Abstract
Close follow-up of patients with liver cirrhosis has led to increased detection of hepatocellular carcinoma (HCC) at an early stage, especially with magnetic resonance imaging (MRI) innovations. We report the case of a 70-year-old man, with a recent history of liver cirrhosis due to chronic hepatitis C virus (HCV) complicated by hepatocellular carcinoma (HCC), and for whom trans-arterial chemoembolization (TACE) was planned, as the patient was assigned Child B7 at admission. Angiography performed during the first TACE cycle shows not only the “tumor blush” corresponding to previously detected HCC but also an additional small foci of HCC uptake seen within a large dysplastic nodule giving the appearance of “nodule-within-nodule.” Early detection of hepatocellular carcinoma improves prognosis. Hence, it is essential to be aware of all early aspects of HCC, including the nodule-within-nodule appearance on cross-sectional imaging, and also in angiography, as in this case.
Keywords: Nodule-within-nodule, Early HCC, Dysplastic nodule, Chemoembolization
Abbreviations: HCV, hepatitis C virus; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; MRI, magnetic resonance imaging; TACE, trans-arterial chemoembolization; BP, blood pressure; PR, Pulse rate; RR, respiratory rate; AST, aspartate aminotransferase; ALT, Alanine amino-transferase; NR, normal range; PT, prothrombin time; LDH, lactate dehydrogenase; Alpha-FP, alpha-fetoprotein; CBC, complete blood count; HIV, human immunodeficiency virus; DSA, Digital subtraction angiography; RAHA, right anterior hepatic artery; HCV-Ab, HCV antibodies. T1-WI, T1-weighted images; T2-WI, T2-weighted images
Introduction
Hepatocellular carcinoma (HCC) is the most common primary cancer of the liver [1]. Unlike other tumors that arise in a normal tissue background, HCC occurs in the context of a change in the liver field, characterized by the replacement of liver parenchyma by fibrosis, scarring, and nodular regeneration [2]. Therefore, hepatocarcinogenesis should be viewed as a continuum, rather than a series of discrete states [2].
Hepatocellular carcinoma differs from most neoplasms by the fact that it is often diagnosed on the basis of imaging features alone, due to the early enhancement of these tumors, with a portal and late washout, so that an early diagnosis can be made by abdominal cross-sectional imaging [3]. The particularity of our case is the detection of an early HCC within a dysplastic nodule during TACE of an already known HCC. this finding was subsequently confirmed by a liver MRI.
Case presentation
A 70-year-old man, known to be hypertensive and diabetic type 2 on metformin, was referred to the gastroenterology department for investigation of liver cirrhosis which was discovered during gallbladder cure surgery a month ago. His overall condition on admission was generally good; the temperature was 36.4 (oral), blood pressure (BP) of 135/75, pulse rate (PR) of 69, and respiratory rate (RR) of 15. There was no anemia, jaundice, or lower-limb edema. Abdominal examination revealed a surgical scar in right hypochondrium, soft abdomen on palpation, and flank dullness on percussion. The liver and spleen were not palpable.
The Laboratory investigations revealed a modest increase in liver function tests as aspartate aminotransferase (AST) level of 109 IU/L (2.7x N), Alanine amino-transferase (ALT) level of 77 IU/L (1.4xN), total bilirubin of 9 micormol/l (normal range NR: 5.1 and 17 µmol/l), direct bilirubin of 6 mmol/L (NR < 5.1 µmol/l), alkaline phosphatase of 170 IU/L (NR: 68-220 IU/L) and serum albumin of 29 mg/l (NR:35 to 55 mg/l). Prothrombin Time (PT) was at 78% (NR: > 85%), lactate dehydrogenase (LDH) increased at 848 IU/L (NR: 180-430 IU/L). Alpha-fetoprotein was normal at 7.6 (NR: 10-20 ng/ml), such as complete blood count (CBC), renal function tests, and hydro-electrolytic tests.
Serological tests for hepatitis B and HIV were negative, while HCV antibodies (HCV-Ab) and HCV-RNA were positive (viral load of 6,222,570 copies/ml). Ascites puncture did not reveal any infection of the ascitic fluid and the ascites protein level was decreased to 12 g/l (NR>25). A nasofibroscopy was made revealing grade 1 esophageal varices with portal hypertensive gastropathy.
Abdominal ultrasonography showed global atrophy of the liver with parenchymal heterogeneity and irregular liver surface, it showed also a low echoic lesion of about 20 mm in diameter at the junction of VII-VIII segments. On MRI this lesion exhibited typical features relative to an HCC. On the basis of the clinical and biological results and according to the Child-Pugh classification, the patient was graded B7, which makes him a good candidate for super-selective chemoembolization.
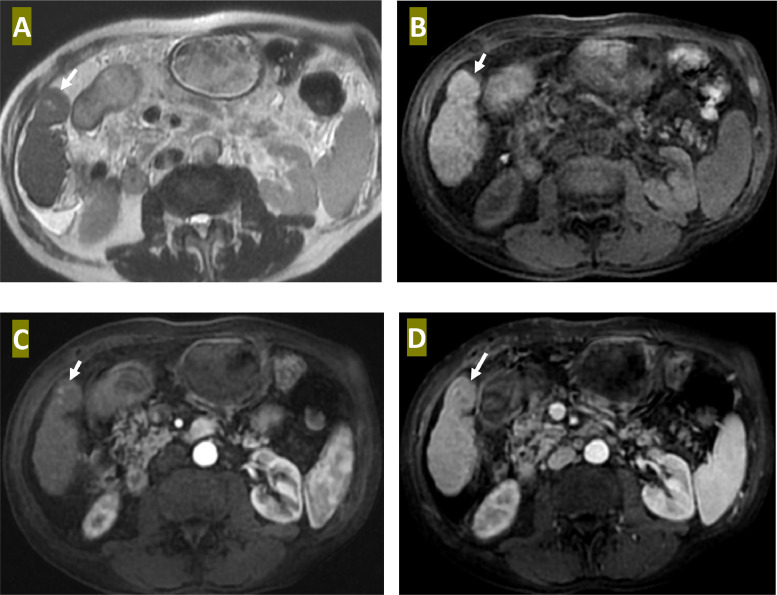
Angiography during TACE revealed that there was not only the well-established HCC but also a foci of small uptake within a large dysplastic nodule (Fig. 1). These angiographic findings were also evident on abdominal MRI (Fig. 2).
Fig. 1.
DSA during TACE. Selective angiography of the anterior branch of the right anterior hepatic artery (RAHA) revealed a tumor blush in segment VIII as a progressive increased arterial uptake, compatible with well-known HCC (circle 1). Other foci of increased uptake in segment V (circle 3) were seen within a dysplastic nodule (circle 2).
Fig. 2.
Liver MRI: Axial T2 WI (A), Axial T1 WI (B), Arterial phase axial T1 WI (C) a, and portal venous phase axial T1 WI (D) reveal a lesion of isosignal to parenchymal liver on T1 and T2WI (arrow) with visualization of 2 small foci of high T2 signal within it. These 2 lesions exhibit intense enhancement in arterial phase with a washout at portal phase, while the dysplastic nodule is always isosignal on all angio-MR phases.
The patient was embolized with a mixture containing an anticancer agent (doxorubicin) and lipiodol using the Seldinger technique, and a follow-up CT scan was made 6 weeks later demonstrating significant lipiodol fixation relative to a good therapeutic response. There was no new suspect lesion elsewhere (Fig. 3).
Fig. 3.
Noncontrast axial CT performed 6 weeks after chemoembolization shows lipiodol uptake in the HCC of segment VIII (circle), and a small uptake within the nodule of segment VI (arrow).
Both small and well-known HCCs have been well controlled for 1 year and 6 months post first TACE cycle, without notable complications.
Discussion
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world, and its incidence is growing in many countries [1]. The leading risk factor for the development of HCC is liver cirrhosis, especially those related to chronic viral hepatitis. (HVC/HBC) [1].
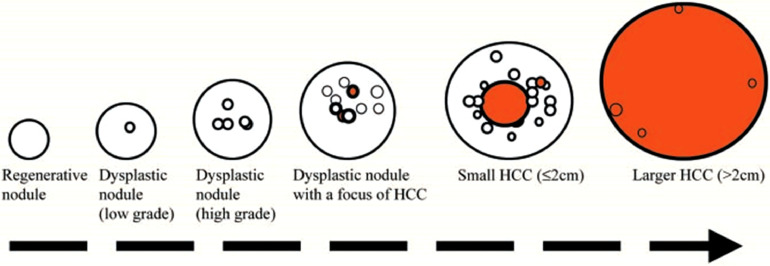
The cirrhotic liver contains regenerative nodules as well as dysplastic nodules and hepatocellular carcinoma [2]. Understanding the progression from benign to dysplastic and malignant nodules makes it easier to understand the complexity of these nodules described in cirrhotic livers, especially with pulse sequences of resonance imaging (MR) [3] (Fig. 4).
Fig. 4.
Stepwise pathway of carcinogenesis for HCC in cirrhosis. One or more regenerative nodules may show signs of atypia (O) and change into dysplastic nodules. Atypia within dysplastic nodules can progress further and give rise to small and large HCCs ().
At some stage during the carcinogenesis process of HCC (most likely when regenerative nodules transform into dysplastic nodules), the formation of new tumor vessels (tumor angiogenesis, neovascularization) occurs [3]. This neovascularization is very important not only in the transformation of regenerative nodules into dysplastic nodules and small HCC but also for the sustained growth of HCC. In addition, neovascularization within HCC can be used for early detection and characterization of these lesions by imaging [4].
Imaging features
Generally, regenerative nodules exhibit a low signal on T2-WI, a variable signal on T1-WI, and no enhancement in the arterial phase of dynamic MRI [4]. HCC is a focal liver lesion with high signal intensity on T2-WI and variable signal intensity on T1-WI; it shows intense enhancement during arterial phase and wash-out in portal and/or late phases [4,5].
The signal intensity and enhancement features of dysplastic nodules are not well established. Due to the gradual transition from a regenerative nodule to a low-grade dysplastic nodule, to a high-grade dysplastic nodule, and finally, to a small HCC and a large HCC, the hepatocytes within liver nodules are undergoing many changes that may not be reflected in their signal intensity or vascularity. Hence, MR imaging sequences cannot reliably distinguish between regenerative and dysplastic nodules [5,6].
High-grade dysplastic nodules and small HCC may look like a nodule within a nodule.
On MRI, especially if a focus of HCC arises from a regenerative nodule or a low-grade dysplastic nodule. On T2-weighted images, it may be a large nodule with low signal intensity, with one or more internal foci with higher signal intensity. On T1-wi images, these lesions typically show the clearly low signal intensity of a large nodule, with internal foci that are isointense to the liver [5].
On MR imaging, it is important to recognize HCC when it is still small because the tumor is aggressive and has a rapid doubling time [5,7].
Treatment
Surgical resection for early HCC is associated with a 5-year survival and recurrence-free survival rates of 93% and 47%, respectively [8]. The survival rate of patients with early HCC is 54%, which is significantly better than for patients with conventional HCC. In contrast, TACE is not a good indication for early HCC as no necrosis of lesions is histopathologically confirmed [8]. This is mainly due to immature neovascularization within the lesions. For nodule-in-nodule type HCC, quick treatment is required as it proceeds rapidly to advanced HCC [9].
Conclusion
Only a few cases are reported in the literature concerning the development of early HCC within a regenerating or dysplastic nodule known as the “nodule-within-nodule” pattern. With the innovation of MRI, more cases will be reported that can be grouped into case series and thus we can anticipate benign nodules that have imaging features that can predict a later malignant transformation and thus require close surveillance.
Our case has also the particularity of illustrating the interest in initial angiogram before embolization, which shows foci of abnormal uptake initially visible on the cross-sectional imaging.
Acknowledgments
Patient consent
I, the author of the article “Early hepatocellular carcinoma developed within dysplastic nodule as nodule-within-nodule appearance: Case report with literature review” approve that the patient gives her consent for information be to published in Radiology Case Reports.
Footnotes
Competing Interests: The authors declare no conflicts.
References
- 1.Takahashi H, Mori K, Sekino Y, Okumura T, Hiyama T, Fukuda K, et al. Angiographic findings in patients with hepatocellular carcinoma previously treated using proton beam therapy. J Oncol. 2019;2019 doi: 10.1155/2019/3580379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEvoy SH, McCarthy CJ, Lavelle LP, Moran DE, Cantwell CP, Skehan SJ, et al. Hepatocellular carcinoma: illustrated guide to systematic radiologic diagnosis and staging according to guidelines of the American Association for the Study of Liver Diseases. Radiographics. 2013;33(6):1653–1668. doi: 10.1148/rg.336125104. [DOI] [PubMed] [Google Scholar]
- 3.Hussain SM, Zondervan PE, IJzermans JNM, Schalm SW, de Man RA, Krestin GP, et al. Benign versus malignant hepatic nodules: MR imaging findings with pathologic correlation. Radiographics. 2002;22(5):1023–1036. doi: 10.1148/radiographics.22.5.g02se061023. [DOI] [PubMed] [Google Scholar]
- 4.Takayasu K, Muramatsu Y, Mizuguchi Y, Moriyama N, Ojima H. Imaging of early hepatocellular carcinoma and adenomatous hyperplasia (dysplastic nodules) with dynamic CT and a combination of CT and angiography: experience with resected liver specimens. Intervirology. 2004;47.3-5:199–208. doi: 10.1159/000078473. [DOI] [PubMed] [Google Scholar]
- 5.Hussain SM., Semelka RC, Mitchell DG. MR imaging of hepatocellular carcinoma. Magn Reson Imaging Clin. 2002;10.1:31–52. doi: 10.1016/s1064-9689(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 6.Choi B.I., Takayasu K, Han MC. Small hepatocellular carcinomas and associated nodular lesions of the liver: pathology, pathogenesis, and imaging findings. AJR Am J Roentgenol. 1993;160.6:1177–1187. doi: 10.2214/ajr.160.6.8388618. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell D.G., Rubin R, Siegelma, E.S, Burk Jr, Rifkin M. Hepatocellular carcinoma within siderotic regenerative nodules: appearance as a nodule within a nodule on MR images. Radiology. 1991;178.1:101–103. doi: 10.1148/radiology.178.1.1845784. [DOI] [PubMed] [Google Scholar]
- 8.Takayasu K, et al. Response of early-stage hepatocellular carcinoma and borderline lesions to therapeutic arterial embolization. AJR Am J Roentgenol. 1993;160.2:301–306. doi: 10.2214/ajr.160.2.8380949. [DOI] [PubMed] [Google Scholar]
- 9.Sadek AG, Mitchell D.G, Siegelman E.S, Outwater E.K, Matteucci T, Hann H.W, et al. Early hepatocellular carcinoma that develops within macroregenerative nodules: growth rate depicted at serial MR imaging. Radiology. 1995;195.3:753–756. doi: 10.1148/radiology.195.3.7754006. [DOI] [PubMed] [Google Scholar]