Summary
Ambient air pollutants are health hazards to children. This study comprised 773,504 emergency department visits (EDVs) at 0–14 years of age with respiratory diseases in southern China. All air pollutants were positively associated with EDVs of total respiratory diseases, especially pneumonia. NO2, PM10, and PM2.5 had intraday effects and cumulative effects on asthma EDVs. The effect of SO2, PM10, and PM2.5 on pneumonia EDVs was stronger in girls than in boys. The effect of NO2 on acute upper respiratory tract infection EDVs was greater in children aged 0–5 years old; however, the effect of PM10 on acute upper respiratory tract infection EDVs was greater in the 6–14 years group. In a two-pollutant model, NO2 was associated with bronchitis and pneumonia, and PM10 was associated with acute upper respiratory tract infection. In this time-series study, NO2 and PM10 were risk indicators for respiratory diseases in children.
Subject areas: Environment, Exposure, Atmospheric science, Environmental health, Pollution
Graphical abstract
Highlights
-
•
Air pollution associates with children emergency visits for respiratory diseases
-
•
NO2 and PM10 are risk indicators for respiratory diseases in children
-
•
Young children are more sensitive to gaseous pollutants
-
•
School-age children are more sensitive to PM10
Environment; Exposure; Atmospheric science; Environmental health; Pollution
Introduction
Air pollution has become an important public health issue for most cities around the world. Recently, a systematic analysis for the Global Burden of Disease in 204 countries and regions from 1990 to 2019 reported that, among children 0–9 years old, the largest increase in risk exposure was ambient particulate matter air pollution (GBD 2019 Risk Factors Collaborators 2020). There has been a pronounced increase in the literature on the adverse effects of ambient air pollution on children’s health (Lee 2021; Bates 1995).
Children are susceptible to the adverse health effects of air pollution due to their immature immune systems, their underdeveloped lung and metabolic systems, and the co-occurrence of infection with respiratory pathogens (Lee 2021). In addition, children are proportionally more exposed than adults to ambient air pollution because they have high ventilation rates and mouth breathing and spend more time outdoors for playing (Gouveia et al., 2018). The consequences of exposure to air pollution during early life include impaired lung function and an increased risk of respiratory illness (Xiao et al., 2016).
Evidence is mounting that ambient air pollution exposure is significantly associated with respiratory diseases in children (Goldizen et al., 2016). The prior studies have demonstrated a significant impact of air pollution on infants and children in terms of morbidity and mortality, which is manifested primarily as a range of respiratory problems (Gouveia et al., 2018; Jakubiak-Lasocka et al., 2015). In this regard, the findings from daily ambient air pollution concentrations and hospital admission counts showed that all ambient air pollutants were positively associated with pneumonia hospitalizations, and all pollutants other than carbon monoxide were positively associated with hospitalizations for bronchitis and asthma in children (Nhung et al., 2018). Exposure to fine particles (PM2.5) is also associated with outpatient visits for acute lower respiratory infections, pneumonia, and acute bronchiolitis/asthma of children under 5 years old (Davila Cordova et al., 2020). Childhood respiratory diseases may be partially preventable with better control of environmental contaminants (Hasunuma et al., 2014; Bayer-Oglesby et al., 2005).
Previous studies on air pollution and respiratory diseases were mainly concentrated in polluted western cities and northern industrial cities in China, such as Beijing, Jinan, Shijiazhuang, and Lanzhou (Zhang et al., 2019; Liu et al., 2019; Song et al., 2018; Ma et al., 2020). However, little has been documented about such effects in children in other cities where the pollution levels have received limited attention, especially less industrialized cities in the south of China. Furthermore, studies on outdoor air pollution and emergency department visits (EDVs), especially in children in Chinese cities, remain limited, although the associations between ambient air pollution and daily hospitalizations and outpatient visits have been well described (Liu et al., 2017; Zhang et al., 2019).
In the present study, we conducted a time-series study to investigate the association between five ambient air pollutants (sulfur dioxide [SO2], nitrogen dioxide [NO2], ozone [O3], inhalable particles [PM10], and PM2.5) and EDVs with respiratory diseases stratified by acute upper respiratory tract infection, bronchitis, pneumonia, and asthma in children in Guangzhou, southern China.
Results
Demographic characteristics
Table 1 shows the descriptive statistics for daily EDVs due to respiratory diseases in Guangzhou in the study period of 2012–2015. During the four-year study period, 773,504 respiratory disease emergency department encounters met our inclusion criteria. Among all encounters, patients were 58.5% (n = 452,106) boys and 41.5% (n = 321,398) girls, and they were 68.0% (n = 526,133) 0–5 years old and 32.0% (n = 247,371) 6–14 years old. There were 334,215 EDVs (boys: 57.2%; 0–5 years old: 63.8%) for acute upper respiratory tract infection, 127,874 EDVs (boys: 61.5%; 0–5 years old: 77.4%) for bronchitis, 16,403 EDVs (boys: 58.8%; 0–5 years old: 79.0%) for pneumonia, and 7,683 ED EDVs (boys: 68.9%; 0–5 years old: 27.3%) for asthma.
Table 1.
Descriptive statistics of daily emergency department visits of children with respiratory diseases in Guangzhou, 2012–2015
| Daily hospital admissions | Mean | SD | Min | P25 | Median | P75 | Max | Summary(%) |
|---|---|---|---|---|---|---|---|---|
| Total respiratory diseases | ||||||||
| Total | 529.4 | 215.4 | 107.0 | 373.0 | 496.0 | 667.0 | 1391.0 | 773504 |
| Boy | 309.4 | 124.6 | 56.0 | 221.0 | 290.0 | 390.0 | 808.0 | 452106(58.5%) |
| Girl | 220.0 | 92.2 | 41.0 | 152.0 | 204.0 | 278.0 | 606.0 | 321398(41.5%) |
| 0–5 years old | 360.1 | 147.2 | 67.0 | 253.0 | 335.0 | 459.0 | 918.0 | 526133(68.0%) |
| 6–14 years old | 169.3 | 77.4 | 40.0 | 111.0 | 156.0 | 207.0 | 534.0 | 247371(32.0%) |
| Acute upper respiratory infection | ||||||||
| Total | 228.8 | 109.6 | 44.0 | 146.0 | 206.0 | 291.0 | 736.0 | 334215 |
| Boy | 130.9 | 62.6 | 18.0 | 84.0 | 117.0 | 167.0 | 415.0 | 191228(57.2%) |
| Girl | 97.9 | 48.2 | 19.0 | 61.00 | 88.0 | 126.0 | 348.0 | 142987(42.8%) |
| 0–5 years old | 146.0 | 68.9 | 26.0 | 94.0 | 130.0 | 189.0 | 478.0 | 213377(63.8%) |
| 6–14 years old | 82.7 | 46.8 | 16.0 | 49.0 | 73.0 | 102.0 | 357.0 | 120838(36.2%) |
| Bronchitis | ||||||||
| Total | 87.5 | 40.1 | 8.0 | 60.0 | 82.0 | 110.0 | 244.0 | 127874 |
| Boy | 53.9 | 24.7 | 5.0 | 37.0 | 51.0 | 68.0 | 155.0 | 78674(61.5%) |
| Girl | 33.7 | 16.6 | 2.0 | 22.0 | 31.0 | 43.0 | 115.0 | 49200(38.5%) |
| 0–5 years old | 67.8 | 32.6 | 7.0 | 45.0 | 63.0 | 85.0 | 208.0 | 98989(77.4%) |
| 6–14 years old | 19.8 | 10.0 | 0.0 | 12.0 | 18.0 | 25.0 | 63.0 | 28885(22.6%) |
| Pneumonia | ||||||||
| Total | 11.23 | 6.74 | 0.00 | 6.00 | 10.00 | 15.00 | 46.00 | 16403 |
| Boy | 6.60 | 4.30 | 0.00 | 3.00 | 6.00 | 9.00 | 28.00 | 9643(58.8%) |
| Girl | 4.63 | 3.26 | 0.00 | 2.00 | 4.00 | 6.00 | 21.00 | 6760(41.2%) |
| 0–5 years old | 8.87 | 5.82 | 0.00 | 5.00 | 8.00 | 12.00 | 43.00 | 12960(79.0%) |
| 6–14 years old | 2.36 | 1.96 | 0.00 | 1.00 | 2.00 | 3.00 | 21.00 | 3443(21.0%) |
| Asthma | ||||||||
| Total | 5.26 | 3.36 | 0.00 | 3.00 | 5.00 | 7.00 | 24.00 | 7683 |
| Boy | 3.62 | 2.59 | 0.00 | 2.00 | 3.00 | 5.00 | 17.00 | 5290(68.9%) |
| Girl | 1.64 | 1.50 | 0.00 | 1.00 | 1.00 | 2.00 | 10.00 | 2393(31.1%) |
| 0–5 years old | 1.43 | 1.42 | 0.00 | 0.00 | 1.00 | 2.00 | 8.00 | 2094(27.3%) |
| 6–14 years old | 3.83 | 2.69 | 0.00 | 2.00 | 3.00 | 5.00 | 18.00 | 5589(72.7%) |
Air pollutants and meteorology
Table 2 summarizes levels of air pollutants, temperature, and relative humidity for the period 2012–2015 in Guangzhou. Over the entire study period, SO2, NO2, O3, PM10, and PM2.5 concentration averaged 18.0 μg/m3, 55.6 μg/m3, 79.9 μg/m3, 65.9 μg/m3, and 47.5 μg/m3, respectively. And daily mean temperature and relative humidity was 23.2°C and 78.0%.
Table 2.
Descriptive statistics of air pollutants and meteorological factors in Guangzhou, 2012–2015
| Data | Mean | SD | Min | P25 | Median | P75 | Max |
|---|---|---|---|---|---|---|---|
| Air pollutants concentrations | |||||||
| SO2 (μg/m3) | 18.0 | 9.3 | 1.5 | 11.5 | 16.3 | 23.0 | 55.5 |
| NO2 (μg/m3) | 55.6 | 22.5 | 10.3 | 39.0 | 51.0 | 67.8 | 169.0 |
| O3 (μg/m3) | 79.9 | 53.2 | 2.5 | 37.3 | 70.8 | 110.3 | 296.5 |
| PM10 (μg/m3) | 65.9 | 32.5 | 5.0 | 41.0 | 59.5 | 83.5 | 244.8 |
| PM2.5 (μg/m3) | 47.5 | 25.6 | 6.8 | 28.0 | 42.5 | 60.5 | 193 |
| Meteorologic measures | |||||||
| Temperature (°C) | 23.2 | 6.4 | 5.5 | 18.2 | 24.9 | 28.3 | 33.5 |
| Relative humidity (%) | 78.0 | 11.1 | 31.0 | 72.0 | 79.0 | 86.0 | 100.0 |
Table 3 presents the correlations among the air pollutants, temperature, and relative humidity, combined across all of the sites. Daily means of NO2, PM10, and PM2.5 were strongly correlated with each other (Pearson correlation coefficients ranged from 0.74 to 0.90). SO2 was relatively highly correlated with NO2/PM10/PM2.5 (r = 0.57, 0.58, and 0.55, respectively). Maximal 8-h mean O3 was weakly correlated with PM10 and PM2.5 (r = 0.25 and 0.19). The temporal correlations between air pollutants and meteorological factors were low and negatively (0.09 ≤ |r| ≤ 0.32), except O3 (r = 0.59 and −0.46).
Table 3.
Spearman’s correlations between air pollutants and meteorological factors in Guangzhou, 2012–2015
| SO2 | NO2 | O3 | PM10 | PM2.5 | Temperature | Relative humidity | |
|---|---|---|---|---|---|---|---|
| SO2 | 1.00 | 0.57a | 0.25a | 0.58a | 0.55a | 0.09a | −0.10a |
| NO2 | 1.00 | 0.01 | 0.74a | 0.76a | −0.32a | −0.02 | |
| O3 | 1.00 | 0.25a | 0.19a | 0.59a | −0.46a | ||
| PM10 | 1.00 | 0.90a | −0.17a | −0.29a | |||
| PM2.5 | 1.00 | −0.29a | −0.27a | ||||
| Temperature | 1.00 | 0.07a | |||||
| Relative humidity | 1.00 |
Significant difference (p < 0.01).
Single pollutant
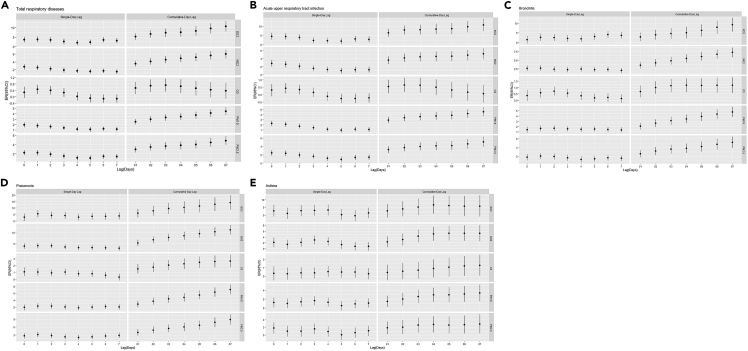
Figure 1 indicates the estimated effect of each pollutant on EDVs of total respiratory diseases, acute upper respiratory tract infection, bronchitis, pneumonia, and asthma in single-pollutant model and the specific values of ER and its 95% CI are shown in the additional material (Tables S1–S5). For both the single-day and cumulative-day lag effect, SO2, NO2, PM10, and PM2.5 had a positive and statistically significant association with total respiratory diseases, acute upper respiratory tract infection, and bronchitis EDVs (Figures 1A–1C). All air pollutants had significant associations with EDVs of pneumonia for both the single-day and cumulative-day lag effect, except O3 at lag 7 days (Figure 1D). NO2, PM10, and PM2.5 had a positive and statistically significant association with asthma EDVs at lag 0 days and for the cumulative-day lag effect (Figure 1E).
Figure 1.
The estimated effect of air pollutants on respiratory EDVs of total children in single-pollutant model
(A) total respiratory disease; (B) acute upper respiratory infection; (C) bronchitis; (D) pneumonia; (E) asthma; see also Tables S1–S5.
Gender difference
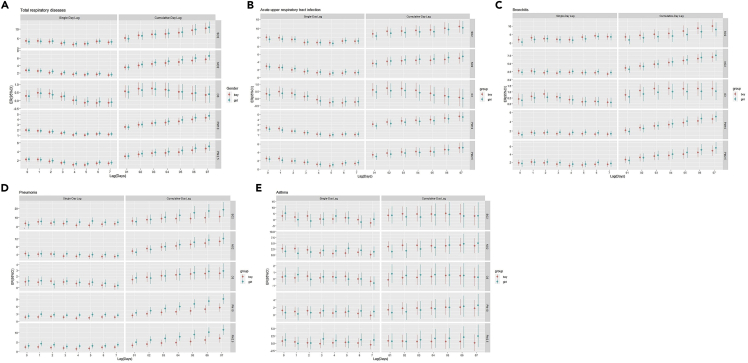
Figure 2 and Tables S6–S10 indicate the percentage changes for respiratory EDVs associated with per 10 μg/m3 increase of air pollutants varied by sex group. The effect estimates of NO2, PM10, and PM2.5 on total respiratory diseases in girls were slightly higher than in boys for the cumulative-day lag effect (Figure 2A). The effect estimates of SO2 and O3 on acute upper respiratory tract infection in boys were slightly higher than in girls for the cumulative-day lag effect (Figure 2B). The effect estimates of air pollutants on bronchitis for the cumulative-day lag effect, SO2, and O3 were slightly higher in boys than in girls; however, NO2, PM10, and PM2.5 were slightly higher in girls than in boys (Figure 2C). The effect estimates of SO2, PM10, and PM2.5 on pneumonia were significantly higher in girls than in boys for the cumulative-day lag effect (p < 0.05; Figure 2D). The effect estimates of NO2 on asthma were higher in boys than in girls for the cumulative-day lag effect (Figure 2E).
Figure 2.
Percentage changes for respiratory emergency room visits associated with per 10 μg/m3 increase in air pollutants by gender subgroup
(A) total respiratory disease; (B) acute upper respiratory infection; (C) bronchitis; (D) pneumonia; (E) asthma; see also Tables S6–S10.
Age difference
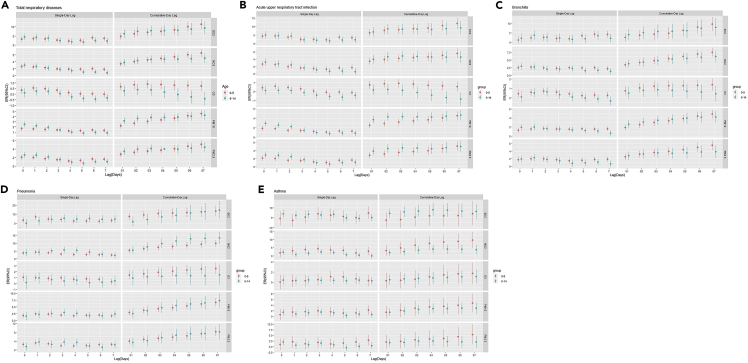
Figure 3 and Tables S11–S15 indicate the percentage changes for respiratory EDVs associated with per 10 μg/m3 increase of air pollutants varied by age group. The effect estimates of NO2 and O3 on total respiratory diseases and acute upper respiratory tract infection were significantly higher in the 0–5 years age group than in the 6–14 years age group, but PM10 was significantly higher in the 6–14 years age group than in the 0–5 years age group (p < 0.05; Figures 3A and 3B). The effect estimates of O3, PM10, and PM2.5 on bronchitis were significantly higher in the 0–5 years age group than in the 6–14 years age group at lag 7 days (p < 0.05; Figure 3C). The effect estimates of NO2 on pneumonia were significantly higher in the 6–14 years age group than in the 0–5 years age group at lag 4, lag 5, and lag 05 days (p < 0.05; Figure 3D). The effect estimates of NO2 on asthma were significantly higher in the 0–5 years age group than in the 6–14 years age group at lag 7 days (p < 0.05; Figure 3E).
Figure 3.
Percentage changes for respiratory emergency room visits associated with per 10 μg/m3 increase in air pollutants by age subgroup
(A) total respiratory disease; (B) acute upper respiratory infection; (C) bronchitis; (D) pneumonia; (E) asthma; see also Tables S11–S15.
Multiple-pollutant model
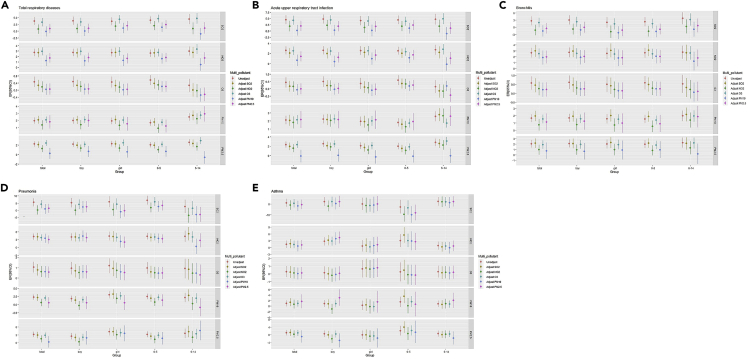
A multiple-pollutant model was fitted by selecting the optimal lag time of the pollutants for respiratory disease by different genders and age groups. Figure 4 and Tables S16–S20 present the percentage changes for respiratory EDVs associated with per 10 μg/m3 increase of air pollutants from two-pollutant models with adjustment for SO2, NO2, O3, PM10, and PM2.5. When introducing NO2, PM10, or PM2.5 into the single-pollutant model, the joint air pollution effects robust decreased, due to control for confounding. The joint effects of PM10 on total respiratory diseases were greater in the 6–14 years age group than in the 0–5 years age group (p < 0.05; Figure 4A). After adjusting for PM2.5, the effect estimates of O3 on acute upper respiratory tract infection remained statistically higher in the 0–5 years age group than in the 6–14 years age group (p < 0.05; Figure 4B). After adjusting for SO2 and NO2, the effect estimates of PM10 on acute upper respiratory tract infection remained statistically higher in the 6–14 years age group than in the 0–5 years age group (p < 0.05; Figure 4B). After adjusting for O3, the effect estimates of PM2.5 on acute upper respiratory tract infection were statistically higher in the 6–14 years age group than in the 0–5 years age group (p < 0.05; Figure 4B). After adjusting for O3, the effect estimates of PM10 on pneumonia remained statistically higher in girls than in boys (p < 0.05; Figure 4D). After adjusting for SO2, the effect estimates of PM2.5 on pneumonia remained statistically higher in girls than in boys (p < 0.05; Figure 4D). The joint effects of NO2 on asthma remained statistically higher in boys than in girls (p < 0.05; Figure 4E). After adjusting for SO2, the effect estimates of NO2, PM10, and PM2.5 on asthma were statistically higher in the 0–5 years age group than in the 6–14 years age group (p < 0.05; Figure 4E).
Figure 4.
For each 10 μg/m3 increase in pollutant concentration in the multiple-pollutant model, the ER (and 95% CI) of emergency visits for respiratory diseases of the total population aged 0–14, boy, girl, children aged 0–5 and 6–14 subgroup
(A) total respiratory disease; (B) acute upper respiratory infection; (C) bronchitis; (D) pneumonia; (E) asthma; see also Tables S16–S20.
Sensitivity analysis
Figure S1 and Table S21 illustrate the ER and 95% CI for every 10 μg/m3 increase in pollutant concentration from the models with varying degrees of freedom per year. Sensitivity analysis showed that the effect of O3 on the 6–14 years age group was still not statistically significant, which was consistent with the results of the O3 single-pollutant model. With an increase in the intensity of the smooth trend of the control time, the overall effect of pollutants showed a gradually decreasing trend, but the fluctuation was not large and remained statistically significant.
Discussion
This is the largest population-based study on the acute effects of ambient air pollutants on respiratory health in children in China to date as shown in Figure 5. All ambient air pollutants were positively associated with EDVs of total respiratory diseases, especially pneumonia. NO2, PM10, and PM2.5 had intraday effects and cumulative effects on asthma EDVs. Our findings suggest exposures to NO2, PM10, and PM2.5 may transiently increase risk of asthma EDVs in children within 24 h. The effect of SO2, PM10, and PM2.5 on pneumonia EDVs was stronger in girls than in boys. The effects of NO2 on acute upper respiratory tract infection EDVs were greater in the 0–5 years age group; however, the effect of PM10 on acute upper respiratory tract infection EDVs was greater in the 6–14 years age group. The significant effects of NO2 and PM10 remained in the two-pollutant models. In a two-pollutant model, NO2 was associated with bronchitis and pneumonia, and PM10 was associated with acute upper respiratory tract infection. Air pollution the day before an emergency call contributed most to the number of respiratory disease cases. Air pollution could successfully predict daily pediatric EDVs.
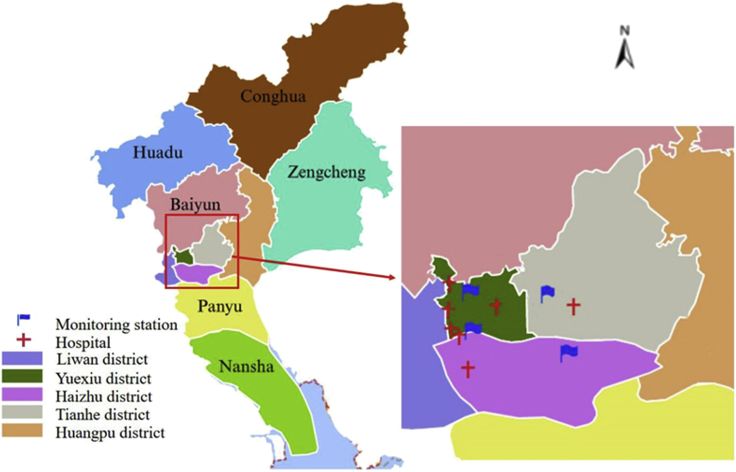
Figure 5.
Locations of 7 hospitals and 4 air pollution monitoring stations in Guangzhou, China in the study
It is worth noting that studies have demonstrated the effects of air pollutants on respiratory diseases at a specific location for public health assessments; therefore, the results might be affected by local air pollutant concentrations, components, climate conditions, and population susceptibility. The present study was conducted in children in Guangzhou, which is representative of the mild air pollution found in cities in southern China. The average concentrations of PM10 and PM2.5 were higher than the air quality guideline (AQG) recommendations of the Chinese government (65.9 vs. 50 μg/m3 and 47.5 vs. 35 μg/m3, respectively), while the concentrations of SO2 (18.0 μg/m3 vs. 50 μg/m3), NO2 (55.6 μg/m3 vs. 80 μg/m3), and O3 (79.9 μg/m3 vs. 100 μg/m3) reached the AQG standards. Compared to previous studies conducted in heavily polluted cities such as Shijiazhuang, Jinan, and Lanzhou, the average air pollutant concentration in Guangzhou was relatively low. An observed association between air pollution and EDVs was consistent with other studies that demonstrated an increased risk for respiratory disease associated with air pollution (Zhang et al., 2019; Liu et al., 2019; Song et al., 2018; Ma et al., 2020).
Epidemiological studies have consistently demonstrated the acute adverse health effects of air pollution exposure on EDVs for pediatric asthma (Liu et al., 2021; Mazenq et al., 2017; Hwang et al., 2017; Gleason et al., 2014). Some studies have demonstrated the main air pollutants associated with all-cause EDVs (Zhang et al., 2020; Chen et al., 2017a, 2017b) and respiratory disease EDVs (Song et al., 2021; Cheng et al., 2021a). Besides, we also investigated the effect estimates of SO2, NO2, O3, PM10, and PM2.5 on respiratory diseases with the stratification of diseases, such as acute upper respiratory tract infection, bronchitis, pneumonia, and asthma cases. Some differences were observed between air pollutants and diseases (Cheng et al., 2021b).
We found sensitivity for gender in the association between ambient air pollutants and EDVs in children in this study. Our study identified stronger associations of SO2, PM10, and PM2.5 with EDVs for pneumonia in girls than boys, which is consistent with published studies on the acute effects of ambient air pollution on outpatient visits for respiratory disease (Ma et al., 2020; Zhu et al., 2017). In several gender-specific analyses, boys and girls were both susceptible to air pollutants, and sex was not a significant modifier since no significant difference was observed between them (Zhou et al., 2019; Bai et al., 2018). In contrast to our findings, some prior studies have found that the risk of air pollution on respiratory hospital visits is higher in boys than that in girls (Liu et al., 2019; Luong et al., 2020).
In the age-stratified analysis, the impacts of NO2 on acute upper respiratory tract infection and asthma were more significant in children aged <5 years, but the impact of PM10 on acute upper respiratory tract infection was more significant in children aged 6–14 years. One possible reason is that children younger than 5 years old are more vulnerable to gas pollutants such as NO2 than older children. It is generally recognized that this high degree of vulnerability among younger children can be attributed to their immature lungs, higher breathing rate, and predominantly oral breathing characteristics, which increases their exposure and susceptibility to respiratory infections. These factors, combined with the underdeveloped immune function, may add together to make infants and younger children more susceptible to gas air pollutants (Ma et al., 2020). Children in the 6–14 years age group start to participate in more outdoor activities, and their awareness of self-protection against air pollution is still weak. They have higher ventilation rates and mouth breathing, which may pull PM10 deeper into children’s lungs and, in combination with an immature respiratory system, clearance of PM10 is slower and more difficult (Ma et al., 2020). It is also proposed that children aged 6–17 years are more vulnerable to PM2.5 exposure (Liu et al., 2019), and the effect of PM10 and PM2.5 on total respiratory hospital outpatients is higher in older children (Song et al., 2018).
Similar to previous studies, a certain lag effect and a cumulative effect of air pollutants on short-term effects with EDVs were revealed in the present study. The maximum effect occurred on the day or the day before EDVs. A 0–1 day lag effect of SO2, NO2, PM10, and PM2.5 on EDVs for total respiratory diseases was found. It has been reported that the maximum effects of NO2, SO2, and PM10 on the risk of hospital admissions for respiratory diseases occur on the day of admission (Phung et al., 2016). A non-linear artificial neural network model showed a statistically significant difference between PM2.5 exposure and hospital admission by 1 lag day (Polezer et al., 2018). In this study, we observed that a 10 μg/m3 increase in O3 daily concentrations increased the respiratory disease EDV daily numbers with 2, 3, 4, or 5 days cumulative effects (lag0–1, lag0–2, lag0–3, and lag0–4, respectively). Recently, a retrospective study also showed a positive association between the cumulative effects of 6 days (lag0–5) for O3 and lower respiratory diseases in children (Zhu et al., 2017).
It was not difficult to find that the cumulative risk estimate of each pollutant from the single-pollutant model was higher than that from the two-pollutant models. One general observation was the tendency of air pollutant estimates to shrink in two-pollutant models, due to the control for confounding. The finding of the very stable coefficients seen for NO2 and PM10 across all two-pollutant models is remarkable in the case of total respiratory diseases, whereas PM10 was significant for acute upper respiratory tract infection and NO2 for bronchitis and pneumonia. These rather stable NO2 and PM10 findings for respiratory diseases are a clear argument for the use of NO2 and PM10 as markers of ambient air pollution in health impact assessments for respiratory diseases in children.
Our study has four strengths. First, this is the largest sample size study to date on the impact of air pollution on children’s EDVs for respiratory diseases. Second, in this study, the diseases were classified in detail, i.e. acute upper respiratory tract infection, bronchitis, pneumonia, and asthma, whereas previous studies on EDVs mainly focused on asthma-related diseases or on all respiratory diseases. Third, in this study, air pollution data from multiple air monitoring points and emergency data from multiple medical centers were used. Fourth, this study analyzed air pollution and respiratory disease in a city without heavy air pollution in southern China.
In summary, this study shows that short-term exposure to ambient air pollutants significantly increases the risk of EDVs from total respiratory diseases in children, especially for pneumonia in girls. Children less than 5 years of age are more sensitive to gaseous pollutants like NO2, whereas older children are more vulnerable to respiratory damage by particulate matter such as PM10. Our results also demonstrate that NO2 and PM10 are indicators of air pollution risk, since the estimated effects of NO2 and PM10 were stable in the multiple-pollutant models. Precautions and protective measures and efforts to reduce exposure to air pollution should be strengthened, especially for children.
Limitations of the study
First, we cannot measure air pollution through personal air samples, and we did not take into account the impact of indoor air pollution. Second, the definition of outcomes in this study eventually relies on the diagnosis and ICD10 coding at the time of discharge, where misclassification of the type of respiratory disease might happen, particularly in the youngest age group. The varying magnitude of misclassifications of clinical diagnosis and ICD10 codes may have introduced bias.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| R software | The R Core Team | https://www.r-project.org/ |
| mgcv package | Mu et al. (2021) | https://rdocumentation.org/packages/mgcv/versions/1.8-31/topics/mgcv.package |
| ggplot2 package | Postma and Goedhart (2019) | https://rdocumentation.org/packages/ggplot2/versions/3.3.2 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Prof. Kefang Lai (klai@163.com).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Study area
Guangzhou, located in the Pearl River Delta (PRD), the capital of Guangdong Province, is the largest city in southern China and an important commercial and financial center with a population in the millions.
Method details
EDVs data
Data on EDVs were collected from seven large general top-level hospitals in Guangzhou between January 1, 2012 and December 31, 2015 (1,461 days). All seven hospitals were located in the densely populated central urban area of Guangzhou and provided 24-h accident and emergency services for residents. Each hospital has its own information system that recorded patient demographic information, home address, symptoms, diagnosis and the date of the emergency. From these hospitals, we obtained data on EDVs for children aged 0–14 years. We limited the study area to four urban districts in Guangzhou, so patients who lived outside these four urban districts were excluded from our study (Figure 5).
We checked the recorded symptoms for each case to further confirm its classification, and collected according to the International Classification of Disease revision 10 (ICD10) for diseases of the respiratory system (ICD10: J00∼J99). Cause-specific respiratory hospitalizations were identified based on the ICD-10 codes: acute upper respiratory tract infection (ICD-10: J00-J06), bronchitis (ICD-10: J20), pneumonia (ICD-10: J18) and asthma (ICD-10: J45). Unclear or undiagnostic cases were excluded.
Air pollution and meteorological data
Air pollution data for SO2, NO2, O3, PM10 and PM2.5 from January 1, 2012 to December 31, 2015 were obtained from the Guangzhou Environmental Monitoring Central Station, including four general monitoring stations that were scattered in the study area. The daily concentrations of air pollutants were calculated as the 24 h mean concentration, except for O3, which was calculated as the 8-h mobile mean concentration. During the study period of 1,461 days, there were 12 days (0.8%) with missing data for SO2, 15 days (1.0%) with missing data for NO2, 21 days (1.4%) with missing data for PM10 and PM2.5, and 25 days (1.7%) with missing data for O3. We accounted for the missing data with the linear interpolation method. We imputed missing data by replacing a missing concentration on a specific day with the average of the previous and next day values, if they are both available. If this was not the case, we replaced missing concentration with the first available value going back or forth. In this study, we used the composite (i.e., mean of the value for the four fixed monitoring stations) air pollutant concentrations for SO2, NO2, O3, PM10 and PM2.5.
Meteorological data, including daily mean temperature (°C) and relative humidity (RH, %) were obtained for the same study period from the National Oceanic and Atmospheric Administration (NOAA).
Quantification and statistical analysis
A generalized additive model (GAM), based on a quasi-Poisson distribution, was used to perform a time series analysis on EDVs for respiratory diseases. Natural cubic spline (ns) functions were applied to control for time trends, as well as weather elements (i.e., daily mean temperature and RH). The core model also adjusted for public holidays and the day of the week (DOW). In this study, by changing the degrees of freedom of the natural cubic spline function, observing the change of image and combining with the Akaike’s information criterion (AIC) value, we could comprehensively judge the degrees of freedom (df). So, a natural cubic spline with 7 df per year was used to control for seasons and long-term trends, and a df of 6 and 5 was used for temperature and RH, respectively. Public holidays and DOW were used as dummy variables. This model is described as follows:
where Yt is the daily count of EDVs for respiratory on day t; X is the daily concentration of a given air pollutant; β is regression coefficient; ns is the natural cubic spline; time indicates long-term trends and seasonality using calendar time (days); humidity is the daily mean relative humidity; temp is the daily mean temperature.
According to the regression coefficient (β) obtained by the model, the excess risk (ER) was reported as a percentage increase with a 95% confidence interval (95% CI) in daily EDVs for a 10 μg/m3 increase in air pollution concentrations. The equations of ER and 95%CI are shown below:
where se is the standard error.
Subgroup analysis was conducted according to gender or age (0–5 years old or 6–14 years old). The data of each subgroup was introduced into the model, and the effects of air pollutants on different gender and age subgroups were obtained. In order to verify whether there were statistically significant differences between subgroups, the 95%CI for the differences among subgroups were calculated by the following equation:
Here, Q(ˆ)1 and Q(ˆ)2 refer to the estimates for the two categories (e.g., boys and girls), and SE(ˆ)1 and SE(ˆ)2 refer to standard errors.
Finally, we conducted three analyses to explore the sensitivity of main results. First, the lag effects of air pollutants were performed with different lag days, including single-day lags (from lag 0 to lag 7) and cumulative-day lags (from lag 01 to lag 07). Single-day lags were air pollutant concentrations of the same day (lag 0) and the past one to seven days (lag 1–7). Cumulative-day lags were moving-average air pollutant concentrations of the same day and the previous days; for example, lag 02 indicated air pollutant concentrations of a three-day moving-average of that day and the previous two days. Second, two-pollutant models were fitted to evaluate the confounding effects of SO2, NO2, O3, PM10 and PM2.5. In the multiple-pollutant models, the pollutant corresponding to the maximum single-lag day is used as the research variable, and other pollutants with the same lag time are introduced. Third, we used alternative df for calendar days (5–9 per year).
Before conducting the model analyses, Spearman’s rank correlation analysis was used to identify the correlation of different air pollutants and meteorological factors. All the statistical analyses were conducted with R, version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) and the “mgcv” package. The results of the statistical tests were two-sided, with values of p < 0.05 considered statistically significant.
Acknowledgments
This work was supported by the Open Project of State Key Laboratory of Respiratory Disease (No. SKLRD20160P010 (K.L.)); the Incubative Project for Innovation Team of Guangzhou Medical University (No. 2017-159 (K.L.)).
Author contributions
M.H. Conceptualization, Methodology, Formal analysis, Data curation, Writing - original draft. Y.Z. Methodology, Validation, Writing - original draft. Y.C. Validation, Data curation, Writing - review & editing. N.Z. Writing - review & editing. K.L. Conceptualization, Funding acquisition, Project administration, Writing - review & editing.
Declaration of interests
The authors declare no conflicts of interest or financial interests.
Published: September 16, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.104879.
Supplemental information
Data and code availability
Data: The authors declare that all data supporting the findings of this study are available within the article or available from the corresponding author upon reasonable request.
Code: This study did not generate any code.
Other items: Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Bates D.V. The effects of air pollution on children. Environ. Health Perspect. 1995;103(Suppl 6):49–53. doi: 10.1289/ehp.95103s649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer-Oglesby L., Grize L., Gassner M., Takken-Sahli K., Sennhauser F.H., Neu U., Schindler C., Braun-Fahrländer C. Decline of ambient air pollution levels and improved respiratory health in Swiss children. Environ. Health Perspect. 2005;113:1632–1637. doi: 10.1289/ehp.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L., Su X., Zhao D., Zhang Y., Cheng Q., Zhang H., Wang S., Xie M., Su H. Exposure to traffic-related air pollution and acute bronchitis in children: season and age as modifiers. J. Epidemiol. Community Health. 2018;72:426–433. doi: 10.1136/jech-2017-209948. [DOI] [PubMed] [Google Scholar]
- Chen G., Li S., Zhang Y., Zhang W., Li D., Wei X., He Y., Bell M.L., Williams G., Marks G.B., et al. Effects of ambient PM(1) air pollution on daily emergency hospital visits in China: an epidemiological study. Lancet Planet. Health. 2017;1:e221–e229. doi: 10.1016/S2542-5196(17)30100-6. [DOI] [PubMed] [Google Scholar]
- Chen G., Zhang Y., Zhang W., Li S., Williams G., Marks G.B., Jalaludin B., Abramson M.J., Luo F., Yang D., et al. Attributable risks of emergency hospital visits due to air pollutants in China: a multi-city study. Environ. Pollut. 2017;228:43–49. doi: 10.1016/j.envpol.2017.05.026. [DOI] [PubMed] [Google Scholar]
- Cheng B., Ma Y., Wang H., Shen J., Zhang Y., Guo L., Guo Y., Li M. Particulate matter pollution and emergency room visits for respiratory diseases in a valley Basin city of Northwest China. Environ. Geochem. Health. 2021;43:3457–3468. doi: 10.1007/s10653-021-00837-x. [DOI] [PubMed] [Google Scholar]
- Cheng J., Su H., Xu Z. Intraday effects of outdoor air pollution on acute upper and lower respiratory infections in Australian children. Environ. Pollut. 2021;268(Pt A) doi: 10.1016/j.envpol.2020.115698. [DOI] [PubMed] [Google Scholar]
- Davila Cordova J.E., Tapia Aguirre V., Vasquez Apestegui V., Ordoñez Ibarguen L., Vu B.N., Steenland K., Gonzales G.F. Association of PM2.5 concentration with health center outpatient visits for respiratory diseases of children under 5 years old in Lima, Peru. Environ. Health. 2020;19:7. doi: 10.1186/s12940-020-0564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2019 Risk Factors Collaborators Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldizen F.C., Sly P.D., Knibbs L.D. Respiratory effects of air pollution on children. Pediatr. Pulmonol. 2016;51:94–108. doi: 10.1002/ppul.23262. [DOI] [PubMed] [Google Scholar]
- Gouveia N., Junger W.L., ESCALA investigators Effects of air pollution on infant and children respiratory mortality in four large Latin-American cities. Environ. Pollut. 2018;232:385–391. doi: 10.1016/j.envpol.2017.08.125. [DOI] [PubMed] [Google Scholar]
- Gleason J.A., Bielory L., Fagliano J.A. Associations between ozone, PM2.5, and four pollen types on emergency department pediatric asthma events during the warm season in New Jersey: a case-crossover study. Environ. Res. 2014;132:421–429. doi: 10.1016/j.envres.2014.03.035. [DOI] [PubMed] [Google Scholar]
- Hasunuma H., Ishimaru Y., Yoda Y., Shima M. Decline of ambient air pollution levels due to measures to control automobile emissions and effects on the prevalence of respiratory and allergic disorders among children in Japan. Environ. Res. 2014;131:111–118. doi: 10.1016/j.envres.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Hwang S.L., Lin Y.C., Lin C.M., Hsiao K.Y. Effects of fine particulate matter and its constituents on emergency room visits for asthma in southern Taiwan during 2008-2010: a population-based study. Environ. Sci. Pollut. Res. Int. 2017;24:15012–15021. doi: 10.1007/s11356-017-9121-3. [DOI] [PubMed] [Google Scholar]
- Jakubiak-Lasocka J., Lasocki J., Badyda A.J. The influence of particulate matter on respiratory morbidity and mortality in children and infants. Adv. Exp. Med. Biol. 2015;849:39–48. doi: 10.1007/5584_2014_93. [DOI] [PubMed] [Google Scholar]
- Lee J.T. Review of epidemiological studies on air pollution and health effects in children. Clin. Exp. Pediatr. 2021;64:3–11. doi: 10.3345/cep.2019.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li Y., Li J., Liu Y., Tao N., Song W., Cui L., Li H. Association between ambient PM2.5 and children's hospital admissions for respiratory diseases in Jinan, China. Environ. Sci. Pollut. Res. Int. 2019;26:24112–24120. doi: 10.1007/s11356-019-05644-7. [DOI] [PubMed] [Google Scholar]
- Liu L., Liu C., Chen R., Zhou Y., Meng X., Hong J., Cao L., Lu Y., Dong X., Xia M., et al. Associations of short-term exposure to air pollution and emergency department visits for pediatric asthma in Shanghai, China. Chemosphere. 2021;263 doi: 10.1016/j.chemosphere.2020.127856. [DOI] [PubMed] [Google Scholar]
- Liu Y., Xie S., Yu Q., Huo X., Ming X., Wang J., Zhou Y., Peng Z., Zhang H., Cui X., et al. Short-term effects of ambient air pollution on pediatric outpatient visits for respiratory diseases in Yichang city, China. Environ. Pollut. 2017;227:116–124. doi: 10.1016/j.envpol.2017.04.029. [DOI] [PubMed] [Google Scholar]
- Luong L.T.M., Dang T.N., Thanh Huong N.T., Phung D., Tran L.K., Van Dung D., Thai P.K. Particulate air pollution in Ho Chi Minh city and risk of hospital admission for acute lower respiratory infection (ALRI) among young children. Environ. Pollut. 2020;257 doi: 10.1016/j.envpol.2019.113424. [DOI] [PubMed] [Google Scholar]
- Ma Y., Yue L., Liu J., He X., Li L., Niu J., Luo B. Association of air pollution with outpatient visits for respiratory diseases of children in an ex-heavily polluted Northwestern city, China. BMC Publ. Health. 2020;20:816. doi: 10.1186/s12889-020-08933-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazenq J., Dubus J.C., Gaudart J., Charpin D., Nougairede A., Viudes G., Noel G. Air pollution and children's asthma-related emergency hospital visits in southeastern France. Eur. J. Pediatr. 2017;176:705–711. doi: 10.1007/s00431-017-2900-5. [DOI] [PubMed] [Google Scholar]
- Mu J., Zeng D., Fan J., Liu M., Yu S., Ding W., Zhang S. Associations between air pollution exposure and daily pediatric outpatient visits for dry eye disease: a time-series study in Shenzhen, China. Int. J. Public Health. 2021;66 doi: 10.3389/ijph.2021.1604235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nhung N.T.T., Schindler C., Dien T.M., Probst-Hensch N., Perez L., Künzli N. Acute effects of ambient air pollution on lower respiratory infections in Hanoi children: an eight-year time series study. Environ. Int. 2018;110:139–148. doi: 10.1016/j.envint.2017.10.024. [DOI] [PubMed] [Google Scholar]
- Phung D., Hien T.T., Linh H.N., Luong L.M.T., Morawska L., Chu C., Binh N.D., Thai P.K. Air pollution and risk of respiratory and cardiovascular hospitalizations in the most populous city in Vietnam. Sci. Total Environ. 2016;557–558:322–330. doi: 10.1016/j.scitotenv.2016.03.070. [DOI] [PubMed] [Google Scholar]
- Polezer G., Tadano Y.S., Siqueira H.V., Godoi A.F.L., Yamamoto C.I., de André P.A., Pauliquevis T., Andrade M.d.F., Oliveira A., Saldiva P.H.N., et al. Assessing the impact of PM2.5 on respiratory disease using artificial neural networks. Environ. Pollut. 2018;235:394–403. doi: 10.1016/j.envpol.2017.12.111. [DOI] [PubMed] [Google Scholar]
- Postma M., Goedhart J. PlotsOfData-A web app for visualizing data together with their summaries. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Lu M., Zheng L., Liu Y., Xu P., Li Y., Xu D., Wu W. Acute effects of ambient air pollution on outpatient children with respiratory diseases in Shijiazhuang, China. BMC Pulm. Med. 2018;18:150. doi: 10.1186/s12890-018-0716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Jiang L., Wang S., Tian J., Yang K., Wang X., Guan H., Zhang N. The impact of main air pollutants on respiratory emergency department visits and the modification effects of temperature in Beijing, China. Environ. Sci. Pollut. Res. Int. 2021;28:6990–7000. doi: 10.1007/s11356-020-10949-z. [DOI] [PubMed] [Google Scholar]
- Xiao Q., Liu Y., Mulholland J.A., Russell A.G., Darrow L.A., Tolbert P.E., Strickland M.J. Pediatric emergency department visits and ambient Air pollution in the U.S. State of Georgia: a case-crossover study. Environ. Health. 2016;15:115. doi: 10.1186/s12940-016-0196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Morisaki H., Wei Y., Li Z., Yang L., Zhou Q., Zhang X., Xing W., Hu M., Shima M., et al. Characteristics of air pollutants inside and outside a primary school classroom in Beijing and respiratory health impact on children. Environ. Pollut. 2019;255(Pt 1) doi: 10.1016/j.envpol.2019.113147. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Fang J., Mao F., Ding Z., Xiang Q., Wang W. Age- and season-specific effects of ambient particles (PM1, PM2.5, and PM10) on daily emergency department visits among two Chinese metropolitan populations. Chemosphere. 2020;246 doi: 10.1016/j.chemosphere.2019.125723. [DOI] [PubMed] [Google Scholar]
- Zhu L., Ge X., Chen Y., Zeng X., Pan W., Zhang X., Ben S., Yuan Q., Xin J., Shao W., et al. Short-term effects of ambient air pollution and childhood lower respiratory diseases. Sci. Rep. 2017;7:4414. doi: 10.1038/s41598-017-04310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Wang T., Zhou F., Liu Y., Zhao W., Wang X., Chen H., Cui Y. Ambient air pollution and daily hospital admissions for respiratory disease in children in Guiyang, China. Front. Pediatr. 2019;7:400. doi: 10.3389/fped.2019.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data: The authors declare that all data supporting the findings of this study are available within the article or available from the corresponding author upon reasonable request.
Code: This study did not generate any code.
Other items: Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.