-month observational period. These findings might suggest that rhFGF-2 exhibits remarkable potential for bone formation, independent of the space-maintaining parameters, although rhFGF-2 is a gel-formed material. However, a previous study reported that defect morphology (3-wall or 1-2-wall defect), significantly affected the amount of radiographic bone fill two years after periodontal regeneration therapy using rhFGF-2 [13]. This discrepancy may be due to differences in observation periods, sample sizes, and methods employed to assess radiographic bone regeneration. Therefore, further studies with larger populations and longer observation periods are necessary to obtain definitive results. In our study, age and the presence of degree I or II furcation involvement had no significant effect on RBD reduction at three years postoperatively. These results were consistent with a previous large-scale study on periodontal regenerative therapy using EMD [19].
The present study has several limitations. First, the standardization of radiographic evaluation of the bony defects using radiographic stents for consistent angulation was not performed despite post hoc compensation. Standardization of the radiograph and three-dimensional bony defect assessment using computed tomography imaging should be applied in future studies. Second, the same clinician performed the re-evaluations and surgery, who was not blinded, which could have induced a bias towards the postoperative PPD. To overcome this, PPD and clinical attachment gain should be examined using patient-specific stents by blinded examiners who are not involved in the surgery. Third, the participants in this study were patients at a university hospital and may represent a different population than that of the general dental clinics. Patients visiting a university hospital are likely to demonstrate a higher health consciousness and greater compliance towards oral health maintenance than those in the local primary care clinics. Consideration should be given to the fact that the presented findings may vary depending on the subject population and their compliance with dental treatment.
5. Conclusion
Periodontal regenerative therapy of intrabony defects using rhFGF-2 is effective at promoting bone regeneration over a three-year period. The radiographic bone defect depth at baseline influences bone regeneration, whereas the bone defect angle, number of bony walls, and presence of furcation involvement do not impact bone regeneration using rhFGF-2.
Declarations of competing interest
None declared.
Acknowledgments
The authors thank the staff of Department of Periodontology of TMDU for their assistance with data collection. This work was supported by a Grant-in-Aid for Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (grant number 20K18497 for RM and 20K18571 for SF).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Papapanou P.N., Wennström J.L. The angular bony defect as indicator of further alveolar bone loss. J Clin Periodontol. 1991;18:317–322. doi: 10.1111/j.1600-051x.1991.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 2.Kao R.T., Nares S., Reynolds M.A. Periodontal regeneration – intrabony defects: a systematic review from the AAP Regeneration Workshop. J Periodontol. 2015;86:S77–S104. doi: 10.1902/jop.2015.130685. [DOI] [PubMed] [Google Scholar]
- 3.Murakami S. Periodontal tissue regeneration by signaling molecule(s): what role does basic fibroblast growth factor (FGF-2) have in periodontal therapy? Periodontol. 2011;56:188–208. doi: 10.1111/j.1600-0757.2010.00365.x. 2000. [DOI] [PubMed] [Google Scholar]
- 4.Kao R.T., Murakami S., Beirne O.R. The use of biologic mediators and tissue engineering in dentistry. Periodontol. 2009;50:127–153. doi: 10.1111/j.1600-0757.2008.00287.x. 2000. [DOI] [PubMed] [Google Scholar]
- 5.Murakami S., Takayama S., Kitamura M., Shimabukuro Y., Yanagi K., Ikezawa K., et al. Recombinant human basic fibroblast growth factor (bFGF) stimulates periodontal regeneration in class II furcation defects created in beagle dogs. J Periodontal Res. 2003;38:97–103. doi: 10.1034/j.1600-0765.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 6.Takayama S., Murakami S., Shimabukuro Y., Kitamura M., Okada H. Periodontal regeneration by FGF-2 (bFGF) in primate models. J Dent Res. 2001;80:2075–2079. doi: 10.1177/00220345010800121001. [DOI] [PubMed] [Google Scholar]
- 7.Mayahara H., Ito T., Nagai H., Miyajima H., Tsukuda R., Taketomi S., et al. In vivo stimulation of endosteal bone formation by basic fibroblast growth factor in rats. Growth Factors. 1993;9:73–80. doi: 10.3109/08977199308991583. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T., Hanada K., Tamura M., Shibanushi T., Nigi H., Tagawa M., et al. Stimulation of endosteal bone formation by systemic injections of recombinant basic fibroblast growth factor in rats. Endocrinology. 1995;136:1276–1284. doi: 10.1210/endo.136.3.7867582. [DOI] [PubMed] [Google Scholar]
- 9.Shujaa Addin A., Akizuki T., Hoshi S., Matsuura T., Ikawa T., Fukuba S., et al. Biodegradable gelatin/beta-tricalcium phosphate sponges incorporating recombinant human fibroblast growth factor-2 for treatment of recession-type defects: a split-mouth study in dogs. J Periodontal Res. 2017;52:863–871. doi: 10.1111/jre.12456. [DOI] [PubMed] [Google Scholar]
- 10.Fukuba S., Akizuki T., Matsuura T., Okada M., Nohara K., Hoshi S., et al. Effects of combined use of recombinant human fibroblast growth factor-2 and β-tricalcium phosphate on ridge preservation in dehiscence bone defects after tooth extraction: a split-mouth study in dogs. J Periodontal Res. 2021;56:298–305. doi: 10.1111/jre.12818. [DOI] [PubMed] [Google Scholar]
- 11.Fukuba S., Akizuki T., Hoshi S., Matsuura T., Shujaa Addin A., Okada M., et al. Comparison between different isoelectric points of biodegradable gelatin sponges incorporating β-tricalcium phosphate and recombinant human fibroblast growth factor-2 for ridge augmentation: a preclinical study of saddle-type defects in dogs. J Periodontal Res. 2019;54:278–285. doi: 10.1111/jre.12628. [DOI] [PubMed] [Google Scholar]
- 12.Saito A., Bizenjima T., Takeuchi T., Suzuki E., Sato M., Yoshikawa K., et al. Treatment of intrabony periodontal defects using rhFGF-2 in combination with deproteinized bovine bone mineral or rhFGF-2 alone: a 6-month randomized controlled trial. J Clin Periodontol. 2019;46:332–341. doi: 10.1111/jcpe.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoki H., Bizenjima T., Seshima F., Sato M., Irokawa D., Yoshikawa K., et al. Periodontal surgery using rhFGF-2 with deproteinized bovine bone mineral or rhFGF-2 alone: 2-year follow-up of a randomized controlled trial. J Clin Periodontol. 2021;48:91–99. doi: 10.1111/jcpe.13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khoshkam V., Chan H.L., Lin G.H., Mailoa J., Giannobile W.V., Wang H.L., et al. Outcomes of regenerative treatment with rhPDGF-BB and rhFGF-2 for periodontal intra-bony defects: a systematic review and meta-analysis. J Clin Periodontol. 2015;42:272–280. doi: 10.1111/jcpe.12354. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura M., Akamatsu M., Kawanami M., Furuichi Y., Fujii T., Mori M., et al. Randomized placebo-controlled and controlled non-inferiority phase III trials comparing Trafermin, a recombinant human fibroblast growth factor 2, and enamel matrix derivative in periodontal regeneration in intrabony defects. J Bone Miner Res. 2016;31:806–814. doi: 10.1002/jbmr.2738. [DOI] [PubMed] [Google Scholar]
- 16.Sculean A., Donos N., Schwarz F., Becker J., Brecx M., Arweiler N.B. Five-year results following treatment of intrabony defects with enamel matrix proteins and guided tissue regeneration. J Clin Periodontol. 2004;31:545–549. doi: 10.1111/j.1600-051X.2004.00518.x. [DOI] [PubMed] [Google Scholar]
- 17.Sculean A., Kiss A., Miliauskaite A., Schwarz F., Arweiler N.B., Hannig M. Ten-year results following treatment of intra-bony defects with enamel matrix proteins and guided tissue regeneration. J Clin Periodontol. 2008;35:817–824. doi: 10.1111/j.1600-051X.2008.01295.x. [DOI] [PubMed] [Google Scholar]
- 18.De Ry S.P., Roccuzzo A., Lang N.P., Sculean A., Salvi G.E. Long-term clinical outcomes of periodontal regeneration with enamel matrix derivative: a retrospective cohort study with a mean follow-up of 10 years. J Periodontol. 2022;93:548–559. doi: 10.1002/JPER.21-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikami R., Mizutani K., Shioyama H., Matsuura T., Aoyama N., Suda T., et al. Influence of aging on periodontal regenerative therapy using enamel matrix derivative: a 3-year prospective cohort study. J Clin Periodontol. 2022;49(2):123–133. doi: 10.1111/jcpe.13552. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama Y., Matsuda H., Itoh S., Iwai Y., Takai H., Mezawa M., et al. Impact of adjunctive procedures on recombinant human fibroblast growth factor-2-mediated periodontal regeneration therapy: a retrospective study. J Periodontol. 2021;92:983–994. doi: 10.1002/JPER.20-0481. [DOI] [PubMed] [Google Scholar]
- 21.Hamp S.E., Nyman S., Lindhe J. Periodontal treatment of multirooted teeth. Results after 5 years. J Clin Periodontol. 1975;2:126–135. doi: 10.1111/j.1600-051x.1975.tb01734.x. [DOI] [PubMed] [Google Scholar]
- 22.Mizutani K., Shioyama H., Matsuura T., Mikami R., Takeda K., Izumi Y., et al. Periodontal regenerative therapy in type 2 diabetes patients using minimally invasive surgical technique with enamel matrix derivative under 3-year observation: a prospective cohort study. J Periodontol. 2021;92(9):1262–1273. doi: 10.1002/jper.20-0590. [DOI] [PubMed] [Google Scholar]
- 23.Steffensen B., Webert H.P. Relationship between the radiographic periodontal defect angle and healing after treatment. J Periodontol. 1989;60:248–254. doi: 10.1902/jop.1989.60.5.248. [DOI] [PubMed] [Google Scholar]
- 24.Tsitoura E., Tucker R., Suvan J., Laurell L., Cortellini P., Tonetti M. Baseline radiographic defect angle of the intrabony defect as a prognostic indicator in regenerative periodontal surgery with enamel matrix derivative. J Clin Periodontol. 2004;31:643–647. doi: 10.1111/j.1600-051X.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- 25.Cortellini P., Prato G.P., Tonetti M.S. The modified papilla preservation technique. A new surgical approach for interproximal regenerative procedures. J Periodontol. 1995;66:261–266. doi: 10.1902/jop.1995.66.4.261. [DOI] [PubMed] [Google Scholar]
- 26.Cortellini P., Prato G.P., Tonetti M.S. The simplified papilla preservation flap. A novel surgical approach for the management of soft tissues in regenerative procedures. Int J Periodontics Restor Dent. 1999;19:589–599. [PubMed] [Google Scholar]
- 27.Cortellini P., Tonetti M.S. Clinical concepts for regenerative therapy in intrabony defects. Periodontol. 2000 2015;68:282–307. doi: 10.1111/prd.12048. [DOI] [PubMed] [Google Scholar]
- 28.Li F., Yu F., Xu X., Li C., Huang D., Zhou X., et al. Evaluation of recombinant human FGF-2 and PDGF-BB in periodontal regeneration: a systematic review and meta-analysis. Sci Rep. 2017;7:65. doi: 10.1038/s41598-017-00113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nibali L., Sultan D., Arena C., Pelekos G., Lin G.H., Tonetti M. Periodontal infrabony defects: systematic review of healing by defect morphology following regenerative surgery. J Clin Periodontol. 2021;48:100–113. doi: 10.1111/jcpe.13381. [DOI] [PubMed] [Google Scholar]
- 30.Tonetti M.S., Pini-Prato G., Cortellini P. Periodontal regeneration of human intrabony defects. IV. Determinants of healing response. J Periodontol. 1993;64:934–940. doi: 10.1902/jop.1993.64.10.934. [DOI] [PubMed] [Google Scholar]
- 31.Cortellini P., Carnevale G., Sanz M., Tonetti M.S. Treatment of deep and shallow intrabony defects. A multicenter randomized controlled clinical trial. J Clin Periodontol. 1998;25:981–987. doi: 10.1111/j.1600-051x.1998.tb02402.x. [DOI] [PubMed] [Google Scholar]
- 32.Klein F., Kim T.S., Hassfeld S., Staehle H.J., Reitmeir P., Holle R., et al. Radiographic defect depth and width for prognosis and description of periodontal healing of infrabony defects. J Periodontol. 2001;72:1639–1646. doi: 10.1902/jop.2001.72.12.1639. [DOI] [PubMed] [Google Scholar]
- 33.Tonetti M.S., Prato G.P., Cortellini P. Factors affecting the healing response of intrabony defects following guided tissue regeneration and access flap surgery. J Clin Periodontol. 1996;23:548–556. doi: 10.1111/j.1600-051x.1996.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 34.Garrett S., Loos B., Chamberlain D., Egelberg J. Treatment of intraosseous periodontal defects with a combined adjunctive therapy of citric acid conditioning, bone grafting, and placement of collagenous membranes. J Clin Periodontol. 1988;15:383–389. doi: 10.1111/j.1600-051x.1988.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 35.Cosyn J., Cleymaet R., Hanselaer L., De Bruyn H. Regenerative periodontal therapy of infrabony defects using minimally invasive surgery and a collagen-enriched bovine-derived xenograft: a 1-year prospective study on clinical and aesthetic outcome. J Clin Periodontol. 2012;39:979–986. doi: 10.1111/j.1600-051X.2012.01924.x. [DOI] [PubMed] [Google Scholar]
- 36.Parashis A.O., Polychronopoulou A., Tsiklakis K., Tatakis D.N. Enamel matrix derivative in intrabony defects: prognostic parameters of clinical and radiographic treatment outcomes. J Periodontol. 2012;83:1346–1352. doi: 10.1902/jop.2012.110551. [DOI] [PubMed] [Google Scholar]